Chapter 3 Implant Basics
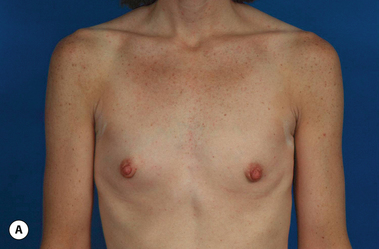
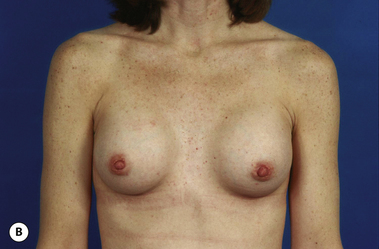
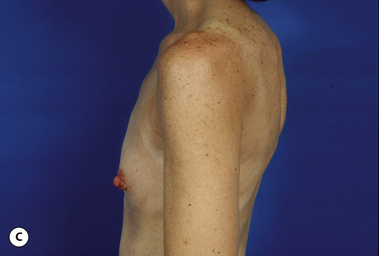
There are many factors that combine to determine the quality of the aesthetic result obtained when an implant is placed under the breast. Some of these factors are relatively fixed and are therefore subject to limited control by the surgeon. Perhaps the most important variable that falls into this category is the nature of the pre-existing soft tissue framework of the breast. However, one variable that is most decidedly under the control of the surgeon is the nature of the implant that is chosen to perform the procedure. By intelligently choosing the correct combination of implant characteristics, the results obtained after breast augmentation can be optimized as much as possible. Therefore, while other decisions that are made when planning a breast augmentation such as incision location and pocket placement are without question of great importance, choosing the ‘right’ breast implant is one of the most critical decisions to be made in breast augmentation.
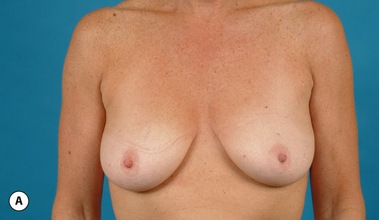
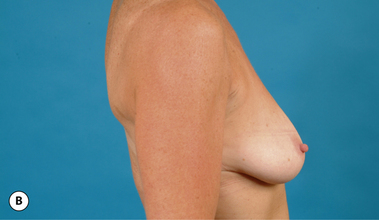
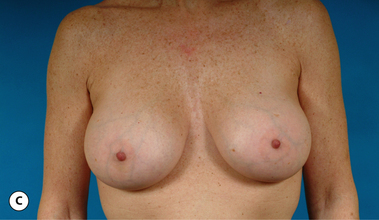
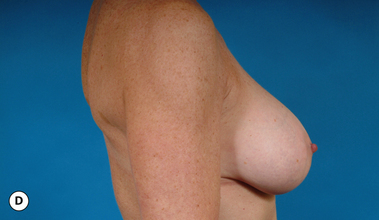
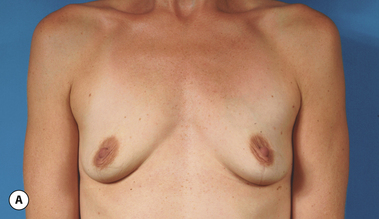
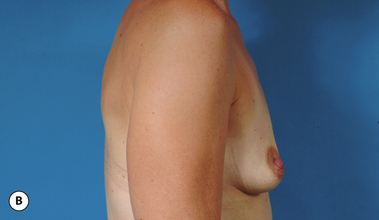
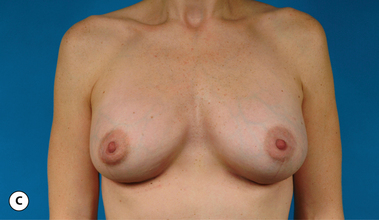
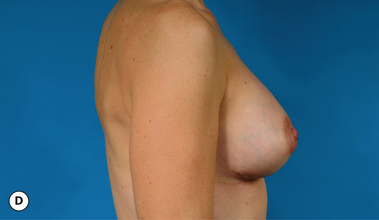
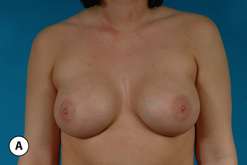
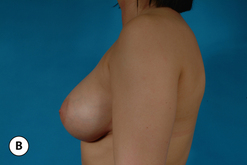
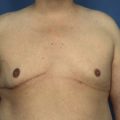
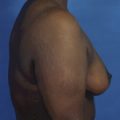
It bears emphasizing that the effect of the implant on the ultimate result after breast augmentation is directly related to the thickness of the soft tissue cover of the breast. In patients where there is a relatively thick layer of parenchyma and fat, essentially any breast implant will provide an acceptable result if the proper volume is provided. There is no reason to use, for instance, a shaped device in these patients as the advantage of the anatomic shape is overwhelmed by the volume of the surrounding soft tissue and the subtleties of the shaped concept are so obscured as to be rendered inconsequential. In fact, when the volume of the breast implant ends up providing 50% or less of the overall volume of the breast, any shaping advantage afforded by an anatomic device, or any more subtle ‘feel’ effect associated with, for instance, a silicone gel implant, ends up being obscured by the volume of the native breast. As the percentage of overall breast volume that the implant provides increases, the effect that the shape, size and consistency of the chosen breast implant has on the final result becomes more pronounced. Generally speaking, when the implant provides more than 75% of the volume of the breast, variables such as shape, fill material and projection begin to have an increasingly noticeable effect on the overall result (Figures 3.1, 3.2).
Implant Construction
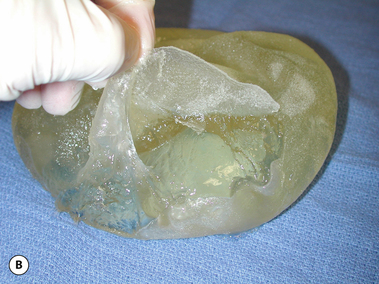
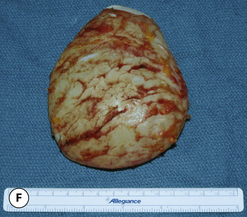
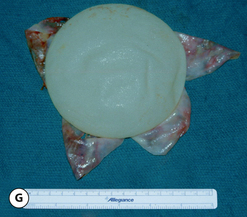
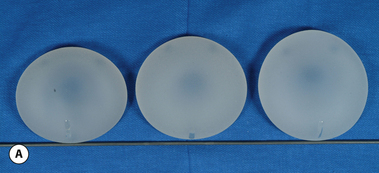
The outer shell is made of a silicone rubber that is cured onto a form called a mandril. The mandril is specifically constructed to create an implant of a specific size and shape. For a given implant design, there are generally whole families of mandrils which maintain the basic shape but exhibit a wide range of graduated dimensions and volumes. Either the mandrils are dipped into liquid silicone or the silicone is sprayed on to apply a thin coat of the material to the mandril. This thin layer of liquid silicone is allowed to dry and the mandril is then dipped again. After a series of dips, the silicone layer that is now attached to the mandril has a uniform thickness sufficient to hold reliably a given volume, the configuration of which is determined by the shape and dimensions of the mandril that made the shell. The flexible silicone shell is cut free from the dipping rod attached to the mandril and the elastic form is stretched and pulled off. For saline implants, the circular defect created by freeing the shell from the mandril is patched and a small fill valve is inset into the patch to complete the construction of the shell. For silicone gel implants, the shell is filled with a prescribed volume of the chosen gel and the defect is then patched to seal the gel inside the shell (Figure 3.3 A–E).
Implant Behavior
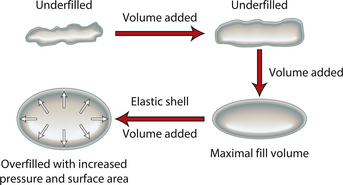
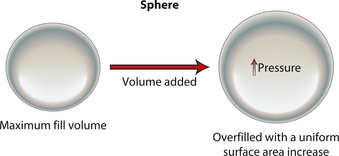
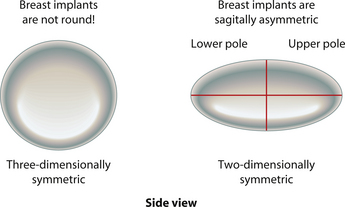
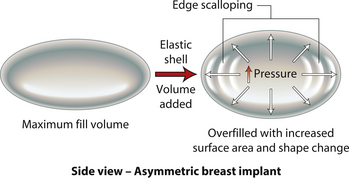
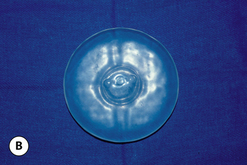
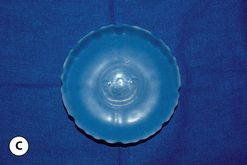
When an implant shell is completely empty, the outer shell is wrinkled and the shape of the device is correspondingly distorted. As volume is added to the implant, the wrinkles are filled out and the shape progressively improves until, eventually, a volume is reached where the shell is completely filled and, without any other outside influences, the shape of the implant exactly approximates the shape of the mandril that made the device. However, because the outer silicone rubber shell is elastic, it is possible to continue to fill the implant to a point beyond the fill volume determined by the mandril. As a result, the surface area of the implant expands slightly and the intraluminal pressure of the device increases (Figure 3.4). It is at this point that the physics-based relationships between surface area and volume become important. Very simply, for a given volume, the smallest surface area that can contain that volume assumes the shape of a sphere. Put another way, a sphere describes a surface area to volume relationship that is maximally efficient. This explains why breast implants appear rounded when they are subjected to the forces of capsular contracture. Because the volume of the device is fixed, as the surface area of the capsule decreases due to the contracture, the relationship between the surface area of the capsule and the volume of the implant becomes more efficient and gradually assumes the shape of a sphere (Figure 3.5 A,B). Therefore, with a perfectly spherical implant shell, overfilling would result in an increased intraluminal pressure and a symmetrically realized increase in the surface area of the implant with no distortion in the shape of the device (Figure 3.6). Essentially, all that would result would be a slightly bigger sphere that felt a little firmer. However, breast implants are not spherical in shape and it is very important to realize that what are commonly referred to as round implants are round only in two dimensions. Typically, in the horizontal and vertical planes, the devices are symmetric. However, in the sagittal plane, the dimensions of the implant are different and, in that regard, what is typically referred to as a round implant is actually shaped (Figure 3.7). Although this typical ‘round’ implant does not approach the degree of shape that the more standard ‘anatomical’ implant does, it is shaped nonetheless as it has asymmetrical dimensions in one plane and therefore functions as an asymmetric device. This asymmetry in shell design has implications in how the implant responds to filling. As the implant proceeds from an empty state to one of progressive inflation, the shape will improve until that point is reached where the exact volume that corresponds to that of the mandril is reached. This is the optimal fill volume for the implant. As this optimal fill volume is exceeded and the implant becomes overfilled, the surface area of the device increases slightly and the pressure inside the device increases. As continued filling proceeds, the ability of the outer shell to stretch to accommodate the increasing volume becomes overwhelmed and the increase in the surface area of the implant tails off rapidly such that the surface area measurement becomes more or less fixed. At this point, the laws of surface area to volume physics become applied and the device gradually becomes more spherical and the projection in the sagittal plane increases and the vertical height and horizontal width diminish. Because the contour of the radius of the device is more aggressively shaped as compared to the smoother dome-like contour of the front and back of the implant, the ability of the elastomer in this part of the shell to stretch smoothly to re-accommodate the increasing volume of the implant is limited. Therefore, as the projection of the implant increases, the change in the shape of the device cannot be smoothly accommodated along the radius of the device and stress risers form at the implant edge. This is a well-recognized phenomenon noted particularly in saline implants and this edge distortion is known as ‘scalloping’ (Figures 3.8–3.10, (uDVD clip 1.05)). As the implant is overfilled even further and becomes more spherical, the edge scalloping becomes more severe. Recalling the example of capsular contracture, where the volume remains fixed and the surface area gradually decreases, here in the case of an overfilled implant, it is the surface area which becomes more or less fixed and the volume which increases. In either instance, it is the physics of adding of fluid to a confined space that ultimately governs the resulting shape of a breast implant.
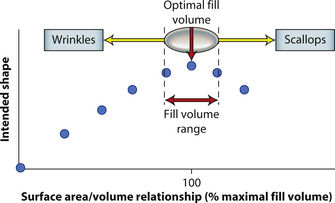
By applying these concepts, it is possible to gain a better understanding of implant behavior with regard to, in particular, saline implants. Figure 3.11 represents a stylized graph showing implant shape on the Y axis and degree of implant fill on the X axis. Initially, the implant is empty and the shape is an unfilled shell. Then, as fluid is added to the device, the wrinkles in the device are filled out and the shape gradually improves until the optimal fill volume, or the fill volume determined by the implant mandril, is reached. As further filling proceeds, scallops develop at the edge of the implant and the shape begins to deteriorate. Therefore, with the optimal fill volume as the apex of the desired shape, there is a small window of fill volume variability either side of which will still allow an acceptable shape to be created in the implant. Falling too far short of the optimal fill volume will result in progressively prominent wrinkling in the shell and exceeding the optimal fill volume by too great a degree will result in scalloping of the implant edge (Figure 3.11). This fill volume window has long been recognized by plastic surgeons and manufacturers and it is generally accepted that the optimal fill volume of a saline implant can be exceeded by roughly 10% without creating an obvious shape distortion. Such flexibility in implant filling is one of the advantages afforded by the use of saline implants. By differentially filling an implant of the same basic size and dimension without causing a significant distortion in the shape of the device, patients who present with mild asymmetries in breast size can be treated effectively without the potentially troubling need to resort to implants of different base diameters.
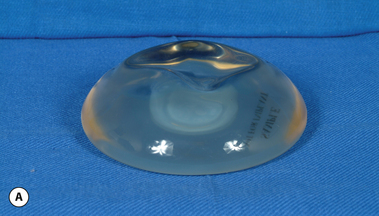
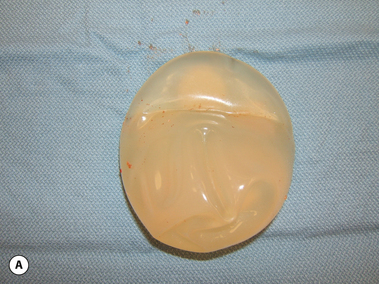
These principles are equally applicable to ‘round’ silicone gel implants. However, when comparing the characteristics of saline versus silicone gel implants, it is important to recognize two major differences. First, the volume of a silicone gel implant is fixed, a feature that actually simplifies the use of these devices to a certain extent. Second, every silicone gel implant, whether it be a moderate-, moderate plus- or high-profile device, is underfilled relative to the optimal implant volume created by the mandril. This mismatch in fill volume relative to the available surface area of the device, along with the thicker consistency of the gel, results in an implant which has a decidedly softer feel than a properly filled saline implant. When a silicone gel implant is placed on a flat surface, the degree to which it is underfilled can be assessed by noting the variable degree of collapse of the central part of the shell in the middle of the device (Figure 3.12 A,B). This central shell collapse can be temporarily corrected by gently placing an evenly applied force to the surface of the implant. The implant then assumes a properly filled appearance without deformation. A similar phenomenon occurs when the implant is placed upright 90 degrees. The underfilled device will collapse in the upper pole, sometimes markedly, and this collapse can be partially overcome by applying force to the anterior surface of the device (Figure 3.13 A,B). In situ, this external force is applied to the implant by the surrounding soft tissue framework of the breast. Therefore, the outward appearance of a breast augmented with an underfilled fixed-volume silicone gel implant will depend upon the degree of underfilling of the device as modified by the nature of the external forces applied by the soft tissue framework of the breast.
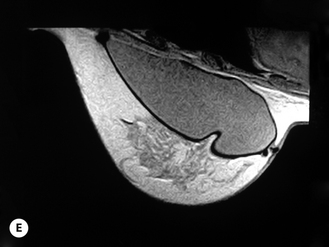
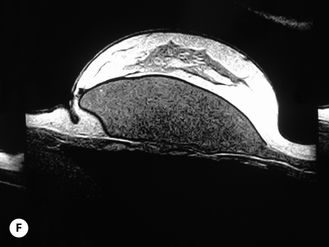
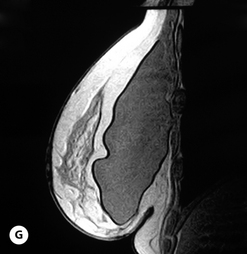
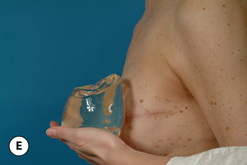
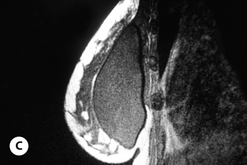
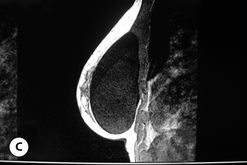
Given these variables, it can be deduced that saline-filled devices must be filled to a level at or near the optimal fill volume of the device lest the resulting surface irregularities which would develop, either wrinkles or scallops, become visible through the breast. These surface irregularities become even more pronounced when what appears to be a properly filled saline implant is placed upright 90 degrees. In this position, the pressure of the saline falling to the lower pole of the breast creates collapse of the upper pole with pronounced folding and wrinkling being the result (Figure 3.14). Applying force to the surface of the device, as happens with a relatively constricted soft tissue envelope, will counteract the tendency for the saline to fall to the bottom of the shell and upper pole distortion will tend to be corrected. However, in a lax skin envelope, if the soft tissue cover is thin enough, these irregularities develop unchecked and implant distortion with visible contour irregularities in the breast can potentially be seen. Silicone gel implants are under less severe constraints and can be underfilled without causing the same degree of potential surface irregularity. This is due to the fact that the denser consistency of the gel and the softer elasticity of a silicone gel implant shell combine to form softer wrinkles, which create edges which are less sharp than in saline devices. As a result, a fixed-volume, underfilled, silicone gel implant can settle to the bottom of a breast pocket and can fold and wrinkle according to the dictates of the overlying soft tissue framework to assume a shape other than that imparted to the shell by the shape of the mandril. Simply stated, the wrinkles associated with a standard round silicone gel implant tend to be much softer than those which form in saline devices and result in visible surface irregularities only in the thinnest of patients. In actuality, this shape is variably and at times markedly anatomical, a fact which has been documented in supine, prone and most importantly upright magnetic resonance imaging (MRI) evaluations of patients with fixed-volume silicone gel implants in place (Figure 3.15 A–G). When the soft tissue envelope is sufficiently lax, the underfilled gel device settles to the bottom of the pocket and forms folds and wrinkles in a patient-specific fashion to create, along with the overlying breast tissue, the final shape of the resulting augmented breast. This is actually a very powerful way to use a breast implant as each device is molded into a custom-made shape specific for each patient. When the volume and eventual configuration of the round gel implant complements the native breast well, very aesthetic results can be reliably and consistently obtained in breast augmentation.
However, in assessing the shape of an in situ breast implant, the presence of wrinkles in the implant shell and what effect these wrinkles might have in both the short and the long term must be carefully considered. As long as these wrinkles form softly enough to allow the implant to settle into a smooth anatomic shape and cannot be seen through the soft tissue cover of the breast, they are well tolerated. However, over time, wrinkles eventually create weak points in the implant shell and may well be the major etiologic cause of ultimate implant failure (Figure 3.16 A,B). It is here that the anatomically shaped implant concept offers particular advantage. Because most of the mass of an anatomic implant is centered in the lower part of the device, when the implant is placed upright, the magnitude of the distorting forces across the peripheral upper edge of the implant is minimized. As a result, anatomically shaped implants are inherently less likely to wrinkle or otherwise develop a shape distortion than ‘round’ devices, a fact that can afford the surgeon a greater ability to control the shape of the upper pole of the breast (Figure 3.17 A–F). This tendency to maintain shape when placed upright is further enhanced when the fill material has enough structural integrity to support the implant shell. Both saline and standard viscosity gel do not tend to provide strong enough support to the upper pole of an anatomically shaped implant shell and wrinkles and folds can form when these devices are placed under a lax skin envelope. As such, these types of devices are subject to potential fold flaw failure with rupture over time. However, with the newer cohesive gels, which have an enhanced level of viscosity, strong structural support is provided to the entire implant shell and these devices do maintain their shape well, even when placed upright under a lax skin envelope (Figure 3.18 A,B). To support the outer shell further, anatomically shaped cohesive gel devices are filled to a level which much more closely approximates the optimal fill volume determined by the anatomically shaped mandril which made the device. As a result, these types of anatomically shaped cohesive gel devices are very resistant to wrinkle or fold formation and therefore are associated with very low rupture rates (Figure 3.19 A–C).
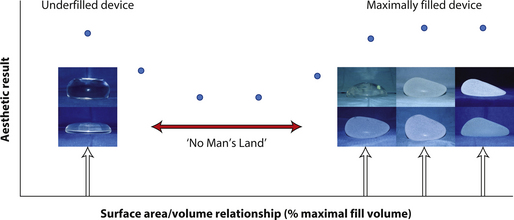
Figure 3.20 represents a graphical summary of these concepts. The quality of the aesthetic result is represented on the Y axis and the percentage of filling of the implant relative to the optimal fill volume is represented on the X axis. The left side of the graph demonstrates that the result provided by a relatively underfilled round gel implant with a less cohesive gel consistency which falls to the bottom of the pocket and assumes an anatomic shape is generally very aesthetic. Thinking about it another way, it is the breast that shapes the implant, which is a very versatile and powerful way to use a device. At the other end of the graph is the result provided by a completely filled, anatomically shaped cohesive gel implant, which can also provide a very aesthetic result. In this instance, it is most decidedly the implant which is shaping the breast, which is also an excellent strategy for using a breast implant. It must be noted, however, that when using the anatomically shaped, cohesive gel devices, a premium is placed on accurately matching an implant of the proper dimensions to the basic measurements obtained from the patient. Because the implant does not conform at all to pressure from the overlying skin envelope, it must be matched well to the existing soft tissue framework to provide the best result. For this reason, anatomically shaped cohesive gel devices are generally less forgiving with regard to sizing errors than their smooth round gel counterparts.
Special note must be made regarding the center portion of the graph. Here, the aesthetic quality of the result has been denoted as being less favorable as compared with the results obtained using the devices noted on either end. The reason for this is related to both the fluid characteristics of the filling material and the shape of the implant shell. As noted earlier, underfilled round implants will form wrinkles when placed upright under the breast. When the implant is a silicone gel device made with a gel that has a soft consistency, the wrinkles tend to fold smoothly and are much less likely to create a visible surface irregularity. Therefore, although the potential for fold flaw failure in these devices is present, experience has shown that these implants can last for decades without rupturing. However, with saline implants, the wrinkles that form tend to be more sharply demarcated and create a sharper edge in the implant shell. The same is true for any type of round or anatomically shaped cohesive gel device that is underfilled. If the stiffness of the gel is not sufficient to support the shell, very sharply demarcated folds will form particularly across the upper pole. Not only does this pose a risk for potentially creating a visible contour deformity in the breast, but the folded shell edge will weaken over time, ultimately leading to implant rupture. One strategy that can be utilized to combat this tendency for a saline implant to wrinkle in the upper pole is to overfill it slightly to help ensure that the volume of the upper pole will be maintained when the device is placed upright (Figure 3.21 A–C). Caution must be used in these circumstances, however, as, while this strategy may help reduce the tendency for the implant to demonstrate upper pole wrinkling, it also tends to create a rounded appearance that might become objectionable in thinner patients.
Surface Texturing of Breast Implants – Historical Perspective
It was not long after the introduction of the very first generation of silicone gel implants that an anatomically shaped version became available. Intuitively it made sense, even in those early days of implant experience, that if the device was anatomically shaped, a more aesthetic result could be created using such a device. The problem of rotation was recognized early on and one of the first anatomically shaped devices available for general use was manufactured with a series of dacron patches attached to the back. These patches incited a tenacious tissue ingrowth response which locked the device into position and prevented rotation. Unfortunately, these implants were also associated with a capsular contracture rate which approached 100%. The dacron patch was thought to be at least partially responsible for this unacceptably high capsular contracture rate and this device fell from favor (Figure 3.22 A–D). During this time period from the late 1960s and into the 1970s, other design modifications were investigated including the development of saline devices, double-chambered devices and various types of gel implants, most with a ‘round’ shape, and the concept of an anatomically shaped implant became a distant concern. Ultimately, an implant coated with a thin layer of polyurethane foam was developed in an attempt to alter the surface interaction of the device with the surrounding capsule (Figure 3.23 A–C). The resulting capsular contracture rate associated with these devices was very low and ultimately several different designs became available including a round version known as the Meme implant and an anatomic version known as the Replicon (Figure 3.23 D,E). Since that time, several other manufacturers have developed similar devices. This was a critical moment in implant design as it represented one of the first attempts to merge the concept of a using a shaped device with a textured surface that promoted capsular ingrowth. By using this strategy, the shaped device becomes locked into position such that it cannot rotate postoperatively, a critical factor in effectively using a shaped implant. The early shaped polyurethane-coated devices were only mildly anatomic and still maintained a fair amount of upper pole fullness. As a result, early efforts to improve the anatomic shape provided by the devices included a technique of implant ‘stacking’. This involved using a round polyurethane-coated base implant and then positioning, or ‘stacking’, a smaller round polyurethane-coated device on the lower pole of the base implant in an attempt to increase the overall lower pole projection of the breast. The friction provided by the polyurethane coating, along with the tissue ingrowth which occurred along the surface of the devices, tended to maintain the position of the two implants on top of one another to create an overall anatomic appearance.
The mechanism of action for the reduced rate of capsular contracture associated with the use of polyurethane-coated implants was eagerly investigated. It had been demonstrated previously that the polyurethane foam lattice network of the polyurethane foam-coated implant underwent gradual degradation over time and that, histologically, multinucleated giant cells could be identified in microscopic sections of capsules from patients augmented or reconstructed with polyurethane foam-coated devices. Small defects could be seen in the foam structure indicative of this gradual degradation. Eventually, over a span of up to 20 years, it has been demonstrated that the foam covering is completely digested away, converting the device into a smooth-walled silicone gel implant (see Figure 3.23 F,G). Coincident with this complete removal of the foam covering has been the development of a contracture in some patients. As a result, it is generally accepted that this mild chronic inflammatory response was either responsible for, or indicative of, a process which prevented the capsule from contracting. Once this foam lattice network was completely removed, the device became simply a smooth round gel implant with an associated rate of capsular contracture indicative of any smooth round gel device. As a by-product of investigation into this process of gradual foam degradation, claims were made that certain breakdown products of the metabolism of the foam were carcinogenic. Although later shown to be of no significant consequence, the results of these claims led to the discontinuation of polyurethane foam-coated implants from production, at least in the USA. Also, the design and use of anatomically shaped implants was sharply curtailed due to the loss of the ability to ensure the devices would maintain their proper orientation.
Biocell
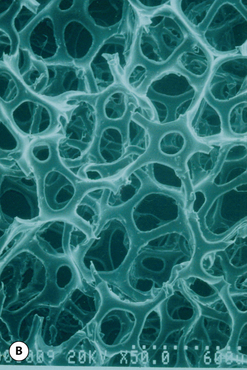
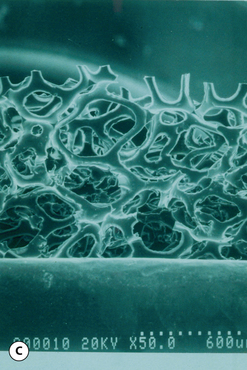
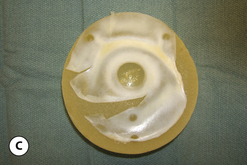
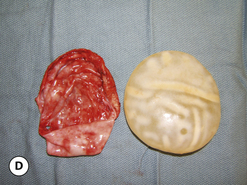
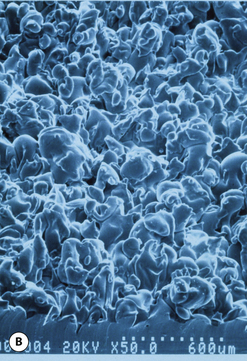
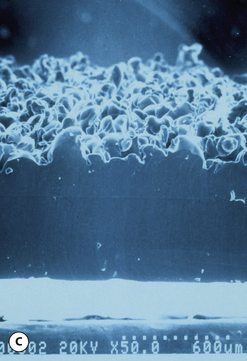
This surface texture is manufactured by layering salt granules into a tacky implant shell and then washing them out once the shell has completely cured. The result is an open pore lattice network that creates a roughened surface to the device (Figure 3.24 A–C). Because this is the most aggressive of the textures, actual ingrowth of the capsule can occasionally be demonstrated, particularly when used with tissue expanders, which creates an interesting Velcro-like effect once the device is removed and the capsule is forcibly separated away from the textured surface (Figure 3.24 D). Although the effect of textured surfaces on the rate of capsular contracture remains controversial, there is no doubt that, when textured ingrowth of the capsule occurs, the device is locked into position and cannot rotate. This finding greatly facilitated the subsequent development of a new generation of anatomically shaped tissue expanders and implants and was perhaps one of the most important advances in implant technology in the modern era. Unfortunately, ingrowth of the capsule into the Biocell textured surface is an unpredictable occurrence. Certainly with the anatomically shaped tissue expanders used today, as the device is expanded, the shell of the expander is forced into the capsule as the pressure in the expander increases with each inflation. As a result, it is very common to be able to demonstrate textured ingrowth into the surface of the device when the expander is removed and replaced with the permanent implant. Even here, however, ingrowth is very often incomplete and portions of the surface of the expander do not demonstrate any ingrowth. But there is usually enough to stabilize the anatomic expander in position and nearly every tissue expander manufactured today has some type of textured surface to help stabilize expander position and orientation. With anatomically shaped implants, however, the compressive forces around the implant are invariably less vigorous and there is less impetus for the capsule to grow into the Biocell textured surface. As a result, textured ingrowth is found much less commonly in both round and anatomically shaped implants.
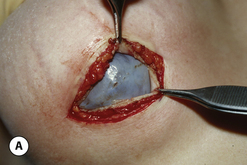
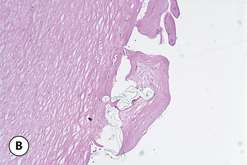
One interesting observation noted with Biocell textured devices is the occasional formation of a ‘double capsule’ around the implant. Inside the capsule that forms in association with the surrounding soft tissue of the pocket is a second pseudocapsule that becomes densely adherent to the textured surface of the device (Figure 3.25 A). This inner capsule has a smooth outer surface and demonstrates solid ingrowth into the textured surface that must be peeled away from the implant to be effectively removed. Histologically, this tissue is characterized by a multilaminate scaffold of collagen within which is interspersed a sparse population of fibroblasts (Figure 3.25 B). This is an extremely interesting finding given that the inner capsule is a separate tissue layer that maintains no direct link to the surrounding capsule and therefore has no apparent source of vascular supply. It can be postulated that these cells have a very low metabolic requirement and are surviving on diffusional sources of oxygen and energy alone. One possible etiology for the formation of the inner capsule may be related to seroma formation in the pocket around the implant, although exactly how the fibroblasts are able to populate the surface of the device and then proliferate enough to form a distinct fibrous layer remains unknown. In most instances, the development of an inner capsule is a benign occurrence that only partially involves the surface of the implant and, as such, simply represents an area where the outer capsule cannot grow into the textured surface of the device (Figure 3.25 C). Occasionally, however, the implant becomes completely surrounded by the fibrous layer of the inner capsule and since the interspace between the two very smooth capsules is mildly lubricated, the implant can rotate and flip in the pocket with ease. As a result, when double capsule formation occurs in association with a shaped implant, the risk of undesirable implant rotation becomes greater (Figure 3.25 D,E).
Siltex
This surface texture is manufactured by pressing a layer of polyurethane foam into a thin tacky sheet of silicone rubber which has been affixed to the shell of an otherwise smooth implant. In this fashion, an imprint of the irregular polyurethane foam surface is reproduced on the surface of the textured device. This design was meant to duplicate the pore size of the interstices of the native polyurethane foam and thus, theoretically, duplicate the positive effect polyurethane has on reducing the rate of capsular contracture. The net effect of this technique is a less aggressive texture which does not promote tissue ingrowth (Figure 3.26 A–C). The effect of this texture, and also, the Biocell texture when ingrowth does not occur, is to provide a rough surface that tends to stabilize the implant and inhibit sliding of the implant across the smooth surface of the capsule. It is analogous to the effect the ridges present in fingertips have on grasping ability. By creating a rough surface on the device, sufficient friction is created to inhibit implant rotation and, along with other factors such as pocket dimensions, help keep an anatomically shaped tissue expander or implant properly oriented.
Capsular Contracture
Although textured surfaces can assist in preventing implant rotation, the major impetus for designing the various types of textures was to mimic the effect that polyurethane foam had on capsular contracture. Since their introduction, many reports have focused on this very issue and, at this point, the question of whether or not textured surfaces reduce the rate of capsular contracture remains unanswered. Despite the differences which have been described between the two types of textures, several well-designed studies appear to show a reduction in the rate of capsular contracture using both textured surfaces. Conversely, there are other studies which demonstrate no protective effect. To accept the hypothesis that surface texturing of a silicone shell will prevent capsular contracture, a viable mechanism of action must be presented. One theory is based on the fact that the capsule becomes disorganized as it grows into the interstices of the textured surface and this somehow inhibits the ability of the capsule to contract. In fact, the collagen layers outside the zone of ingrowth become layered as in any other capsule, rendering this hypothesis questionable at best. Also, I have seen many instances of a severe capsule formed around a Biocell textured device where there was near complete ingrowth of the inner layers of the capsule into the textured surface, with no apparent protective effect on the development of the contracture. Additionally, if textured ingrowth was responsible for reducing the capsular contracture rate, then the Siltex surface should not be associated with any similar effect since it does not promote tissue ingrowth. Yet, several studies show a positive effect with the Siltex surface. Finally, the effect of polyurethane foam was to create a low-grade inflammatory response in the capsule. Somehow, this observation was linked to reduction in the rate of capsular contracture. There is no such low-grade inflammatory response in the capsule which forms around a textured implant. Looking at all these data as objectively as possible, it is difficult to conclude with certainty what effect surface texturing has on the rate of capsular contracture. Currently, it is my opinion that textured surfaces do not impact significantly on the rate of capsular contracture. Future studies hopefully will shed further light on this important question.
Implant Types
Round
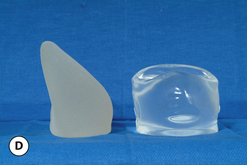
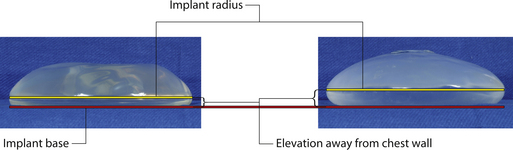
As noted previously, what are commonly referred to as ‘round’ implants are actually shaped devices that are asymmetrical in the sagittal plane. For the purposes of this discussion, ‘round’ will refer to any device in which the horizontal width and vertical height are the same and the shape of the implant on either side of these reference lines is symmetrical. With this as a basic definition, there are a whole host of different round devices available from several different manufacturers. Between the different styles of round devices, there are subtle differences in the shape of the peripheral margins of these various implants. In some, the radius of the peripheral edge lies midway up the overall vertical height of the device. Others keep the apex of this peripheral margin closer to the base of the implant (Figure 3.27). This variable is controlled by the shape of the mandril that made the shell. Although this difference is subtle, such design features can provide an advantage. By using implants that have a peripheral radius located closer to the base of the implant, a less dramatic contour change is created between the implant and the chest wall, which can provide a more natural-appearing result with less of a contour break in the upper pole of the breast in thin patients, or patients with ptosis.
In order to understand the effect of projection on the performance of a breast implant, it is helpful to analyze it in conjunction with the other two variables which govern implant design, namely volume and dimension. This will be discussed in more detail in Chapter 4, which deals with breast augmentation. But to summarize, when volume is held constant for a particular implant, as projection increases from low to mid to high, the base diameter of the device decreases (Figure 3.28 A,B). This makes intuitive sense since more of the volume is concentrated in the central portion of the device, to provide the increased projection, therefore there can be less to distribute peripherally, which results in a decrease in the horizontal and vertical dimension of the implant. Therefore, for a given implant volume, there will be three categories of projection to choose from and, as projection increases, the base diameter of the implant decreases. This basic relationship has several implications for use in aesthetic breast surgery.
Wrinkling
A moderate-profile implant will have a relatively widened width and height as compared to the projection. In essence, it is more dramatically ‘shaped’ than a high-profile device, where the measured width, height and projection are relatively more closely aligned. As a result, when the moderate-profile device is placed upright, it has a greater tendency to collapse and wrinkle than the high-profile implant. Where this functions as an advantage is in the breast which has a lax skin envelope. Here, in the ideal setting, the moderate-profile implant will fold, wrinkle and finally settle to the bottom of the pocket, assuming the shape dictated by the influences of the overlying soft tissue framework. As was noted previously, this is a very versatile way to use a breast implant, as long as the wrinkles are not visible and do not appreciably weaken the shell. If, however, after breast augmentation, folds and wrinkles are visible in the breast, using a high-profile device may be one potential solution as the tendency to form prominent folds and wrinkles is less for a high-profile device in the upright position.
Anatomically Shaped Saline Implants
With the advent of silicone texturing of the outer shell of breast implants and the realization that surface texturing could stabilize the shaped devices into position came renewed interest in the development of anatomically shaped breast implants. From an historical perspective, this timeline coincided with the United States government’s review of the safety data regarding silicone gel implants, which ultimately resulted in severe restrictions being placed on the use of these devices. As this Food and Drug Administration (FDA) review proceeded over the ensuing years, implant design modifications were focused on saline-filled devices, which resulted in several different types of anatomically shaped saline implants being developed (Figure 3.29 A,B). Each of these devices was constructed using an anatomically shaped mandril with varying degrees of shape. Although initially received with enthusiasm, these devices fell from favor and failed to generate sustained positive results for several reasons.
Shape
The same filling issues discussed with regard to round devices are applicable for anatomically shaped saline devices. There is a filling window below which wrinkles form and above which edge scalloping results. However, the fact that the device was constructed to present and maintain a specific orientation altered these dynamics slightly. As has been noted, when a saline implant is placed upright, the very minimally cohesive fluid drops to the bottom of the device and any degree of underfilling promptly results in collapse of the upper pole. In fact, even if the device is filled to the optimal fill volume, in the upright position there continues to be a mild fluid shift inferiorly, which can result in loss of volume in the upper pole with collapse. Therefore, to counteract completely any tendency for the implant to deform in the upright position, a mild overfilling strategy is optimal. This tends to work against the shaped concept as a mild bulging of the upper pole of the implant can result and a common complaint associated with the use of these devices is that the patients demonstrate too much upper pole fullness (Figure 3.30 A–D). Also, any degree of overfilling of the implant tends to create a firmer breast than was perhaps normally found with round saline implants or silicone gel devices. This phenomenon was more evident with the taller height versions of the anatomic saline implants, with shorter height devices being somewhat immune to these problems. Because of the reduced vertical dimension, the fluid shifts were less marked in the shorter implants, making them easier to use. However, one unfortunate outcome of this experience, which was the first experience many surgeons had with shaped devices, was the impression that the shaped concept was not valid and offered no particular advantage in aesthetic or reconstructive breast surgery. The simple fact was that, for a variety of reasons, these early shaped saline implants were not anatomic enough to demonstrate effectively the improved results that are possible with the use of properly shaped devices.
Anatomically Shaped Gel Implants
As noted previously, because the implant maintains its shape and does not conform to the pressures applied by the overlying soft tissue framework, it is now the implant that is shaping the breast and there is less room for error in appropriately picking an implant for a given patient than with smooth round gel devices. Therefore, it was recognized that a wider selection of devices would be required to meet the needs of a variety of clinical situations. As a result, each manufacturer offers a matrix of devices which vary in height, projection and the degree of cohesiveness of the silicone gel (Figure 3.31 A,B).
Implant height
For many surgeons, this is a new variable to consider when it comes to picking a specific implant for a patient. Previously, with round devices, the height was a default dimension and was simply the same measurement as the width. With anatomically shaped cohesive gel devices, the height can and should be carefully matched to the patient’s body habitus and breast size to avoid overfilling of the upper pole of the breast. Simple stated, smaller patients with smaller breasts do better with low- to mid-height devices, whereas taller patients with broader chests are better candidates for full-height devices.