Immunosuppression in Ocular Surface Stem Cell Transplantation
Introduction
Patients with bilateral limbal stem cell deficiency or severe unilateral limbal stem cell deficiency, who are not candidates for autologous transplantation alone, require allograft tissue to restore their limbal stem cell population.1,2 Limbal tissue with its greater load of Langerhans cells has a high presence of antigens, compared to clear corneal avascular tissue.3 This is relevant in living-related conjunctival limbal allograft (LR-CLAL) and in keratolimbal allograft (KLAL) where a small rim of vascular conjunctiva in addition to rich limbal tissue is transplanted directly adjacent to recipient blood vessels. In addition, eyes with severe ocular surface disease not only have a deficiency in limbal stem cells, but also suffer from chronic conjunctival inflammation (Table 46.1) which is a poor predictor for limbal stem cell transplantation success.4
Table 46.1

Patients with Stage IIb and IIc disease often have concomitant conjunctival scarring, decreased aqueous and tear production, potential for ocular surface keratinization, and have the poorest prognosis for surgical rehabilitation.1,4
(Modified from Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc 1996;94:677–743; and Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea 1996;15:549–556.)
In solid organ transplantation, aggressive systemic immunosuppression is understood to be crucial to the survival of the allograft and we have learned a great deal, especially from our renal transplant colleagues. Therefore, a strong emphasis must be placed on the importance of long-term multiagent systemic immunosuppression in maintaining graft survival and a clear, stable ocular surface following limbal stem cell transplantation5–7 in addition to topical therapy. In one study, 31 eyes of 23 patients with aniridic keratopathy underwent limbal stem cell replacement using KLAL from cadaver donors5 Nineteen (90.5%) of 21 eyes receiving systemic immunosuppression obtained a stable ocular surface, whereas only four (40.0%) of 10 eyes not receiving systemic immunosuppression achieved ocular surface stability (p < 0.01). Another study of 12 eyes from 10 patients who underwent KLAL with prolonged systemic immunosuppression (mean 52.7 months) improved the outcomes in eyes with total limbal stem cell deficiency.6 The largest study of 225 eyes from 136 patients after ocular surface stem cell transplantation (OSST) using a protocol of long-term systemic immunosuppression (mean 42.1 months) demonstrated a stable ocular surface in 105 (77.2%) patients.7 It should be noted that immunosuppression is necessary in all patients who receive limbal allografts, including those who receive HLA-matched living-related tissue.8
Topical Immunosuppression
Following limbal allograft transplantation, all patients begin topical immunosuppression using corticosteroids and cyclosporine at a respective four times a day and two times a day dosing. This regimen is maintained on a tapered dose of twice daily or once daily, depending on the degree of ocular surface inflammation, indefinitely. Previously, corticosteroids such as 1% prednisolone acetate and compounded 2% cyclosporine A were effective for eyes undergoing OSST. However, with the advent of 0.05% difluprednate ophthalmic emulsion (Durezol, Alcon Inc., Fort Worth, TX), a more potent steroid that has shown similar clinical results at half the dose when compared to prednisolone acetate,9 and cyclosporine 0.05% emulsion (Restasis, Allergan, Irvine, CA), these two agents used at doses of four times daily and two times daily, respectively, now represent the mainstay of topical immunosuppression regimens after allograft transplantation. In patients with controlled ocular surface inflammation and steroid-induced elevated intraocular pressures, topical loteprednol (Lotemax, Bausch and Lomb, Rochester, NY) may be used instead.
Systemic Immunosuppression
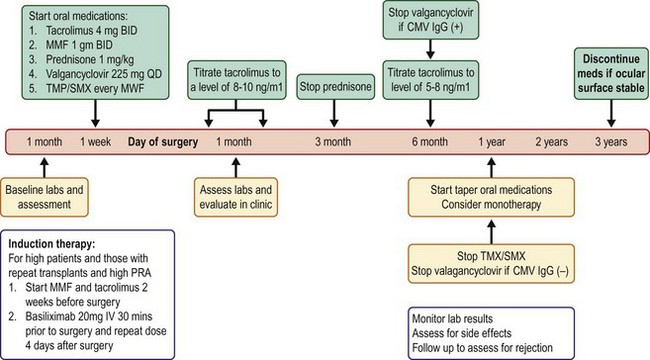
In the earlier years of using systemic immunosuppression with OSST, oral prednisone, cyclosporine (Neoral; Novartis, East Hanover, NJ), and azathioprine (Imuran; GlaxoSmithKline, Philadelphia, PA) were used. The current protocol (Fig. 46.1) now includes tacrolimus (Prograf; Astellas Pharma US, Inc, Deerfield, IL), mycophenolate mofetil (MMF, Cellcept; Hoffmann La Roche, Nutley, NJ), and a shorter course of prednisone. The use of these three medications in combination was affirmed as the mainstay of immunosuppression in renal transplantation with the ELiTE (Efficacy Limiting Toxicity Elimination)-Symphony randomized control study.10
Corticosteroids such as prednisone, prednisolone acetate, loteprednol, and difluprednate, are still the most rapid immunosuppressant available. They exert their effects by non-specifically inhibiting many aspects of the inflammatory response, including blocking the synthesis of prostaglandins and reducing the number of circulating T lymphocytes. Tacrolimus is similar to cyclosporine (both are calcineurin inhibitors that inhibit T-lymphocyte activation by binding to FK-506 and cyclophilin regulatory proteins, respectively), but the adverse effects of gingival hyperplasia and hirsutism are not seen with tacrolimus, making it more tolerable for patients.11 Azathioprine and MMF are antimetabolites that inhibit the proliferation of both T and B lymphocytes. Large clinical trials involving renal transplant patients have shown a lower incidence of acute graft rejection in patients receiving MMF versus azathioprine (when both groups received steroids and cyclosporine).12
A Steroid-Minimizing Protocol
In the current systemic immunosuppression protocol that is advocated in OSST (see Fig. 46.1), oral prednisone is initiated at a dose of 1 mg/kg/day, and the dose is tapered over 1 to 3 months, depending on the level of inflammation. Oral tacrolimus is initiated at 4 mg twice daily and oral MMF at 1 g twice daily. The dose of tacrolimus is decreased around 6 months and MMF after 12 months, depending on the degree of ocular surface inflammation and corneal epithelium stability. Patients are on 225 mg of valganciclovir (Valcyte; Hoffmann La Roche, Nutley, NJ) daily and trimethoprim/sulfamethoxazole (Bactrim, single strength; Mutual Pharmaceutical Company, Philadelphia, PA) one tablet every Monday, Wednesday, and Friday, for prophylaxis against cytomegalovirus and Pneumocystis carinii, respectively. Once a patient and potential donor are matched in terms of their ABO blood typing (if the donor is a living relative), then the patient’s immunosuppression regimen is tailored and individualized based on their immunological risk stratification. Details of when and which pre- and post-OSST investigations are ordered to assess a patient’s baseline health status and to monitor for therapeutic drug levels and adverse events are outlined in Box 46.1. For the first 6 months after limbal allograft surgery, target serum tacrolimus trough levels should be between 8 and 10 ng/mL. Thereafter, a trough level of 5 to 8 ng/mL is targeted for 12–18 months after surgery.
Individualization of the Protocol
There are local factors innate to the diseased eye and donor and recipient immunological factors involved in tailoring a patient’s systemic immunosuppression regimen. High-risk eyes are those with stage 2B or 2C disease: total limbal stem cell deficiency and conjunctival inflammation with associated conjunctival scarring, decreased aqueous tear production, and high likelihood of ocular surface keratinization.4 Examples of ocular conditions in this category would be severe Stevens – Johnson syndrome, ocular cicatricial pemphigoid, and severe chemical injuries. Risk stratification also includes ABO blood type, donor type (living relative versus cadaver), tissue human leukocyte antigen (HLA) type, panel reactive antigen (PRA) percentage, donor-specific antigen (DSA) detection, repeat transplantation failure, or need for repeat OSST (Table 46.2). The primary antigens recognized by the immune system include blood type and HLA proteins. Blood type ABO antigens have been detected primarily on epithelial cells. Class 1 antigens (HLA-A, B, and C) are expressed by all nucleated cells in the body and are found on the corneal epithelium, stromal keratocytes, and endothelial cells. Class II antigens (HLADR, DQ, and DP) are present mainly on antigen-presenting cells, such as macrophages and Langerhans cells. Cross-matching is used to determine whether the recipient has antibodies against the potential donor. A negative cross-match is desired and a positive cross-match increases the risk of graft rejection and poor graft survival. Serum PRA testing is done before transplantation to determine the amount of HLA antibodies a patient has against a group of potential donors from the general community. This test is performed on all repeat procedures to quantify the risk of rejection after repeat procedures.
Table 46.2
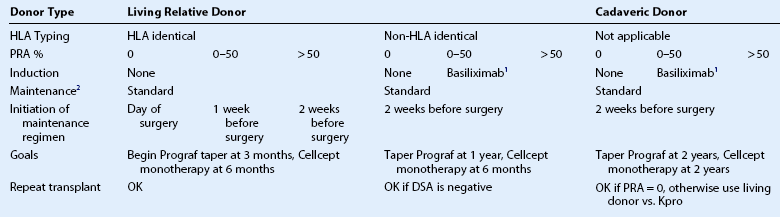
1Basiliximab (Simulect) 20 mg IV is given 30 minutes prior to transplantation with a second dose given 4 days after transplantation.
2Standard maintenance protocol includes oral prednisone 1 mg/kg, tacrolimus (FK-506 or Prograf) 4 mg twice daily, MMF (Cellcept) 1 g twice daily, oral valganciclovir (Valcyte) 225 mg daily, and trimethoprim/sulafamethoxazole (Bactrim single strength) 1 tablet on Mondays, Wednesdays, Fridays, or dapsone 100 mg orally daily if the patient has a sulfa allergy. The higher the percentage of PRA, panel reactive antibodies, the harder it is to find a match and the patient is more likely to reject. Transplants are at higher risk of failure if DSA, donor-specific antibodies, is positive.
(Modified from Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year Experience. Cornea 2012;31:655-61.)
Patients with a high PRA percentage undergo further DSA testing. DSA detection makes it possible to screen for the presence of host antibodies directed against the specific HLA antigens of the donor. Repeat transplantation is advisable only if DSA is negative (Table 46.2). The closeness of the match between the donor and recipient influences the risk of acute rejection, the amount of immunosuppressive drugs needed to prevent rejection, and ultimately, the long-term survival of the graft.
Basiliximab is a systemic immunosuppressive agent that binds to special receptors on activated T lymphocytes to prevent T-lymphocyte replication and B-lymphocyte activation. Inhibition of B lymphocytes prevents antibody production that normally would bind to foreign transplanted tissue to stimulate an immune response. In renal transplant patients at high risk for rejection, induction with basiliximab has been shown to be effective, well tolerated, easy to administer, and cost effective;13 thus, in OSST, if the recipient does not have identical HLA proteins to the donor and has a high PRA, then induction with basiliximab is given (see Fig. 46.1 and Table 46.2). In this group of higher-risk OSST patients, maintenance systemic immunosuppression is initiated 2 weeks before surgery and tapering is not attempted until later: the tacrolimus dosage is decreased starting beyond 6 months and MMF monotherapy is not attempted until beyond 1 year (see Fig. 46.1).
Minimizing Side Effects
Our 10 years of experience with systemic immunosuppression in OSST has shown that it is safe and effective for patients with severe ocular surface disease when followed by an ophthalmologist and transplant medicine specialist.7 Severe adverse events occurred infrequently in our patient population (1.5%), and there were no deaths and no secondary tumors in this series of 136 patients with mean follow-up time of 54 months.
A systematic review of the steroid-sparing systemic immunosuppression regimen for OSST patients used at the Cincinnati Eye Institute and University of Cincinnati has reported the regimen to be safe (Table 46.3).7 There were no deaths or secondary tumors observed in this large, long-term retrospective study of relatively young patients (mean age, 43.6 years). Severe adverse events (MI, pulmonary embolus) occurred infrequently in this population (1.5%). Furthermore, morbidity in KLAL patients receiving combined MMF and tacrolimus resulted in significantly fewer systemic adverse effects than renal transplant patients because renal transplant patients have significantly more comorbidities.14 Thus, the importance of evaluating immunosuppression-associated risks in the context of interpreting a patient’s pre-existing co-morbidities should be emphasized.
Table 46.3
Adverse Events Reported in 136 Patients (225 Eyes) after Ocular Surface Stem Cell Transplantation7
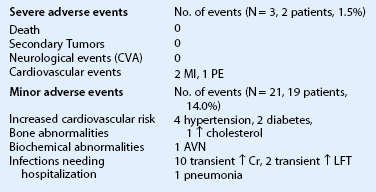
(Modified from Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year Experience. Cornea 2012;31:655-61.)
General Considerations
Patients on immunosuppressive therapy require particular attention, compared to routine ophthalmology patients. Prior to starting therapy it is recommended that the patient undergoes evaluation by a primary care physician or internist and that the following are determined: pregnancy status, tuberculosis exposure, infectious serology testing, as well as appropriate baseline laboratory tests (see Box 46.1). Absolute contraindications for systemic immunosuppression are patients with a history of malignancy less than 5 years, non-adherence with clinical or laboratory follow-up or a history of noncompliance with medications, and significant co-morbidities, such as diabetes, uncontrolled hypertension, renal insufficiency, congestive heart failure, and other organ failure. Systemic immunosuppression is recommended for healthy patients less than 60 years of age and usually not recommended for patients more than 70 years of age. Patients between the ages of 60 and 70, depending on their overall health, and patients less than 10 years of age may receive systemic immunosuppression with mild modifications to the regimen.
References
1. Holland, EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743.
2. Biber, JM, Skeens, HM, Neff, KD, et al. The cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
3. Niederkorn, JY. Effect of cytokine-induced migration of Langerhans cells on corneal allograft survival. Eye. 1995;9(Pt 2):215–218.
4. Holland, EJ, Schwartz, GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–556.
5. Holland, EJ, Djalilian, AR, Schwartz, GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–130.
6. Liang, L, Sheha, H, Tseng, SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol. 2009;127:1428–1434.
7. Holland, EJ, Mogilishetty, G, Skeens, HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year Experience. Cornea. 2012;31:655–661.
8. Rao, SK, Rajagopal, R, Sitalakshmi, G, et al. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology. 1999;106:822–828.
9. Donnenfeld, ED. Difluprednate for the prevention of ocular inflammation postsurgery: an update. Clinical Ophthalmology. 2011;5:811–816.
10. Ekberg, H, Tedesco-Silva, H, Demirbas, A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575.
11. Vanrenterghem, YF. Which calcineurin inhibitor is preferred in renal transplantation: tacrolimus or cyclosporine? Curr Opin Nephrol Hypertens. 1999;8:669–674.
12. Mycophenolate mofetil in renal transplantation: 3-year results from the placebo-controlled trial. European Mycophenolate Mofetil Cooperative Study Group. Transplantation. 1999;68:391–396.
13. Chapman, TM, Keating, GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63:2803–2835.
14. Alloway, R, Mogilishetty, G, Cole, L, et al. Ocular surface transplant recipients experience minimal immunosuppression complications: implications for composite tissue transplants. Am J Transplant. 2007;1058:419b.