Immunoglobulins
Structure
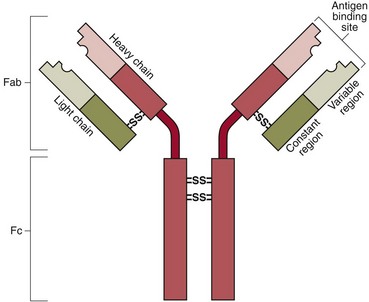
All immunoglobulins have the same basic structure and consist of two identical ‘light’ and two identical ‘heavy’ polypeptide chains, held together by disulphide bridges (Fig 26.1). The light chains may be either of two types: kappa or lambda. The heavy chains may be of five types: alpha, gamma, delta, epsilon and mu. The immunoglobulins are named after their heavy chain type, as IgA, IgG, IgD, IgE and IgM.
The molecules are characterized by two functional areas:
 The Fab, or variable end is the area that recognizes and binds to the antigen.
The Fab, or variable end is the area that recognizes and binds to the antigen.
 The Fc end is responsible for interaction with other components of the immune system, e.g. complement and T-helper cells.
The Fc end is responsible for interaction with other components of the immune system, e.g. complement and T-helper cells.
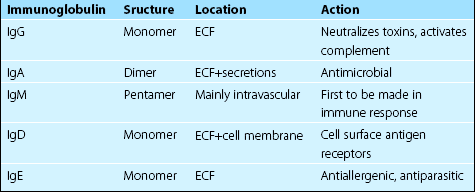
The various classes of immunoglobulins have different tertiary structure and functions (Table 26.1). The major antibodies in the plasma are IgG, IgA and IgM.
Electrophoresis of serum proteins
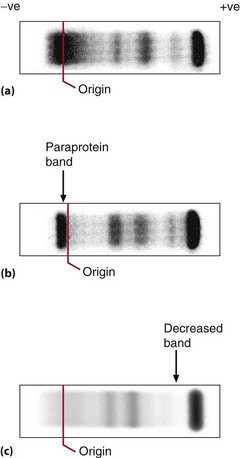
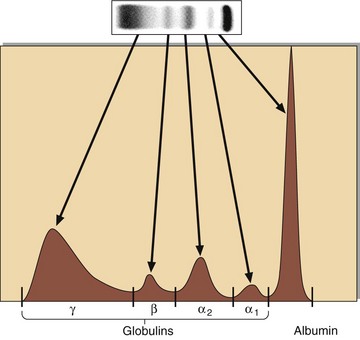
Electrophoresis may be carried out to study a number of protein abnormalities. The normal pattern is shown in Figure 26.2(a). Immunoglobulins are detected primarily in the gamma globulin area on electrophoresis. Electrophoresis can show gross deficiency or excess of immunoglobulins and the presence of discrete bands (paraproteins) (Fig 26.2, b and c). Serum should be used for electrophoresis, as the fibrinogen of plasma (consumed during clotting) gives a discrete band that can easily be mistaken for a paraprotein. A quantitative measure of each protein class may be obtained by scanning the electrophoresis strip (Fig 26.3).
Measurement
Immunoglobulins may be measured in a number of ways, the necessity for the request often being triggered by an observed increase in the ‘globulin’ fraction (p. 50). If an abnormality is detected, then the particular type of immunoglobulin, or indeed of light or heavy chains where these are produced alone, may be confirmed by immunofixation or quantitatively by other means.
Increased immunoglobulins
Paraproteins
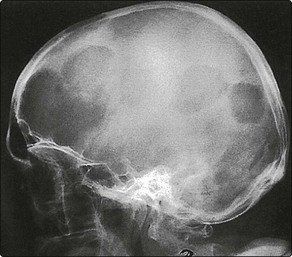
Myeloma is characterized by osteolytic lesions (Fig 26.4), and bone pain is often the presenting symptom. In the face of increasing synthesis of abnormal immunoglobulins, other bone marrow functions are reduced, and there is a decline in red and white cell and platelet formation and decreased production of normal immunoglobulins. Anaemia and susceptibility to infection are the consequences. Treatment of myeloma involves the use of bone marrow suppressive drugs. The increased serum paraprotein may cause renal damage leading to renal failure. Hypercalcaemia is also a feature of myeloma.
Deficiencies or absence of immunoglobulins
Deficiencies or absence of immunoglobulins can occur as a result of infection, genetic abnormalities or the effects of therapy (Table 26.2). Where the situation is irreversible, replacement therapy has been used, either by addition of immunoglobulin-rich plasma or by the transplantation of bone-marrow-containing competent plasma cells.
Table 26.2
Causes of hypogammaglobulinaemia
| Type | Specific causes |
| Physiological | Levels of IgA and IgM are low at birth |
| Genetic | Bruton’s X-linked agammaglobulinaemia |
| Severe combined immunodeficiency (SCID) | |
| Acquired | Malnutrition |
| Malignancy | |
| Infections, e.g. HIV, measles | |
| Immunosuppressant drugs, e.g. azathioprine, ciclosporin |