Immunizations for Travel
The Centers for Disease Control and Prevention (CDC) Travelers’ Health website provides resources for locating a pretravel vaccination clinic. The site also provides links to state health departments and the Yellow Fever Vaccination Clinic Registry that lists facilities approved to provide yellow fever vaccinations (http://wwwnc.cdc.gov/travel/contentTravelClinics.aspx).
The CDC provides travel health information to address the many different health risks a traveler may face with electronic access through its website (http://www.cdc.gov/travel). This site offers information to assist travelers in deciding the vaccines, medications, and other measures necessary to prevent illness and injury during international travel. CDC Health Information for International Travel (“The Yellow Book”) is an excellent resource for travel health and is available in a searchable online version on the CDC Traveler’s Health website at http://www.cdc.gov/yellowbook.
Routine Immunizations
Routine immunizations are those customarily given in childhood and updated in adult life. The vaccines currently recommended in childhood include those against tetanus, diphtheria, pertussis, varicella, measles-mumps-rubella, poliovirus, Haemophilus influenzae type b, hepatitis A, and hepatitis B. The recommended immunization schedules for persons ages 0 to 6 years and 7 to 18 years are published each year as approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/vaccines/recs/acip), the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org). A complete list of routine childhood and adult immunization recommendations and immunization schedules can be found at http://www.cdc.gov/vaccines/schedules/index.html.
1. Diphtheria, Tetanus, and Pertussis: Primary immunization in young children is accomplished with a combination vaccine containing acellular pertussis antigen. The tetanus and diphtheria toxoids (Td) vaccine classically used for booster doses in older children and adults has no pertussis component and a lower dose of diphtheria antigen. Absence of a pertussis booster for adults has led to waning immunity and susceptibility to pertussis. Booster doses of Td vaccine given at 10-year intervals are recommended to maintain immunity. For adventure and other travelers to remote locations who might sustain open wounds and be unable to safely obtain a tetanus booster, a Td booster could be given after 5 years. Particularly for travelers to areas of the world where diphtheria remains a risk (e.g., most countries of Africa, Asia, and the Middle East, countries of the former Soviet Union, and focal areas of Latin America), care must be taken to recognize that the last “tetanus” booster might really have been tetanus toxoid alone without the diphtheria component. If the person has no record or recollection of immunization, it is advisable to obtain this vaccine before traveling. If there is any doubt about whether or not an adult received the primary series, three doses of Td should be administered; the first dose and second dose should be separated by 4 weeks and the third dose should be given 6 to 12 months later.
2. Measles, mumps, and rubella: Travelers to developing countries are at risk for acquiring measles. If travelers have received two doses of measles vaccine, usually given in childhood as the measles-mumps- rubella (MMR) vaccine, they are protected. Persons born after 1956 should receive a second dose of measles vaccine, which is now available only as MMR. Adults who have not received two doses of the measles vaccine and who do not have a documented history of infection or immunity should receive two doses of MMR vaccine separated by at least 28 days. MMR vaccine is a live attenuated virus preparation. It is contraindicated in pregnancy and in immunocompromised persons.
3. Poliovirus: Poliomyelitis vaccine is usually not boosted after childhood in the United States except for anticipated high-risk exposure through work or travel to areas where polio is endemic. An inactivated (killed) virus polio vaccine (IPV) regimen is recommended for a primary immunization series given before 18 years of age. IPV is also recommended for booster doses in people 18 years and older because of a higher risk of complications associated with the live oral vaccine in older individuals. Polio has been nearly eradicated worldwide, except for India. All traveling adults should have received a primary course of the polio vaccine.
4. H. influenzae type B: Immunization is acquired in childhood. The risk for invasive H. influenzae disease, including meningitis, is greatest in children younger than 7 years old, and the infection is common among children in the developing world. Traveling children should be kept up-to-date according to standard pediatric vaccination schedules.
5. Influenza: Influenza vaccine is recommended for all health care workers and for international travelers because prolonged air travel and exposure to crowded or extreme environments create a predisposition to infection. Other considerations are based on conventional recommendations regarding underlying health and age (e.g., all people older than 65 years and those with chronic lung, heart, or kidney disease or with impaired immunity). The current influenza vaccine is not protective against avian influenza A (H5N1), which has caused outbreaks, including fatalities, in Southeast Asia and China. Travelers to locations where avian influenza might be transmitted should avoid visits to markets or farms or other activities that might bring them into close contact with fowl.
6. Pneumococcal: Pneumococcal vaccine polyvalent is administered intramuscularly as a 0.5-mL single dose and is recommended for all adults 65 years or older (this recommendation may be lowered to 50 years of age) and in younger individuals with conditions that increase the risk for invasive pneumococcal disease. Specific indications for pneumococcal vaccination in adults younger than 65 years include the following:
a. Persons 19 to 64 years of age with chronic cardiovascular disease (including congestive heart failure and cardiomyopathy), chronic pulmonary disease (including asthma and chronic obstructive pulmonary disease), diabetes mellitus, alcoholism, chronic liver disease, and cigarette smoking
b. Individuals with functional (e.g., sickle cell disease) or anatomic asplenia
c. Immunocompromised individuals 19 years or older, including those with human immunodeficiency virus (HIV) infection, malignancy (including leukemia, lymphoma, multiple myeloma, and other cancers), chronic renal failure, or those receiving immunosuppressive drugs
Recommended Vaccines for Travelers (Table 49-1)
Table 49-1
Vaccines and Immunoglobulin for Adult Travelers Who Completed Childhood Immunizations
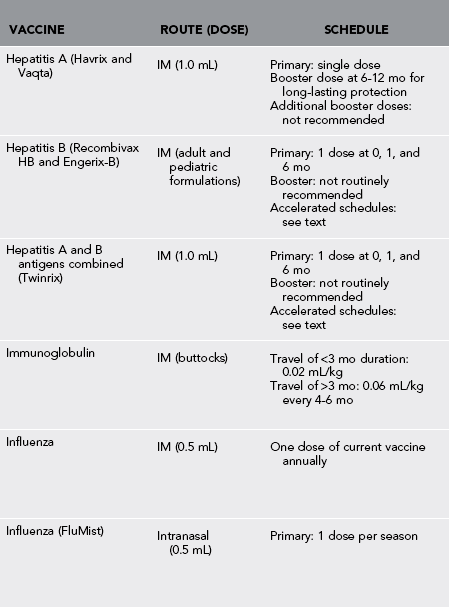
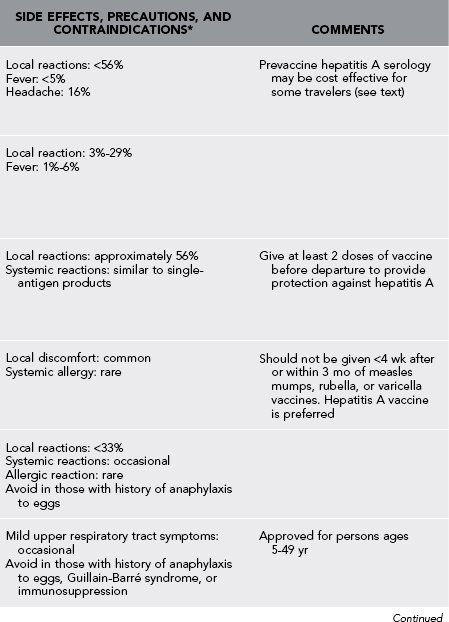
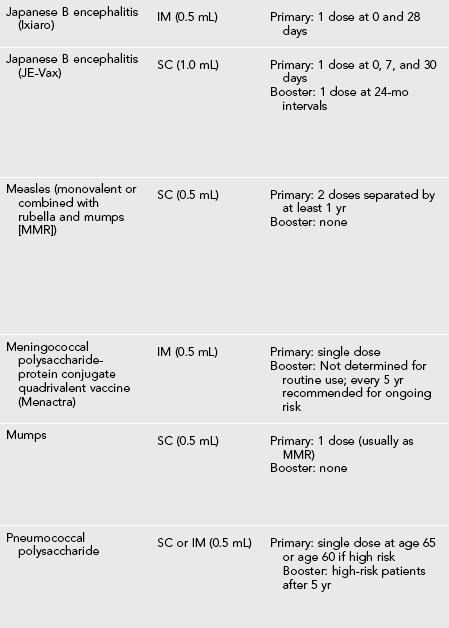



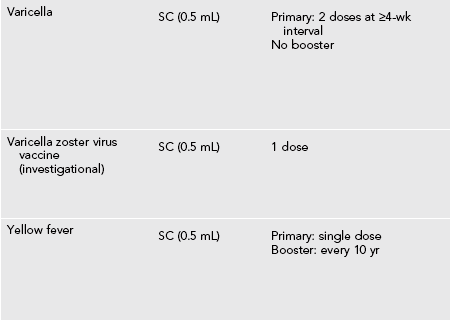
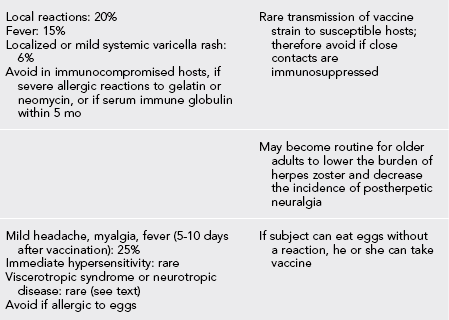
*Moderate or severe acute illness with or without fever or a serious reaction to a previous dose is a contraindication to all vaccines.
Modified from Centers for Disease Control and Prevention: Health Information for International Travel 2012 (http://wwwnc.cdc.gov/travel/page/yellowbook-2012-home.htm); and Hill DR, Ericsson CD, Pearson RD, et al: Guidelines for the practice of travel medicine, Clin Infect Dis 43:1499, 2006.
Hepatitis B
Meningococcus
Vaccine protection against meningococcal meningitis is recommended for long-term travelers to the sub-Saharan “meningitis belt” of Africa. Short-term travelers to this region should receive vaccine if they will travel during the dry season (December to June) or have extensive contact with local people. Meningococcal vaccine is required for travel to Saudi Arabia during the time of the annual Hajj and Umrah religious pilgrimages. Regardless of travel, the classic recommendation has been that young adults who will live in school dormitories, persons with complement deficiencies, persons who will have prolonged contact with a local population such as in a refugee camp, and persons with surgical or functional asplenia should be vaccinated. Travelers to regions where outbreaks are occurring should be vaccinated. Check the CDC website (http://www.cdc.gov/travel) to determine where epidemic disease is occurring.
Tuberculosis (BCG Vaccine)
Vaccine Summary
1. Like other live attenuated vaccines, BCG vaccine is contraindicated in persons with immunosuppression caused by congenital conditions, chemotherapy, radiation therapy, HIV infection, or another condition resulting in impaired immune response. Pregnancy is considered a relative contraindication.
2. Epidemiologic data suggest that the vaccine may be more useful in protecting children from disseminated extrapulmonary complications of tuberculosis than in protecting adults from primary pulmonary infection.
3. Occasionally children in families going abroad for extended residence are requested by the receiving country to provide proof of BCG vaccination to qualify for a visa.
4. A BCG vaccine is commercially available in the United States and is approved by the American Academy of Pediatrics Committee on Infectious Diseases for use in children traveling to live in areas in which tuberculosis is prevalent or there is a likelihood of exposure to adults with active or recently arrested tuberculosis.
5. Persons immunized with BCG vaccine test positive on PPD skin tests for many years afterward, regardless of the degree of protection conferred by the vaccine.
Required Travel Vaccines
Yellow fever is a viral infection transmitted by Aedes aegypti mosquitoes in equatorial South America and Africa. The vaccine for yellow fever is highly immunoprotective. Several countries require proof of yellow fever vaccine before entry. The CDC recommends consideration of yellow fever vaccine before travel in countries at risk for yellow fever virus transmission, listed in Table 49-2, and vaccination is required for entry into the countries listed in Table 49-3.
Table 49-2
Countries With Risk for Yellow Fever Virus Transmission

From Centers for Disease Control and Prevention: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/yellow-fever.htm. Courtesy Centers for Disease Control and Prevention.
Table 49-3
Countries That Require Proof of Yellow Fever Vaccination From All Arriving Travelers1
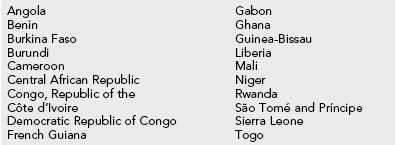
1Country requirements for yellow fever vaccination are subject to change at any time; therefore, CDC encourages travelers to check with the destination country’s embassy or consulate before departure.
From Centers for Disease Control and Prevention: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/yellow-fever.htm. Courtesy Centers for Disease Control and Prevention.
Vaccine Summary
1. The vaccine strain is an attenuated live virus and well tolerated.
2. It is administered as a single injection.
3. Significant antibody levels are achieved in more than 95% of persons vaccinated.
4. Booster interval is every 10 years, although persistent antibody titers have been detected 30 to 40 years after vaccination.
5. The vaccine is not recommended for infants younger than 9 months because of postvaccination encephalitis.
6. It is also not recommended during pregnancy unless the risk for yellow fever is thought to be greater than the risk for adverse effects from the vaccine.
7. Other contraindications are immunosuppression or a history of severe allergy to eggs.
8. Vaccine-associated viscerotropic disease has been reported in a small number of first-time recipients of yellow fever vaccine. This may be associated with thymic dysfunction and/or age older than 60 years.
9. Another method for reducing the risk for yellow fever (or any mosquito-borne disease) is liberal use of mosquito repellent and netting in endemic areas.