Chapter 23 Immediate Hypersensitivity (Type I)
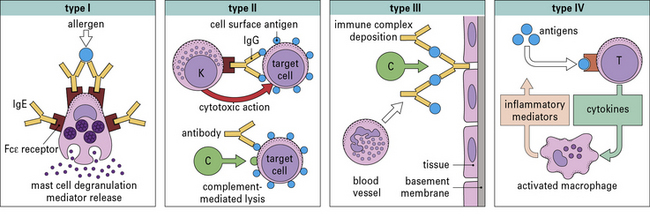
• The classification of hypersensitivity reactions is based on the system proposed by Coombs and Gell.
• Historical observations have shaped our understanding of immediate hypersensitivity. The severity of symptoms depends on IgE antibodies, the quantity of allergen, and also a variety of factors that can enhance the response including viral infections and environmental pollutants.
• Most allergens are proteins.
• Production of IgE depends on genotype. In genetically predisposed individuals, IgE production occurs in response to repeated low-dose exposure to inhaled allergens such as dust mite, cat dander, or grass pollen.
• Allergens are the antigens that give rise to immediate hypersensitivity and contribute to asthma rhinitis or food allergy.
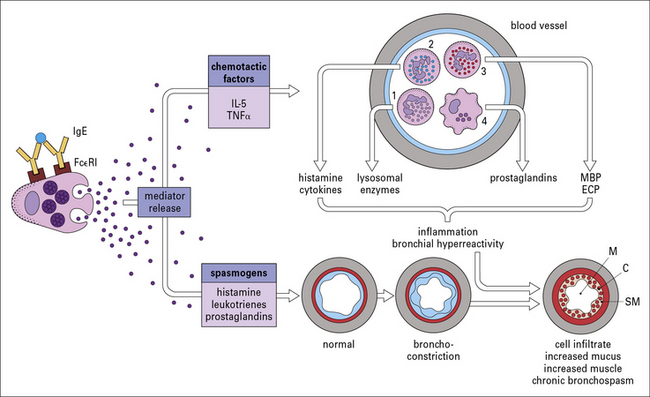
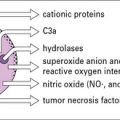
• Mast cells and basophils contain histamine. IgE antibodies bind to a specific receptor, FcεRI, on mast cells and basophils. This Fc receptor has a very high affinity, and when bound IgE is cross-linked by specific allergen, mediators including histamine, leukotrienes, and cytokines are released.
• Multiple genes have been associated with asthma in different populations. Multiple genetic loci influence the production of IgE, the inflammatory response to allergen exposure, and the response to treatment. Polymorphisms have been identified in the genes, in promoter regions, and in the receptors for IgE, cytokines, leukotrienes, and the β2-receptors.
• Skin tests are used for diagnosis and as a guide to treatment.
• Several different pathways contribute to the chronic symptoms of allergy.
• Immunotherapy can be used for hayfever and anaphylactic sensitivity.
• New approaches are being investigated for treating allergic disease.
Classification of hypersensitivity reactions
• Immediate (Type I) hypersensitivity responses are characterized by the production of IgE antibodies against foreign proteins that are commonly present in the environment (e.g. pollens, animal danders, or house dust mites) and can be identified by wheal and flare responses to skin tests which develop within 15 minutes.
• Antibody-mediated (Type II) hypersensitivity reactions occur when IgG or IgM antibodies are produced against surface antigens on cells of the body. These antibodies can trigger reactions either by activating complement (e.g. autoimmune hemolytic anemia) or by facilitating the binding of natural killer cells (see Chapter 24);.
• Immune complex diseases (Type III hypersensitivity) involve the formation of immune complexes in the circulation that are not adequately cleared by macrophages or other cells of the reticuloendothelial system. The formation of immune complexes requires significant quantities of antibody and antigen (typically microgram quantities of each). The classical diseases of this group are systemic lupus erythematosus (SLE), chronic glomerulonephritis, and serum sickness (see Chapter 25).
• Cell-mediated reactions (Type IV hypersensitivity) are those in which specific T cells are the primary effector cells (see Chapter 26). Examples of T cells causing unwanted responses are:
The original Coombs and Gell classification is shown in Figure 23.1.
Historical perspective on immediate hypersensitivity
• human anaphylaxis had no familial characteristics (unlike most of the other allergic diseases); and
• natural exposure to inhaled allergens did not cause anaphylaxis or urticaria.
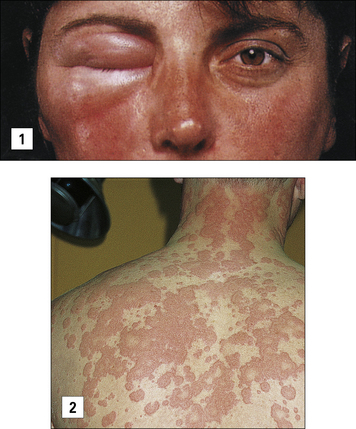
Anaphylaxis may also occur as a result of eating an allergen such as peanut or shellfish, or following the rupture of hydatid cysts with the rapid release of parasite antigens (Fig. 23.2).
Characteristics of type I reactions
Most allergens are proteins
Substances that can give rise to wheal and flare responses in the skin and to the symptoms of allergic disease are derived from many different sources (see http://www.allergen.org/). When purified they are almost all found to be proteins and their sizes range from 10–40 kDa. These proteins are all freely soluble in aqueous solution, but have many different biological functions including digestive enzymes, carrier proteins, calycins, and pollen recognition proteins.
Any allergen can be described or classified by its source, route of exposure, and nature of the specific protein (Fig. 23.3).
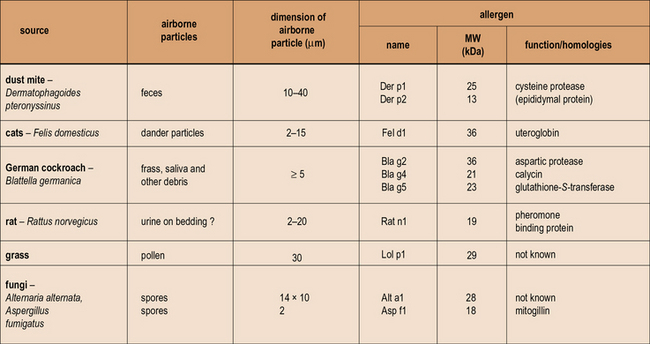
Fig. 23.3 Properties of allergens
Patients who become ‘allergic’ to one of the well-recognized sources of allergens have actually produced an IgE antibody response to one or more of the proteins produced by mites, trees, grass, cats, or fungi. The proteins are predominantly water soluble with a molecular weight (MW) ranging from 10–40 kDa. In many cases the function of the proteins is known, but it is not clear whether function such as enzymic activity alters the ability of these proteins to induce an allergic response. The properties of the particles carrying these allergens are very important because they influence both how much becomes airborne, and also where the allergen is deposited in the respiratory tract. The dimensions of the particles airborne vary from ≤2 μm for Aspergillus or Penicillium spores to ≥20 μm for mite fecal pellets and some pollen grains. (Sizes are given as diameter in μm. For a full list of allergens see http://www.allergen.org/.)
IgE is distinct from the other dimeric immunoglobulins
IgE is distinct from the other dimeric immunoglobulins because it has:
• an extra constant region domain;
• a different structure to the hinge region; and
• binding sites for both high- and low-affinity IgE receptors, FcεRI and FcεRII, respectively (see Fig. 3.19).
The properties of IgE can be separated into three areas:
• the characteristics of the molecule including its half-life and binding to IgE receptors;
• the control of IgE and IgG antibody production by T cells; and
• the consequences of allergen cross-linking IgE on the surface of mast cells or basophils.
The half-life of IgE is short compared with that of other immunoglobulins
• serum IgE has a much shorter half-life than other isotypes (~2 days compared with 21–23 days for IgG);
• IgE is produced in small quantities and is only produced in response to a select group of antigens (allergens and parasites); and
• IgE antibodies are sequestered on the high-affinity receptor on mast cells and basophils.
Q. What fundamental reason explains the low production of IgE?
A. The class switch from IgM to IgE happens infrequently and is controlled by T cells. The position of the IgE constant region gene is towards the distal end of the Ig gene stack (see Fig. 9.17), but the position alone cannot explain the infrequency of the switch to IgE.
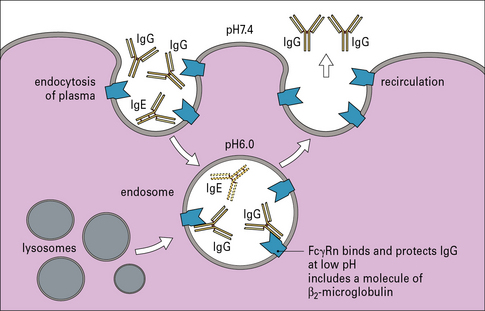
Serum is constantly being taken up by endocytosis. Many macromolecules including IgE degrade in the endosome. One major exception is IgG, which is protected by binding to the neonatal Fc gamma receptor, FcγRn (Fig. 23.4).
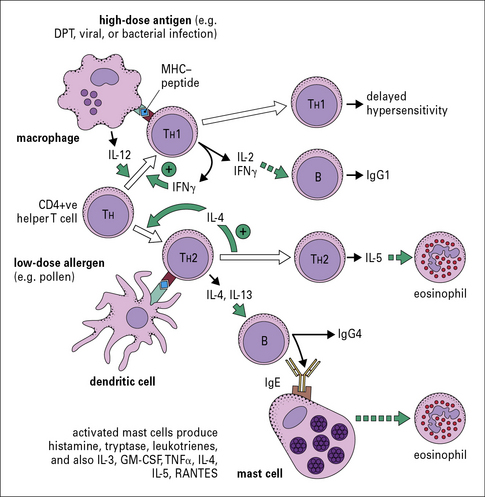
T cells control the response to inhalant allergens
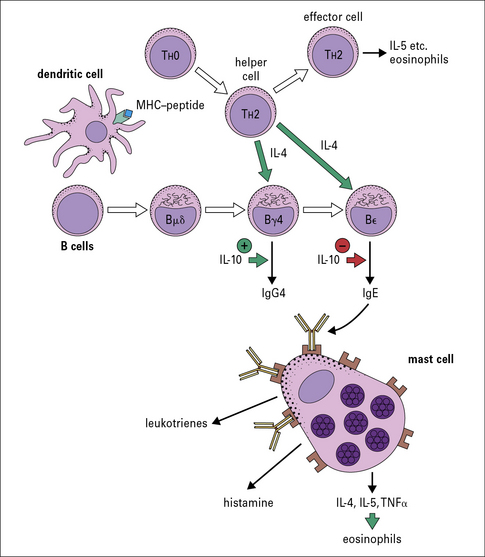
IgE production is dependent on TH2 cells
T cells that suppress TH2 responses including IgE production:
• act predominantly by producing interferon-γ (IFNγ); and
• are produced when the animal (e.g. mouse, rat, or rabbit) is primed in the presence of Freund’s complete adjuvant.
The main cytokines that are specifically relevant to a TH1 response include:
By contrast, the primary cytokines relevant to a TH2 response are:
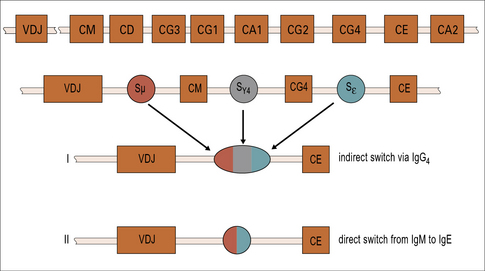
Both IgE and IgG4 are dependent on IL-4
The genes for immunoglobulin heavy chains are in sequence on chromosome 14. The gene for ε occurs directly following the gene for γ4. Both of these isotypes are dependent on IL-4 and they may be expressed sequentially (Fig. 23.6).
Recently, it has been shown that children raised in a house with a cat can produce an IgG response, including IgG4 antibody, without becoming allergic. A modified TH2 response (increased IgG4 and decreased IgE) therefore represents an important mechanism of tolerance to allergens (Fig. 23.7). IgG4 antibody responses without IgE antibody are a feature of immunity/tolerance to insect venom, rat urinary allergens, and food antigens as well as cat allergens.
Characteristics of allergens
Allergens have similar physical properties
The primary characterization of allergens relates to their route of exposure. The routes includes:
The inhalant allergens cause hayfever, chronic rhinitis, and asthma
Allergens can only become airborne in sufficient quantity to cause an immune response or symptoms when they are carried on particles. Pollen grains, mite fecal particles, particles of fungal hyphae or spores, and animal skin flakes (or dander) are the best defined forms in which allergens are inhaled (Fig. 23.8).
In each case it is possible to define the approximate particle size and the quantity of protein on the particle as well as the speed with which the proteins in the particle dissolve in aqueous solution (see Fig. 23.3).
Small quantities of inhalant allergen cause immediate hypersensitivity
Only a small number of food proteins are common causes of allergic responses
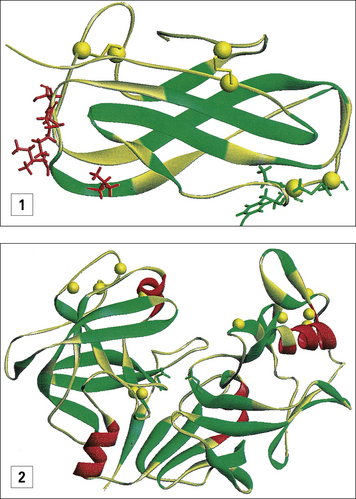
IgE binding sites can be identified on the tertiary structure of allergens
Many different allergens have been cloned, and for a few the tertiary structure is now known either from X-ray crystallography (e.g. Bet v1), by nuclear magnetic resonance (e.g. Der p2), or by modeling relationships to known homologs (Fig. 23.w1).
Desensitization can be used to control type I hypersensitivity
• allergen molecules modified in vitro by formaldehyde or glutaraldehyde (allergoids);
Therapeutic trials have been carried out with peptides from ragweed pollen antigens and the cat allergen Fel d1. The results show that peptide recognition is restricted by the HLA-DR type of the patient, which means that a wide range of peptides are necessary for treatment. In addition, there is clear evidence that peptides can produce a significant response in the lungs (Fig. 23.9) indicating that T cells in the lung can contribute to an asthmatic response.
Mediators released by mast cells and basophils
• basophils are circulating polymorphonuclear leucocytes that are not present in normal tissue, but can be recruited to a local site by cytokines released from either T cells or mast cells;
• mast cells cannot be identified in the circulation, but are present in connective tissue and at mucosal surfaces throughout the body.
Mast cells in different tissues are morphologically and cytogenetically distinct.
• in the rabbit the histamine content of the peripheral blood is almost all in platelets;
• in the mouse there are few if any circulating basophils; and
• in rats the degranulation of mast cells appears to be one granule at a time.
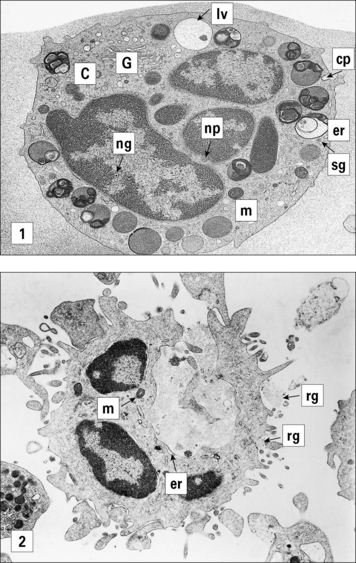
By contrast, in human mast cells and basophils the granules fuse with the exterior membrane and release their contents as a solution. The membrane of the granule then becomes part of the plasma membrane (Fig. 23.10).
Mast cells in different tissues have distinct granule proteases
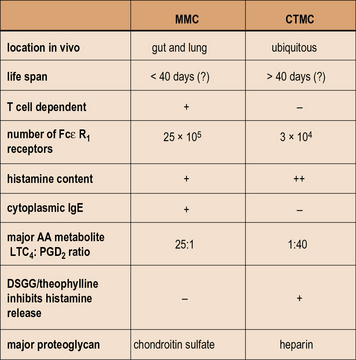
Mast cells were originally identified by Ehrlich who named them based on the distinctive, tightly packed granules. (Mast means well fed, or fattening, in German.) Mast cells in different tissues can be distinguished by staining for proteases, and the content of these enzymes may be relevant to their role in allergic diseases. The granule proteases of mast cells have been cloned and sequenced and are distinct for two types of mast cell (Fig. 23.11):
• mucosal mast cells are characterized by the presence of tryptase without chymase (MCT);
• by contrast, connective tissue mast cells contain both chymase and tryptase (MCTC).
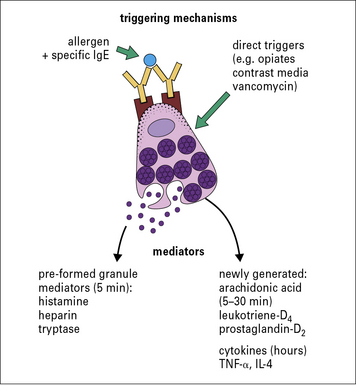
Cross-linking of FcεRI receptors results in degranulation
The process of degranulation in human mast cells and basophils involves fusing of the membrane of the granules containing histamine with the plasma membrane (see Fig. 23.10). The granule contents rapidly dissolve and are secreted, leaving behind a viable degranulated or partially degranulated cell. This process is initiated in most cases by cross-linking of two specific IgE molecules by their relevant allergen.
When two IgE receptors (FcεRI) are cross-linked, signal transduction through the γ chains of the receptor (see Fig. 23.12) leads to influx of calcium, which initiates both degranulation and the synthesis of newly formed mediators (Fig. 23.12).
In allergic individuals mast cells can be recruited to the skin and to the nose
In allergic individuals mast cell recruitment has been demonstrated both:
Genetic associations with asthma
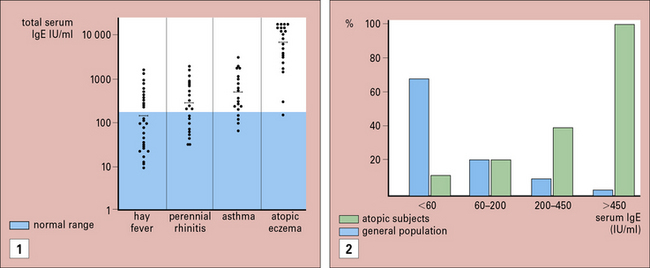
Systematic studies of allergic diseases are complicated because the phenotypes for diseases, such as hayfever and asthma, are not well defined and depend on the approach used to make the diagnosis. Although on average, total IgE values increase progressively from normal, in hay fever, asthma, and atopic dermatitis, the individual values vary widely (Fig. 23.13).
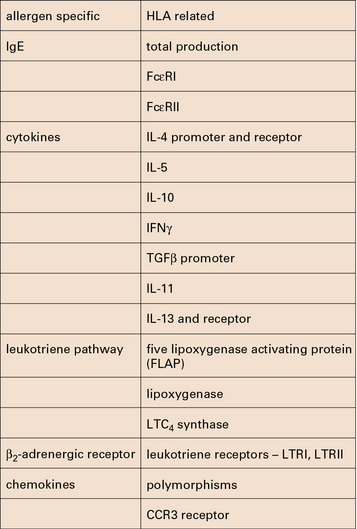
If a candidate gene is identified, it is possible to examine the gene for polymorphisms that link to asthma. However, a brief consideration of the possible targets (Fig. 23.14) makes it clear how complex the analysis of asthma is likely to be, and indeed is proving to be. Typical examples include polymorphisms of the promoter region for IL-4 and polymorphisms of the gene for IL-5.
Skin tests for diagnosis and to guide treatment
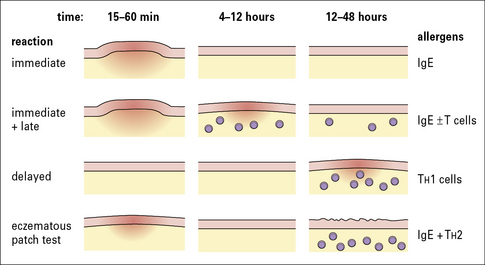
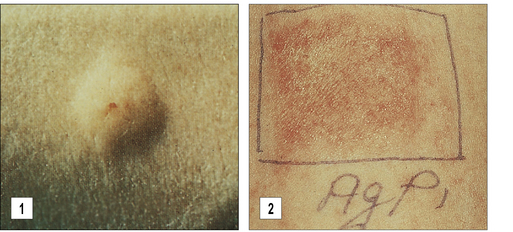
The primary method for diagnosing immediate hypersensitivity is skin testing. The characteristic response is a wheal and flare (Figs 23.15 and 23.16):
• the wheal is caused by extravasation of serum from capillaries in the skin, which occurs as a direct effect of histamine and is accompanied by pruritus (also a direct effect of histamine);
• the larger erythematous flare is mediated by an axon reflex.
• a prick test, in which a 25-gauge needle or a lancet is used to introduce ~0.2 μL of extract into the dermis;
Positive skin tests are common
Late skin reactions probably include several different events
Late reactions probably include several different events:
• the direct effects of prostaglandins, leukotrienes, and cytokines released by mast cells following the initial release of histamine;
• infiltration of lymphocytes, eosinophils, basophils, and neutrophils into the local site mediated by chemokines and other cytokines released from mast cells;
• release of products from the infiltrating cells. In general, these events are occurring in parallel over a period of hours.
True delayed skin test responses (i.e. without an immediate response) are:
Pathways that contribute to the chronicity of allergic diseases
• the time course is too long;
• there is a cellular infiltrate in these tissues; and
• there are major differences in disease between patients who have apparently similar titer and specificity of IgE in their serum.
• Local recruitment of mast cells and basophils, combined with increased ‘releasability’ of these cells, allows an increased response to the same allergen challenge – this mechanism plays a major role in the increased symptoms in the nose during the pollen seaseon.
• Release of leukotrienes, chemokines and cytokines from mast cells, or basophils; these mediators can have direct effects on blood vessels and smooth muscles. In addition IL-5, tumor necrosis factor (TNFα), and chemokines are each thought to contribute to the recruitment of inflammatory cells.
• T cells can be recruited to local tissues and can release a wide range of cytokines which have direct inflammatory effects.
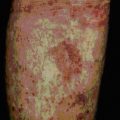
Atopic dermatitis and the atopy patch test
• the time course of the disease is chronic;
• injection of allergen into the skin causes a wheal and flare response, and doesn’t consistently cause eczema;
• the disease is multi-factorial, including a role for food allergy, skin infection, genetic variations in skin barrier function (based on filaggrin), and also inhalant allergens.
Epidermal spongiosis and a dermal infiltrate are features of a positive patch test
• by local intradermal injections;
• by applying a patch of allergen on gauze that stays on the skin for 2 days; or
• by fixing a chamber containing allergen over a denuded area of skin.
The cellular infiltrate includes eosinophils, basophils, and lymphocytes.
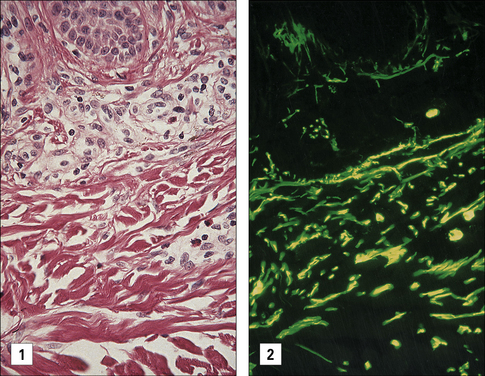
With persistent allergen at a site (i.e. 6 days), the eosinophils degranulate locally. This is in keeping with the evidence that the skin of patients with eczema contains large quantities of the eosinophil granule major basic protein (MBP), even though very few whole eosinophilic cells are visible (Fig. 23.17).
Allergens contribute to asthma
The causal role of bee venom in anaphylaxis or grass pollen in seasonal hayfever is obvious because:
• the epidemiological evidence that positive skin tests or serum IgE antibodies are a major risk factor for asthma;
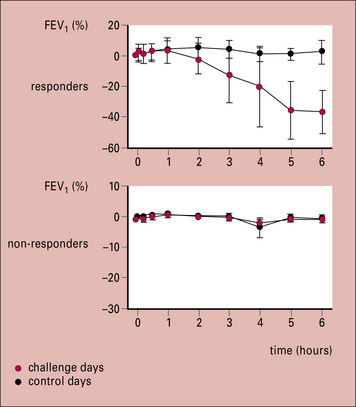
• bronchial challenge with nebulized extracts can produce both rapid bronchospasm, within 20 minutes, and a late reaction in 4–8 hours, which is characterized by renewed mediator production and a cellular infiltrate;
• reduced exposure to allergens can lead to decreased symptoms and decreased non-specific bronchial reactivity – this avoidance can be achieved either by moving patients to an allergen-free unit or by controlling exposure in the home.
Bronchi in the lungs of patients with asthma are characterized by increased mast cells, lymphocytes of the TH2 type, eosinophils, and products of eosinophils. In addition, there is increased mucus production secondary to goblet cell hyperplasia, epithelial desquamation, and collagen deposition below the basement membrane. These changes are a reflection of chronic inflammation, and it is generally considered that eosinophils play a major role in these events (Fig. 23.18).
BAL analysis after allergen challenge demonstrates mast cell and eosinophil products
Furthermore, MBP is present in biopsies of the lungs and can produce epithelial change typical of asthma in vitro (Fig. 23.19).
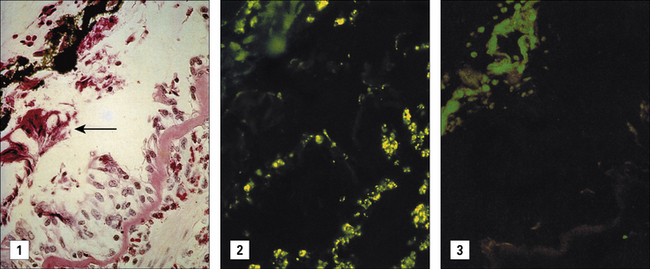
Fig. 23.19 Localization of MBP in the lung of a severe asthmatic
(Courtesy of Dr G Gleich, reprinted from J. Allergy Clin. Immunol. 1982;70:160–169, with permission from American Academy of Allergy Asthma and Immunology.)
Evidence for inflammation of the lungs of patients with asthma is indirect
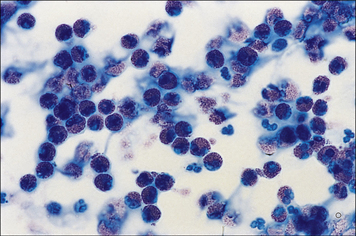
• peripheral blood or nasal smear eosinophils are increased in most patients presenting with an acute episode of asthma (Fig. 23.20);
• nasal secretions may contain increased ECP and IL-8 (CXCL8).
Treatments for type I hypersensitivity
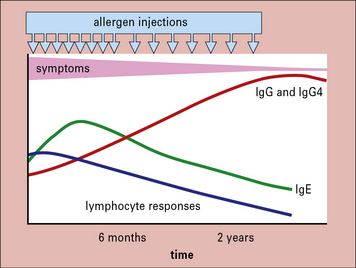
Immunotherapy is an effective treatment for hayfever and anaphylactic sensitivity to venom
The response to treatment includes:
• an increase in serum IgG antibodies;
• a striking decrease in the response of peripheral blood T cells to antigen in vitro; and
Over a longer period of time there is a progressive decrease in IgE antibodies in the serum (Fig. 23.21).
• decreased local recruitment of mast cells and basophils;
• decreased recruitment of eosinophils to the nose or lungs;
• increased IgG including IgG4 antibodies with progressive decreases in IgE – the IgG antibodies may act as blocking antibodies by binding allergen before it cross-links IgE on mast cells.
Modified forms of allergen-specific immunotherapy
Adjuvants can shift the immune response away from a simple TH2 response
Other forms of immune based non-specific therapy
Recombinant soluble IL-4R can block the biological activity of IL-4
• recombinant soluble IL-4 receptor (sIL-4R). Treatment with sIL-4R has proved moderately effective in clinical trials of allergic asthma. The mechanism is that sIL-4R binds to IL-4 before it can react with the receptor on T cells or B cells, and thus blocks its biological activity. However, it is less clear which of the many actions of IL-4 is relevant to the clinical effects:
The efficacy of sIL-4R provides indirect evidence for the role of T cells in allergic disease.
Some new treatment approaches may not be practical
The primary treatment of allergic disease is based on:
• pharmacological management including disodium cromoglycate, theophyline, leukotriene antagonists, and local corticosteroids; and
Critical thinking: Severe anaphylactic shock (see p. 442 for explanations)
| Investigation | Result (normal range) |
|---|---|
| hemoglobin (g/dL) | 14.2 (11.5–16.0) |
| white cell count (× 109/L) | 7.5 (4.0–11.0) |
| neutrophils (× 109/L) | 4.4 (2.0–7.5) |
| eosinophils (× 109/L) | 0.40 (0.04–0.44) |
| total lymphocytes (× 109/L) | 2.4 (1.6–3.5) |
| platelet count (× 109/L) | 296 (150–400) |
| serum immunoglobulins IgG (g/L) IgM (g/L) IgA (g/L) IgE (IU/mL) |
10.2 (5.4–16.1) 0.9 (0.5–1.9) 2.1 (0.8–2.8) 320 (3–150) |
| RAST bee venom wasp venom |
class 4 class 0 |
| skin prick tests | grade (0–5) |
| bee venom (10 μg/mL) | 3+ |
Akdis C.A., Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999;13:603–609.
Ali F.R., Kay A.B., Larche M. Airway hyperresponsiveness and bronchial mucosal inflammation in T cell peptide-induced asthmatic reactions in atopic subjects. Thorax. 2007;62:750–757.
Beaven M.A., Metzger H. Signal transduction by Fc receptors: the FcεRI case. Immunol Today. 1993;14:222–226.
Borish L., Rosenwasser L. TH1/TH2 lymphocytes: doubt some more. J Allergy Clin Immunol. 1997;99:161–164.
Chung C.H., Mirakhur B., Chan E., et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117.
Commins S.P., Satinover S.M., Hosen J., et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433.
Coyle A.J., Wagner K., Bertrand C., et al. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996;183:1303–1310.
Ege M.J., Mayer M., Normand A.C., et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709.
Galli S.J. New concepts about the mast cell. N Engl J Med. 1993;328:257–265.
Geha R.F. Regulation of IgE synthesis in humans. J Allergy Clin Immunol. 1992;90:143–150.
Haselden B.M., Kay A.B., Larch M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894.
Miller J.S., Schwartz L.B. Human mast cell proteases and mast cell heterogeneity. Curr Opin Immunol. 1989;1:637–642.
Montford S., Robinson H.C., Holgate S.T. The bronchial epithelium as a target for inflammatory attack in asthma. Clin Exp Immunol. 1992;22:511–520.
Platts-Mills T.A.E., Vervloet D., Thomas W.R., et al. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–S24.
Platts-Mills T.A.E., Vaughan J.W., Squillace S., et al. Sensitisation, asthma and a modified TH2 response in children exposed to cat allergen. Lancet. 2001;357:752–756.
Prausnitz C., Kustner H. Gell P.G.H., Coombes R.R.A. Clinical aspects of immunology. Oxford: Blackwell Scientific Publications; 1962:808–816.
Sporik R., Holgate S.T., Platts-Mills T.A.E., Cogswell J.J. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507.
Wan H., Winton H.L., Soeller C., et al. Der p1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133.
Wark P.A., Johnston S.L., Bucchieri F., et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947.
Wide L., Bennich H., Johansson S.G.O. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;ii:1105.