Imaging Techniques
Conventional Radiography
Conventional radiography is the creation of a projection image with the use of x-rays to view parts of the human body. This primary, cost-effective imaging method currently is the modality used most frequently and widely in clinical practice for evaluation of the respiratory system in pediatric patients. Conventional radiography is associated with a very low level of ionizing radiation exposure (in the range of 0.01 to 0.02 mSv for chest posteroanterior [PA] radiography).1,2 Soft tissue neck study and chest radiography, the two conventional radiographic imaging techniques used most frequently to assess the pediatric respiratory system, are discussed in the following sections.
Soft Tissue Neck Study
Inspiratory stridor resulting from an upper airway obstruction is the most frequent indication of a soft tissue neck study in pediatric patients.3 The common causes of inspiratory stridor include croup, epiglottitis, a foreign body, and an upper airway mass in infants and children. To obtain optimal diagnostic quality, the neck of the patient should be extended and images should be obtained at full inspiration. The standard soft tissue neck radiographic views consist of both an anteroposterior (AP) view and a lateral view. An additional expiratory lateral view of the neck subsequently may be obtained for assessment of subglottic stenosis, which can be beneficial in differentiating a fixed large airway disorder (e.g., subglottic stenosis) from a dynamic large airway disorder (e.g., tracheomalacia).
Chest Radiography
Chest radiography is the imaging study obtained most frequently to evaluate the respiratory system in pediatric patients. Immobilization is usually required in uncooperative infants and young children (<5 years) to achieve consistent image quality by decreasing motion artifacts and position deviations.4 Chest radiography is obtained during quiet inspiration in uncooperative infants and young children and during full inspiration in cooperative older pediatric patients. The standard chest radiographic views consist of the AP view in infants and young children (<5 years) and both the AP and PA views in older children, in addition to a lateral view. AP, PA, and lateral views of the chest can be obtained with the patient in the supine or erect position. Exposure parameters of the chest radiography should be appropriately optimized. However, unnecessary radiation to nonthoracic structures such as the lower neck, proximal upper extremity, and upper abdomen should be avoided by using appropriate collimation and shielding.5,6
In contrast to a radiographic screen-film system, image acquisition and display are decoupled in digital radiography, which allows increased versatility of image manipulations.7,8 One of the most commonly used image processing techniques in digital chest radiography is unsharp mask filtering or edge enhancement, which facilitates the detection of thoracic fine linear abnormalities or structures such as pneumothorax, vascular catheters, and the interlobar fissures, even in newborns.9 Recently, optimization of digital radiographic techniques has been emphasized to reduce potentially unnecessary overexposure to pediatric patients.6,10 The increased likelihood of overexposure in digital radiography, the so-called dose creep, is attributed not only to the lack of a standardized exposure index but also to the difficulty in recognizing overexposed radiographs by radiologic technologists and radiologists. Fortunately, a new standardized exposure index for digital radiography was proposed recently.11
Optimized techniques of digital chest radiography in children often are different from those in adults. For instance, automatic exposure control and grids are not particularly helpful in small pediatric patients. Additional views such as expiratory, decubitus, and oblique views sometimes are necessary to clarify abnormalities detected on standard AP or PA views or to solve unanswered clinical questions. Expiratory views may be used to confirm air trapping due to an underlying airway obstruction. The lateral decubitus view generally is used to detect freely shifting pleural effusion or air; it is used infrequently to demonstrate an air-fluid level in an intraparenchymal cavitary lesion. In addition, the lateral decubitus view may be used to demonstrate air trapping in the dependent lung or to clarify poorly defined lung opacities in the nondependent lung in uncooperative infants and young children.12,13 Oblique views may be helpful for evaluating rib, soft tissue, hilar, carinal, and peripheral lung abnormalities.
Fluoroscopy
Fluoroscopy can be used to evaluate dynamic large airway and lung abnormalities, such as airway obstruction, air trapping, and diaphragmatic palsy/paralysis. Use of fluoroscopic techniques and equipment should be optimized to minimize exposure of both pediatric patients and operators to harmful ionizing radiation by following the “imaging gently” and “step lightly” principles.14
Fluoroscopy-Guided Airway Study
A fluoroscopy-guided airway study may be helpful in demonstrating dynamic airway abnormalities, such as laryngomalacia, tracheomalacia, obstructive sleep apnea, and vocal cord dysfunction syndrome.15–17 Additionally, a fixed airway disorder such as stenosis or stricture also can be identified and differentiated from a dynamic airway disorder. Compared with endoscopic procedures for evaluating large airways, a fluoroscopy-guided airway study generally is inexpensive and less invasive.18 In recent years, the diagnostic role of the fluoroscopy-guided airway study has begun to be replaced by dynamic airway CT with a low radiation dose technique.19 Dynamic airway CT is more accurate than a fluoroscopy-guided airway study for evaluation of the location, degree, and extent of both the dynamic and fixed airway abnormalities. Furthermore, dynamic airway CT can provide additional information about other intrathoracic structures, which is a valuable benefit.19
Ultrasound
Ultrasound is a valuable and widely used imaging modality for evaluating the lungs and pleura, particularly in pediatric patients, because it is widely available, relatively easy to perform, and does not expose patients to radiation.20,21 Its real-time evaluation capability and portability are important additional benefits. Furthermore, crucial information regarding associated vascular structures or underlying blood flow also can be obtained with color Doppler ultrasound.20,21 Although chest radiography or CT is the main imaging modality of choice for evaluating the lungs and pleura, the complementary use of ultrasound may provide clinically relevant information in patients with certain conditions.20,21
Evaluation of Lungs
The main clinical indication for ultrasound of the lungs in pediatric patients is to characterize a peripheral lung opacity (i.e., parenchymal versus pleural disease) that has been detected with chest radiography.20 Frequent underlying etiologies include atelectasis, consolidation, lung necrosis, a lung abscess, congenital lung lesions, and a primary or metastatic lung neoplasm (Fig. 49-1).20,21 For evaluation of these abnormalities, conventional chest radiography should be carefully reviewed to localize the area of interest so the clinical question can be specifically answered with the ultrasound evaluation.20,21 Ultrasound evaluation of the lungs typically is performed with the patient in the supine or upright position. However, to improve the visualization in some selected situations, the lateral decubitus view or the supraclavicular and/or suprasternal notch view may be beneficial. Optimal views can be achieved by placing a pillow or blanket on the dependent side or behind the shoulder to help extend the neck of the patient.20
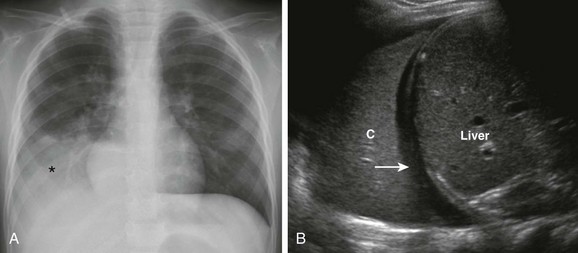
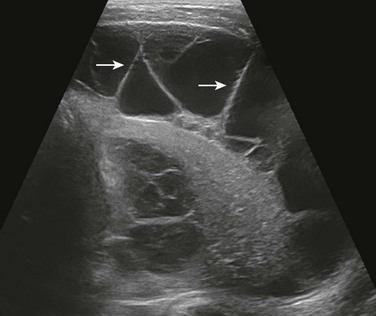
Figure 49-1 Plain radiography and ultrasound in a 3-year-old girl with fever, cough, and respiratory distress.
A, Frontal chest radiograph shows an opacity (asterisk) projecting over the right lower lung zone. B, Longitudinal ultrasound view of the right hemithorax demonstrates a consolidated lung (C), small effusion (arrow), and liver.
The choice of ultrasound transducer depends primarily on three factors: the age of the patient, the size of the patient, and the location of the abnormality.20,21 Although curved or linear array transducers typically are used to evaluate peripheral lung opacity, transducers that are smaller in size, such as sector or vector transducers, may be necessary for imaging in infants or young children who have a small available acoustic window (such as between the ribs). High-frequency transducers (e.g., 7.5 to 15.0 MHz) are helpful in evaluating the chest in infants and young children who do not have a substantial amount of subcutaneous fat because they provide higher resolution ultrasound images with a limited ability for soft-tissue penetration.20 Conversely, use of low-frequency transducers (e.g., <5 MHz) may be necessary when evaluating older children who have a large amount of subcutaneous fat because these transducers provide better soft tissue penetration, although the ultrasound image resolution is decreased.20 Color Doppler ultrasound is useful for evaluating lung lesions with underlying vascular structures in cases of pulmonary sequestration or blood flow in cases involving a neoplasm.
Evaluation of Pleura
Ultrasound can be useful in differentiating pleural fluid from atelectasis and/or consolidation when the diagnosis is equivocal on the basis of conventional radiography. Furthermore, ultrasound is more sensitive and accurate than conventional radiography or CT for characterizing pleural fluid, which may be simple or complex (Fig. 49-2).20 Ultrasound evaluation of patients in both the erect and lateral decubitus positions can be useful for differentiating between freely flowing and loculated pleural fluid. In addition, ultrasound can visualize the internal debris, septations, and pleural thickening often associated with parapneumonic collections. Such complex pleural effusion often requires a drainage procedure that can be facilitated with the guidance of ultrasound.
Computed Tomography
CT is a valuable cross-sectional imaging study for evaluating pediatric airways and lungs, mainly because its images provide excellent air-tissue contrast. The development of multidetector CT (MDCT), which can provide a short scan time with a decreased rate of sedation, has increased the role of CT in the assessment of pediatric airways and lungs.22 Respiratory motion artifacts often degrade CT image quality in infants and young children who breathe freely during the scan. These artifacts can be reduced with the use of general anesthesia, and controlled ventilation.23,24 These techniques also can be used to control either the inspiratory or expiratory phases of respiration.23–25 In addition, recently introduced high-pitch dual-source spiral CT scanning is very helpful in reducing motion artifacts on chest CT scans of children who breathe freely during the procedure.26
Reducing the CT radiation dose while maintaining diagnostic image quality is of critical importance in pediatric patients because of the greater radiosensitivity and longer life expectancy of children, coupled with increasing CT use. Low-dose, body size–adapted chest CT protocols using variable tube voltages and tube currents have been established, usually on the basis of body weight.27,28 However, several recent studies have demonstrated that cross-sectional dimensions provide better CT dose adaptation to body habitus.29–31 A practical pediatric chest CT protocol based on a volume CT dose index individually determined by cross-sectional area and mean density of the body recently was developed for daily clinical use.32 The tube current always should be modulated if applicable because this modulation allows the CT dose to be reduced substantially without degrading image quality.33–35 Electrocardiogram-triggered sequential scanning may be used in CT of the chest in children to achieve fewer motion artifacts and a lower radiation dose.24,36
The image quality of CT of the chest in children may be further improved by adjusting kilovolt levels and reconstruction algorithms as follows: a low kilovolt level for enhanced CT and a high kilovolt level for unenhanced CT, a high-frequency reconstruction algorithm for lung evaluation, and a standard reconstruction algorithm for mediastinal evaluation. In addition to axial CT images, postprocessed and reconstructed images such as multiplanar reformatted (MPR) and three-dimensional (3D) images considerably increase diagnostic accuracy of pediatric chest CT (Fig. 49-3).37 CT scanning with thinner collimation (<1 mm) offers better quality MPR and 3D images. Various visualization techniques including maximum intensity projection, minimum intensity projection, volume rendering, and virtual bronchoscopy can help further visualize thoracic abnormalities (see Fig. 49-3).
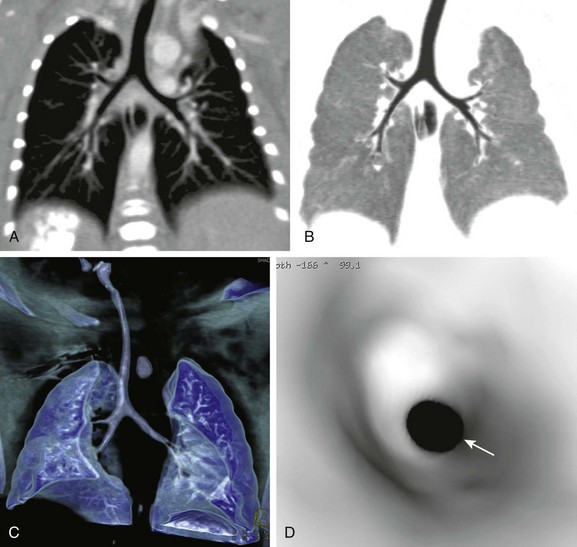
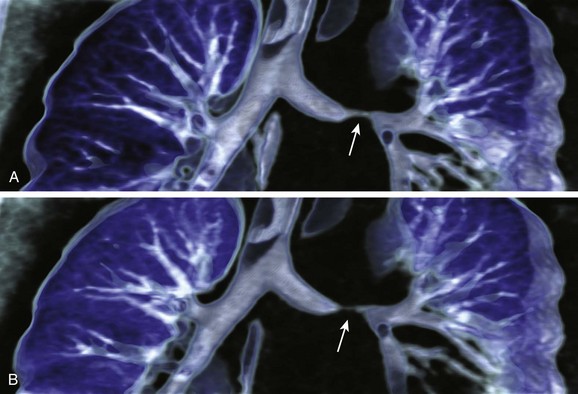
Figure 49-3 Multiplanar reformatted and three-dimensional (3D) computed tomography (CT) imaging.
A, A coronal thin-slab CT image shows normal relationships between the airway and the pulmonary artery, indicating the thoracic situs solitus. B, A coronal minimum-intensity projection CT image shows anatomic details of the central airways. C, A volume-rendered CT image shows the 3D appearance of the lungs and airways. D, A virtual bronchoscopic image providing an endoscopic view of the trachea shows a concentric narrowing (arrow) from a tracheal web.
Evaluation of Airways
Anatomic details of the pediatric airways can be imaged with CT.38,39 However, small airways distal to the subsegmental level (i.e., airways <0.5 to 1.5 mm in diameter) often are invisible on CT because of their size. CT is less invasive than bronchoscopy in evaluating pediatric airways and provides cross-sectional imaging without superimposition. Various airway abnormalities, including fixed or dynamic obstruction, bronchiectasis, and wall thickening, can be diagnosed with CT. MRI may be used to evaluate large airways with comparable diagnostic accuracy, but MRI is clearly inferior to CT in evaluating small airways because of lower spatial resolution and a lower signal/noise ratio.
Static Airway CT Study
A static airway CT study is performed mainly to assess a fixed airway narrowing or stenosis. Axial CT data are acquired in infants and young children while they breathe freely and in older children while they hold their breath at the end of an inspiration (Fig. 49-3, C). Anatomic details of the airway typically are well visualized, even in free-breathing studies, because of the fast scan speed of MDCT (>16 row MDCT). The extent and cause of airway abnormalities can be better evaluated with MPR and 3D CT images than with axial CT images alone.
Dynamic Airway Computed Tomographic Study
A paired inspiratory and expiratory CT study is performed primarily for the dynamic evaluation of a large airway disorder, most commonly tracheobronchomalacia.19 Expiratory CT is performed with the low radiation dose technique to minimize the dose delivered during this dual-phase study (Table 49-1). The resultant effective dose from this paired study typically is in the range of 3.5 to 7.5 mSv.19 Expiratory CT can be obtained with two different respiratory maneuvers: (1) holding the breath at the end of an expiration during end-expiratory CT and (2) forced exhalation during dynamic expiratory CT. In uncooperative infants and young children, general anesthesia with intubation, controlled ventilation, or respiratory triggering can be used.19,23,24,39
Table 49-1
Tube Current and Kilovoltage by Patient Weight for Central Airway Multidetector Computed Tomography
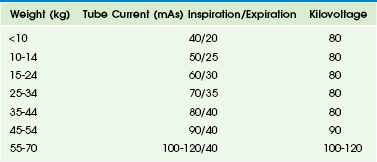
From Lee EY, Boiselle PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation, Radiology. 2009;252(1):7-22.
Cine Airway Computed Tomographic Study
In cine airway CT, axial sequential scans are acquired continuously at predefined noncontiguous slices with high resolution and use of the low radiation dose technique throughout the respiratory cycle.40 This technique allows changes in the cross-sectional area and shape of the airway to be demonstrated throughout the entire respiratory cycle, thus making the diagnosis of tracheobronchomalacia possible. To achieve the high temporal resolution required for cine airway CT, the fastest gantry rotation speed is used. The total scan time should be tailored to a range of one to two respiratory cycles to minimize the radiation dose. As a result, the radiation dose of cine airway CT is quite low—in the range of 0.2 mSv to 0.3 mSv.24 Cine airway CT is performed in infants and young children as they breathe freely or with a coughing maneuver in cooperative older children.19,41 Coughing exaggerates the expiratory collapse of the airway because it elicits a higher intrathoracic extra-airway pressure than does normal or forced exhalation, and thus it is the preferred technique for a cine airway CT study. In addition to dynamic airway evaluation, cine airway CT also can be used to detect trapping of air in the lung, which often is a secondary sign of underlying small airway disease.40
Four-Dimensional Airway Computed Tomographic Study
Increased longitudinal coverage (e.g., 4 cm for 64-section CT and 16 cm for 320-section CT) of modern MDCT makes a four-dimensional (4D) airway CT study feasible.42 As in a cine airway CT study, an axial sequential CT scan is continuously acquired without table movement throughout the respiration cycle in 4D airway CT. The major difference from cine airway CT is the real-time volumetric coverage of almost the entire central airway in infants and young children (Fig. 49-4).
Evaluation of Lungs
CT is valuable in evaluating not only the air spaces (which may be either opaque or hyperlucent) but also interstitial and vascular abnormalities and parenchymal nodules or masses in the lung. In addition, mediastinal, hilar, and chest wall abnormalities also can be assessed. However, soft tissue contrast resolution of CT is inferior to that of MRI. Furthermore, MRI is better than CT in evaluating intraspinal and paraspinal regions. However, expiratory CT is necessary to identify air trapping accurately.40,43
To obtain proper expiratory CT data, patient cooperation is required. In uncooperative infants and young children, lateral decubitus CT may be used as an alterative.44 In this position, dependent and nondependent lungs show different lung volumes that mimic inspiration and expiration, respectively (Fig. 49-5, A). However, a potential drawback of the lateral decubitus CT technique is the need to change the position of the patient, which may awaken the patient, thus making interpretation difficult because of considerable respiratory misregistration between the dependent and nondependent lungs and exaggerated respiratory motion artifacts in the nondependent lung. Cine CT in the supine position can overcome some of the limitations of lateral decubitus CT (Fig. 49-5, B and C).40 Recently, dual-energy chest CT has been used in pediatric patients to assess regional lung perfusion and ventilation.45–51 By using 3D CT data, quantitative analysis to determine lung volume or density can be performed.
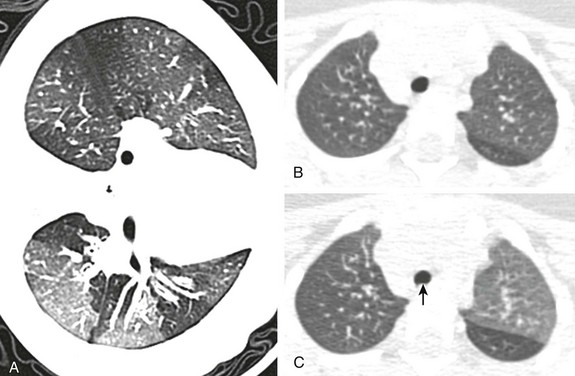
Figure 49-5 Expiratory computed tomography (CT) techniques in children breathing freely.
A, A left side down lateral decubitus CT scan shows geographic hyperlucent areas due to trapping of air, the so-called mosaic lung attenuation, in the dependent left lung of a child with bronchiolitis obliterans. Inspiratory (B) and expiratory (C) cine CT images show the normal left upper lobe and the abnormal right upper and left lower lobes with trapping of air. The normal posterior indentation of the trachea (arrow) is noted at expiration (C).
Routine Chest Computed Tomographic Study (Without or with Intravenous Contrast)
Routine chest CT is performed without or with intravenous administration of an iodinated contrast agent, depending on the clinical questions being addressed. As previously mentioned, tube voltage should be determined with the use of an iodinated contrast agent. High tube voltage should be used for an unenhanced chest CT scan, whereas low tube voltage should be selected for an enhanced chest CT scan. The use of precontrast and postcontrast (dual-phase) chest CT should be avoided in pediatric patients as much as possible to minimize exposure to radiation. Virtual unenhanced CT imaging using the dual-energy technique may substitute for real unenhanced CT imaging, thus reducing radiation dose.51
High-Resolution Computed Tomographic Study
High-resolution CT (HRCT) originally was developed in the 1980s as a special technique in which noncontiguous thin sections, approximately 1 mm in slice thickness, are acquired at 7- to 20-mm intervals depending on the size of the patient.52 To illustrate fine details of the lung anatomy and pathology, a high spatial frequency reconstruction algorithm is used. This classic HRCT study has been used principally as a follow-up imaging study of diffuse lung abnormalities, such as interstitial lung disease or cystic fibrosis, while delivering a very low radiation dose. The recent development of MDCT allows contiguous thin sections of the entire lung to be viewed with a reasonably low radiation dose and MPR capabilities, which changes the technical concept of HRCT of the lung. MDCT with thin collimation (<1 mm), single spiral CT scanning can provide both thick sections (for a routine chest CT with standard reconstruction algorithm) and thin sections (for HRCT of the lung with high spatial frequency reconstruction algorithm) without additional scanning radiation exposure. It has been reported that reconstructed HRCT images from volumetric MDCT acquisition have significantly less motion artifact than images obtained with traditional axial HRCT acquisition in pediatric patients.52–55 Nonetheless, the classic HRCT technique still may be used in pediatric patients because of the overall decreased radiation with this CT technique compared with MDCT volumetric data acquisition.
Magnetic Resonance Imaging
Lack of ionizing radiation exposure and excellent soft tissue contrast are advantages of MRI. Different tissue characteristics can be evaluated with T1- and T2-weighted imaging and, more recently, balanced steady-state free precession imaging. Fat saturation may be added to the pulse sequence if necessary. The use of gadolinium contrast agents can further improve tissue characterization on T1-weighted imaging. In addition, a variety of functional thoracic assessments including perfusion, ventilation, and respiratory mechanics can be performed with MRI.56,57 Water diffusivity or cellularity of thoracic masses may be evaluated with diffusion-weighted imaging (Fig. 49-6). Hyperpolarized gas MRI allows excellent static and dynamic visualization of the lung and airway and may overcome the limitations of proton MRI of the thorax. However, hyperpolarized gas MRI has markedly limited availability, which is the main obstacle to clinical use and the primary reason it remains in the research realm. It should be noted that chest MRI examination cannot be performed in some circumstances (e.g., when contraindications for MRI exist, such as the presence of a permanent cardiac pacemaker, the presence of a cardioverter defibrillator, or claustrophobia).
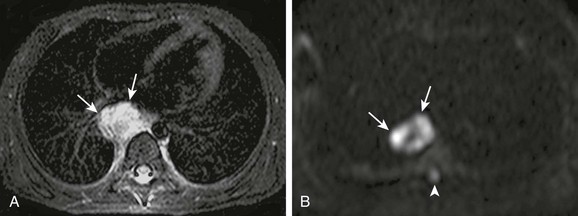
Figure 49-6 Diffusion-weighted magnetic resonance imaging (MRI).
A, Axial T2-weighted chest MRI using a short tau inversion recovery sequence shows a metastatic neuroblastoma involving a paraesophageal lymph node (arrows). B, On axial diffusion-weighted MRI (b-value, 800 s/mm2), hyperintense areas are predominant in the lesion (arrows), indicating restricted water diffusion or hypercellularity. Of note, the normal spinal cord (arrowhead) appears modestly hyperintense.
Evaluation of Airways
Central airways and their relationships to adjacent cardiovascular structures can be assessed with noncontrast black-blood MRI (Fig. 49-7).58 Tracheobronchomalacia can be diagnosed with real-time dynamic airway MRI. However, it is preferable to perform airway evaluations with CT. The delineation of peripheral small airways on MRI is somewhat limited because of relatively low spatial resolution.
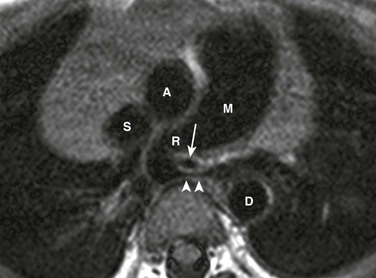
Figure 49-7 Black-blood magnetic resonance imaging (MRI).
An axial T1-weighted, electrocardiogram-triggered chest MRI using spin-echo echo-planar imaging and the black-blood technique shows an intimate relationship between the left pulmonary artery sling and congenital tracheal stenosis (arrow), the so-called “sling-ring” complex. Note a severe focal stenosis (arrowheads) of the left pulmonary artery between the trachea and the spine. M, main pulmonary artery; R, right pulmonary artery; A, ascending aorta; D, descending aorta; S, superior vena cava.
Evaluation of Lungs
Despite the inherent limitations of lung MRI that were previously mentioned, a study showed that lung nodules of a diameter larger than 3 to 4 mm can be detected with MRI with use of 1.5T systems.59 Contrast-enhanced time-resolved magnetic resonance angiography offers not only anatomic details of pulmonary vessels but also hemodynamics of pulmonary circulation. Because contrast bolus timing is not needed and less motion artifact occurs because of high temporal resolution (e.g., <1 second for the entire lung), time-resolved MR angiography often replaces static high-resolution MR angiography in pediatric patients.60 Lung perfusion MRI can be obtained with either the contrast-enhanced or non–contrast-enhanced technique (Fig. 49-8). Real-time dynamic chest MRI with the patient breathing freely may be used to demonstrate normal and abnormal respiratory dynamics.61 Regional ventilation may be assessed with oxygen-enhanced MRI or hyperpolarized gas (3helium or 129xenon) MRI. Diffusion-weighted imaging using hyperpolarized gas enables calculation of the size of peripheral air spaces. Hyperpolarized xenon MRI has an additional capability of assessing diffusing capacity in the lung. A preliminary study recently reported a promising free-breathing proton MRI technique without any contrast agents that demonstrated both lung perfusion and lung tissue density in a single data acquisition by means of Fourier decomposition.62 Although MRI may provide anatomic and functional information of the lung without radiation, CT generally is preferred in pediatric patients, mainly because of its high spatial resolution.
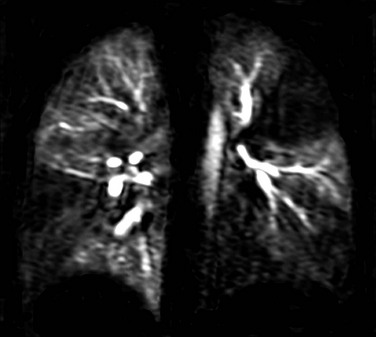
Figure 49-8 Contrast-enhanced lung perfusion magnetic resonance imaging.
A coronal image obtained from time-resolved magnetic resonance angiography shows maximal contrast enhancement in the normal lung areas and multiple lung perfusion defects. The signal outside the lung is suppressed by means of subtraction.
Nuclear Medicine Studies
Many nuclear medicine techniques have an important advantage over anatomic imaging methods in some clinical settings because they have the ability to evaluate physiologic processes. Several radionuclide techniques are commonly are used to evaluate pediatric diseases of the respiratory system or chest wall, as listed in Table 49-2. Most nuclear medicine studies are performed with an intravenous injection of a radiopharmaceutical agent. The biodistribution and subsequent route of clearance of the tracer determine the amount and organ distribution of radiation exposure to the patient. Many nuclear medicine procedures carry inherently higher radiation exposure to the patient than plain film radiographic procedures, but the radiation exposure is comparable to or less than that accompanying CT examinations. It is important to use the lowest possible injected tracer dosage that allows good-quality images to be recorded. Although accepted standard adult radiopharmaceutical dosages often are established, dosage determination in children is best calculated on the basis of weight, which in turn is derived from the adult reference administered activity.63 In recent years, considerable efforts have been made to ensure that pediatric dosages are as low as possible while remaining practical. Current recommendations are presented in Table 49-2.
Table 49-2

Modified from Gelfand MJ, Parisi MT, Treves ST. Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines, J Nucl Med. 2011;52:318-322.
Lung Perfusion and Ventilation Study
Lung perfusion imaging, although largely supplanted by CT techniques for the diagnosis of pulmonary embolus, remains an important technique for the evaluation of lung physiology and pathology. The incidence of pulmonary embolism in the pediatric population is lower than in the adult population, but occasionally perfusion imaging is used with patients who have significant risk factors to evaluate for an embolus when a contraindication to the administration of radiographic contrast material exists. Nuclear ventilation and perfusion imaging is much more commonly used to evaluate relative function in diseases in which knowledge of physiology helps to guide clinical care. Both relative ventilation and perfusion can be quantitated, which permits treatment planning and follow-up evaluation in a variety of clinical entities such as lung malformations resulting from congenital lobar emphysema and congenital pulmonary airway malformations (Fig. 49-9). The quantitative analysis of relative lung perfusion and ventilation also is useful in preoperative cases before pneumonectomy to evaluate for the estimated postsurgical lung capacity.64
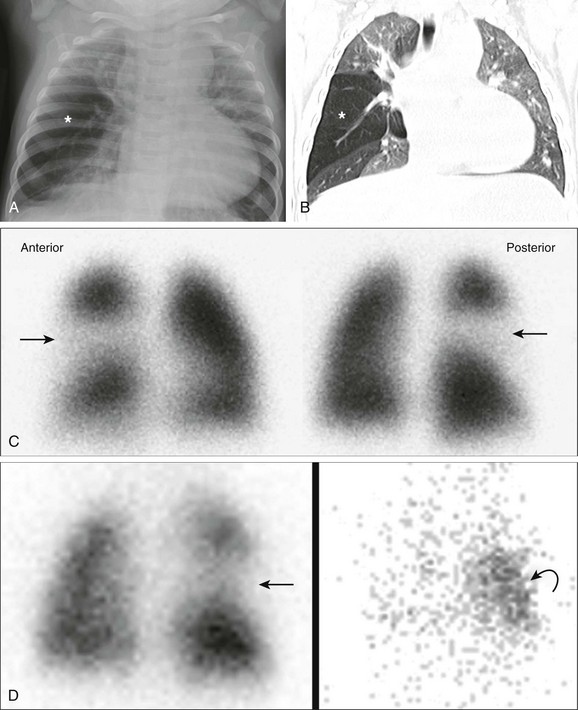
Figure 49-9 Multimodality imaging of a 7-month-old girl with congenital lobar emphysema.
A, A frontal chest radiograph shows hyperlucency (asterisk) in the right mid to lower lung zone. B, A coronal lung window computed tomography image demonstrates hyperinflation and oligemia of the right middle lobe (asterisk), consistent with congenital lobar emphysema. C, A perfusion lung scan shows no perfusion to the right middle lobe (arrow) on the anterior view (Anterior) and posterior view (Posterior). D, A posterior image from a ventilation scan on the left showing initial decreased ventilation to the right middle lung (straight arrow) with air trapping in the same area of the later images on the right (curved arrow).
Patients with congenital heart disease frequently undergo ventilation and perfusion imaging to quantitate relative perfusion between the right and left lung at baseline and after catheter intervention (Fig. 49-10).
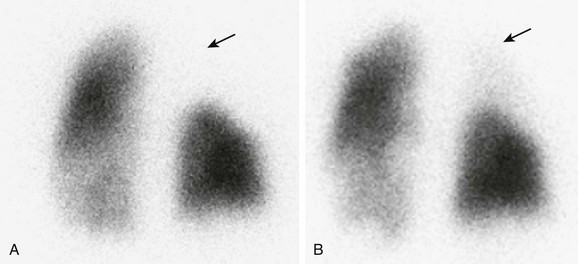
Figure 49-10 Nuclear medicine perfusion imaging in a 20-year-old man with tetralogy of Fallot.
A, A posterior view of the lung scan shows decreased perfusion to the right upper lung (arrow). B, After placement of a right upper pulmonary artery stent, the perfusion study now shows some perfusion to the right upper lung (arrow).
The perfusion study is performed with technetium-99m (99mTc) macroaggregated albumin (MAA). The dosage in pediatrics is based on patient weight (Table 49-2). In cases of congenital heart disease when differential lung perfusion is evaluated, a specially prepared radiopharmaceutical agent with a low concentration of particles of MAA is recommended. The tracer is always injected with the patient supine so a hydrostatic gradient is not present from lung apex to base. Imaging can be performed with the patient either upright or supine, but supine imaging typically is performed in children. If imaging is being performed to evaluate pulmonary physiology, typically only anterior and posterior planar views are acquired. The differential lung perfusion is then calculated by drawing a region of interest around each lung and calculating the geometric mean (i.e., the square root of the product of anterior and posterior views) of counts in each lung. If the perfusion scan is being performed to evaluate for a pulmonary embolism, additional anatomic information is recorded, with a standard of eight planar images (i.e., anterior, posterior, left and right lateral, right anterior oblique, left anterior oblique, right posterior oblique, and left posterior oblique views). Alternatively, single photon emission computed tomography (SPECT) imaging of the lungs can be performed. When evaluating for a possible pulmonary embolus, a correlation should be made with a chest radiograph obtained within 24 hours of the study.
Salivagram
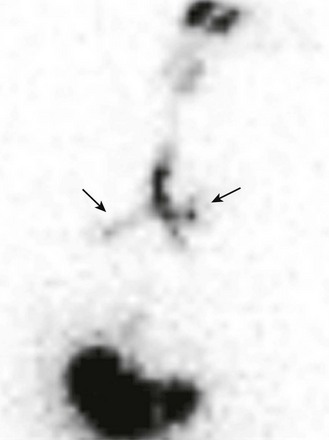
Radionuclide evaluation for salivary aspiration (i.e., a salivagram) offers a safe and easily performed test with minimal radiation exposure to the patient. The sensitivity of the salivagram has been reported to range between 26% and 73%.65–67 The salivagram can be performed with the administration of a small drop (approximately 100 µL) of 99mTc sulfur colloid (300 µCi [11.1 MBq]) that is placed in the oral cavity while the patient is lying in a supine position in the imaging bed. After administration of the radiopharmaceutical agent, the patient is allowed to swallow normally and posterior planar imaging of the mouth, chest, and upper abdomen is obtained dynamically for 60 minutes. Imaging is acquired using a low-energy, high-resolution or ultra–high-resolution collimator, with consecutive dynamic images recorded every 30 seconds for a total of 120 frames. A positive salivagram for aspiration shows the radiopharmaceutical agent entering the bronchi (Fig. 49-11).
Neuroendocrine Imaging Study
Neuroendocrine tumors can be imaged very effectively with use of iodine-123–labeled metaiodobenzylguanidine (123I MIBG). The two tumors most commonly evaluated with this tracer are pheochromocytomas and neuroblastomas (Fig. 49-12). Although other tumors such as ganglioneuromas, carcinoid tumors, and medullary thyroid carcinoma also can be imaged with this tracer, they show a lower sensitivity of detection. MIBG is a norepinephrine analog, and its uptake in malignancy can be used both to evaluate a primary tumor and metastasis at presentation and to evaluate tumor response after treatment. It is important to review the patient’s medications before imaging because a number of pharmaceutical agents have the potential to block the tumor uptake of MIBG. These agents include preparations that contain ephedrine and its derivatives, along with neuroleptic drugs, tricyclic antidepressants, and central nervous system stimulants. The MIBG dose always contains a small amount of unlabeled radioiodine that will localize within the thyroid gland, thus increasing local radiation exposure. Patient radiation dosimetry can be reduced by administering nonradioactive iodine to block this uptake before the procedure. The increased circulating nonradioactive iodine reduces subsequent extraction of radioactive iodine and is easily achieved by administering a strong solution of potassium iodide (1 drop three times a day) beginning 1 day before the injection and continuing for 3 days after the tracer injection.
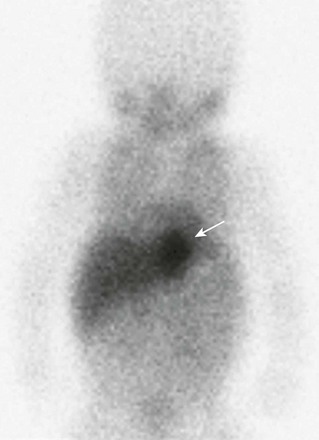
Figure 49-12 Neuroendocrine tumor imaging in a 4-week-old girl with a left-sided thoracic mass that was first detected on prenatal imaging.
A 123I metaiodobenzylguanidine study shows intense uptake by the mass (arrow). The patient underwent surgical resection of the mass. The histology result was consistent with neuroblastoma.
Bone Scan
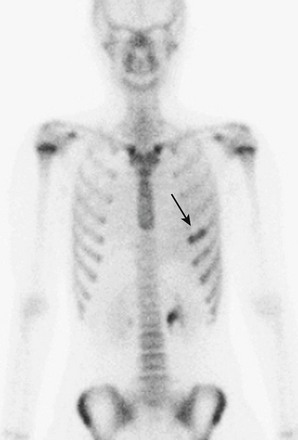
A bone scan is used frequently in the evaluation of the thoracic skeleton, such as in the evaluation of chest wall pain (Fig. 49-13). 99mTc-labeled methylenediphosphonate is the most common radiopharmaceutical agent used for bone scans. This tracer is administered intravenously, and imaging is performed from 2 to 4 hours after administration, using a whole body sweep (anterior and posterior) or individual images of the area of interest. SPECT imaging also can be performed. This technique is particularly useful in the evaluation of the ribs and spine. When evaluating a patient for suspected osteomyelitis, initial blood flow images are recorded immediately after the administration of tracer, in addition to the standard delayed imaging.
Positron Emission Tomography
Positron emission tomography (PET) is useful in evaluating many primary and metastatic intrathoracic tumors in pediatric patients (Fig. 49-14). Pediatric tumors commonly evaluated with PET include Hodgkin lymphoma, non-Hodgkin lymphoma, rhabdomyosarcoma, osteosarcoma, Ewing sarcoma, neuroblastoma, and other less common malignancies.
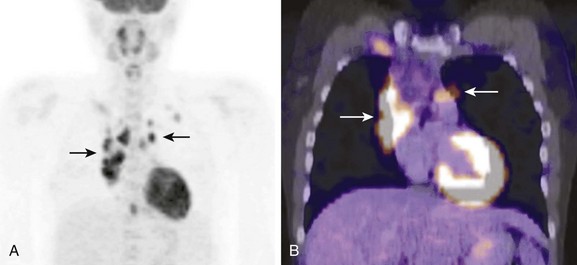
Figure 49-14 Positron emission tomography (PET) imaging in a 19-year-old with newly diagnosed Hodgkin disease.
A, A coronal PET image shows multiple sites of abnormal uptake in the anterior mediastinum (arrows). B, Fusion of a PET and computed tomography (CT) image shows the abnormal area of uptake corresponding to the enlarged lymph nodes on CT (arrows).
Another important aspect of pediatric PET studies is the high incidence of metabolically active brown fat activation compared with the adult population. The presence of brown fat increases the number of equivocal studies.68 Warming patients at 24° C before and during the FDG uptake phase decreases the incidence of brown fat activation significantly.69
In the evaluation of tumors, PET provides an advantage over anatomic imaging because it detects viable tissue. Use of PET in the evaluation of Hodgkin lymphoma has been shown to increase the detection of metastases when compared with CT alone.70
Goo, HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010;11:4–18.
John, SD, Swischunk, LE. Stridor and upper airway obstruction in infants and children. Radiographics. 1992;12:625–643.
Lee, EY, Greenberg, SB, Boiselle, PM. Multidetector computed tomography of pediatric large airway diseases: state-of-the-art. Radiol Clin North Am. 2011;49(5):869–893.
Lee, EY. Advancing CT and MR imaging of the lungs and airways in children: imaging into practice. Pediatr Radiol. 2008;38:S208–S212.
Treves, ST, Baker, A, Fahey, FH, et al. Nuclear medicine in the first year of life. J Nucl Med. 2011;52(6):905–925.
Willis, CE. Optimizing digital radiography of children. Eur J Radiol. 2009;72:266–273.
References
1. Linet, MS, Kim, KP, Rajaraman, P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. 2009;39(suppl 1):S4–S26.
2. Mettler, FA, Jr., Huda, W, Yoshizumi, TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263.
3. John, SD, Swischunk, LE. Stridor and upper airway obstruction in infants and children. Radiographics. 1992;12:625–643.
4. Kohda, E, Tsutsumi, Y, Nagamoto, M, et al. Revisit image control for pediatric chest radiography. Radiat Med. 2007;25:60–64.
5. Soboleski, D, Theriault, C, Acker, A, et al. Unnecessary irradiation to non-thoracic structures during pediatric chest radiography. Pediatr Radiol. 2006;36:22–25.
6. Willis, CE. Computed radiography: a higher dose? Pediatr Radiol. 2002;32:745–750.
7. MacMahon, H, Vyborny, C. Technical advances in chest radiography. AJR Am J Roentgenol. 1994;163:1049–1059.
8. Willis, CE. Optimizing digital radiography of children. Eur J Radiol. 2009;72:266–273.
9. Goo, HW, Kim, HJ, Song, KS, et al. Using edge enhancement to identify subtle findings on soft-copy neonatal chest radiographs. AJR Am J Roentgenol. 2001;177:437–440.
10. Don, S. Pediatric digital radiography summit overview: state of confusion. Pediatr Radiol. 2011;41:567–572.
11. Seibert, JA, Morin, RL. The standardized exposure index for digital radiography: an opportunity for optimization of radiation dose to the pediatric population. Pediatr Radiol. 2011;41:573–581.
12. Capitanio, MA, Kirkpatrick, JA. The lateral decubitus film. An aid in determining air-trapping in children. Radiology. 1972;103:460–462.
13. Kaufman, AS, Kuhns, LR. The lateral decubitus view: an aid in evaluating poorly defined pulmonary densities in children. AJR Am J Roentgenol. 1977;129:885–888.
14. Strauss, KJ, Kaste, SC. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—a white paper executive summary. Pediatr Radiol. 2006;36(suppl 2):110–112.
15. Berg, E, Naseri, I, Sobol, SE. The role of airway fluoroscopy in the evaluation of children with stridor. Arch Otolaryngol Head Neck Surg. 2008;134:415–418.
16. Thakkar, K, Yao, M. Diagnostic studies in obstructive sleep apnea. Otolaryngol Clin N Am. 2007;40:785–805.
17. Nastasi, KJ, Howard, DA, Raby, RB, et al. Airway fluoroscopic diagnosis of vocal cord dysfunction syndrome. Ann Allergy Asthma Immunol. 1997;78:586–588.
18. Donnelly, LF, Strife, JL, Myer, CM, 3rd. Is sedation safe during dynamic sleep fluoroscopy of children with obstructive sleep apnea? AJR Am J Roentgenol. 2001;177:1031–1034.
19. Lee, EY, Boiselle, PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation. Radiology. 2009;252:7–22.
20. Lee, EY, Siegel, MJ. Paediatric chest. In Allan PL, Baxter GM, Weston MJ, eds.: Clinical ultrasound, 3rd ed, London: Churchill Livingstone Elsevier, 2011.
21. Coley, BD. Chest sonography in children: current indications, techniques, and imaging findings. Radiol Clin North Am. 2011;49(5):825–846.
22. Lemos, AA, Siegel, MJ, Rossi, G, et al. Single- versus multidetector-row CT: comparison of sedation rates, conventional angiograms and motion artifacts in young children following liver transplantation. Radiol Med. 2006;111:911–920.
23. Long, FR, Williams, RS, Adler, BH, et al. Comparison of quiet breathing and controlled ventilation in the high-resolution CT assessment of airway disease in infants with cystic fibrosis. Pediatr Radiol. 2005;35:1075–1080.
24. Goo, HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010;11:4–18.
25. Mueller, KS, Long, FR, Flucke, RL, et al. Volume-monitored chest CT: a simplified method for obtaining motion-free images near full inspiratory and end expiratory lung volumes. Pediatr Radiol. 2010;40:1663–1669.
26. Lell, MM, May, M, Deak, P, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011;46:116–123.
27. Yang, DH, Goo, HW. Pediatric 16-slice CT protocol: radiation dose and image quality. J Korean Radiol Soc. 2008;59:333–347.
28. Kim, JE, Newman, B. Evaluation of a radiation dose reduction strategy for pediatric chest CT. AJR Am J Roentgenol. 2010;194:1188–1193.
29. Boone, JM, Geraphty, EM, Seibert, JA, et al. Dose reduction in pediatric CT: a rational approach. Radiology. 2003;228:352–360.
30. Nyman, U, Ahl, TL, Kristiansson, M, et al. Patient-circumference-adapted dose regulation in body computed tomography. A practical and flexible formula. Acta Radiol. 2005;46:396–406.
31. Jung, YY, Goo, HW. The optimal parameter for radiation dose in pediatric low dose abdominal CT: cross-sectional dimensions versus body weight. J Korean Radiol Soc. 2008;58:169–175.
32. Goo, HW. Individualized volume CT dose index determined by cross-sectional area and mean density of the body to achieve uniform image noise of contrast-enhanced pediatric chest CT obtained at variable kV levels and with combined tube current modulation. Pediatr Radiol. 2011;41:839–847.
33. Goo, HW, Suh, DS. Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol. 2006;36:344–351.
34. Goo, HW, Suh, DS. The influences of tube voltage and scan direction on combined tube current modulation: a phantom study. Pediatr Radiol. 2006;36:833–840.
35. Peng, Y, Li, J, Ma, D, et al. Use of automatic tube current modulation with a standardized noise index in young children undergoing chest computed tomography scans with 64-slice multidetector computed tomography. Acta Radiol. 2009;50:1175–1181.
36. Pauls, S, Aschoff, AJ, Whal, J, et al. Multi-detector row CT: is prospective electrocardiographic triggering improving the detection of small pulmonary tumors? Acad Radiol. 2005;12:614–619.
37. Siegel, MJ. Multiplanar and three-dimensional multi-detector row CT of thoracic vessels and airways in the pediatric population. Radiology. 2003;229:641–650.
38. Papaioannou, G, Young, C, Owen, CM. Multidetector row CT for imaging the paediatric tracheobronchial tree. Pediatr Radiol. 2007;37:515–529.
39. Long, FR. Imaging evolution of airway disorders in children. Radiol Clin North Am. 2005;43:371–389.
40. Goo, HW, Kim, HJ. Detection of air trapping on inspiratory and expiratory phase images obtained by 0.3-sec cine-CT in the lungs of free breathing young children. AJR Am J Roentgenol. 2006;187:1019–1023.
41. Shin, JH, Goo, HW. Tracheomalacia in infants and children: detection by free-breathing cine CT (abstract VP32-11). In: Program in brief of the 96th Scientific Assembly and Annual Meeting of Radiological Society of North America. Chicago: Radiological Society of North America; 2010.
42. Wagnetz, U, Roberts, HC, Chung, T, et al. Dynamic airway evaluation with volume CT: initial experience. Can Assoc Radiol. 2010;61:90–97.
43. Lee, EY, Tracy, DA, Bastos, M, et al. Expiratory volumetric MDCT evaluation of air trapping in pediatric patients with and without tracheomalacia. AJR Am J Roentgenol. 2010;194:1210–1215.
44. Choi, SJ, Choi, BK, Kim, HJ, et al. Lateral decubitus HRCT: a simple technique to replace expiratory CT in children with air trapping. Pediatr Radiol. 2002;32:179–182.
45. Goo, HW. Initial experience of dual-energy lung perfusion CT using dual-source CT system in children. Pediatr Radiol. 2010;40:1536–1544.
46. Chae, EJ, Seo, JB, Goo, HW, et al. Xenon ventilation CT with a dual-energy technique of dual-source CT: initial experience. Radiology. 2008;248:615–624.
47. Goo, HW, Chae, EJ, Seo, JB, et al. Xenon ventilation CT using a dual-source dual-energy technique: dynamic ventilation abnormality in a child with bronchial atresia. Pediatr Radiol. 2008;38:1113–1116.
48. Goo, HW, Yang, DH, Hong, SJ, et al. Xenon ventilation CT using dual-source and dual-energy technique in children with bronchiolitis obliterans: correlation of xenon and CT density values with pulmonary function test results. Pediatr Radiol. 2010;40:1490–1497.
49. Goo, HW, Yang, DH, Kim, N, et al. Collateral ventilation to congenital hyperlucent lung lesions assessed on xenon-enhanced dynamic dual-energy CT: an initial experience. Korean J Radiol. 2011;12:25–33.
50. Goo, HW, Yu, J. Redistributed regional ventilation after the administration of a bronchodilator and demonstrated on xenon-inhaled dual-energy CT in a patient with asthma. Korean J Radiol. 2011;12:386–389.
51. Johnson, TR, Krauss, B, Sedlmair, M, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510–1517.
52. Brody, AS. Imaging considerations: interstitial lung disease in children. Radiol Clin North Am. 2005;43:391–403.
53. Ha, HI, Goo, HW, Seo, JB, et al. Effects of high-resolution CT of the lung using partial versus full reconstruction on motion artifacts and image noise. AJR Am J Roentgenol. 2006;187:618–622.
54. Kelly, DM, Hasegawa, I, Borders, R, et al. High-resolution CT using MDCT: comparison of degree of motion artifacts between volumetric and axial methods. AJR Am J Roentgenol. 2004;182:757–759.
55. Bastos, M, Lee, EY, Strauss, KJ, et al. Motion artifacts on high-resolution CT images of pediatric patients: comparison of volumetric and axial CT methods. AJR Am J Roentgenol. 2009;193:1414–1418.
56. Puderbach, M, Kauczor, HU. Can lung MR replace CT? Pediatr Radiol. 2008;38:S439–S451.
57. Lee, EY. Advancing CT and MR imaging of the lungs and airways in children: imaging into practice. Pediatr Radiol. 2008;38:S208–S212.
58. Yedururi, S, Guillerman, RP, Chung, T, et al. Multimodality imaging of tracheobronchial disorders in children. Radiographics. 2008;28:e29.
59. Biederer, J, Hintze, C, Fabel, M. MRI of pulmonary nodules: technique and diagnostic value. Cancer Imaging. 2008;19:125–130.
60. Chung, T. Magnetic resonance angiography of the body in pediatric patients: experience with a contrast-enhanced time-resolved technique. Pediatr Radiol. 2005;35:3–10.
61. Lee, CH, Goo, JM, Kim, YT, et al. The clinical feasibility of using non-breath-hold real-time MR-echo imaging for the evaluation of mediastinal and chest wall tumor invasion. Korean J Radiol.. 2010;11:37–45.
62. Bauman, G, Puderbach, M, Deimling, M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn Reson Med. 2009;62:656–664.
63. Gelfand, MJ, Parisi, MT, Treves, ST. Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med. 2011;52:318–322.
64. Mineo, TC, Schillaci, O, Pompeo, E, et al. Usefulness of lung perfusion scintigraphy before lung cancer resection in patients with ventilatory obstruction. Ann Thorac Surg. 2006;82:1828–1834.
65. Bar-Sever, Z, Connolly, LP, Treves, ST. The radionuclide salivagram in children with pulmonary disease and a high risk of aspiration. Pediatr Radiol. 1995;25(suppl 1):S180–S183.
66. Levin, K, Colon, A, DiPalma, J, et al. Using the radionuclide salivagram to detect pulmonary aspiration and esophageal dysmotility. Clin Nucl Med. 1993;18:110–114.
67. Cook, SP, Lawless, S, Mandell, GA, et al. The use of the salivagram in the evaluation of severe and chronic aspiration. Int J Pediatr Otorhinolaryngol. 1997;41:353–361.
68. Bar-Sever, Z, Keidar, Z, Ben-Barak, A, et al. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging. 2007;34:630–637.
69. Zukotynski, KA, Fahey, FH, Laffin, S, et al. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging. 2009;36:602–606.
70. Schaefer, NG, Hany, TF, Taverna, C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast-enhanced CT? Radiology. 2004;232:823–829.