CHAPTER 6 Imaging Surveillance for Locally Recurrent Disease
Effective palliative, as well as curative, treatment is now available for patients with recurrent breast cancer. Optimal therapy is dependent on an accurate assessment of the location and extent of recurrent disease, which dramatically influences treatment decision making. Gene expression analysis has been used to identify patients at increased risk for distant recurrence.1,2 There is also the suggestion that expression profiling may be helpful in identifying patients at greater risk for local recurrence.3 This information can potentially be used to tailor the frequency and types of imaging modalities used to monitor these patients.
Most often, the imaging assessment of recurrent disease extent requires use of both anatomic modalities, such as mammography, ultrasound, CT, and MRI, and molecular or metabolic imaging, utilizing positron emission tomography (PET) or combination PET/CT. While fluorodeoxyglucose (FDG) PET is a highly accurate method for evaluating the whole body for recurrence (both local and distant), anatomic and molecular imaging should be viewed as complementary. Hathaway and associates demonstrated the value of combining PET with MRI nearly a decade ago.4
The superb spatial resolution of CT and MRI is of great value in defining the size, shape, and precise location of suspected metastases, whether locoregional or distant. However, anatomically based imaging modalities may be suboptimal in separating active tumor from treatment-related changes. FDG PET can be very helpful in this situation. Additionally, whole-body PET can often change a patient’s stage; confirmation of local recurrence only may direct the treating physician toward a more aggressive, curative-intent approach (e.g., surgery, localized radiation, or both). One study showed that FDG PET changed the clinical stage in 36% of patients and led to a change in therapy in 58%.5
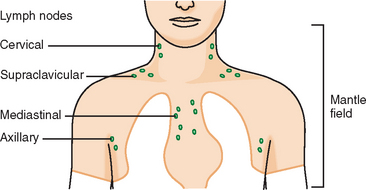
As with any tumor, knowledge of the typical biologic behavior and predictable sites of recurrence is helpful in focusing attention on specific regions. In patients treated with mastectomy, axillary node dissection, and chemotherapy, the most frequent sites of locoregional recurrence are the chest wall and supraclavicular nodes.6 Remote sites may include the mediastinum, internal mammary nodes, and most commonly, the skeleton.7
LOCOREGIONAL
Locoregional recurrence may occur in up to 22% of patients undergoing breast conservation surgery and radiation.8 Breast cancer 10-year local recurrence rates in women with negative surgical margins who undergo lumpectomy and radiotherapy may be as low as 10% or less.9–12 Although overall recurrence rates are low, higher recurrence rates have been reported in select patient groups, such as those with high-grade ductal carcinoma in situ (DCIS).13 In addition, recurrence rates in younger women and in women who do not undergo radiation therapy have been reported as high as 35%.14
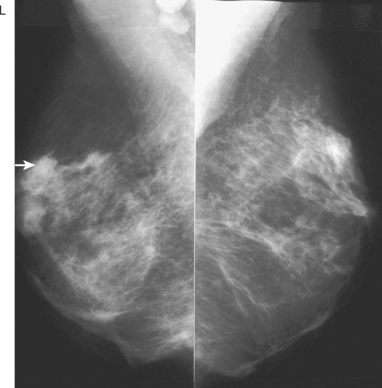
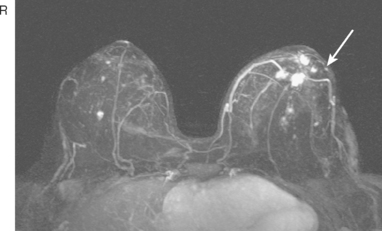
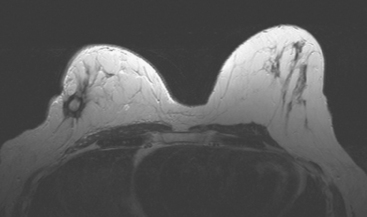
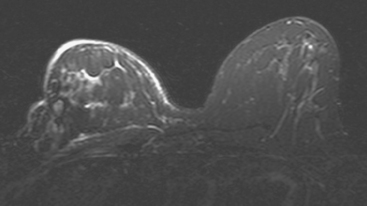
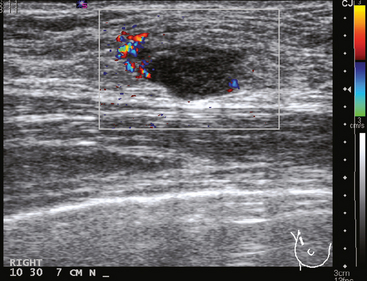
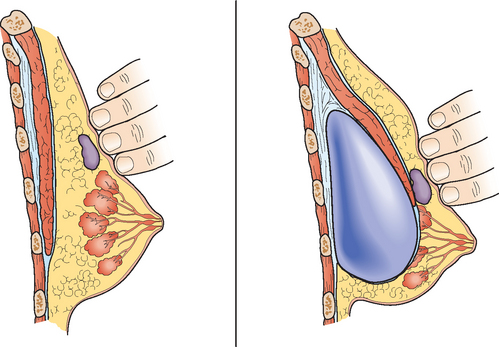
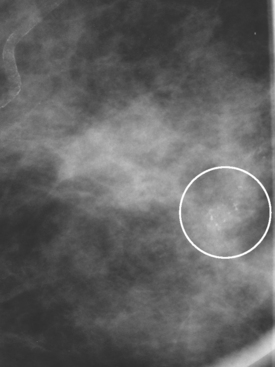
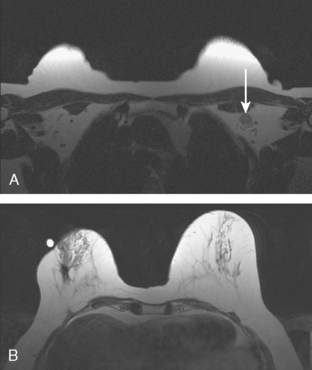
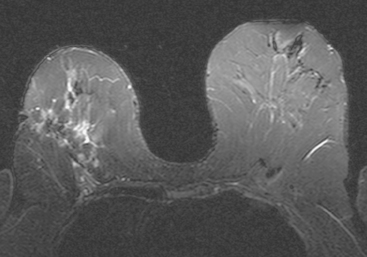
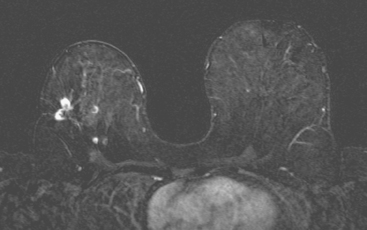
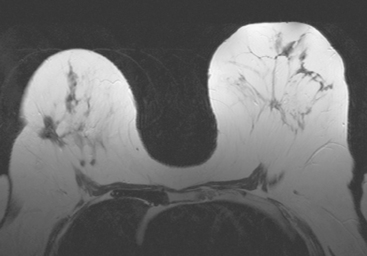
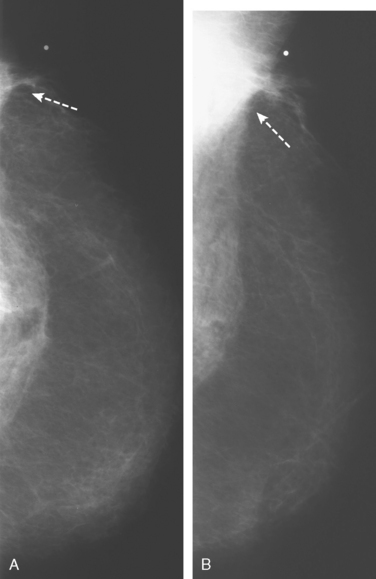
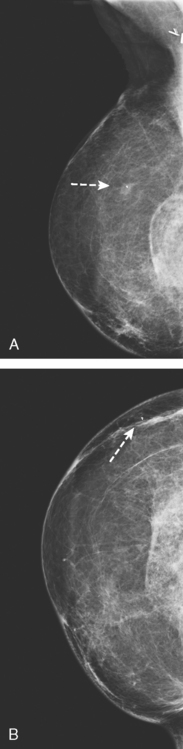
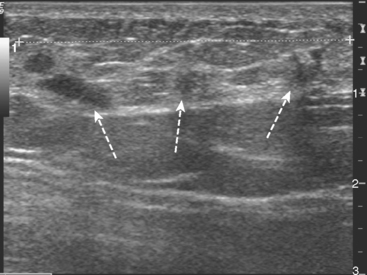
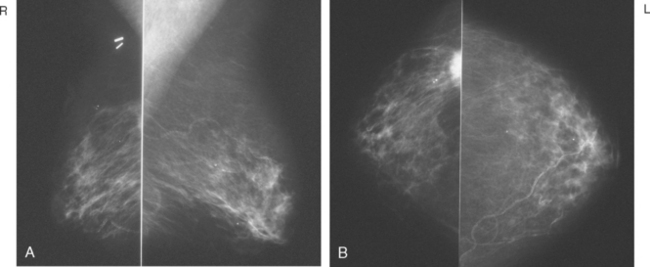
Serial surveillance mammography, as well as diagnostic mammography when there is clinical suspicion of breast recurrence, remains the cornerstone of the imaging approach to previously treated breast cancer patients (Figure 1). Complementary studies include breast ultrasound and MRI, as discussed in Chapter 3 (Figures 2 and 3). Cases presented in this chapter illustrate the spectrum of imaging manifestations of locally recurrent breast cancer.
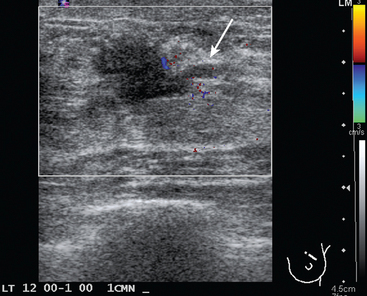
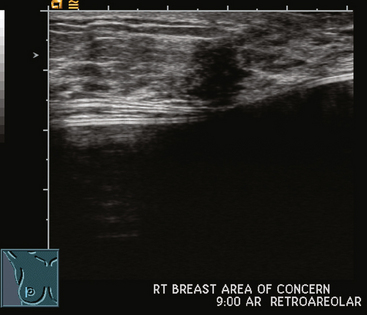
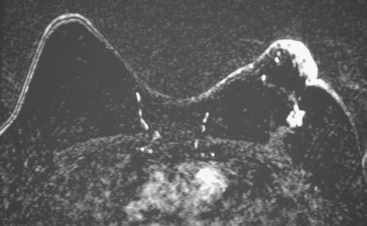
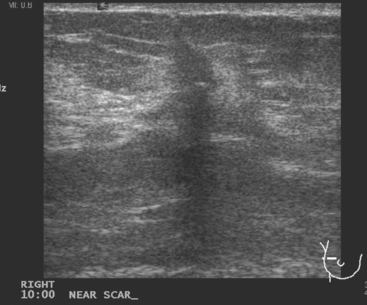
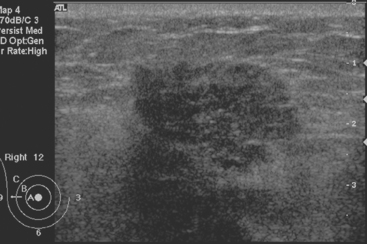
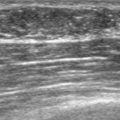
FIGURE 2 Ultrasound of the same patient in Figure 1 shows one of multiple solid masses identified by ultrasound. This mass has particularly suspicious sonographic features: it is solid and irregularly marginated with no definable capsule, and a tail of suspected ductal extension can be seen extending to the right (arrow).
Local failure most commonly occurs in or near the lumpectomy bed, in the same quadrant, and may manifest in a manner similar to the initial presentation (e.g., microcalcifications developing where calcified DCIS has previously been treated). Such changes arising in a lumpectomy bed under surveillance need careful analysis, such as with magnification views, to differentiate from similar-appearing, treatment-related changes (e.g., calcification due to fat necrosis). In some cases, differentiation based on morphology may not be possible, and tissue sampling will be required.
Palpable lumps developing in the breast, axilla, or chest wall of a patient previously treated for breast cancer raise the specter of recurrent disease, but not infrequently are due to treatment-related mimics, such as fat necrosis masses. Depending on location, these can be evaluated with some combination of mammography, ultrasound, and tissue sampling. At times, such mimics are sufficiently characteristic that imaging alone can make the diagnosis, but histologic sampling may be necessary for confirmation.
Mammographic follow-up is warranted in patients who have undergone subcutaneous mastectomy because of the considerable amount of remaining breast tissue. The yield from mammographic surveillance of the breast after standard mastectomy or skin-sparing mastectomy and reconstruction has been questioned. Because most recurrences occur in the skin and subcutaneous tissues, they can usually be detected on physical examination. The overall annual recurrence rate of T1 or T2 tumors following mastectomy has been reported to be 1% to 2% during the first 5 years.15 Annual mammographic evaluation following mastectomy and transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction has been advocated to screen for any residual breast tissue and to detect recurrences before they are palpable.16
The timing for mammographic follow-up after breast conserving therapy has not been standardized. At a minimum, a 6-month follow-up mammogram should be performed to establish the patient’s baseline after surgery. The incidence of a recurrence in the ipsilateral breast 20 years after surgery is 14.3% among women who undergo irradiation after lumpectomy and 39.2% among those who undergo lumpectomy without irradiation.17
CHEST
Pathologic involvement of the internal mammary lymph nodes and mediastinal lymph nodes is best determined by PET.18 Although both areas are evaluable by CT, reliance on size alone has been shown to be inferior to assessment of metabolic activity in patients with non–small cell lung cancer (histologic gold standard).19 In one series of 73 patients, FDG PET was found to be twice as accurate as CT in identifying involved internal mammary or mediastinal nodes.20
BONE
Bone metastases are covered in depth in Chapter 8. Imaging options include plain radiographs, CT, MRI, nuclear medicine bone scans, and PET. Plain radiographs are readily available, are relatively inexpensive, expose the patient to a low level of radiation, require no patient preparation, and are completed rapidly. Typically, a minimum 30% reduction in bone mineralization is necessary before a radiograph becomes abnormal. Additionally, radiographs provide limited coverage. These factors reduce sensitivity both at the site of concern and for distant disease. Most commonly, bone radiographs are performed in an effort to improve the specificity of a nuclear bone scan. Even this function is being replaced by the utilization of CT or MRI for scintigraphic correlation. Both of these modalities dramatically improve diagnostic accuracy, compared with plain radiographs, in bone and surrounding soft tissue. However, the screening method of choice remains the nuclear medicine bone scan. It is a whole-body scan, delivers low radiation, and is exquisitely sensitive to changes in bone metabolism. PET and PET/CT utilizing F-18 FDG and sodium fluoride (NaF), are covered in greater detail in Chapter 8. Preliminary data suggest an advantage for PET over bone scintigraphy in the identification of skeletal metastases (especially lytic metastases). NaF, also a positron-emitting pharmaceutical, is a bone-specific agent, whereas FDG PET allows a whole-body (bone and soft tissue) evaluation.
DISTANT METASTASES
The confirmation and quantitation of distant metastases has both therapeutic and prognostic implications. Imagers are often asked to provide answers to the following questions: (1) Are distant metastases present? (2) Is the disease focal or widespread? (3) What are the prognostic implications? (4) In the absence of symptoms, with elevated serum tumor markers, where is the disease? Since the answers to questions 1 to 3 are applicable to most patients under investigation for recurrence, in many centers, PET has become the modality of choice to search for breast cancer metastases following initial therapy. Whole-body surveys have shown FDG PET to be both sensitive and specific for detection of distant disease.19–22
1 van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009.
2 Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-2826.
3 Mamounas E, Tang G, Bryant J, et al. Association between the 21-gene recurrence score assay (RS) and risk of locoregional failure in node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20 [abstract]. Presented at the San Antonio Breast Cancer Symposium 28th Annual Meeting, December 10, 2005.
4 Hathaway PB, Mankoff DA, Maravilla KR, et al. The value of combined FDG-PET and magnetic resonance imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology. 1998;210(3):807-814.
5 Yap CS, Seltzer MA, Schiepers C, et al. Impact of whole-body 18-FDG PET on staging and managing patients with breast cancer; the referring physician’s perspective. J Nucl Med. 2001;42(9):1334-1337.
6 Katz A, Strom EA, Bucholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817-2827.
7 Eubank WB, Mankoff DA. Current and future uses of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2004;34(3):224-240.
8 Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg. 2005;189(2):229-235.
9 Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259-267.
10 Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30-39.
11 Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000;6:28-33.
12 Provenzano E, Hopper JL, Giles GG, et al. Histological markers that predict clinical recurrence in ductal carcinoma in situ of the breast: an Australian population-based study. Pathology. 2004;36:221-229.
13 Borg MF. Breast-conserving therapy in young women with invasive carcinoma of the breast. Australas Radiol. 2004;48:376-382.
14 Scott WJ, Gobar LS, Terry JD, et al. Mediastinal lymph node staging of nonsmall cell lung cancer: a prospective comparison of computed tomography and positron emission tomography. J Thorac Cardiovasc Surg. 1996;111(3):642-648.
15 Kroll SS, Schusterman MA, Tadjalli HE, et al. Risk of recurrence after treatment of early breast cancer with skin-sparing mastectomy. Ann Surg Oncol. 1997;4:193-197.
16 Helvie MA, Bailey JE, Roubidoux MA, et al. Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology. 2002;224:211-216.
17 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
18 Eubank WB, Mankoff DA, Takasugi J, et al. 18 Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19(15):3516-3523.
19 Lonneux M, Borbath L, Berliere M, et al. The place of whole-body PET/FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging. 2000;3:45-49.
20 Hubner KF, Smith GT, Thie JA, et al. The potential of F-18-FDG/PET in breast cancer: detection of primary lesions, axillary lymph node metastases, or distant metastases. Clin Positron Imaging. 2000;3:197-205.
21 Gallowitsch HJ, Kresnik E, Gasser J, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003;38:250-256.
22 Kamel EM, Wyss MT, Fehr MK, et al. [18F]-fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol. 2003;129(3):147-153.
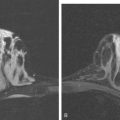
CASE 1 Postoperative scar with hematoma on MRI
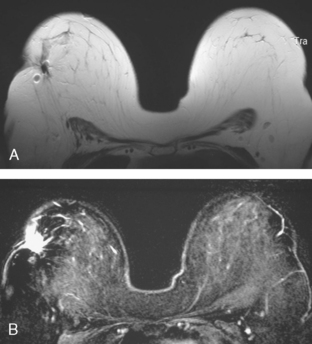
A 47-year-old woman with dense breasts was evaluated with a breast MRI 1 year after being treated for stage I, T1N0M0 right breast cancer. Her tumor was a 1.5-cm infiltrating ductal carcinoma (IDC) with mucinous and clear cell features, without angiolymphatic invasion, estrogen receptor and progesterone receptor positive, with margins clear for invasion and notable only for a focal close (1 mm) inferior medial margin for ductal carcinoma in situ (DCIS). Five sentinel lymph nodes were negative. She had been treated with partial mastectomy, four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) chemotherapy, and radiation therapy, with a boost to the lumpectomy bed, and was taking tamoxifen. See, Case 8 in Chapter 1 for the imaging features of this patient’s breast cancer at initial diagnosis.
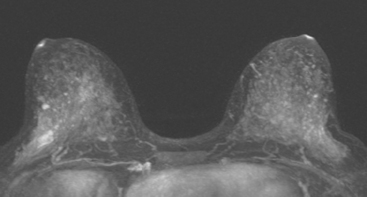
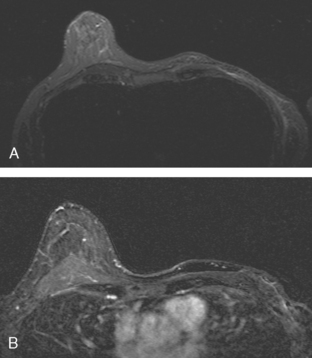
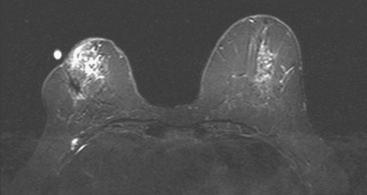
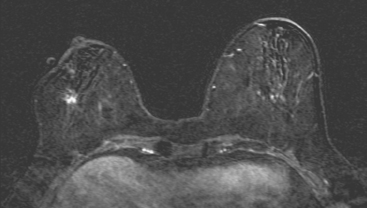
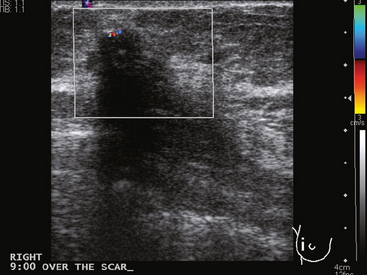
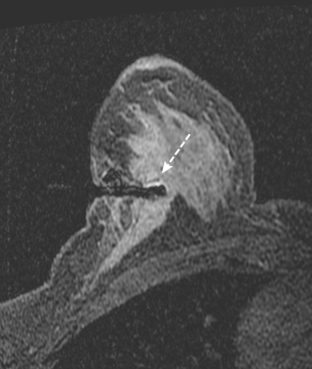
Because of her extreme breast density, surveillance breast MRI and ultrasound were obtained 1 year after breast cancer treatment. At the lumpectomy level, findings of a small residual hematoma were seen at the scar level on MRI (Figures 1, 2, and 3). The ultrasound correlate is seen in Figure 4.
TEACHING POINT
Most postoperative fluid collections are seromas, which display fluid signal on MRI (low on T1 and high signal on short tau inversion recovery (STIR) and T2). Hematomas may be differentiated from seromas by recognition of blood product signal intensity. In this example, bright signal seen on unenhanced T1-weighted sequences is methemoglobin, and the hypointense rim, best seen on gradient echo sequences, is characteristic of hemosiderin.
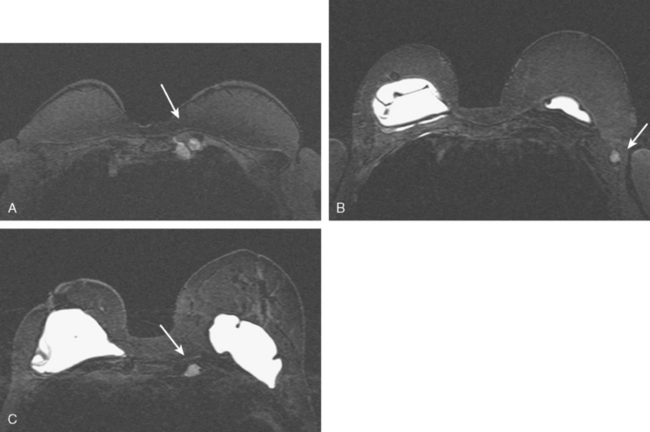
CASE 2 Changes from recent bilateral mastectomy and tissue expander placement
A 36-year-old woman noted a palpable right upper outer quadrant (UOQ) breast mass. Breast imaging evaluations showed a corresponding 1-cm hypoechoic, solid, vascular, sonographically indeterminate breast mass. Ultrasound-guided biopsy identified infiltrating ductal carcinoma (IDC) (Figure 1).
The past medical history was notable for prior mantle radiation for Hodgkin’s disease 12 years before. Preoperative breast MRI evaluation showed the known IDC as a 1.1-cm, early enhancing mass with washout, with a 4-mm suspected satellite nodule adjacent. A separate, lobular 5 × 3-mm possible intramammary lymph node was noted in the lateral breast. The left breast showed no focal or concerning findings (Figures 2 and 3).
The patient elected to undergo bilateral mastectomies, with tissue expanders placed. The right mastectomy specimen contained a 0.9-cm IDC, with high-grade ductal carcinoma in situ (DCIS) extending 5 mm beyond the invasive carcinoma, estrogen receptor negative, HER-2/neu negative. Atypical ductal hyperplasia was identified in multiple quadrants, as well as found in the left mastectomy specimen. The margins were negative. Four right axillary lymph nodes showed no evidence of disease.
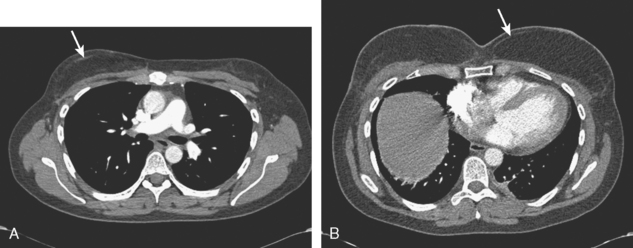
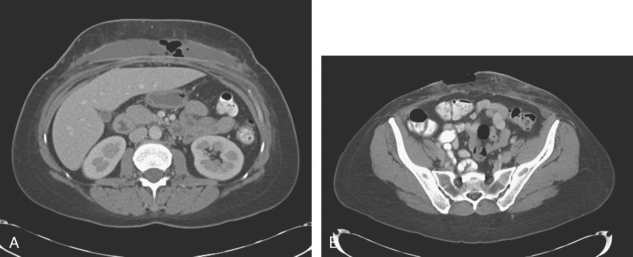
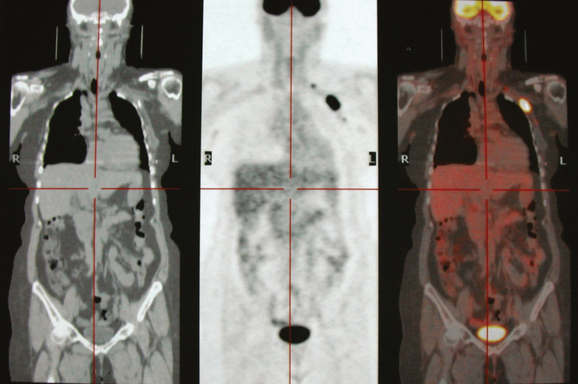
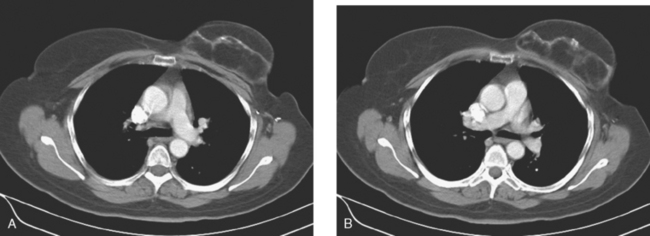
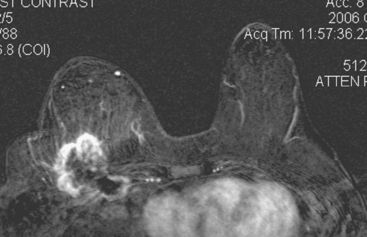
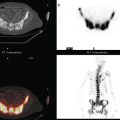
Staging evaluations with positron emission tomography (PET) and CT obtained before chemotherapy showed chest wall changes attributable to surgery, as well as normal variant endometrial activity (Figures 4, 5, and 6).
TEACHING POINTS
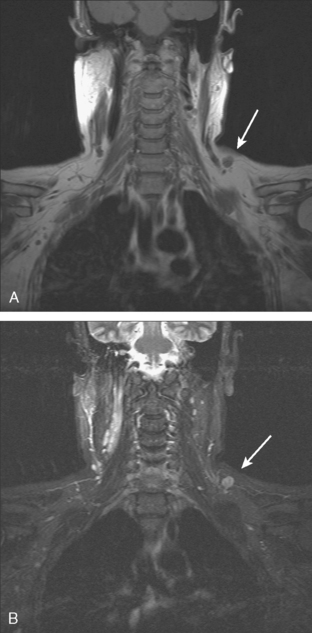
Younger patients treated for lymphoma with mantle radiation are at increased risk for a second malignancy (the incidence is 10% to 20% at 20 years of follow-up). Mantle radiation includes the neck, supraclavicular, infraclavicular, axillary, mediastinal, and hilar regions (Figure 7). The unshielded upper outer quadrants of the breasts receive the highest doses.
In women, if a second malignancy develops, it is most commonly breast cancer.
The normal, early postoperative findings of mastectomy with tissue expander placement are demonstrated here. A described, normal variant source of fluorodeoxyglucose (FDG) activity on whole-body PET is also seen here in the pelvis. Endometrial activity is most commonly seen in premenopausal patients during the first (menstruation) or third (ovulation) week of the menstrual cycle. If endometrial activity is identified on PET in a postmenopausal patient, this warrants further investigation, because endometrial carcinoma can manifest this way.
Estabrook A, Giron G. Treatment of unusual malignant neoplasias and clinical presentations. In: Roses DF, editor. Breast Cancer. 2nd ed. Philadelphia: Churchill Livingstone; 2005:705-706.
Lerman H, Metser U, Grisaru D, et al. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266-271.
Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465-475.
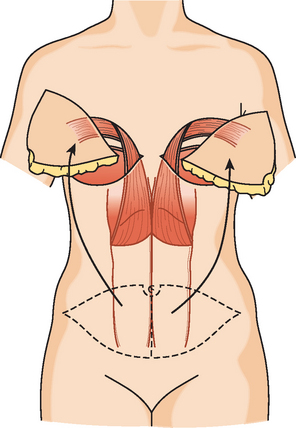
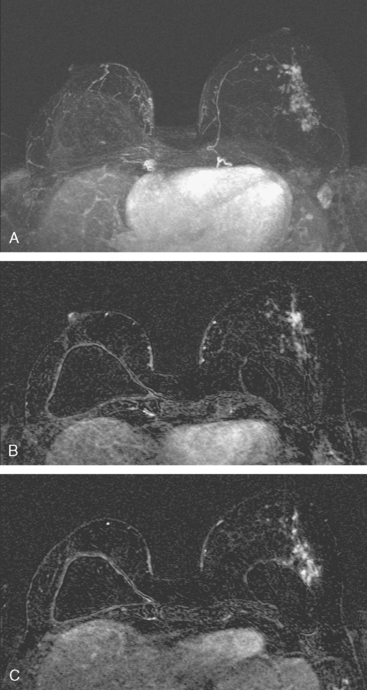
CASE 3 Normal CT appearance of bilateral TRAM flap reconstruction and post–TRAM flap abdominal complications
A 38-year-old woman elected to undergo prophylactic left mastectomy and bilateral transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction 10 months after finishing radiation therapy for a right stage II, T2N1, 3-cm infiltrating ductal carcinoma (IDC), estrogen receptor and progesterone receptor negative, and HER-2/neu negative-, with 3 of 22 involved axillary lymph nodes. She had been treated with right mastec-tomy with clear margins, chemotherapy with four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) and four cycles of paclitaxel (Taxol), and right chest wall, supraclavicular, and posterior axillary boost radiation therapy (see Case 18 in Chapter 3 for the presenting imaging features and initial staging of this patient).
The patient had undergone genetic testing subsequent to her diagnosis and treatment and proved to be BRCA-1 positive. Her family history was of premenopausal breast cancer in a maternal aunt. As a consequence, she decided to undergo hysterectomy and oophorectomy, which was performed at the same time as prophylactic left mastectomy and bilateral TRAM flap reconstruction (Figures 1, 2, and 3). Her left breast mastectomy specimen was benign.
The patient’s postoperative course was complicated by development of abdominal wound infection (Figure 4), requiring débridement twice of infected, necrotic tissue and intravenous antibiotic therapy, as well as anticoagulation for pulmonary embolism.
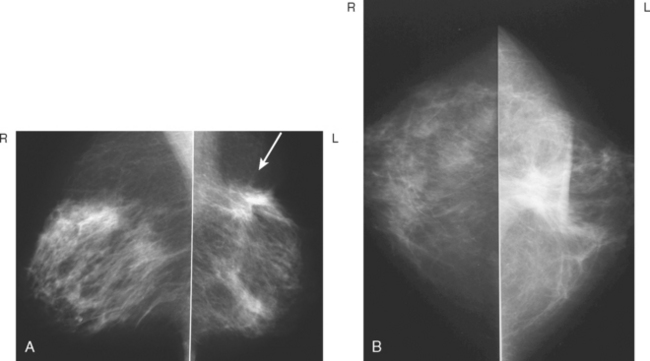
CASE 4 Recurrent DCIS presenting as new microcalcifications
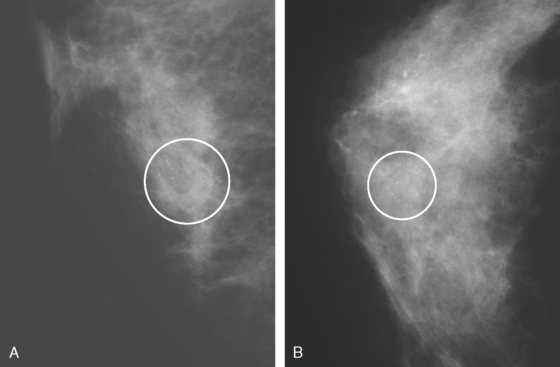
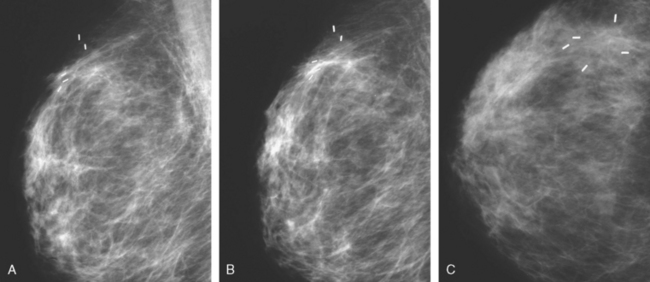
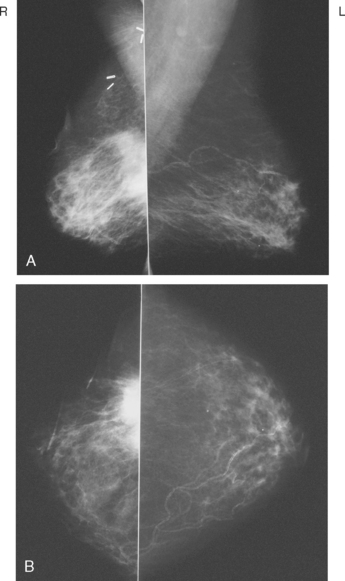
A 53-year-old woman, 2 years postmenopausal, underwent routine mammographic screening. A small cluster of faint, new, indeterminate microcalcifications was identified on the left (Figure 1), in the lower inner quadrant. The patient was unable to tolerate stereotactic biopsy and underwent surgical excision after needle localization. Pathology showed a 1-cm ductal carcinoma in situ (DCIS) lesion (with necrosis, cribriform, solid, and focal micropapillary forms), estrogen receptor negative, HER-2/neu positive, with a focal (0.2 cm) microinvasive component. The margins were close.
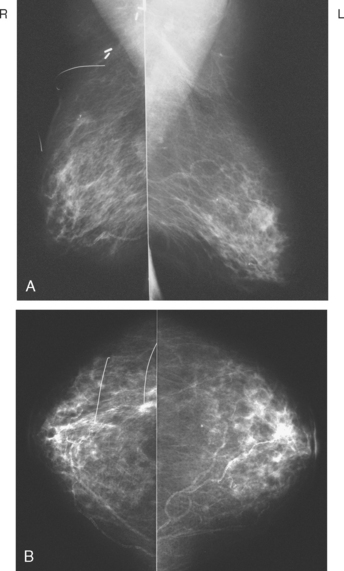
Eleven months after radiation therapy, routine mammographic surveillance showed new clustered left microcalcifications, which had developed since a prior study 6 months before (Figures 2 and 3). These were sampled stereotactically, and identified as DCIS.
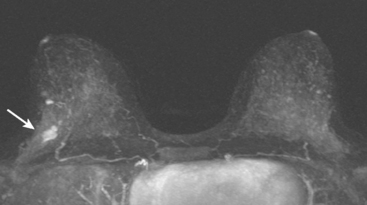
Mastectomy was performed. The histology showed a 6-mm focus of high-grade DCIS with comedo necrosis and negative margins. One examined axillary lymph node was negative. Breast MRI was obtained 1 month postoperatively to evaluate the opposite side, given the patient’s unusual clinical course (Figure 4). No concerning findings were seen.
Clinically, the patient was doing well 22 months after mastectomy.
TEACHING POINTS
This patient had an unusual clinical course, with rapid development of calcified DCIS only 11 months after undergoing treatment for DCIS with lumpectomy and radiation. In retrospect, perhaps breast MRI might have been useful in the initial evaluation of this patient’s extent of disease. The use of breast MRI with DCIS diagnoses, to look for invasive disease or to assess the extent of DCIS, is considered controversial and is far from universal in practice. However, because it is known that DCIS is often noncalcified, and not accurately mapped by mammography when noncalcified, and because DCIS on core biopsy is not infrequently upstaged on excision to micro or frank invasion, cogent arguments can be made to consider the use of breast MRI in initial staging of DCIS as well as invasive cancer.
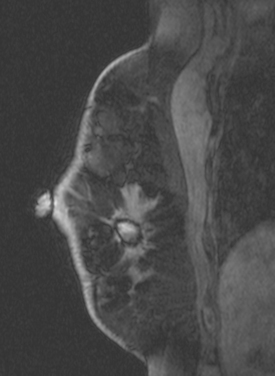
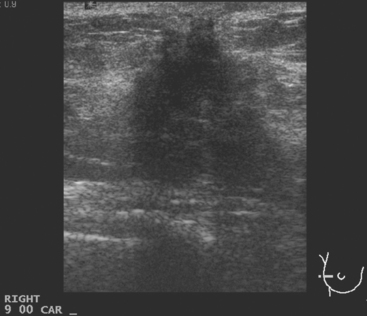
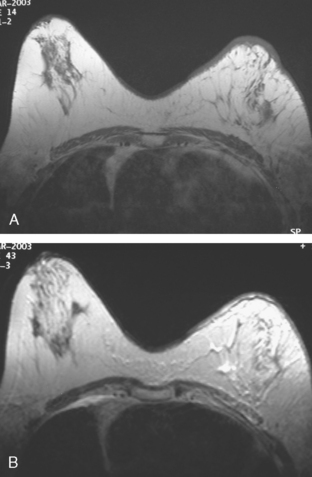
CASE 5 Recurrent DCIS detected on surveillance MRI
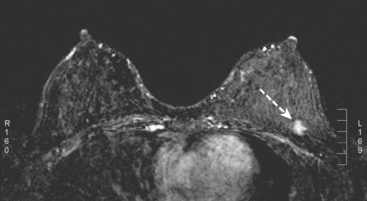
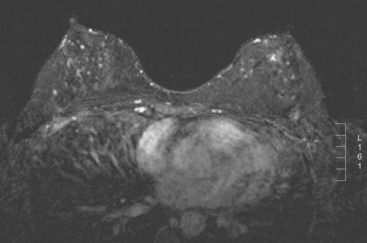
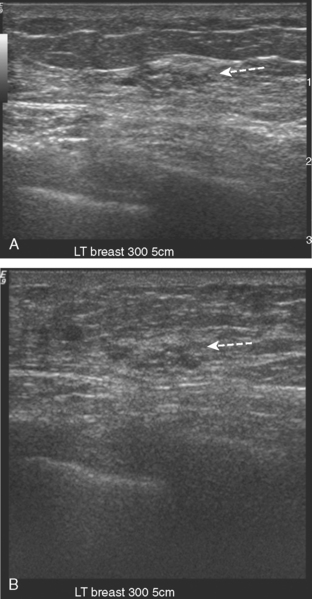
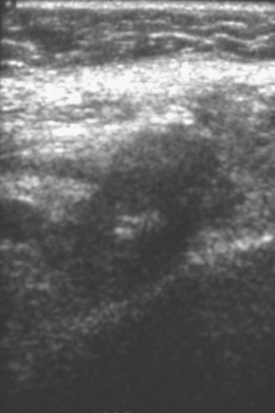
A 51-year-old woman, with prior left breast ductal carcinoma in situ (DCIS) treated with lumpectomy only, underwent annual screening MRI. The MRI demonstrated a developing 1.2-cm focal area of enhancement in the left breast, lateral to the patient’s prior lumpectomy site (Figures 1 and 2). Mammography showed no abnormality. Ultrasound demonstrated a small area of altered echotexture that appeared to correspond to the abnormal finding on MRI (Figure 3). Ultrasound-guided core needle biopsy identified intermediate-grade DCIS. The patient was treated with surgical excision and radiation therapy.
TEACHING POINTS
This case demonstrates the ability of MRI to detect in-breast recurrence that is occult on mammography and nearly occult on ultrasound. Without the level of suspicion generated by the MRI finding or the MRIs to guide directed ultrasound evaluation, it is unlikely that the cancer recurrence in this patient would have been detected on whole-breast survey ultrasound. The 10-year local recurrence rates in patients who undergo lumpectomy with negative margins and radiation therapy have been reported to be as low as 10% or less. However, some patients are at increased risk for local recurrence. These include younger patients, patients with an extensive intraductal component, and patients with close or positive surgical margins. MRI may be a useful adjuvant to screening mammography in these patients. In addition, MRI aids in surveillance of patients who undergo no radiation therapy or partial- rather than whole-breast irradiation.
Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30-39.
Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000;6:28-33.
Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259-267.
CASE 6 ADH/DCIS found on breast MRI obtained to evaluate silicone implant integrity in a breast conservation therapy (BCT) patient
A 62-year-old woman, with a prior history of T1cN0M0 right breast cancer 13 years earlier, underwent surveillance mammography. She had silicone implants, which had been placed 9 years before, which replaced earlier implants. Her breast cancer had been treated with partial mastectomy, radiation, and chemotherapy. She had only been able to tolerate tamoxifen for 1 year.
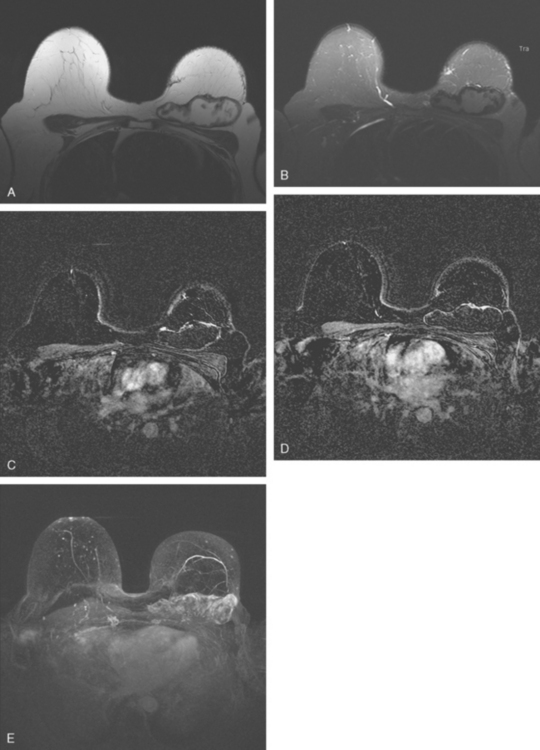
Her mammogram suggested possible implant rupture, with new findings of a right suspected silicone granuloma and new increased density of left axillary lymph nodes. Breast implant MRI was performed to assess implant integrity. Because of her prior history of breast cancer, a hybrid protocol was performed to assess for implant rupture and to evaluate the parenchyma with enhanced, subtracted, dynamic technique. Enlarged left axillary and internal mammary lymph nodes were identified (Figure 1). They were bright on a “silicone-only” sequence (STIR with water saturation), indicating the lymph nodes contained silicone. Rupture of the right breast implant was noted, and postsurgical distortion was seen of the right breast. No abnormal enhancement was seen on the right, but the left breast showed a large (6 cm), lateral expanse of multinodular, clumped, morphologically concerning enhancement (Figure 2). Breast ultrasound was performed to see if a correlate could be identified for biopsy, but none was seen. The left axilla was evaluated and showed “snowstorm” shadowing from an enlarged lymph node, characteristic of silicone (Figure 3).
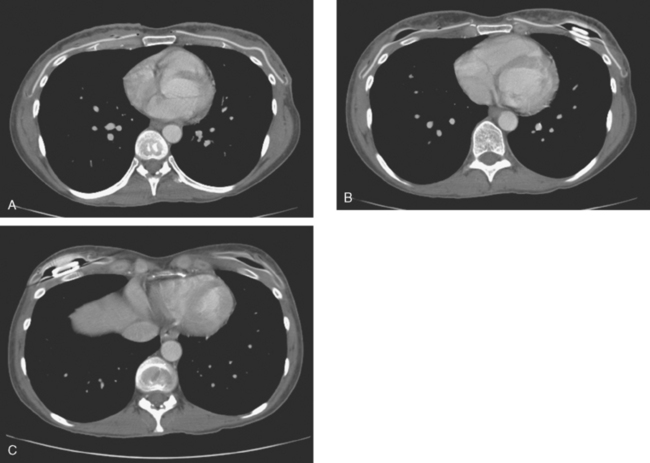
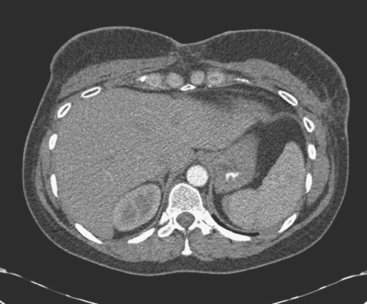
The patient was evaluated for possible left breast conservation therapy. However, because of the 6-cm extent of disease suggested by MRI, it seemed likely that clear margins might not be attained. Since her right breast implant was ruptured, the patient elected to undergo removal of both implants, with bilateral skin-sparing mastectomies and placement of tissue expanders. A left sentinel lymph node sampling and limited axillary dissection was performed, with 5 nodes obtained containing silicone, but no malignancy. No malignancy was found in either breast. In the left lateral breast, fibrocystic changes with sclerosing adenosis, in-traductal papilloma, and focal areas of atypical ductal hyperplasia were identified. A chest CT obtained postoperatively showed enlarged internal mammary and pericardial lymph nodes, as well as the appearance of tissue expanders (Figure 4).
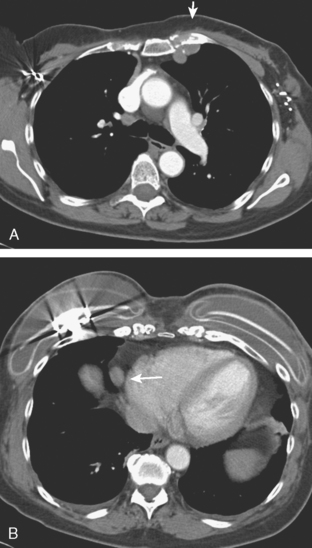
FIGURE 4 Images from a contrast-enhanced chest CT (A, above; B, below) show surgical clips in both axilla. Soft tissue density at the left axillary level is postoperative. Adjacent enlarged left internal mammary lymph nodes correspond to those seen in Figure 1A on MRI. In B, small pleural effusions are seen. Bilateral tissue expanders are in place. An enlarged pericardial lymph node is seen in B (arrow).
TEACHING POINTS
Breast cancer patients who have undergone reconstruction with silicone implants may present with imaging findings that result from implant failure, but mimic breast cancer recurrence. In this example, routine mammographic surveillance of a patient previously treated with breast conservation raised the question of loss of implant integrity. Breast implant MRI is performed unenhanced, and does not generally provide diagnostic information about the breast parenchyma. Breast implant protocols rely on high-resolution, sagittal and axial, T2-weighted sequences to assess for intracapsular rupture. The presence of extracapsular silicone is sought using a sequence in which only silicone is bright, such as the STIR with water saturation sequence used here. Silicone-containing lymph nodes within the field of view will be bright, as demonstrated here. In this case, characteristic ultrasound findings confirming silicone in the left axilla were found subsequently. Presumably, even the enlarged pericardial lymph node seen on chest CT is due to uptake of silicone. Unfortunately, only time (longitudinal follow-up) or histologic sampling can confirm that the nodal enlargement is due to silicone. PET imaging in this circumstance could potentially be misleading because silicone-containing lymph nodes may well be hypermetabolic, owing to the intense granulomatous response silicone incites (see Case 10 in Chapter 10).
Because this patient had a known history of breast cancer, and because the presence of implants hampers mammographic evaluation, a “hybrid” breast MRI protocol was utilized, with sequences obtained for evaluation of the implants, as well as enhanced, subtracted, dynamic breast parenchymal imaging. The enhanced portion of the study showed unexpectedly intense and segmental clumped enhancement. The pattern is morphologically concerning, with a segmental distribution of tiny enhancing nodules. The MRI differential includes infiltrating lobular carcinoma, DCIS, and atypical ductal hyperplasia (ADH). When no sonographic correlate was identified, MRI-guided biopsy was performed. This could be safely performed because most of the enhancement extends anterior to the implant. The histology of DCIS fit the morphologic features of the abnormality, which appeared extensive by MRI. Complete excision of the area of enhancement with a satisfactory cosmetic result and clear margins would be difficult. Because the patient now had confirmation of implant failure as well, bilateral skin-sparing mastectomy and implant removal were performed. Not surprisingly, the right mastectomy specimen was negative for malignancy. Surprisingly, no additional malignancy was found in the left mastectomy specimen, only ADH.
Could this case have been handled differently? If ADH had been obtained on initial MRI-guided sampling, that would also have required excision because there is a known incidence of undersampling and upgrading of ADH lesions to DCIS between core and excisional biopsies. Excision of such an extensive area of enhancement would have been technically difficult to accomplish with a good cosmetic result. This case does reaffirm that MRI findings need histologic proof before being acted on, but difficulty can arise from the inherent limitations of sampling. See Case 16 in Chapter 3 for a similar case of a “borderlands” breast lesion.
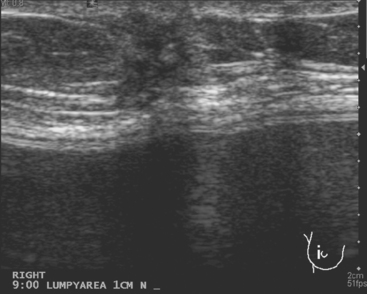
CASE 7 New palpable chest wall lump postmastectomy with implant reconstruction, excisional biopsy–proven recurrent IDC
Ultrasound showed a 6-mm, irregularly marginated mass, disrupting the normal architecture planes (Figure 1). This was surgically excised, identifying recurrent infiltrating ductal carcinoma (IDC), estrogen receptor and progesterone receptor positive and HER-2/neu negative, involving the subcutaneous adipose tissue and skeletal muscle of the chest wall.
Staging evaluations with positron emission tomography (PET)/CT and bone scan showed no additional sites of recurrent breast cancer. The patient underwent excision, with removal of the implant, and axillary dissection. No residual cancer was found in the breast, and 1 of 11 lymph nodes showed involvement (largest focus was 0.8 cm).
TEACHING POINTS
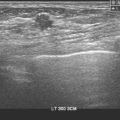
Implants hamper mammographic evaluation of breast tissue. However, the stretching and thinning of breast tissue over an implant can actually make lumps more easily palpable and can aid sonographic evaluation as well (Figure 2).
The sonographic appearance of this small mass was certainly suspicious: it is as tall as wide, with irregular margins and disruption of tissue planes. Of course, not every new, suspicious sonographic mass developing in a patient with previous breast cancer represents recurrent breast cancer. See Figure 3 (Case 9 in Chapter 7) for a very similar-appearing finding that proved to be fibrosis and foreign body giant cell reaction in a previously treated breast cancer patient with recently placed implants.
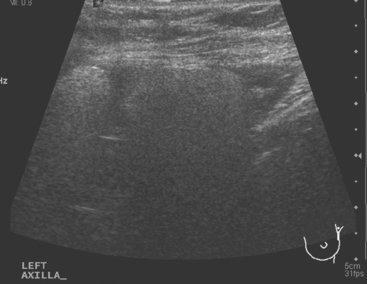
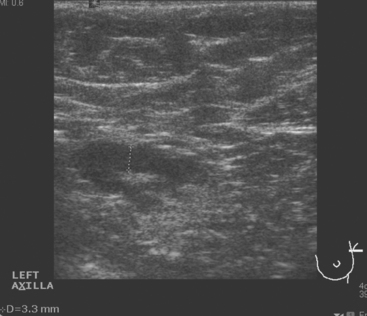
CASE 8 Axillary recurrence, presentation with pain
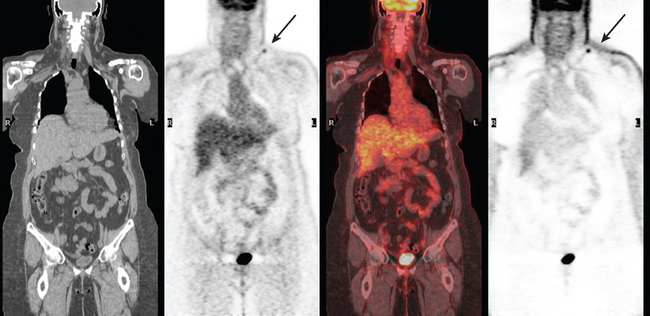
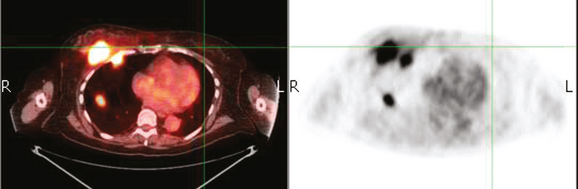
A 50-year-old woman presented with a 6-month history of progressive left axillary and shoulder pain, and suspicion of recurrent breast cancer. She had undergone a left modified radical mastectomy 13 years before, with subsequent reconstruction with an implant. She was also treated at the time of initial diagnosis with Cytoxan, methotrexate, 5-fluorouracil (CMF) chemotherapy for nodepositive disease, with 6 of 25 lymph nodes involved. She reportedly had undergone a positron emission tomography (PET)/CT scan at an outside facility showing left axillary and supraclavicular hypermetabolism, but no films were available initially to substantiate this. She had been advised at the outside facility to undergo radiation and begin tamoxifen based on the PET/CT evidence of recurrence, but was transferring care. She had no tissue diagnosis of recurrence at the time of transfer of care. She was referred for ultrasound-guided biopsy following a diagnostic ultrasound.
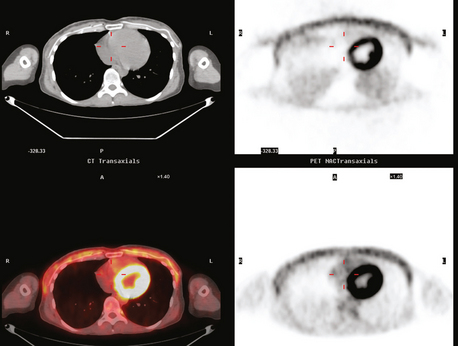
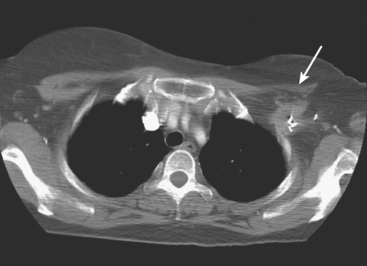
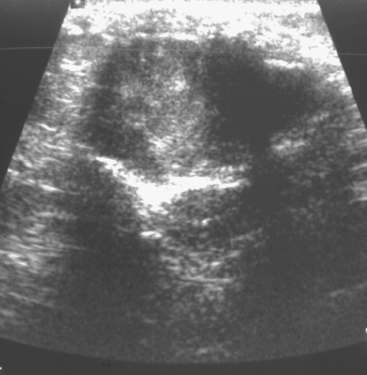
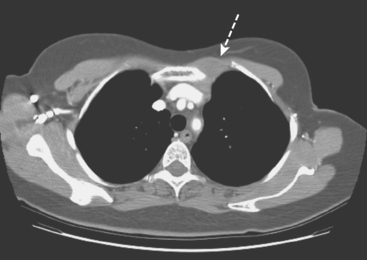
On ultrasound, an ill-defined, deep, hypoechoic mass was seen in the axilla (Figure 1). Hyperechoic foci were noted within this, suggesting coarse calcifications or metal. Because the patient presented without any supporting imaging or reports, she was sent for a contrast-enhanced chest CT scan to better define the nature of the axillary findings and her anatomy. Contrast enhanced CT of the chest showed clips from prior lymphadenectomy in the left axilla, surrounded by mass-like soft tissue (Figure 2), which seemed to correlate with the sonographic findings. Based on this, a 14-gauge ultrasound-guided core needle biopsy was performed, confirming infiltrating ductal carcinoma (IDC), estrogen receptor positive, progesterone receptor negative.
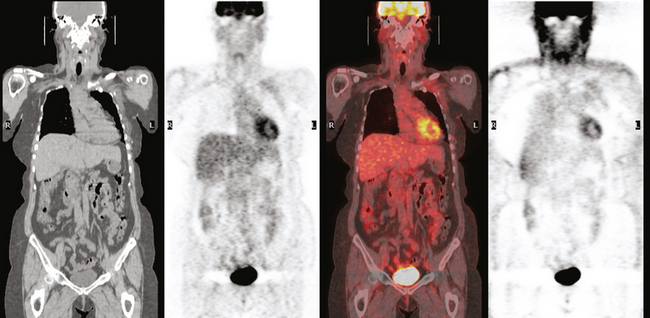
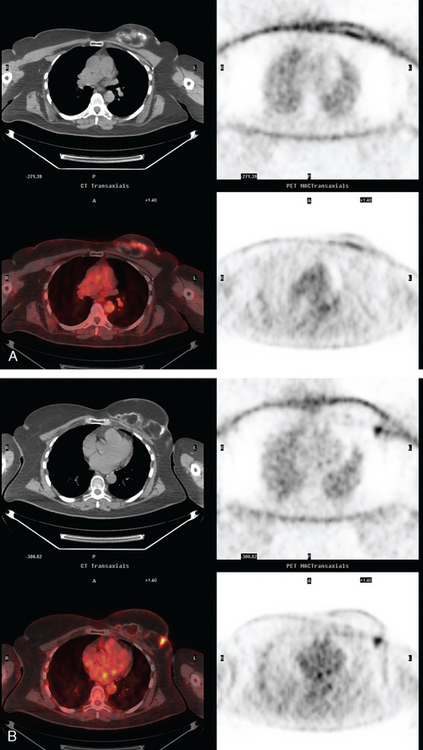
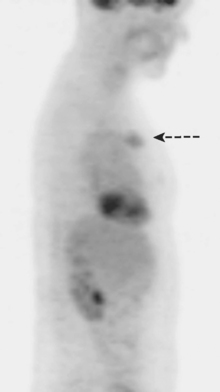
Subsequently, images from her outside PET scan became available, showing hypermetabolism in the left axilla, corresponding to the ultrasound and CT abnormalities (Figure 3).
The patient was treated with radiation therapy, with relief of her pain, and started on anastrozole (Arimidex). Repeat PET/CT showed resolution of the abnormalities noted previously (Figures 4 and 5).
TEACHING POINTS
It is always easier to find your way if you have a map. In this case, the patient presented for biopsy without any supporting documents or images. She gave a history of an abnormal recent PET scan, but no images were then available. Evaluation with ultrasound showed concerning but difficult to define axillary findings. Chest CT was obtained to better delineate her anatomy, and made clearer the significance of the ultrasound findings. The echogenic foci were surgical clips from prior lymphadenectomy, making the surrounding soft tissue bulk highly suspicious for recurrence.
CASE 9 Parasternal recurrence, draining to contralateral axilla (role of lymphoscintigraphy)*
A 43-year-old woman with a history of treated left breast cancer 3 years before developed a growing palpable and tender left parasternal nodule, which on physical examination was noted to be firm and fixed to the chest wall. Excisional biopsy confirmed infiltrating ductal carcinoma (IDC), thought to be recurrent disease in a lower internal mammary lymph node.
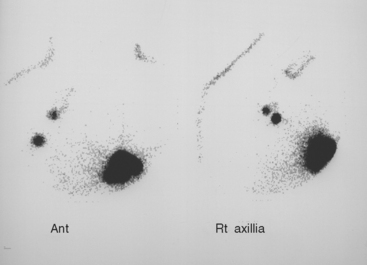
The recurrence at the left lower inner quadrant parasternal level had been operatively excised because of its fixation to the chest wall, and margins of this excision were involved. Before re-excising the proven recurrence, lymphoscintigraphy was obtained to assess the drainage pattern, and showed drainage to the contralateral axilla (Figure 1). Right axillary sentinel lymph node sampling showed several foci of subcapsular metastatic ductal carcinoma, measuring up to 2 mm. The re-excised proven recurrence site contained a 1-cm poorly differentiated IDC, as well as several areas of intramammary metastases.
CASE 10 Multicentric recurrent breast cancer with skin involvement
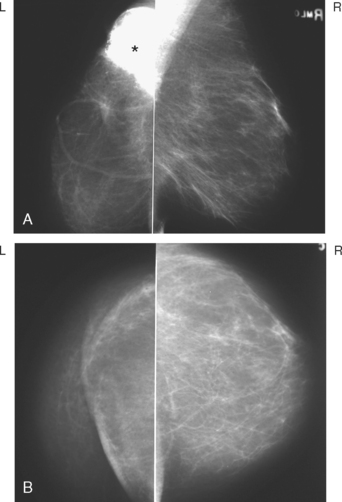
A 54-year-old woman was noted to have skin induration 3 years after left lumpectomy and radiation therapy for breast cancer. Her original tumor presented as a small cluster of microcalcifications (Figure 1). Stereotactic biopsy established the diagnosis of infiltrating ductal carcinoma (IDC), with extensive high-grade ductal carcinoma in situ (DCIS). Her tumor was stage IIB, T2N1, with one of nine lymph nodes involved.
The patient underwent mammographic evaluation to assess the clinically noted skin thickening (Figure 2). Postsurgical scarring was noted, but no concerning change was seen on mammography.
Breast MRI was obtained to evaluate for clinically suspected recurrence (Figures 3, 4, and 5). At surgery, an initial excisional biopsy was performed. Frozen section showed carcinoma in the specimen and overlying skin. A simple mastectomy was performed. The specimens showed a 1.6-cm, high-grade invasive carcinoma with multiple additional small foci of tumor and extensive vascular invasion, as well as involvement of the upper dermis of the skin. The tumor was estrogen receptor negative, progesterone receptor positive, and HER-2/neu positive.
CASE 11 Physical examination change and abnormal enhancement of a 4-year-old lumpectomy scar; pathology-proven fat necrosis mimicking tumor bed recurrence
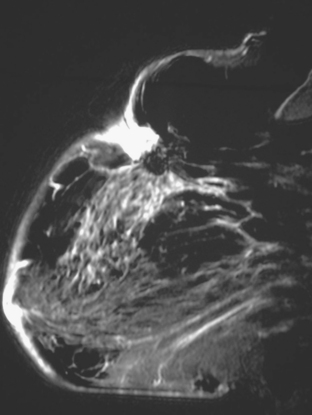
Ultrasound showed no definite abnormality, and mammography 3 months before had been stable (Figure 1). MRI was requested to evaluate for possible recurrence. It showed a spiculated, progressively enhancing, 3-cm mass in the lumpectomy bed, suspicious for recurrence (Figures 2 and 3).
Palpation-guided biopsy by the surgeon showed only fatty fibrous breast tissue.
Subsequent surgical excision removed an elliptical, 5-cm specimen consisting of skin and the underlying fibrofatty tissue and containing a stellate scar. The pathology showed fat necrosis, reactive inflammation and fibrosis, and no carcinoma. Postoperative healing was complicated by development of a chronic draining open wound. Six months later, with the wound continuing to drain and failing to heal completely, the patient underwent completion mastectomy. Pathology of the specimen showed no invasive or in situ carcinoma. A healing, inflamed biopsy cavity with metaplastic squamous epithelial lining and a cutaneous fistula was found, as well as fat necrosis, fibrosis, and active inflammation at the prior biopsy site.
CASE 12 Enhancing scar, suspicious for recurrence; contralateral axillary nodal presentation of new occult breast primary
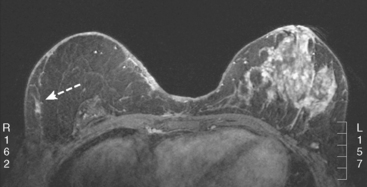
Breast MRI identified concerning findings bilaterally, including progressive enhancement of the right lumpectomy scar and enlargement of a left axillary lymph node (Figures 1, 2, and 3).
Ultrasound was obtained for correlation. At the level of the right lumpectomy scar site, dense shadowing was noted. There was a very hypoechoic mass–like component with vascularity, which was targeted for ultrasound-guided core biopsy (Figure 4). Pathology showed dense collagenous stroma and no carcinoma.
Left axillary lymph node ultrasound-guided fine-needle aspiration of a lymph node with cortical mantle thickening (Figure 5) showed rare atypical cells. It was repeated, with atypical ductal epi-thelial cells obtained. Surgical excision was recommended.
The assessment was that this was a new axillary presentation of left breast cancer. Breast MRI was repeated to look for a primary but did not identify one (Figure 6). Bilateral diagnostic mammograms were also negative (Figure 7). Positron emission tomography (PET)/CT was also negative.
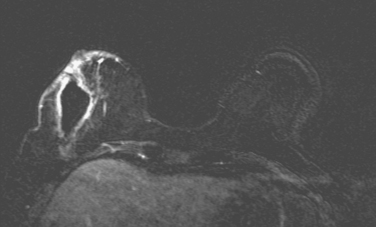
FIGURE 6 Repeat breast MRI, 5 months after breast MRI in Figures 1 to 3 and 3 weeks after surgery to excise the right lumpectomy scar and sample a left axillary lymph node. Enhanced, subtracted, axial image at the level of the excised scar shows 3-week-old postsurgical changes, including a large seroma at the surgical site, with a thin, enhancing rim and generalized skin enhancement.
The patient was treated with six cycles of Taxotere, Adriamycin, Cytoxan (TAC) chemotherapy and radiation therapy to the left breast and peripheral lymphatics (supraclavicular and posterior axillary boost). Repeat breast MRI 4 months after completion of radiation therapy shows expected left breast radiation changes, and evolution of the right breast postsurgical changes (Figure 8).
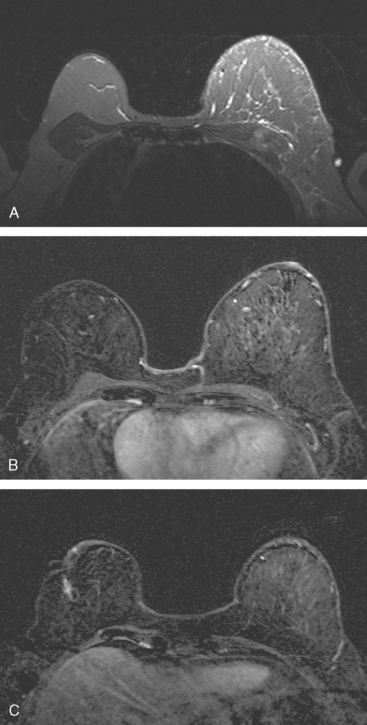
FIGURE 8 Breast MRI, 4 months after completion of left breast radiation therapy and 1 year after breast MRI in Figure 6. A, Axial STIR shows the left breast to be larger than the right. There is a diffuse increase in high signal reticulation and skin thickening on the left from radiation therapy. Note also the increased signal of the pectoralis and chest wall musculature. B, Enhanced subtracted view of both breasts shows low-level increased diffuse enhancement of the left breast compared with the right, also attributable to radiation. C, Enhanced, subtracted view of both breasts at the level of the previously excised right lumpectomy scar. The seroma present previously has resorbed, leaving only an enhancing linear scar.
TEACHING POINTS
A normal scar, especially a 14-year-old one, should not enhance. Enhancement of postsurgical scars is expected to largely resolve by 18 months, so new enhancement at a lumpectomy scar (a change from a previous MRI) appropriately raises concern for recurrence. Of course, not all new enhancement represents recurrent breast cancer. Fat necrosis and inflammation at areas of prior tissue trauma from surgery or radiation can develop years after treatment, and raise the question of recurrence. Histologic sampling with imaging guidance is appropriate. If the results do not indicate malignancy and the sampling was thorough, a benign result offering a reasonable alternative diagnosis may allow the patient to be followed. Additional tissue injury from sending a patient to surgery has to be carefully considered in a previously radiated field. Histologic proof of recurrent malignancy is a clear indication for a more aggressive approach. If there is not proof of malignancy, the risk-to-benefit ratio has to be carefully weighed. In this case, the patient’s scar was excised, and she healed uneventfully. See Case 11 in this chapter, a similar case in which fat necrosis complicating a scar led to a patient having to undergo a histologically unnecessary mastectomy for wound management.
Typical early (first 3 to 6 months after treatment) postradiation therapy findings are demonstrated here. They include skin thickening, edema, and enhancement, as well as parenchymal edema and reticulation. These findings are well seen on fluid-sensitive sequences (STIR or fat-saturated, T2-weighted), and peak at 3 to 6 months. Mild generalized increased parenchymal enhancement compared with the untreated opposite side can be detected in the first months after treatment and is demonstrated here. This low-level enhancement is not sufficiently intense to interfere with detection of clinically relevant disease. These changes are the result of acute tissue reaction to the ionizing radiation.
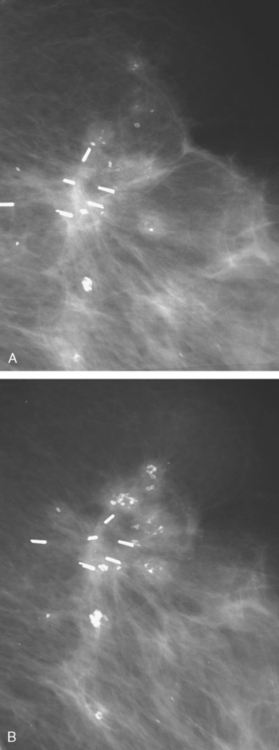
CASE 13 Proven multicentric fat necrosis mimicking multicentric recurrence
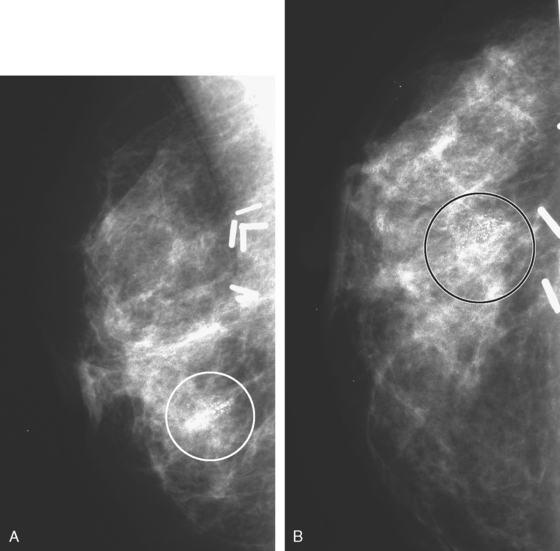
Mammographic evaluations showed postsurgical changes, with increasing density with associated coarse dystrophic calcifications (Figures 1 and 2). MRI was recommended for evaluation of the palpable abnormality. This was interpreted as suspicious for multifocal recurrence in the same quadrant as the prior lumpectomy (Figures 3, 4, 5, and 6). Ultrasound-guided biopsy was recommended.
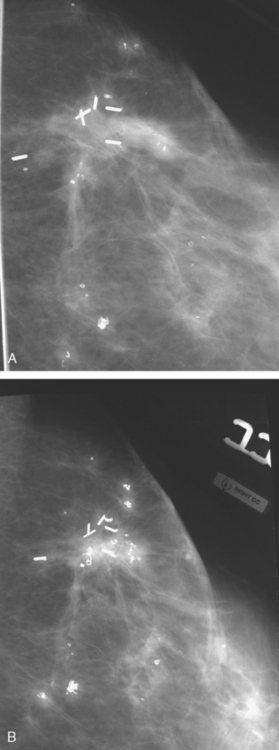
FIGURE 2 A and B, Craniocaudal magnification views of the lumpectomy scar, 6 months apart, performed at the same time as in Figure 1. Contracting density at the level of the clips is seen developing between the two exams, with coarse associated calcification. More peripheral small nodular densities also display coarse, chunky, progressive calcification.
Ultrasound showed multiple sites of intense shadowing (Figure 7). Core needle biopsy of a representative region returned benign histology of fat necrosis with fibrosis and microcalcification. Subsequent mammographic and clinical follow-up has been stable, with no evidence of recurrence 2 years after biopsy.
TEACHING POINTS
Not everything that enhances intensely in a lumpectomy bed is a recurrence. As on other breast imaging modalities, fat necrosis can be a perfect mimic of breast cancer on MRI (see Case 8 in Chapter 7 and Case 11 in this chapter). In this case, the mammographic changes are well within the described spectrum of fat necrosis changes in the setting of a radiated postlumpectomy scar. The scar shows contraction and increased density over time. Small nodular opacities in the vicinity show progressive coarse, dystrophic calcifications. The MRI findings are clearly abnormal, but correlation with the mammographic findings does suggest fat necrosis as a potential diagnosis. Of course, histologic sampling is required before consideration of the next course of action. In this case, the original breast MRI interpretation of the findings as highly suspicious for recurrent malignancy (without offering any additional differential possibilities) led to concern for possible sampling error when the ultrasound-guided histology of fat necrosis was obtained.
CASE 14 TRAM flap recurrence on mammography
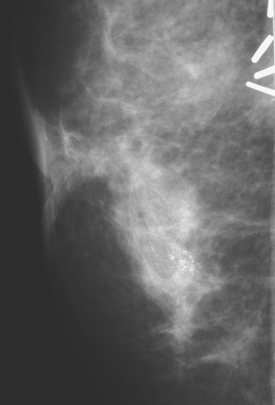
A 68-year-old woman, 5 years after left breast mastectomy and transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction, presented with a palpable mass at the lateral aspect of the left TRAM reconstruction (Figure 1). Subsequent core needle biopsy confirmed recurrent infiltrating ductal carcinoma.
CASE 15 Residual carcinoma in TRAM flap–reconstructed neobreast
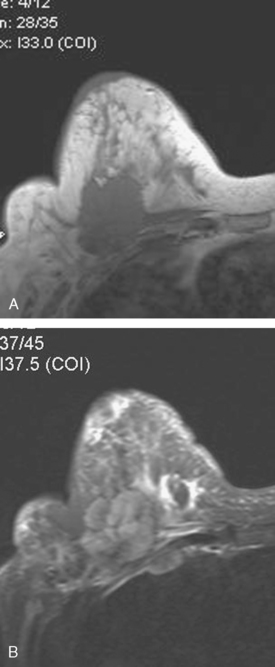
A 54-year-old woman presented with a palpable mass within the upper outer right breast. The patient was referred to a surgeon for evaluation. Needle biopsy of the palpable mass revealed ductal carcinoma in situ (DCIS). The patient underwent a preoperative MRI, which demonstrated an extensive area of enhancement in the lateral right breast, suggesting extensive intraductal disease (Figure 1). Mammography and ultrasound failed to demonstrate with certainty the extensive findings seen on MRI. Therefore, MRI-guided biopsy was performed for surgical planning to establish the extent of disease (Figure 2). The MRI-guided biopsy of a site distant to the known right DCIS confirmed additional DCIS. Because of this, the patient underwent right mastectomy with transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction. Pathology showed close margins for the mastectomy specimen. Six months later, the patient had a follow-up MRI that was interpreted as normal (Figure 3). On mammography 1 year after mastectomy, the small marker clip from MRI biopsy was noted in the lateral aspect of the TRAM flap (Figure 4). Ultrasound evaluation of the area of the clip demonstrated multiple small nodules (Figure 5). Core needle biopsy revealed both DCIS and invasive ductal carcinoma. The patient was treated with surgical resection and radiation therapy.
TEACHING POINTS
This case illustrates the role of mammography in surveillance for residual or recurrent disease in patients who undergo mastectomy and TRAM flap reconstruction. This recurrence was not initially detected on follow-up MRI. This case also highlights the fact that even with complete mastectomy, some breast tissue or carcinoma may be left behind, despite negative surgical margins. For that reason, mammographic surveillance of the mastectomy side may be beneficial. The presence of the MRI-guided biopsy marker clip on the postmastectomy mammogram, from a biopsy positive for DCIS, indicated the patient’s disease had not been completely excised. Ultrasound showed suspicious findings for residual disease and served for biopsy guidance. MRI should be used as an adjuvant to mammography for surveillance rather than a replacement. In cases with close or positive surgical margins, MRI cannot exclude small or microscopic foci of residual disease.
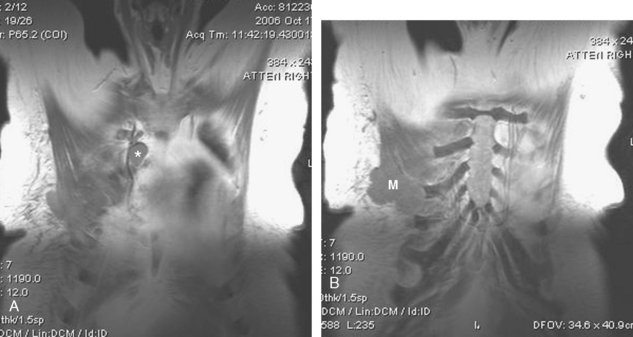
CASE 16 TRAM flap reconstruction with fat necrosis and supraclavicular lymphadenopathy, simulating recurrent disease
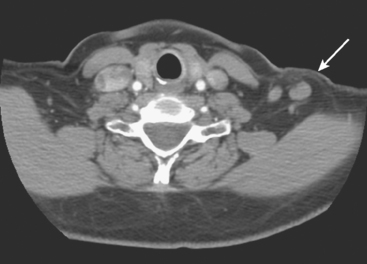
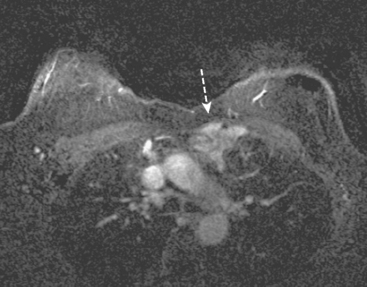
A 57-year-old woman was suspected to have recurrent breast cancer, based on palpable left supraclavicular adenopathy, 2 years after left mastectomy and TRAM flap reconstruction for recurrent left breast cancer. Her original cancer had been a poorly differentiated, stage 1, node-negative, estrogen receptor– and progesterone receptor–negative tumor, which was treated with lumpectomy, four cycles of doxorubicin (Adriamycin) plus cyclophosphamide (Cytoxan) (AC) and radiation therapy 2.5 years before recurring.
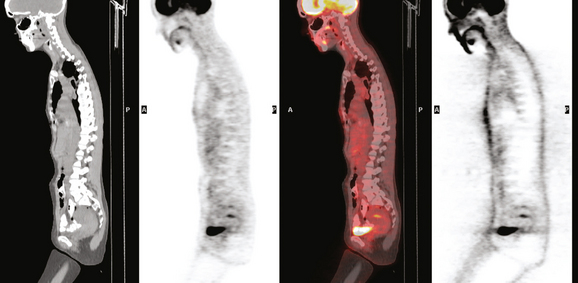
Two years after undergoing mastectomy and TRAM flap reconstruction, the patient was noted on physical examination to have a 2-cm firm left supraclavicular lymph node. It was confirmed by MRI (Figure 1). Referral to an ear, nose, and throat specialist for fine-needle aspiration (FNA) resulted in development of arm paresthesias during the procedure, which was stopped. The patient was later referred for CT-guided biopsy. This obtained a fragment of a benign lymph node, with no morphologic evidence of metastatic carcinoma. The patient was followed clinically, and underwent positron emission tomography (PET)/CT about 1.5 years after the lymph node was first noted. Two hypermetabolic foci were identified on PET/CT, one in the reconstructed neo-breast and one corresponding with the left supraclavicular node (Figures 2, 3, 4, and 5). The findings were interpreted as consistent with recurrent breast malignancy. Breast MRI was obtained subsequently and showed a rim-enhancing mass with fatty and soft tissue elements at the posterior aspect of the reconstructed breast (Figure 6). This was thought to be secondary to the TRAM flap surgery, with fat necrosis. The patient had not had a mammogram since the surgery, so mammography was obtained for correlation (Figures 7 and 8). This showed rim and coarse fat necrosis–type calcifications (CT images provided for correlation, Figures 9 and 10). Subsequent ultrasound-guided biopsy confirmed fat necrosis with dense fibrosis and calcification (Figure 11). The left supraclavicular lymph node was excised, was benign, with nonspecific granulomatous lymphadenitis, and presumably was reactive and secondary to the breast process.
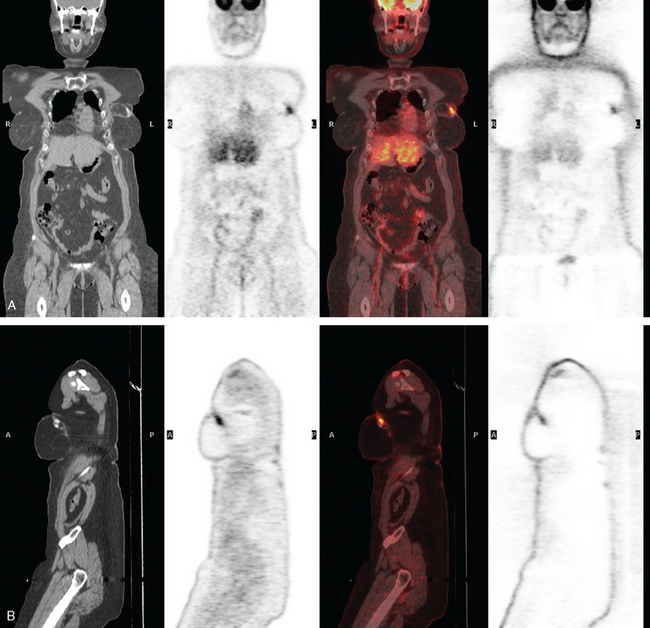
FIGURE 3 Coronal (A) and sagittal (B) views through the focal activity within the left neo-breast.
(from left to right: CT, PET, fused PET/CT, NAC PET).
TEACHING POINTS
The fat necrosis findings in this case are a florid, dramatic example, but nonetheless display many of the typical features of fat necrosis lesions. Fat necrosis is a nonsuppurative, inflammatory process of aseptic saponification of fat, induced by blood and tissue lipase after trauma. The trauma can be surgical, as in this case; and surgery, especially extensive surgery, is the most common cause of fat necrosis. Other commonly encountered etiologies are blunt or penetrating trauma and radiation therapy. Other insults, such as breast infection or spontaneous subareolar duct rupture, are less common causes of fat necrosis. When symptomatic, fat necrosis most commonly is detected clinically as a painless, palpable breast mass. The imaging appearance ranges from characteristically benign (oil cysts) to confounding. On both clinical and imaging grounds, fat necrosis can be indistinguishable from malignancy and may require sampling for differentiation. Fat necrosis can be considered the “great mimic” of breast cancer because it can present as calcifications, spiculated masses, or architectural distortion. See Cases 11 and 13 in this chapter and Case 8 in Chapter 7 for additional examples. The most reliable mammographic finding for the diagnosis is an oil cyst, resulting from liquefaction of fat. Calcifications forming in response to fat necrosis can be fairly characteristic when mature, being coarse and dystrophic and forming eggshell or rim-like forms, often on the margins of oil cysts. Of course, developing fat necrosis calcifications can often be indeterminate when first detected. The decision to biopsy newly identified calcifications forming in a surgical bed depends on numerous factors, including how the prior cancer manifested (mass versus microcalcifications), the morphology of the calcifications, and the time course since surgery, with fat necrosis most commonly developing within 3 years after completion of therapy. Histologically, fat necrosis lesions show fat cell death, with vacuole formation, with surrounding fibroblasts, lipid-laden macrophages, and multinucleated giant cells.
Review of the imaging features of this lesion delineates many of the imaging manifestations of fat necrosis. The mass is fatty centrally, with characteristic density on CT and signal intensity on MRI. The soft tissue component is peripheral and rim-like, and the calcifications are coarse and dystrophic. The enhancement associated with the mass on MRI corresponds to the peripheral fibrosis component. The sonographic appearance is of a complex and heterogeneous solid mass but is well within the spectrum described as potential ultrasound appearances of fat necrosis masses in a series by Soo and colleagues. The ultrasound findings in this study ranged from cystic to complex to solid-appearing masses, with circumscribed or irregular margins, often with distortion of the normal adjacent breast anatomy. The ultrasound appearances of 31 fat necrosis masses in 23 patients analyzed in this report categorized them as solid in 15, complex with mural nodules in 7, complex with echogenic bands in 4, anechoic with posterior acoustic enhancement in 2, anechoic with shadowing in 2, and without a visible mass in 1. Evolution of the sonographic appearance was noted over time of four of six masses that were followed, with complex lesions either becoming increasingly solid in three or increasingly cystic in one. These six fat necrosis masses, which were followed with ultrasound, either stayed unchanged in size over time (two cases) or decreased in size (four cases).
Devon RK, Rosen MA, Mies C, et al. Breast reconstruction with a transverse rectus abdominis myocutaneous flap: spectrum of normal and abnormal MR imaging findings. RadioGraphics. 2004;24(5):1287-1299.
LePage MA, Kazerooni EA, Helvie MA, et al. Breast reconstruction with TRAM flaps: normal and abnormal appearances at CT. RadioGraphics. 1999;19:1593-1603.
Soo MS, Kornguth PJ, Hertzberg BS, et al. Fat necrosis in the breast: sonographic features. Radiology. 1998;206:261-269.
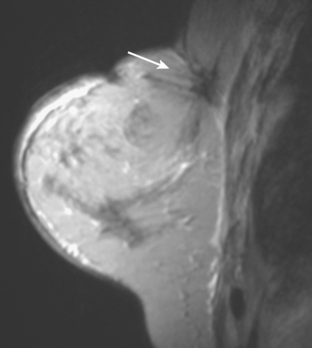
CASE 17 Chest wall recurrence detected on surveillance MRI
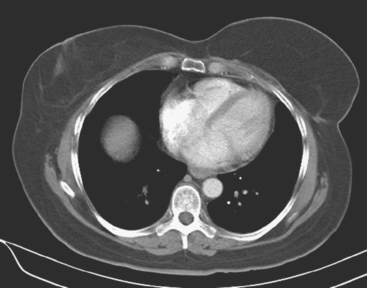
A 72-year-old woman, 7 years after treatment with lumpectomy and radiation therapy for left breast infiltrating ductal carcinoma, underwent annual screening MRI. The MRI demonstrated an abnormal area of enhancement involving the anterior chest wall, suspicious for chest wall recurrence (Figure 1). CT and positron emission tomography (PET) scans were performed to further evaluate the finding. The CT scan confirmed the findings (Figure 2), and the PET scan showed increased uptake of the abnormality (Figure 3). Subsequent needle biopsy confirmed the suspected diagnosis of chest wall recurrence, and the patient was treated with radiation therapy.
TEACHING POINTS
This case illustrates the ability of MRI to detect recurrent disease in patients treated for breast carcinoma. In addition to in-breast recurrences, the anterior chest wall, mediastinum, and axilla can be evaluated on MRI. PET/CT may help to improve diagnostic confidence and accuracy in evaluating the extent of recurrent disease. It also plays an important role in the diagnosis of bone metastases, providing information regarding the presence or absence of structural skeletal lesions on CT as well as their degree of metabolic activity.
Fueger BJ, Weber WA, Quon A, et al. Performance of 2-deoxy-2-[F-18]fluoro-D glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol. 2005;7:369-376.
Isasi CR, Moadel R, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat. 2005;90:105-112.
Tastumi M, Cohade C, Mourtzikos M, et al. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging. 2006;33:254-262.
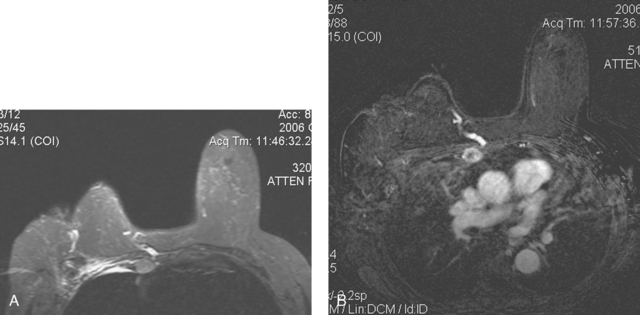
CASE 18 Recurrent IDC and radiation-induced pleomorphic sarcoma, with chest wall invasion*
On physical examination, the right lateral breast was indurated and fixed to the chest wall. Her mammogram showed suspicious increased density deep to the level of her scar (Figures 1, 2, and 3). Ultrasound showed a corresponding, heterogeneous, hypoechoic mass with irregular margins (Figure 4). Ultrasound-guided core needle biopsy showed recurrent ductal carcinoma and a very atypical spindle cell proliferation, with atypia in excess of that expected from radiation damage. This component was thought to be a pleomorphic sarcoma, probably radiation induced.
Subsequent evaluations included breast MRI; chest, abdomen, and pelvis CT scans; and positron emission tomography (PET)/CT. The breast MRI showed the mass to be large, with rim enhancement and washout. The mass penetrated and invaded the chest wall and was accompanied by a large internal mammary lymph node (Figures 5, 6, 7, and 8). Both the mass and the internal mammary lymph node were intensely hypermetabolic on PET, as was a right lower lobe nodule (Figure 9).
TEACHING POINTS
Locally recurrent breast cancer may be heralded by a change in physical examination or may be first identified by a change on imaging. Local recurrences in patients previously treated with breast conservation most commonly occur within the lumpectomy bed. Contraction of a postsurgical scar can be expected as a normal evolution in the appearance of a previously treated breast. However, increased size or density of a scar should be cause for concern and further evaluation. Enhanced breast MRI can be very helpful in differentiating between a scar and recurrent disease, although occasionally, a complicated scar, such as by fat necrosis, can convincingly mimic recurrent disease (see Case 11 in this chapter).
The dual histology in this case is also unusual. The ultrasound-guided core biopsy specimens were compared with the original breast primary and were morphologically similar, representing recurrent invasive poorly differentiated ductal carcinoma. However, a component with atypical spindle cell proliferation was noted, in excess of that expected in a radiation-damaged field, which was interpreted by outside consultation as a postirradiation pleomorphic sarcoma. Radiation-induced sarcomas are a rare, but recognized, late potential complication of radiation therapy in breast conservation patients. See Case 9 in Chapter 13 for an additional example and discussion of this issue.