Chapter 34 Imaging Patients with Chest Pain in the Emergency Department
INTRODUCTION
At the other end of the spectrum are patients with atypical and noncardiac chest discomfort who can be safely sent home. However, these patients represent only a small minority of patients with potential acute coronary syndrome (ACS). The majority of patients presenting with chest pain have an initial evaluation that is insufficient to either diagnose or exclude myocardial ischemia, and thus further evaluation is required. This group accounts for nearly two-thirds of ED chest pain patients.1,2
Standard tools and techniques currently used for the evaluation of all chest pain patients include the ECG, the history, and myocardial markers. The ECG is the first test performed for the evaluation of patients with potential myocardial ischemia. Although the presence of ischemic ECG findings can identify patients who will benefit from more aggressive pharmacologic and interventional treatment, diagnostic ECG changes are present in only a minority of patients with ACS.3 The history can be useful for risk stratifying patients into higher- and lower-risk groups, but in most cases, the substantial overlap prevents it use for determining who can be safely discharged from the ED.3
Another key component in the evaluation of chest pain patients are myocardial markers, particularly the troponins, which are considered the gold standard for diagnosing MI. Current recommendations are that all patients presenting with chest pain in the ED should undergo cardiac biomarker sampling, with repeat or serial sampling 6 to 8 hours later in those with initially negative markers (class I), with troponin being the preferred marker.3 However, as with the ECG, there are important limitations. First, all markers have a time-dependent rise and fall from their release to detection in the bloodstream, which takes at least 2 to 4 hours after MI onset. Second, by definition, markers of necrosis require myocardial damage to be released; therefore, they will be negative in patients who have ischemia only.
Because of these limitations, the standard evaluation process for patients who have a nonischemic ECG and a low-risk presentation typically involves admission to a chest pain observation area where serial biomarkers are performed to exclude MI. If negative, the patient subsequently undergoes provocative testing (typically treadmill testing without imaging) and is discharged if negative. If any part of the evaluation is positive, the patient is admitted for further evaluation. This approach has limitations because a significant minority of patients have an intermediate likelihood of coronary artery disease, or an abnormal rest ECG, or are unable to exercise adequately,4 limiting the number of chest pain patients eligible for an observational admission.
ACUTE MYOCARDIAL PERFUSION IMAGING
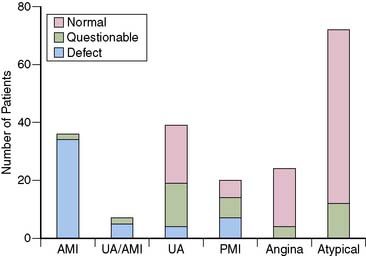
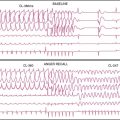
The first studies to evaluate the ability of rest MPI to identify ACS in chest pain patients were performed in the 1970s. Wackers et al.5 found a high diagnostic accuracy when planar thallium-201 (201Tl) imaging was performed in 203 patients admitted for possible MI. Images were abnormal in all 34 patients who had MI, as well as in 27 of the 47 (58%) patients who had unstable angina. (Fig. 34-1). In contrast, none of the 98 patients diagnosed with stable angina or atypical chest pain had abnormal studies. Others, also using 201Tl in chest pain patients, subsequently confirmed these results.6
Although imaging of chest pain patients using 201Tl appeared to be a promising tool, a number of limitations precluded its widespread adoption. Planar imaging has a lower sensitivity for detecting small areas of ischemia and ischemia in the posterior distribution, an area not often associated with diagnostic ECG changes. Because of its energy characteristics, relatively rapid redistribution, and imaging limitations in large patients, 201Tl is not an optimal agent for acute imaging. Although some attempted to overcome the logistical problems by using portable gamma cameras placed in the ED,7,8 these efforts were unsuccessful.
The development of single-photon emission computed tomography (SPECT) imaging, in combination with the availability of the technetium-labeled myocardial perfusion agents and their superior image quality, subsequently led to observational studies, followed by clinical trials demonstrating the utility of acute ED MPI. Technetium-99m (99mTc)-labelled sestamibi and tetrofosmin are taken up by the myocardium in proportion to blood flow, similar to thallium, but they lack significant redistribution.9 Therefore, patients can be injected while experiencing symptoms and imaged up to several hours later, making it possible to perform high-quality SPECT imaging outside of the ED setting in the absence of dedicated equipment.
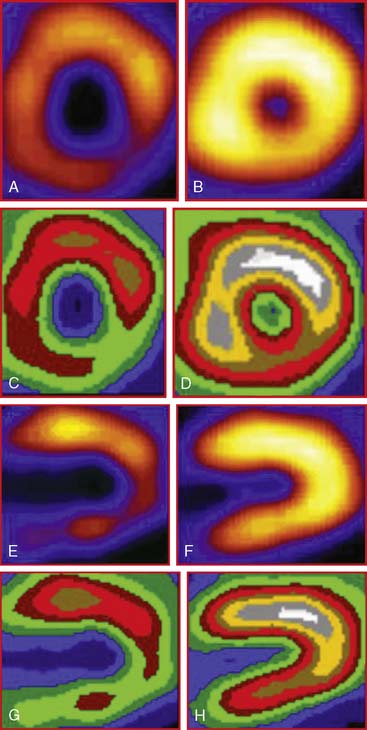
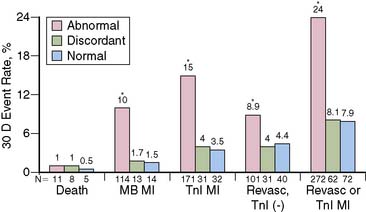
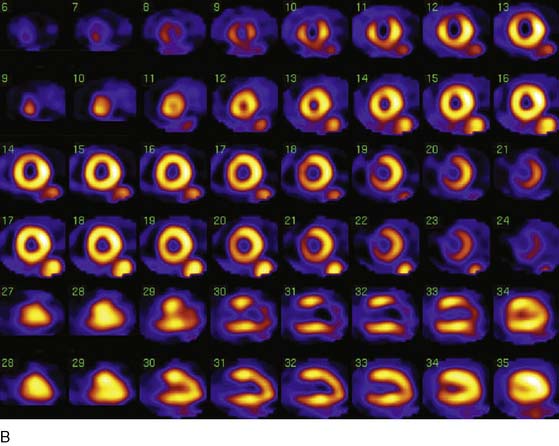
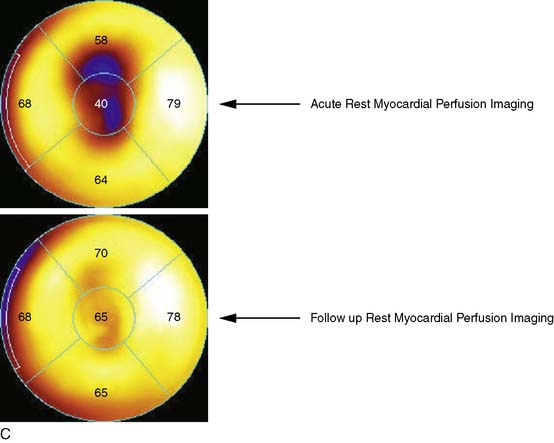
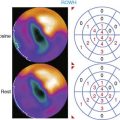
The favorable energy and dosimetry of 99mTc allows for gated reconstructions, an advantage not available at that time with 201Tl imaging. The ability to quantitate ejection fraction10 adds an additional prognostic component, identifying patients who may have unsuspected systolic dysfunction. More important, correlating wall motion and thickening with perfusion defects can be used to determine whether a defect reflects true ischemia or infarction or is the result of artifact or tissue attenuation.11 In the setting of acute infarction or ischemia, wall motion is typically abnormal; in contrast, an apparent perfusion defect in the presence of normal wall motion and thickening on gated SPECT usually indicates an artifact such as tissue attenuation (Fig. 34-2). The ability to perform simultaneous wall motion and perfusion significantly improves specificity12 and is particularly valuable in the acute setting where serial images are not available. Theoretically a patient could be injected during symptoms, and if imaged after symptom resolution, the wall-motion abnormality could resolve, and the defect could be misinterpreted as resulting from attenuation rather than ischemia. However, this appears to be infrequent in practice. Kontos et al. reported that for 2286 consecutive patients who were admitted for exclusion of ischemia following acute rest MPI, the proportion of patients who had troponin I (TnI) elevations (4.0 vs 3.5%), creatine kinase (CK)-MB MI (1.7 vs 1.5%), or who underwent revascularization (5.5 vs 5.2%), was not different between those who had normal perfusion and function and those who had perfusion defects but had normal wall motion in that area. In contrast, those who had perfusion defects associated with abnormal wall motion were significantly more likely to have TnI elevations (15%), CK-MB MI (10%), and undergo revascularization (17%) (Fig. 34-3).12 A potential explanation is that the severity of ischemia that occurs with an ACS event is associated with underlying myocardial stunning13,14 and persistence of wall motion abnormalities.
The combination of perfusion and wall motion has prognostic value. In a recent multicenter study reported by Kaul et al., perfusion plus regional function provided significantly greater diagnostic and prognostic value when compared to perfusion alone for predicting outcomes in 163 patients with possible ACS.15
DIAGNOSTIC VALUE
Sensitivity in Acute Myocardial Infarction
The first studies to demonstrate the high diagnostic accuracy using SPECT imaging with sestamibi for evaluating chest pain patients were performed in select populations of admitted patients. In a proof-of-concept study, Christian et al. performed early MPI in 14 patients without ST-segment elevation (most of whom had ST-segment depression) who were diagnosed with MI and who later underwent coronary angiography.16 Abnormal studies were found in 13 of the 14 patients. The amount of myocardium at risk averaged 20% ± 15% (range 2% to 53%) of the left ventricle, and half had either the left circumflex or a ramus branch as the culprit artery.
In one of the first studies to evaluate ED patients, Hilton et al. imaged 102 patients who presented to the ED with typical angina and a nonischemic ECG.17 Only 1 of the 70 patients with a normal MPI had an event, compared to 2 of the 15 with an equivocal MPI (13%), and 12 of the 17 (71%) with abnormal MPI. In a multivariate analysis, the only independent predictor of cardiac events was abnormal MPI.
Numerous subsequent studies that included single-center and multicenter observational studies, as well as a randomized trial, were performed in large numbers of heterogenous patients undergoing an ED chest pain evaluation. They consistently found a high sensitivity (90% to 100%) for detecting acute MI (Table 34-1). Because sensitivity is not perfect, the results of acute MPI cannot be interpreted in isolation but must be placed in context with the clinical evaluation. However, infarcts that are present despite normal acute MPI are typically small, non-Q-wave infarcts and associated with an uncomplicated clinical course.
Table 34-1 Diagnostic Accuracy of Rest Myocardial Perfusion Imaging in Patients with Acute Chest Pain Syndrome and Normal or Nonischemic Rest Electrocardiograms
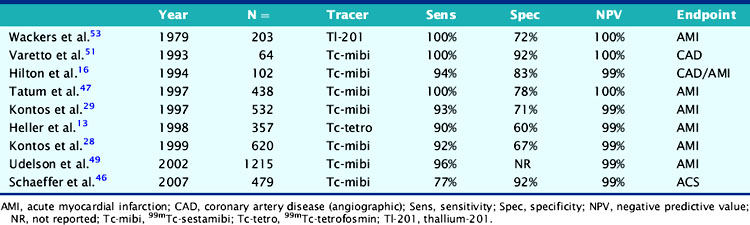
In contrast to most diagnostic tools that have not been evaluated in a randomized trial, the ability to safely discharge a patient after negative acute MPI was confirmed in the Emergency Room Assessment of Sestamibi for Evaluating Chest Pain (ERASE) study.18 A total of 2475 patients were randomized to routine care or ED MPI, in which patients were injected with sestamibi in the ED and then underwent acute imaging, with the results called back to the ED physician.18 All patients, whether admitted or discharged, underwent subsequent marker analysis and further diagnostic evaluation with either stress testing or coronary angiography. There was no difference between the two groups, in the percentage of ACS patients with MI (97% versus 96%) or unstable angina (83% versus 81%) who were admitted with one MI patient from each group discharged from the ED. However, there was a significantly lower admission rate and a higher rate of direct discharge from the ED in the ED MPI arm of the study compared to the standardized care arm. Importantly, this was a consistent effect seen at six of the seven institutions involved in the trial.
These results were confirmed in a subsequent smaller randomized trial. Candell-Riera et al. randomized 222 low-risk chest pain patients to either acute rest MPI or conventional management in the ED. Fewer patients undergoing rest MPI were admitted (18% versus 33%; P < 0.03), and overall ED time was shorter (13 ± 6 versus 16 ± 9 hours; P < 0.01).19
A limitation of the mentioned studies is that acute MPI was performed predominantly in low-risk patients, so relatively small numbers of patients who had MI were included in any one study, resulting in an imprecise estimate of sensitivity. To address this, Kontos et al. analyzed results from 141 consecutive patients who underwent ED MPI and were subsequently diagnosed with CK-MB MI.20 Overall sensitivity was 89% (95% CI: 83% to 94%), consistent with prior studies but with smaller confidence intervals. Similar to prior studies, patients with negative MPI had small MIs, with an average peak CK of 313 ± 227 U/L, compared to 590 ± 620 U/L (P < 0.001) in those with positive MPI. In addition, nonsignificant disease was found in the majority of patients with negative MPI who underwent coronary angiography.
Acute Coronary Syndrome Without Myocardial Infarction
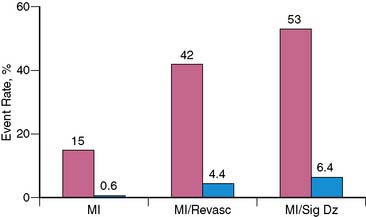
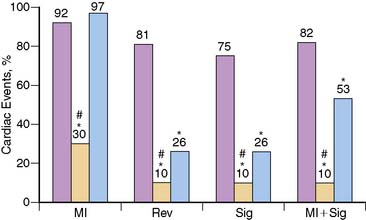
Although specificity of acute MPI appears low when MI is the only end point considered, this in part relates to one of its important advantages, the ability to identify patients who have ischemia alone. Bilodeau et al.21 imaged 45 patients without a history of MI who were admitted for unstable angina. The presence of a perfusion defect had a high accuracy (sensitivity 96%, specificity 79%) for predicting angiographic coronary disease in patients injected during an episode of pain, compared to a sensitivity of only 65% for the ECG. In another study Kontos et al. found that in 532 patients admitted after acute rest MPI in the ED, acute MI, as assessed by CK-MB elevations, was present in only 15% of patients with positive MPI (Fig. 34-4).22 However, the majority of patients with positive MPI had an end point consistent with ACS, including acute MI, subsequent revascularization, or significant coronary disease (>70% stenosis) on coronary angiography. Considering these as positive end points improved positive predictive value to 53%.
NEGATIVE PREDICTIVE VALUE AND PROGNOSIS
The negative predictive value in clinical studies has been consistently high and has typically exceeded 99% (see Table 34-1).18,23–26 The high negative predictive value for excluding significant ischemia allows effective identification of those who can be evaluated in lower-intensity settings other than the coronary care unit (CCU) or who can be discharged home. Patients with negative MPI have a low risk for both short- and long-term cardiac complications as well. Hilton et al.17 found that patients with normal MPI had an excellent prognosis, with no late events at 90-day follow-up. Similarly, Tatum et al. reported that patients with negative acute MPI had a cardiac event rate of only 3% during the subsequent year.26
INCREMENTAL DIAGNOSTIC VALUE
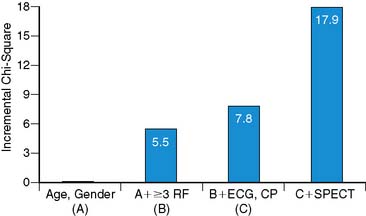
In the current cost-conscious environment, even though a new test may offer high diagnostic value, an additional requirement before adoption is that it also provides significant incremental value over the current standard of care. Kontos et al. found that in a multivariate analysis, abnormal MPI was the most important independent predictor of MI or revascularization in 532 patients who underwent acute ED MPI.22 Similarly, Heller et al. found that that abnormal SPECT was the most important multivariate predictor of MI in 357 patients who underwent acute MPI. In addition, acute MPI added significant incremental diagnostic value after consideration of demographic, clinical, and ECG variables (Fig. 34-5).23
In an interesting intent-to-treat survey study, Knott et al. performed acute MPI in 120 ED patients.27 The requesting physician completed a questionnaire before imaging, asking what the proposed management would be had the test not been available. They found there would have been a 34% reduction in overall hospital admissions and a 59% reduction in planned CCU admissions. Interestingly, overall CCU admissions were not reduced, because 17 patients initially considered low risk were admitted to the CCU after MPI was found to be abnormal, indicating a more appropriate utilization of resources.
COST-EFFECTIVENESS
Despite the application of relatively complex and expensive technology, ED MPI can be cost effective if the number of patients admitted is decreased.23,28,29,30 Several observational studies have confirmed that cost reductions occur when rest MPI is used as an integral part of patient management. In addition, a preliminary analysis from the ERASE study confirms that using ED MPI as a key part of the initial diagnostic strategy was indeed cost-effective, since costs were reduced a mean $70 per patient.31
Costs are reduced by a number of mechanisms. One obvious mechanism is discharging more low-risk patients directly from the ED rather than the patient being admitted or undergoing a more prolonged observation. Second, by identifying patients with atypical symptoms and a nonischemic ECG who have MI, inappropriate discharges can be averted. A third mechanism is more appropriate selection of diagnostic procedures, reducing the rate of coronary angiography in low-risk patients.27,28
Comparison with Troponin
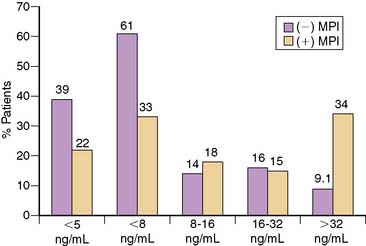
Because most of the studies evaluating the use of acute MPI were performed in the 1990s, CK or CK-MB were typically used to diagnose MI. However, because of its high sensitivity and specificity for detecting myocardial necrosis, current guidelines recommend that cardiac troponin should be the diagnostic standard for MI.32 Since approximately 3% to 4% of the left ventricle must be ischemic to allow detection by MPI,33 it would be expected that use of a more sensitive cardiac marker such as troponin would identify more patients who have necrosis than would acute MPI. This has been supported by a number of small studies that reported that although the sensitivity of MPI was high, it was significantly lower than that of TnI or TnT.25,34 In a larger study, Kontos et al. analyzed outcomes in 319 patients who were initially considered low risk for ACS and underwent acute rest MPI as part of standard chest pain evaluation protocol. They subsequently were found to have elevated TnI values,35 thus meeting the American College of Cardiology/European Society of Cardiology (ACC/ESC) definition for MI. A total of 77 patients had negative MPI, giving a sensitivity of only 76%. However, more than half (n = 47 [61%]) had a peak CK-MB of < 8 ng/mL and therefore did not meet the previous CK-MB MI definition (Fig. 34-6). Patients with negative MPI had significantly lower peak CK-MB values (15 ± 25 ng/mL versus 45 ± 78 ng/mL; P < 0.001), had higher ejection fractions (56 ± 15% versus 47 ± 13%; P < 0.01) and were more likely to have nonsignificant disease (45% versus 30%; P < 0.001) than those with positive MPI.
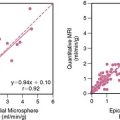
Rather than being considered as competing diagnostic tools, markers and MPI offer complementary information. Despite the higher sensitivity of troponin, rest MPI has some important advantages over using markers alone. By definition, myocardial markers are abnormal only in patients with necrosis and therefore are negative in patients who have ischemia alone. However, in both settings, perfusion will be abnormal. Second, after the onset of infarction, it typically takes a few hours before markers of necrosis can be detected in the bloodstream. Given the additional time for laboratory processing and reporting (blood-to-brain time),36 this may be as long as 4 to 6 hours after MI onset. In contrast, despite the time required for imaging and processing, acute MPI results can be available within 1 to 2 hours after injection. Thus, the sensitivity of MPI is significantly higher than that of the initial troponin. These two advantages were demonstrated in a study of 620 consecutive patients who underwent serial marker sampling with TnI and CK-MB after having acute ED MPI performed. The sensitivity of TnI and MPI were similar for identifying patients who had CK-MB MI. However, sensitivity of MPI was higher when compared to the initial TnI, and was superior for identifying patients who underwent revascularization or who had significant disease on coronary angiography (Fig. 34-7).25
RISK AREA
An important prognostic parameter provided by acute MPI is that it provides quantitative information on the ischemic risk area in ACS patients. The most important determinant of infarct size is the ischemic risk zone, or the amount of myocardium in jeopardy.37 The ischemic risk area is a validated measure of prognosis; patients with larger defects have a worse long-term prognosis.38,39 The ischemic risk area in patients with non-ST-elevation ACS is often large, even in the absence of ischemic ECG changes. Kontos et al.40 reported that in a group of 87 patients diagnosed with MI after undergoing ED acute MPI, the ischemic risk area ranged from 0% to 62% of the left ventricle, with a mean risk area of 18% ± 11%. These results were similar to those reported by Christian et al.16 in the initial group of 14 patients studied. Patients with normal ECGs at the time of presentation had risk areas similar to those of patients with abnormal but nonischemic ECGs (16% ± 12% versus 19% ± 12%; P = 0.25).41
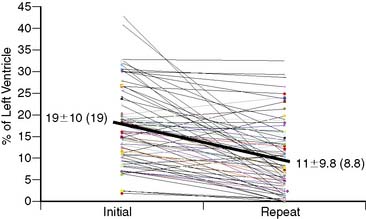
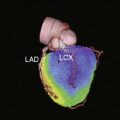
In a follow-up study, 69 patients who had acute MPI in the ED were diagnosed with MI and subsequently underwent repeat MPI.42 The initial risk area was 19% ± 9.7% of the left ventricle. Only two patients had a risk area = 6%. When performed, revascularization occurred more than 9 hours after presentation (mean time 3.0 ± 3.5 days [median 2 days]). Repeat MPI was performed a median of 5 days after acute MPI. The amount of salvage averaged 45% ± 34%, although variations in area at risk, final infarct size, and degree of salvage were high (see Fig. 34-8). Forty-six patients (67%) had significant salvage, defined as a greater than 25% decrease in defect size, 32 (46%) of whom had over 50% salvage (see Fig. 34-7). In those who had significant salvage, the initial defect size was 19% ± 10% (median 16%), which decreased to 6.5% ± 5.1% (5.6%) on repeat imaging. An example of a patient with significant salvage is shown in Figure 34-9. Fifty patients (73%) had EF measured on the initial and repeat images. Initial mean EF was 49% ± 12% (median 50%), which showed a trend toward improvement on repeat imaging by increasing to 53% ± 10% (P = 0.06; median 54%). Fifty percent of patients had an abnormal EF at the time of initial imaging, whereas only 34% had an abnormal EF on repeat MPI. EF increased significantly in patients who had significant salvage, from 50% ± 14% (51%) to 55% ± 7.7% (55%) (15% increase; P < 0.01) but was unchanged in patients who did not have significant salvage, from 50% ± 14% (51%) to 49% ± 13% (52%) (2% decrease; P = NS). Consistent with prior studies, the area at risk was substantial, was similar to that of patients with inferior ST-elevation MI,42,43 and occurred despite late revascularization, outside the time frame typically considered necessary for myocardial salvage. A case with significant salvage is shown in Figure 34-9.
One explanation for this is that MI in these patients is often due to occlusion of the left circumflex coronary artery. Because it usually supplies the lateral and posterior walls of the left ventricle, thus areas not well represented by the surface ECG,44 infarcts from this artery are much less likely to have ischemic ECG changes. In a study of consecutive patients with MI, left circumflex occlusion was associated with ST-segment elevation less than 50% of the time, and diagnostic ECG changes occurred significantly less frequently than infarctions resulting from occlusion of the left anterior descending or right coronary artery.44 O’Keefe found that risk area was not significantly different in patients who had left circumflex occlusion associated with ST-segment elevation, left circumflex occlusion without ST elevation, and right coronary artery occlusion.43
OTHER ISSUES
Radiopharmaceuticals
The limitations of thallium as an imaging agent for acute chest pain evaluation have been discussed. However, one alternative in some institutions is that in the chest pain–free patient, rest thallium rather than technetium is used.45 If negative, the patient can then undergo immediate stress MPI, an option not available if a high-dose-technetium injection had been used instead.
Although most studies were performed with sestamibi, comparable results have been obtained with tetrofosmin. In a multicenter study, Heller et al. found a sensitivity of 90% in 357 patients who underwent acute MPI.23 Negative predictive value (NPV) was equally high (99%), with only two patients who had small non-Q-wave MIs having negative acute MPI. In the study in which 319 patients had TnI elevations, sensitivity of acute MPI with sestamibi (75%) and tetrofosmin (80%) was similar, as was the proportion who had CK-MB MI (84% versus 87%).35
Timing of Tracer Injection
For optimal diagnostic accuracy, it is important to inject a radiotracer as soon as possible after presentation and prior to symptom resolution. Although it would be expected that as time progresses after resolution of symptoms that the diagnostic yield of rest MPI in patients with ischemia alone would decrease, this has not been a consistent finding. Studies in which patients were injected within 6 hours of symptom resolution have not found a significant decrease in diagnostic accuracy.21,22,46,47 Kontos et al. found that when patients were injected within 6 hours of symptoms, sensitivity was similar for identifying patients who had MI, revascularization, or significant coronary disease between those with and without symptoms who were injected.22
However, the longer the symptom-free interval, the more likely rest MPI will be negative. Wackers et al.47 performed 201Tl scintigraphy in 98 patients admitted with chest pain who had MI excluded. Sensitivity was 57% when patients were imaged within 6 hours of the last anginal symptoms; however, it decreased to only 8% when patients were imaged after 12 hours. Bilodeau et al.21 found that the sensitivity of MPI for detection of coronary artery disease was 96% in 45 patients injected with tracer during chest pain. When the same patients were reinjected later while pain free, the sensitivity had decreased to 65%. In both cases, the sensitivity was significantly higher than that of the initial ECG.
One explanation relates to the difference in the underlying mechanisms causing perfusion defects in ACS patients as compared to those undergoing stress testing. Rather than causing true ischemia, stress perfusion defects result from flow heterogeneity between areas supplied by coronary arteries with and without significant stenoses. The perfusion tracer is injected at the time of maximal flow imbalance, with a rapid return of coronary flow to baseline once the patient stops exercising. In contrast, in ACS patients, perfusion defects result from the combination of intermittent thrombotic occlusion and vasoconstriction in the setting of complex coronary morphology,48 resulting in marked decreased coronary blood flow49 and persistently decreased regional myocardial perfusion.48 Because regional hypoperfusion is one of the first steps in the ischemic cascade, symptoms of chest discomfort are often a late clinical manifestation, so regional hypoperfusion will frequently be present even in the absence of symptoms.
Perfusion defects may also result from distal embolization of a proximal thrombus, leading to downstream microvascular obstruction.50 In a study of 75 patients who underwent sestamibi injection during rotational atherectomy, a procedure in which distal embolization of microparticles is frequent, perfusion defects were present in 65% of patients.50
Another potential mechanism was reported in an interesting study of 40 patients who had a percutaneous intervention. Fram et al. found that perfusion abnormalities persisted in patients injected with 99mTc-sestamibi at varying time intervals after balloon inflation, although the size of the perfusion defect decreased as the time interval after the procedure increased.51 They hypothesized that the pharmacodynamics of sestamibi are dependent on both membrane and mitochondrial functional integrity, which may be depressed as a result of lingering metabolic alterations, especially in high-energy metabolites, which occur after transient ischemia. In summary, the sensitivity of acute MPI will be dependent on a number of factors, including the time interval after symptom cessation, the severity of ischemia, the severity of the underlying degree of stenosis, and presence or absence of necrosis.
One new imaging agent, β-methyl-p-[123I]-iodophenyl pentadecanoic acid (BMIPP), which relies on the change from free fatty acid to glucose metabolism in the heart, may offer advantages for identifying ACS in chest pain–free patients. Free fatty acids are the preferred substrate for high-energy ATP production in the normal myocardium.52 In the setting of myocardial ischemia, suppression of fatty acid metabolism and a switch to glucose utilization occur. This switch in metabolism, known as metabolic stunning, offers advantages for imaging the chest-pain patient in whom symptoms have resolved, a group of patients who represent a significant proportion of ED chest-pain patients. BMIPP is a methyl branched-chained fatty acid that is not easily metabolized and thus is retained in myocardial cells.53 When labeled with 123I, iodofiltic acid provides excellent images of the myocardium.
The feasibility of BMIPP imaging was recently evaluated in a pilot study in which 105 patients presenting with possible ACS were injected with BMIPP within 30 hours of symptom cessation.54 Quantitative BMIPP imaging plus initial diagnosis increased sensitivity for identifying ischemia by 30% (from 54% to 84%; P = 0.003) and for ACS by 17% (from 83% to 100%; P = NS), without significantly changing specificity. Further studies are in progress that will more fully evaluate the potential of this technique.
Special Populations
Another group of patients in whom ED rest MPI can be useful are those presenting with cocaine-associated chest pain. In the absence of ischemic ECG changes or known coronary disease, the risk of ACS is low.55 In a study of 216 consecutive patients with chest pain after recent cocaine use who underwent ED MPI, only 5 patients (2.3%) had abnormal studies, including 2 with acute MI.56 None of the 38 patients with normal MPI had subsequently acute MI by biomarkers after admission to the CCU, and only 7% of the 67 patients undergoing subsequent stress MPI had reversible myocardial perfusion defects. At 30-day follow-up, there were no cardiac events in patients with normal rest MPI. This indicates that rest MPI can be used as an alternative to either hospital or observation admission.
LIMITATIONS OF ACUTE MYOCARDIAL PERFUSION IMAGING
Acute MPI has some limitations when used to assess chest pain patients. Acute MI, acute ischemia, and prior MI all cause perfusion defects, and differentiation is not possible based on the images alone. However, patients with prior MI are at higher risk for acute events and are usually not candidates for primary triage to an outpatient evaluation. Sensitivity for identifying necrosis is imperfect for MPI, since at least 3% to 5% of the left ventricle must be ischemic for a defect to be visible. However, many patients who have negative rest MPI despite marker elevations have nonsignificant disease on coronary angiography35 and are therefore at low risk for short-term adverse outcomes, although aggressive risk-factor modification would still be indicated. Therefore, rather than being seen as competitive diagnostic tools for evaluating ED chest-pain patients, markers and MPI should be considered complementary.
Some of these perceived limitations have led to consideration of other techniques for evaluating ED chest-patients and include coronary computed tomographic angiography (CTA). Improvements in technology have dramatically increased overall sensitivity and specificity, and have reduced the number of coronary segments that cannot be accurately assessed. Using current 64-slice technology, CTA has a reported sensitivity and specificity that exceeds 90% and a negative predictive value of 99%.57,58 This high negative predictive value of CTA, because of its ability to rapidly exclude clinically significant coronary artery disease, has the potential to more efficiently diagnose and triage ED chest pain patients.
A randomized trial of low-risk patients with acute chest pain evaluated by either early CTA or a standard diagnostic protocol has been reported.59 Patients randomized to immediate CTA were eligible for discharge with normal or minimally abnormal results (<25%), patients with severe stenosis (>70%) were referred for immediate invasive angiography, and patients with intermediate-grade stenosis underwent additional stress testing. Among patients randomized to CTA, 75% could be triaged by CTA alone. Importantly, of the 67% of CTA patients who were discharged immediately, no major adverse cardiac events occurred over the next 6 months. Overall, the diagnostic accuracy of CTA was 94%, and the negative predictive value was 100%. However, a number of limitations were demonstrated in this study: 46% of potential subjects were excluded for a reason that would have precluded imaging, and 24% of those who did undergo CTA had to have further diagnostic testing with stress MPI because of equivocal CTA results.
INCORPORATION INTO EMERGENCY DEPARTMENT CHEST-PAIN EVALUATION
The consistently favorable results of observational and clinical trials on the efficacy of acute rest MPI in the ED have formed the basis of current recommendations and guidelines (Table 34-2).60 The recommended patient selection criteria are similar to those used for admission to a chest-pain observation unit. Patients should be low risk (no ischemic ECG changes or history of coronary disease) and hemodynamically stable. The optimal use of MPI as a triage tool is in patients who will be discharged home and have stress testing on an outpatient basis if imaging is negative.61
Table 34-2 Guidelines for the Use of Acute Rest Myocardial Perfusion Imaging in the Emergency Department

In low-risk patients injected during symptoms, the presence of normal rest images makes an ACS unlikely, and in the younger patient, subsequent stress testing may not be necessary. If the likelihood of having coronary artery disease based on clinical variables is relatively low and the rest ECG normal, rest MPI can be followed by standard ECG exercise testing. However, a significant proportion of patients have an intermediate likelihood of coronary artery disease and an abnormal rest ECG, or are unable to adequately exercise.4 In these patients, stress testing with imaging, either echocardiography or (more commonly) SPECT imaging, using exercise or pharmacologic stress would be appropriate.
The ability to have imaging available during all hours is a potential logistic issue. In a study from Schaeffer et al. addressing this issue, patients presenting from 12 am to 6 am were injected with sestamibi, and imaging was delayed until that morning.62 There was no difference in diagnostic accuracy in patients who waited to be imaged compared to those who presented during other time periods.
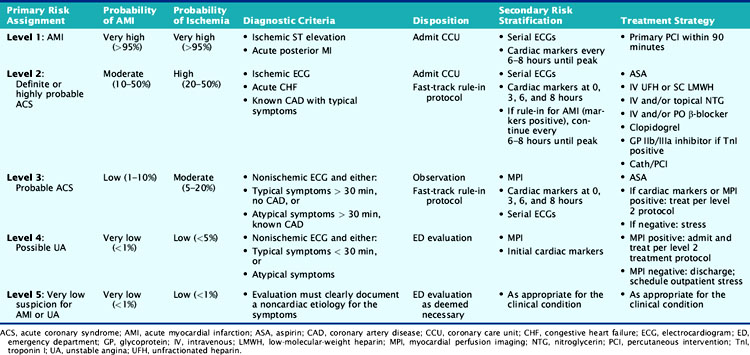
One of the first programs to incorporate rest MPI as a diagnostic strategy for ED chest-pain patients was at Virginia Commonwealth University Medical Center (formerly Medical College of Virginia). In contrast to most chest-pain programs, the systematic chest-pain protocol developed and implemented is designed for all chest pain patients, with MPI used for the evaluation of lower-risk patients (Table 34-3).26 All patients presenting to the ED with chest pain or other symptoms consistent with myocardial ischemia undergo rapid evaluation with assignment to a triage risk level, which is based on the probability of having MI or ischemia as derived from clinical and ECG variables. After the initial evaluation, patients thought to be at high risk (ST-segment elevation [level 1], ST-segment depression, or ischemic T-wave inversion [level 2]) or those with known coronary disease experiencing typical symptoms (level 2) are admitted directly to the CCU. Patients considered low to moderate risk for ACS (e.g., absence of ischemic ECG changes or history of coronary disease) undergo further risk stratification using acute rest MPI.26 Level 3 patients are admitted as observation patients and undergo a rapid rule-in protocol. Level 4 patients are evaluated in the ED. If images are either negative or unchanged from previous studies, patients are discharged home and scheduled for outpatient stress testing. If MPI is positive, they are admitted and advanced to the level 2 treatment protocol.
Table 34-3 Initial Triage and Treatment Algorithm Used at Virginia Commonwealth University for Evaluation of Patients with Possible Myocardial Ischemia
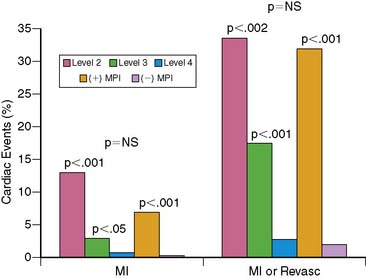
This simple risk-stratification scheme accurately separates patients into high-, intermediate-, and low-risk groups. The ability of MPI to further risk stratify lower-risk patients was confirmed; outcomes in patients with positive MPI were similar to those in the high-risk level 2 patients (Fig. 34-10).26 Close collaboration of the ED, CCU, and nuclear medicine staff can lead to patients without prior MI who have large perfusion defects at the time of imaging being successfully triaged directly to coronary angiography and revascularization.
Fesmire and colleagues have used a similar protocol with a slight modification.45 Patients presenting with chest pain are evaluated similar to the MCV protocol. Patients who present without chest pain undergo rest SPECT thallium imaging. If images are negative, subsequent stress SPECT 99mTc-sestamibi imaging is immediately performed. If rest images are abnormal, the patient is reevaluated for possible ACS prior to stress testing.
1. Faroghi A., Kontos M.C., Jesse R.L., Roberts C.S., Tatum J.L., Ornato J.P. Are cardiac risk factors predictive in low risk emergency department chest pain patients who have chest pain? J Am Coll Cardiol. 2000;35:379A.
2. Lee T.H., Goldman L. Evaluation of the patient with acute chest pain. N Engl J Med. 2000;342:1187-1195.
3. Anderson J.L., Adams C.D., Antman E.M., Bridges C.R., Califf R.M., Casey D.E.Jr, et al. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1-e157.
4. Zalenski R.J., Rydman R.J., McCareen M., Roberts R.R., Jovanovic B., Das K., et al. Feasibility of a rapid diagnostic protocol for an emergency department chest pain unit. Ann Emerg Med. 1997;29:99-108.
5. Wackers F.J., Lie K.I., Liem K.L., Sokole E.B., Samson G., van der Schoot J., et al. Potential value of thallium-201 scintigraphy as a means of selecting patients for the coronary care unit. Br Heart J. 1979;41:111-117.
6. Van der Wieken L.R., Kan G., Belfer A.J., Visser C.A., Jaarsma W., Lie K.I., et al. Thallium-201 scanning to decide CCU admission in patients with non-diagnostic electrocardiograms. Int J Cardiol. 1983;4:285-299.
7. Henneman P.L., Mena I.G., Rothstein R.J., Garrett K.B., Pleyto A.S., French W.J. Evaluation of patients with chest pain and nondiagnostic ECG using thallium-201 myocardial planar imaging and technetium-99m first-pass radionuclide angiography in the emergency department. Ann Emerg Med. 1992;21:545-550.
8. Mace S.E. Thallium myocardial scanning in the emergency department evaluation of chest pain. Am J Emerg Med. 1989;7:321-328.
9. Okada R.D., Glover D., Gaffney T., Williams S. Myocardial kinetics of technetium-99m-hexakis-2-methoxy-2-methylpropyl-isonitrile. Circulation. 1988;77:491-498.
10. Germano G., Kiat H., Kavanagh P.B., Moriel M., Mazzanti M., Su H.T., et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138-2147.
11. DePuey E.G., Rozanski A. Using gated technetium-99m-sestamibi SPECT to characterize fixed myocardial defects as infarct or artifact. J Nucl Med. 1995;36:952-955.
12. Kontos M.C., Hinchman D., Cunningham M., Miller J.J., Cherif J., Nixon J.V. Comparison of contrast echocardiography with single-photon emission computed tomographic myocardial perfusion imaging in the evaluation of patients with possible acute coronary syndromes in the emergency department. Am J Cardiol. 2003;91:1099-1102.
13. Kloner R.A., Jennings R.B. Consequences of brief ischemia: Stunning, preconditioning, and their clinical implications. Circulation. 2001;104:2981-2989. Part 1
14. Kloner R.A., Jennings R.B. Consequences of brief ischemia: Stunning, preconditioning, and their clinical implications. Circulation. 2001;104:3158-3159. Part 2
15. Kaul S., Senior R., Firschke C., Wang X.Q., Lindner J., Villanueva F.S., et al. Incremental value of cardiac imaging in patients presenting to the emergency department with chest pain and without ST-segment elevation: a multicenter study. Am Heart J. 2004;148:129-136.
16. Christian T.F., Clements I.P., Gibbons R.J. Noninvasive identification of myocardium at risk in patients with acute myocardial infarction and nondiagnostic electrocardiograms with technetium-99m-Sestamibi. Circulation. 1991;83:1615-1620.
17. Hilton T.C., Fulmer H., Abuan T., Thompson R.C., Stowers S.A., Fulmer H., et al. Ninety-day follow-up of patients in the emergency department with chest pain who undergo initial single-photon emission computed tomography perfusion scintigraphy with technetium 99m-labeled sestamibi. J Nucl Cardiol. 1996;3:308-311.
18. Udelson J.E., Beshansky J.R., Ballin D.S., Feldman J.A., Griffith J.L., Heller G.V., et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia. JAMA. 2002;288:2693-2700.
19. Candell-Riera J., Oller-Martinez G., Pereztol-Valdes O., Castell-Conesa J., Aguade-Bruix S., Garcia-Alonso C., et al. Early myocardial perfusion gated-SPECT in patients with chest pain and non-diagnostic ECG in the emergency department. Rev Esp Cardiol. 2004;57:225-233.
20. Kontos M.C., Kurdziel K., McQueen R., Arrowood J.A., Jesse R.L., Ornato J.P., et al. Comparison of 2-dimensional echocardiography and myocardial perfusion imaging for diagnosing myocardial infarction in emergency department patients. Am Heart J. 2002;143:659-667.
21. Bilodeau L., Theroux P., Gregoire J., Gagnon D., Arsenault A. Technetium-99m sestamibi tomography in patients with spontaneous chest pain: correlations with clinical, electrocardiographic and angiographic findings. J Am Coll Cardiol. 1991;18:1684-1691.
22. Kontos M.C., Jesse R.L., Schmidt K.L., Ornato J.P., Tatum J.L. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997;30:976-982.
23. Heller G.V., Stowers S.A., Hendel R.C., Herman S.D., Daher E., Ahlberg A.W., et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011-1017.
24. Hilton T.C., Thompson R.C., Williams H.J., Saylors R., Fulmer H., Stowers S.A. Technetium-99m sestamibi myocardial perfusion imaging in the emergency room evaluation of chest pain. J Am Coll Cardiol. 1994;23:1016-1022.
25. Kontos M.C., Jesse R.L., Anderson F.P., Schmidt K.L., Ornato J.P., Tatum J.L. Comparison of myocardial perfusion imaging and cardiac troponin I in patients admitted to the emergency department with chest pain. Circulation. 1999;99:2073-2078.
26. Tatum J.L., Jesse R.L., Kontos M.C., Nicholson C.S., Schmidt K.L., Roberts C.S., et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med. 1997;29:116-123.
27. Knott J.C., Baldey A.C., Grigg L.E., Cameron P.A., Lichtenstein M., Better N. Impact of acute chest pain Tc-99m sestamibi myocardial perfusion imaging on clinical management. J Nucl Cardiol. 2002;9(3):257-262.
28. Kontos M.C., Schmidt K.L., McCue M., Rossiter L.F., Jurgensen M., Nicholson C.S., et al. A comprehensive strategy for the evaluation and triage of the chest pain patient: a cost comparison study. J Nucl Cardiol. 2003;10:284-290.
29. Radensky P.W., Hilton T.C., Fulmer H., McLaughlin B.A., Stowers S.A. Potential cost effectiveness of initial myocardial perfusion imaging for assessment of emergency department patients with chest pain. Am J Cardiol. 1997;79:595-599.
30. Weissman I.A., Dickinson C.Z., Dworkin H.J., O’Neill W.W., Juni J.E. Cost-effectiveness of myocardial perfusion imaging with SPECT in the emergency department evaluation of patients with unexplained chest pain. Radiology. 1996;199:353-357.
31. McGuire D.K., Hudson M.P., East M.A., Dyke C.K., Cantor W.J., Battle J., et al. Highlights from the American Heart Association 72nd Scientific Sessions: November 6 to 10, 1999. Am Heart J. 2000;139:359-370.
32. Alpert J.S., Thygesen K., Bassand J.P., et al. Myocardial infarction redefined–A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36:959-969.
33. O’Connor M.K., Hammell T., Gibbons R.J. In vitro validation of a simple tomographic technique for estimation of percentage myocardium at risk using methoxyisobutyl isonitrile technetium 99m (sestamibi). Eur J Nucl Med. 1990;17:69-76.
34. Duca M.D., Giri S., Wu A.H., Morris R.S., Cyr G.M., Ahlberg A., et al. Comparison of acute rest myocardial perfusion imaging and serum markers of myocardial injury in patients with chest pain syndromes. J Nucl Cardiol. 1999;6:570-576.
35. Kontos M.C., Fratkin M.J., Jesse R.L., Anderson F.P., Ornato J.P., Tatum J.L. Sensitivity of acute rest myocardial perfusion imaging for identifying patients with myocardial infarction based on a troponin definition. J Nucl Cardiol. 2004;11:12-19.
36. Novis D.A., Jones B.A., Dale J.C., Walsh M.K. A College of American Pathologists Q-Probes study of 7020 troponin and 4368 creatine kinase-MB determinations in 159 institutions. Arch Pathol Lab Med. 2004;128:158-164.
37. Reimer K.A., Jennings R.B., Cobb F.R., Murdock R.H., Greenfield J.C.Jr, Becker L.C., et al. Animal models for protecting ischemic myocardium: results of the NHLBI Cooperative Study. Comparison of unconscious and conscious dog models. Circ Res. 1985;56:651-665.
38. Miller T.D., Christian T.F., Hopfenspirger M.R., Hodge D.O., Gersh B.J., Gibbons R.J. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334-341.
39. Miller T.D., Hodge D.O., Sutton J.M., Grines C.L., O’Keefe J.H., DeWood M.A., et al. Usefulness of technetium-99m sestamibi infarct size in predicting posthospital mortality following acute myocardial infarction. Am J Cardiol. 1998;81:1491-1493.
40. Kontos M.C., Kurdziel K.A., Ornato J.P., Schmidt K.A., Jesse R.L., Tatum J.L.A. nonischemic electrocardiogram does not always predict a small myocardial infarction: Results with acute myocardial perfusion imaging. Am Heart J. 2001;141:360-366.
41. Kontos M.C., Kurdziel K.A., Ornato J.P., Jesse R.L., Tatum J.L. Myocardial salvage in patients with non-ST-elevation myocardial infarction determined by myocardial perfusion imaging. Am J Cardiol. 2005;95:398-401.
42. Klarich K.W., Christian T.F., Higano S.T., Gibbons R.J. Variability of myocardium at risk for acute myocardial infarction. Am J Cardiol. 1999;83:1191-1195.
43. O’Keefe J.H.Jr, Sayed-Taha K., Gibson W., Christian T.F., Bateman T.M., Gibbons R.J. Do patients with left circumflex coronary artery–related acute myocardial infarction without ST-segment elevation benefit from reperfusion therapy? Am J Cardiol. 1995;75:718-720.
44. Huey B.L., Beller G.A., Kaiser D.L., Gibson R.S. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol. 1988;12:1156-1166.
45. Fesmire F.M., Hughes A.D., Stout P.K., Wojcik J.F., Wharton D.R. Selective dual nuclear scanning in low-risk patients with chest pain to reliably identify and exclude acute coronary syndromes. Ann Emerg Med. 2001;38:207-215.
46. Varetto T., Cantalupi D., Altieri A., Orlandi C. Emergency room technetium-99m sestamibi imaging to rule out acute myocardial ischemic events in patients with nondiagnostic electrocardiograms. J Am Coll Cardiol. 1993;22:1804-1808.
47. Wackers F.J., Lie K.I., Liem K.L., Sokole E.B., Samson G., van der Schoot J.B., et al. Thallium-201 scintigraphy in unstable angina pectoris. Circulation. 1978;57:738-742.
48. Emre A., Ersek B., Gursurer M., Aksoy M., Siber T., Engin O., et al. Angiographic and scintigraphic (perfusion and electrocardiogram- gated SPECT) correlates of clinical presentation in unstable angina. Clin Cardiol. 2000;23:495-500.
49. Topol E.J., Weiss J.L., Brinker J.A., Brin K.P., Gottlieb S.O., Becker L.C., et al. Regional wall motion improvement after coronary thrombolysis with recombinant tissue plasminogen activator: importance of coronary angioplasty. J Am Coll Cardiol. 1985;6:426-433.
50. Koch K.C., vom Dahl J., Kleinhans E., Klues H.G., Radke P.W., Ninnemann S., et al. Influence of a platelet GPIIb/IIIa receptor antagonist on myocardial hypoperfusion during rotational atherectomy as assessed by myocardial Tc-99m sestamibi scintigraphy. J Am Coll Cardiol. 1999;33(4):998-1004.
51. Fram D.B., Azar R.R., Ahlberg A.W., Gillam L.D., Mitchel J.F., Kiernan F.J., et al. Duration of abnormal SPECT myocardial perfusion imaging following resolution of acute ischemia: an angioplasty model. J Am Coll Cardiol. 2003;41:452-459.
52. Taegtmeyer H. Metabolism: The lost child of cardiology. J Am Coll Cardiol. 2000;36:1386-1388.
53. Goodman M.M., Kirsch G., Knapp F.F.Jr. Synthesis and evaluation of radioiodinated terminal p-iodophenyl substituted a- and b-methyl-branched fatty acids. J Med Chem. 1984;27:390-397.
54. Kontos M.C., Goldman J.L., Li Y., Kacena K., LaFrance N., Babich J.W., Udelson J.E. Iodofiltic acid I 123 (BMIPP) SPECT provides incremental diagnostic value over initial clinical information in emergency department patients with suspected acute coronary syndrome. Circulation. 2008;118(Suppl):S579.
55. Hollander J.E., Hoffman R.S., Gennis P., Fairweather P., DiSano M.J., Schumb D.A., et al. Prospective multicenter evaluation of cocaine-associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330-339.
56. Kontos M.C., Schmidt K.L., Nicholson C.S., Ornato J.P., Jesse R.L., Tatum J.L. Myocardial perfusion imaging with technetium-99m sestamibi in patients with cocaine-associated chest pain. Ann Emerg Med. 1999;33:639-645.
57. Budoff M.J., Dowe D., Jollis J.G., Gitter M., Sutherland J., Halamert E., Scherer M., Bellinger R., Martin A., Benton R., Delago A., Min J.K. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724-1732.
58. Meijboom W.B., Meijs M.F., Schuijf J.D., Cramer M.J., Mollet N.R., van Mieghem C.A., Nieman K., van Werkhoven J.M., Pundziute G., Weustink A.C., de Vos A.M., Pugliese F., Rensing B., Jukema J.W., Bax J.J., Prokop M., Doevendans P.A., Hunink M.G., Krestin G.P., de Feyter P.J. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135-2144.
59. Goldstein J.A., Gallagher M.J., O’Neill W.W., Ross M.A., O’Neil B.J., Raff G.L. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863-871.
60. Wackers F.J., Brown K., Heller G., Kontos M.C., Tatum J.L., Udelson J., et al. American Society of Nuclear Cardiology position statement on radionuclide imaging in patients with suspected acute ischemic syndromes in the emergency department or chest pain center. J Nucl Cardiol. 2002;9:246-250.
61. Klocke F.J., Baird M.G., Lorell B.H., Bateman T.M., Messer J.V., Berman D.S., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation. 2003;108:1404-1418.
62. Schaeffer M.W., Brennan T.D., Hughes J.A., Gibler W.B., Gerson M.C. Resting radionuclide myocardial perfusion imaging in a chest pain center including an overnight delayed image acquisition protocol. J Nucl Med Technol. 2007;35:242-245.