CHAPTER 37 Imaging of Coronary Revascularization
Coronary Stents and Bypass Grafts
NONINVASIVE EVALUATION OF CORONARY STENTS
Since Dr. Andreas Gruentzig performed the first successful percutaneous transluminal coronary angioplasty (PTCA) in 1977,1 this procedure has become commonplace with more than 550,000 PTCA procedures performed in the United States in 2000. In the late 1980s Dr. Julio Palmaz invented the bare-metal stent (BMS). In 1993, randomized clinical trials demonstrated decreased angiographic restenosis rates (defined as >50% narrowing of a previously stented site) compared with PTCA alone—ushering in the era of elective stent implantation.2 Currently, more than 80% of patients undergoing percutaneous coronary intervention also receive intracoronary stents (Fig. 37-1).3 Intracoronary stent implantation is not, however, without risk with two major complications—occlusion secondary to thrombosis and restenosis.4 Many patients undergo repeat angiography to ascertain the presence of these complications; however, given the invasive nature of coronary angiography and its potential complications, a noninvasive technique for detection of these complications would be clinically important.
In-Stent Restenosis and Thrombosis—Etiology, Prevalence, Pathophysiology
Restenosis after angioplasty can result from early vessel recoil, late constrictive remodeling (also called negative remodeling), and neointimal proliferation. Elastic recoil occurs nearly instantaneously, secondary to passive recoil of elastic media. Late constrictive remodeling occurs within 1 to 6 months and is secondary to increased collagen in the extracellular matrix and adventitial thickening.1 Intracoronary stents prevent these mechanisms (acute recoil and constrictive remodeling) (Fig. 37-2).
Unfortunately, stent-induced vascular injury causes increased neointimal hyperplasia/proliferation. The pathophysiology includes proliferation and migration of smooth muscle cells and extracellular matrix formation.1 In-stent restenosis, the principal problem after angioplasty, has a clinical incidence of 20% to 40% for bare-metal stents.5,6 Although several characteristics of high-risk populations have been described as clinical predictors, the likelihood of restenosis in a particular patient remains largely unpredictable.6,7
Drug-eluting stents (DES) have revolutionized the treatment of coronary artery disease through marked reduction of in-stent restenosis.8 The components of these stents can be divided into a platform (the stent), a carrier (usually a polymer), and an agent (a drug) to prevent restenosis (Fig. 37-3).2 The advantage of using a stent as a delivery system is that it allows for local delivery of the drug and averts the need for higher systemic doses. The biology of vascular cells and the cell cycle is being used as a target to prevent restenosis and current agents used in drug-eluting stents all interfere with the cell cycle in some manner.1 Numerous trials have established that sirolimus and paclitaxel drug-eluting stents markedly reduce the incidence of in-stent restenosis. DES have reduced restenosis rates to less than 10% at a 12-month follow-up.6,9,10
Although drug-eluting stents have significantly reduced restenosis events, its very effectiveness has led to increased rates of an uncommon but potentially very severe complication, in-stent thrombosis. To understand this phenomenon, it is important to be aware of risk factors for development of in-stent thrombosis. Persistent, slow coronary blood flow such as occurs with dissection or hypoperfusion, exposure of the blood to prothrombotic subendothelial constituents, such as tissue factor or to the stent itself, and failure to suppress platelet adhesion/aggregation at a time of prothrombotic risk predispose the patient to in-stent thrombosis.11
A probable explanation for an increased risk of thrombosis in DES compared to BMS after cessation of antiplatelet therapy is delayed arterial healing.11 Normally after vascular injury, the vessel wall undergoes a number of changes including migration and hypertrophy of vascular smooth muscle cells. During this period, endothelial cells colonize the surface of the stent and regain their normal function.12 DES cause incomplete neointimal coverage and delay endothelialization of the stent. Subsequently, there is eventual propagation of thrombi over the surface of the stent.
The reported incidence of thrombosis of bare-metal stents 1 month postprocedure has ranged from 0.5% to 2.5% in clinical trials. Bare-metal stent thrombosis usually occurs within the first 24 to 48 hours or much less commonly within the first month after stent placement. Eighty percent of stent thrombosis events have been found to occur within the first 2 days and events occurring more than 1 week after the procedure were rare.13 Stent thrombosis within 1 year of implantation occurs with similar frequency between DES and BES; however beyond 1 year, there is a small but significant increased risk of very late stent thrombosis in DES.14 Because of this increased risk, extension of antiplatelet therapy is recommended.
The decision to place a drug-eluting rather than a bare-metal stent therefore rests on clinical grounds and evaluation of the benefits and risks of these stents. The lower rate of repeat target revascularization with DES must be weighed against the cost of longer term antiplatelet therapy to prevent stent thrombosis, the risk of bleeding, and the complications of noncompliance with drug therapy (Fig. 37-4).
Clinical Presentation
Restenosis following angioplasty and stent implantation is classically considered a benign process in which the typical patient presents with exertional angina.14 Importantly, however, an appreciable proportion of these patients present with an acute coronary syndrome. One study from the Cleveland Clinic demonstrated that 9.5% of patients with restenosis presented with acute myocardial infarction and 26.4% presented with unstable angina.15
Stent thrombosis is particularly concerning because of its potential catastrophic consequences. As stents are often placed in proximal segments of major coronary arteries, thrombotic occlusion usually manifests as severe ischemia or myocardial infarction. One study demonstrated that 70% of cases of stent thrombosis manifested as acute myocardial infarction.8,16 Mortality rates are very high ranging from 11% to 15% for BMS thromboses and 25% to 45% with DES thrombosis. The higher mortality with DES thrombosis has been suggested to be secondary to a combination of abrupt thrombotic events and decreased collateral formation.14 Again, the risk of coronary artery stent thrombosis and its frequent consequences of myocardial infarction or death are minimized by the use of dual antiplatelet therapy. Patient compliance is also an important issue when determining the type of stent to use.
Imaging Techniques and Findings
Radiography
To date, the majority of patients with chest pain after coronary artery stent placement undergo catheter angiography. This invasive method, however, has the disadvantage of moderate-to-high cost and the possibility of severe complications. Therefore, a noninvasive alternative for the assessment of stented segments in these patients would be highly desirable.17 To that end, attempts have been made to noninvasively assess coronary artery stents with varying degrees of success.
Ultrasound
Transcatheter intravascular ultrasound (IVUS) imaging is another invasive technique in which a miniaturized ultrasound transducer, mounted on the tip of catheter, is inserted directly into a vessel to produce in-vivo real time assessment of the vascular lumen as well as plaque composition. IVUS has been used during stent placement with the promise that it will improve the clinical outcome of stent placement via reduction of incomplete expansion and incomplete apposition of the stent to the vessel wall. Also, IVUS allows for the measurement of minimal stent area which has been found to predict angiographic and clinical restenosis.18
MRI
The coronary arteries can be evaluated using cardiac MRI techniques; however, coronary magnetic resonance angiography (MRA) is currently not as easily performed or as fast as coronary computed tomographic angiography (CTA). A number of unsolved problems limit MRI reliability. Long acquisition times, the small size of the coronary arteries, and cardiac and respiratory artifacts causing nonevaluable segments hamper the clinical implementation of coronary MRA.19
Furthermore, although multiple studies have demonstrated that MRI performed less than 8 weeks after coronary stent placement is safe,20–22 local susceptibility artifact leads to signal voids/artifacts at the site of the stent on MR images. These artifacts can be substantial and preclude direct MR evaluation of in-stent and persistent coronary patency.23
CT
Noninvasive assessment using computed tomography to assess in-stent stenosis has been attempted since the era of electron beam CT (EBCT). Early studies with EBCT could not directly visualize in-stent restenosis and quantification was not possible.24,25
Multidetector CT (MDCT) has several advantages over EBCT; of note, MBCT has increased spatial resolution and improved signal-to-noise ratio.17 Early MDCT scanning with four-detector scanners were inadequate for stent interpretation with inability to visualize the majority of the stent lumen.17,26 Subsequent studies using 16-, 40-, and 64-detector scanners have had mixed results. Most studies demonstrated high sensitivity and specificity but in general ignored nonevaluable segments from analysis. The number of stents found nonevaluable (up to 54% of 232 stents in a study by Gilard and colleagues), is a major limitation of this analysis. The negative predictive value was generally greater than 95%; however, the positive predictive value ranged from 29% to 78%.6,27–34
Hamon and colleagues performed a meta-analysis of 15 studies analyzing the diagnostic capabilities of 16-, 40-, and 64-detector CT scanners in comparison with invasive coronary angiography for detection of in-stent stenosis. 13% of 1175 stents in these studies were nonevaluable. After exclusion of these stents from analysis, they found a sensitivity of 84% and a specificity of 91%.35 The positive-predictive value (PPV) was almost uniformly low secondary to nonevaluable stents being classified as in-stent restenosis (ISR) for statistical analysis. An additional meta-analysis of 16- to 64-detector row CT scanners performed by Sun and colleagues demonstrated similar results. Although there was the hope that 64-detector row CT scanners would be more accurate than slower 16-detector row CT scanners, both meta-analyses demonstrated equivalent sensitivity and specificity.35,36 Kumbani and coworkers recently performed a meta-analysis of 14 studies solely using 64-detector row CT scanners for detection of ISR. Overall sensitivity was 91%, specificity was 91%, PPV was 68%, and the negative-predictive value (NPV) was 98%. Nine percent of 1447 stents were deemed nonevaluable with decreased performance with inclusion of these stents. Only five studies in the analysis included stents less than 3 mm.37 Given that a large number of stents in the general population are less than 3 mm, this further decreases the utility of MDCT in nonselected stent patients.
A post-hoc analysis of 75 stents in 52 patients from the Core-64 trial, the first multicenter international, single-blinded study to determinate the accuracy of 64-detector row CT MDCT, was performed to assess the accuracy of MDCT in detecting in-stent restensosis. This analysis demonstrated less favorable results than the previous meta-analyses. Only 48 of 75 stents (64%) were considered evaluable and there was an overall accuracy of 77.1% for detection of 50% in-stent restenosis of evaluable stents. The PPV and NPV was 57.1% and 80.5%, respectively. A quantitative approach using in-stent and peri-stent attenuation didn’t improve accuracy. The researchers attribute the poor performance of MDCT to the fact that 80% of stents were <3 mm with secondary problems of calcifications, motion artifact, and blooming artifact.38
In general, there is increased evaluability and improved detection of in-stent restenosis with larger stents and unfavorable results with stents less than 3 mm diameter. High heart rates, calcification, and increased body mass index decreased the accuracy of MDCT.32 When considered in the evaluation, thicker stent struts also reduced the evaluability of the stents.36
Dual-source CT scanners have improved temporal resolution compared with single source scanners and promise to potentially minimize motion artifacts that limit in-stent evaluation. Pugliese and associates evaluated a dual-source CT scanner for evaluation of instant restenosis in 100 patients with 178 stents. Nine of the stents were uninterpretable (all of which were smaller than 2.75 mm in diameter) secondary to high-density artifacts obscuring the stent lumen. Sensitivity, specificity, PPV, and NPV in detecting >50% restenosis was 94%, 92%, 77%, and 98%, respectively. The diagnostic performance of the dual source CT scanner at heart rates less than 70 bpm did not differ significantly from its performance with faster heart rates. The stent diameter was important as the sensitivity was 100% in >3 mm diameter stents but dropped to 84% in <2.75 mm stents. Similarly the specificity and the PPV dropped precipitously as the stent diameter decreased. This study demonstrated that the dual-source CT scanner was able to negate the effect of heart rate on evaluation of stent stenosis. The NPV was very good (97% to 98%), even in patients with fast heart rates. Stent diameter was the most important feature in this study.39
In the evaluation of the coronary arteries in a patient with history of percutaneous coronary intervention and stent placement, a clinical history with specific locations of stent placement is of utmost importance. Given that concentric calcification can mimic a stent and not all stents are uniformly recognized on MDCT;40 a history of stent placement allows for a more accurate interpretation—understanding that even with the most current scanners, evaluation of in-stent restenosis remains challenging.
CT scanners with 64 or more detectors are, in general, currently considered the standard for coronary CTA because the reduced breath-holding time afforded by these scanners is better tolerated by patients owing to minimization of motion artifacts.7,41 Metallic stents cause beam hardening on CT imaging resulting in stent struts appearing thicker (or “bloomed”) than they really are with secondary overlap of the vessel lumen and underestimation of the in-stent luminal diameter. In addition, this artifact can cause streaky dark bands which can simulate stenosis (Fig. 37-5).42 Calcification of the vessel wall near or at the outer surface of an implanted stent also contributes to beam hardening. Partial volume averaging is another artifact inherent in cross-sectional imaging and particularly problematic given the small caliber of the coronary arteries.7,43
Sharp filters and thin slices (0.5 to 0.6 mm) reduce blooming and partial volume averaging for improved assessment of stent patency.44 Studies have demonstrated that dedicated edge-enhancing convolution kernels decrease severity of blooming artifacts, resulting in superior depiction of the stent lumen.7 The disadvantages of these filters are an increase in image noise. The most appropriate filter must be chosen to account for this trade-off.
Analysis
When these technical factors are optimized and the data are obtained, the stent lumen should be evaluated using multiplanar reformation (MPR) and cross-sectional/short axis images. Appropriate display window settings must be considered to most accurately evaluate these images. Wide window settings (e.g., width 1500 HU) and center level of 300 HU have been recommended to further decrease image interpretation difficulties with blooming artifact.7
When deemed evaluable, the in-stent lumen should be check for in-stent restenosis. The level and quality of enhancement should be ascertained and any in-stent filling defects should be noted. Homogeneous high attenuation in the stent similar to the attenuation in the proximal or distal reference vessel implies normal flow (Figs. 37-6 and 37-7). Different grades of restenosis similar to those used by Gasper and colleagues and Ehara and associates27,34 can be used (Figs. 37-8 and 37-9), including mild neointimal proliferation (<50% stenosis), significant (>50% restenosis), severe (>75% stenosis)/possible occlusion, and definitive occlusion (100% occlusion).
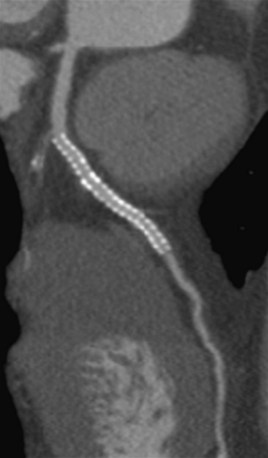
 FIGURE 37-6 Homogeneous contrast attenuation within LAD stent demonstrates lack of significant in-stent restenosis.
FIGURE 37-6 Homogeneous contrast attenuation within LAD stent demonstrates lack of significant in-stent restenosis.
(Courtesy of Dr. Sablayrolles, Saint-Denis France.)
The perigraft vasculature should also be analyzed as restenosis at stent borders and is reported to occur frequently.45 No contrast distal to the stent indicates definitive occlusion.46 The presence of contrast distal to the stent does not, however, exclude occlusion secondary to the possibility of collateral vasculature feeding the vessel segments distal to the occluded stent in a retrograde direction (Fig. 37-10). It has also been noted that with stent restenosis, the CT attenuation of vessels distal to the stent is decreased compared with the attenuation of the artery proximal to the stent; however, no specific cutoff point has been ascertained.31
The diagnosis of in-stent thrombosis is much rarer and requires a collaborative clinical history. This is a highly morbid event that results in Q-wave infarction and death in the majority of cases. It is likely to show complete occlusion on cardiac CTA and the angiographic definition is no flow or faint flow beyond the occlusion.47
Synopsis of Treatment Options
The treatment of in-stent restenosis is usually PTCA and adjunctive repeat stenting with DES. Prior to the availability of DES, one major focus of investigation was to treat restenosis with intracoronary radiation. DES, however, is the treatment of choice with greater efficacy. There is currently insufficient evidence to recommend any specific treatment for DES restenosis; however, repeat stenting with DES is currently performed in many institutions after IVUS evaluation and stent expansion by repeat balloon angioplasty.11
Conclusion
Coronary CTA can be considered to rule out in-stent restenosis in carefully selected patients such as in calm, thin patients with slow, stable heart rates who have large diameter stents.48 Its routine use, however, is currently not recommended secondary to its low positive-predictive value and frequent poor visualization of the lumen.35,49,50 A multi-societal consensus statement that included the American College of Cardiology Foundation and American College of Radiology rates cardiac CT for the indication of evaluation of in-stent restenosis after percutaneous coronary intervention (PCI) as inappropriate, assigning it an appropriateness score of 2 on a 1 to 9 scale, with 9 being considered the highest reasonable and acceptable indication.51 There is a consensus that only stents with diameter of over 3 mm are routinely interpretable. Unfortunately, in routine clinical settings many patients are treated with smaller stents. Even though the dual-source CT scanners can certainly reduce or possibly eliminate the limitation of motion artifacts, further increased spatial resolution appears necessary before reliable imaging of most stents for restenosis becomes suitably accurate for routine use.
CORONARY ARTERY BYPASS ASSESSMENT
Background
Surgical revascularization is well recognized as a modern achievement in therapy for advanced coronary artery disease. Although the concept of coronary bypass grafting for occlusive disease was originally proposed by Carrel in 1910,52 Drs. Michael DeBakey and Edward Garrett are credited with the first successful saphenous vein graft (SVG) bypass in 1964. Performed as a salvage for a complicated left anterior descending coronary endarterectomy, the patient did well postoperatively and demonstrated continued patency of the SVG graft when restudied 8 years later.53 That same year, Kolesov performed the first sutured mammary-artery-coronary bypass.54 It wasn’t until the end of the decade that broader acceptance and use of internal mammary grafting took place.
Within a decade from these initial advances, coronary bypass operations had a tremendous impact on therapy for atherosclerotic disease. The National Center for Health Statistics report 450,000 coronary revascularization procedures performed in 2006.3 Continued improvements in operative techniques have allowed increasingly challenging patients to be operated on with success.
Saphenous Vein Grafts
Saphenous venous grafts were first successfully used in a CABG operation in 1964.55 Although resistant to spasm versus their arterial counterparts, the use of SVGs is limited by a higher occurrence of intimal hyperplasia and atherosclerotic changes after exposure to systemic blood pressure, resulting in lower graft patency rates.56,57 Saphenous vein grafts are attached proximally from the ascending aorta to the coronary artery distal to the diseased coronary lesion(s). The SVG can be sutured directly to the anterior portion of the ascending aorta or can be attached with an anastomotic device.
Occlusive failures of saphenous vein grafts are well documented and have been extensively investigated.58–63 A large angiographic study of bypass graft patency (n = 5065, 91% venous, 9% arterial) found 88% of grafts to be patent perioperatively, 81% to be patent at 1 year, and 75% of grafts to be patent at 5 years.61 Further declines in patency to 50% were observed in 353 grafts examined more than 15 years after revascularization. Nevertheless, continued improvements in surgical techniques, combined with concomitant antiplatelet or anticoagulant agents, and lipid-lowering therapy have allowed SVGs to remain a main choice for surgical bypass.
Arterial Grafts
Despite early success with internal mammary grafts, widespread acceptance came nearly a decade later. Compared with the saphenous vein, the internal mammary artery (IMA) has unique biologic characteristics that enable it to resist atherosclerosis and maintain high patency rates. The IMA has a nonfenestrated internal elastic lamina and lacks vaso vasorum inside the vessel wall, which tends to protect against intimal hyperplasia and cellular migration.63 In addition, the medial layer of IMA is thin and is limited in muscle cells, resulting in a decreased tendency for maladaptive vasoconstriction.64
Advantages of IMA conduits over SVGs include decreased postoperative mortality, improved cardiac event-free survival rates, and improved long-term patency rates well above 90% at 10 years.62,63 Because of its proximity to the left anterior descending (LAD) artery and favorable patency rates, the left IMA (LIMA) is most commonly used as an in situ graft to revascularize the LAD or diagonal artery, supplying the anterior or anterolateral cardiac wall.
Other Arteries
The profound benefits afforded by IMA grafting have given foundation to utilization of arterial conduits as coronary bypass grafts including the right IMA, right gastroepiploic artery, radial artery, and inferior epigastric arteries. The use of right gastroepiploic and inferior epigastric arteries in CABG procedures has been limited because of the need to extend the median sternotomy to expose the abdominal cavity. Although the use of these arteries increases surgical time and technical difficulty of the surgery, these arteries can be used as a free graft to perform total arterial revascularization.65,66 In rare instances, the right gastroepiploic artery may be used in situ for revascularization to the posterior descending artery.
Computed Tomography
The ability of CT to characterize the patency of bypass grafts has been discussed since the 1990s60,67–69 with first-generation (four- and eight-detector) MDCT scanners. A recent meta-analysis demonstrated that obstructive bypass graft disease can be detected by using at least a 16-detector row CT with a high diagnostic accuracy, with a sensitivity of 98%, a specificity of 97%, a positive-predictive value of 93%, and a negative-predictive value of 99%.70
The advent of ECG gating and improved subsecond data acquisition has given rise to near complete suppression of cardiac motion with superior subcentimeter spatial resolution. In particular, 64-detector row CT devices, available since 2005, have not only shown the ability to acquire high-quality images, but also have demonstrated impressive accuracy in evaluating coronary artery stenosis and bypass graft patency in a larger cohort of patients.71
The increased number of detectors lead to increased temporal resolution (e.g., 83 msec) and spatial resolution (e.g., 0.4 × 0.4 × 0.4 mm3) and reduction of both cardiac and respiratory motion artifacts, leading to improved ability to assess graft stenosis and occlusion.13 Newer three-dimensional (3D) image processing algorithms and advanced volumetric visualization techniques can provide improved evaluation of grafts in multiple planes using various projections (Fig. 37-11).
Ropers and colleagues were among the first to compare bypass graft imaging with 64-detector MDCT compared to conventional catheter angiography and reported sensitivity of 97% and specificity of 98% of MDCT for detecting graft occlusion.72 Other subsequent studies using 64-detector MDCT have reported sensitivity and specificity values of 93.3% and 100% and 91.4% and 100%, respectively, for graft occlusion and significant luminal stenosis (>50% luminal narrowing).73–76
The protocol for CT bypass imaging is similar to that for coronary CTA. One important difference is that the scan should be extended superiorly to include the origins of the internal mammary arteries. As is routine, heart rate control and proper breathing instructions are critical to performing a diagnostic study. Development of a new generation of scanner technology such as cardiac freeze-frame technique, dual-source CT, and flat-panel CT promise further improvements in temporal resolution and the ability to acquire slices with a gantry rotation time of about 100 ms, potentially reducing the problems of breath-holding, motion artifact, and artifacts related to variations of heart rate during the scan.77 The role of bypass assessment with MDCT holds great potential to replace conventional catheter angiography in the near future as MDCT continues to evolve.
Magnetic Resonance Imaging
Coronary imaging with MRA can be performed using two-dimensional (2D) or 3D techniques. Cardiac synchronization with ECG gating is necessary to minimize degradation of images owing to cardiac motion. Motion of the diaphragm also presents a limitation. Different techniques, such as a breath-hold technique versus a free breathing with navigator echo-based technique, have been used to overcome these issues.78 For a 2D coronary MRA using the breath-hold technique, the patient may be required to do many breath-holds for 16 to 20 seconds (and even longer for a 3D coronary MRA).79,80
Few studies have focused on the ability of MR to assess coronary bypass grafts. Brenner and coworkers evaluated 85 patients after an average of 7 days post CABG and were able to assess saphenous and arterial grafts with 94% specificity and 90% sensitivity.81 Langerak and colleagues focused only on venous grafts and demonstrated a sensitivity and specificity of 65% to 82% and 82%, respectively, for the detection of a graft stenosis >50% and 73% and 80% to 87% for a graft stenosis >70%.82
Cardiac MRA is particularly limited in visualizing IMA bypass grafts. This is primarily due to the smaller diameters of arterial versus venous grafts (1 to 3 mm compared to the 3 to 6 mm), as well as the imaging artifacts arising from surgical metal clips. Several early studies failed to evaluate IMA grafts simply due to the suboptimal image quality.83,84 Notwithstanding these results, Knoll and colleagues85 and Wintersberger and associates86 compared the assessment of IMA grafts with MRA versus conventional angiography. Both studies were able to determine IMA graft patency with MRI with a sensitivity of 94%
Despite these initial investigations, MR imaging of bypass grafts remains difficult in patients with atrial fibrillation and tachycardia. Furthermore, extremely agitated or claustrophobic patients cannot be examined. Whole heart SSFP coronary magnetic resonance angiography has emerged as another promising MR technique that has already shown promise in evaluating native coronary arteries.87–90 However, its value in assessment of CABG is yet to be determined.
Analysis
Postoperative Evaluation
It is important to follow the anatomic course of the bypass graft and note the patency of the graft at its expected target. The LIMA extends from its origin at the subclavian artery and courses through the anterior mediastinum along the right ventricular outflow tract after being separated surgically from its original position in the left parasternal region. Infrequently, sequential distal anastomoses, with side-to-side and end-to-side anastomoses to the diagonal and LAD arteries, respectively, or involving separate sections of the LAD artery, are performed (Figs. 37-12 and 37-13).
Complications
Graft Thrombosis and Occlusion
Bypass graft failures are classified either as early or late following CABG surgery. During the early phase, usually within 1 month after CABG surgery, the most common cause of graft failure is thrombosis from platelet dysfunction at the site of focal endothelial damage during surgical harvesting and anastomosis.91
Additionally, other factors (such as the hypercoagulability state of the patient and the high-pressure distention or stretching of the venous graft, with its intrinsically weaker antithrombotic features) further initiate early venous graft failure, resulting in a 3% to 12% occlusion rate within 1 month postoperatively.92
Late-phase venous graft failure is due primarily to progressive changes related to systemic blood pressure exposure. One month after surgery; the venous graft starts to undergo neointimal hyperplasia.92 Although this process does not produce significant stenosis, it is the foundation for later development of graft atheroma. Beyond 1 year, atherosclerosis is the dominant process, resulting in graft stenosis and occlusion (Fig. 37-14).
Arterial grafts, such as IMA grafts, are resistant to atheroma development. Late IMA graft failure is more commonly due to progression of atherosclerotic disease in the native coronary artery distal to the graft anastomosis (Fig. 37-15).93
CT angiography can delineate multiple findings associated with graft stenosis and occlusion. Calcified and noncalcified atherosclerotic plaque is readily identified, and the calculation of the extent of graft narrowing is straightforward. Occlusion can be determined by nonvisualization of a vessel known to have been used for surgical grafting. In many instances, the most proximal part of an occluded aortocoronary graft fills with contrast, creating a small outpouching or “nubbin” from the ascending aorta, allowing a diagnosis (Fig. 37-16). A residual low attenuation structure, or “ghost”, of part of the occluded graft may be visible. Acute or chronic graft occlusion can sometimes be differentiated by the diameter of the bypass graft (Fig. 37-17). In chronic occlusion, the diameter is usually reduced from scarring, as compared with acute occlusion in which the diameter is usually enlarged (Fig. 37-18).
Graft Aneurysm
There are two types of bypass graft aneurysms: true aneurysms and pseudoaneurysms. True aneurysms are usually found 5 to 7 years after CABG surgery and are related to atherosclerotic disease.94 Alternatively, occurrences of pseudoaneurysms are more variable, although these lesions are usually found at the anastomotic site. Pseudoaneurysm cases that are found earlier may be related to infection or tension at the anastomotic site, resulting in suture rupture. In late-onset pseudoaneurysms, similar to true aneurysms, atherosclerotic changes likely played a role.95
Currently, there is no clear guideline for surgical repair. Measurement of an aneurysm >2 cm has been a cause for concern. Graft aneurysms can lead to various complications, including compression and mass effect on adjacent structures, thrombosis and embolization of the bypass graft leading to an acute coronary event, formation of fistula to the right atrium and ventricle, sudden rupture leading to hemothorax, hemopericardium, or death.94
Summary
KEY POINTS
 The newer generations of CT scanners have demonstrated increased accuracy for detection of in-stent restenosis, however artifacts still prevent it from being routinely used except in select cases.
The newer generations of CT scanners have demonstrated increased accuracy for detection of in-stent restenosis, however artifacts still prevent it from being routinely used except in select cases. Long-term patency of saphenous vein grafts is considerably less than with arterial grafts. The most common cause of early saphenous graft failure is thrombosis. Beyond 1 year, atherosclerosis is the dominant process, resulting in graft stenosis and occlusion.
Long-term patency of saphenous vein grafts is considerably less than with arterial grafts. The most common cause of early saphenous graft failure is thrombosis. Beyond 1 year, atherosclerosis is the dominant process, resulting in graft stenosis and occlusion.1 Dobesh PP, Stacy ZA, Ansara AJ, Enders JM. Drug-eluting stents: a mechanical and pharmacologic approach to coronary artery disease. Pharmacotherapy. 2004;11:1554-1577.
2 Serruys PW, Kutryk MJB, Ong ATL. Coronary-artery stents. N Engl J Med. 2006;354(5):483-495.
3 Writing group membersLloyd-Jones D, Adams R, Carnethon M, et al Heart Disease and Stroke Statistics—2009 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Circulation, 119 3, 2009. e21-e181.
4 Schneider DB, Dichek DA. Intravascular stent endothelialization. A goal worth pursuing? Circulation. 1997;95(2):308-310.
5 Wolf F, Feuchtner GM, Homolka P, et al. In vitro imaging of coronary artery stents: are there differences between 16- and 64-slice CT scanners? Eur J Radiol. 2008;68(3):465-470.
6 Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur. Heart J. 2006;27(21):2567-2572.
7 Pugliese F, Cademartiri F, van Mieghem C, et al. Multidetector CT for visualization of coronary stents. Radiographics. 2006;26(3):887-904.
8 Shimohama T, Honda Y, Fitzgerald PJ. The risks and benefits of drug-eluting stents. Am Heart Hosp J. 2007;5(3):146-150.
9 Morice M, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780.
10 Raja SG, Dreyfus GD. Efficacy and safety of drug-eluting stents: current best available evidence. J Card Surg. 2006;21(6):605-612.
11 Cutlip D Coronary artery stent thrombosis: general issues [Internet] In Basow D, (ed). UpToDate. Up To Date. Waltham, Mass. 2009 [cited 2010 Jan 21] Available from http://www.uptodateonline.com/online/content/topic.do?topicKey=correvas/7394&source=see_link
12 Stouffer G, Todd J Percutaneous Transluminal Coronary Angioplasty: eMedicine Cardiology Available from http://emedicine.medscape.com/article/161446-overview
13 Cutlip D, Levin T Intracoronary stent restenosis [Internet] In Basow D, (ed). UpToDate. UpToDate, Waltham, Mass. 2009. Available from http://www.uptodateonline.com/online/content/topic.do?topicKey=correvas/7394&source=see_link
14 Steinberg DH, Waksman R. Drug-eluting stent thrombosis vs. bare metal stent restenosis: finding the lesser of two evils. Am Heart Hosp J. 2007;5(3):151-154.
15 Chen MS, John JM, Chew DP, et al. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 2006;151(6):1260-1264.
16 Harper RW. Drug-eluting coronary stents—a note of caution. Med J Aust. 2007;186(5):253-255.
17 Maintz D, Grude M, Fallenberg EM, et al. Assessment of coronary arterial stents by multislice-CT angiography. Acta Radiol. 2003;44(6):597-603.
18 Baim DS. Grossman’s Cardiac Catheterization, Angiography, and Intervention, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
19 Cademartiri F, Palumbo AA, Maffei E, et al. Noninvasive imaging of coronary arteries with 64-slice CT and 1.5T MRI: challenging invasive techniques. Acta Biomed. 2007;78(1):6-15.
20 Gerber TC, Fasseas P, Lennon RJ, et al. Clinical safety of magnetic resonance imaging early after coronary artery stent placement. J Am Coll Cardiol. 2003;42(7):1295-1298.
21 Schroeder AP, Houlind K, Pedersen EM, et al. Magnetic resonance imaging seems safe in patients with intracoronary stents. J Cardiovasc Magn Reson. 2000;2(1):43-49.
22 Syed MA, Carlson K, Murphy M, et al. Long-term safety of cardiac magnetic resonance imaging performed in the first few days after bare-metal stent implantation. J Magn Reson Imaging. 2006;24(5):1056-1061.
23 Manning WJ, Nezafat R, Appelbaum E, et al. Coronary magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2007;15(4):609-637. vii
24 Pump H, Möhlenkamp S, Sehnert CA, et al. Coronary arterial stent patency: assessment with electron-beam CT. Radiology. 2000;214(2):447-452.
25 Knollmann FD, Möller J, Gebert A, et al. Assessment of coronary artery stent patency by electron-beam CT. Eur Radiol. 2004;14(8):1341-1347.
26 Krüger S, Mahnken AH, Sinha AM, et al. Multislice spiral computed tomography for the detection of coronary stent restenosis and patency. Int J Cardiol. 2003;89(2-3):167-172.
27 Gaspar T, Halon DA, Lewis BS, et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol. 2005;46(8):1573-1579.
28 Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94(4):427-430.
29 Carbone I, Francone M, Algeri E, et al. Non-invasive evaluation of coronary artery stent patency with retrospectively ECG-gated 64-slice CT angiography. Eur Radiol. 2008;18(2):234-243.
30 Sheth T, Dodd JD, Hoffmann U, et al. Coronary stent assessability by 64 slice multi-detector computed tomography. Catheter Cardiovasc Interv. 2007;69(7):933-938.
31 Das KM, El-Menyar AA, Salam AM, et al. Contrast-enhanced 64-section coronary multidetector CT angiography versus conventional coronary angiography for stent assessment. Radiology. 2007;245(2):424-432.
32 Nakamura K, Funabashi N, Uehara M, et al. Impairment factors for evaluating the patency of drug-eluting stents and bare metal stents in coronary arteries by 64-slice computed tomography versus conventional coronary angiography. Int J Cardiol. 2008;130(3):349-356.
33 Cademartiri F, Schuijf JD, Pugliese F, et al. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J Am Coll Cardiol. 2007;49(22):2204-2210.
34 Ehara M, Kawai M, Surmely J, et al. Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography: comparison with invasive coronary angiography. J Am Coll Cardiol. 2007;49(9):951-959.
35 Hamon M, Champ-Rigot L, Morello R, et al. Diagnostic accuracy of in-stent coronary restenosis detection with multislice spiral computed tomography: a meta-analysis. Eur Radiol. 2008;18(2):217-225.
36 Sun Z, Davidson R, Lin CH. Multi-detector row CT angiography in the assessment of coronary in-stent restenosis: a systematic review. Eur J Radiol. 2009;69(3):489-495.
37 Kumbhani DJ, Ingelmo CP, Schoenhagen P, et al. Meta-analysis of diagnostic efficacy of 64-slice computed tomography in the evaluation of coronary in-stent restenosis. Am J Cardiol. 2009;103(12):1675-1681.
38 Wykrzykowska JJ, Arbab-Zadeh A, Godoy G, et al. Assessment of in-stent restenosis using 64-MDCT: analysis of the CORE-64 Multicenter International Trial. AJR Am J Roentgenol. 2010;194(1):85-92.
39 Pugliese F, Weustink AC, Van Mieghem C, et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart. 2008;94(7):848-854.
40 Gilard M, Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart. 2006;92(1):58-61.
41 Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46(3):552-557.
42 Lewis BS, Halon DA. Integrating multidetector computed tomography into clinical practice: computed tomography scanning shows its metal. J Am Coll Cardiol. 2007;49(9):960-962.
43 Nakanishi T, Kayashima Y, Inoue R, et al. Pitfalls in 16-detector row CT of the coronary arteries. Radiographics. 2005;25(2):425-438.
44 Hoffmann U, Ferencik M, Cury RC, Pena AJ. Coronary CT angiography. J. Nucl. Med. 2006;47(5):797-806.
45 Schuijf JD, Pundziute G, Jukema JW, et al. Evaluation of patients with previous coronary stent implantation with 64-section CT. Radiology. 2007;245(2):416-423.
46 Oncel D, Oncel G, Karaca M. Coronary stent patency and in-stent restenosis: determination with 64-section multidetector CT coronary angiography—initial experience. Radiology. 2007;242(2):403-409.
47 Orford JL, Lennon R, Melby S, et al. Frequency and correlates of coronary stent thrombosis in the modern era: analysis of a single center registry. J Am Coll Cardiol. 2002;40(9):1567-1572.
48 Van Mieghem CAG, Cademartiri F, Mollet NR, et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation. 2006;114(7):645-653.
49 Herzog C, Zangos S, Zwerner P, et al. CT of coronary artery disease. J Thorac Imaging. 2007;22(1):40-48.
50 Schroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29(4):531-556.
51 Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475-1497.
52 Carrel AVIII. On the experimental surgery of the thoracic aorta and heart. Ann Surg. 1910;52(1):83-95.
53 Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA. 1973;223(7):792-794.
54 Kolesov VI, Potashov LV. [Surgery of coronary arteries]. Eksp Khir Anesteziol. 1965;10(2):3-8.
55 Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA. 1973;223(7):792-794.
56 Bourassa MG, Fisher LD, Campeau L, et al. Long-term fate of bypass grafts: the Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation. 1985;72(6 Pt 2):V71-78.
57 Campeau L, Lespérance J, Corbara F, et al. Aortocoronary saphenous vein bypass graft changes 5 to 7 years after surgery. Circulation. 1978;58(3 Pt 2):I170-175.
58 Bourassa MG. Fate of venous grafts: the past, the present and the future. J Am Coll Cardiol. 1991;17(5):1081-1083.
59 Campeau L, Enjalbert M, Lespérance J, et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med. 1984;311(21):1329-1332.
60 Engelmann MG, Knez A, von Smekal A, et al. Non-invasive coronary bypass graft imaging after multivessel revascularisation. Int J Cardiol. 2000;76(1):65-74.
61 FitzGibbon G, Kafka H, Leach A, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616-626.
62 Loop F, Lytle B, Cosgrove D, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
63 Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916-931.
64 Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45-68.
65 Buche M, Schroeder E, Gurné O, et al. Coronary artery bypass grafting with the inferior epigastric artery. Midterm clinical and angiographic results. J Thorac Cardiovasc Surg. 1995;109(3):553-559.
66 Pym J, Brown PM, Charrette EJ, et al. Gastroepiploic-coronary anastomosis. A viable alternative bypass graft. J Thorac Cardiovasc Surg. 1987;94(2):256-259.
67 Nieman K, Oudkerk M, Rensing BJ, et al. Coronary angiography with multi-slice computed tomography. Lancet. 2001;357(9256):599-603.
68 Enzweiler CNH, Kivelitz DE, Wiese TH, et al. Coronary artery bypass grafts: improved electron-beam tomography by prolonging breath holds with preoxygenation. Radiology. 2000;217(1):278-283.
69 HA J, Cho S, Shim W, et al. Noninvasive evaluation of coronary artery bypass graft patency using three-dimensional angiography obtained with contrast-enhanced electron beam CT. Am J Roentgenol. 1999;172(4):1055-1059.
70 Hamon M, Lepage O, Malagutti P, et al. Diagnostic performance of 16- and 64-section spiral ct for coronary artery bypass graft assessment: meta-analysis. Radiology. 2008;247(3):679-686.
71 Jones CM, Athanasiou T, Dunne N, et al. Multi-detector computed tomography in coronary artery bypass graft assessment: a meta-analysis. Ann Thorac Surg. 2007;83(1):341-348.
72 Ropers D, Ulzheimer S, Wenkel E, et al. Investigation of aortocoronary artery bypass grafts by multislice spiral computed tomography with electrocardiographic-gated image reconstruction. Am J Cardiol. 2001;88(7):792-795.
73 Ropers D, Pohle F, Kuettner A, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006;114(22):2334-2341.
74 Onuma Y, Tanabe K, Chihara R, et al. Evaluation of coronary artery bypass grafts and native coronary arteries using 64-slice multidetector computed tomography. Am. Heart J. 2007;154(3):519-526.
75 Meyer TS, Martinoff S, Hadamitzky M, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol. 2007;49(9):946-950.
76 Malagutti P, Nieman K, Meijboom WB, et al. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: evaluation of grafts and coronary arteries. Eur. Heart J. 2007;28(15):1879-1885.
77 Andreini D, Pontone G, Ballerini G, et al. Bypass graft and native postanastomotic coronary artery patency: assessment with computed tomography. Ann Thorac Surg. 2007;83(5):1672-1678.
78 Dhawan S, Dharmashankar KC, Tak T. Role of magnetic resonance imaging in visualizing coronary arteries. Clin Medicine Res. 2004;2(3):173-179.
79 Danias PG, Edelman RR, Manning WJ. Coronary MR angiography. Cardiol Clin. 1998;16(2):207-225.
80 Foo TK, Saranathan M, Hardy CJ, Ho VB. Coronary artery magnetic resonance imaging: a patient-tailored approach. Top Magn Reson Imaging. 2000;11(6):406-416.
81 Brenner P, Wintersperger B, von Smekal A, et al. Detection of coronary artery bypass graft patency by contrast enhanced magnetic resonance angiography. Eur J Cardiothorac Surg. 1999;15(4):389-393.
82 Langerak SE, Vliegen HW, de Roos A, et al. Detection of vein graft disease using high-resolution magnetic resonance angiography. Circulation. 2002;105(3):328-333.
83 Rubinstein RI, Askenase AD, Thickman D, et al. Magnetic resonance imaging to evaluate patency of aortocoronary bypass grafts. Circulation. 1987;76(4):786-791.
84 Theissen P, Sechtem U, Langkamp S, et al. [Noninvasive assessment of aortocoronary bypass using magnetic resonance tomography]. Nuklearmedizin. 1989;28(6):234-242.
85 Knoll P, Bonatti G, Pitscheider W, et al. [The value of nuclear magnetic resonance tomography in evaluating the patency of aortocoronary bypass grafts]. Z Kardiol. 1994;83(6):439-445.
86 Wintersperger BJ, Engelmann MG, von Smekal A, et al. Patency of coronary bypass grafts: assessment with breath-hold contrast-enhanced MR angiography—value of a non-electrocardiographically triggered technique. Radiology. 1998;208(2):345-351.
87 So NM, Lam WW, Li D, et al. Magnetic resonance angiography of coronary arteries with a 3-dimensional magnetization-prepared true fast imaging with steady-state precession sequence compared with conventional coronary angiography. Am Heart J. 2005;150(3):530-535.
88 Maintz D, Ozgun M, Hoffmeier A, et al. Whole-heart coronary magnetic resonance angiography: value for the detection of coronary artery stenoses in comparison to multislice computed tomography angiography. Acta Radiol. 2007;48(9):967-973.
89 Katoh M, Spuentrup E, Stuber M, et al. R. Flow targeted 3D steady-state free-precession coronary mr angiography: comparison of three different imaging approaches [Internet]. Invest Radiol. 2009 Oct. Available from http://www.ncbi.nlm.nih.gov/pubmed/19858729
90 Sakuma H, Ichikawa Y, Chino S, et al. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48(10):1946-1950.
91 Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616-626.
92 Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916-931.
93 Douglas JS. Percutaneous approaches to recurrent myocardial ischemia in patients with prior surgical revascularization. Semin Thorac Cardiovasc Surg. 1994;6(2):98-108.
94 Memon A, Huang RI, Marcus F, et al. Saphenous vein graft aneurysm: case report and review. Cardiol Rev. 2003;11(1):26-34.
95 Dubois CL, Vandervoort PM. Aneurysms and pseudoaneurysms of coronary arteries and saphenous vein coronary artery bypass grafts: a case report and literature review. Acta Cardiol. 2001;56(4):263-267.


 FIGURE 37-1
FIGURE 37-1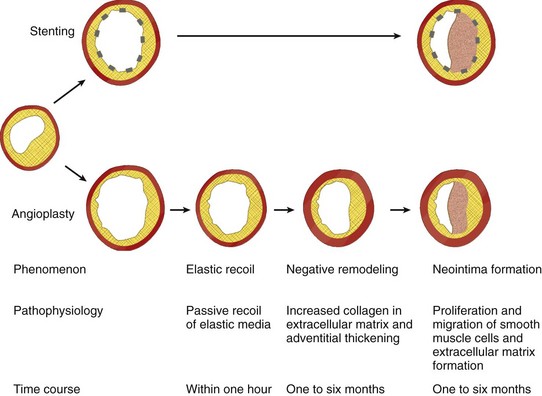
 FIGURE 37-2
FIGURE 37-2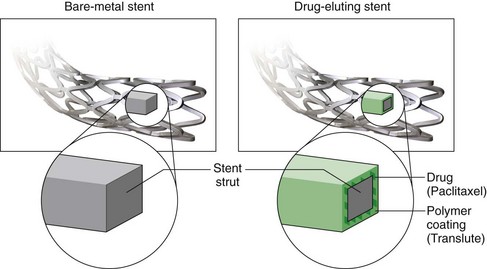
 FIGURE 37-3
FIGURE 37-3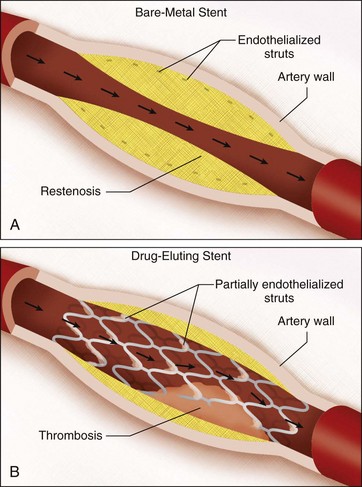
 FIGURE 37-4
FIGURE 37-4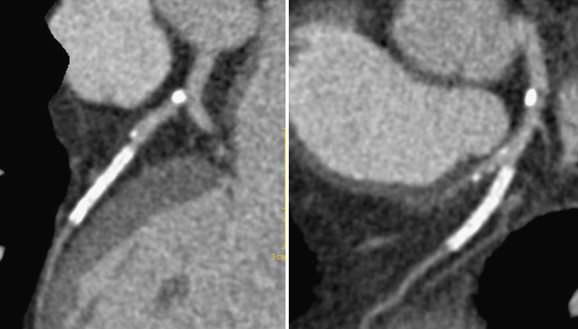
 FIGURE 37-5
FIGURE 37-5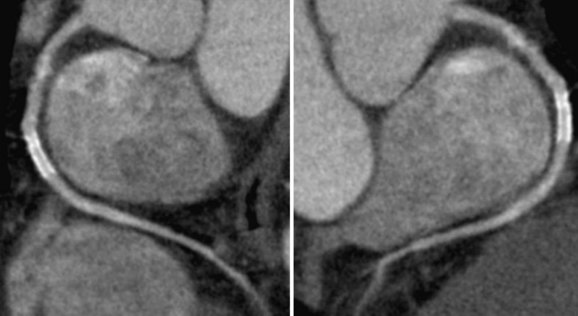
 FIGURE 37-7
FIGURE 37-7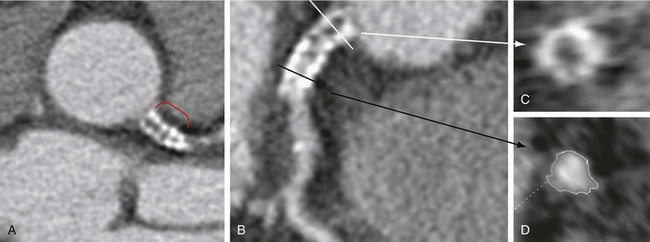
 FIGURE 37-8
FIGURE 37-8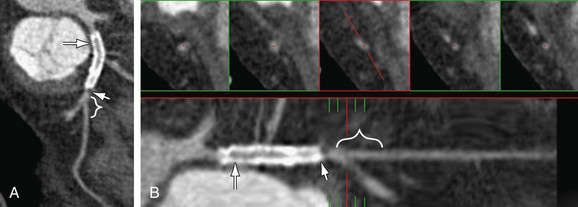
 FIGURE 37-9
FIGURE 37-9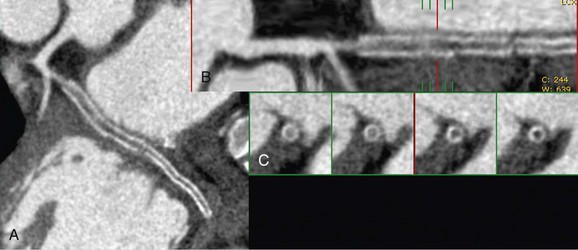
 FIGURE 37-10
FIGURE 37-10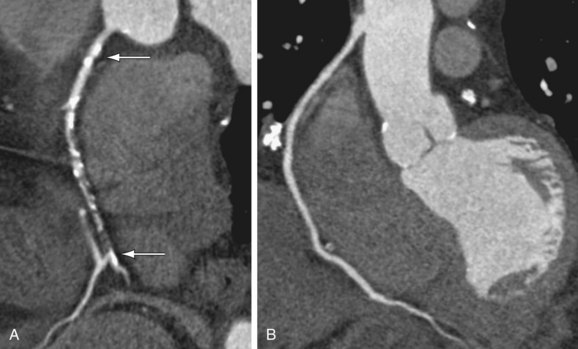
 FIGURE 37-11
FIGURE 37-11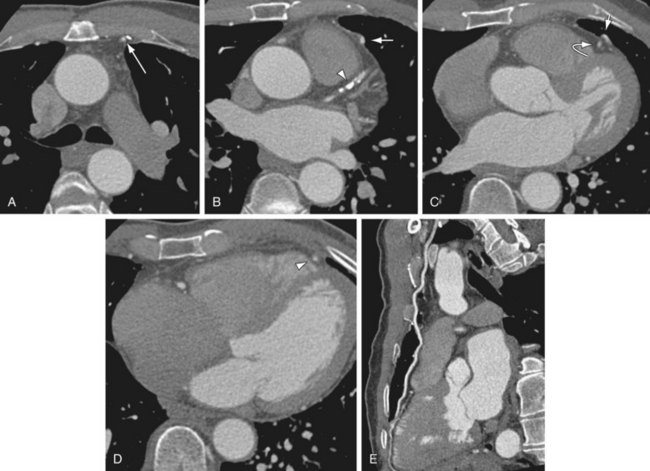
 FIGURE 37-12
FIGURE 37-12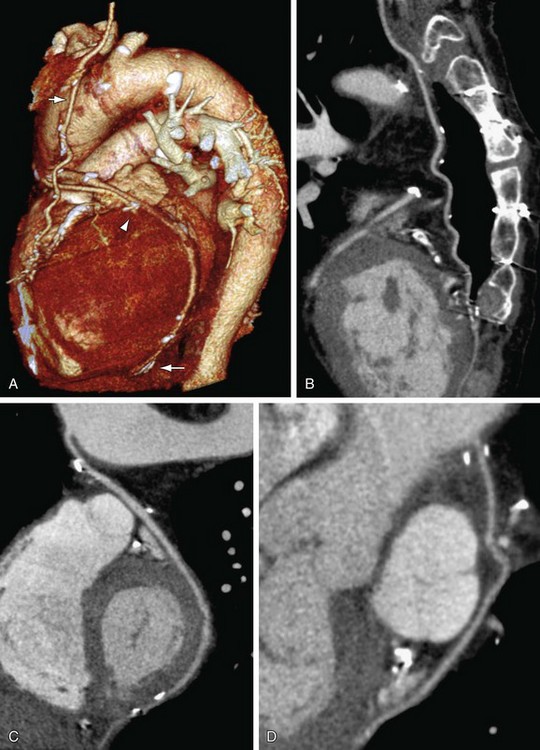
 FIGURE 37-13
FIGURE 37-13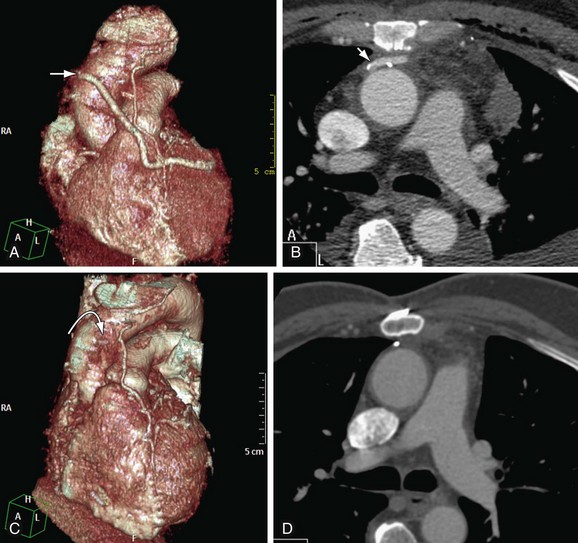
 FIGURE 37-14
FIGURE 37-14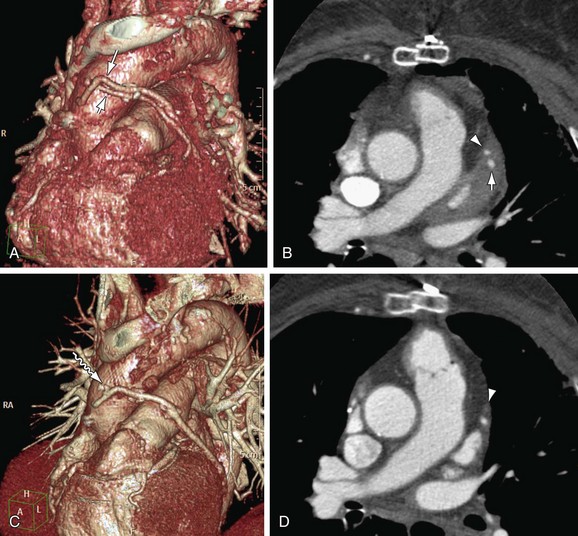
 FIGURE 37-15
FIGURE 37-15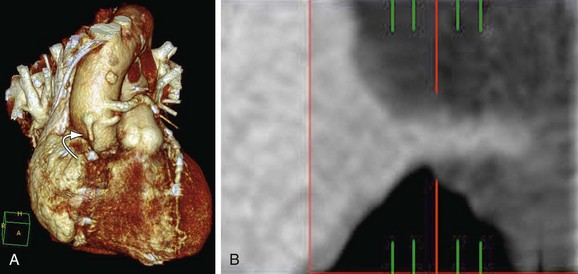
 FIGURE 37-16
FIGURE 37-16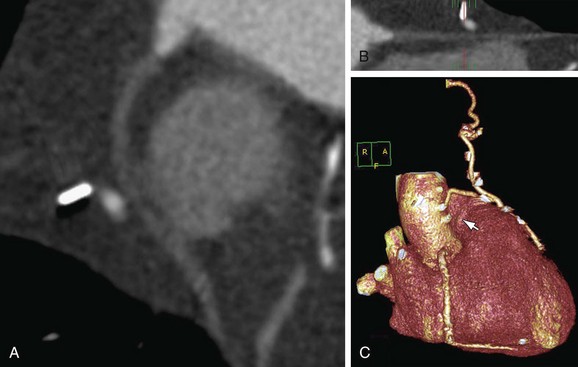
 FIGURE 37-17
FIGURE 37-17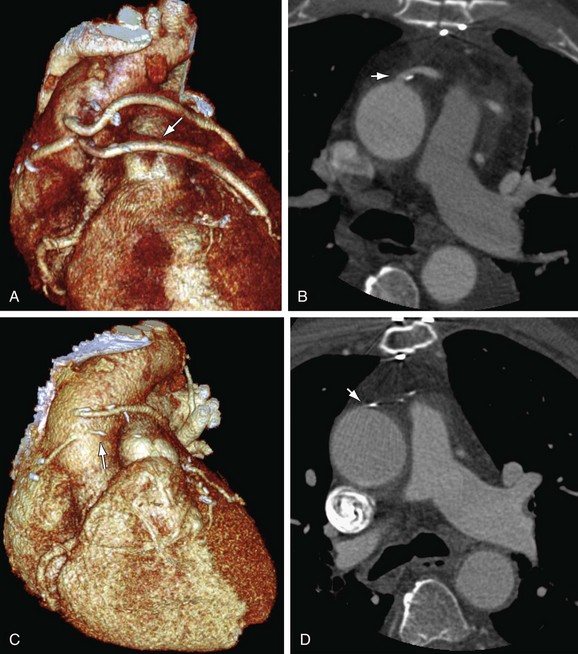
 FIGURE 37-18
FIGURE 37-18



