CHAPTER 31 Imaging of Atrial Fibrillation Intervention
DEFINITION
AF, a supraventricular tachyarrhythmia, is the most common cardiac arrhythmia with an overall prevalence of 0.4% to 1%.1 AF manifests clinically with an “irregularly irregular” heart rate, lack of P waves on ECG, and heart rates of up to 200 beats/min. To guide treatment options, the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) has issued guidelines classifying AF into paroxysmal, persistent, and permanent.2 Paroxysmal AF is defined as AF lasting less than 7 days and terminating spontaneously. Persistent AF lasts longer than 7 days and requires cardioversion to achieve normal sinus rhythm. Permanent AF lasts longer than 1 year. When AF occurs in individuals younger than 60 years and without underlying risk factors, it is termed lone AF.
PREVALENCE AND EPIDEMIOLOGY
AF affects approximately 2.3 million people in the United States and more than 6 million people in Europe.3 Its prevalence increases with age. Although AF is rare in children, in adults, the incidence and prevalence double every 10 years after age 50. The lifetime risk of developing AF is one in four after age 40.
Epidemiologic studies have linked AF to underlying structural heart disease, particularly mitral valvular disease, hypertension, and coronary artery disease.4 Age, diabetes, smoking, obesity, male sex, and white race are also independently associated with increased risk of AF.1,2 The epidemics of obesity and chronic heart failure are expected to contribute to an increased incidence of AF. The total annual cost for treatment of AF is estimated at $6.65 billion.5 AF results in approximately 350,000 hospitalizations, 5 million office visits, and 276,000 emergency department visits annually in the United States.
PATHOPHYSIOLOGY OF ATRIAL FIBRILLATION AND PULMONARY VEINS
The pathophysiology of AF is complex and not fully understood. AF is initiated by rapid firing of ectopic foci or re-entrant wavelets most often (94%) originating from the pulmonary veins.6 Other sources of ectopic foci have been identified in superior vena cava, coronary sinus, ligament of Marshal, crista terminalis, body of left atrium and right atrium, and more distal pulmonary veins.7–9 Pathologic studies have shown the extension of myocardial sleeves into pulmonary veins.10 A sleeve of myocardium surrounds the proximal aspect of the pulmonary vein and when measured from the venoatrial junction extends 0.2 to 1.7 cm out of the pulmonary veins with the longest sleeves associated with the superior pulmonary veins. The longest extensions occur in the following order: left superior pulmonary vein, right superior pulmonary vein, left inferior pulmonary vein, and right inferior pulmonary vein.11 Haissaguerre and colleagues6 showed a similar rank order when identifying ectopic foci for ablation. More than 90% of these ectopic beats arise from pulmonary veins; 50% arise from the left superior pulmonary vein alone. The posterior left atrium and pulmonary veins have become the focus of attention in treatment efforts of AF. When initiated, an abnormal atrial substrate maintains AF in susceptible individuals. Current AF treatments strive to isolate electrically the originating foci in the pulmonary veins from the atrial substrate and to modify the substrate.
MANIFESTATIONS OF DISEASE
Clinical Presentation
AF prevents ventricular preload and is associated with increased cardiovascular morbidity or mortality.12 It is an independent risk factor for stroke, increasing the risk by threefold to fivefold.4,13 It is also associated with increased risk of systemic embolism. The source of the embolism is left atrial appendage (LAA) in most patients.14 AF is associated with an increase in overall mortality in multiple studies.15,16
Clinically Relevant Anatomy
The left atrium can be divided into four components: the septum, the vestibule, the appendage, and the venous portion. The walls of the left atrium are described as superior, posterior, left lateral, septal, and anterior.17 The anterior wall behind the transverse pericardial sinus posterior to the aorta is very thin, averaging 2 mm in thickness.18 The thickest portion of the left atrium is the superior wall, averaging 4.5 ± 0.6 mm in thickness.18
The interatrial septum comprises the foramen ovale. The foramen ovale is a flap valve that typically fuses by early adulthood. The prevalence of patent foramen ovale is reported to be 25%.19 The foramen ovale is the only portion of the septum that can be traversed without risk of injury to the sinoatrial nodal artery or exiting the heart.18
Most of the left atrium is smooth-walled. The exception is the LAA, which is derived from the primitive atrium and contains multiple pectinate muscles and a trabeculated surface. The LAA is a common source of atrial thrombi because of relative stasis of blood flow in this region in AF and the narrow neck of the appendage. Accessory atrial appendages (also termed roof pouch) have been reported in 0.06% of patients; these are of unclear clinical significance, but they may result in discontinuity of a roof ablation line if unrecognized (Fig. 31-1).20,21
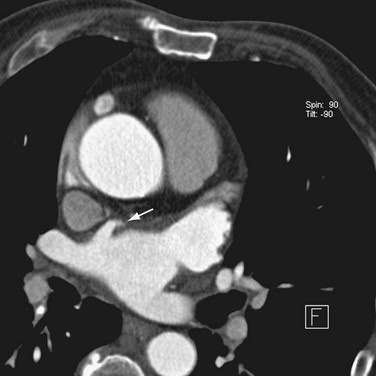
 FIGURE 31-1 Accessory LAA. A focal outpouching (arrow) is seen anterior to the right superior pulmonary vein.
FIGURE 31-1 Accessory LAA. A focal outpouching (arrow) is seen anterior to the right superior pulmonary vein.
The venous component comprises most of the left atrium. Classic left atrial anatomy consists of bilateral superior and inferior pulmonary veins and is present about two thirds of the time (Fig. 31-2).22,23 Accessory pulmonary veins are named for the lobes or segments they drain. Common anatomic variants include a conjoined left pulmonary trunk, which bifurcates to form the left superior pulmonary vein and left inferior pulmonary vein (under incorporation) and has been reported as a consistent source of arrhythmogenic atrial ectopy (Fig. 31-3).24,25 An accessory right middle pulmonary vein is the most common variant, occurring 20% to 30% of the time; it originates from the intervenous saddle and is typically 1 cm or less in diameter (Fig. 31-4).26 The next most common accessory vein drains the superior segment of the right lower lobe independently. One of the most striking accessory veins is the “top vein” (3%), which can drain the posterior segment right upper lobe or superior segment right lower lobe, and is particularly important to describe for patients being considered for the Wolf mini-Maze procedure; this unusual location is blind to the thoracoscopist (Fig. 31-5).27,28 Overincorporation of the right inferior pulmonary vein commonly results in multiple orificial branches and has been reported in 66% to 99% of cases (Fig. 31-6).29 Rarely, the inferior pulmonary veins can be conjoined resulting in an inferior truncus (Fig. 31-7).30
The sinoatrial node is found along the course of the sinoatrial artery in the subepicardium. This subepicardial location makes the node more vulnerable to select cardiac surgery and pericardial disease.31 The node artery arises from the right coronary artery in 66% of cases, arises from the left circumflex artery in 27%, and has a dual supply in 6%.32 Most arise from the proximal right coronary artery and course along the anterior interatrial groove toward the cavoatrial junction (Figs. 31-8 and 31-9).
CLINICAL TREATMENT OF ATRIAL FIBRILLATION
Traditionally, treatment of AF has included electrical or chemical cardioversion followed by long-term antiarrhythmic therapy. This strategy is unsatisfactory in maintenance of sinus rhythm, with less than 50% of patients being in sinus rhythm after 1 to 2 years.33 It also has the accompanying disadvantage of requiring lifelong anticoagulation. Maintenance of sinus rhythm is important in younger active individuals to decrease the incidence of stroke, and in older patients with heart failure, the loss of the “atrial kick” affects overall cardiac output.
In an effort to improve treatment efficacy, a surgical procedure called the Cox Maze procedure was developed; this procedure results in isolation of the pulmonary veins and compartmentalization of the atria.34 Multiple incisions in both atria result in scar tissue that reduces the amount of atrial tissue between scars to below the critical re-entry circuit size, preventing AF. A thoracoscopic variant of this procedure, the Wolf mini-Maze, was developed in an effort to prevent the more morbid median sternotomy necessitated by the Cox-Maze procedure.35 It is a bilateral video-assisted thoracoscopic off-pump procedure. A curved bipolar radiofrequency (RF) ablation device is used to create bilateral, transmural, linear lesions around an atrial cuff of the right and left pulmonary veins achieving electrical isolation (Fig. 31-10). In addition, a staple excision of the LAA is performed. Relevant to the Wolf mini-Maze procedure, the surgeon is “blind” to the anatomy of the posterior wall of the left atrium. Accessory veins in this region are particularly vulnerable to surgical mishap.28
Catheter-based procedures have been developed that aim at treating the triggers and the substrate for the AF. Ablation involves the purposeful devitalization of arrhythmogenic myocardial tissue to treat arrhythmias. This devitalization may be accomplished using cryotherapy or, more commonly, RF current to induce a thermal injury. RF lesions are typically 3 to 6 mm in diameter and 3 mm deep.36 More recently, use of externally irrigated catheters has increased in an effort to improve lesion delivery and reduce char formation at the catheter tip, which decreases thermal efficiency.37 The choice of catheter and energy delivery varies among different centers with an emphasis on smaller, lower energy systems. The most recent ACC/AHA/ESC guideline recommends ablation of AF in symptomatic patients who have not responded to medical therapy.2,38 Currently, ablation is performed in a wider array of patients, however, particularly in patients with congestive heart failure, in whom ablation of AF has been shown to improve ventricular systolic function.39
The first contemporary approach to AF ablation was performed by Haissaguerre and colleagues6 in 1994 by point ablation of distal pulmonary vein foci. This procedure was moderately successful, although it was complicated by a high percentage of pulmonary vein stenosis. Since then, multiple approaches have been developed, including segmental isolation of pulmonary veins and circumferential ablation, a stepwise approach that requires additional ablation lines in the roof of the left atrium, mitral annulus and isthmus, and coronary sinus and isolation of the superior vena cava (Fig. 31-11). Ablation of continuous atrial fractionated signals and autonomic ganglia around the pulmonary veins has also been tried.38,40–43 The most common technique includes isolation of pulmonary veins using a circumferential extraostial ablation 1 to 2 cm on the atrial side of the pulmonary veins and isolation of the posterior left atrium.38,44,45 Additional ablation lines may be created in the posterior left atrium, termed the roof ablation line, and at the mitral isthmus.
Left atrium function is partially preserved with the surgical Maze procedure.46,47 With catheter ablation techniques targeting the posterior left atrium, the pulmonary vein orifices, and the mitral isthmus line, 30% to 40% of left atrial surface area may be ablated.48 Multidetector CT evaluation of left atrial transport after circumferential RF ablation of paroxysmal AF found a decrease in left atrium function, although it is unclear whether this impairment is severe enough to predispose to thrombus formation.49 More recently, Takahashi and coworkers45 reported recovery of left atrium function with stepwise catheter ablation for chronic AF. Patient selection may also be important because pulmonary vein electrical isolation has been shown to be more effective in paroxysmal than in persistent AF.50
Imaging Techniques
The anatomy of the pulmonary veins can be delineated during the procedure by retrograde contrast venography in conjunction with intracardiac ultrasonography.51 More often, a CT or MRI examination of the left atrium is performed before the procedure.52
Multidetector Computed Tomography
These examinations are reviewed as comprehensive cardiac CT angiography. The pulmonary veins are reported based on standard nomenclature, and two-dimensional diameter orifice measurements are defined at the venoatrial junction. Many authors report the distance to the first bifurcation (trunk length), although in our experience, this is less important now that electrophysiologists are performing circumferential extraostial ablations. We report all orificial pulmonary veins, defined as branches that occur within 5 mm of the venoatrial junction. It has been shown that ablation within 5 mm of the ostium of a pulmonary vein or first bifurcation increases risk of stenosis after the procedure.50,53 All accessory veins must be identified and reported; these veins are frequently less than 1 cm in diameter, increasing the risk of stenosis if they are unrecognized before ablation. In addition, the left atrium is fully described, including a left atrial diameter measurement defined in transesophageal echocardiographic terms as the distance from the posterior wall of the left atrium abutting the esophagus to the anterior wall of the left atrium.
An evaluation for LAA thrombus must be performed because LAA thrombus is an absolute contraindication to cardioversion and RF ablation. Although transesophageal echocardiography has been the gold standard in the detection of LAA thrombus, 64-row multidetector CT reliably excludes LAA thrombus (Fig. 31-12).54 Using a ratio threshold density of the LAA to the ascending aorta of greater than 0.75 had 100% negative predictive value in excluding LAA thrombus.55 Occasionally, the equivalent of spontaneous echocardiographic contrast is identified as failure to opacify the LAA on first pass of contrast material.54 Sluggish flow through the narrow neck of the LAA and loss of atrial contractility can result in poor mixing of blood that may mimic a clot in the LAA. Delayed images can be performed if this is recognized at the time of the scan to show diffusion of contrast material into the tip of the LAA (Fig. 31-13).56
Patent foramen ovale can be identified as a fine linear hypodensity paralleling the foramen (Fig. 31-14).57 Atrial septal aneurysms are defined as a bulge of the interatrial septum of greater than 15 mm into either atrium. This defect has been associated with strokes and migraine headaches.58
The course of the esophagus should be defined relative to the pulmonary veins and is crucial information that assists the electrophysiologist when performing circumferential RF ablation. Although the course of the esophagus varies, it often lies close to, or parallels, the ostia of the left pulmonary veins. The walls of the left atrium and esophagus are thin (often <5 mm in thickness), and the left inferior pulmonary vein can often course immediately adjacent to the esophagus, making thermal injury to the esophagus a possible complication of RF ablation (Fig. 31-15).59 Esophageal mobility of greater than or equal to 2 cm has been reported in most patients under conscious sedation, so real-time imaging of the esophagus is not replaced by cross-sectional imaging.60 The electrophysiology cardiologist typically passes an esophageal stethoscope as a marker of esophageal location at fluoroscopy throughout the RF ablation.
Certain anatomic variants are important to identify before the procedure, including persistent left superior vena cava and partial anomalous pulmonary venous return. In persistent left superior vena cava, the anomalous vein drains to the coronary sinus with a course along the left lateral wall running along the ligament of Marshall. This is in the expected location for a typical pulmonary vein ablation line. A fistula at this level would create a right-to-left shunt (Fig. 31-16). Partial anomalous pulmonary venous return must be identified to avoid confusion at fluoroscopy (Fig. 31-17). In addition, based on the anomalous draining vein, associated defects may be present. Right upper lobe partial anomalous pulmonary venous return is frequently associated with sinus venosus atrial septal defect.
It is crucial to evaluate these examinations similar to other cardiac CT angiography examinations for unsuspected ancillary findings. In a review of patients before Wolf mini-Maze procedure, Meyer and colleagues28 reported a high incidence of ancillary findings, including mitral stenosis, coronary artery disease, pulmonary nodules, and pleural abnormalities. Pleural abnormalities are particularly important to identify before thoracoscopic procedures because unfettered access to the pleural space may be an issue. Evaluation of the coronary arteries in AF patients is challenging, but not impossible with 64-row (or more) multidetector CT if the patients are evaluated in end-systole.61,62 More recent advances with the improved temporal resolution of dual source imaging may result in reliable evaluation of the coronary arteries in AF patients.63,64
Magnetic Resonance Imaging
As an alternative to CT, MRI evaluation of the left atrium and pulmonary veins may be performed.65 This examination characteristically is divided into several sequences: an ECG gated fat-suppressed two-dimensional fast spin-echo sequence for morphology, an ECG gated single-slice cine mode single breath-hold sequence such as steady-state free precession (SSFP) to show cyclical blood flow, and a three-dimensional gadolinium-enhanced MR angiography sequence such as a breath-hold three-dimensional fast spoiled gradient-recalled-echo imaging sequence (Fig. 31-18).66 It has been shown more recently that a three-dimensional noncontrast free breathing MR angiography examination using SSFP can adequately evaluate pulmonary veins in patients unable to suspend respiration or at high risk for contrast complications.67 Lickfett and associates68 reported a 32.5% change in pulmonary vein ostial diameters during the cardiac cycle with the largest diameter in late diastole and the minimum diameter occurring in early systole. One potential limitation of pulmonary vein imaging with MRI is the contraindication to imaging patients with pacemakers and defibrillators.
Image Fusion
Using these volumetric data sets, a three-dimensional model of the left atrium can be reconstructed. This model can be referenced in the electrophysiology laboratory for catheter manipulation. More recent innovations have merged three-dimensional electroanatomic mapping systems with the three-dimensional cardiac model to permit tracking of catheter movement and more precise anatomic localization of ablation points. Three mapping technologies are in common use. The first uses electromagnetic positioning established by means of a locator pad consisting of three ultra-low emitting coil magnets placed below the patient, a reference external catheter usually positioned posterior to the patient, and the mapping catheter with a magnetic field sensor. The location of the sensor is determined from the intersection of the theoretical spheres whose radii are the distances measured by the sensor.69 A second system uses cutaneous pads to generate electrical fields that track the catheter.70 Finally, a third approach involves the use of a multielectrode balloon to track catheter position.71
With all these systems, an anatomic shell is created by moving the catheter along the walls of the cardiac chamber and recording multiple catheter positions. Electrograms and points of interest (e.g., anatomic markers, ablation points) can be represented on the shell. Image fusion is usually achieved with a point registration algorithm using fiducial points gathered during mapping at fixed anatomic locations, such as pulmonary vein–left atrium junctions; this is later refined by the process of surface registration minimizing the distance between the two surfaces.72,73 The integrated image provides a map of RF ablation points and real-time catheter localization (Fig. 31-19). Multiple studies have evaluated these registration methods and shown clinically acceptable accuracy.74–83
The major obstacles to optimal registration seem to be inherent inaccuracies of mapping systems, effect of respiration and heart rate, the effect of indentation caused by the mapping catheter on the real-time map, limitation of rigid registration algorithms, lack of accurate anatomic landmarks, and differences in chamber size and alignment between the time of image acquisition and mapping.73,83,84 Lickfett and associates68 showed a mean phasic change in ostial position of 7.2 mm. In a nonrandomized study, decreased fluoroscopy times were reported along with improved clinical outcomes when AF ablations were performed with image integration software.85
Postprocedure Imaging
In a survey of 9000 procedures, Cappato and colleagues86 found an overall rate of major complications of 6% associated with AF catheter ablation procedures. Complications include death, stroke, cardiac tamponade, venous stenosis, and atrioesophageal fistula.
Pulmonary Venous Stenosis
Pulmonary venous stenosis is reported in 1.5% to 42.4% of patients after RF ablation for AF.86 This complication was considerably more common when point ablations were performed within the pulmonary veins. Current extraostial techniques combined with lower energy delivery, decreased ablation temperatures, and intracardiac echocardiography result in an incidence of moderate to severe stenosis of less than 1.4% at centers experienced in these techniques.87 Nevertheless, pulmonary vein stenosis (Fig. 31-20) may result in focal edema, veno-occlusive disease, parenchymal hemorrhage, venous thrombosis, and venous infarcts. Pulmonary vein balloon angioplasty and stenting can be used for symptomatic severe stenosis.
Atrioesophageal Fistula
Atrioesophageal fistula is a rare but potentially devastating complication of left atrium RF ablation procedures with a reported 50% mortality. The increased prevalence of this complication of RF ablation for AF and its increased frequency are thought to be due to the increased use of circumferential pulmonary vein ablation and posterior left atrium ablation instead of point ablations.87,88 In a prospective study using endoscopic analysis of the esophagus after pulmonary vein ablation, 53% of patients had erythema 24 hours after pulmonary vein ablation, and 18% had focal necrosis/ulceration.89 Transesophageal echocardiography and esophagoscopy are contraindicated if this diagnosis is suspected because of air insufflation. CT is the study of choice, and shows infiltration of the mediastinal fat and subtle fluid collections between the posterior wall of the left atrium and the esophagus (Fig. 31-21).90
Recurrent Atrial Fibrillation
The left atrial isthmus has been implicated in recurrent AF. The left atrial isthmus is the region between the left inferior pulmonary vein orifice and the posteroinferior mitral annulus, and is typically longer in AF patients.91 The precise anatomic relationships of the adjacent coronary sinus and left circumflex artery should be determined. Injury to the circumflex coronary artery has been reported with atrial isthmus line ablation.92 Circumferential pulmonary vein ablation effectively eliminates AF, but may result in organized atrial tachycardias, of which 90% are re-entrant and related to gaps in the ablation lines.93 Early studies suggest that myocardial delayed enhancement MRI may be an effective noninvasive tool for identifying ablation-related atrial scarring.94,95
REPORTING: INFORMATION FOR REFERRING PHYSICIANS
KEY POINTS
 AF is the most common cardiac arrhythmia, initiated by rapid firing of ectopic foci or re-entrant wavelets most often originating from the pulmonary veins.
AF is the most common cardiac arrhythmia, initiated by rapid firing of ectopic foci or re-entrant wavelets most often originating from the pulmonary veins. Common pulmonary vein anatomic variants include a conjoined left pulmonary trunk and an accessory right middle lobe pulmonary vein.
Common pulmonary vein anatomic variants include a conjoined left pulmonary trunk and an accessory right middle lobe pulmonary vein.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100-1105.
Cronin P, Sneider MB, Kazerooni EA, et al. MDCT of the left atrium and pulmonary veins in planning radiofrequency ablation for atrial fibrillation: a how-to guide. AJR Am J Roentgenol. 2004;183:767-778.
Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48:854-906.
Lacomis JM, Wigginton W, Fuhrman C, et al. Multi-detector row CT of the left atrium and pulmonary veins before radiofrequency ablation for atrial fibrillation. RadioGraphics. 2003;23:S35-S48.
Saremi F, Krishnan S. Cardiac conduction system: anatomic landmarks relevant to interventional electrophysiologic techniques demonstrated with 64-detector CT. RadioGraphics. 2007;27:1539-1567.
1 Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469-473.
2 Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48:854-906.
3 Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949-953.
4 Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84:131R-138R.
5 Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348-356.
6 Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666.
7 Yamada T, McElderry HT, Doppalupadi H, et al. Catheter ablation of focal triggers and drivers of atrial fibrillation. J Electrocardiol. 2008;41:138-143.
8 Saksena S, Skadsberg ND, Rao HB, et al. Biatrial and three-dimensional mapping of spontaneous atrial arrhythmias in patients with refractory atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:494-504.
9 Li J, Wang L. Catheter ablation of atrial fibrillation originating from superior vena cava. Arch Med Res. 2006;37:415-418.
10 Saito T, Waki K, Becker AE. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000;11:888-894.
11 Nathan H, Eliakin M. The junction between the left atrium and the pulmonary veins. Circulation. 1966;34:412-422.
12 Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042-1046.
13 Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
14 Aschenberg W, Schluter M, Kremer P, et al. Transesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol. 1986;7:163-166.
15 Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long term risks associated with atrial fibrillation: 20-year follow-up of the Renfrow/Paisley study. Am J Med. 2002;113:359-364.
16 Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695-703.
17 McAlpine WA. Heart and Coronary Arteries. Berlin: Springer-Verlag; 1975.
18 Ho SY, Sanchez-Quintana D, Cabrera JA, et al. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:1525-1533.
19 Anderson RH, Brown NA, Webb S. Development and structure of the atrial septum. Heart. 2002;88:104-110.
20 Vanovermeire OM, Duerinckx AJ. Accessory appendages if the left atrium as seen during 64-slice coronary CT angiography. Presented at the Scientific Sessions at the Syllabus Society of Thoracic Radiology. 2006. March 13
21 Wongcharoen W, Tsao HM, Wu MH, et al. Morphologic characteristics of the left atrial appendage, roof, and septum: implications for the ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:951-956.
22 Lacomis J, Schwartzman D, Wigginton W, et al. 3D-multidetector CT and variations in pulmonary venous and left atrial anatomy: implications in atrial fibrillation patients undergoing ablative therapy. Radiology. 2002;S225:631.
23 Marom EM, Herndon JE, Kim YH, et al. Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology. 2004;230:824-829.
24 Mansour M, Holmvang G, Sosnovik D, et al. Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:387-393.
25 Schwartzman D, Bazaz R, Nosbisch J. Common left pulmonary vein: a consistent source of arrhythmogenic atrial ectopy. J Cardiovasc Electrophysiol. 2004;15:560-566.
26 Tsao HM, Wu MH, Yu WC, et al. Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:1353-1357.
27 Lickfett L, Kato R, Tandri H, et al. Characterization of a new pulmonary vein variant using magnetic resonance angiography: incidence, imaging and interventional implications of the “right top pulmonary vein.”. J Cardiovasc Electrophysiol. 2004;15:538-543.
28 Meyer CA, Hall JE, Mehall JR, et al. Impact of preoperative 64-slice CT scanning on mini-Maze atrial fibrillation surgery. Innovations. 2007;2:169-175.
29 Perez-Lugones A, Schwartzman PR, Schweikert R, et al. Three-dimensional reconstruction of pulmonary veins in patients with atrial fibrillation and controls: morphological characteristics of different veins. Pacing Clin Electrophysiol. 2003;26:8-15.
30 Sra J, Malloy A, Shah H, et al. Common ostium of the inferior pulmonary veins in a patient undergoing left atrial ablation for atrial fibrillation. J Interv Card Electrophysiol. 2006;15:203.
31 James TN. The sinus node. Am J Cardiol. 1977;40:965-986.
32 Saremi F, Abolhoda A, Ashikyan O, et al. Arterial supply to sinuatrial and atrioventricular nodes: imaging with multidetector CT. Radiology. 2008;246:99-109.
33 van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
34 Cox JL, Ad N, Palazzo T, et al. Current status of the maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:15-19.
35 Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:797-802.
36 Saremi F, Krishnan S. Cardiac conduction system: anatomic landmarks relevant to interventional electrophysiologic techniques demonstrated with 64-detector CT. RadioGraphics. 2007;27:1539-1567.
37 Macle L, Jais P, Weerasooriya R, et al. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:1067-1073.
38 Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816-861.
39 Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373-2383.
40 Wright M, Haissaguerre M, Knecht S, et al. State of the art: catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2008;9:583-592.
41 O’Neill MD, Jais P, Hocini M, et al. Catheter ablation for atrial fibrillation. Circulation. 2007;116:1515-1523.
42 Nademanee K, Schwab M, Porath J, et al. How to perform electrogram-guided atrial fibrillation ablation. Heart Rhythm. 2006;3:981-984.
43 Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619-2628.
44 Sanders P, Hocini M, Jais P, et al. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation: long-term clinical outcome. Eur Heart J. 2007;28:1862-1871.
45 Takahashi Y, O’Neill MD, Hocini M, et al. Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J Am Coll Cardiol. 2007;49:1306-1314.
46 Lonnerholm S, Blomstrom P, Nilsson L, et al. Atrial size and transport function after Maze III procedure for paroxysmal atrial fibrillation. Ann Thorac Surg. 2002;73:107-111.
47 Albirini A, Scalia GM, Murray RD, et al. Left and right atrial transport function after the Maze procedure for atrial fibrillation: an echocardiographic Doppler follow-up study. J Am Soc Echocardiogr. 1997;10:937-945.
48 Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004;110:3036-3042.
49 Lemola K, Desjardins B, Sneider M, et al. Effect of left atrial circumferential ablation for atrial fibrillation on left atrial transport function. Heart Rhythm. 2005;2:923-928.
50 Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077-1081.
51 Callans DJ, Wood MA. How to use intracardiac echocardiography for atrial fibrillation ablation procedures. Heart Rhythm. 2007;4:242-245.
52 Lacomis JM, Pealer K, Fuhrman CR, et al. Direct comparison of computed tomography and magnetic resonance imaging for characterization of posterior left atrial morphology. J Interv Card Electrophysiol. 2006;16:7-13.
53 Scharf C, Sneider M, Case I, et al. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol. 2003;14:150-155.
54 Kim YY, Klein AL, Halliburton SS, et al. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J. 2007;154:1199-1205.
55 Patel A, Au E, Donegan K, et al. Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm. 2008;5:253-260.
56 Saremi F, Channual S, Gurudevan SV, et al. Prevalence of left atrial appendage pseudothrombus filling defects in patients with atrial fibrillation undergoing coronary computed tomography angiography. J Cardiovasc CT. 2008;2:164-171.
57 Saremi F, Attai SF, Narula J. 64 multidetector CT in patent foramen ovale. Heart. 2007;93:505.
58 Mugge A, Daniel WG, Angermann C, et al. Atrial septal aneurysm in adult patients: a multi-center study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785-2792.
59 Lemola K, Sneider M, Desjardins B, et al. Computed tomographic analysis of the anatomy of the left atrium and esophagus: implications for left atrial catheter ablation. Circulation. 2004;110:3655-3660.
60 Good E, Oral H, Lemola K, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:2107-2110.
61 Sato T, Anno H, Kondo T, et al. Applicability of ECG-gated multislice helical CT to patients with atrial fibrillation. Circulation J. 2005;69:1068-1073.
62 Strub WM, Vagal A, Meyer C. Optimizing coronary artery imaging in patients with atrial fibrillation with ECG-gated 64 MDCT. AJR Am J Roentgenol. 2007;189:W50-W51.
63 Oncel D, Oncel G, Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology. 2007;245:703-711.
64 Wolak A, Gutstein A, Cheng VY, et al. Dual-source coronary computed tomography angiography in patients with atrial fibrillation: initial experience. J Cardiovasc CT. 2008;2:172-180.
65 Lacomis J, Schwarzman D, Fuhrman C, et al. Ablation imaging of left atrial and pulmonary venous anatomy in atrial fibrillation patients: comparison of 3D multidetector CT and magnetic resonance angiography. Radiology. 2002;S225:626.
66 Ghaye B, Szapiro D, Dacher JN, et al. Percutaneous ablation for atrial fibrillation: the role of cross-sectional imaging. RadioGraphics. 2003;23:S19-S33.
67 Singhal A, Tomasian A, Sassani A, et al. 3D noncontrast free breathing MR angiography (MRA) of pulmonary veins by steady state free precession (SSFP) technique. Annual Meeting of the American Roentgen Ray Society Meeting, Washington, DC, April 15, 2008.
68 Lickfett L, Dickfeld T, Kato R, et al. Changes of pulmonary vein orifice size and location throughout the cardiac cycle: dynamic analysis using magnetic resonance cine imaging. J Cardiovasc Electrophysiol. 1999;10:136-144.
69 Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart: in vitro and in vivo accuracy results. Circulation. 1997;95:1611-1622.
70 Takahashi Y, Rotter M, Sanders P, et al. Left atrial linear ablation to modify the substrate of atrial fibrillation using a new nonfluoroscopic imaging system. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S90-S93.
71 Schilling RJ, Peters NS, Davies DW. Feasibility of a noncontact catheter for endocardial mapping of human ventricular tachycardia. Circulation. 1999;99:2543-2552.
72 Dong J, Calkins H, Solomon SB, et al. Integrated electroanatomic mapping with three-dimensional computed tomographic images for real-time guided ablations. Circulation. 2006;113:186-194.
73 Sra J, Ratnakumar S. Cardiac image registration of the left atrium and pulmonary veins. Heart Rhythm. 2008;5:609-617.
74 Bertaglia E, Bransolino G, Zoppo F, et al. Integration of three-dimensional left atrial magnetic resonance images into a real-time electroanatomic mapping system: validation of a registration method. Pacing Clin Electrophysiol. 2008;31:273-282.
75 Dong J, Dalal D, Scherr D, et al. Impact of heart rhythm status on registration accuracy of the left atrium for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1269-1276.
76 Richmond L, Rajappan K, Voth E, et al. Validation of computed tomography image integration into the EnSite NavX mapping system to perform catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:821-827.
77 Zhong H, Lacomis JM, Schwartzman D. On the accuracy of CartoMerge for guiding posterior left atrial ablation in man. Heart Rhythm. 2007;4:595-602.
78 Dong J, Dickfeld T, Dalal D, et al. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:459-466.
79 Ector J, De Buck S, Adams J, et al. Cardiac three-dimensional magnetic resonance imaging and fluoroscopy merging: a new approach for electroanatomic mapping to assist catheter ablation. Circulation. 2005;112:3769-3776.
80 Fahmy TS, Mlcochova H, Wazni OM, et al. Intracardiac echo-guided image integration: optimizing strategies for registration. J Cardiovasc Electrophysiol. 2007;18:276-282.
81 Kistler PM, Earley MJ, Harris S, et al. Validation of three-dimensional cardiac image integration: use of integrated CT image into electroanatomic mapping system to perform catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:341-348.
82 Sra J, Krum D, Hare J, et al. Feasibility and validation of registration of three-dimensional left atrial models derived from computed tomography with a noncontact cardiac mapping system. Heart Rhythm. 2005;2:55-63.
83 Sra J, Krum D, Malloy A, et al. Registration of three-dimensional left atrial computed tomographic images with projection images obtained using fluoroscopy. Circulation. 2005;112:3763-3768.
84 Noseworthy PA, Malchano ZJ, Ahmed J, et al. The impact of respiration on left atrial and pulmonary venous anatomy: implications for image-guided intervention. Heart Rhythm. 2005;2:1173-1178.
85 Kistler PM, Rajappan K, Jahngir M, et al. The impact of CT images integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:1093-1101.
86 Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100-1105.
87 Lacomis JM, Goitein O, Deible C, et al. CT of the pulmonary veins. J Thorac Imaging. 2007;22:63-76.
88 Pappone C, Oral J, Santinelli V, et al. Atrioesophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724-2726.
89 Schmidt M, Nolker G, Marschang H, et al. Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace. 2008;10:205-209.
90 Schley P, Gulker H, Horlitz M. Atrio-esophageal fistula following circumferential pulmonary vein ablation: verification of diagnosis with multislice computed tomography. Europace. 2006;8:189-190.
91 Chiang SJ, Tsao HM, Wu MH, et al. Anatomic characteristics of the left atrial isthmus in patients with atrial fibrillation: lessons from computed tomographic images. J Cardiovasc Electrophysiol. 2006;17:1274-1278.
92 Takahashi Y, Jais P, Hocini M, et al. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol. 2005;16:1104-1107.
93 Chae S, Oral H, Good E, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50:1781-1787.
94 Reddy VY, Schmidt EJ, Holmvang G, et al. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J Cardiovasc Electrophysiol. 2008;19:434-437.
95 Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690-695.

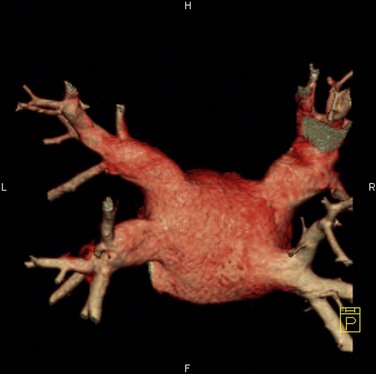
 FIGURE 31-2
FIGURE 31-2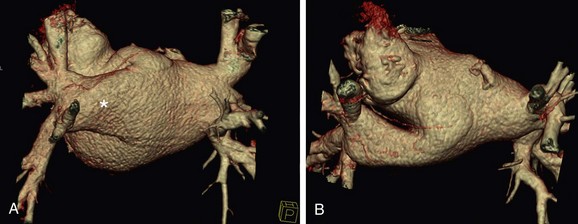
 FIGURE 31-3
FIGURE 31-3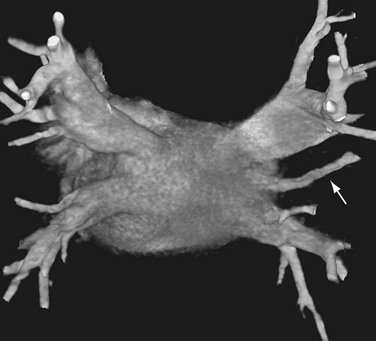
 FIGURE 31-4
FIGURE 31-4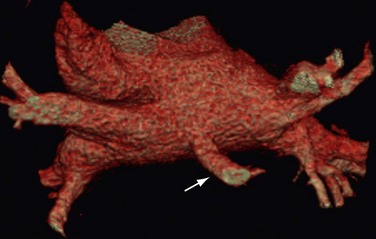
 FIGURE 31-5
FIGURE 31-5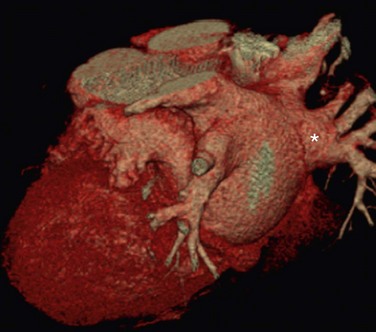
 FIGURE 31-6
FIGURE 31-6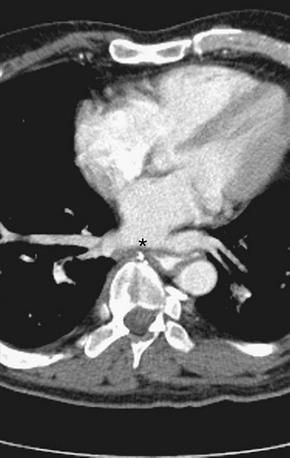
 FIGURE 31-7
FIGURE 31-7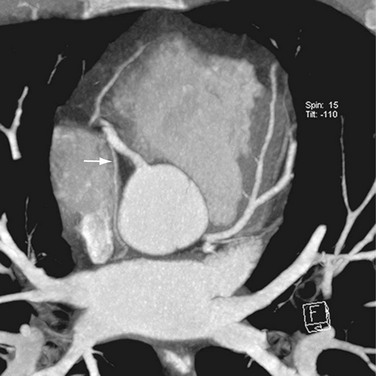
 FIGURE 31-8
FIGURE 31-8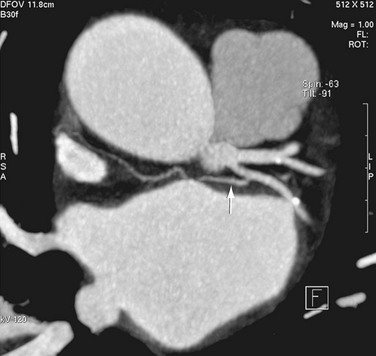
 FIGURE 31-9
FIGURE 31-9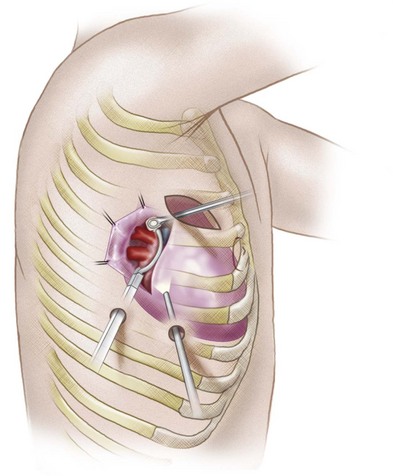
 FIGURE 31-10
FIGURE 31-10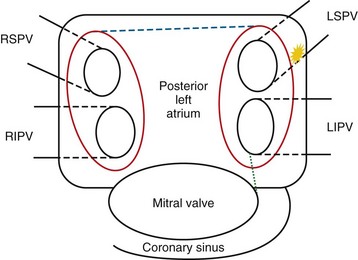
 FIGURE 31-11
FIGURE 31-11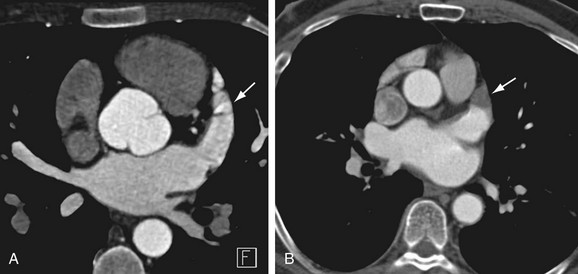
 FIGURE 31-12
FIGURE 31-12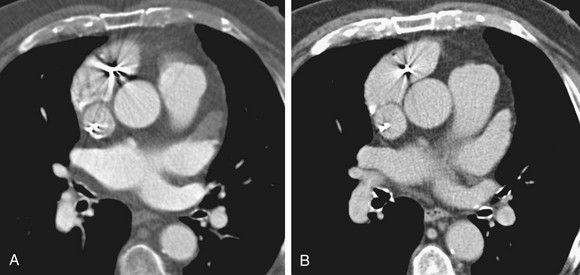
 FIGURE 31-13
FIGURE 31-13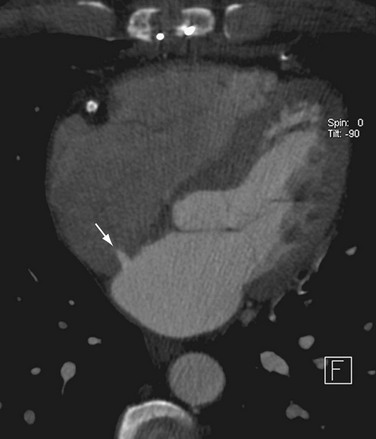
 FIGURE 31-14
FIGURE 31-14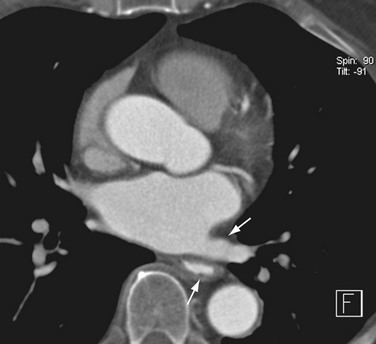
 FIGURE 31-15
FIGURE 31-15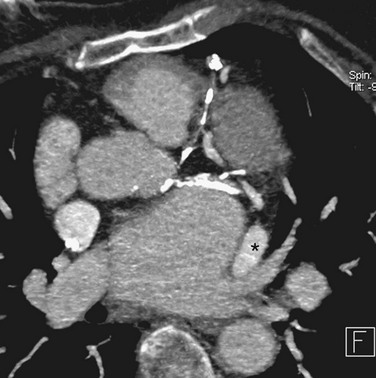
 FIGURE 31-16
FIGURE 31-16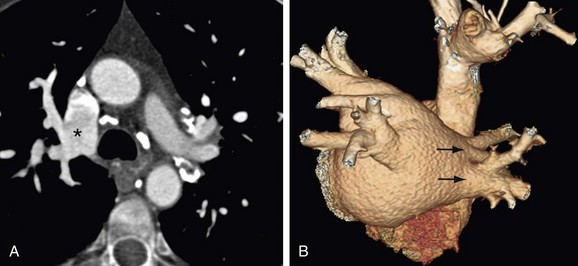
 FIGURE 31-17
FIGURE 31-17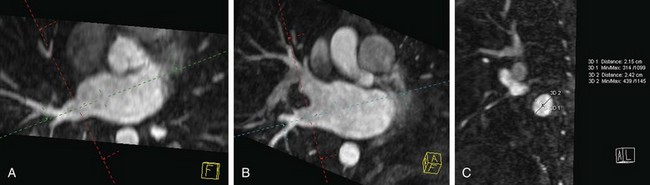
 FIGURE 31-18
FIGURE 31-18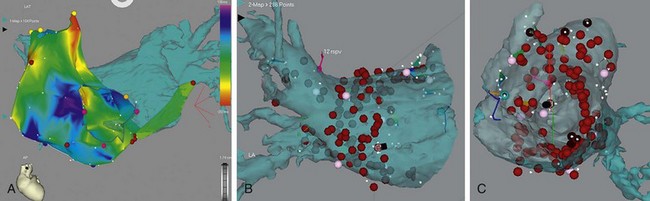
 FIGURE 31-19
FIGURE 31-19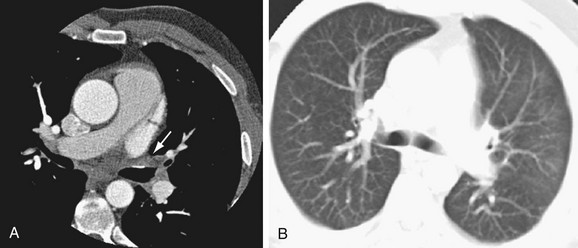
 FIGURE 31-20
FIGURE 31-20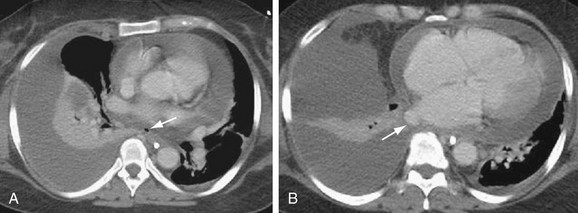
 FIGURE 31-21
FIGURE 31-21



