Chapter 26 Imaging in Women
INTRODUCTION
Despite recent declines in the rates of cardiovascular deaths in women, cardiovascular disease (CVD) is the leading cause of mortality in women.1 In the United States, more than 500,000 women each year will die of CVD, mostly coronary artery disease (CAD), more than in men (Fig. 26-1).1 This is not just a disease of aging women. Ischemic heart disease is the number one killer of women at all ages, and the mortality for younger women is greater than for men. Women younger than age 75 have higher in hospital mortality post myocardial infarction (MI), and until the age of 60 have higher 2-year post-MI mortality.2,3 There is a gap between these staggering statistics and a woman’s perception of her own health risk. With campaigns aimed at increasing awareness of the risk of heart disease in women, the recognition of heart disease as a women’s issue has improved, but in an American Heart Association (AHA) survey, only 13% of women identified CVD as their own greatest risk.4 Only 38% of these women reported that they had had a discussion with their physician about their risk for CVD, thus providing an opportunity for education and assessment.4
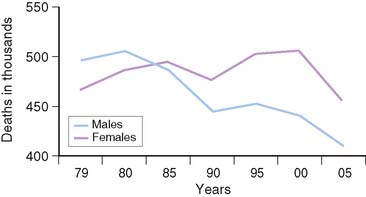
Figure 26-1 Cardiovascular disease mortality trends for males and females, United States (1979–2005).
(From NCHS and NHLBI.)
Women often experience typical symptoms of CAD, but there are gender differences in both how women present with acute coronary syndromes and the symptoms they report. Despite the fact that women present more commonly with unstable angina than with ST elevation MI, they have a higher mortality than men.5–9 Women appear to have less plaque rupture leading to ST elevation MI, and more plaque erosion resulting in unstable angina. In autopsy studies, women have less evidence of obstructive disease until the 7th decade of life.10 Two-thirds of women have fatal MI without recognized prodromal symptoms as their initial presentation of CAD.1 There is evidence that there are gender differences in pain perception, and this may contribute to the lack of specificity of typical anginal symptoms in women.11,12 Contributing to the challenge in diagnosing CAD in women is the variability in the reporting of chest pain as the predominant symptom in women who present with CAD. In one study, Milner et al. reviewed 550 individuals (41% women) who had evidence of ischemia or MI and found that both men and women reported chest pain with equal frequency.13 Women, however, were more likely to report an increased number of symptoms that were atypical, such as mid back pain, nausea, vomiting, dyspnea, palpitations, and indigestion. This increased symptomatology likely contributes to the difficulty in obtaining an accurate diagnosis of CAD in women. Other studies support the higher frequency of atypical symptoms and less chest pain prior to presentation as well as at the time of diagnosis. Women were more likely to describe back pain, jaw pain, rest pain, pain related to mental stress, and pain that awoke them from sleep.14–16 In a recent study, 515 women were surveyed 4 to 6 months post MI, and 43% did not describe any chest discomfort at the time of their MI.17 They did, however, report atypical prodromal symptoms, including unusual fatigue, sleep disturbance, and shortness of breath during the month before their MI, with only a minority reporting chest discomfort (29.7%).17 This variation in presentation and symptomatology has contributed to the difficulty in diagnosing heart disease in women and missed opportunities to diagnose and treat women with CAD.
Traditional risk factors for CAD are similar in men and women, but certain risk factors such as dyslipidemia and the presence of diabetes play a more important role in women. Elevated LDL levels play a central role in the development of CAD in both men and women, as evidenced by the linear relationship between LDL levels and risk for CAD. This is particularly true in women younger than 65 years of age.18–20 For women, the risk associated with elevated triglycerides has been shown to be an independent predictor of risk for CAD.21 Additionally, low HDL levels in women convey a greater risk than in men. For every 1 mg/dL increase in HDL, the risk of CAD decreases 3% in women, compared to 2% in men.22 Notably, the risk for death from CAD was more than two times higher in women with HDL levels below 50 mg/dL compared to those with levels above 60 mg/dL.18 Postmenopausal lipid levels are more atherogenic, with higher LDL and triglyceride levels and lower HDL levels.23,24
Although diabetes has a prevalence of 5% to 10% in both men and women, the risk for CVD is markedly higher in women. In a 20-year follow-up of the Framingham data, nondiabetic women had half the mortality rate of men.25 Those with diabetes had death rates equal to men, a disproportionate increase in mortality.
Metabolic syndrome, characterized by central obesity, hypertension, impaired glucose tolerance, low HDL-C, and elevated triglycerides affects almost a quarter of U.S. women.26 This dysmetabolic state increases the risk for the development of atherosclerosis and subsequent cardiovascular events.27 In women with suspected ischemia, Marroquin and investigators for the Women’s Ischemia Syndrome Evaluation (WISE) showed that there was a greater burden of atherosclerotic disease in those with metabolic syndrome compared to normal-metabolic women with angiographic evidence of disease (Fig. 26-2).27 This evidence illustrates the increased risk associated with the dysmetabolic state and alerts us to focus on preventive strategies in women at risk.
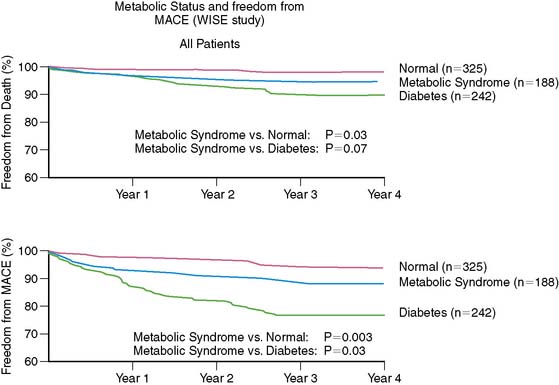
Figure 26-2 Event-free survival by metabolic status.
(From Marroquin OC, Kip KE, Kelley DE, et al., for the Women’s Ischemia Syndrome Evaluation Investigators: Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: A report from the Women’s Ischemia Syndrome Evaluation, Circulation 109:714–721, 2004.)
While public awareness regarding the detrimental effects of tobacco appears to be increasing, the Surgeon General reported in 2001 that the overall rate of cigarette use in women is on the rise, particularly in younger, less educated women.28 The Nurses’ Health Study showed increased cardiovascular mortality with cigarette use, which declined after 10 years with smoking cessation.29,30 Age-adjusted mortality rates were substantially increased in women with diabetes who also smoked. In comparison to nonsmokers, the relative risk (RR) of smokers of 1 to 14 cigarettes a day was 0.92.29 This risk increased in a dose-dependent manner to an RR of 1.95 for those with a history of over 50 pack-years.29 For diabetic patients who smoked more than 15 cigarettes a day, the RR of a cardiovascular event was 7.67.30
DIAGNOSING CORONARY ARTERY DISEASE IN WOMEN
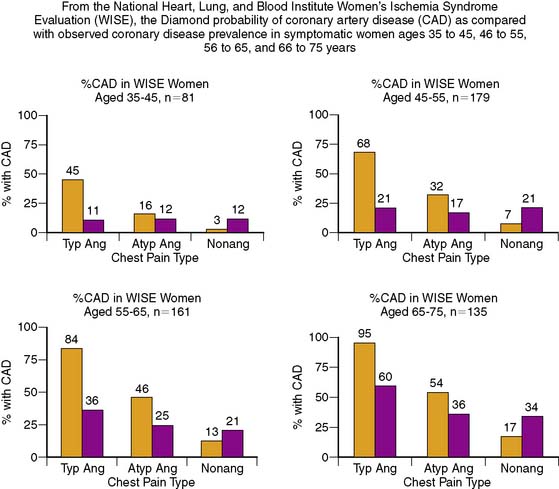
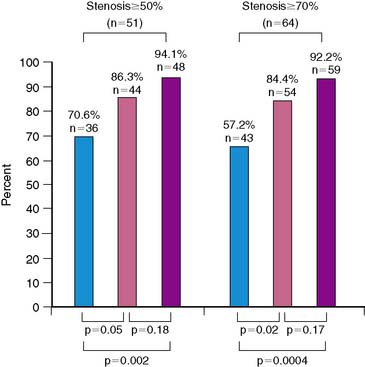
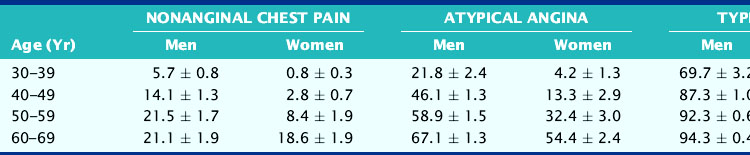
Diagnosis of CAD can be challenging in women, given the lower prevalence of obstructive disease, greater symptomatology, and lower functional capacity. Establishing the pretest likelihood of disease in a women is key in the diagnosis of CAD, balancing the probability of false positives when the disease prevalence is low with the need to avoid unnecessary additional and/or invasive testing. Diamond and Forrester analyzed the pretest probability of CAD as well as sensitivity and specificity in patients undergoing exercise electrocardiography.31 The prevalence of coronary disease by angiography was stratified into asymptomatic, nonanginal, atypical, and typical angina categories. Table 26-1 demonstrates interaction of gender and age with type of symptoms. Using this type of strategy can help further refine the likelihood of disease and hence direct the most appropriate testing, such as cardiac catheterization for high-risk individuals and exercise tolerance testing for low-risk individuals. In the WISE study, this strategy was found to overestimate the degree of obstructive disease (Fig. 26-3).32
Table 26-1 The Effect of Age and the Presence of Symptoms on the Probability of Underlying Coronary Artery Disease in Men and Women
There is growing recognition that women can have anginal symptoms, evidence of ischemia by exercise or noninvasive testing, but no evidence of obstructive disease at the time of cardiac catheterization. In the WISE study, women who had evidence of ischemia on phosphorus-31 nuclear magnetic resonance spectroscopy stress testing and no evidence of obstructive CAD had higher rates of hospitalization for anginal symptoms, repeat catheterization, and overall costs.32 Additional information from the WISE study showed that coronary microvascular dysfunction is seen in almost half of women with chest pain and nonobstructive disease.33 This group of women present a challenge in both diagnosis and treatment.
Exercise Tolerance Testing
In symptomatic women in whom the presence of CAD is uncertain, exercise tolerance testing (ETT) is the simplest and least expensive testing modality. In certain populations, such as women with a low likelihood of CAD and low disease prevalence, this test is appropriate and is supported by current guidelines. In this group, a negative exercise electrocardiographic response with normal hemodynamics has been associated with a high negative predictive value.34
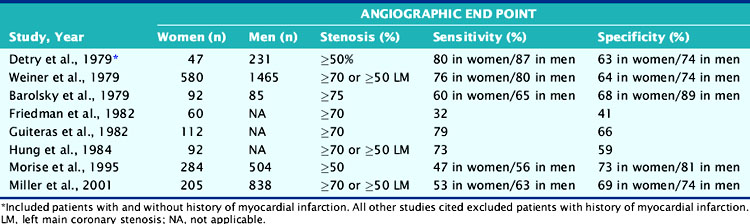
A major limitation of ETT is its compromised diagnostic accuracy (Table 26-2). A meta-analysis reported by Kwok and colleagues reported 15 studies with a sensitivity of 61% (range, 46% to 79%) and specificity of 69% (range, 51% to 86%).35 These testing results suggest a limited value of ETT alone in the appropriate diagnosis of CAD in women. Additionally, the increased age in women on presentation is concomitant with functional impairment. This results in reduced exercise capacity and inability to complete a diagnostic stress test. Other factors contribute to ETT being suboptimal: resting ST-T wave changes in hypertensive women, lower electrocardiogram (ECG) voltage, and hormonal factors.36–41 For example, endogenous estrogen has a digoxin-like effect, resulting in a false-positive ECG response, particularly midcycle when estrogen levels are highest.42 In an effort to determine the most cost-effective way to identify women at high risk for CVD, the What is the Optimal Method for Ischemia Evaluation in Women (WOMEN) Study is being conducted with intermediate- and high-risk women with chest pain or equivalent symptoms suggestive of ischemic heart disease. The study’s objective is to determine whether exercise ECG has the same negative predictive value for risk detection as gated myocardial perfusion single-photon emission computed tomography (SPECT) in women.43 Currently, use of the Framingham Risk Score (FRS) to predict the presence of disease may underestimate the degree of atherosclerosis. In the Multi-Ethnic Study of Atherosclerosis (MESA), 32% of women classified as low risk by FRS had evidence of coronary artery calcium indicative of atherosclerotic disease.44 These women also had higher rates of coronary artery disease and cardiovascular events.
Table 26-2 Sensitivity and Specificity of Exercise Tolerance Testing in the Diagnosis of Coronary Artery Disease
Women with diabetes are of particular concern. It has been recognized that such patients are at heightened risk for premature as well as accelerated atherosclerosis, MI, and cardiac death.1 It has also been reported that ECG is less reliable in diabetic patients,45 so in diabetic women, ETT alone may be particularly misleading (see Chapter 22).
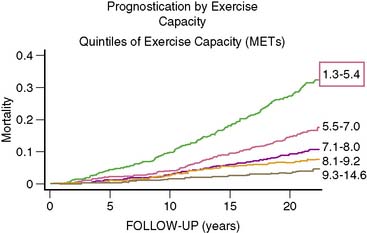
Despite these limitations, if exercise testing is to be used in women, interpretation of the test should include factors in addition to ST-T segment depression.46,47 This should include parameters such as changes in ST/heart rate and the Duke Treadmill Score. Despite the described limitations, it should be also noted that the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines recommend exercise testing in women with intermediate likelihood of CAD.48 Generally, optimal diagnostic stress testing is assumed when a patient achieves 85% or greater of predicted maximal heart rate. However, in deconditioned women, a hyperexaggerated response may yield a rapid and marked increase in heart rate. The test should be continued, and if a female patient cannot achieve at least 5 metabolic equivalents (METs) of exercise, they should be considered a candidate for pharmacologic myocardial perfusion imaging.38 Women with lower functional capacity have been found to have higher prevalence of cardiovascular risk factors, angiographic evidence of CAD, and adverse events compared to those with higher functional capacity.49 Functional capacity is associated with better prognostic value than electrocardiographic evidence of ischemia.50 Exercise capacity is an important predictor of cardiovascular outcome in women, and unless functional capacity is significantly limited, all stress testing should be done with exercise (Fig. 26-4).38
Stress Myocardial Perfusion Imaging in Women
Stress myocardial perfusion imaging (MPI) was first developed to offset the limitations of ETT in the accurate diagnosis and location of CAD in patients. Using exercise as the stress modality, radionuclide perfusion imaging with thallium (Tl)-201 has been shown to have on average a sensitivity of 83% and specificity of 88% using planar imaging.51 This represents approximately 20% to 25%, improvement in diagnostic accuracy in comparison to ETT alone. However, a considerable number of laboratories are now using SPECT and technetium (Tc)-99m-based imaging agents. SPECT imaging studies have been shown to be more accurate than planar imaging in the diagnosis of CAD and in separating single-vessel from multivessel disease.51
Of considerable importance, Stratman and colleagues demonstrated for all levels of exercise that Tc-99m-sestamibi SPECT imaging was significantly more sensitive in the detection of CAD than ECG with exercise data alone.52 Several studies have noted improved diagnosis of multivessel disease in comparison with planar methods. Unfortunately, few studies are available in women alone.
Pharmacologic Myocardial Perfusion Imaging (See Chapter 15)
An important advantage of MPI is the ability to assess patients unable to complete adequate exercise. For such patients unable to achieve 85% maximally predicted heart rate, the diagnostic utility of the exercise ECG falls precipitously.53–54 In such circumstances, MPI with pharmacologic stress provides an important and diagnostically useful alternative (see Chapter 14).
It has been estimated that 35% to 40% of all stress MPI is performed with pharmacologic stress. The most common pharmacologic type is that of vasodilator stress with either dipyridamole or adenosine. Pharmacologic vasodilator stress MPI has been shown to be comparable between dipyridamole and adenosine using either planar or SPECT imaging.55 Similar results have been reported with Tc-99m imaging agents.56
For women, Amanullah and colleagues found high sensitivity and moderate specificity ranges with adenosine in women despite their symptom complex. The sensitivity and specificity in nonanginal versus anginal symptoms was 93% and 69% versus 92% and 83%, respectively.56 They then compared sensitivity and specificity with respect to pretest probability of CAD. The sensitivity ranged from 82% and increased to 95% with a high-likelihood status. Given the fact that woman’s symptoms are more difficult to decipher clinically, these data support the use of adenosine MPI in evaluating symptomatic patients for CAD. Studies using another vasodilator, dipyridamole, have shown a sensitivity of 87% in women for detecting single-vessel disease.55 A retrospective review of women and men who underwent dipyridamole with 99mTc-sestamibi and cardiac catheterization surprisingly demonstrated an improved sensitivity for detecting left anterior descending (LAD) disease in women compared to men.57 These findings suggest the diagnostic accuracy of pharmacologic stress MPI is high regardless of the agent and should be used in women unable to exercise.
Dobutamine is an alternative for women with contraindications to vasodilator stress (reactive airway disease). This agent depends on a chronotropic response, and data suggest a similar accuracy to vasodilators. However, as with exercise, the accuracy of dobutamine is heart-rate dependent.58 In general, then, both exercise and pharmacologic stress are superior to exercise ECG alone. In a review of the literature, Leppo found the diagnostic accuracy, including dobutamine, to be equivalent.59
GENDER-RELATED CHALLENGES IN THE DIAGNOSIS OF CORONARY ARTERY DISEASE
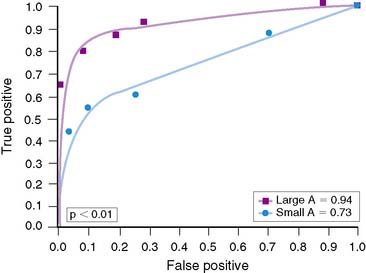
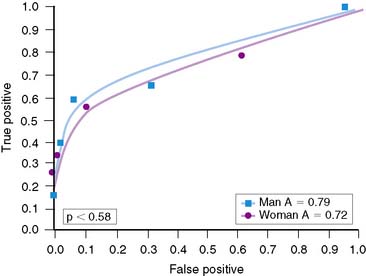
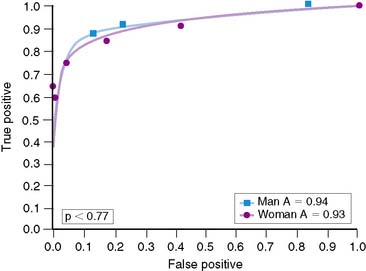
Overall, accuracy with exercise MPI in women appears to be similar to that in men.37,38 However, the identification of single-vessel CAD in women may be lower than in men.60 Reasons for this are not clear, although the difference may relate to heart size. Hansen and colleagues analyzed men and women who underwent exercise 201Tl stress tests.60 Women were statistically more likely to have smaller chamber sizes. When chamber volumes were compared, larger chamber sizes correlated with better diagnostic accuracy (Fig. 26-5). When men and women with smaller chamber sizes were compared, diagnostic accuracy was similar but less than in those with larger chambers (Figs. 26-6 and 26-7). Whether there is a true gender difference within the smaller heart subset is unclear, owing to fewer numbers of men with small hearts. Speculation by the authors was made regarding the oversight of a lesion in a smaller heart, inasmuch as it may be less likely to be visualized in a small heart compared to a large heart. Thus, heart size may be a factor in diagnostic accuracy and appears to be an issue more frequently in women. Regardless of this limitation, data have shown a higher accuracy of exercise MPI for single-vessel disease over exercise alone.37
Attenuation artifact is a critical issue in the accurate diagnosis of CAD in patients, especially women. It is well recognized that attenuation artifact in nuclear cardiology procedures is commonplace, affecting overall accuracy and primary specificity. Common areas of attenuation artifact are in the anterior and inferior walls (see Chapter 5). Attenuation artifact is often observed equally in stress and rest images, but reversible attenuation is also possible. For women, attenuation artifact is of considerable importance because it can commonly occur in the anterior wall from breast, as well as the inferior wall for multiple reasons, including pharmacologic stress (liver). Prior to the past several years, this resulted in reduced specificity, even in comparison with stress echocardiography.35
A meta-analysis compared exercise-ECG stress MPI and stress echo in 21 studies involving 4113 women.35 While stress MPI was clearly superior to ETT alone, a reduced specificity in relation to stress echocardiography was suggested. Of importance, the perfusion studies included in the analysis were antiquated, not incorporating modern approaches to resolve attenuation artifact. It does point out the necessity of resolving attenuation artifact using appropriate and often newer techniques.
Solutions to attenuation artifact have centered on three approaches: prone imaging, ECG-gated SPECT imaging, and attenuation correction. The first, prone imaging, requires acquisition of SPECT perfusion data while the patient is in a prone position, and data are compared to supine imaging. The assumption is that inferior attenuation from diaphragm will be noted in the supine position “normalized” by prone imaging. Recent documentation supports this claim,61 although it is unclear whether this can be applied to attenuation artifact from other sources such as liver or breast. It is theoretically valuable for both fixed or reversible attenuation artifacts in the inferior wall. It is also not clear how often an incomplete solution results from prone imaging.
ECG-gated SPECT imaging has also been used to distinguish attenuation artifact from CAD. Applied to fixed defects only, it is assumed that if wall motion is normal in the area of the perfusion abnormality, the perfusion abnormality is due to attenuation artifact. Conversely, if wall motion is abnormal, this is consistent with CAD, either stunned myocardium or prior MI. Taillefer and colleagues demonstrated the improved specificity in diagnosing coronary disease in women, utilizing gating to help differentiate attenuation from stunned or scarred myocardium.40 In addition, two other studies involving 170 patients have confirmed the value of this technique in women.34,52 In the study by Taillefer et al., the specificity of gated SPECT imaging was 92%, compared with 67% with nongated thallium studies in the same patients.40 These results were both higher than the confidence intervals in regard to the specificity in the previously mentioned meta-analysis. This supports the enhanced diagnostic accuracy of gated SPECT imaging in women (Fig. 26-8).
A more recent approach to attenuation artifact uses attenuation correction with transmission line sources (usually gadolinium-100; see Chapters 6 and 7). This solution differs from ECG-gated SPECT in that both fixed and reversible attenuation artifact can be evaluated. Recent data from Bateman et al. and Links et al. demonstrated improved specificity in comparison with ECG-gated SPECT imaging in both genders.62,63 Bateman reported an improved specificity for both genders and an improved normalcy rate in women.63
In summary, the use of stress MPI for the diagnosis of CAD in women is very important and provides substantially higher accuracy than ETT alone.64 Using pharmacologic stress in appropriate patients is of considerable value in women, given the fact that CAD is more prevalent in a population less likely to complete adequate exercise protocols. One limitation in comparison to men may be the ability to identify single-vessel disease in women, although further studies are necessary. Finally, attenuation artifact is a very important issue, and such solutions as ECG-gated SPECT imaging or attenuation correction are extremely important to use when assessing female patients. A very useful algorithm for the evaluation of women with suspected or known coronary disease is illustrated in Figure 26-9.38
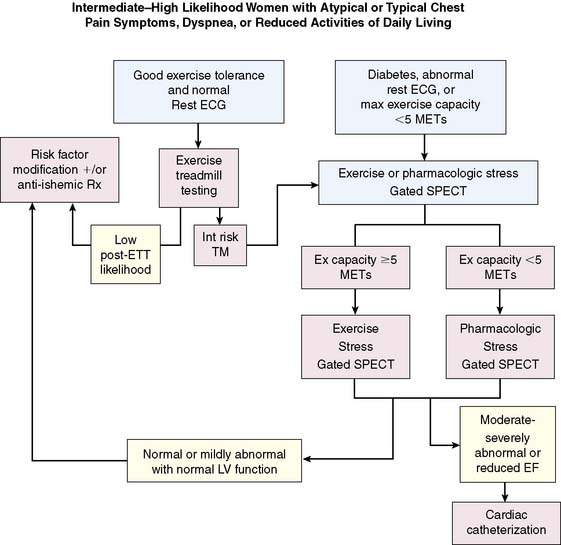
Figure 26-9 Recommended algorithm for the evaluation of women with suspected or known coronary disease.
(From Mieres JH, Shaw LJ, Hendel RC, et al: A report of the American Society of Nuclear Cardiology Task Force on Women and Heart Disease [writing group on perfusion imaging in women], J Nucl Cardiol 10:95–101, 2003. Reprinted with permission from the American Society of Nuclear Cardiology.)
RISK STRATIFICATION IN WOMEN (See Chapter 16)
The previous sections presented evidence that stress MPI, particularly with modern techniques like ECG-gated SPECT imaging and attenuation correction, can provide high diagnostic accuracy. Of equal importance is the ability to categorize a patient’s risk of cardiac events such as nonfatal MI or cardiac death. MPI results have been shown in multiple investigations including over 22,000 patients to provide a powerful role in risk stratification (see Chapters 15 and 16). Data suggest that patients with normal stress MPI, particularly with exercise as the stress, have a low risk of cardiac events.65,66 This risk is essentially the same as a normal population. Conversely, patients with abnormal studies65 have a 12-fold higher annualized risk of a cardiac event69 based on a review of 14 studies (Fig. 26-10). The general concept proposed is the size and severity of the perfusion abnormality is also related to risk of coronary events (Figs. 26-11 and 26-12).67–70 Cardiac survival is also predicted by the number of ischemic vascular territories (Fig. 26-13).70,72 These findings may help direct therapies. For example, those with normal studies most likely do not need further testing unless symptoms continue. Patients with low-risk images (one-vessel or mild perfusion abnormalities) may be adequately treated with aggressive medical therapies. An analysis by O’Keefe and colleagues supports this position, demonstrating excellent outcomes in patients with low-risk scans being treated solely with medical approaches.73 Finally, patients with large or severe abnormalities are candidates for both revascularization and aggressive medical therapies.
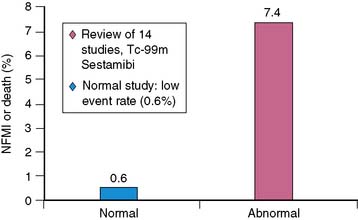
Figure 26-10 Prognostic significance of a normal study.
(From Iskander S, Iskandrian AE: Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging, J Am Coll Cardiol 32:57–62, 1998.)
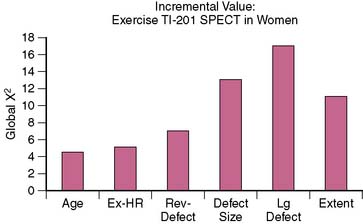
Figure 26-11 Incremental value of exercise thallium-201 SPECT in women.
(From Pancholy SB, Fattah AA, Kamal AM, et al: Independent and incremental prognostic value of exercise thallium single-photon emission computed tomographic imaging in women, J Nucl Cardiol 2(2 Pt 1):110–116, 1995.)
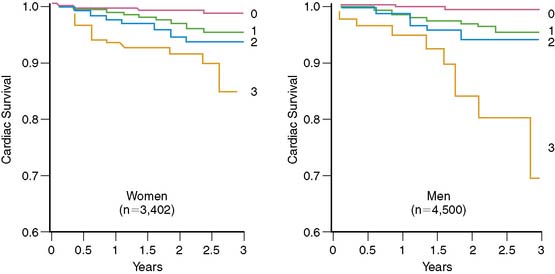
Figure 26-12 Cardiac survival by number of SPECT vascular territories with ischemia in women and men.
(From Marwick TH, Shaw LJ, Lauer MS, et al: The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group, Am J Med 106:172–178, 1999.)
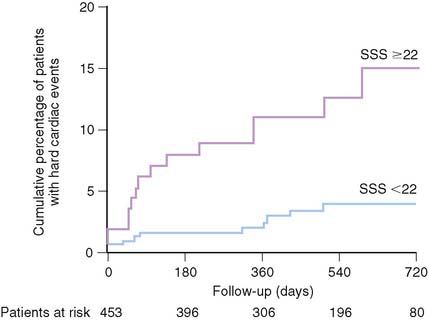
Figure 26-13 Relationship between extent of ischemia and cardiac events.
(From Ladenheim ML, Pollock BH, Rozanski A, et al: Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease, J Am Coll Cardiol 7:464–471, 1986.)
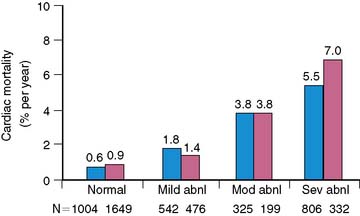
The prognostic value of stress MPI has also been accumulating for women. Data in over 8000 women suggest the cardiac event rate in patients with normal stress myocardial perfusion results is less than 1%.71,74–80 This low event rate has been found even in patients in whom a high pretest likelihood of CAD is present. Similarly, the revascularization in women with a normal perfusion study was very low. Similar to the general population, the size and severity of the perfusion abnormality also is associated with an increase in the higher cardiac event rate.71,74–80 This appears to be true even in patients with a high pretest likelihood. As the extent of the perfusion abnormality increases, the rates for both MI and cardiac death increase four- to sixfold. In women, both the size of the defect (summed stress score [SSS] > 22) and ejection fraction (EF) (<52%) are predictive of cardiac death, MI, or ventricular fibrillation (see Fig. 26-13).81 An SSS of higher than 14 was predictive of all cardiac events, including revascularization, in a series of 453 consecutive female patients. Additional data from nearly 900 patients referred for SPECT further support the prognostic role of EF in women, with high event rates occurring in those with lower EFs.82 Women with hard events had larger end-systolic volume index (63 versus 35 mL/m2) and end-diastolic volume index (106 versus 76 mL/m2) compared to men, as well as lower EFs (<44%).82 Referral for catheterization appears to have shifted more recently and appears to be based on imaging results rather than solely upon the gender of the patient.79
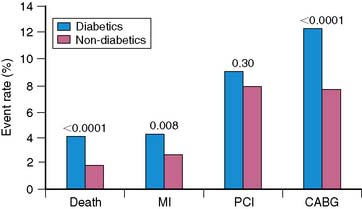
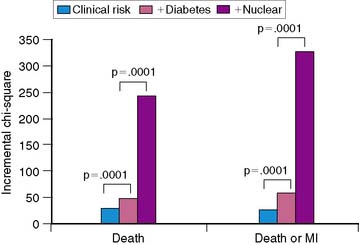
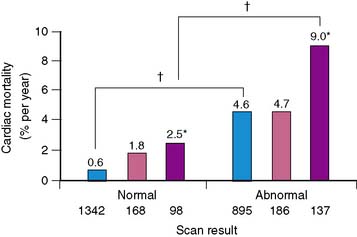
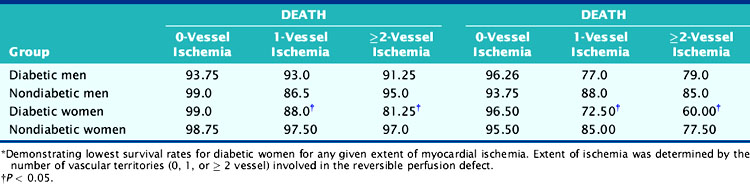
Women with diabetes mellitus constitute a very high-risk population, even in comparison to men with the same disorder.83–85 Several studies have now demonstrated the prognostic value of SPECT MPI (Fig. 26-14).73,86,87 Given the severity of perfusion abnormality, the presence of diabetes mellitus was associated with a significantly higher event rate when compared with men (Table 26-3 and Fig. 26-15).87 In this multivariable analysis, gender was no longer an independent predictor of coronary events, whereas severity of the perfusion abnormality was. In addition to the clinical risk assessment and the presence of diabetes mellitus, the addition of nuclear imaging added significantly to risk stratification (Fig. 26-16). Beyond the presence or absence of diabetes mellitus, the type of diabetes also played a role in the overall prognosis (Fig. 26-17).86
Table 26-3 Kaplan-Meier 3-Year Survival Rate for Patients With and Without Diabetes Stratified by Gender*
1. American Heart Association and National Center on Health Statistics. Heart Disease and Stroke Statistics–2004 Update. Dallas, TX: National Heart, Lung and Blood Institute, 2004.
2. Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ: Sex differences in 2-year mortality after hospital discharge for myocardial infarction, Ann Intern Med 134:173–181
3. Robinson K., Conroy R.M., Mulcahy R., Hickey N. The 15-year prognosis of a first acute coronary episode in women. Eur Heart J. 1992;13:67-69.
4. Mosca L., Ferris A., Fabunmi R., Robertson R.M. Tracking women’s awareness of heart disease: An American Heart Association National Study. Circulation. 2004;190:573-579.
5. Lerner D.J., Kannel W.B. Patterns of coronary artery disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383-390.
6. Hochman J.S., McCabe C.H., Stone P.H., et al. Outcome and profile of women and men presenting with acute coronary syndromes: A report from TIMI IIIB. J Am Coll Cardiol. 1997;30:141-148.
7. Hochman J.S., Tamis J.E., Thompson T.D., et al. Sex, clinical presentation and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341: 226-2321.
8. Marrugat J., Sala J., Malia R., et al. Mortality differences between men and women following first myocardial infarction. JAMA. 1998;2801:1405-1409.
9. Chang W.C., Kaul P., Westerhout C.M., et al. Impact of sex on long-term mortality from acute myocardial infarction vs unstable angina. Arch Intern Med. 2003;163:2476-2484.
10. Shaw L.J., Shaw R.E., Radford M., et alfor the ACC-National Cardiovascular Data Registry. Sex and ethnic differences in the prevalence of significant and severe coronary artery disease in the ACC-National Cardiovascular Data Registry. Circulation. 110, 2004. SIII800
11. Rollman G.B., Lauterbacher S., Jones K.S: Sex and gender differences in responses to experimentally induced pain in humans. In Fillingham R.B., editor. Sex, Gender and Pain, vol 17. Seattle: International Association for the Study of Pain, 2001.
12. Sheps D.S., McMahon R.P., Light K.C., et al. Low hot pain threshold predicts shorter time to exercise-induced angina: results from the psychophysiological investigations of myocardial ischemia (PIMI) study. J Am Coll Cardiol. 1999;33:1855-1862.
13. Milner K.A., Funk M., Richards S., Wilmes R.M., Vaccarino V., Krumholz H.M. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399.
14. Culic V., Eterovic D., Dinko M., Rumboldt Z., Izet H. Gender differences in triggering of acute myocardial infarction. Am J Cardiol. 2000;85:753-756.
15. Goldberg R.J., O’Donnell C., Yarzebski J., Bigelow C., Savageau J., Gore J.M. Sex differences in symptom presentation associated with acute myocardial infarction: A population-based perspective. Am Heart J. 1998;136:189-195.
16. Kudenchuk P.J., Maynard C., Martin J.S., Wirkus M., Weaver W.D. Comparison of presentation, treatment and outcomes of acute myocardial infarction in men versus women (the Myocardial Infarction Triage and Intervention Registry). Am J Cardiol. 1996;78:9-14.
17. McSweeney J.C., Cody M., O’Sullivan P., Elberson K., Moser D.K., Garvin B.J. Women’s early warning symptoms of acute myocardial infarction. Circulation. 2004;108:2619-2623.
18. Manolio T.A., Pearson T.A., Wenger N.K., Barrett-Connor E., Payne G.H., Harlan W.H. Cholesterol and heart disease in older persons and women: Review of an NHLBI workshop. Ann Epidemiol. 1992;2:161-176.
19. Stamier J., Wentworth D., Neaton J.D. for the MRFIT Research Group: Is relationship between serum cholesterol and risk for premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256:2823-2828.
20. Gordon T., Castelli W.P., Hjortland M.C., Kannel W.B., Dawber T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707-714.
21. Castelli W.P. Cholesterol and lipids in the risk of coronary artery disease- the Framingham Heart Study. Can J Cardiol. 1988;4:5A-10A.
22. Gordon D.J., Probstfield J.L., Barrison R.J., et al. High-density lipoprotein cholesterol and cardiovascular disease in men and women. Four prospective American studies. Circulation. 1989;79:8-15.
23. Stevenson J.C., Crook D., Godsland I.F. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83-90.
24. Jenner J.L., Ordovas J.M., Lamon-Fava S., et al. Effects f age, sex and menopausal status on plasma lipoprotein(a) levels. The Framingham Offspring Study. Circulation. 1993;87:1135-1141.
25. Hu F.B., Stampfer M.J., Solomon C.G., et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women. Arch Intern Med. 2001;161:1717-1723.
26. Ford E.S., Giles W.H., Dietz Wh. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356-359.
27. Marroquin O.C., Kip K.E., Kelley D.E., et al. for the Women’s Ischemia Syndrome Evaluation Investigators: Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: A report from the Women’s Ischemia Syndrome Evaluation. Circulation. 2004;109:714-721.
28. Survey by ADA and AHA, 2001.
29. Al Delaimy W.K., Willett W.C., Manson J.E., et al. Smoking and mortality among women with type 2 diabetes: The Nurses’ Health Study cohort. Diabetes Care. 2001;24(12):2043-2048.
30. Al Delaimy W.K., Manson J.E., Solomon C.G., et al. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med. 2002;162(3):273-279.
31. Diamond G.A., Forrester J.S. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350-1358.
32. Shaw L.J., Bairey Merz C.N., Pepine C.J., et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4-S20.
33. Reis S.E., Holubkov R., Conrad Smith A.J., et al. Coronary microvascular dysfunction in women is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735-741.
34. Santana-Boado C., Candell-Riera J., Castell-Conesa J., et al. Diagnostic accuracy of technetium-99m-MIBI myocardial SPECT in women and men [commentary]. J Nucl Med. 1998;39(5):751-755.
35. Kwok Y., Kim C., Grady D., et al. Meta-analysis of exercise testing to detect coronary artery disease in women [commentary]. Am J Cardiol. 1999;83(5):660-666.
36. Arruda-Olson A.M., Juracan E.M., Mahoney D.W., et al. Prognostic value of exercise echocardiography in 5,798 patients: Is there a gender difference? J Am Coll Cardiol. 2002;39(4):625-631.
37. Friedman T.D., Greene A.C., Iskandrian A.S., et al. Exercise thallium-201 myocardial scintigraphy in women: Correlation with coronary arteriography. Am J Cardiol. 1982;49(7):1632-1637.
38. Mieres J.H., Shaw L.J., Hendel R.C., et al. A report of the American Society of Nuclear Cardiology Task Force on Women and Heart Disease (writing group on perfusion imaging in women). J Nucl Cardiol. 2003;10(1):95-101.
39. Schmermund A., Erbel R., Silber S. Age and gender distribution of coronary artery calcium measured by four-slice computed tomography in 2,030 persons with no symptoms of coronary artery disease. Am J Cardiol. 2002;90(2):168-173.
40. Taillefer R., DePuey E.G., Udelson J.E., et al. Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG-gated SPECT) in detecting coronary artery disease in women. J Am Coll Cardiol. 1997;29(1):69-77.
41. White M.P. Pharmacologic stress testing: Understanding the options. J Nucl Cardiol. 1999;6(6):672-675.
42. Morise A.P., Dalal J.N., Duval R.D. Value of a simple measure of estrogen status for improving the diagnosis of coronary artery disease in women. Am J Med. 1993;94(5):491-496.
43. Mieres J.H., Shaw L.J., Hendel R.C., et al. The WOMEN Study: What is the Optimal Method for Ischemia Evaluation in WomeN? A multi-center, prospective, randomized study to establish the optimal method for detection of coronary artery disease (CAD) risk in women at an intermediate-high pretest likelihood of CAD: study design. J Nucl Cardiol. 2009;16:105-112.
44. Lakoski S.G., Greenland P., Wong N.D., et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score. The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2007;167(22):2437-2442.
45. Nesto R.W., Phillips R.T., Kett K.G., et al. Angina and exertional myocardial ischemia in diabetic and nondiabetic patients: assessment by exercise thallium scintigraphy [erratum appears in Ann Intern Med 108(4):646, 1988]. Ann Intern Med. 1988;108(2):170-175.
46. Alexander K.P., Shaw L.J., Shaw L.K., et al. Value of exercise treadmill testing in women [erratum appears in J Am Coll Cardiol 1999 Jan;33(1):289]. J Am Coll Cardiol. 1998;32(6):1657-1664.
47. Okin P.M., Kligfield P. Gender-specific criteria and performance of the exercise electrocardiogram. Circulation. 1995;92(5):1209-1216.
48. Gibbons R.J., Balady G.J., Bricker J.T., et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40(8):1531-1540.
49. Wessel T.R., ARant C.V., Olson M.B., et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292:1179-1187.
50. Gulati M., Pandy D.K., Arnsdorf M.F., et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation. 2003;108:1554-1559.
51. Goodgold H.M., Rehder J.G., Samuels L.D., et al. Improved interpretation of exercise Tl-201 myocardial perfusion scintigraphy in women: Characterization of breast attenuation artifacts. Radiology. 1987;165(2):361-366.
52. Stratmann H.G., Williams G.A., Wittry M.D., et al. Exercise technetium-99m sestamibi tomography for cardiac risk stratification of patients with stable chest pain. Circulation. 1994;89(2):615-622.
53. Heller G.V., Ahmed I., Tilkemeier P.L., et al. Comparison of chest pain, electrocardiographic changes, and thallium-201 scintigraphy during varying exercise intensities in men with stable angina pectoris. Am J Cardiol. 1999;68:569-574.
54. Heller G.V., Ahmed I., Tilkemeier P.L., et al. Influence of exercise intensity upon the presence, distribution and size of thallium-201 defect. Am Heart J. 1992;123:909-916.
55. Navare S.M., Kapetanopoulos A., Heller G.V. Pharmacologic radionuclide myocardial perfusion imaging. Curr Cardiol Rep. 2003;5(1):16-24.
56. Amanullah A.M., Kiat H., Friedman J.D., et al. Adenosine technetium-99m sestamibi myocardial perfusion SPECT in women: Diagnostic efficacy in detection of coronary artery disease. J Am Coll Cardiol. 1996;27(4):803-809.
57. Travin M.I., Katz M.S., Moulton A.W., et al. Accuracy of dipyridamole SPECT imaging in identifying individual coronary stenoses and multivessel disease in women versus men. J Nucl Cardiol. 2000;7(3):213-220.
58. Shehata A.R., Gillam L.D., Mascitelli V.A., et al. Impact of acute propranolol administration on dobutamine-induced myocardial ischemia as evaluated by myocardial profusion imaging and echocardiography. Am J Cardiol. 1997;80:268-272.
59. Leppo J.A. Comparison of pharmacologic steroagents. J Nucl Cardiol. 1996;3(6 Pt 2):522-526.
60. Hansen C.L., Crabbe D., Rubin S. Lower diagnostic accuracy of thallium-201 SPECT myocardial perfusion imaging in women: An effect of smaller chamber size. J Am Coll Cardiol. 1996;28(5):1214-1219.
61. Hayes S.W., De Lorenzo A., Hachamovitch R., et al. Prognostic implications of combined prone and supine acquisitions in patients with equivocal or abnormal supine myocardial perfusion SPECT. J Nucl Med. 2003;44:1633-1640.
62. Bateman T.M., Heller G.V., Johnson L.L., et al. Does attenuation correction add value to non-attenuation corrected ECG-gated technetium-99m sestamibi SPECT? [abstract]. J Nucl Cardiol. 2003;10:S91.
63. Links J.M., DePuey E.G., Taillefer R., et al. Attenuation correction and gating synergistically improve the diagnostic accuracy of myocardial perfusion SPECT. J Nucl Cardiol. 2002;9(2):183-187.
64. Iskandrian A.E., Heo J., Nallamothu N. Detection of coronary artery disease in women with use of stress single-photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol. 1997;4(4):329-335.
65. Iskander S., Iskandrian A.E. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol. 1998;32(1):57-62.
66. Gibbons R.J., Hodge D.O., Berman D.S., et al. Long-term outcome of patients with intermediate-risk exercise electrocardiograms who do not have myocardial perfusion defects on radionuclide imaging. Circulation. 1999;100(21):2140-2145.
67. Heller G.V., Herman S.D., Travin M.I., et al. Independent prognostic value of intravenous dipyridamole with technetium-99m sestamibi tomographic imaging in predicting cardiac events and cardiac-related hospital admissions. J Am Coll Cardiol. 1995;26(5):1202-1208.
68. Galassi A.R., Azzarelli S., Tomaselli A., et al. Incremental prognostic value of technetium-99m-tetrofosmin exercise myocardial perfusion imaging for predicting outcomes in patients with suspected or known coronary artery disease. Am J Cardiol. 2001;88(2):101-106.
69. Vanzetto G., Ormezzano O., Fagret D., et al. Long-term additive prognostic value of thallium-201 myocardial perfusion imaging over clinical and exercise stress test in low to intermediate risk patients: Study in 1137 patients with 6-year follow-up. Circulation. 1999;100(14):1521-1527.
70. Ladenheim M.L., Pollock B.H., Rozanski A., et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 1986;7(3):464-471.
71. Pancholy S.B., Fattah A.A., Kamal A.M., et al. Independent and incremental prognostic value of exercise thallium single-photon emission computed tomographic imaging in women. J Nucl Cardiol. 1995;2(2 Pt 1):110-116.
72. Marwick T.H., Shaw L.J., Lauer M.S., et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106(2):172-178.
73. O’Keefe J.H.Jr, Bateman T.M., Ligon R.W., et al. Outcome of medical versus invasive treatment strategies for non-high-risk ischemic heart disease [commentary]. J Nucl Cardiol. 1998;5(1):28-33.
74. Cacciabaudo J.M., Hachamovitch R. Stress myocardial perfusion SPECT in women: Is it the cornerstone of the noninvasive evaluation? [commentary]. J Nucl Med. 1998;39(5):756-759.
75. Hachamovitch R., Berman D.S., Kiat H., et al. Effective risk stratification using exercise myocardial perfusion SPECT in women: Gender-related differences in prognostic nuclear testing. J Am Coll Cardiol. 1996;28(1):34-44.
76. Machecourt J., Longere P., Fagret D., et al. Prognostic value of thallium-201 single-photon emission computed tomographic myocardial perfusion imaging according to extent of myocardial defect. Study in 1,926 patients with follow-up at 33 months. J Am Coll Cardiol. 1994;23(5):1096-1106.
77. Stratmann H.G., Tamesis B.R., Younis L.T., et al. Prognostic value of dipyridamole technetium-99m sestamibi myocardial tomography in patients with stable chest pain who are unable to exercise. Am J Cardiol. 1994;73(9):647-652.
78. Amanullah A.M., Kiat H., Hachamovitch R., et al. Impact of myocardial perfusion single-photon emission computed tomography on referral to catheterization of the very elderly. Is there evidence of gender-related referral bias. J Am Coll Cardiol. 1996;28(3):680-686.
79. Travin M.I., Duca M.D., Kline G.M., et al. Relation of gender to physician use of test results and to the prognostic value of stress technetium 99m sestamibi myocardial single-photon emission computed tomography scintigraphy. Am Heart J. 1997;134(1):73-82.
80. Barrett-Connor E.L., Cohn B.A., Wingard D.L., et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. [erratum appears in JAMA 1991 Jun 26;265(24):3249]. JAMA. 1991;265(5):627-631.
81. America Y.G., Bax J.J., Boersma E., et al. The additive prognostic value of perfusion and functional data assessed by quantitative gated SPECT in women. J Nucl Cardiol. 2009;16:10-19.
82. Wexler O., Yoder S.R., Elder J.L., et al. Effect of gender on cardiovascular risk stratification with ECG gated SPECT left ventricular volume indices and ejection fraction. J Nucl Cardiol. 2009;16:28-37.
83. Heyden S., Heiss G., Bartel A.G., et al: Sex differences in coronary mortality among diabetics in Evans County, Georgia, J Chron Dis, 33(5): 265-273, 1980. Kannel W.B., McGee D.L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care. 2(2):120-126, 1979.
84. Pan W.H., Cedres L.B., Liu K., et al. Relationship of clinical diabetes and asymptomatic hyperglycemia to risk of coronary heart disease mortality in men and women. Am J Epidemiol. 1986;123(3):504-516.
85. Giri S., Shaw L.J., Murthy D.R., et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105(1):32-40.
86. Berman D.S., Kang X., Hayes S.W., et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41(7):1125-1133.
87. Mora S., Redberg R., Cui Y., et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20 year follow-up of the Lipid Research Clinics Prevalence Study. JAMA. 2003;290:1600-1607.