Chapter 31 Imaging in Patients Receiving Cardiotoxic Chemotherapy
INTRODUCTION
Of the many chemotherapeutic agents used in the treatment of malignancies, the anthracycline doxorubicin is well established for its therapeutic efficacy, despite its potential cardiotoxicity. Doxorubicin (Adriamycin) has been available for more than 30 years.1,2 Although a number of other anthracycline derivatives are available, doxorubicin is the most extensively used agent. Currently, it is used widely in the treatment of breast and gynecologic malignancies, lymphoma, and lung cancer. The cardiotoxicity associated with doxorubicin can be both acute and chronic.3 Acute cardiotoxicity is transient and without permanent consequences. It involves hypotension, pericarditis, tachycardia, or other arrhythmias. Chronic cardiotoxicity is of greater concern because it can cause progressive left ventricular dysfunction leading to clinical congestive heart failure, which in its most severe form can be fatal.
Some of the earliest clinical paradigms for nuclear cardiology techniques for monitoring and assessing therapeutic efficacy involved doxorubicin and its cardiotoxicity. Remarkably, techniques first reported in the late 1970s continue to be relevant.4 This chapter focuses on nuclear cardiology approaches to following patients receiving potentially cardiotoxic chemotherapy. It focuses on global left ventricular function monitoring, which is the most widely used clinical approach, and also discusses alternative nuclear and nonnuclear monitoring strategies.
MECHANISMS OF CARDIOTOXICITY
The most commonly accepted view of the mechanism of doxorubicin cardiotoxicity involves a combination of oxidative stress, or free radical production by the metabolites of doxorubicin, coupled with sensitivity of the myocardium to the cytotoxic effects of oxidative stress.5,6 Generated free radicals are usually toxic, reacting with a number of cell constituents and leading to lipid peroxidation, depletion of specific peptides, and damage to nucleic acids. In addition, mammalian myocardium, in contrast to other organs, is relatively deficient in enzymatic free radical scavengers that can potentially reverse the process. Doxorubicin metabolites also may have direct effects on these enzymes. In addition, doxorubicin can bind to cellular ionic iron, resulting in a complex that is highly toxic to intracellular proteins and membrane lipids.7
Alternative mechanisms involve calcium overload, release of vasoactive amines, decrease in cardiac muscle protein gene expression, and induction of apoptosis.8–12
PATHOLOGY
The myocardial damage associated with doxorubicin toxicity involves myofibrolysis, cytoplasmic vacuolization as a result of ballooning of sarcoplasmic reticulum, and degeneration of nuclei and mitochondria.13 Such changes ultimately result in substantial fibrosis. Minor grades of change are usually seen, even after lower doses. Higher grades are associated with impending congestive heart failure.14 A grading system for evaluating histopathologic changes based on the findings of endomyocardial biopsy has been described (Table 31-1). Intrinsic relationships between the severity of histopathologic abnormality and the degree of left ventricular dysfunction are poor. Even though there is an approximate linear relationship between histopathologic changes and cumulative dose, there is such marked variation that specific relationships cannot be readily established in individual patients.15 Further, sampling of biopsy material, because of the small sample size, may lead to inadequate identification of a more severe problem. On the other hand, the reverse can occur, and the degree of dysfunction can be overestimated based on a sampling error as a result of the nonuniform pathologic process.
Table 31-1 Histopathologic Grading of Doxorubicin Cardiotoxicity
| Grade 0 | No abnormality |
| Grade 1 | Minimal number of cells (<5% of total cells in each block) with early changes (early myofibrillar loss or distended sarcoplasmic reticulum) |
| Grade 1.5 | Small groups of cells involved (5%-15% of total number), some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 2 | Groups of cells involved 16%-25% of total number, some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 2.5 | Groups of cells involved 26%-35% of total number, some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 3 | Diffuse cell damage (>35% of total number of cells) with marked changes (total loss of contractile elements, loss of organelles, mitochondrial and nuclear degeneration) |
Adapted from Billingham ME, Mason JW, Bristow MR, Daniels JR: Anthracycline cardiomyopathy monitored by morphologic changes, Cancer Treat Rep 62:865–872, 1978.
INCIDENCE OF CARDIOTOXICITY
Although the severity and occurrence of clinical cardiotoxicity are dose dependent, there is considerable variability in individual susceptibility. As a result, some patients may experience substantial left ventricular dysfunction at relatively lower doses, whereas others may tolerate high cumulative doses with minimal if any effects. Usually, doses of more than 450 to 500 mg/m2 result in increased occurrence of cardiotoxicity. Older studies reported an incidence of heart failure of approximately 2% at a cumulative dose of 300 mg/m2 or less and 7% at a dose of 550 mg/m2. The incidence rose to more than 20% at a cumulative dose of more than 700 mg/m2.16,17 No specific genetic or biochemical markers to predict susceptibility have been identified. However, retrospective analysis shows that certain clinical risk factors are associated with a predilection for doxorubicin cardiotoxicity, including antecedent cardiac disease, age, mediastinal radiation, concomitant cyclophosphamide therapy, concomitant paclitaxel therapy, and, more recently, concomitant therapy with monoclonal antibody against epidermal growth factor (HER2).3
CLINICAL COURSE
Left ventricular dysfunction may occur without identifiable cardiac symptoms before clinical congestive heart failure develops. Left ventricular dysfunction induced by doxorubicin is usually irreversible. However, in a significant number of patients, improvement (and even normalization) has occurred after discontinuation of doxorubicin therapy or institution of appropriate therapy for congestive heart failure.18–20 If treatment with doxorubicin is continued after asymptomatic left ventricular dysfunction occurs, a progressive and rapid decline in ventricular performance leading to the clinical manifestation of congestive heart failure is likely.20 It is unusual for patients who have completed a course of doxorubicin chemotherapy to have ventricular dysfunction and heart failure after discontinuation of therapy.11 However, this phenomenon is seen in a relatively small proportion of patients.
MONITORING FOR CARDIOTOXICITY
Radionuclide angiocardiography, initially by first-pass techniques and more recently by equilibrium techniques, remains the standard for assessing potential cardiotoxicity (see Chapter 12).19 Neither clinical examination nor electrocardiography or more standard clinical laboratory evaluation is efficacious in this regard. The use of radionuclide angiocardiography for measuring left ventricular ejection fraction as the functional index of cardiac performance was established in a variety of clinical settings. Although this measure is not a pure index of contractility and is influenced by preload and afterload, it has excellent accuracy and reproducibility.21 It can be performed in virtually all patients and is not limited by acoustic window artifacts that can affect ultrasound studies. The technique is fully automated22 and well standardized and provides the level of reproducible quantitative data necessary for reliable serial application.
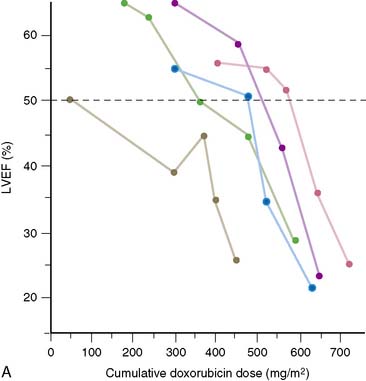
In 1979, Alexander and colleagues4 initially described 55 patients monitored with serial first-pass quantitative radionuclide angiocardiography at rest. They observed a significant relationship between the cumulative dose of doxorubicin and the development of ventricular dysfunction. Five patients in this series had heart failure; all five had an ejection fraction of less than 30% when symptoms occurred (Fig. 31-1). Based on this initial experience, guidelines were suggested.
Schwartz and colleagues, nearly a decade later, reported a larger experience with the monitoring of 1487 patients over a 7-year period.19 Based on this experience, the initial guidelines were slightly amended. These guidelines remain in place today (Table 31-2). In patients with a normal baseline ejection fraction (>50%), moderate toxicity was defined to include an end point of a decline of more than 10% in absolute ejection fraction, with a final ejection fraction of less than 50%.
Table 31-2 Guidelines for Monitoring Doxorubicin Cardiotoxicity by Serial Radionuclide Angiography
| Baseline evaluation: Baseline radionuclide angiocardiography at rest is done to estimate left ventricular ejection fraction (LVEF) before the start of doxorubicin therapy or before 100 mg/m2 of doxorubicin has been given. |
| Subsequent evaluations: Subsequent studies are done 3 weeks after the indicated last dose of doxorubicin and before consideration of the next dose, at the following intervals: |
Adapted from Schwartz RG, Zaret B: Diagnosis and treatment of drug-induced myocardial disease. In Muggia FC, Speyer JL (eds): Cardiotoxicity of Anticancer Therapy, Baltimore: Johns Hopkins University Press, 1992, pp 173–197.
Somewhat modified guidelines have been applied to patients with abnormal baseline ventricular function.23 Initially it was believed that administration of doxorubicin to such patients was contraindicated. In this setting, more frequent monitoring is useful (see Table 31-2). In our initial experience, no identifiable etiology was noted in approximately a third of patients with abnormal baseline function.19 This observation underscores the importance of obtaining baseline measurements in patients before instituting therapy.
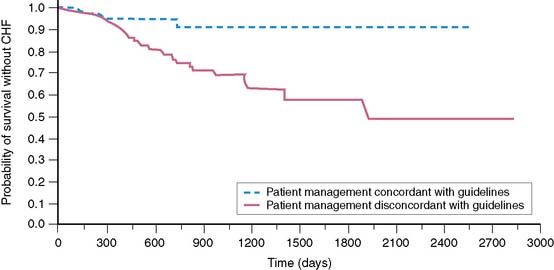
In the experience of Schwartz and colleagues,19 clinical congestive heart failure developed in 46 of 282 (16%) high-risk patients. The total cumulative dosage of doxorubicin that precipitated heart failure varied widely and ranged from 75 to 1095 mg/m2. Clinical congestive heart failure was mild in 46% of patients, moderate in 41%, and severe in 11%. In only one patient could death be attributed to congestive heart failure. There was no worsening of heart failure during 1 year of follow-up. In that study, high-risk patients whose management was in accordance with the guidelines specified in Table 31-2 had a very low of incidence of mild clinical congestive heart failure (2 of 70 patients). In contrast, 20% of patients who were not managed according to guidelines based on serial monitoring had clinical signs of congestive heart failure (Fig. 31-2).
Schwartz and colleagues19 also compared the experience in community versus university hospitals and found no difference in any of the outcomes. Initial results showed that measures of resting global ventricular function obtained serially clearly provided a means of monitoring patients receiving chemotherapy. Earlier it was suggested that sensitivity could be improved by adding exercise stress to the resting study. Although earlier findings were conflicting with respect to the value of exercise ventricular function and outcome,24 for several reasons, we believe that data support serial studies in the resting state alone. First, resting studies show excellent sensitivity for detecting problems. Second, patients with malignancy may have excessive discomfort with exercise and may be at risk for pathologic fracture. Third, the reproducibility of exercise over time may be difficult due to systemic factors such as anemia, general debilitation, and changes in blood pressure. Fourth, exercise responses may be nonspecific, particularly in the elderly.
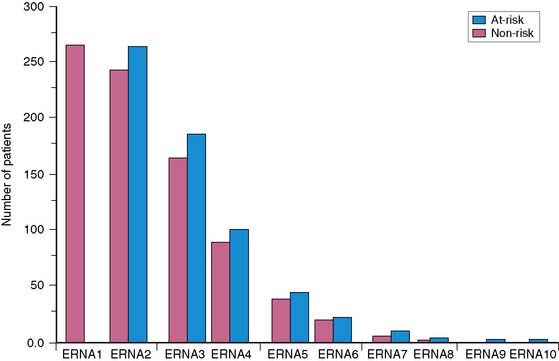
Based on clinical experience obtained in the 1980s and 1990s, the use of serial ventricular function monitoring for assessing patients receiving doxorubicin has become clinically routine. However, it is unclear whether earlier guidelines carry the same importance as they did previously. To evaluate this question, Mitani and colleagues25 reported a retrospective clinical study at our institution in which 265 patients with malignancy were followed with serial left ventricular ejection fraction measurements. Studies were obtained with serial equilibrium radionuclide angiocardiography on at least two occasions. Each patient underwent an average of 3.3 ± 1.3 studies (Fig. 31-3). Follow-up was complete in 93% of patients. In this retrospective analysis, patients with normal ventricular function at baseline and a 10% or greater absolute decrease in left ventricular ejection fraction, to a final value of 50% or less during doxorubicin therapy, were considered to be “at risk” for heart failure. Over an average follow-up of more than 2 years, seven patients (2.6%) had some form of congestive heart failure (Fig. 31-4). Within the same time frame, 90 patients (34%) died of cancer-related causes. There were no deaths due to heart failure. Comparison of the at-risk group with a low-risk group of patients who did not meet these criteria showed a higher incidence of heart failure, a lower baseline ejection fraction (with the lowest ejection fraction at its lower level), and a high rate of cancer-related deaths in the at-risk group. In 34 patients, doxorubicin was discontinued during the monitoring period. In 20 of these patients (59%), discontinuation was based on ejection fraction criteria for toxicity.
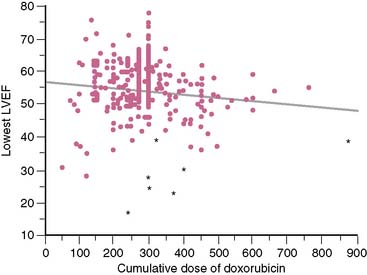
A cost analysis showed that the overall expenditure for serial equilibrium radionuclide angiocardiogram studies was less than the estimated 1-year cost of care for the additional estimated cases of congestive heart failure that theoretically would have developed without monitoring but were prevented by routine monitoring of left ventricular ejection fraction.25,26 It was concluded that serial monitoring of left ventricular ejection fraction by radionuclide techniques is both appropriate and cost-effective for predicting and preventing congestive heart failure in this patient cohort. This study showed only a weak inverse relationship between the lowest ejection fraction and the cumulative dose achieved (Fig. 31-5). In addition, congestive heart failure occurred at varying doses, some of which were relatively low, further emphasizing the need for careful monitoring in these patients, even early in the course of chemotherapy.
Other nuclear imaging approaches involving the myocardium include indium-111-antimyosin imaging, a marker of myocardial necrosis, and iodine-123-methyliodobenzylguanidine (MIBG), which measures adrenergic neuronal uptake.27–32 Antimyosin antibody imaging uptake showed high sensitivity but low specificity. Its uptake lacks specificity and is observed in most patients receiving intermediate doses of doxorubicin, even in the absence of left ventricular dysfunction. This technique is no longer available for clinical use in the United States. Data have been obtained primarily in experimental animals.
In conclusion, serial monitoring of radionuclide measures of global left ventricular function continues to provide clinically relevant, time-tested data for monitoring patients for doxorubicin cardiotoxicity. This approach remains a standard clinical procedure in our institution and constitutes the largest current indication for the performance of radionuclide angiocardiography. In patients who achieve long-term success with doxorubicin treatment of malignancy, it will be important to show that late-developing left ventricular dysfunction does not become a clinical problem. Similarly, few data are available on the short- and long-term results in children with malignancy monitored with the same approach.33 Nevertheless, serial monitoring of left ventricular ejection fraction as a well-standardized measure of global left ventricular function with radionuclide techniques remains a valuable clinical procedure.
TRASTUZUMAB (HERCEPTIN) CARDIOTOXICITY
Trastuzumab was approved by the U.S. Food and Drug Administration (FDA) for clinical use in 1998 and is indicated for the treatment of metastatic breast carcinoma in women with tumors that overexpress the HER2 (human epidermal growth factor receptor 2) protein.34 Trastuzumab is a humanized monoclonal antibody against HER2. The drug binds to the extracellular juxtamembrane domain of HER2 and inhibits proliferation and survival of HER2-dependent tumors.35 This overexpression of HER2 occurs in 20% to 25% of breast tumors. For the drug to be chemotherapeutically useful, either HER2 overexpression must be demonstrated by immunohistochemistry, or amplification of the gene must be detected by fluorescence in situ hybridization (FISH). The development of this chemotherapeutic agent represents a prime example of translational research in which molecular mechanisms are first defined and then used as the basis for developing new therapeutic approaches.35
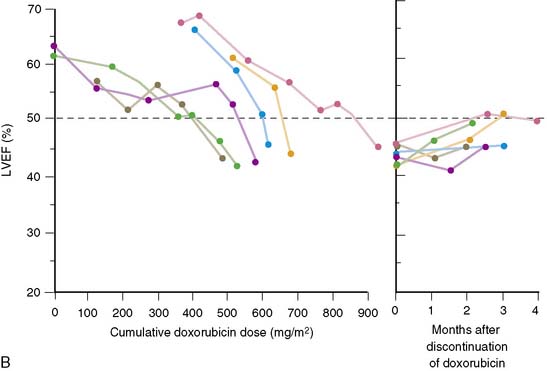
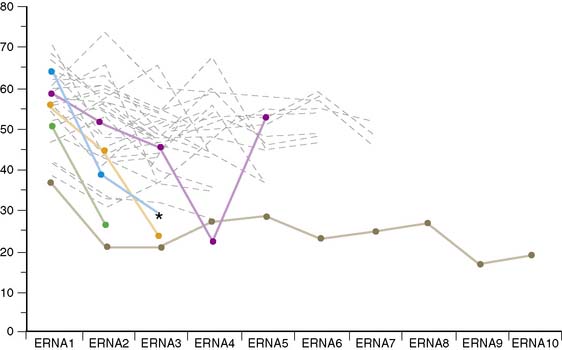
The HER2 gene plays an important role in embryonic and postnatal heart function. It is also involved in blunting the effects of stress signaling pathways as well as apoptosis. Several large clinical trials have demonstrated the efficacy of trastuzumab treatment in patients with HER2-positive breast cancer. However, in the course of these studies, the side effects of decreased cardiac function and clinical congestive heart failure have been recognized.36–42 Although there has not been complete standardization of specific protocols for the monitoring for cardiotoxicity, equilibrium radionuclide angiocardiography (ERNA) has played a major role in evaluating and following such patients. The cardiotoxicity of trastuzumab stands in direct contrast to that of doxorubicin (Table 31-3). Whereas cardiotoxicity with doxorubicin may stabilize and can be detected relatively early in its course, underlying damage appears to be permanent. Trastuzumab cardiotoxicity has a reasonably high likelihood of reversal within 2 to 4 months if medication is discontinued. Doxorubicin effects are generally cumulative and dose related, whereas those of trastuzumab are not. The overall mechanism of doxorubicin toxicity is likely free radical formation and oxidative stress, whereas with trastuzumab the mechanism involves blocked ErbB2 signaling. Permanent changes are detected with electromicrosopy and routine histology in doxorubicin toxicity but are not seen with trastuzumab. Rechallenge with doxorubicin is associated with a high likelihood of recurrent ventricular dysfunction that may result in progressively severe heart failure and death. In contrast, increasing data demonstrate that rechallenge is possible with trastuzumab, without further decline in ventricular function.
| Doxorubicin | Trastuzumab | |
|---|---|---|
| Clinical profile | Myocardial damage permanent | Likely reversible if drug stopped (2–4 months) |
| Histologic changes | ||
| No histologic changes | ||
| Dose relationship | Dose related, cumulative | Not dose related |
| Likely mechanism | Oxidative stress | Blocked ErbB2 signaling |
| Rechallenge | Likely progressive cardiac dysfunction | Increasing data showing rechallenge may be safe |
| Monitoring of ventricular function | Well-established means of following patient’s experience | Provides valuable data but less clinical than with doxorubicin |
Adapted from Ewer MS, Lippman SM: Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity, J Clin Oncol 23:2900–2902, 2005.
1. Blum R.H., Carter S.K. Adriamycin: A new anticancer drug with significant clinical activity. Ann Intern Med. 1974;80:249-259.
2. Young R.C., Ozols R.F., Myers C.E. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305:139-153.
3. Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. 2000;7:53-62.
4. Alexander J., Dainiak N., Berger H.J., et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med. 1979;300:278-283.
5. Singhal P.K., Iliskovic N., Li T., et al. Adriamycin cardiomyopathy: Pathophysiology and prevention. FASEB J. 1997;11:931-936.
6. Gianni L., Myers C.E. The role of free radical formation in the cardiotoxicity of anthracycline. In: Muggia F.M., Green M.D., Speyer J.L., editors. Cancer Treatment and the Heart. Baltimore: Johns Hopkins University Press; 1992:9-46.
7. Hershko C., Pinson A., Link G. Prevention of anthracycline cardiotoxicity by iron chelation. Acta Hematol. 1996;95:87-92.
8. Halili-Rutman I., Hershko C., Link G., et al. Inhibition of calcium accumulation by the sarcoplasmic reticulum: A putative mechanism for the cardiotoxicity of Adriamycin. Biochem Pharmacol. 1997;54:211-214.
9. Harada H., Cusack F.J., Olson R.D., et al. Taurine deficiency and doxorubicin: Interaction with the cardiac sarcolemmal calcium pump. Biochem Pharmacol. 1990;39:745-751.
10. Ito H., Miller S.C., Billingham M.E., et al. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1990;87:4275-4279.
11. Jeyaseelan R., Poizat C., Wu H.Y., et al. Molecular mechanisms of doxorubicin-induced cardiomyopathy: Selective suppression of Reiske iron-sulfur protein, ADP/ATP translocase, and phosphofructokinase genes is associated with ATP depletion in rat cardiomyocytes. J Biol Chem. 1997;272:5828-5832.
12. Zhang J., Clark J.R., Herman E.H., et al. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: Differential effects in heart, kidney and intestine and inhibition by ICRF-187. J Mol Cell Cardiol. 1996;28:1931-1943.
13. Billingham M.E., Mason J.W., Bristow M.R., et al. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62:865-872.
14. Bristow M.R., Mason J.W., Billingham M.E., et al. Dose-effect and structure-function relationship in doxorubicin cardiomyopathy. Am Heart J. 1981;102:709-718.
15. Isner J.M., Ferrans V.J., Cohen S.R., et al. Clinical and morphologic cardiac findings after anthracycline chemotherapy: Analysis of 64 patients studied at necropsy. Am J Cardiol. 1983;51:1167-1174.
16. Young R.C., Ozols R.F., Myers C.E. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305:139-153.
17. Lefrak E.A., Pitha J., Rosenheim S., et al. A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer. 1973;32:302-314.
18. Saini J., Rich M.W., Lyss A.P. Reversibility of severe left dysfunction due to doxorubicin cardiotoxicity: Report of three cases. Ann Intern Med. 1987;106:814-816.
19. Schwartz R.G., McKenzie B., Alexander J., et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy: Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109-1118.
20. Schwartz R.G., Zaret B.L. Diagnosis and treatment of drug induced myocardial disease. In: Muggia F.C., Speyer J.L., editors. Cardiotoxicity of Anticancer Therapy. Baltimore: Johns Hopkins University Press; 1992:173-197.
21. van Royen N., Jaffe C.C., Krumholz H.M., et al. Comparison and reproducibility of visual echocardiographic and quantitative radio-nuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843-850.
22. Lee F.A., Fetterman R., Zaret B.L., et al. Rapid radionuclide derived systolic and diastolic cardiac function using cycle-dependent background correction and Fourier analysis. In: Proceedings of Computers in Cardiology. Linkoping, Sweden: IEEE Computer Society; 1985:443-446.
23. Choi B.W., Berger H.J., Schwartz P.E., et al. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. Am Heart J. 1983;106:638-643.
24. Palmeri S.T., Bonow R.O., Myers C.E., et al. Prospective evaluation of doxorubicin cardiotoxicity by rest and exercise radionuclide angiography. Am J Cardiol. 1986;58:607-613.
25. Mitani I., Jain D., Joska T.M., et al. Doxorubicin cardiotoxicity: Prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10:132-139.
26. Schulman K.A., Mark D.B., Califf R.M. Outcomes and costs within a disease management program for advanced congestive heart failure. Am Heart J. 1998;135:S282-S292.
27. Estorch M., Carrio I., Berna L., et al. 111In-Antimyosin scintigraphy after doxorubicin therapy in patients with advanced breast cancer. J Nucl Med. 1990;31:1965-1969.
28. Carrio I., Lopez-Pousa A., Estorch M., et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J Nucl Med. 1993;34:1503-1507.
29. Jain D., Zaret B.L. Antimyosin cardiac imaging: Will it play a role in the detection of doxorubicin cardiotoxicity? [editorial]. J Nucl Med. 1990;31:1970-1974.
30. Carrio I., Estorch M., Berna L., et al. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med. 1995;36:2044-2049.
31. Lekakis J., Prassopoulos V., Athanassiadis P., et al. Doxorubicin-induced cardiac neurotoxicity: Study with iodine 123-labeled metaiodobenzylguanidine scintigraphy. J Nucl Cardiol. 1996;3:37-41.
32. Takano H., Ozawa H., Kobayashi I., et al. Myocardial sympathetic dysinnervation in doxorubicin cardiomyopathy. J Nucl Cardiol. 1996;27:49-55.
33. Goorin A.M., Chauvenet A.R., Perez-Atayda A.R., et al. Initial congestive heart failure, six to ten years after doxorubicin chemotherapy for childhood cancer. J Pediatr. 1990;116:144-147.
34. Hudis C.A. Trastuzumab: Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51.
35. Chien K.R. Herceptin and the heart: A molecular modifier of cardiac failure. N Eng J Med. 2006;354:789-790.
36. Keefe D.L. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592-1600.
37. Perez E.A., Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004;22:322-329.
38. Ewer M.S., Lippman S.M. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity? J Clin Oncol. 2005:2900-2902.
39. Ewer M.S., Vooletich M.T., Durand J.-B., et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820-7826.
40. Guarnei V., Lenihan D.J., Valero V., et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center Experience. J Clin Oncol. 2006;24:4107-4115.
41. Telli M.L., Hunt S.A., Carlson R.W., et al. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525-3533.
42. Suter T.M., Procter M., van Veldhuisen D.J., et al. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-3865.