Chapter 27 Imaging for Preoperative Risk Stratification
INTRODUCTION
The evaluation of preoperative cardiac risk in patients undergoing noncardiac surgery has been a challenging and important topic over the past 25 years. The incidence of perioperative cardiac mortality has declined in recent years, but the prevalence of both coronary artery disease (CAD) and noncardiac surgical procedures in the United States1,2 is predicted to significantly increase over the next 30 years. Therefore, preoperative evaluation for cardiac risk will continue to be an important issue for surgeons, cardiologists, and medical consultants.2–6
This chapter will review the use of nuclear cardiology myocardial perfusion imaging (MPI) for preoperative and long-term risk stratification, including recommendations from the recently revised 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery2 for which patients benefit from preoperative imaging. The recommendations for preoperative risk assessment in the current Guidelines are also included in the recent American College of Cardiology/American Heart Association SPECT (single-photon emission computed tomography) Appropriateness criteria.7 Although controlled prospective randomized clinical trials are lacking, there are many retrospective reports and a few meta-analyses that demonstrate the utility of MPI in this evaluation process. We will focus on the evaluation of ischemia and left ventricular (LV) function, both of which have been shown to have significant prognostic utility for cardiac events such as myocardial infarction (MI) or cardiac death8 in patients with CAD. The issues of perioperative medical therapy and revascularization will also be reviewed.
The 2007 revised Guidelines divide preoperative clinical cardiac risk assessment into five steps.2 Overall clinical risk is determined by individual patient risk factors, the risk of the surgical procedure, and the patient’s functional capacity. This initial clinical evaluation can determine which patients may warrant further risk stratification with stress MPI.
STEP THREE: DETERMINE WHETHER THE PLANNED PROCEDURE IS LOW-RISK SURGERY
The type of surgery has an important impact on perioperative risk. In Table 27-1,2 the major types of surgical procedures are divided into high-, intermediate-, and low-risk groups. High-risk surgery, with a combined perioperative MI/cardiac death rate greater than 5%, includes major vascular, aortic, and peripheral vascular surgery that often warrants preoperative assessment with MPI. Intermediate-risk surgery includes intrathoracic, intraperitoneal, orthopedic, prostate, and head/neck surgery. Low-risk surgery includes superficial, endoscopic, breast, cataract, and ambulatory surgery. An important change in the 2007 Guidelines is the recognition that preoperative MPI rarely changes management of patients undergoing low-risk surgery, even in patients with clinical risk factors and poor functional capacity. Therefore, low-risk procedures can generally be performed without additional preoperative imaging.
| High Surgical Risk: |
| Aortic and other major vascular surgery |
| Peripheral vascular surgery |
| Intermediate Surgical Risk: |
| Carotid endarterectomy |
| Head and neck surgery |
| Intraperitoneal and intrathoracic surgery |
| Orthopedic surgery |
| Prostate surgery |
| Low Surgical Risk: |
| Endoscopic procedures |
| Superficial procedures |
| Cataract surgery |
| Breast surgery |
| Ambulatory surgery |
STEP FOUR: ASSESS FUNCTIONAL CAPACITY
A key factor in the Guidelines for predicting perioperative cardiac risk is the patient’s functional capacity, which can be estimated from a careful history of daily activity or determined with exercise stress testing. The Duke Activity Status Index9 can be used to approximate the metabolic equivalents (METs) for many daily activities. If the patient can exceed 4 METs with daily activity without symptoms (climbs one to two flights of stairs, performs own housework, or exercises regularly), then that patient will typically have sufficient cardiovascular reserve to tolerate the stress of surgery. The 2007 revised Guidelines recognize that stress MPI is unlikely to alter management in asymptomatic patients with good functional capacity. In contrast, symptomatic patients or those with very limited functional capacity may have poor cardiovascular reserve, with worse perioperative and long-term outcomes after noncardiac surgery.
STEP FIVE: ASSESS CLINICAL RISK IN SYMPTOMATIC PATIENTS OR PATIENTS WITH POOR/UNKNOWN FUNCTIONAL CAPACITY
Although the clinical question that is often posed to the medical or cardiac consultant is whether or not to “clear” the patient for noncardiac surgery, the Guidelines2 suggest an overall conservative approach to the use of expensive tests and interventions. The real challenge is in symptomatic or mildly symptomatic patients who are being evaluated for elective surgical procedures. A detailed medical history, physical examination, and electrocardiogram (ECG) should include information about angina, prior MI, CHF, symptomatic arrhythmia, diabetes, peripheral vascular disease, and prior history of coronary angiography or revascularization procedures. Even in the presence of known CAD, the risk of perioperative death or MI is less than 1% in patients who have undergone revascularization within the previous 4 years.10 It is also reasonable to proceed to surgery if the patient has had a recent coronary angiogram or stress test that reveals favorable results with no change in clinical symptoms. However, the presence of cardiac symptoms or prior cardiovascular disease, as already noted, should alert the consulting physician to consider further noninvasive testing. This decision to consider further testing such as stress MPI in patients with poor functional capacity can be made based on clinical risk factors and surgical risk.
Clinical Risk Score
Since the initial report by Goldman et al.11 demonstrated the utility of a clinical risk score for prediction of perioperative risk, several authors have published similar clinical risk scores based on the presence of clinical cardiovascular disease, diabetes mellitus (DM), renal disease, or other medical comorbidities.11–13 The 2007 Guidelines incorporate the Revised Clinical Risk Index developed by Lee et al.13
Lee et al.13 evaluated a total of 4315 patients undergoing elective noncardiac surgery. They derived a simple clinical index of six factors that could predict perioperative cardiac events: The presence of high-risk surgery, a history of CAD, history of CHF, history of cerebrovascular disease, diabetes treated with insulin, and a serum creatinine above 2 mg/dL constitute a useful index for risk assessment. In a validation population, the authors noted that when zero to one of these six factors was present (74% of all patients), the postoperative cardiac event rate was less than 1%. In contrast, the event rate in patients with any two factors was 7% (18% of all patients), and in those patients with three or more factors (8% of all patients), the event rate was 11%. Therefore, the Revised Clinical Risk Index classified three-quarters of the patients as low risk. A decision to consider further testing such as stress MPI can be made based on the presence of the five clinical risk factors together with surgical risk (the sixth risk factor).
Surgical Risk
In addition to the patient’s clinical cardiac risk, the risk of the planned surgery (see Table 27-1) should be considered in the decision for preoperative MPI testing. As discussed, current Guidelines now recommend proceeding directly to low-risk surgery without preoperative imaging. High-risk vascular surgery or intermediate-risk surgery often warrants preoperative assessment with MPI when the patient’s clinical risk is increased and functional capacity is poor.
Vascular Surgery
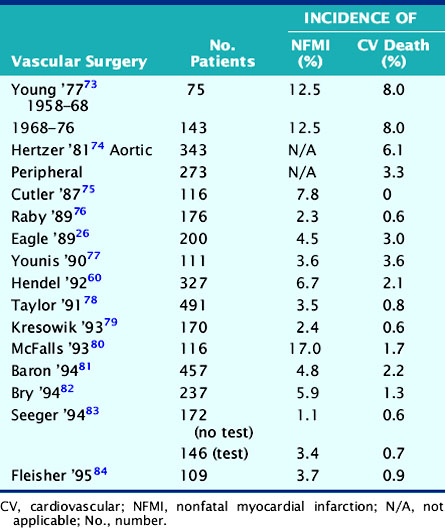
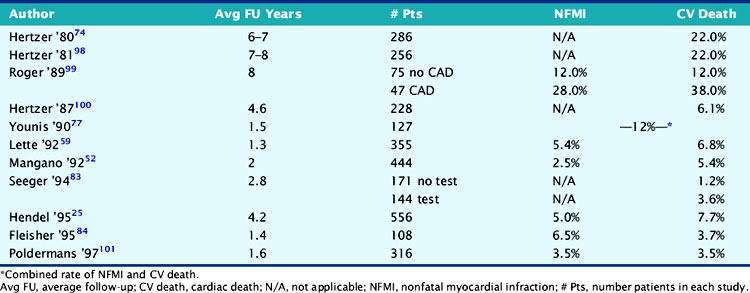
Assessment of cardiac risk is of particular importance prior to elective peripheral vascular surgery, owing to the high prevalence (˜60%) of coexisting coronary artery disease.14,15 The incidence of nonfatal MI or cardiac death in this population is summarized in Table 27-2. These data show that although vascular procedures are considered high-risk surgery, the perioperative cardiac death rate for vascular surgery has fallen to less than 2% as a result of improved preoperative assessment and perioperative management. However, these data also show that although the rate of nonfatal perioperative MI has also decreased, it remains two- to threefold higher than perioperative cardiac death. Therefore, it appears that while preoperative assessment and perioperative management have resulted in a lower perioperative death rate, the ability to predict and prevent perioperative MI is more difficult. The observation that nonfatal perioperative MI is a powerful predictor of late cardiac events16–18 suggests the need to combine preoperative risk assessment with longer-term coronary management.
Transplant Surgery
The question of routine screening for all patients undergoing renal transplantation is primarily an issue of long-term cardiac prognosis. There is little immediate risk in performing transplant surgery, but it is clear that cardiac morbidity and mortality can have a significant impact on long-term postoperative survival. This seems to be especially true for patients with diabetes19 and can result in a policy of routine noninvasive stress imaging or coronary angiography in many patients before transplantation. Heston et al.20 have shown that an “expert system” using clinical risk predictors and thallium stress testing achieved an overall accuracy of 89% in predicting 4-year cardiac mortality among 189 renal transplant candidates. Other studies21,22 show that if there are no clinical risk predictors, such as a history of CHF, angina, insulin-dependent diabetes, age older than 50 years, or an abnormal ECG (excluding LV hypertrophy), no further cardiac evaluation is needed prior to renal transplantation.
In patients being evaluated for cardiac risk prior to liver transplantation, there has been little published experience. A study from Kryzhanovski and Beller23 suggests that perioperative cardiac risk is too low to warrant routine stress MPI or radionuclide angiography prior to liver transplantation. There were no cardiac events in 63 liver transplant procedures, and only one patient had a high-risk scan. Therefore, cardiac evaluations should be used in this patient subgroup only when there is clear evidence of coronary disease or when clinical risk factors are present.
THE DECISION TO OBTAIN PREOPERATIVE TESTING: A SHORTCUT APPROACH
After initial clinical assessment, a large group of patients with good functional capacity or those undergoing low-risk surgery can proceed to surgery without further testing. However, there remains a group of patients with clinical evidence of cardiovascular disease and poor functional capacity whose risk is uncertain or increased. Table 27-3 presents a shortcut approach to a large number of patients in whom the decision to recommend testing prior to surgery can be difficult. Basically, if all of the three listed factors are true, then the Guidelines suggest that noninvasive cardiac testing can be considered as part of the preoperative evaluation.
RISK ASSESSMENT WITH PREOPERATIVE STRESS IMAGING
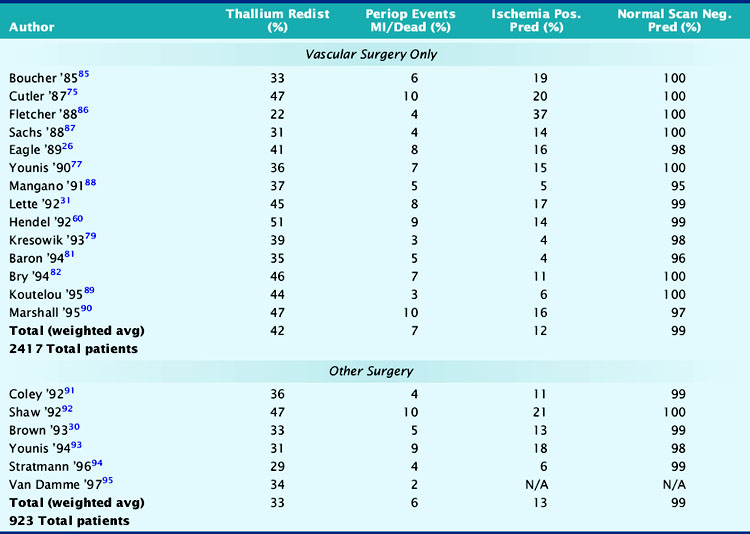
The utility of vasodilator (dipyridamole or adenosine) MPI imaging in more than 3000 patients is summarized in Table 27-4. In the upper section, all studies involved vascular surgery, and the incidence of thallium redistribution was 42%. The overall positive predictive accuracy of a reversible perfusion defect (ischemia) for prediction of perioperative MI or cardiac death is 12%. In the vascular surgery population, in which the prevalence of CAD is 60% to 70%, 38% (930/2417) of the patients have a normal stress perfusion scan. The average negative predictive accuracy is 99%, which indicates that a normal stress perfusion study is a powerful prognostic indicator.
The lower section of Table 27-4 summarizes similar results for patients with nonvascular surgery. Most patients in this section were studied because of increased clinical risk of CAD, and the overall event rate of 6% was similar to the vascular surgery group. It is interesting to note that although the incidence of thallium redistribution was somewhat lower, the positive and negative predictive values are similar to those noted in the vascular surgery group.
Shaw et al. reviewed the utility of preoperative noninvasive testing in a large meta-analysis.24 These authors reviewed stress imaging studies from 1985 to 1994 in which either dipyridamole thallium (n = 1994) or dobutamine echocardiography (n = 455) was used for perioperative risk stratification. Reversible perfusion defects were noted in 26% of patients, and perioperative nonfatal MI or cardiovascular death occurred in 9% of these cases. In contrast, 430 (22%) patients had normal perfusion scans with an event rate of 1.4%. Similar prognostic utility was noted in the dobutamine echocardiography studies. New wall-motion abnormalities during dobutamine-induced stress were noted in 39% of the patients, and 11% of these patients had a major cardiac event. In the 270 (61%) patients without new regional wall-motion abnormalities, the event rate was 0.4%. The authors of this meta-analysis concluded that (1) reversible perfusion defects have significant positive predictive accuracy, but the overall accuracy depends on the prevalence of CAD and clinical risk factors; (2) dobutamine-induced wall-motion abnormalities predict adverse outcomes, but the relatively small population size yields wider confidence limits; (3) the use of semiquantitative image analysis for MPI should improve its prognostic utility; and (4) fixed defects predict long-term cardiac events with an accuracy equal to reversible defects for perioperative events.
The issue of a possible gender difference in perioperative risk stratification was studied by Hendel et al.25 This study of 567 vascular surgery patients showed that, overall, perioperative and long-term cardiac events were similar for both men and women. Clinical predictors of cardiac risk were less useful in women versus men. ECG ST-segment depression with dipyridamole infusion predicted increased perioperative risk for men but not for women. An important finding was that multivariate analysis showed thallium redistribution to be an independent predictor of risk for both men and women.
Several authors have evaluated the use of clinical risk factors to identify the intermediate, risk patients who most benefit from preoperative stress MPI. Eagle et al.26 evaluated the utility of preoperative dipyridamole thallium MPI in 200 patients undergoing vascular surgery. In the 32% of patients at low clinical risk, with no DM or clinical risk factors for CAD, the perioperative rate of cardiac death, perioperative MI, pulmonary edema, or unstable angina was 3.1%. Thallium redistribution stratified the 54% of patients at intermediate risk (one or two risk factors) into a low-risk group with a perioperative event rate equal to that for patients with no risk factors and a high-risk group with a perioperative event rate of 30%. Therefore, these data support the current recommendation to reserve preoperative stress MPI for patients with clinical risk factors.
L’Italien et al. showed similar results in a multicenter analysis of over 1081 patients undergoing vascular surgery.27 In this study, clinically low-risk patients with a 3% perioperative event rate and high-risk patients with a 19% event rate were not more accurately risk stratified by preoperative dipyridamole thallium MPI. However, clinical assessment classified 51% of the patients as being at intermediate surgical risk, and stress thallium MPI effectively separated more than 80% of these intermediate-risk patients into low-risk or high-risk groups.
In a prospective clinical trial of patients undergoing abdominal aortic surgery, Vanzetto et al.28 identified patients at increased clinical risk based on the presence of two or more of the following predictors: age older than 70 years; history of MI, angina, CHF, DM, hypertension with LV hypertrophy; or a resting ECG that shows Q waves or ST-segment ischemia. Of 457 patients, 69% were classified as low risk and underwent surgery without preoperative stress testing. There was a 4% event rate in patients with one risk factor and a rate of approximately 2% in patients without any CAD risk predictors. One hundred forty-seven (32%) of the patients were classified as increased risk, and subsequently, 134 of these 147 patients underwent surgery after dipyridamole SPECT thallium scans. On the basis of these clinical criteria alone, 9% of the higher-risk patients had cardiac events. The perioperative rate of cardiac death or MI was 13% in patients with abnormal perfusion scans, compared to 1.9% in those with normal MPI. It is also important to add that Vanzetto et al.28 performed a multivariate analysis that showed that the number of ischemic segments was the single best predictor of perioperative events.
In another study of preoperative cardiac risk assessment, Bartels et al.29 evaluated a strategy that emulated the ACC/AHA recommendations for cardiac risk. Clinical risk classifications were assigned to 201 patients who were to undergo major vascular surgery. Approximately 10% of the patients were defined as high risk based on the presence of major clinical predictors of cardiac risk, 40% at intermediate risk, and the remaining 50% at low risk. All the low-risk patients and the intermediate-risk patients (52%) with a functional capacity of greater than 5 METs based on a questionnaire (Duke Activity Status Index)9 proceeded directly to major aortic surgery without further testing. The remaining intermediate-risk patients (48%) who had a functional capacity of less than 5 METs on the questionnaire underwent noninvasive testing (40%) or intensified medical care (60%) before surgery. In the high-risk group, approximately half underwent noninvasive testing, and the other half received intensified medical treatment. Subsequently, five (6%) intermediate risk and two (9%) high-risk patients underwent preoperative coronary angiography, resulting in one coronary bypass procedure and two cancellations of further elective surgery. Cardiac events occurred in 5% of the high-risk group and in 9% of the intermediate-risk group patients. The low-risk group had an event rate of 2%.
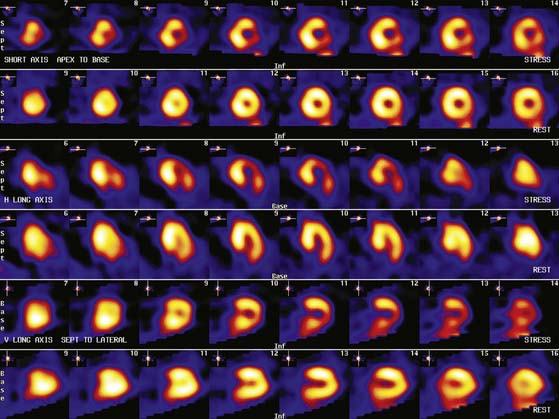
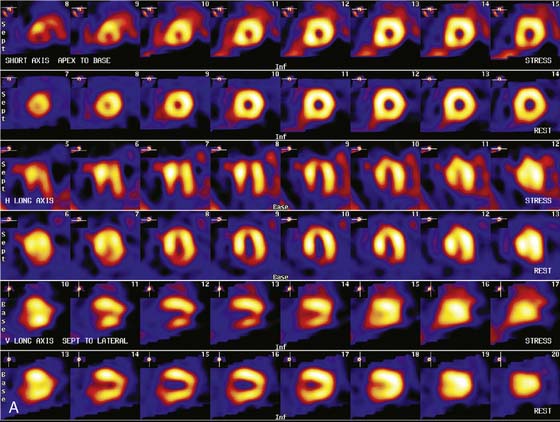
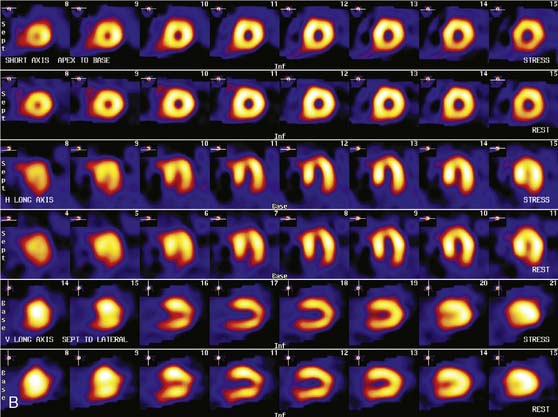
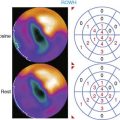
Patients with a mildly abnormal preoperative MPI study can generally undergo surgery with medical treatment for coronary disease. However, when preoperative stress MPI indicates very high risk of a perioperative cardiac complication, the Guidelines recommend evaluation with coronary angiography. Identification of patients at the highest cardiac risk with stress MPI is based on detection of a large area of multivessel ischemia together with markers of LV dysfunction such as LV dilation, increased thallium lung uptake, and reduced left ventricular ejection fraction (LVEF). Brown et al.30 showed that ischemic defect size detected with dipyridamole thallium MPI correlated with surgical risk. A large reversible scan defect and DM identified patients at highest risk for perioperative cardiac events. Lette et al.31 also found that large perfusion defects in multiple coronary territories or transient LV dilation with dipyridamole identified high-risk patients with a 52% incidence of perioperative cardiac death or MI. In contrast, the 63% of the patients with normal MPI or small apical scan defects and normal LV cavity size had a perioperative event rate of only 1.3%. In the study of SPECT thallium MPI prior to aortic aneurysm surgery discussed earlier, Vanzetto et al.28 showed that the best multivariate predictor of perioperative cardiac death or MI was the number of ischemic segments detected by dipyridamole SPECT MPI. A classification of perioperative risk based on MPI results is shown in Table 27-5, with specific patient examples shown in Figures 27-1 and 27-2.
Table 27-5 Prognostic Gradient of Nuclear Stress Nuclear Perfusion Imaging
| Very Low Risk: |
| Normal stress perfusion study with normal LVEF |
| Low Risk: |
| Stress-induced or fixed perfusion defects of small size with normal LVEF |
| Intermediate Risk: |
| Stress-induced or fixed perfusion defects of moderate size without LV dilation or increased thallium-201 lung uptake |
| Mildly to moderately depressed resting LVEF 35%-49% |
| High Risk: |
| Large stress-induced perfusion defect (particularly if anterior) |
| Multiple stress-induced perfusion defects of moderate size |
| Large, fixed perfusion defect with LV dilation or increased thallium-201 lung uptake |
| Moderate stress-induced perfusion defect with LV dilation, increased thallium-201 lung uptake, or diabetes mellitus |
| Severely depressed LVEF < 35% |
LVEF, left ventricular ejection fraction; LV, left ventricular.
Based in part on summary recommendations from International Nuclear Cardiology Retreat96 and ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction.97
PERIOPERATIVE MEDICAL TREATMENT
For patients found to be at increased perioperative risk, medical therapy should be considered. While Mangano et al.32 showed no reduction in perioperative risk with atenolol treatment, Poldermans et al.33 found a marked reduction in risk with bisoprolol treatment. Recent studies of perioperative β-blockade in diabetic34 and vascular surgery35 patients have shown no clear benefit. The multicenter Perioperative Ischemic Evaluation (POISE) Trial36 showed conflicting results. Perioperative metoprolol treatment resulted in an overall reduction in perioperative cardiac events (driven primarily by a reduction in MI) but significantly increased perioperative stroke and death. Recommendations for the use of perioperative β-blockers range from the view of no clear benefit37–39 to the view that all vascular surgery patients should receive β-blockers and omit preoperative stress imaging.40 Two recent meta-analyses also showed contradictory results about the benefit of perioperative β-blockade.41,42 Further research on the role of perioperative β-blockade is warranted, but the 2006 ACC/AHA focused update on perioperative β-blocker therapy43 recommends perioperative β-blockers for most patients undergoing vascular surgery and for other surgical patients with CAD or multiple cardiac risk factors.
The benefit of perioperative statin therapy has been addressed in two studies. A case-control study found significantly lower mortality in vascular surgery patients who were treated with perioperative statins.44 A randomized trial of atorvastatin in vascular surgery patients showed an early and sustained reduction in postoperative mortality.45
CORONARY ANGIOGRAPHY AND REVASCULARIZATION
PCI has not been clearly shown to reduce perioperative mortality. There is a high cardiac risk if noncardiac surgery is performed within 4 to 6 weeks after bare metal stenting and concern about risk within 12 months after treatment with a drug-eluting stent.46–48 While balloon angioplasty is sometimes considered as an alternative to avoid the risk of stent thrombosis, perioperative risk early after angioplasty may be similar to that with a coronary stent.49 The Guidelines recommend delaying elective noncardiac surgery for at least 14 days after balloon angioplasty, 30 to 45 days after bare metal stenting, and 365 days after a drug-eluting stent (Fig. 27-3).2
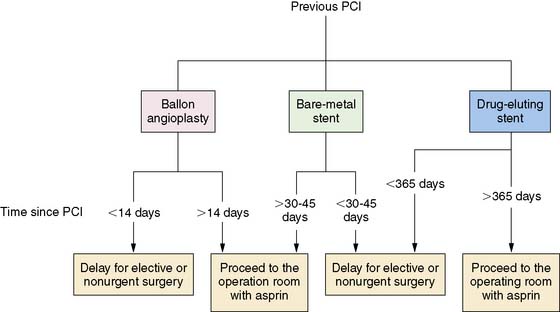
Figure 27-3 Recommended timing of noncardiac surgery after prior percutaneous coronary intervention (PCI). Reproduced, with permission, from Fleisher et al.2
(From Fleisher LA, Beckman JA, Brown KA, et al: ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery] developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery [erratum appears in J Am Coll Cardiol 50:e242, 2007], J Am Coll Cardiol 50:e159-e241, 2007.)
The Coronary Artery Revascularization Prophylaxis (CARP) trial showed no benefit of revascularization over optimal medical management in most patients undergoing noncardiac vascular surgery (Fig. 27-4)50 when the highest-risk patients with left main CAD or severe LV dysfunction were excluded. Although there is some uncertainty about the subgroup of patients with multiple clinical risk factors and a large area of ischemia detected with preoperative imaging,50,51 most patients at increased cardiac risk should be managed with appropriate medical therapy, with coronary angiography and revascularization reserved for the highest-risk patients who will benefit from improvement in long-term prognosis.
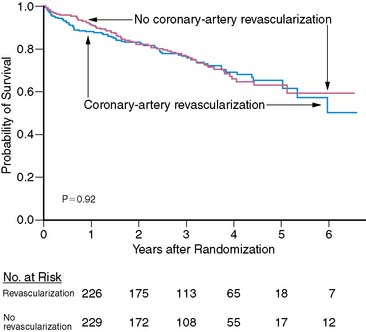
Figure 27-4 Survival of patients undergoing vascular surgery in the Coronary Artery Revascularization Prophylaxis (CARP) trial.50 There was no significant difference in survival with or without preoperative revascularization.
Reproduced, with permission, from McFalls et al.50 Copyright © 2004 Massachusetts Medical Society. All rights reserved.
LONG-TERM PROGNOSIS AFTER NONCARDIAC SURGERY
When performing a preoperative consultation for cardiac risk, assessment and management of long-term prognosis may be even more important than short-term perioperative cardiac risk, particularly in vascular surgery patients. Table 27-6 summarizes late cardiac events that occurred in a population dominated by vascular surgery procedures. It appears that long-term cardiac mortality has been decreasing over the past decade, as noted previously in Table 27-2 for perioperative events, but CAD still correlates with a worse long-term prognosis. Cardiac risk factors should be appropriately treated (hypertension, diabetes, hyperlipidemia, smoking, ischemia, heart failure). This evaluation and recommendations should be communicated to the patient and the primary care physician so that it becomes part of long-term medical care. This is especially important for any patient who sustains a severe ischemic episode, heart failure, or nonfatal MI in the perioperative period, since all of these events carry high risk for future cardiac events over the next 2 to 4 years.52
In a review of the vascular surgery literature from 1975 through 1987, Hertzer53 noted that late mortality in patients with aortic aneurysms was 44% in those with probable CAD versus 22% in those without CAD. This review also noted increased long-term mortality among patients having infrainguinal or carotid surgery based on the presence of symptomatic CAD. L’Italien et al.54 also showed that patients undergoing infrainguinal or carotid surgery had significantly lower cumulative survival compared with aortic surgery patients. However, after adjustment for cardiac risk factors, the differences in long-term cardiac event rates for the specific surgical procedures were significantly reduced. Multivariate analysis showed that a history of angina or CHF, DM, fixed dipyridamole thallium defects, and perioperative MI were the best predictors of long-term events. In contrast, survival in patients without any CAD risk factors was above 95% in all three surgical groups. Therefore, these authors concluded that cardiac status, DM, and MPI results were critical predictors for long-term prognosis, whereas the specific type of vascular surgery was less important.
UTILIZING PREOPERATIVE TESTING FOR LONG-TERM PROGNOSIS
There is evidence that both the data collected and the process of evaluation and subsequent workup or intervention (medical or surgical) have resulted in better outcomes and predictions. A recent review of a national Medicare population sample identified a cohort of patients (n = 6895) who underwent elective vascular surgery during a 17-month period in 1991 and 1992. The authors noted a relatively high mortality (14%) at 1 year of follow-up. However, in those patients undergoing preoperative stress testing with or without coronary bypass surgery, the mortality rate was lower (<6%).55 In another follow-up study of peripheral vascular surgery patients (n = 343) for a mean of 40 months, cardiac events were significantly more frequent in those who had an LVEF of less than 35% or ischemia on dipyridamole-thallium imaging.56 Other studies31,57 also confirm the value of semiquantitative analysis of MPI when using these types of preoperative tests to predict future cardiac events. All these studies have the ability to combine an assessment of myocardial ischemia and left ventricular function into a more useful clinical index.
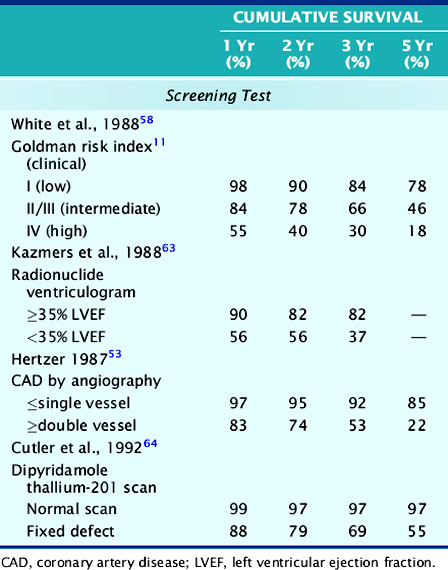
Table 27-7 shows the long-term survival after aortic vascular surgery in different patient studies using various clinical parameters that have demonstrated significant preoperative prognostic utility. The clinical risk index58 can be divided into low, intermediate, and high-risk groups, and there is a significant survival difference between low- and high-risk patients. As expected, the mortality is elevated in the high-risk group, but the low-risk patients have 22% mortality over 5 years. This is the same group of patients who are often sent directly to elective surgery without cardiac testing and typically have low (˜2%) perioperative event rates. It is possible that assessment of functional capacity or use of stress MPI could identify some patients at higher long-term risk, even in this lower-risk group. Intermediate-risk patients also have a fairly high (>50%) mortality over the 5-year follow-up period and may warrant further investigation.
LVEF is another powerful predictor of survival in patients with CAD. Although early studies of MPI utilized indirect measurements of LV function, such as increased LV size31,57,59 and extent of fixed defects,24,60 it is now routine to measure LVEF with gated SPECT imaging.61,62 In Table 27-7, Kazmers et al.63 show that 3-year survival is significantly better in patients with an LVEF above 35% than in those with lower LVEF. However, even patients with preserved LVEF have a mortality of approximately 6% per year. Therefore, having an LVEF above 35% does not necessarily ensure an excellent event-free survival.
The long-term prognostic power of multivessel CAD has been demonstrated by Hertzer.53 In Table 27-7, a summary of data collected after vascular surgery is shown. These investigators noted that patients with two- or three-vessel CAD had a 5-year event-free survival of only 22%. In contrast, those patients with only single-vessel CAD or normal coronary angiograms had a survival of 85%. Although this identifies a relatively low-risk group with a 3% per year cardiac event rate, MPI can also identify these low-risk patients without the need for routine coronary angiography.
A study of long-term prognosis by Cutler et al.64 is presented in Table 27-7. The authors report a very low event rate of less than 1% per year in vascular patients who had a normal stress MPI scan. In contrast, fixed perfusion defects identified patients at higher long-term risk. In a subsequent study,57 Emlein et al. reported that the extent of LV cavity dilation was the most important factor in predicting cardiac death among patients with abnormal MPI scans. These studies clearly show that patients with a normal MPI scan have a very low risk of long-term cardiac events, similar to the data in Table 27-3 that show a high negative predictive value of a normal MPI scan for perioperative events. Therefore, pharmacologic stress MPI can be used to assess appropriate patient populations for both perioperative and long-term events by use of slightly different predictors.
In a study of long-term prognosis of peripheral vascular surgery patients (n = 343) for a mean of 40 months, cardiac events were significantly more frequent in those who had an LVEF of below 35% or ischemia on dipyridamole thallium MPI.56 Other studies57,65 also confirm the value of semiquantitative analysis of MPI to predict long-term cardiac events. These studies combine an assessment of myocardial ischemia and LV function into a more accurate prognostic index.
When vascular or other surgical patients are identified at high long-term risk for cardiac events, medical therapy for coronary disease and appropriate use of revascularization using standard guidelines should be considered. Despite their increased operative mortality with CABG,66 patients with peripheral vascular disease derive equal or greater long-term survival benefit from revascularization. The European Coronary Surgery Study Group showed that in patients with peripheral vascular disease and multivessel CAD, the 5-year survival of 66% was significantly improved to 89% with CABG.67 An analysis of data from the Coronary Artery Surgery Study registry by Rihal et al.68 of patients with combined coronary and peripheral arterial disease showed a significant survival benefit of CABG for patients with three-vessel CAD. The benefit was greatest for patients with reduced LVEF. Landesberg et al.69,70 showed that long-term survival in vascular surgery patients with moderate to severe ischemia detected by stress MPI, long-term survival was significantly improved by revascularization. These authors noted that patients with moderate to severe ischemia who were selected for revascularization had a 5-year survival (74%) similar to that patients with only mild or no perfusion defects. In contrast, those patients with moderate to severe defects who did not undergo revascularization had a 53% 5-year survival.70 Patients with reduced LVEF appeared to derive the greatest benefit.
CONCLUSION: USE OF GUIDELINES FOR COST-EFFECTIVE RISK ASSESSMENT
Data from Almanaseer et al.71 and Froelich et al.72 show that adherence to the Guidelines significantly reduced preoperative stress imaging, perioperative length of stay and cost, and increased use of perioperative β-blockers while maintaining a low rate of perioperative cardiac death and MI.
1. Rosamond W., Flegal K., Furie K., et al. Heart disease and stroke statistics 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25-e146.
2. Fleisher L.A., Beckman J.A., Brown K.A., et al: ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery [erratum appears in J Am Coll Cardiol 50(17):e242, 2007] J Am Coll Cardiol, 50(17):e159-e241, 2007.
3. Mangano D.T., Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med. 1995;333(26):1750-1756.
4. Fleisher L.A., Eagle K.A. Clinical practice. Lowering cardiac risk in noncardiac surgery. N Engl J Med. 2001;345(23):1677-1682.
5. Poldermans D., Hoeks S.E., Feringa H.H. Pre-operative risk assessment and risk reduction before surgery. J Am Coll Cardiol. 2008;51(20):1913-1924.
6. Hoeks S.E., Schouten O., van d V., Poldermans D. Preoperative cardiac testing before major vascular surgery. J Nucl Cardiol. 2007;14(6):885-891.
7. Brindis R.G., Douglas P.S., Hendel R.C., et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol. 2005;46(8):1587-1605.
8. Califf R.M., Armstrong P.W., Carver J.R., D’Agostino R.B., Strauss W.E. Task force 5: Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol. 1996;27:1007-1019.
9. Hlatky M.A., Boineau R.E., Higginbotham M.B., et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Hassan S.A., Hlatky M.A., Boothroyd D.B., et al. Outcomes of noncardiac surgery after coronary bypass surgery or coronary angioplasty in the Bypass Angioplasty Revascularization Investigation (BARI) [commentary]. Am J Med. 2001;110(4):260-266.
11. Goldman L., Caldera D.L., Nussbaum S.R., et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
12. Detsky A.S., Abrams H.B., Forbath N., Scott J.G., Hilliard J.R. Cardiac assessment for patients undergoing noncardiac surgery: A multifactorial clinical risk index. Arch Intern Med. 1986;146:2131-2134.
13. Lee T.H., Marcantonio E.R., Mangione C.M., et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
14. Gersh B.J., Rihal C.S., Rooke T.W., Ballard D.J. Evaluation and management of patients with both peripheral vascular and coronary artery disease. J Am Coll Cardiol. 1991;18:203-214.
15. Hertzer N.R., Beven E.G., Young J.R., et al. Coronary artery disease in peripheral vascular patients: A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223-233.
16. Hollenberg M., Mangano D.T., Browner W.S., London M.J., Tubau J.F., Tateo I.M. Predictors of postoperative myocardial ischemia in patients undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268(2):205-209.
17. Mangano D.T., Browner W.S., Hollenberg M., Li J., Tateo I.M. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268(2):233-239.
18. Kertai M.D., Boersma E., Klein J., van U.H., Bax J.J., Poldermans D. Long-term prognostic value of asymptomatic cardiac troponin T elevations in patients after major vascular surgery. Eur J Vasc Endovasc Surg. 2004;28(1):59-66.
19. Weinrauch L.A., D’Elia J.A., Healy R.W., et al. Asymptomatic coronary artery disease: Angiography in diabetic patients before renal transplantation: Relations of findings to postoperative survival. Ann Intern Med. 1978;88:346-348.
20. Heston T.F., Norman D.J., Barry J.M., Bennett W.M., Wilson R.A. Cardiac risk stratification in renal transplantation using a form of artificial intelligence. Am J Cardiol. 1997;79:415-417.
21. Iqbal A., Gibbons R.J., McGoon M.D., Steiroff S., Frohnert P.T., Velosa J.A. Noninvasive assessment of cardiac risk in insulin-dependent diabetic patient being evaluated for pancreatic transplantation using thallium-201 myocardial perfusion scintigraphy. Transplant Proc. 1991;23:1690-1691.
22. Le A., Wilson R., Douek K., et al. Prospective risk stratification in renal transplant candidates for cardiac death. Am J Kidney Dis. 1994;24:65-71.
23. Kryzhanovski V.A., Beller G.A. Usefulness of preoperative noninvasive radionuclide testing for detecting coronary artery disease in candidates for liver transplantation. Am J Cardiol. 1997;79(7):986-988.
24. Shaw L.J., Eagle K.A., Gersh B.J., Miller D.D. Meta-analysis of intravenous dipyridamole-thallium-201 imaging (1985 to 1994) and dobutamine echocardiography (1991 to 1994) for risk stratification before vascular surgery. J Am Coll Cardiol. 1996;27(4):787-798.
25. Hendel R.C., Chen M.H., L’Italien G.J., et al. Sex differences in perioperative and long-term cardiac event-free survival in vascular surgery patients: An analysis of clinical and scintigraphic variables. Circulation. 1995;91:1044-1051.
26. Eagle K.A., Coley C.M., Newell J.B., et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110:859-866.
27. L’Italien G.J., Paul S.D., Hendel R.C., et al. Development and validation of a Bayesian model for perioperative cardiac risk assessment in a cohort of 1,081 vascular surgical candidates. J Am Coll Cardiol. 1996;27(4):779-786.
28. Vanzetto G., Machecourt J., Blendea D., et al. Additive value of thallium single-photon emission computer tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol. 1996;77:143-148.
29. Bartels C., Bechtel J.F.M., Hossmann V., Horsch S. Cardiac risk stratification for high-risk vascular surgery. Circulation. 1997;95:2473-2475.
30. Brown K.A., Rowen M. Extent of jeopardized viable myocardium determined by myocardial perfusion imaging best predicts perioperative cardiac events in patients undergoing noncardiac surgery. J Am Coll Cardiol. 1993;21:325-330.
31. Lette J., Waters D., Cerino M., Picard M., Champagne P., Lapointe J. Preoperative coronary artery disease risk stratification based on dipyridamole imaging and a simple three-step, three-segment model for patients undergoing noncardiac vascular surgery or major general surgery. Am J Cardiol. 1992;69:1553-1558.
32. Mangano D.T., Layug E.L., Wallace A., Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335:1713-1720.
33. Poldermans D., Boersma E., Bax J.J., et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789-1794.
34. Juul A.B., Wetterslev J., Gluud C., et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ. 2006;332(7556):1482.
35. Yang H., Raymer K., Butler R., Parlow J., Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
36. POISE Study GroupDevereaux P.J., Yang H., et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847.
37. Devereaux P.J., Yusuf S., Yang H., Choi P.T., Guyatt G.H. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing noncardiac surgery based on reliable evidence? CMAJ. 2004;171(3):245-247.
38. Devereaux P.J., Leslie K., Yang H. The effect of perioperative beta-blockers on patients undergoing noncardiac surgery: Is the answer in. Can J Anaesth. 2004;51(8):749-755.
39. Devereaux P.J., Beattie W.S., Choi P.T., et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;331(7512):313-321.
40. Poldermans D., Bax J.J., Schouten O., et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol. 2006;48(5):964-969.
41. Schouten O., Shaw L.J., Boersma E., et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis. 2006;17(2):173-179.
42. Bangalore S., Wetterslev J., Pranesh S., Sawhney S., Gluud C., Messerli F.H. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis [commentary]. Lancet. 2008;372(9654):1962-1976.
43. Fleisher L.A., Beckman J.A., Brown K.A., et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol. 2006;47(11):2343-2355.
44. Poldermans D., Bax J.J., Kertai M.D., et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107(14):1848-1851.
45. Durazzo A.E., Machado F.S., Ikeoka D.T., et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39(5):967-975.
46. Kaluza G.L., Joseph J., Lee J.R., Raizner M.E., Raizner A.E. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35(5):1288-1294.
47. Wilson S.H., Fasseas P., Orford J.L., et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
48. Compton P.A., Zankar A.A., Adesanya A.O., Banerjee S., Brilakis E.S. Risk of noncardiac surgery after coronary drug-eluting stent implantation. Am J Cardiol. 2006;98(9):1212-1213.
49. Leibowitz D., Cohen M., Planer D., et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
50. McFalls E.O., Ward H.B., Moritz T.E., et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351(27):2795-2804.
51. Boersma E., Poldermans D., Bax J.J., et al. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001;285(14):1865-1873.
52. Mangano D.T., Browner W.S., Hollenberg M., Li J., Tateo I.M. Long-term cardiac prognosis following noncardiac surgery. JAMA. 1992;268:233-239.
53. Hertzer N.R. Basic data concerning associated coronary artery disease in peripheral vascular patients. Ann Vasc Surg. 1987;1:616-620.
54. L’Italien G.J., Cambria R.P., Cutler B.S., et al. Comparative early and late cardiac morbidity among patients requiring different vascular surgery procedures. J Vasc Surg. 1995;21:935-944.
55. Fleisher L.A., Eagle K.A., Shaffer T., Anderson G.F. Perioperative- and long-term mortality rates after major vascular surgery: the relationship to preoperative testing in the Medicare population. Anesth Analg. 1999;89(4):849-855.
56. Schueppert M.T., Kresowik T.F., Corry D.C., et al. Selection of patients for cardiac evaluation before peripheral vascular operations. J Vasc Surg. 1996;23(5):802-808.
57. Emlein G., Villegas B., Dahlberg S., Leppo J. Left ventricular cavity size determined by preoperative dipyridamole thallium scintigraphy as a predictor of late cardiac events in vascular surgery patients. Am Heart J. 1996;131:907-914.
58. White G.H., Advani S.M., Williams R.A., Wilson S.E. Cardiac risk index as a predictor of long-term survival after repair of abdominal aortic aneurysm. Am J Surg. 1988;156:103-107.
59. Lette J., Waters D., Champagne P., Picard M., Cerino M., Lapointe J. Prognostic implications of a negative dipyridamole-thallium scan: Results in 360 patients. Am J Med. 1992;92:615-620.
60. Hendel R.C., Whitfield S.S., Villegas B.J., Cutler B.S., Leppo J.A. Prediction of late cardiac events by dipyridamole thallium imaging in patients undergoing elective vascular surgery. Am J Cardiol. 1992;70:1243-1249.
61. Garcia E.V., Bacharach S.L., Mahmarian J.J., et al. Imaging guidelines for nuclear cardiology procedures. Part 1. J Nucl Cardiol. 1996;3:G1-G46.
62. Sharir T., Germano G., Kavanagh P.B., et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100(10):1035-1042.
63. Kazmers A., Cerqueira M.D., Zierler R.E. Perioperative and late outcome in patients with left ventricular ejection fraction of 35% or less who require major vascular surgery. J Vasc Surg. 1988;8:307-315.
64. Cutler B.S., Hendel R.C., Leppo J.A. Dipyridamole-thallium scintigraphy predicts perioperative and long-term survival after major vascular surgery. J Vasc Surg. 1992;15:972-981.
65. Lette J., Waters D., Bernier H., et al. Perioperative and long-term cardiac risk assessment. Ann Surg. 1991;216:192-204.
66. Mullany C.J., Darling G.E., Pluth J.R., et al. Early and late results after isolated coronary artery bypass surgery in 159 patients aged 80 years and older. Circulation. 1990;82(Suppl IV):IV-229-IV-236.
67. European Coronary Surgery Study Group. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet. 1982;2(8309):1173-1180.
68. Rihal C.S., Eagle K.A., Mickel M.C., Foster E.D., Sopko G., Gersh B.J. Surgical therapy for coronary artery disease among patients with combined coronary artery and peripheral vascular disease. Circulation. 1995;91(1):46-53.
69. Landesberg G., Wolf Y., Schechter D., et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after carotid endarterectomy. Stroke. 1998;29(12):2541-2548.
70. Landesberg G., Mosseri M., Wolf Y.G., et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after major vascular surgery. Circulation. 2003;108(2):177-183.
71. Almanaseer Y., Mukherjee D., Kline-Rogers E.M., et al. Implementation of the ACC/AHA guidelines for preoperative cardiac risk assessment in a general medicine preoperative clinic: improving efficiency and preserving outcomes. Cardiology. 2005;103(1):24-29.
72. Froehlich J.B., Karavite D., Russman P.L., et al. American College of Cardiology/American Heart Association preoperative assessment guidelines reduce resource utilization before aortic surgery. J Vasc Surg. 2002;36(4):758-763.
73. Young A.E., Sandberg G.W., Couch N.P. The reduction of mortality of abdominal aortic aneurysm resection. Am J Surg. 1977;134:585-590.
74. Hertzer N.R. Fatal myocardial infarction following abdominal aortic aneurysm resection: Three hundred forty-three patients followed 6–11 years postoperatively. Ann Surg. 1980;192:667-673.
75. Cutler B.S., Leppo J.A. Dipyridamole thallium 201 scintigraphy to detect coronary artery disease before abdominal aortic surgery. J Vasc Surg. 1987;5:91-100.
76. Raby K.E., Goldman L., Creager M.A., et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med. 1989;321:1296-1300.
77. Younis L.T., Aguirre F., Byers S., et al. Perioperative and long-term prognostic value of intravenous dipyridamole thallium scintigraphy in patients with peripheral vascular disease. Am Heart J. 1990;119:1287-1292.
78. Taylor L.M.Jr., Yeager R.A., Moneta G.L., McConnell D.B., Porter J.M. The incidence of perioperative myocardial infarction in general vascular surgery. J Vasc Surg. 1991;15:52-61.
79. Kresowik T.F., Bower T.R., Garner S.A., et al. Dipyridamole thallium imaging in patients being considered for vascular procedures. Arch Surg. 1993;128:299-302.
80. McFalls E.O., Doliszny K.M., Grund F., Chute E., Chesler E. Angina and persistent exercise thallium defects: Independent risk factors in elective vascular surgery. J Am Coll Cardiol. 1993;21:1347-1352.
81. Baron J.F., Mundler O., Bertrand M., et al. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. N Engl J Med. 1994;330:663-669.
82. Bry J.D.L., Belkin M., O’Donnell T.F.Jr, et al. An assessment of the positive predictive value and cost-effectiveness of dipyridamole myocardial scintigraphy in patients undergoing vascular surgery. J Vasc Surg. 1994;19:112-124.
83. Seeger J.M., Rosenthal G.R., Self S.B., Flynn T.C., Limacher M.C., Harward T.R.S. Does routine stress-thallium cardiac scanning reduce postoperative cardiac complications? Ann Surg. 1994;219:654-663.
84. Fleisher L.A., Rosenbaum S.H., Nelson A.H., Jain D., Wackers FJTh, Zaret B.L. Preoperative dipyridamole thallium imaging and ambulatory electrocardiographic monitoring as a predictor of perioperative cardiac events and long term outcome. Anesthesiology. 1995;83:906-917.
85. Boucher C.A., Brewster D.C., Darling C., Okada R., Strauss H.W., Pohost G.M. Determination of cardiac risk by dipyridamole-thallium imaging before peripheral vascular surgery. N Engl J Med. 1985;312:389-394.
86. Fletcher J.P., Antico V.F., Gruenewald S., Kershaw L.Z. Dipyridamole-thallium scan for screening of coronary artery disease prior to vascular surgery. J Cardiovasc Surg. 1988;29:666-669.
87. Sachs R.N., Tellier P., Larmignat P., et al. Assessment by dipyridamole-thallium-201 myocardial scintigraphy of coronary risk before peripheral vascular surgery. Surgery. 1988;103:584-587.
88. Mangano D.T., London M.J., Tubau J.F., et al. Dipyridamole thallium-201 scintigraphy as a preoperative screening test: A reexamination of its predictive potential. Circulation. 1991;84:493-502.
89. Koutelou M.G., Asimacopoulos P.J., Mahmarian J.J., Kimball K.T., Verani M.S. Preoperative risk stratification by adenosine thallium 201 single-photon emission computed tomography in patients undergoing vascular surgery. J Nucl Med. 1995;2:389-394.
90. Marshall E.S., Raichlen J.S., Forman S., Heyrich G.P., Keen W.D., Weitz H.H. Adenosine radionuclide perfusion imaging in the preoperative evaluation of patients undergoing peripheral vascular surgery. Am J Cardiol. 1995;76:817-821.
91. Coley C.M., Field T.S., Abraham S.A., Boucher C.A., Eagle K.A. Usefulness of dipyridamole-thallium scanning for preoperative evaluation of cardiac risk for nonvascular surgery. Am J Cardiol. 1992;69:1280-1285.
92. Shaw L., Miller D.D., Kong B.A., et al. Determination of perioperative cardiac risk by adenosine thallium-201 myocardial imaging. Am Heart J. 1992;124:861-869.
93. Younis L., Stratmann H., Takase B., Byers S., Chaitman B.R., Miller D.D. Preoperative clinical assessment and dipyridamole thallium-201 scintigraphy for prediction and prevention of cardiac events in patients having major noncardiovascular surgery and known or suspected coronary artery disease. Am J Cardiol. 1994;74:311-317.
94. Stratmann H.G., Younis L.T., Wittry M.D., Amato M., Mark A.L., Miller D.D. Dipyridamole technetium-99m sestamibi myocardial tomography for preoperative cardiac risk stratification before major or minor nonvascular surgery. Am Heart J. 1996;132:536-541.
95. Van Damme H., Pierard L., Rigo P.L.R. Cardiac risk assessment before vascular surgery: a prospective study comparing clinical evaluation, dobutamine stress echocardiography, and dobutamine Tc-99m sestamibi tomoscintigraphy. J Cardiovasc Surg. 1997;5:54-64.
96. Beller G.A., Brown K.A., Hendel R.C., Shaw L.J., Williams K.A. Unresolved issues in risk stratification: Chronic coronary artery disease including preoperative testing. J Nucl Cardiol. 1997;4:92-95.
97. Anderson J.L., Adams C.D., Antman E.M., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116(7):e148-e304.
98. Hertzer N.R. Fatal myocardial infarction following lower extremity revascularization: Two hundred seventy-three patients followed six to eleven postoperative years. Ann Surg. 1981;193:492-498.
99. Roger V.L., Ballard D.J., Hallett J.W.Jr, Osmundson P.J., Puetz P.A., Gersh B.J. Influence of coronary artery disease on morbidity and mortality after abdominal aortic aneurysmectomy: A population-based study, 1971–1987. J Am Coll Cardiol. 1989;14:1245-1252.
100. Hertzer N.R., Young J.R., Beven E.G., et al. Late results of coronary bypass in patients with infrarenal aortic aneurysms: The Cleveland Clinic study. Ann Surg. 1987;205:360-367.
101. Poldermans D., Arnese M., Fioretti P.M., et al. Sustained prognostic value of dobutamine stress echocardiography for late cardiac events after major noncardiac vascular surgery. Circulation. 1997;95(1):53-58.