Country
% of IgAN
Spain
24
France
33
Italy
37
Czech Republic
37
UK
39
Estonia
35
Poland
51
Sweden
30
Norway
19
Finland
40
Apart from genetic conditioning, exposure to different environmental factors may be considered, including diet, intestinal microbiota, alcohol intake, or chronic intestinal diseases favoring high intestinal permeability (e.g., celiac disease, which is unevenly distributed in Europe) [12, 13]. Finally different pathogen exposure in various European countries due to the climatic variability might also be considered.
IgAN is a major cause of need of dialysis and transplantation worldwide, with renal survival ranging from 95 % to 77 % at 10 years [2–4, 14]. There is a higher prevalence of patients entering dialysis because of IgAN in Northern European countries in comparison to Southern ones (ERA-EDTA Registry, unpublished data presented at 2013 ERA-EDTA Congress), suggesting various risk factors not only for development of IgAN but also for progression, including a different genetic conditioning for factors favoring the sclerotic progression of renal disease [15]. However, it is also possible that the variability of treatment choice pays a role and this has never been explored before.
In Norway at 10 years, 77 % of patients with IgAN survive to end-stage renal disease (ESRD) [16], while this survival observed after 20 years in most reports from Southern Europe (Spain, France, Italy) [17, 18]. Furthermore in Norway also patients with low progression at 10 years can develop ESRD later on suggesting particularly aggressive forms of IgAN or a failure of treatment.
12.2 Risk Factors for Progression of IgA Nephropathy
There is a need for detecting new risk factors for the progression of IgAN, in order to identify patients deserving a more early and aggressive therapy meanwhile avoiding overtreatment of benign cases who will never lose their renal function. Indeed about 20 % of patients enter during the follow-up persistent remission [19].
The role of pathology lesions detected at renal biopsy as risk factors for the progression of IgAN has been enlightened by the Oxford classification of IgAN study [20–22]. This collaborative work identified four pathological features (mesangial proliferation (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy/interstitial fibrosis (T), resulting in a MEST score) which predicted renal outcome independently from all clinical indicators at the time of renal biopsy and during follow-up. However, the limited number of patients enrolled in the Oxford classification (265 cases) and their heterogeneous origin (from four continents) indicated a need for validation studies. Several validation studies have been published (reviewed in [23]) over the following years with variable results, but all had in common the limitation of investigating small cohorts, with the exception of a Chinese study on 1,026 cases [24]. In small cohorts, different baseline features (age, eGFR, sclerotic lesions, treatments) were likely to condition the final results.
12.3 The VALIGA Cohort Investigated: Patients and Analysis Methods
ERA-EDTA Immunonephrology Working Group VALIGA (Validation Study of the Oxford Classification of IgAN), granted by the ERA-EDTA Immunonephrology Working Group, aimed at investigating a large number of European patients with various clinical presentations encompassing the whole spectrum of IgAN, looking for the prognostic value of the pathology features indicated by the Oxford study [25]. The enrolment criteria allowed also the inclusion of patients who did not enter the original Oxford study, as those presenting at renal biopsy with mild proteinuria (<0.5 g/day) or having an already advanced form of IgAN, with residual glomerular filtration rate (e-FGFR) <30 ml/min/1.73 m2. The VALIGA study had a very successful enrolment accounting for 1,147 patients with IgAN with renal biopsy material available for central review and suitable data sets at renal biopsy and during the follow-up. This study has produced the largest single cohort reported in the worldwide literature on patients with IgAN, comparable only to the Chinese cohort [24].
Clinical data were collected by the coordinating center in Turin, Italy, from 55 nephrology and pathology institutions of 13 European countries (Fig. 12.1) scored by the local pathologists. Each renal biopsy was centrally reviewed in Oxford, UK. Statistical analysis was done in Montreal, Canada. The outcomes (rate of renal function decline and the combined survival from a 50 % reduction of renal function or end-stage renal failure) were detected over a median follow-up of 4.8 years. In patients with an initial proteinuria <0.5 g/day, the outcome considered was the survival from 30 % reduction in eGFR.

Fig. 12.1
European map of the centers participating in the Validation Study of the Oxford Classification of IgAN (VALIGA) in Europe. The study enrolled 1,147 patients with IgA nephropathy from 55 centers from 13 European countries, which were followed for a median of 4.8 years
The VALIGA cohort [25] was well representative of the whole spectrum of the disease. Ethnicity was for 97 % Caucasian, and gender was 73 % men. Patients’ mean age was 36 years; 15 % of the patients were children. At renal biopsy their mean eGFR was 73 ml/min/1.73 m2 and proteinuria 1.3 (0.6–2.6) g/day. Patients were presenting different degrees of proteinuria and various stages of chronic kidney disease at renal biopsy (Fig. 12.2). The mean yearly loss of the eGFR was 1.8 ml/min/1.73 m2/year. The combined end point of 50 % reduction of renal function or end-stage renal failure was reached in 16 % of the cases. The renal function survival from the combined end point was 74 % at 10 years.
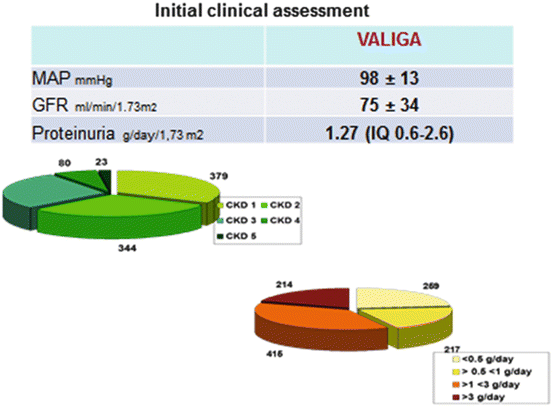

Fig. 12.2
Clinical data at initial assessment of patients with IgA nephropathy enrolled in VALIGA. MAP mean arterial blood pressure, GFR glomerular filtration rate, CKD chronic kidney disease stages
VALIGA patients had mesangial proliferative changes (M1) in 28 % of the cases, endocapillary hypercellularity (E1) in 11 %, segmental glomerulosclerosis (S1) in 70 %, and tubulointerstitial changes (T1 or T2) in 21 %. Moreover 28 % had arteriolar damage and 7 % crescents. The lesions were differently distributed according to baseline proteinuria and renal function, with a clear increase in MEST score in patients with high levels of proteinuria.
12.4 Risk Factors for Progression in the VALIGA Cohort
In the whole VALIGA cohort, the value of the MEST score to predict the rate of renal function decline as well as survival without ESRD or 50 % reduction in initial GFR was validated, except for the E lesion, rare within these patients.
The value of mesangial proliferation was assessed not only in the whole cohort but also in subgroups of patients with early diagnosis and proteinuria <0.5 g/day and even in those with renal biopsy in advanced stages, with eGFR <30 ml/min/1.73 m2. In patients with low proteinuria, mesangial proliferation and endocapillary hypercellularity significantly influenced the survival from progression to levels of proteinuria ≥1 g/day and ≥2 g/day. In patients with reduced renal function at renal biopsy, mesangial proliferation maintained its value as a predictive factor.
12.5 The Effect of Therapy on Progression of VALIGA Patients
The VALIGA cohort gathered 1,147 patients with IgAN from 13 European countries. This large database allowed an analysis on the possible long-term benefits of CS, in patients with any level of GFR, including those patients with an initial eGFR ≤50 ml/min/1.73 m2 [26].
Most of the patients (86 %) received renin-angiotensin system (RAS) blockade and half of them had also immunosuppressive regimens (mostly oral or pulse corticosteroids (CSs), seldom associated with other immunosuppressants including azathioprine, cyclophosphamide, and mycophenolate mofetil) (Fig. 12.3). In the VALIGA cohort, 523 patients (46 %) received corticosteroids (CSs)/immunosuppression and 624 (54 %) did not.
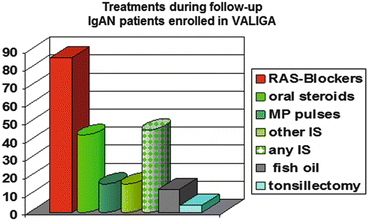

Fig. 12.3
Distribution of treatments in patients with IgA nephropathy enrolled in VALIGA
Since this was a retrospective registry, it reflected the spontaneous attitude for treating patients with IgAN. The European nephrologists chose to treat with CS patients with signs of clinical activity, including high proteinuria and low eGFR, and pathology signs of potentially progressive disease, including high MEST score. However, in spite of most frequent risk factor for progression, the outcome of patients who had received CS was significantly better than those who did not. After therapy eGFR increased and proteinuria decreased, both significantly.
The benefits of CS were further assessed using a propensity score, identifying 184 patients treated with CS and RAS inhibitors to 184 patients using RAS blockers only, who were similar for clinical and pathological data. The renal outcomes of the patients who received CS in addition to RAS blockade resulted to be significantly better than in patients who had RAS inhibition alone as far as rate of renal function decline and proteinuria reduction were concerned [26].
12.6 Differences in Treatment Approaches Within Europe
As mentioned above, the large VALIGA cohort allowed a sub-analysis of subjects with IgAN enrolled in different European countries. We aimed at investigating differences in clinical and pathological features and the influence of immunosuppressive regimen on the final outcome. We considered 247 VALIGA patients enrolled in East-Northern Europe (including the Netherlands, England, Scotland, Estonia, Poland, and Sweden) and 600 cases from South Europe (Italy).
Patients from Northern and Southern Europe had rather similar demographic and clinical features at renal biopsy (Table 12.2). Gender distribution and mean age at renal biopsy were similar (70 and 73 % of males, respectively, in the two cohorts), with a mean age of 34 and 36 years and similar proportion of children (15–19 %). Also baseline eGFR, proteinuria, and mean arterial blood pressure (MAP) were similar in patients with IgAN enrolled in Northern and in Southern Europe (mean eGFR 77 ± 30 and 76 ± 31 ml/min/1.73 m2, median proteinuria 1.5 and 1.8 g/day, MAP 99 and 97 mmHg, respectively). Pathology features were slightly worse in patients enrolled in Northern Europe in comparison to those in Southern Europe for all the MEST scores, with significantly higher S and T scores, in spite of similar mean baseline values of eGFR and proteinuria. The follow-up was 4.8–5.8 years, with median follow-up proteinuria of 1 and 0.7 g/day, respectively, in patients from North and South Europe (Table 12.2).
Table 12.2
Baseline data of patients with IgA nephropathy enrolled in Northern European countries (England, Scotland, Estonia, Poland, and Sweden) and in Southern Europe (Italy)
|
VALIGA
|
VALIGA
|
|
|---|---|---|
|
Northern EU countries
|
Southern EU countries
|
|
|
Number of patients
|
274
|
600
|
|
Male (%)
|
70
|
73
|
|
Age (years)
|
34.3 ± 15
|
36 ± 17.5
|
|
Pediatric subjects (%)
|
15
|
19
|
|
eGFR (ml/min/1.73 m2)
|
77 ± 30
|
76 ± 31
|
|
Proteinuria (g/day)
|
1.5 (0.6–3.1)
|
1.8 (0.5–2.3)
|
|
MAP (mmHg)
|
99 ± 13
|
97 ± 13
|
|
Pathology features
|
||
|
M1 (%)
|
33
|
25
|
|
E1 (%)
|
14
|
9
|
|
S1 (%)
|
74*
|
65
|
|
T1–T2 (%)
|
23+
|
18
|
|
Follow-up
|
||
|
Duration of follow-up (years)
|
6.8 ± 4.6
|
5.8 ± 4
|
|
Follow-up proteinuria (g/day)
|
1 (0.4–1.7)
|
0.7 (0.4–1.5)
|
The treatment options chosen by the nephrologists in different European countries (Table 12.3) were similar in the use of RAS blockade (84 % in both European geographic areas), while there was a huge difference in the use of CS/IS, prescribed to 28 % only of IgAN patients in Northern Europe versus 53 % in Southern Europe.
Table 12.3
Clinical outcome of patients with IgA nephropathy enrolled in Northern European countries (England, Scotland, Estonia, Poland, and Sweden) and in Southern Europe (Italy)
|
VALIGA
|
VALIGA
|
|
|---|---|---|
|
Northern EU countries
|
Southern EU countries
|
|
|
Number of patients
|
247
|
600
|
|
Treatment
|
||
|
RAS blockade (%)
|
84
|
84
|
|
Steroids/immunosuppressors (%)
|
28
|
53
|
|
Outcome
|
||
|
Rate of renal function decline (ml/min/1.73 m2/year)
|
−3.2 ± 6.6*
|
−0.8 ± 6.6
|
|
50 % decrease in eGFR
|
22*
|
9.5
|
|
ESRD
|
16*
|
8.3
|
|
50 % decrease in eGFR or ESRD (%)
|
23.5*
|
11.2
|
The outcome was significantly worse (p < 0.001) in VALIGA patients enrolled in Northern Europe for each parameter investigated (Table 12.3), including the rate of eGFR decline (3.2 versus −0.8 ml/min/1.73 m2/year), 50 % decrease in eGFR (22 % versus 9.5 %), ESRD (16 % versus 8.3 %), and the combined end point of 50 % decrease in eGFR or ESRD (23.5 % versus 11.2 %).
Hence, the unadjusted outcome of patients with IgAN enrolled in VALIGA centers of Northern Europe was significantly worse than what was found in patients from Southern Europe, in spite of similar clinical baseline data, and the most relevant risk factor detected was significantly higher use of CS/IS regimens in patients from Southern Europe.
12.7 Conclusion
The VALIGA study provided a large cohort of patients with IgAN with various clinical presentations and different treatments. It was useful not only to validate the MEST score in predicting the renal function decline but also in getting insight into the possible benefit of immunosuppressive therapy in addition to RAS blockade in comparison to RAS blockade as monotherapy. The major novelty in the results from the two publications from VALIGA study [25, 26] includes the value of mesangial and endocapillary hypercellularity particularly in early stages of IgAN and the protective effects on renal functional decline of CS/IS treatments added on RAB blockade in comparison to RAS inhibition alone.
In Europe the frequency of IgAN and the rate of progression to end-stage renal failure greatly differ in various countries, with eastward and northward. Among the factors which may be responsible for this variability, like genetic as well as environmental factors, differences in treatment approaches to IgAN could be taken into account. From the VALIGA cohort, we observed that CS/IS treatment, mostly associated with RAS inhibition, was frequently adopted in Southern Europe, while Northern Europe tended to use RAS blockade alone. A difference in clinical outcome of patients with IgAN was observed between these two geographical European areas in association with different treatment regimens.
Acknowledgments
The study was granted by the first research call of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) in 2009.
Appendix
VALIGA Steering Committee
Daniel Cattran, Terence H Cook, Rosanna Coppo, John Feehally, Ian SD Roberts, and Stéphan Troyanov
VALIGA Nephrologists
Tesar V, Maixnerova D (Prague, Czech Republic), Lundberg S (Stockholm, Sweden), Gesualdo L (Foggia-Bari, Italy), Emma F, Fuiano L (Rome, Italy), Beltrame G, Rollino C (Turin, Italy), Coppo R, Amore A, Camilla R, Peruzzi L. (Turin, Italy), Praga M (Madrid, Spain), Feriozzi S, Polci R (Viterbo, Italy), Segoloni G, Colla L (Turin, Italy), Pani A, Angioi A, Piras L (Cagliari, Italy), Feehally J (Leicester, United Kingdom), Cancarini G, Ravera S (Brescia, Italy), Durlik M (Warsaw, Poland), Moggia E (Cuneo, Italy), Ballarin J (Barcelona, Spain), Di Giulio S (Rome, Italy), Pugliese F, Serriello I (Rome, Italy), Caliskan Y, Kilicaslan I (Istanbul, Turkey), Locatelli F, Del Vecchio L (Lecco, Italy), Wetzels JFM, Peters H (Nijmegen, The Netherlands), Berg U (Huddinge, Sweden), Carvalho F, da Costa Ferreira AC (Lisbon, Portugal), Maggio M (Lodi, Italy), Wiecek A (Katowice, Poland), Ots-Rosenberg M (Tartu, Estonia), Magistroni R (Modena, Italy), Topaloglu R, Bilginer Y (Ankara, Turkey), D’Amico M (Como, Italy), Stangou M (Thessaloniki, Greece), Giacchino F (Ivrea, Italy), Goumenos D, Kalliakmani P, Gerolymos M (Patras, Greece), Galesic K (Zagreb, Croatia), Geddes C (Glasgow, United Kingdom), Siamopoulos K, Balafa O (Ioannina, Greece), Galliani M (Rome, Italy), Stratta P, Quaglia M (Novara, Italy), Bergia R, Cravero R (Biella, Italy), Salvadori M, Cirami L (Florence, Italy), Fellstrom B, Kloster Smerud H (Uppsala, Sweden), Ferrario F, Stellato T (Monza, Italy), Egido J, Martin C (Madrid, Spain), Floege J, Eitner F (Aachen, Germany), Lupo A, Bernich P (Verona, Italy), Menè P (Rome, Italy), Morosetti M (Ostia, Italy), van Kooten C, Rabelink T, Reinders MEJ (Leiden, The Netherlands), Boria Grinyo JM (Barcelona, Spain), Cusinato S, Benozzi L (Borgomanero, Italy), Savoldi S, Licata C (Ciriè, Italy), Mizerska Wasiak M (Warsaw, Poland), Martina G, Messuerotti A (Chivasso, Italy), Dal Canton A, Esposito C, Migotto C (Pavia, Italy), Triolo G, Mariano F (Turin, Italy), Pozzi C (Cinisello B., Italy), and Boero R (Turin, Italy)
VALIGA Pathologists
Mazzucco G (Turin, Italy), Giannakakis C (Rome, Italy), Honsova E (Prague, Czech Republic), Sundelin B (Stockholm, Sweden), Di Palma AM (Foggia-Bari, Italy), Ferrario F (Monza, Italy), Gutiérrez E (Madrid, Spain), Asunis AM (Cagliari, Italy), Barratt J (Leicester, United Kingdom), Tardanico R (Brescia, Italy), Perkowska-Ptasinska A (Warsaw, Poland), Arce Terroba J (Barcelona, Spain), Fortunato M (Cuneo, Italy), Pantzaki A. (Thessaloniki, Greece), Ozluk Y (Istanbul, Turkey), Steenberger E (Nijmegen, The Netherlands), Soderberg M (Huddinge, Sweden), Riispere Z (Tartu, Estonia), Furci L (Modena, Italy), Orhan D (Ankara, Turkey), Kipgen D (Glasgow, United Kingdom), Casartelli D (Lecco, Italy), Galesic Ljubanovic D (Zagreb, Croatia), Bertoni E (Florence, Italy), Cannata Ortiz P (Madrid, Spain), Groene HJ (Heidelberg, Germany), Stoppacciaro A (Rome, Italy), Bajema I, Bruijn J (Leiden, The Netherlands), Fulladosa Oliveras X (Barcelona, Spain), Maldyk J (Warsaw, Poland), and Ioachim E (Ioannina, Greece)
References
1.
Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;20(368):2402–14.CrossRef
2.
Radford Jr MG, Donadio Jr JV, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207.PubMed
3.
4.
Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, Itabashi M, Takei T, Uchida K, Nitta K. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9(3):e01756. doi:10.1371/journal.pone.0091756.CrossRef
5.
6.
7.
8.
Zaza G, Bernich P, Lupo A; ‘Triveneto’ Register of Renal Biopsies (TVRRB). Incidence of primary glomerulonephritis in a large North-Eastern Italian area: a 13-year renal biopsy study. Nephrol Dial Transplant. 2013;2:367–72.
9.
Verde E, Quiroga B, Rivera F, López-Gómez JM. Renal biopsy in very elderly patients: data from the Spanish Registry of Glomerulonephritis. Am J Nephrol. 2012;3:230–7.CrossRef
10.
11.
Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8(6):e1002765. doi:10.1371/journal.pgen.1002765. Epub 2012 Jun 21. PubMed PMID: 22737082; PubMed Central PMCID: PMC3380840.CrossRefPubMedPubMedCentral
12.
13.
14.
15.
Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013;64:339–56.CrossRefPubMedPubMedCentral
16.
17.
18.
Gutiérrez E, Hernández E, Morales E, Praga M. Immunoglobulin therapy and IgA nephropathy. Nefrologia. 2007;27:765–6.PubMed
19.
Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, Olea T, Martínez-Ara J, Segarra A, Bernis C, García A, Goicoechea M, García de Vinuesa S, Rojas-Rivera J, Praga M, Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN). Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol. 2012;23:1753–60.CrossRefPubMedPubMedCentral
20.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45.CrossRef
21.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56.CrossRef
22.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, Feehally J, Roberts IS, Amore A, Alpers CE, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator SN, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo AB, Geddes CC, Groene HJ, Haas M, SI H, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–7.CrossRef
23.
24.
Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, Chen N, Pan X, Fu P, Hu Z, Wang L, Fan Q, Zheng H, Zhang D, Wang Y, Huo Y, Lin H, Chen S, Sun S, Wang Y, Liu Z, Liu D, Ma L, Pan T, Zhang A, Jiang X, Xing C, Sun B, Zhou Q, Tang W, Liu F, Liu Y, Liang S, Xu F, Huang Q, Shen H, Wang J, Shyr Y, Phillips S, Troyanov S, Fogo A, Liu ZH. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–20.CrossRefPubMed
25.
Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A, on behalf of the VALIGA study of the ERA-EDTA Immunonephrology Working Group. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–36.CrossRefPubMedPubMedCentral
26.
Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, Roberts IS, Cattran D, Coppo R; on behalf of the VALIGA study of the ERA-EDTA Immunonephrology Working Group. Corticosteroids in IgA Nephropathy: a retrospective analysis from the VALIGA Study. J Am Soc Nephrol. 2015 Feb 12; pii: ASN.2014070697. [Epub ahead of print] PubMed
Conflict of Interest
The authors declare that they have no conflict of interest.