CHAPTER 23 Idiopathic Scoliosis
Idiopathic scoliosis is the most common cause of spinal deformity; 80% of all scoliosis cases are due to idiopathic scoliosis. Before arriving at the diagnosis of idiopathic scoliosis in a patient, other causes, such as congenital, neuromuscular (developmental or acquired), functional, inflammatory or infectious, pathologic, and intraspinal, have to be discounted. Ponseti and Friedman1 first described early-onset scoliosis in 1950. Dickson2 expounded further on that concept and proposed that idiopathic scoliosis be divided into early (0 to 5 years old) and late onset (>5 years old), based on spinal growth velocity noted in these two age groups. Presently, idiopathic scoliosis is divided into three categories based on chronologic age: infantile (birth to 2 years + 11 months), juvenile (3 years to 9 years + 11 months), and adolescent (10 years to 17 years + 11 months).3 The radiographic diagnosis necessitates measuring the coronal plane angle, using the Cobb method, as equal to or greater than 10 degrees. Patients with curves less than 10 degrees are considered to have spinal asymmetry.4,5
Epidemiology
Infantile and juvenile scoliosis are less prevalent than adolescent idiopathic scoliosis. Infantile idiopathic scoliosis is more common in Europe, constituting less than 1% of idiopathic scoliosis cases in the United States, and tends to comprise left-sided thoracic curves, typically occurring in boys. More recent reviews suggest that there might be a decline in its incidence.6 Juvenile cases are typically diagnosed at age 7 years in girls and 5 years in boys and account for about 10% to 20% of idiopathic scoliosis cases.3 In contrast to infantile idiopathic scoliosis, juvenile cases tend to occur predominantly in girls and tend to comprise right-sided curves. Between the ages of 3 and 6 years, there seems to be a similar distribution between boys and girls, however, again becoming predominant in girls after age 6 years.
Adolescent idiopathic scoliosis is more prevalent than other types of idiopathic scoliosis. Among adolescents, the prevalence of 10-degree curves is less than 3%, with about 5% of curves showing progression of greater than 30 degrees.4 This prevalence decreases as a function of curve magnitude, however, to about 0.3% to 0.5% and 0.1% in curves measuring 20 degrees and 40 degrees.7 The prevalence of curves greater than 10 degrees is higher among girls, with a 4 : 1 ratio of girls to boys.8
Etiology
Infantile scoliosis occurs roughly in 1 of 10,000 births. Possible causes are thought to occur from intrauterine molding or postnatal pressure on the spinal column from supine positioning while sleeping. Other etiologic factors that have been considered in idiopathic scoliosis include dysfunction in proprioception to maldevelopment in central pattern generators in the spinal cord9–11 and connective tissue, hormonal, and muscle structural changes.12 More recent reports in the literature strongly suggest a genetic link. The growth spurt noted among adolescents seems to play a role in progression, as a critical buckling load is reached on the existing curve as the spine grows. In a review of the literature, Kouwenhoven and Castelein13 concluded many factors may play a role in the initiation and progression of adolescent idiopathic scoliosis at a certain age. The literature suggests, however, that in the observed deformation of the spine, genetics and the unique mechanics of the fully erect posture, which is exclusive to humans, play an important role.
Genetics
The genetics of idiopathic scoliosis seem to be similar in all idiopathic groups. The incidence rate is 11% among first-degree relatives, 2.4% among second-degree relatives, and 1.4% among third-degree relatives.14 Concordance among monozygotic and dizygotic twins has been reported to range from 73% to 92% and 36% to 63%.15,16 In 2007, Anderson and colleagues17 published a population-based study taken from the Danish Twin Registry in which 46,418 twins were registered. From the 34,944 respondents, the concordance rate for monozygotic twins was 13% versus 0% for dizygotic twins. In 2007, Gao and colleagues18 published a report providing evidence of linkage and association with 8q12 loci. Through further investigation, they discovered the first gene (CHD7) associated with a susceptibility to idiopathic scoliosis. In 2008, Kulkarni and colleagues19 mapped a developmentally critical CHD gene to 15q26.1 and when taken together with CHD7, this suggested a possible role for CHD2 in the embryonic development of the spine. More recently in 2009, Gurnett and colleagues20 published a report of a single multigenerational family in which adolescent idiopathic scoliosis and pectus excavatum segregated as an autosomal dominant condition. Through linkage analysis, the investigators identified a genetic locus for the two conditions on chromosome 18q. Although genetic mapping for idiopathic scoliosis is still in its infancy, great strides are being made to help clinicians better understand the etiology and prevalence of this condition.
Natural History
About 90% of infantile curves show spontaneous resolution, especially among infants who are younger than 1 year when idiopathic scoliosis is diagnosed.6,21 The curves that typically progress are double curves with a thoracic component. Insight into the curves that can progress can be obtained from the rib-vertebral angle difference (RVAD) and “phase of the rib head.”22 In terms of phase of the rib head, if the head and neck of the convex rib of the vertebral body at the apex of the curve does not overlap the vertebral body, it is termed phase I; if it does overlap, it is termed phase II. In terms of the latter, curves with RVAD greater than 20 degrees or phase II angles are very likely to progress. Phase II angles are almost certain to progress; RVAD calculation is unnecessary when a curve is noted to be phase II type. Juvenile curves less than 25 degrees also can spontaneously resolve. These curves tend to progress, however, if they are discovered before age 6 years and idiopathic scoliosis is diagnosed or if they are greater than 30 degrees. About 70% of juvenile curve types tend to progress. The RVAD has not proved to be useful as a predictor of curve progression.
Many factors are considered in the natural history of adolescent idiopathic scoliosis. Growth potential, skeletal maturity, curve magnitude, and curve location are important considerations when assessing progression of adolescent idiopathic scoliosis. Although family history, gender, and rotation do not seem to have an effect on progression, growth potential seems to have a significant effect. Peak height growth seems to correlate best with, and is a better predictor of, progression than skeletal maturity. In terms of biomechanics, this likely is secondary to attaining vertebral column height, which leads to a critical buckling load and greater bending moments with eventual curve progression. In girls, peak height growth seems to occur 6 to 12 months before the onset of menarche. In boys, peak height growth seems to correlate with the closure of triradiate cartilages. Larger or double curves tend to progress more than single curves. Additionally, curves can progress after skeletal maturity; thoracic curves greater than 50 degrees and thoracolumbar/lumbar curves greater than 30 degrees can progress on average 1 degree per year.23,24
Evaluation
History and Physical Examination
A complete history and physical examination is completed, and any family history of scoliosis is noted. With infantile and juvenile cases in particular, a thorough prenatal and birth and developmental history is obtained. In adolescent cases, growth spurt history, if any at the time of presentation, is noted. This information is imperative in determining peak growth velocity and its implications on curve progression. Symptoms of pain or weakness and how the patient perceives his or her appearance relative to the deformity are especially important with adolescent idiopathic scoliosis.25 Age at onset of menarche and voice changes in boys are noted as well because they are likely predictors of growth potential and possible curve progression.
Trunk shift is evaluated with the patient standing and the hips and knees fully extended. The relationship of the patient’s head to the pelvis is also noted in evaluating the overall coronal and sagittal balance. Any shoulder, breast, or pelvic asymmetry is noted.26 Curve rotation is assessed by performing an Adams forward-bend test and is quantified with a scoliometer. This assessment is modified in infants by laying the patient on the examiner’s knee. This test also helps assess the rigidity of the curve, which is an important factor in terms of prognostication. Leg-length discrepancy and pelvic obliquity are evaluated. Alternatively, a sitting forward test can be performed. The latter maneuver can also help rule out plagiocephaly and developmental hip dysplasia, especially in infants. When leg-length discrepancy is the likely cause of the deformity, a shoe lift is used to reevaluate the patient to determine if the curve corrects.
A thorough neurologic examination is performed. The neurologic examination includes all cranial nerves; motor strength; reflexes (including abdominal reflexes), often associated with Chiari malformations; sensory modalities; and gait.27 Finally, other possible causes of scoliosis, such as congenital, neuromuscular, and syndromic types, must be ruled out. Infection, neoplasms, and spondylolisthesis also must be discounted.
Radiographic Evaluation
Posteroanterior and lateral 36- × 14-inch long cassette views including bending films—with appropriately placed bolsters—for further curve classification and planning bracing or surgical intervention are obtained, and the Cobb angles are measured.5 Curves greater than 20 degrees in infants and children, neurologic symptoms in all patients with idiopathic scoliosis, and left-sided, sharp angular or irregular curve patterns require further investigation, including screening total spine magnetic resonance imaging (MRI).28,29 When anomalies of the nervous system are present on MRI, a neurosurgical consultation is indicated.
Classification Systems
There is no formal curve classification system for infantile or juvenile scoliosis. The first treatment-based adolescent idiopathic scoliosis classification was developed in 1983 by King and colleagues.30 They reported on a series of 405 patients with adolescent idiopathic scoliosis, developing a uniplanar system based on thoracic curves and analyzing only the coronal plane. This system allowed for surgical planning and helped in assessing whether or not a King II curve could be selectively fused.31 Interobserver and intraobserver reliability of this traditional thoracic classification system is fair at best, however.32,33 Additionally, coronal decompensation has been reported after selective fusions.
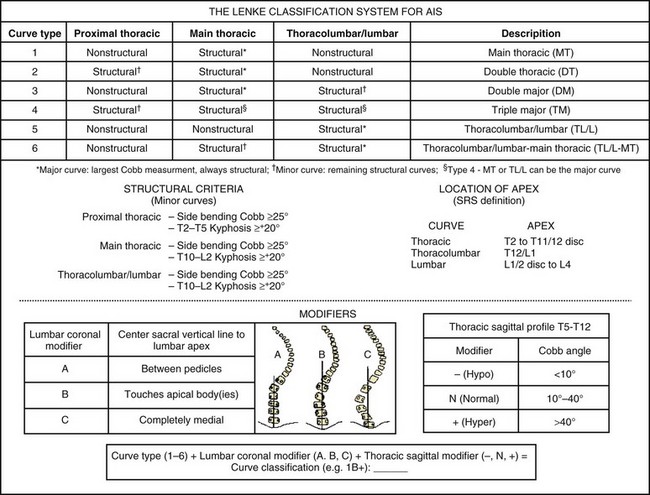
Lenke and colleagues34 developed a comprehensive, practical two-dimensional classification system in 2001, which included the sagittal plane as well (Fig. 23–1). The Lenke curve classification includes not only thoracic curves, but also thoracolumbar/lumbar curve patterns. Additionally, this classification allows the surgeon to assess curves in coronal and sagittal planes, and it has been proven reliable in terms of interobserver and intraobserver reliability.32,33 Its definition of the structural characteristics of a proximal thoracic curve has been deemed reliable, leading to shorter proximal fusions when this curve pattern is nonstructural.35 The Lenke classification allows a stricter structural curve evaluation, permitting a more objective analysis of when a given curve would tolerate a selective fusion leading to a balanced outcome.36,37 The latter is clinically significant because one of the most important principles in preventing postoperative decompensation is proper identification of curve patterns, including curves that tolerate selective fusion.31,38 This three-tiered classification combines a curve type (1 through 6) and coronal lumbar and sagittal thoracic modifiers to produce a triad comprehensive curve classification (e.g., 1A−).
More recently, the addition of a lower end vertebra (LEV) modifier has been proposed for the Lenke classification. The LEV of the lowest structural curve was found to be a highly reliable vertebral landmark, and comparison of the LEV with a selected lowest instrumented vertebra (LIV) is an important consideration in the overall evaluation of the surgical treatment of adolescent idiopathic scoliosis.39 A more detailed postoperative assessment of the distal fusion length is theoretically allowed by comparing the LIV with the LEV. Such a modifier aids in a more thorough evaluation of the Lenke curve classification (e.g., 5CN-L3 when L3 is the LEV of the curve being classified).
Three-Dimensional Classification
Although the Lenke classification system has criteria in the coronal and the sagittal planes that have an impact classification, it still has shortcomings because scoliosis is a known three-dimensional deformity, and the axial or transverse plane is not a component of this system. A task force of the Scoliosis Research Society has been charged with developing a clinically useful three-dimensional analysis and ultimate classification of scoliosis.40 The key factor in this assessment would be the plane and maximal curvature, which is the three-dimensional deformity that occurs as the spine translates and rotates out of the normal sagittal profile in scoliosis deformities. It is expected that in the next decade much more information will be provided so that three-dimensional analysis and classification will become a standard for all practitioners.
Nonoperative Treatment Options and Indications
Three fundamental treatment options exist for idiopathic scoliosis: observation, bracing and casting, and surgery. These treatment modalities are based on the natural history of idiopathic scoliosis or the potential or probability of curve progression.4,41 Many modalities have been proposed to slow or halt curve progression, such as electrical stimulation and physical therapy; however, none of these modalities have been scientifically proven to be a viable alternative in the treatment of scoliosis.42,43
Observation
Most infantile curves are left-sided; these curves have been known to resolve spontaneously up to 90% of the time, but they can progress.41 Deciphering which curves will progress can be guided by the RVAD and the relationship of the apical rib head to the vertebral body (i.e., phase I or II).22 Typically, curves less than 20 degrees are expectantly followed every 6 to 8 months. Infants with curves less than 25 degrees and RVAD less than 20 degrees and children with curves less than 25 degrees should be followed clinically and radiographically every 6 months. Treatment is instituted for curves greater than 25 degrees. Treatment is also started for a progression of 5 degrees or greater in two consecutive visits or 10 degrees or greater in one follow-up visit. Juvenile curves more often require operative intervention, however.
Bracing and Casting
Bracing44 is the nonoperative treatment of choice in small but progressive scoliosis in growing children and teens. In about 75% of cases, bracing can control the curve and avoid progression, rendering the curve small enough so that the risk of progression after growth is unlikely.42 In a younger child, whose growth potential remains a significant issue, bracing allows for continued growth until the patient requires eventual operative treatment because of curve progression. With infantile cases, molded casting followed by bracing used to be the mainstay in nonoperative management. Bracing and casting of these patients comes with potential consequences, however, that include pulmonary restriction, which can have future ramifications.45,46 Sanders and colleagues47 found serial casting to be beneficial in the treatment of infantile scoliosis. They reported that curves less than 60 degrees often fully corrected in infants if casting was started before age 20 months.
Bracing is usually started the first office visit when the patient is skeletally immature (Risser ≤2) and presents with a 25- to 40-degree curve. Several brace options exist, and deciding which brace to use depends on the apex of the curve and physician preference. Curves with an apex above T6 would likely require the use of a Milwaukee (cervicothoracolumbosacral orthosis) brace.48 Conversely, curves with apices at T7 or below and above L2 do well in a Boston underarm thoracolumbosacral orthosis brace, and these braces are more socially acceptable because of the lack of a cervical extension. The Charleston bending brace is an option if the child is noncompliant to brace-wear. This brace is typically worn at night, and some studies have shown its efficacy.49,50 The efficacy of a brace seems to depend on the length of time the brace is worn.51 When bracing is initiated and pad placement is deemed appropriate, patient follow-up occurs every 4 to 6 months, with in-brace radiographic evaluation and appropriate fitting adjustments made when necessary.
Modifications to the standard thoracolumbosacral orthosis include variations of the Chêneau brace (Jacques Chêneau, Münster, Germany) and the SpineCor dynamic brace (SpineCorporation, Chesterfield, UK). The Chêneau 2000 orthosis allows for a greater amount of initial correction by using a hypercorrected mold and pads, which provide derotational forces.52 This brace is the first that uses the theory of expansion to allow for active correction by respiratory movements.53 The SpineCor54 and TrIAC (Boston Brace International, Avon, MA) are nonrigid braces. They work by using straps, which correspond to a specific correcting movement depending on the curve pattern, producing a progressive positional change, dynamic curve correction, and appropriate muscle balance.
Operative Intervention
Operative intervention is usually recommended for patients whose curves progress despite nonoperative management.54 In infants, operative intervention is controversial; it is occasionally performed in infants with curves greater than 45 degrees or thoracolumbar/lumbar curves greater than 40 degrees. Children are typically more prone to curve progression and are more likely to require operative intervention. Other patients who are likely to benefit from operative intervention are skeletally immature patients with adolescent idiopathic scoliosis with a greater than 40- to 45-degree curve and mature patients with curves greater than 50 degrees.23,55
Surgical Techniques
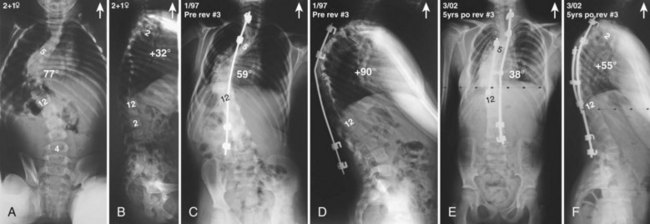
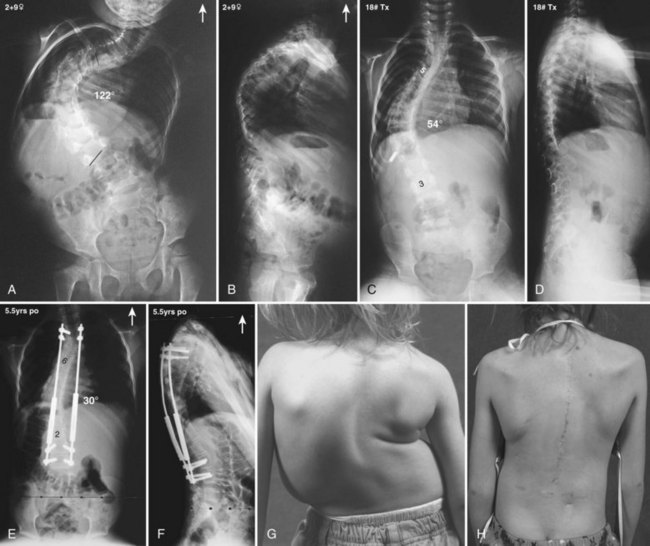
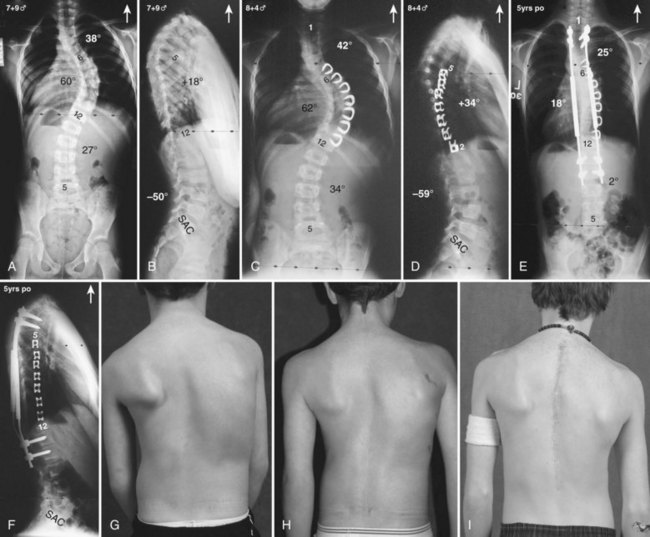
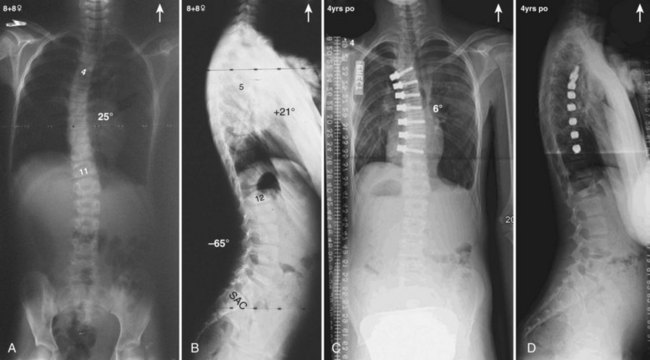
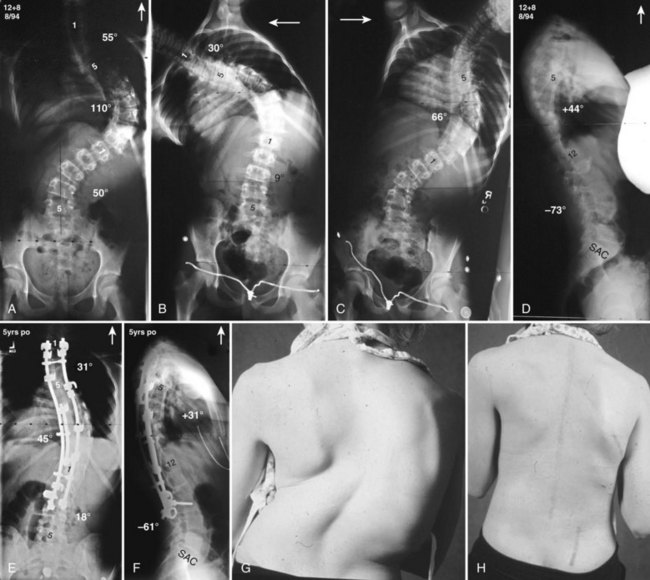
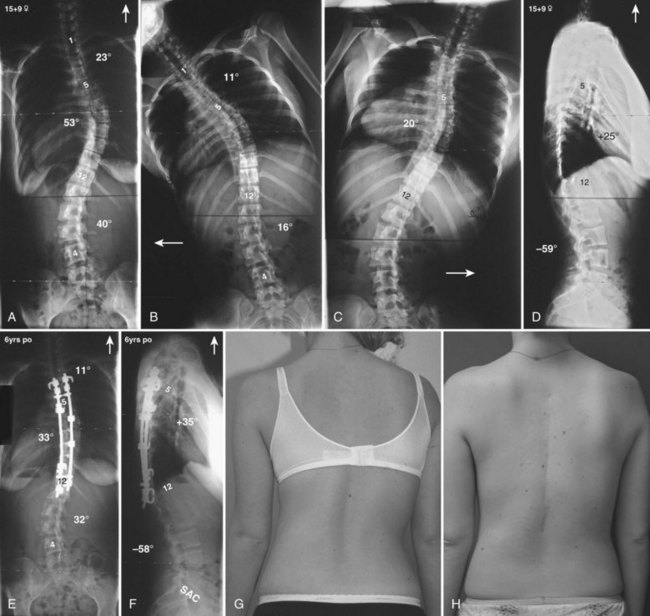
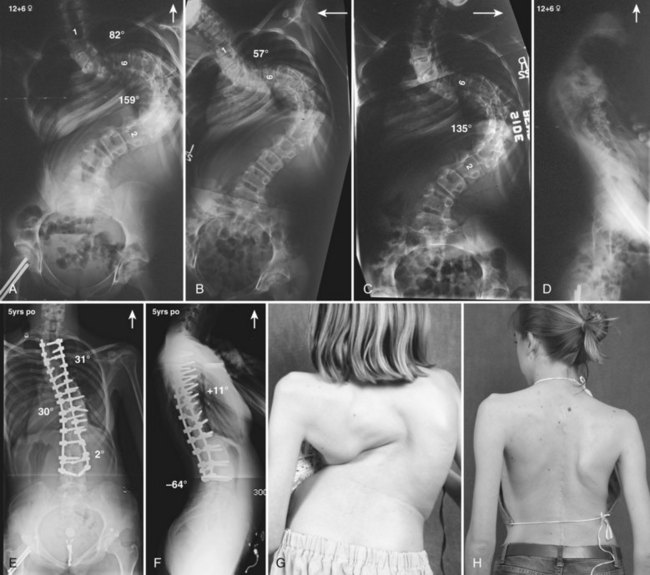
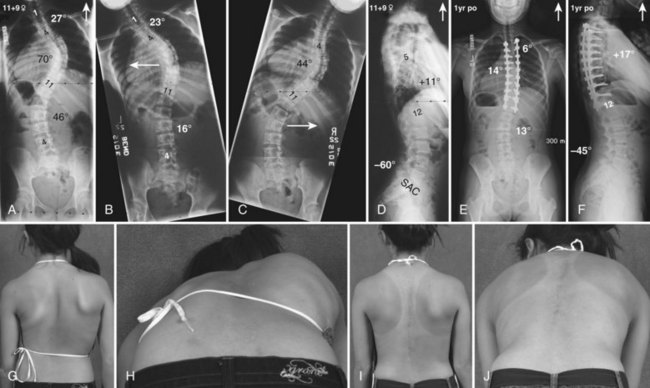
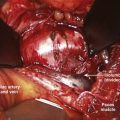
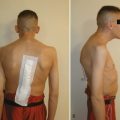
Currently, anterior-only, posterior-only, and circumferential procedures are the mainstay of surgical treatment options.57 The prevalence of anterior-only and circumferential procedures has declined with a concomitant increase in posterior-only procedures. Surgeons have become aware more recently that early intervention with a definitive anteroposterior fusion for progressive infantile and juvenile curves leads to loss of trunk height development, which can lead to chest wall and lung underdevelopment.58,59 This problem has promoted innovative techniques to try to control progressive curves surgically without definitive fusion, which include epiphysiodesis,60,61 growing rod placement (Figs. 23-2 and 23-3),62 intervertebral stapling (Fig. 23–4),86 spinal tethering (Fig. 23–5),66 and the vertical expandable prosthetic titanium rib67; the last-mentioned is used more in progressive early-onset scoliosis in which rib and chest wall deformities can be quite severe.64
Many surgeons today still prefer to perform an anterior approach in younger patients when there is risk of crankshaft development and especially for thoracolumbar and lumbar major curves (Lenke type 5). With the introduction of pedicle screws, a posterior approach has shown numerous benefits over an anterior procedure, such as better maintenance of the obtained correction, providing more powerful corrective forces, three-column control, and obviating the need for anterior releases and thoracoplasties.68–74 In addition, a posterior approach avoids the negative consequences of chest cage disruption and pulmonary compromise that can result from an open anterior approach (Fig. 23–6).75–77 In addition, the thoracic aorta is at risk if an anteriorly placed screw penetrates the vertebral cortex on the opposite side.78,79
According to Tis and colleagues,80 with advances in anterior instrumentation, surgeons theoretically should see a reduction in the rate of rod breakage, pseudarthrosis, and sagittal decompensation and improved correction rates. These authors concluded that open anterior spinal fusion surgery is a safe method for the treatment of thoracic adolescent idiopathic scoliosis. At 5-year follow-up, they reported good coronal and sagittal correction of the main thoracic and compensatory thoracolumbar/lumbar curves, but they also reported that pulmonary function was mildly decreased as with any procedure in which a thoracotomy is performed. Tis and colleagues80 also concluded that in skeletally immature patients, an open anterior spinal fusion can increase kyphosis; however, newer techniques used in their series seemed to limit progressive kyphosis, which has been noted in previously published reports.
Adolescent curves can typically be surgically treated via an anterior or posterior approach (or both) with instrumentation and fusion.77 Anterior fusion levels typically extend from end-to-end vertebrae, as measured with the Cobb technique. Short fusions above and below the apex, depending on whether the apex is a disc or a vertebra, have been advocated for flexible thoracolumbar curves.81 In this technique, if the apex is a vertebral body, the discs above and below the apex are included in the fusion. If the apex is a disc, the two discs above and below the apex are included in the fusion. Brodner82 predicted fusion levels based on the supine-pull (“stretch”) films, ensuring a thorough release is performed to obtain a “bone-on-bone” fusion. Anterior structural grafts have been used to counter the kyphogenesis associated with anterior instrumentation.77
Thoracoscopic procedures have been shown to provide advantages over open anterior thoracotomy procedures.81 Kishan and colleagues85 showed that anterior thoracoscopy had fewer adverse affects on pulmonary function. Sucato and colleagues86 found that adding a thoracoscopic release performed in the prone position to a posterior instrumentation and fusion offered the advantages of minimally invasive surgery and did not require repositioning to perform the posterior procedure. In addition, when double-lung ventilation is used, acute pulmonary complications are significantly reduced. A steep learning curve is required, however, and these techniques have diminished in popularity owing to the proliferation of pedicle screw constructs.
Determining proximal and distal fusion levels is paramount in preventing decompensation because choosing the incorrect levels is the main reason for postoperative decompensation.26,87 Adding-on is another phenomenon that can result if a fusion is stopped “short.” Suk and colleagues88 reported 5-year results of 203 patients in which they found that adding-on occurred in 17 patients who were fused on average two levels short of the neutral vertebra.
Distal fusion levels are based on the understanding and determination of the end, neutral, and stable vertebrae of the distal structural curve that is to be included in the fusion.89 Current correction techniques employing pedicular fixation and derotation maneuvers allow for preservation of distal fusion levels, however. When fusing to a vertebra cephalad to the stable vertebra, the intended LIV must touch the central sacral vertical line (CSVL), not have a significant rotation (Nash-Moe grade ≤1.5), and the disc below this proximal vertebra must be parallel or closed on the convexity. In placing thoracic screws, it is essential to follow sequential steps at every screw placement.68,69 With small pedicles, time should be taken to expand the pedicle to accommodate a screw.90 Although the authors advocate the use of pedicle screws whenever possible, when employing hook and rod segmental instrumentation, it is imperative to reverse hook orientation where the discs are reversed in orientation to maintain coronal and sagittal balance.91 Based on the lumbar modifier, attention must also be paid to the degree of tilt left on the LIV when carrying out selective fusions.
A rough estimate of the degree of tilt to be left on the LIV is equal to the remaining tilt on a preoperative supine film, with judicious use of intraoperative radiographs. This tilt can be assessed on intraoperative full-length radiographs92; this is imperative in allowing accommodation of the structural component of the lumbar curves, especially with selective fusions.37 The lower endplate of the LIV should be horizontal for type A lumbar modifier curves, a mild tilt should be left on type B curves, and an appropriate degree of tilt should be left on the LIV for type C curves. Clinical assessment of the deformity cannot be overemphasized because it plays as important a role as the radiographic findings when deciding whether to perform a selective fusion.
Selective Fusions
The Lenke classification system allows a more objective way to decide when to perform selective fusions in patients with adolescent idiopathic scoliosis, especially with type C curve patterns.37,88 The Lenke system has stricter criteria that define the structural characteristics of individual curves, leading to an objective analysis when choosing which curves can be selectively fused without ensuing clinical imbalance.33 Radiographic parameters aid in objective analysis of the structural nature of each curve relative to the other, such that if parameters match, both curves usually require surgical attention.54,93 Relative Cobb angle measurements and apical vertebral rotation and apical vertebral translation ratios of the thoracic and thoracolumbar/lumbar curves are important in such analysis and decision processes.38,39
Skeletal immaturity and clinical appearance are additional important factors.31,33,87 The magnitude of the rib and lumbar prominences is important during the clinical analysis and decision making for selective fusion (e.g., is the patient willing to accept a moderate lumbar hump when contemplating a selective thoracic fusion and vice versa). One cannot overlook the thoracolumbar sagittal profile because this can lead to curve misclassification and incorrect operative management. As previously noted, based on the lumbar modifier, attention must be paid to the degree of tilt left on the LIV when carrying out selective thoracic fusions, allowing accommodation of the structural lumbar curve (Fig. 23–7).
Selective anterior fusions of major thoracolumbar/lumbar curves associated with minor and partially structural thoracic curves in Lenke 5C and 6C curves can occasionally be considered, provided that the thoracic curve is less than 50 degrees, the thoracic curve bends out to 20 degrees or less, the thoracolumbar/lumbar-to-thoracic Cobb ratio is 1.25 or greater, and the triradiate cartilages are closed.36 Additionally, such selective fusions should not be undertaken when shoulder depression ipsilateral to the thoracolumbar/lumbar curve exists, the patient is highly skeletally immature, or a clinically unacceptable rib hump is present. To prevent decompensation, if the lumbar curve bends out less than the thoracic curve, the lumbar curve should not be overcorrected because the thoracic curve likely would not compensate to achieve postoperative balance.87 One study showed an average spontaneous correction of 14 degrees or 36% improvement of the thoracic curve when selective thoracic fusion was performed.37
Adjuncts to Correction
Direct Vertebral Rotation
In the past, curves greater than 75 degrees, curves with less than 50 degrees of correction, and curves needing a thoracoplasty have required anterior releases. With the use of modern techniques of multisegmental pedicle screw fixation and the addition of DVR techniques, safe and effective procedures demonstrating greater coronal and sagittal realignment along with acceptable cosmesis without the need for an anterior procedure have been reported.94–96 In the thoracic region, the DVR helps derotate the spine and significantly decreases the rib prominence (Fig. 23–8). Care must be taken to use stiffer rods, prebent in the sagittal profile to prevent inducing hypokyphosis in the thoracic spine. It also helps to obtain better three-dimensional correction in the thoracolumbar/lumbar component of Lenke double major curves and to minimize the LIV tilt/horizontal angle.94
Osteotomies
Sagittal imbalance can generally be divided into two types.97 In type I, the patient is in global positive balance owing to compensating for loss of segmental lordosis or kyphosis. In type II, loss of segmental lordosis or kyphosis is not compensated, leading to global imbalance. When scoliosis is associated with type I, multiple Ponté osteotomies can be performed at the site of maximal loss of segmental kyphosis. Mobility of the anterior column is required. In revision cases, these can be done asymmetrically with the widest portion of the osteotomy at the convexity or at the site of a previous fusion mass. The surgeon must pay attention when performing these osteotomies because the patient can be pitched into the concavity, possibly creating global coronal imbalance.98 Type II sagittal imbalance is best managed by the use of pedicle subtraction osteotomy because longer lever arms and greater degrees of correction are often warranted, especially with sharp angular deformities. Anterior column mobility is not required, however, because one pivots the correction anterior to the osteotomy site.
Similar to sagittal imbalance, coronal imbalance can be classified as type A or B. In type A, the shoulders and pelvis are tilted in the opposite direction, whereas in type B, they tilt in the same direction.97 Typically, single-plane deformities—type A—can be addressed with a single pedicle subtraction osteotomy; additionally, multiple or asymmetrical pedicle subtraction osteotomies can be used when dealing with stiff or kyphoscoliotic cases. Simple trigonometric calculations at the vertebral body where the osteotomy is going to be performed permit precise determination of the angle of bony resection required for global balance.99 Type B deformities likely require vertebral column resections.
Vertebral column resections can be performed via a combined anterior and posterior approach (i.e., circumferentially) or from a posterior-only approach (Fig. 23–9).100 Compromised pulmonary function lends consideration, however, to performing a posterior-only approach. The surgeon must balance the potential pulmonary compromise of the patient with the understanding that the extracavitary approach (i.e., posterior-only) requires a higher level of surgical understanding and is technically more demanding.101,102 As with all things, adherence to safety is the most important principle, and if the surgeon is uncomfortable with a particular approach or technique, a referral should be made. This correction modality is appropriate in the setting of congenital cases, multiplanar or kyphoscoliotic stiff curves, curves previously fused circumferentially, and cases of global imbalance. In the last-mentioned situation, attention must be paid to the shoulders and pelvic tilt direction.
Minimally Invasive Techniques
Minimally invasive spine surgery is a popular concept that uses imaging, retraction, and implant technologies to help surgeons locate the exact area on which they are to operate. This type of procedure is done through incisions of less than 1 inch long; damage to surrounding muscles and other tissues is avoided, which rapidly increases the healing and reduces recovery time. It also uses technology to perform the surgery more efficiently. One example is a video-assisted thoracoscopic procedure. According to Newton and colleagues,81 this procedure can be used for an anterior thoracic release or to achieve deformity correction via rod-screw constructs. Because of the small incisions made and the muscle-splitting technique used, a reduction in chest wall disruption and subsequent lung volume decrease, as is the case with an open thoracotomy approach, was noted. This procedure offers comparable curve correction and a faster return to presurgical function.84–86
Complications
Complications can occur during any of the treatment stages—preoperative, intraoperative, or postoperative.103 During the preoperative period, inappropriate curve classification and inadequate surgical planning can lead to inappropriate surgical decisions. It is essential to ensure that when performing a selective fusion, appropriate structural curve criteria are met.87 Choosing inappropriate fusion levels can also be included in the category of preoperative complications, which is discussed later.
Intraoperative neurophysiologic monitoring with somatosensory evoked potentials and motor evoked potentials helps alert the surgeon to any impending intraoperative spinal cord neurologic deficit.104 These deficits typically occur from spinal cord distraction, from overcorrection, from vascular compromise, or, rarely, directly from instrumentation. If the somatosensory evoked potentials and motor evoked potentials decline past warning criteria, the surgeon should implement a course of action that includes ensuring that the irrigation being used is of adequate temperature, keeping adequate mean arterial blood pressure elevated at a minimum greater than 80 to 90 mm Hg, and reversing instrumentation or spinal correction to the prewarning criteria state. Appropriate-dosed steroid boluses are also considered in accordance with the National Acute Spinal Cord Injury Study (NASCIS) III protocol if motor evoked potentials do not return to baseline within a reasonable time. A wake-up test should also be performed to assess true upper and lower neurologic function. In addition to adhering to the proven sequential technique of free hand screw placement, pedicle screw stimulation provides an added safety measure.68,69 Judicious use of intraoperative imaging can also be employed, especially with significant deformities.
Postoperative complications can arise from delayed consequences of technical errors, neurovascular compromise, medical comorbidities, and wound infections. Although perioperative antibiotics are commonly used, when wound infections do occur, they generally are treated aggressively with wound irrigation and débridement. Instrumentation well seated on the spine is always left in place; however, the decision to remove or maintain the bone graft is defined by the individual case and surgeon preference. In addition, removal of instrumentation can lead to loss of curve correction and decompensation.105 Additionally, at final closure, heat-stable, powder antibiotics can be placed inside the wound (deep and superficial to the fascia), and long-term parenteral antibiotics are provided based on the results of intraoperative wound cultures and sensitivities. With delayed or late infections, the instrumentation is initially removed and later replaced because the deformity can progress as the fusion mass is subject to repeated bending forces.105,106 Also, the fusion mass is inspected further, and any pseudarthrosis noted is repaired at the reinstrumentation stage.
Summary
Pearls and Pitfalls
1 Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80:1097-1106.
2 Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169-1181.
3 Lenke LG, Betz RR, Clements D, et al. Curve prevalence of a new classification of operative adolescent idiopathic scoliosis: Does classification correlate with treatment? Spine (Phila Pa 1976). 2002;27:604-611.
4 Sanders AE, Baumann R, Brown H, et al. Selective anterior fusion of thoracolumbar/lumbar curves in adolescents: When can the associated thoracic curve be left unfused? Spine (Phila Pa 1976). 2003;28:706-713.
5 Edwards CC2nd, Lenke LG, Peelle M, et al. Selective thoracic fusion for adolescent idiopathic scoliosis with C modifier lumbar curves: 2- to 16- year radiographic and clinical results. Spine (Phila Pa 1976). 2004;29:536-546.
1 Ponseti IV, Friedman B. Prognosis in idiopathic scoliosis. J Bone Joint Surg Am. 1950;32:381-395.
2 Dickson RA. Conservative treatment for idiopathic scoliosis. J Bone Joint Surg Br. 1985;67:176-181.
3 James JI. Idiopathic scoliosis: The prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36:36-49.
4 Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine (Phila Pa 1976). 1986;11:773-776.
5 Cobb JR. Outline for the study of scoliosis. Instr Course Lect. 1948;5:261-275.
6 Fernandes P, Weinstein SL. Natural history of early onset scoliosis. J Bone Joint Surg Am. 2007;89(suppl 1):21-33.
7 Kane WJ, Moe JH. A scoliosis-prevalence survey in Minnesota. Clin Orthop Relat Res. 1970;69:216-218.
8 Pring ME, Wenger DR. Adolescent deformity. In: Bono CM, Garfin SR, editors. Spine Orthopedic Surgery Essentials. Philadelphia: Lippincott Williams & Wilkins; 2004:163-164.
9 Moreau A, Wang DS, Forget S, et al. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29:1772-1781.
10 Azeddine B, Letellier K, Wang da S, et al. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:45-52.
11 Yamamoto H: A postural dysequilibrium as an etiological factor in idiopathic scoliosis [abstract O]. In Programs and Abstracts of the 17th Annual Meeting of the Scoliosis Research Society, Denver, 1982, p 52.
12 Machida M, Dubousset J, Imamura Y, et al. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine (Phila Pa 1976). 1993;18:1609-1615.
13 Kouwenhoven JW, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: Review of the literature. Spine (Phila Pa 1976). 2008;33:2989-2998.
14 Risser JC, Norquist DM, Cockrell BRJr, et al. The effect of posterior spine fusion on the growing spine. Clin Orthop Relat Res. 1966;46:127-139.
15 Carr AJ. Adolescent idiopathic scoliosis in identical twins. J Bone Join Surg Br. 1990;72:1077.
16 Kesling LK, Reinker KA. Scoliosis in twins: A meta-analysis of the literature and report of six cases. Spine (Phila Pa 1976). 1997;22:2009-2014.
17 Anderson MO, Thomsen K, Kyvik KO. Adolescent idiopathic scoliosis in twins: A population-based survey. Spine (Phila Pa 1976). 2007;32:927-930.
18 Gao X, Gordon D, Zhang D, et al. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am J Hum Genet. 2007;80:957-965.
19 Kulkarni S, Nagarajan P, Wall J, et al. Disruption of chromodomain helicase DNA binding protein 2 (CHD2) causes scoliosis. Am J Med Genet. 2008;146A:1117-1127.
20 Gurnett CA, Alaee F, Bowcock A, et al. Genetic linkage localizes an adolescent idiopathic scoliosis and pectus excavatum gene to 18q. Spine (Phila Pa 1976). 2009;34:E94-E100.
21 Diedrich O, von Strempel A, Scholz M, et al. Long-term observation and management of resolving infantile idiopathic scoliosis: A 25-year follow-up. J Bone Joint Surg Br. 2002;84:1030-1035.
22 Mehta MH. The rib-vertebra angle in the early diagnosis between resolving and progressive infantile scoliosis. J Bone Joint Surg Br. 1972;54:230-243.
23 Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:447-455.
24 Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: Long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63:702-712.
25 Lonstein JE. Patient evaluation. In: Bradford DS, Lonstein JE, Moe JH, et al, editors. Moe’s Textbook of Scoliosis and Other Spinal Deformities. 2nd ed. Philadelphia: WB Saunders; 1987:47-88.
26 Li M, Gu S, Ni J, et al. Shoulder balance after surgery in patients with Lenke Type 2 scoliosis corrected with the segmental pedicle screw technique. J Neurosurg Spine. 2009;10:214-219.
27 Muhonen MG, Menezes AH, Sawin PD, et al. Scoliosis in pediatric Chiari malformations without myelodysplasia. J Neurosurg. 1992;77:69-77.
28 Dobbs MB, Lenke LG, Szymanski DA, et al. Prevalence of neural axis abnormalities in patients with infantile scoliosis. J Bone Joint Surg Am. 2002;84:2230-2234.
29 Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity: Is a magnetic resonance image screening necessary? Spine (Phila Pa 1976). 1998;23:206-210.
30 King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:1302-1313.
31 Lenke LG, Bridwell KH, Baldus C, et al. Preventing decompensation in King Type II curves treated with Cotrel-Dubousset instrumentation: Strict guidelines for selective thoracic fusion. Spine (Phila Pa 1976). 1992;17:274S-281S.
32 Schroeder TM, Blanke KM, Vaughan V, et al: Validation of radiographic software to determine Lenke classification [abstract 54]. In Programs and Abstracts of the 40th Annual Meeting of the Scoliosis Research Society, Miami, 2005, p 93.
33 Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80:1097-1106.
34 Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169-1181.
35 Cil A, Pekmezci M, Yazici M, et al: The validity of Lenke’s criteria for defining structural proximal thoracic curves in patients with adolescent idiopathic scoliosis [abstract 74]. In Programs and Abstracts of the 40th Annual Meeting of the Scoliosis Research Society, Miami, 2005, p 120.
36 Sanders AE, Baumann R, Brown H, et al. Selective anterior fusion of thoracolumbar/lumbar curves in adolescents: When can the associated thoracic curve be left unfused? Spine (Phila Pa 1976). 2003;28:706-713.
37 Edwards CC2nd, Lenke LG, Peelle M, et al. Selective thoracic fusion for adolescent idiopathic scoliosis with C modifier lumbar curves: 2- to 16- year radiographic and clinical results. Spine (Phila Pa 1976). 2004;29:536-546.
38 Lenke LG, Bridwell KH: Achieving coronal balance using Cotrel-Dubousset instrumentation (C-D.I.). In 8th Proceeding of the International Congress on Cotrel-Dubousset Instrumentation. Montpellier, Sauramps Medical, 1991, pp 27-32.
39 Lenke LG, Kuklo TR, Sucato DJ, et al: Comparison of the lower-end vertebra (LEV) to the lowest instrumented vertebra (LIV) in adolescent idiopathic scoliosis: A role for the addition of an LEV modifier to the Lenke Classification System [abstract 131]. In Programs and Abstracts of the 13th International Meeting on Advanced Spine Techniques (IMAST), Athens, 2006, p 121.
40 Sangole A, Aubin CE, Labelle H, et al. The central hip vertical axis (CHVA): A reference axis for the Scoliosis Research Society three-dimensional classification of idiopathic scoliosis. Spine (Phila Pa 1976). 2010;35:E530-E534.
41 Weinstein SL. Idiopathic scoliosis: Natural history. Spine (Phila Pa 1976). 1986;11:780-783.
42 Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis: A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am. 1995;77:815-822.
43 Stone B, Beekman C, Hall V, et al. The effect of an exercise program on change in curve in adolescents with minimal idiopathic scoliosis: A preliminary study. Phys Ther. 1979;59:759-763.
44 Fayssoux RS, Cho RH, Herman MJ. A history of bracing for idiopathic scoliosis in North America. Clin Orthop Relat Res. 2010;468:654-664.
45 Kennedy JD, Robertson CF, Olinsky A, et al. Pulmonary restrictive effect of bracing in mild idiopathic scoliosis. Thorax. 1987;42:959-961.
46 Katsaris G, Loukos A, Valavanis J, et al. The immediate effect of a Boston brace on lung volumes and pulmonary compliance in mild adolescent idiopathic scoliosis. Eur Spine J. 1999;8:2-7.
47 Sanders JO, D’Astous J, Fitzgerald M, et al. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop. 2009;29:581-587.
48 Blount WP, Schmidt AC, Keever ED, et al. The Milwaukee brace in the operative treatment of scoliosis. J Bone Joint Surg Am. 1958;40:511-525.
49 Trivedi JM, Thomson JD. Results of Charleston bracing in skeletally immature patients with idiopathic scoliosis. J Pediatr Orthop. 2001;21:277-280.
50 Clin J, Aubin CE, Parent S, et al. A biomechanical study of the Charleston brace for the treatment of scoliosis. Spine (Phila Pa 1976). 2010;35:E940-E947.
51 Price CT, Scott DS, Reed FRJr, et al. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston bending brace: Long-term follow-up. J Pediatr Orthop. 1997;17:703-707.
52 Kotwicki T, Chêneau J. Biomechanical action of a correction brace of thoracic idiopathic scoliosis: Chêneau 2000 orthosis. Disabil Rehabil Assist Technol. 2008;3:146-153.
53 Rigo M, Negrini S, Weiss H, et al. SOSORT consensus paper on brace action: TLSO biomechanics of correction (investigating the rationale for force vector selection). Scoliosis. 2006;1:11.
54 Szwed A, Kolban M, Jaloszewski M. Results of SpineCor dynamic bracing for idiopathic scoliosis. Ortop Traumatol Rehabil. 2009;11:427-432.
55 Reference deleted in proofs.
56 Weinstein SL, Dolan LA, Spratt KF, et al. Health and function of patients with untreated idiopathic scoliosis: A 50-year natural history study. JAMA. 2003;289:559-667.
57 Maruyama T, Takeshita K. Surgical treatment of scoliosis: A review of techniques currently applied. Scoliosis. 2008;18:6.
58 Dobbs MB, Weinstein SL. Infantile and juvenile scoliosis. Orthop Clin North Am. 1999;30:331-341.
59 Lenke LG, Dobbs MB. Management of juvenile idiopathic scoliosis. J Bone Joint Surg Am. 2007;89(suppl 1):55-63.
60 Marks DS, Iqbal MJ, Thompson AG, et al. Convex spinal epiphysiodesis in the management of progressive infantile idiopathic scoliosis. Spine (Phila Pa 1976). 1996;21:1884-1888.
61 Bylski-Austrow DI, Wall EJ, Glos DL, et al. Spinal hemiepiphysiodesis decreased the sizes of vertebral growth plate hypertrophic zone and cells. J Bone Joint Surg Am. 2009;91:854-893.
62 Akbarnia BA, Marks DS, Boachie-Adjei O, et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: A multicenter study. Spine (Phila Pa 1976). 2005;30(17 Suppl):S46-S57.
63 Betz RR, Kim J, D’Andrea LP, et al. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety, and utility study. Spine. 2003;28:S255-S265.
64 Betz RR, Ranade A, Samdani AF, et al. Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine. 2010;35:169-176.
65 Newton PO, Upasani VV, Farnsworth CL, et al. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg. 2008;90:2695-2706.
66 Crawford CHIII, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: A case report. J Bone Joint Surg Am. 2010;92:202-209.
67 Thompson GH, Akbarnia BA, Campbell RMJr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354-361.
68 Lenke LG, Rinella A, Kim Y. Freehand thoracic pedicle screw placement. Semin Spine Surg. 2002;14:48-57.
69 Kim YJ, Lenke LG, Bridwell KH, et al. Free hand pedicle screw placement in the thoracic spine: Is it safe? Spine (Phila Pa 1976). 2004;29:333-341.
70 Good CR, Lenke LG, O’Leary PT, et al. Can posterior-only surgery provide similar radiographic and clinical results as combined anterior (thoracotomy/thoracoabdominal)/posterior approaches for adult scoliosis? Spine (Phila Pa 1976). 2010;35:210-218.
71 Kioschos HC, Asher MA, Lark RG, et al. Overpowering the crankshaft mechanism: The effect of posterior spinal fusion with and without stiff transpedicular fixation on anterior spinal column growth in immature canines. Spine (Phila Pa 1976). 1996;21:1168-1173.
72 Burton DC, Asher MA, Lai SM. Scoliosis correction maintenance in skeletally immature patients with idiopathic scoliosis: Is anterior fusion really necessary? Spine (Phila Pa 1976). 2000;25:61-68.
73 Suk SI, Kim JH, Cho KJ, et al. Is anterior release necessary in severe scoliosis treated by posterior segmental pedicle screw fixation? Eur Spine J. 2007;16:1359-1365.
74 Dobbs MB, Lenke LG, Kim YJ, et al. Anterior/posterior spinal instrumentation versus posterior instrumentation alone for the treatment of adolescent idiopathic scoliotic curves more than 90°. Spine (Phila Pa 1976). 2006;31:2386-2391.
75 Kim YJ, Lenke LG, Bridwell KH, et al. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am. 2005;87:1534-1541.
76 Lonner BS, Auerbach JD, Estreicher MB, et al. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2009;22:551-558.
77 Tis JE, O’Brien MF, Newton PO, et al. Adolescent idiopathic scoliosis treated with open instrumented anterior spinal fusion: Five-year follow-up. Spine (Phila Pa 1976). 2010;35:64-70.
78 Sucato DJ, Kassab F, Dempsey M. Analysis of screw placement relative to the aorta and spinal canal following anterior instrumentation for thoracic idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29:554-559.
79 Maruyama T, Takeshita K, Nakamura K, et al. Spatial relations between the vertebral body and the thoracic aorta in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29:2067-2069.
80 Reference deleted in proofs.
81 Hall JE, Millis MB, Snyder BD. Short segment anterior instrumentation for thoracolumbar scoliosis. In: Bridwell KH, DeWald RL, editors. The Textbook of Spinal Surgery. 2nd ed. Philadelphia: Lippincott-Raven; 1997:665-674.
82 Brodner W, Mun Yue W, Möller HB, et al. Short segment bone-on-bone instrumentation for single curve idiopathic scoliosis. Spine (Phila Pa 1976). 2003;28:S224-S233.
83 Geck MJ, Rinella A, Hawthorne D, et al. Comparison of surgical treatment in Lenke 5C adolescent idiopathic scoliosis: Anterior dual rod versus posterior pedicle fixation surgery: A comparison of two practices. Spine (Phila Pa 1976). 2009;34:1942-1951.
84 Newton PO, Marks M, Faro F, et al. Use of video-assisted thoracoscopic surgery to reduce perioperative morbidity in scoliosis surgery. Spine (Phila Pa 1976). 2003;28:S249-S254.
85 Kishan S, Bastrom T, Betz RR, et al. Thoracoscopic scoliosis surgery affects pulmonary function less than thoracotomy at 2 years postsurgery. Spine (Phila Pa 1976). 2007;32:453-458.
86 Sucato DJ, Erken YH, Davis S, et al. Prone thoracoscopic release does not adversely affect pulmonary function when added to a posterior spinal fusion for severe deformity. Spine (Phila Pa 1976). 2009;34:771-778.
87 Bridwell KH, Lenke LG. Prevention and treatment of decompensation: When can levels be saved and selective fusion be performed in idiopathic scoliosis? Spine State Art Rev. 1994;8:643-658.
88 Suk SI, Lee SM, Chung ER, et al. Selective thoracic fusion with segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis: More than 5-year follow-up. Spine (Phila Pa 1976). 2005;30:1602-1609.
89 O’Brien MF, Kuklo TR, Blanke KM, et al, editors. Radiographic Measurement Manual. Memphis: Medtronic Sofamor Danek, USA, Inc, 2004.
90 Rinella A, Cahill P, Ghanayem A, et al: Thoracic pedicle expansion after pedicle screw placement in a pediatric cadaveric spine: A biomechanical analysis [abstract 35]. In Programs and Abstracts of the 39th Annual Meeting of the Scoliosis Research Society, Argentina, 2004, p 70.
91 Bridwell KH, McAllister JW, Betz RR, et al. Coronal decompensation produced by Cotrel-Dubousset “derotation” maneuver for idiopathic right thoracic scoliosis. Spine (Phila Pa 1976). 1991;16:769-777.
92 Lehman RAJr, Lenke LG, Helgeson MD, et al. Do intraoperative radiographs in scoliosis surgery reflect radiographic result? Clin Orthop Relat Res. 2010;468:679-686.
93 Donaldson S, Stephens D, Howard A, et al. Surgical decision making in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32:1526-1532.
94 Keeler KA, Lehman RA, Lenke LG, et al: Direct vertebral rotation (DVR) in the treatment of thoracolumbar/lumbar adolescent idiopathic scoliosis (AIS): Can it optimize correction when fusing to L3? [abstract 137]. In Programs and Abstracts of the 15th International Meeting on Advanced Spine Techniques, Hong Kong, 2008, p 215.
95 Lee SM, Suk SI, Chung ER. Direct vertebral rotation: A new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29:343-349.
96 Kadoury S, Cheriet F, Beauséjour M, et al. A three-dimensional retrospective analysis of the evolution of spinal instrumentation for the correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18:23-37.
97 Bridwell KH. Adult spinal deformity revision surgery. In: Heary RF, Albert TJ, editors. Spinal Deformity: The Essentials. New York: Thieme Medical Publishers; 2007:240-248.
98 Booth KC, Bridwell KH, Lenke LG, et al. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal balance). Spine (Phila Pa 1976). 1999;24:1712-1720.
99 Ondra SL, Marzouk S, Koski T, et al. Mathematical calculation of pedicle subtraction osteotomy size to allow precision correction of fixed sagittal deformity. Spine (Phila Pa 1976). 2006;31:E973-E979.
100 Bradford DS, Tribus CB. Vertebral column resection for the treatment of rigid coronal decompensation. Spine (Phila Pa 1976). 1997;22:1590-1599.
101 Lenke LG, O’Leary PT, Bridwell KH, et al. Posterior vertebral column resection for severe pediatric deformity: Minimum two-year follow-up of thirty-five consecutive patients. Spine (Phila Pa 1976). 2009;34:2213-2221.
102 Lenke LG, Sides BA, Koester LA, et al. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468:687-699.
103 Lenke LG, Bridwell KH, Erickson MA, et al: Prospective radiographic and clinical outcomes and complications of 756 consecutive operative adolescent idiopathic scoliosis patients [abstract 3]. In Programs and Abstracts of the 44th Annual Meeting of the Scoliosis Research Society, San Antonio, 2009, p 39.
104 Padberg AM, Wilson-Holden TJ, Lenke LG, et al. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery: An accepted standard of care. Spine (Phila Pa 1976). 1998;23:1392-1400.
105 Potter BK, Kirk KL, Shah SA, et al. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2006;31:67-72.
106 Luhmann SJ, Lenke LG, Bridwell KH, et al. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine (Phila Pa 1976). 2009;34:2191-2197.