13 IC-IC Bypasses for Complex Brain Aneurysms
Introduction
EC-IC bypass surgery has been essential in the management of brain aneurysms that are too complex for conventional clipping or endovascular coiling, despite its well-publicized failure to benefit patients with ischemic stroke in the EC-IC Bypass Trial.1,3,5,11,22,27 Revascularization of a territory distal to a giant, dolichoectatic, or thrombotic aneurysm enables the aneurysm to be occluded without risk of ischemic complications, or the parent artery’s blood flow to be reversed or reduced safely. The superficial temporal artery to middle cerebral artery (STA-MCA) bypass was the prototype, and subsequently an array of bypasses was developed with the same concept of redirecting extracranial blood flow from scalp arteries or cervical carotid arteries to the brain, either directly with one anastomosis or with interposition grafts and two anastomoses. In recent years, innovative bypasses have been introduced anecdotally that revascularize intracranial arteries with other intracranial arteries, without contribution from extracranial donor arteries.7–9,15,16,20 These intracranial to intracranial (IC-IC) bypasses are simple, elegant, and more anatomical than their EC-IC counterparts. IC-IC bypasses require no harvest of extracranial donors, spare patients a neck incision, shorten any interposition grafts, are protected within the cranium, and use caliber-matched donor and recipient arteries. These advantages of IC-IC bypasses appeal to experienced bypass surgeons, and their use has increased noticeably. For example, Sekhar and colleagues performed at least 11 IC-IC bypasses in an overall experience with 119 bypasses in 115 patients.26 Similarly, Spetzler and colleagues performed 28 IC-IC bypasses (44%) in an overall experience with 63 bypasses in 61 patients.15
Evolution of bypass surgery for brain aneurysms
Bypass surgery for brain aneurysms began with the introduction and popularization of the STA-MCA bypass by Yasargil.29 This simple bypass revascularized the MCA territory and protected patients from ischemic complications after deliberate arterial occlusion during the treatment of MCA and some ICA aneurysms. Bypass surgery for aneurysms evolved with the development of an array of EC-IC bypasses that used other extracranial donor arteries and interposition grafts connected to proximal donor sites in the neck.2,4,6,10,12–15,17–19,21,23,25,28,30 Even though these second-generation EC-IC bypasses yield excellent results, bypass surgery for aneurysms is evolving further with the development of an array of IC-IC bypasses that eliminates extracranial donor arteries and reconstructs the cerebral circulation in ways that resemble normal cerebrovascular anatomy. In this report, we analyzed this third generation of IC-IC bypasses in a large clinical series, categorized the techniques into four types, and demonstrated aneurysm and patient outcomes comparable to traditional EC-IC bypasses. Aneurysm obliteration rates, bypass patency rates, and neurological results (late GOS and change in GOS) were similar in EC-IC and IC-IC bypass patients, supporting this progression toward intracranial vascular reconstruction.
Bypass demographics
During a 10-year period between November 1997 and November 2007, 1984 aneurysms were treated microsurgically in 1578 patients by the senior author (MTL). Of these patients, 82 (5%) underwent cerebral revascularization surgery as part of the management of an intracranial aneurysm. Overall, there were 50 women and 32 men, with a mean age of 53 years (range, 12–78 years) (Table 13–1). Twenty-one patients presented with subarachnoid hemorrhage (26%). Hunt-Hess Grade III was the most common clinical grade (48%), and four patients presented with poor Hunt-Hess grade. Fifty-six patients (68%) presented with unruptured aneurysms and neurological symptoms, with cranial neuropathy or hemiparesis from mass effect present in 38 patients (68%). Eight patients (14%) presented with transient ischemic attacks or stroke in association with thrombotic aneurysms. Five patients presented with incidental, unruptured aneurysms (6%).
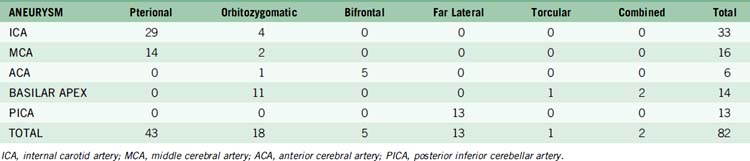
Aneurysm characteristics for IC-IC bypass patients
Aneurysms were distributed throughout the intracranial circulation, with the most common locations being the cavernous ICA (19 aneurysms, 23%), MCA (16 aneurysms, 20%), and posterior inferior cerebellar artery (PICA) (10 aneurysms, 12%) (Table 13–2). The majority of aneurysms were giant in size (46 aneurysms, 56%). Only 15 aneurysms (18%) had saccular morphology, and the remaining 67 aneurysms (82%) had fusiform or dolichoectatic morphology. Thirty-one patients (38%) had thrombotic aneurysms. Eight aneurysms (10%) had been treated endovascularly with coils, of which three were incompletely treated and five were recurrent. Fifteen patients (19%) had multiple aneurysms, with 26 other aneurysms diagnosed.
Reimplantation technique
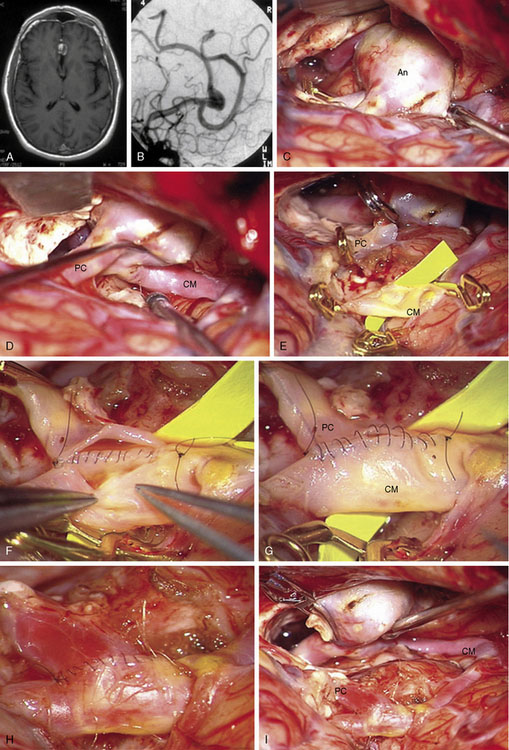
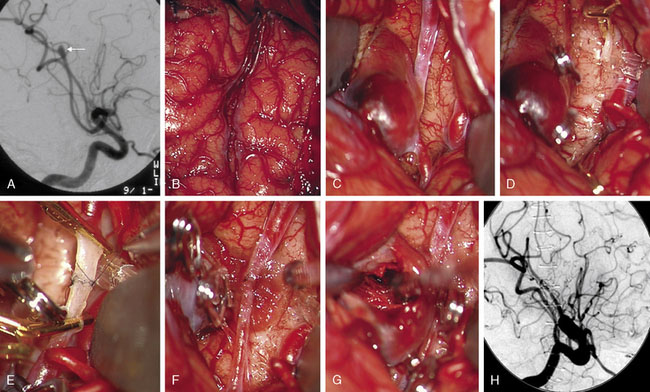
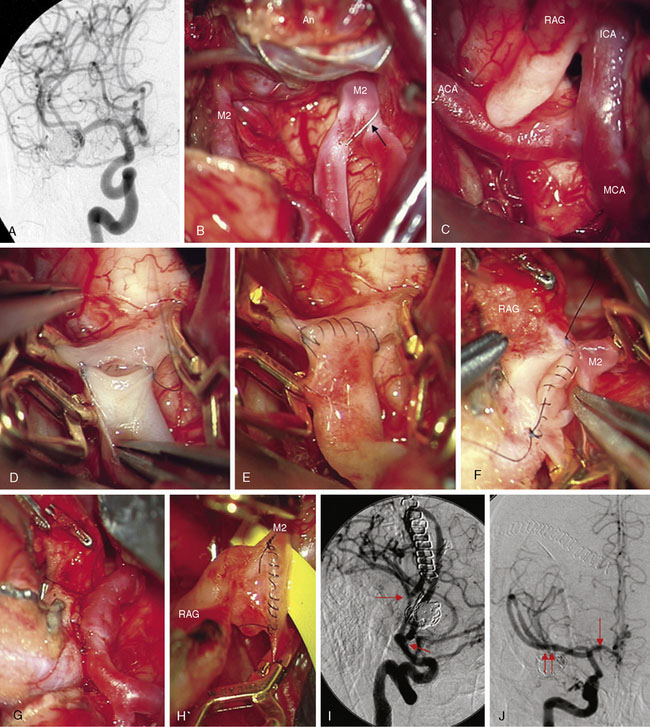
Complex aneurysms with branches that originate from the aneurysm base or side wall can often be reconstructed with tandem clipping techniques that preserve important branch arteries (a fenestrated clip encircling the branch origin and a stacked straight clip closing the fenestration). In cases where clip reconstruction fails, the neck can be clipped to exclude the aneurysm, preserve the parent artery, and sacrifice the branch artery. The occluded branch artery can then be reconstituted with reimplantation onto the parent artery (Figure 13–1). Alternatively, the branch artery can be reimplanted to an adjacent donor artery that is not the parent artery, as long as that donor artery lies in close proximity to the branch (Figure 13–2). Like in situ bypasses, this favorable anatomy occurs with MCA, ACA, and PICA aneurysms. Reimplantation requires one end-to-side anastomosis.
Reanastomosis technique
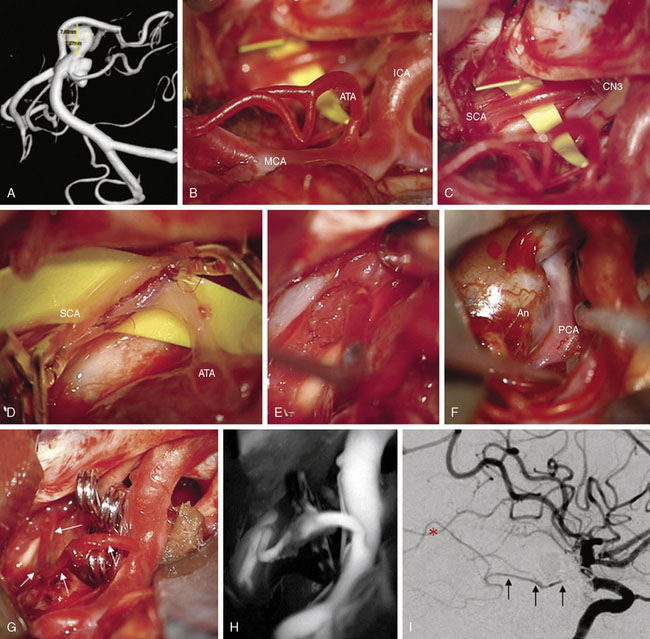
Reanastomosis requires trapping the aneurysm, completely detaching afferent and efferent arteries, and reconnecting cut ends with an end-to-end anastomosis (Figure 13–3). This technique works well with fusiform aneurysms that are small or medium in size. Saccular aneurysms at bifurcations with more than two or more efferent arteries are difficult to reconstruct with primary reanastomosis because the second branch must either be reimplanted or bypassed with an extracranial donor artery. Large and giant aneurysms may be difficult to reanastomose because ends of the parent artery can be widely separated after excising an aneurysm. Mobilizing the ends of afferent and efferent arteries may enable the first stitch to pull them together with minimal tension. If the gap in the parent artery is too long and the tension too great, the suture will tear through the artery wall as it is tightened and ruin the repair. Some large aneurysms in PICA and MCA territories have an unusually redundant parent artery that will allow primary reanastomosis despite their size. Reanastomosis requires one end-to-end anastomosis.
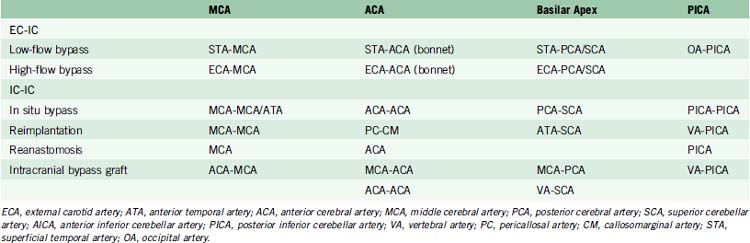
Intracranial bypass technique with grafts
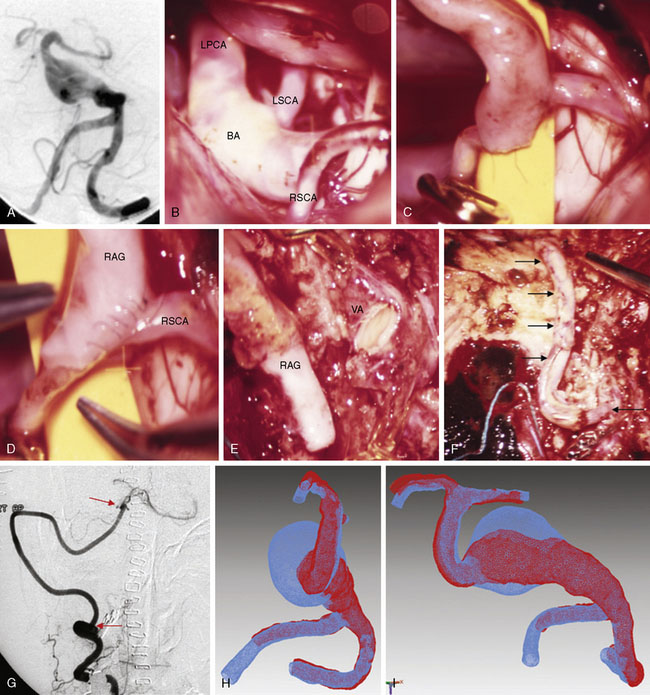
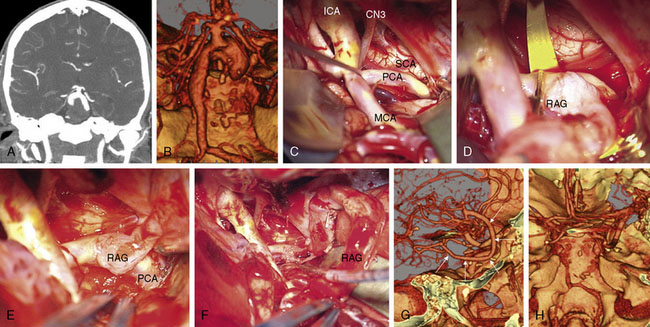
These bypasses use interposition grafts to connect donor and recipient arteries that are entirely intracranial, differentiating them from traditional EC-IC bypasses that utilize extracranial donor arteries (Figures 13-4 through 13-6). In contrast to EC-IC bypasses that use saphenous vein grafts to span from the neck to the Sylvian fissure, intracranial bypass grafts are shorter and radial artery grafts are sufficiently long. Radial artery grafts are preferred over saphenous vein grafts because they are composed of arterial tissue, have higher long-term patency rates, and match the caliber of intracranial arteries. Preoperatively, an Allen’s test with Doppler ultrasound is performed to ensure adequate perfusion of the hand with the ulnar artery and a competent palmar arch.
Selection of the Microsurgical Corridor
As can be expected, surgical approach depends on aneurysm location (see Table 13–2). ICA aneurysms are approached through a pterional craniotomy in most cases, with an orbitozygomatic craniotomy used for additional exposure with giant aneurysms. Similarly, a pterional craniotomy is adequate for most MCA aneurysms, with an orbitozygomatic craniotomy used with two giant aneurysms. ACA aneurysms are exposed through bifrontal craniotomies to access the interhemispheric fissure, with the midline of the head positioned parallel to the floor and angled up 45 degrees to allow gravity to retract the dependent hemisphere. All bypasses for basilar apex aneurysms are performed through orbitozygomatic craniotomies, capitalizing on its additional trans-Sylvian exposure for these deep bypasses. The VA-SCA bypass for basilar trunk aneurysms is performed through a combined far lateral-subtemporal craniotomy. PICA bypasses are performed through far lateral craniotomies, although the PICA-PICA bypass does not require extensive resection of the occipital condyle or much lateral exposure when performed without accessing the aneurysm, as with a staged endovascular occlusion (three patients).
The University of California, San Francisco Bypass Experience
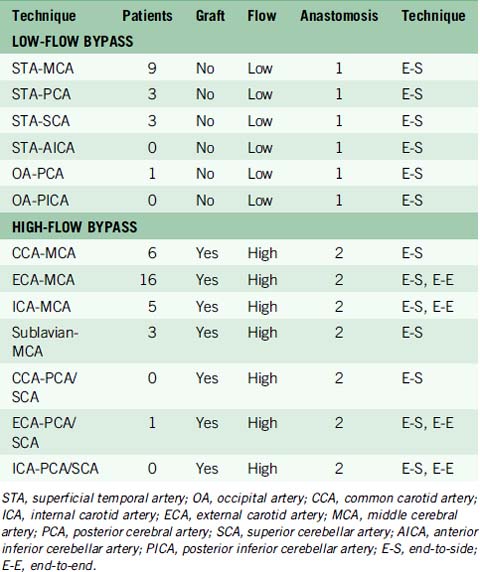
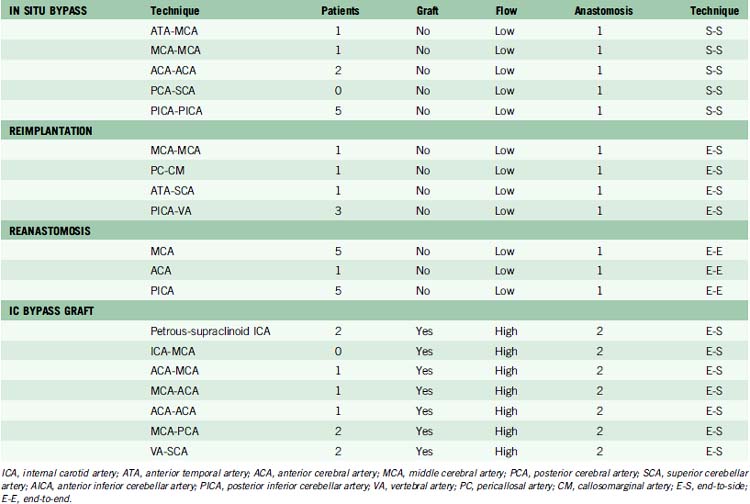
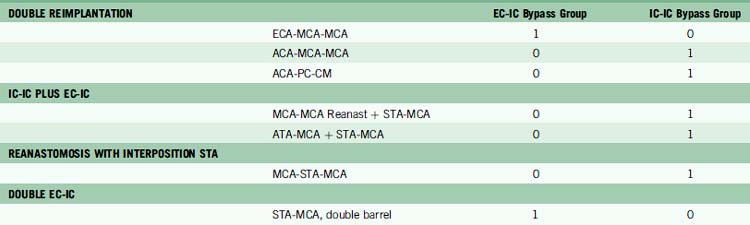
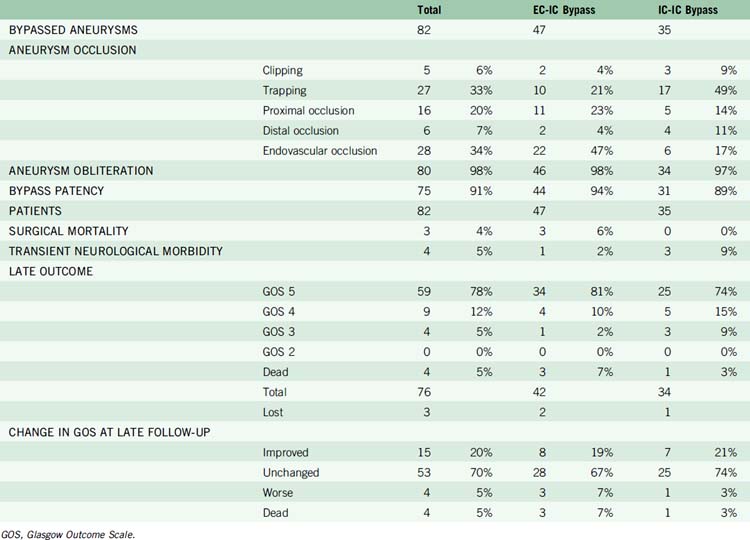
In our institutional experience, of the 82 patients with aneurysms requiring a bypass, 47 patients (57%) received EC-IC bypasses and 35 patients (43%) received IC-IC bypasses.24 EC-IC bypasses included 16 low-flow bypasses with STA donors in 15 patients and OA donor in one patient (Table 13–3). High-flow EC-IC bypasses were performed in 31 patients using saphenous vein grafts in 27 patients and radial artery grafts in four patients. IC-IC bypasses consisted of in situ bypasses in nine patients (26%), reimplantation in six patients (17%), reanastomosis in 11 patients (31%), and intracranial bypass grafts in nine patients (26%) (Table 13–4). Unlike extracranial bypass grafts, intracranial bypass grafts used radial artery more frequently than saphenous vein (six patients vs. three patients, respectively). Seven patients had complex bypass configurations like double reimplantations or additional STA-MCA bypasses (Table 13–5), of which five were categorized as IC-IC bypass patients and two as EC-IC bypass patients.
Aneurysm occlusion was performed during the surgery in 54 patients and consisted of 27 aneurysm trappings (33%), 16 proximal aneurysm occlusions (20%), six distal aneurysm occlusions (7%), and five aneurysm clippings (6%) (Tables 13–6 and 13–7). The remaining 28 patients (34%) underwent staged endovascular occlusion of their aneurysms. Twenty-two of these endovascularly treated patients were in the EC-IC bypass group, reflecting the large number of ICA aneurysms. In contrast, half of the aneurysms in the IC-IC bypass group were trapped during surgery, reflecting the accessibility of these more distally located aneurysms. The small number of aneurysms clipped directly reflects the nonsaccular morphology of these aneurysms. Endovascular staging was typically performed 2 to 3 days after the bypass procedure. Patients were started on aspirin (350 mg qd) immediately after surgery.
Excluding the three surgical mortalities and three additional patients lost to follow-up, final neurological outcomes were assessed in 76 patients (93%). Mean duration of follow-up was 41 months (range, 1–125 months), and did not differ significantly between EC-IC and IC-IC bypass groups (38.6 months and 42.7 months, respectively). Four patients died after hospital discharge, three from complications in rehabilitation and one from delayed growth of a basilar trunk aneurysm with resulting brainstem compression. Three of the four late deaths were in the EC-IC bypass group. Good outcomes (GOS 5 or 4) were measured in 68 patients (90%) overall, and were similar in EC-IC and IC-IC bypass groups (91% and 89%, respectively) (Table 13–7). At late follow-up, 15 patients (20%) were improved and 53 (70%) were unchanged, relative to preoperative neurological condition, excluding lost patients. Changes in outcome by GOS were slightly more favorable in the IC-IC bypass group than the EC-IC bypass group (6% vs. 14% worse or dead in IC-IC vs. EC-IC bypass groups, respectively). Mean final GOS scores reflected a similar trend, with a mean GOS of 4.3 in the EC-IC bypass group and 4.6 in the IC-IC bypass group. Relative to preoperative neurological condition, mean GOS decreased 0.30 in the EC-IC bypass group and increased 0.21 in the IC-IC bypass group.
Conversion of EC-IC to IC-IC
Easy conversion from EC-IC to IC-IC bypass is needed to embrace this progression to intracranial vascular reconstruction. Every current EC-IC bypass can be translated to an IC-IC bypass presented in this clinical experience (Table 13–8). ACA and PICA territories were particularly amenable to intracranial reconstruction, and even though MCA and basilar apex territories were divided between EC-IC and IC-IC techniques, growing experience with A1 ACA-MCA and MCA-PCA bypasses have made them preferred choices for their respective territories.
Bypass selection ultimately depends on an intraoperative assessment of the aneurysm and surrounding anatomy. We typically devise a primary bypass strategy, several contingency strategies (Table 13–8), and make preparations for each (like prepping a graft site). At surgery, there may be several viable options (e.g., PICA-PICA bypass and PICA reimplantation), no options (e.g., P3 segment PCA aneurysm), or serendipitous anatomy presenting unexpected options (e.g., ATA-SCA bypass). We select the bypass that facilitates aneurysm occlusion, restores normal blood flow, and is technically most feasible.
1 Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191-1200.
2 Auguste K.I., Quinones-Hinojosa A., Lawton M.T. The tandem bypass: subclavian artery-to-middle cerebral artery bypass with dacron and saphenous vein grafts. Technical case report. Surg Neurol. 2001;56:164-169.
3 Ausman J.I., Diaz F.G. Critique of the extracranial-intracranial bypass study. Surg Neurol. 1986;26:218-221.
4 Barnett D.W., Barrow D.L., Joseph G.J. Combined extracranial-intracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92-97. discussion 97–98
5 Barnett H.J., Sackett D., Taylor D.W., et al. Are the results of the extracranial-intracranial bypass trial generalizable? N Engl J Med. 1987;316:820-824.
6 Baskaya M.K., Kiehn M.W., Ahmed A.S., et al. Alternative vascular graft for extracranial-intracranial bypass surgery: descending branch of the lateral circumflex femoral artery. Neurosurg Focus. 2008;24:E8.
7 Bederson J.B., Spetzler R.F. Anastomosis of the anterior temporal artery to a secondary trunk of the middle cerebral artery for treatment of a giant M1 segment aneurysm. Case report. J Neurosurg. 1992;76:863-866.
8 Candon E., Marty-Ane C., Pieuchot P., et al. Cervical-to-petrous internal carotid artery saphenous vein in situ bypass for the treatment of a high cervical dissecting aneurysm: technical case report. Neurosurgery. 1996;39:863-866.
9 Evans J.J., Sekhar L.N., Rak R., et al. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004;55:1036-1049.
10 Friedman J.A., Piepgras D.G. Current neurosurgical indications for saphenous vein graft bypass. Neurosurg Focus. 2003;14:e1.
11 Goldring S., Zervas N., Langfitt T. The Extracranial-Intracranial Bypass Study. A report of the committee appointed by the American Association of Neurological Surgeons to examine the study. N Engl J Med. 1987;316:817-820.
12 Hadeishi H., Yasui N., Okamoto Y. Extracranial-intracranial high-flow bypass using the radial artery between the vertebral and middle cerebral arteries. Technical note. J Neurosurg. 1996;85:976-979.
13 Kato Y., Sano H., Imizu S., et al. Surgical strategies for treatment of giant or large intracranial aneurysms: our experience with 139 cases. Minim Invasive Neurosurg. 2003;46:339-343.
14 Langer D.J., Van Der Zwan A., Vajkoczy P., et al. Excimer laser-assisted nonocclusive anastomosis. An emerging technology for use in the creation of intracranial-intracranial and extracranial-intracranial cerebral bypass. Neurosurg Focus. 2008;24:E6.
15 Lawton M.T., Hamilton M.G., Morcos J.J., et al. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83-92. discussion 92–94
16 Lemole G.M.Jr, Henn J., Javedan S., et al. Cerebral revascularization performed using posterior inferior cerebellar artery–posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg. 2002;97:219-223.
17 Mohit A.A., Sekhar L.N., Natarajan S.K., et al. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60:ONS105-ONS122. discussion ONS122–ONS123
18 Morgan M.K., Sekhon L.H. Extracranial-intracranial saphenous vein bypass for carotid or vertebral artery dissections: a report of six cases. J Neurosurg. 1994;80:237-246.
19 Quinones-Hinojosa A., Du R., Lawton M.T. Revascularization with saphenous vein bypasses for complex intracranial aneurysms. Skull Base. 2005;15:119-132.
20 Quinones-Hinojosa A., Lawton M.T. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2005;57:140-145. discussion 140–145
21 Regli L., Piepgras D.G., Hansen K.K. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83:806-811.
22 Relman A.S. The extracranial-intracranial arterial bypass study: what have we learned? N Engl J Med. 1987;316:809-810.
23 Rivet D.J., Wanebo J.E., Roberts G.A., et al. Use of a side branch in a saphenous vein interposition graft for high-flow extracranial-intracranial bypass procedures. Technical note. J Neurosurg. 2005;103:186-187.
24 Sanai N., Zador Z., Lawton M.T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670-683. discussion 683
25 Santoro A., Guidetti G., Dazzi M., et al. Long saphenous-vein grafts for extracranial and intracranial internal carotid aneurysms amenable neither to clipping nor to endovascular treatment. J Neurosurg Sci. 1999;43:237-250. discussion 250–251
26 Sekhar L.N., Natarajan S.K., Ellenbogen R.G., et al. Cerebral revascularization for ischemia, aneurysms, and cranial base tumors. Neurosurgery. 2008;62:SHC1373-SHC1408. discussion SHC1408–SHC1410
27 Sundt T.M.Jr. Was the international randomized trial of extracranial-intracranial arterial bypass representative of the population at risk? N Engl J Med. 1987;316:814-816.
28 Ustun M.E., Buyukmumcu M., Ulku C.H., et al. Radial artery graft for bypass of the maxillary to proximal middle cerebral artery: an anatomic and technical study. Neurosurgery. 2004;54:667-670. discussion 670–671
29 Yasargil M. Anastomosis between Superficial Temporal Artery and a Branch of the Middle Cerebral Artery. Stuttgart: Georg Thieme Verlag, 1969.
30 Zhang Y.J., Barrow D.L., Day A.L. Extracranial-intracranial vein graft bypass for giant intracranial aneurysm surgery for pediatric patients: two technical case reports. Neurosurgery. 2002;50:663-668.