Iatrogenic Causes of Limbal Stem Cell Deficiency
Introduction
Proper functioning of limbal stem cells is crucial to the maintenance of a stable and healthy ocular surface. Limbal stem cells provide a renewal source of corneal epithelial cells and act as a barrier to the extension of conjunctival epithelial cells onto the corneal surface. Limbal stem cell deficiency (LSCD) results in breakdown of the corneal epithelium and poor wound healing, eventually leading to conjunctivalization and opacification of the cornea.1 Symptoms of LSCD include redness, irritation, photophobia, and decreased vision. Early findings on slit lamp examination include corneal neovascularization, pannus, and loss of the palisades of Vogt. As the condition progresses, punctate epithelial keratopathy and frank epithelial defects may develop. Due to decreased wound healing, these epithelial defects may become persistent, which can lead to stromal scarring, ulceration, and perforation. Conjunctivalization of the cornea may occur, where the corneal epithelium is replaced with a conjunctival epithelial phenotype.2 Staining of the conjunctivalized epithelium with fluorescein may occur. If conjunctival stem cells, which are thought to reside in the fornices, are also compromised, the entire ocular surface may become keratinized.3,4
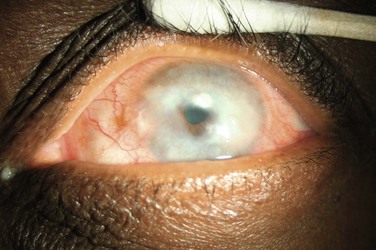
Causes of limbal stem cell deficiency include both inherited and acquired etiologies. Aniridia is a condition in which anterior segment dysgenesis results in a decreased number of limbal stem cells. Aniridic individuals are born with a normal ocular surface. However, as the patient ages, limbal stem cell dysfunction manifests as corneal epitheliopathy which begins in the peripheral cornea and progresses to involve the central cornea, leading to corneal ulceration, scarring and decreased vision. Other congenital causes of LSCD include Peters anomaly and ectodermal dyplasia.1 Autoimmune disorders can also result in limbal stem cell deficiency (Fig. 33.1). Typically, these conditions cause chronic inflammation of the conjunctiva with secondary involvement of the limbus. Stevens–Johnson syndrome, toxic epidermal necrolysis, and ocular cicatricial pemphigoid are all diseases in which chronic conjunctival and limbal inflammation diminishes the stem cell population. Limbal lesions, such as conjunctival or corneal intraepithelial neoplasia (CIN) are also associated with limbal stem cell deficiency.5 Stem cell deficiency is thought to result from replacement of normal stem cells with neoplastic cells. Pterygia have also been associated with LSCD, presumably, due to chronic limbal inflammation.6 Direct trauma to the limbus by alkali, acid, or thermal injury is another common cause of LSCD.3 Other acquired causes of LSCD include infectious causes, such as herpesviruses and trachoma. Recent reports of mustard gas-induced LSCD have also been described.7
The categories above represent the most common causes of LSCD as reported in the literature. However, another group of patients develop LSCD secondary to iatrogenic etiologies, defined as directly resulting from intervention or treatment by a physician or surgeon. Many of these cases are multifactorial, and while they are treated with the same methods as those used for classically described causes of stem cell deficiency, awareness of induced etiologies may be useful for both the early recognition and prevention of LSCD in these patients. This chapter will review examples of iatrogenic causes of LSCD (Box 33.1).
Multiple Ocular Surgery-Induced Iatrogenic Stem Cell Deficiency
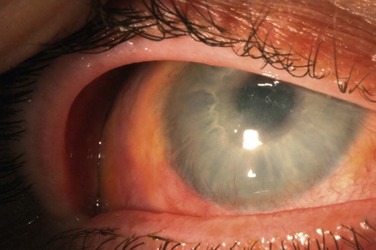
Limbal stem cell deficiency can occur as a result of multiple ocular surgeries (Fig. 33.2). Puangsricharern and Tseng classified limbal stem cell deficiency etiologies into two groups, resulting from either hypofunction or aplasia of stem cells.6 In the latter category, patients had a clear history of limbal stem cell destruction by chemical/thermal burns, Stevens–Johnson syndrome, severe microbial keratitis, contact lens-induced keratopathy, and a previously undescribed group of patients had undergone multiple surgeries or cryotherapies to the limbal region. Patients had clinical signs of LSCD, including corneal vascularization and irregular corneal epithelium, as well as impression cytology demonstrating goblet cell-containing conjunctival epithelial cells on the corneal surface.6
In 1998, Schwartz and Holland described a group of patients with limbal stem cell disease not secondary to a known diagnosis, which they termed ‘iatrogenic limbal stem cell deficiency.’8,9 The patients were similar to the above group in Puangsricharern’s study who had undergone ‘multiple surgeries or cryotherapies of the limbal region.’ Schwartz’s study comprised 14 eyes of 12 patients. All eyes had a history of prior ocular surgery involving the corneoscleral limbus, as well as concurrent external disease, including pterygium, keratoconjunctivitis sicca, rosacea, herpes simplex keratitis, or corneal edema. Eleven eyes had received long-term topical medications. In all eyes, the superior quadrants that corresponded to the areas of prior limbal surgery were affected by corneal scarring and neovascularization. Many of the eyes had undergone multiple ocular surgeries. Nine eyes had undergone prior ICCE, three had ECCE, and nine had penetrating keratoplasties.
All eyes exhibited a chronic, progressive epitheliopathy that began in the peripheral cornea and extended centrally, in a pattern consistent with limbal stem cell deficiency. The epitheliopathy was initially sectoral and located in an area of previous limbal surgery, in contrast to classically described causes of LSCD which involve the entire limbus. The authors noted that the epitheliopathy did not resolve with therapy for dry eye management or with cessation of topical medications, implying a permanent alteration in the stability of the ocular surface. They hypothesized that direct surgical trauma to the limbus resulted in localized stem cell deficiency, resulting in increased susceptibility to further damage from both external disease and toxicity from long-term topical medications. All patients had presumed multifactorial LSCD secondary to prior corneoscleral limbal surgery combined with topical medications.8
Sridhar et al. reported three cases of impression cytology-proven LSCD following limbal surgeries.10 One patient had undergone multiple pterygium surgeries, while the other two had undergone therapeutic penetrating keratoplasties. In all cases, limbal stem cell deficiency was confirmed by detection of goblet cells on the surface of the cornea by impression cytology. Surgical trauma to the limbus was the predisposing factor for the development of LSCD in all three cases, while use of topical medications in cases of therapeutic penetrating keratoplasty was felt to be a contributing factor.
Glaucoma Surgery and Iatrogenic Limbal Stem Cell Deficiency
The development of limbal stem cell deficiency in patients who have undergone glaucoma procedures is multifactorial and, due to a combination of mechanical insult from surgery and toxic injury from antimetabolite and chronic topical medication use.8,11 Antimetabolites, such as 5-fluorouracil and mitomycin C that are used in conjunction with glaucoma surgery are well known to cause corneal toxicity.11–13 5-fluorouracil (5-FU), a cell-cycle-specific antimetabolite, inhibits fibroblast proliferation and is used to prevent scarring and closure of the filtration sites in glaucoma procedures. In the acute phase, the cornea exhibits epithelial toxicity and sloughing following the administration of 5-FU, is likely due to its antimetabolite effect on corneal epithelial progenitor cells. With prolonged and recurrent use of 5-FU, damage to the slower-cycling limbal stem cells can result in corneal conjunctivalization and persistent epithelial breakdown.13 In 2000, Pires et al. reported two cases of iatrogenic limbal stem cell deficiency in post-trabeculectomy patients who had each received over 105 mg 5-FU injections in the postoperative period. Limbal stem cell deficiency was confirmed by impression cytology. One patient with partial limbal stem cell deficiency recovered well after amniotic membrane transplantation while the other, who exhibited total limbal stem cell deficiency, required limbal stem cell transplantation to stabilize the ocular surface.13
Mitomycin C (MMC), a potent DNA cross-linker, is an antimetabolite that targets dividing cells. MMC is usually applied during glaucoma surgery by soaking the drug in sponges and placing them in contact with the conjunctiva near the filtration site. However, this technique can lead to wound leak, due to delayed conjunctival healing.12,14 Sauder and Jonas reported a case series of seven patients who underwent trabeculectomy with a modified technique, in which mitomycin C was administered subconjunctivally prior to opening of the conjunctiva. In this series, three patients developed signs and symptoms of limbal stem cell deficiency, including avascularity of over 50% of the limbal circumference, dry eye, corneal epithelial irregularities, and corneal opacities. The other four patients did not show similar sequelae, but, of note, were each followed for less than 14 months, compared to the three patients with MMC-related complications that were followed for at least 2 years. The authors concluded that subconjunctival MMC in conjunction with trabeculectomy could result in delayed postoperative complications, including late limbal stem cell deficiency, and should therefore be avoided.14
Contact Lens-Induced Iatrogenic Limbal Stem Cell Deficiency
Contact lens-induced keratopathy has long been recognized as a cause of ocular surface disease, due to limbal stem cell deficiency. In 1984, Bloomfield et al. described mild cases of LSCD in contact lens wearers as contact lens-induced keratoconjunctivitis.15 Patients exhibited papillary hypertrophy of the superior tarsus, superior limbal hypertrophy, and superior punctate staining. Severe cases of contact lens-related LSCD, which were classified as contact lens-induced keratopathy, demonstrated superficial punctate keratitis, epithelial irregularities, and a superior ‘V’-shaped vascularized pannus of the cornea in the region of stem cell deficiency.15
Several theories for the development of contact lens-induced keratopathy have been proposed. Bloomfield proposed that mechanical rubbing from poorly fitting contact lenses can damage the limbus and result in limbal stem cell compromise.15 Chronic irritation to the limbal region can cause perilimbal capillaries to grow between the corneal epithelium and Bowman’s membrane, forming a pannus. These capillaries are accompanied by fibroblasts which produce collagen and dissolve Bowman’s membrane, producing a superficial corneal scar and irregular astigmatism. Mechanical pressure from blinking of the upper lid may contribute to superior pannus formation.16 Pannus may also occur from chronic tight lens syndrome17 or from long-term hypoxia from low oxygen permeable soft contact lenses. Signs of tight lens syndrome include corneal edema, punctate epithelial erosions, iritis, and lack of lens movement upon blink. An imprint in the anterior conjunctiva can sometimes be seen upon lens removal.18
In one study, contact lens-induced keratopathy comprised 15% of LSCD cases.19 Another large retrospective study of over 500 soft contact lens wearers identified risk factors for asymptomatic corneal conjunctivalization.20 These patients were predominantly female, myopic, and had been wearing daily disposable soft contact lenses for more than 10 hours per day for 6 or more years. The authors suggested that early diagnosis of the condition, reduced length of contact lens wear, suspension of contact lens use, or refitting with either high oxygen permeability soft or RGP contact lenses, would be useful in decreasing the prevalence of contact lens-related LSCD.20
Toxicity from contact lens solutions can also damage limbal stem cells. Thimerosal is an organic mercurial that was commonly used as an antimicrobial preservative in contact lens disinfecting solutions in the 1980s. Jenkins et al. described chronic corneal epitheliopathy with a history of thimerosal exposure in soft contact lens wearers.16 All patients exhibited corneal changes characterized by epithelial haze and superficial stromal vascularization with significantly decreased visual acuity. In vitro cell studies have shown the cytotoxic effects of thimerosal, which include inhibition of mitotic activity in human corneal epithelial cells, loss of cell membrane integrity, and initiation of mitochondria-mediated and caspase-3-dependent apoptosis in human neurons and fibroblasts.21 Thimerosal toxicity has been reported to cause total limbal stem cell failure with secondary corneal vascularization and opacification.22
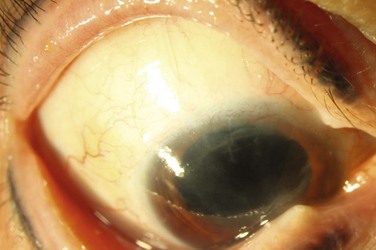
A recent study by Jeng et al. investigated the management of focal limbal stem cell deficiency secondary to long-term contact lens use (Fig. 33.3).23 Nine out of ten patients were women, and superior involvement was present in all eighteen eyes. LSCD was diagnosed based on clinical findings, including the appearance of irregular corneal epithelium seen as dull opaque-appearing tissue of variable thickness originating from the limbus; impression cytology was not available to confirm the diagnosis. Most of the eyes responded to conservative therapy with cessation of contact lens use and administration of artificial tears with corticosteroids; however, one eye underwent conjunctival autograft and another eye underwent amniotic membrane transplantation for more aggressive disease, both with successful visual outcomes.23
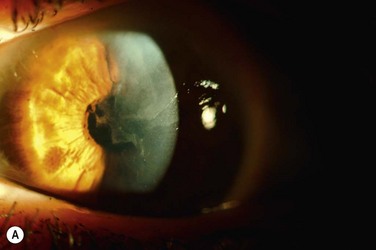

Figure 33.3 (A) Contact lens induced LSCD demonstrating superior wedge-shaped corneal conjunctivalization. (Courtesy Bennie Jeng, MD, reproduced with permission from Wolters Kluwer Health23 Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30:18–23.) (B) Contact lens-induced LSCD with fluorescein stain demonstrating corneal epithelial irregularity. (Courtesy Bennie Jeng, MD, reproduced with permission from Wolters Kluwer Health23 Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30:18–23.)
Iatrogenic Limbal Stem Cell Deficiency Associated with Ocular Surface Tumor Therapy
Ocular surface neoplasias in and of themselves are associated with limbal stem cell deficiency, presumably to the replacement of healthy stem cells with dysfunctional neoplastic cells.5 The treatment of ocular surface neoplasias, including conjunctival intraepithelial neoplasia (CIN), atypical primary acquired melanosis (PAM), and conjunctival melanoma may involve wide surgical excision with cryotherapy to the conjunctiva and limbus, the application of topical adjuvant medications, such as mitomycin C and 5-fluorouracil, or radiation therapy, all of which result in additional insult to the limbal stem cell population.
Limbal stem cell deficiency following the treatment of CIN is likely secondary to a combination of inherent stem cell dysfunction from the neoplastic process and multiple treatment modalities that result in limbal injury. Puangsricharern and Tseng reported LSCD in two patients with CIN who underwent multiple tumor resections and cryotherapies.6 In one case, additional conjunctival scraping for recurrent tumor was performed several years after the initial resection, and the patient subsequently developed superficial pannus and corneal epithelial irregularity. Impression cytology revealed the presence of goblet cells on the corneal surface, indicating LSCD. The patient’s ocular surface stabilized and his visual acuity improved after limbal transplantation from the contralateral eye.6 A recent study by Asoklis et al. demonstrated two cases of partial limbal stem cell deficiency following the excision of limbal tumors with cryotherapy applied to the resected edge of conjunctiva and to the limbus and amniotic membrane grafting. In both cases, there was an extensive limbal invasion of the tumor involving more than one-fourth of the limbus.24
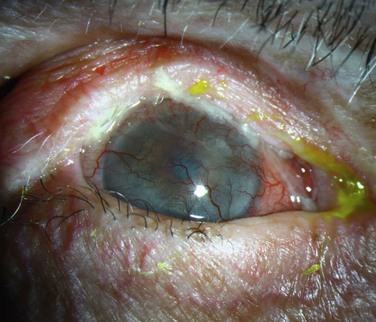
Mitomycin C (MMC) is frequently used in the treatment of ocular surface neoplasias, including CIN, PAM, and conjunctival melanoma, and can result in limbal stem cell deficiency (Fig. 33.4). Dudney et al. first described a case of LSCD in a patient who was treated for conjunctival–corneal intraepithelial neoplasia with a total of 5 week-long courses of topical mitomycin C 0.04%.25 The patient subsequently developed superior stromal haze and nonhealing epithelial defects, presumably secondary to limbal stem cell dysfunction, but was lost to follow-up. In a recent study of 21 patients who underwent MMC therapy for PAM with atypia, five patients were noted to develop limbal stem cell deficiency, diagnosed clinically and confirmed by impression cytology.26 Older age and longer treatment periods were found to be significant risk factors for this complication. Partial resolution of LSCD occurred in three of the patients, as evidenced by normalization of the corneal epithelium and improved visual acuity. Ditta et al. reported LSCD as a complication of long-term MMC therapy in a cohort of patients with conjunctival melanoma. Four out of 15 patients (26.7%) developed clinical signs of stem cell deficiency.27 Russell et al. performed a 10-year review of topical MMC for ocular surface neoplasia, including primary acquired melanosis (PAM), melanoma, corneal–conjunctival intraepithelial neoplasia (CCIN), squamous cell carcinoma (SCC) and sebaceous gland carcinoma (SGC).28 The overall rate of long-term complications was higher than previously reported, at 33%. Seven out of 58 patients developed corneal epithelial changes, due to presumed limbal stem cell deficiency (LSCD), but five of these patients also underwent surgical excision with cryotherapy, which likely contributed to the limbal insult. The remaining two patients had MMC as a primary treatment; thus, it is likely that MMC was the cause of this complication.28
Radiation Therapy-Induced Iatrogenic Limbal Stem Cell Deficiency
Local radiation therapy is known to cause ocular toxicity and visual loss by affecting multiple ocular structures. Plaque radiotherapy for ocular melanomas can result in radiation retinopathy, neovascular glaucoma, and optic neuropathy.29 Radiation can also result in cataract, corneal epithelial toxicity and limbal stem cell deficiency (Fig. 33.5).
In 1996, Fujishima et al. reported a case of temporary corneal stem cell dysfunction after radiation therapy for maxillary cancer.30 The patient received a dose of 61 gy over 44 days, and experienced ocular surface discomfort and vision loss to 20/500. Examination revealed corneal epithelial opacity, and impression cytology demonstrated goblet cells on the cornea. After conservative management with artificial tears and topical antibiotic ointment, the ocular surface improved and 4 months later, visual acuity recovered to 20/40. Repeat impression cytology demonstrated no goblet cells; therefore, the authors concluded that the LSCD had been temporary.
Smith et al. reported a case of permanent LSCD following local radiotherapy for orbital lymphoma in a 31-year-old male patient who received a total of 4600 cGY over 21 sessions.31 Several years later, the patient demonstrated superficial vascularization of the cornea. Immunostaining of impression cytology specimens demonstrated a conjunctival phenotype over portions of the cornea, indicating delayed limbal stem cell deficiency. LSCD can also occur after proton radiotherapy for ocular surface neoplasias, such as conjunctival melanoma.32
Systemic Chemotherapy-Induced Iatrogenic Limbal Stem Cell Deficiency
Limbal stem cell deficiency may arise from the treatment of systemic disease. Ellies et al. reported a case of bilateral corneal epitheliopathy in a patient undergoing systemic chemotherapy for chronic myelocytic leukemia with hydroxyurea.33 Hydroxyurea is a cytotoxic agent that arrests DNA replication. Impression cytology revealed focal LSCD localized to the temporal and inferior portion of the right cornea by exhibiting conjunctival goblet cells and mucins on the cornea and diffuse squamous metaplasia of the left cornea with partial loss of the limbal area. Cessation of hydroxyurea therapy resulted in significant improvement of the ocular surface in both eyes, although the left eye still required keratolimbal allograft with amniotic membrane transplantation for visual rehabilitation.33
Another case of LSCD arising from systemic hydroxyurea use was reported in 2009.34 The patient, who had sickle cell disease, was placed on hydroxyurea for pulmonary hypertension, and developed pterygium-like peripheral corneal neovascularization in both eyes with progressive pannus. Histopathologic analysis of the conjunctiva revealed pingueculae, loss of goblet cells with scar tissue formation, and corneal neovascularization consistent with limbal stem cell deficiency.34
Rare Causes of Iatrogenic Limbal Stem Cell Deficiency
Unusual causes of iatrogenic LSCD have been presented in the literature. Nghiem-Buffet et al. reported one case of stem cell deficiency following phototherapeutic keratectomy (PTK).35 The patient, who had a history of diabetes mellitus and rosacea, underwent PTK for recurrent epithelial erosions. The procedure consisted of complete epithelial debridement and photoablation of the central 8 mm-diameter area of the cornea. He subsequently developed a vascular pannus and underwent excision of the pannus with limbal autografting from the contralateral eye. The diagnosis of LSCD was confirmed by histologic examination of the pannus demonstrating goblet cells within the corneal epithelium.35
Recently, Capella et al. reported a case of LSCD following multiple Avastin injections.36 The patient, who had a history of idiopathic choroidal polypoidal vasculopathy, underwent five sessions of photodynamic therapy, one transpupillary thermal therapy, and seven injections of intravitreal bevacizumab in the superonasal quadrant. He subsequently developed a superior corneal pannus and central epithelial irregularity at the site of the multiple intravitreal injections. LSCD was confirmed by impression cytology. He underwent limbal autograft from the same eye, which resulted in significant improvement of the epitheliopathy and stabilization of the ocular surface.36
It is possible that some iatrogenic causes of LSCD have not yet been identified. UV light exposure has been associated with limbal stem cell deficiency.37,38 A relatively new and increasingly popular early intervention for keratoconus and post-refractive ectasia is corneal collagen cross-linking, which involves the use of UVA irradiation of the cornea after topical application of the photosensitizer riboflavin. Long-term studies are necessary to assess the potential effects of this procedure on limbal stem cells.
Medical Management/Prevention of Iatrogenic Limbal Stem Cell Deficiency
Topical corticosteroids may be useful both to minimize patient discomfort and to control the inflammatory component of LSCD. Corticosteroids may help in the regression of corneal neovascularization. Anti-VEGF agents, such as bevacizumab may also prove beneficial in the management of corneal neovascularization secondary to LSCD. In a small interventional case series by Bock et al., four patients with limbal stem cell deficiency and aggressive corneal neovascularization were treated with topical bevacizumab. All patients showed a decrease in neovascularization, although the response was highly variable. None of the patients experienced adverse corneal side effects.39
Severe cases of LSCD may require surgical intervention with limbal stem cell transplantation.
References
1. Hatch, KM, Dana, R. The structure and function of the limbal stem cell and the disease states associated with limbal stem cell deficiency. Int Ophthalmol Clin. 2009;49:43–52.
2. Lim, P, Fuchsluger, TA, Jurkunas, UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–148.
3. Dua, HS, Saini, JS, Azuara-Blanco, A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Ind J Ophthalmol. 2000;48:83–92.
4. Wei, ZG, Wu, RL, Lavker, RM, et al. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814–1828.
5. Erie, JC, Campbell, RJ, Liesegang, TJ. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 1986;93:176–183.
6. Puangsricharern, V, Tseng, SCG. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485.
7. Javadi, MA, Jafarinasab, MR, Feizi, S, et al. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118:1272–1281.
8. Schwartz, GS, Holland, EJ. Iatrogenic limbal stem cell deficiency. Cornea. 1998;17:31–37.
9. Holland, EJ, Schwartz, GS. Iatrogenic limbal stem cell deficiency. Trans Am Ophthalmol Soc. 1997;95:95–107.
10. Sridhar, MS, Vemuganti, GK, Bansal, AK, et al. Impression cytology-proven corneal stem cell deficiency in patients after surgeries involving the limbus. Cornea. 2001;20:145–148.
11. Schwartz, GS, Holland, EJ. Iatrogenic limbal stem cell deficiency: when glaucoma management contributes to corneal disease. J Glauc. 2001;10:443–445.
12. Hau, S, Barton, K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009;20:131–136.
13. Pires, RTF, Chokshi, A, Tseng, SCG. Amniotic membrane transplantation or conjunctival limbal autograft for limbal stem cell deficiency induced by 5-fluorouracil in glaucoma surgeries. Cornea. 2000;19:284–287.
14. Sauder, G, Jonas, JB. Limbal stem cell deficiency after subconjunctival mitomycin C injection for trabeculectomy. Am J Ophthalmol. 2006;141:1129–1130.
15. Bloomfield, SE, Jakobiec, FA, Theodore, FH. Contact lens induced keratopathy: a severe complication extending the spectrum of keratoconjunctivitis in contact lens wearers. Ophthalmology. 1984;91:290–294.
16. Jenkins, C, Tuft, S, Liu, C, et al. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye. 1993;7:629–633.
17. Arentsen, JJ. Corneal neovascularization in contact lens wearers. Int Ophthalmo Clin. 1986;26:15–23.
18. Achong, RAC. Limbal stem cell deficiency in a contact lens-related case. Clin Eye Vis Care. 1999;11:191–197.
19. Donisi, PM, Rama, P, Fasolo, A, et al. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22:533–538.
20. Martin, R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007;90:26–30.
21. Baskin, DS, Ngo, H, Didenko, VV. Thimerosal induces DNA breaks, caspase-3 activation, membrane damage, and cell death in cultured human neurons and fibroblasts. Toxicol Sci. 2003;74:361–368.
22. Nguyen, DQ, Srinivasan, S, Hiscott, P, et al. Thimerosal-induced limbal stem cell failure: report of a case and review of the literature. Eye Contact Lens. 2007;33:196–198.
23. Jeng, BH, Halfpenny, CP, Meisler, DM, et al. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30:18–23.
24. Asoklis, RS, Damijonaityte, A, Butkiene, L, et al. Ocular surface reconstruction using amniotic membrane following excision of conjunctival and limbal tumors. Eur J Ophthalmol. 2011;21:552–558.
25. Dudney, BW, Malecha, MA. Limbal stem cell deficiency following topical mitomycin C treatment of conjunctival-corneal intraepithelial neoplasia. Am J Ophthalmol. 2004;137:950–951.
26. Lichtinger, A, Pe’er, J, Frucht-Pery, J, et al. Limbal stem cell deficiency after topical mitomycin C therapy for primary acquired melanosis with atypia. Ophthalmology. 2010;117:431–437.
27. Ditta, LC, Shildkrot, Y, Wilson, MW. Outcomes in 15 patients with conjunctival melanoma treated with adjuvant topical mitomycin C: complications and recurrences. Ophthalmology. 2011;118:1754–1759.
28. Russell, HC, Chadha, V, Lockington, D, et al. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010;94:1316–1321.
29. Chan, MD, Melhus, CS, Mignano, JE, et al. Analysis of visual toxicity after gamma knife radiosurgery for treatment of choroidal melanoma: identification of multiple targets and mechanisms of toxicity. Am J Clin Oncol. 2011;34:517–523.
30. Fujishima, H, Shimazaki, J, Tsubota, K. Temporary corneal stem cell dysfunction after radiation therapy. Br J Ophthalmol. 1996;80:911–914. [Epub 1996/10/01].
31. Smith, GT, Deutsch, GP, Cree, IA, et al. Permanent corneal limbal stem cell dysfunction following radiotherapy for orbital lymphoma. Eye. 2000;14:905–907.
32. Wuestemeyer, H, Sauerwein, W, Meller, D, et al. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:438–446.
33. Ellies, P, Anderson, DF, Topuhami, A, et al. Limbal stem cell deficiency arising from systemic chemotherapy. Br J Ophthalmol. 2001;85:373–374.
34. Ding, X, Bishop, RJ, Herzlich, AA, et al. Limbal stem cell deficiency arising from systemic chemotherapy with hydroxycarbamide. Cornea. 2009;28:221–223.
35. Nghiem-Buffet, MH, Gatinel, D, Jacquot, F, et al. Limbal stem cell deficiency following phototherapeutic keratectomy. Cornea. 2003;22:482–484.
36. Capella, MJ, Álvarez de Toledo, J, de la Paz, MF. Limbal stem cell deficiency following multiple intravitreal injections. Archivos de la Sociedad Española de Oftalmología. 2011;86:89–92.
37. Passchier, WF, Bosnjakovic, BFM. Human exposure to ultraviolet radiation : risks and regulations: proceedings of a seminar held in Amsterdam, 23–25 March 1987. Amsterdam; New York, NY, USA: Excerpta Medica. Sole distributors for the USA and Canada, Elsevier Science Pub. Co.; 1987.
38. Zaidi, FH, Bloom, PA, Corbett, MC. Limbal stem cell deficiency: a clinical chameleon. Eye. 2003;17:837–839.
39. Bock, F, Konig, Y, Kruse, F, et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefe’s Arch Clin Exp Ophthalmol. 2008;246:281–284.