Iatrogenic and Traumatic Injuries
Cynthia B. Jensen, Sheila S. Galbraith
Introduction
A variety of untoward events may befall the developing infant while in utero or postpartum. Some of these perinatal problems are inherent in the birth process. Others are related to technologic advances that have become standard obstetric and nursery practice. Although these diagnostic and therapeutic procedures have reduced morbidity and mortality, some also pose a significant risk for iatrogenic complications. Sequelae of iatrogenic complications can also present later in infancy or early childhood. Moreover, iatrogenic and traumatic injuries can occur in older infants. This chapter emphasizes those occurring in neonates and young infants. More extensive discussions of non-accidental injury (more common in older infants) can be found in other textbooks.
Puncture wounds
Amniocentesis
Amniocentesis is currently the most widely used technique for the antenatal diagnosis of genetic disorders. Although routinely a second-trimester procedure, it may also be performed in the third trimester for management of isoimmunization or evaluation of fetal maturity, or late in the first trimester for fetal karyotyping and DNA analysis.1 The risk of damage to the fetus is quite low, particularly in the middle trimester; nevertheless, needle puncture of the skin and sometimes of the underlying structures is a possible complication. Estimates of the incidence of cutaneous scarring ranged as high as 9% in the 1970s; however, with increased experience and the advent of real-time ultrasonography, this figure has dropped to less than 1%.2 Despite the benignity of the procedure, the incidence of fetal injury rises dramatically with an increasing number of needle passages at amniocentesis.
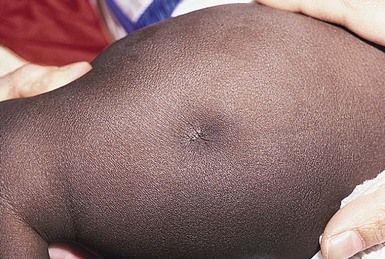
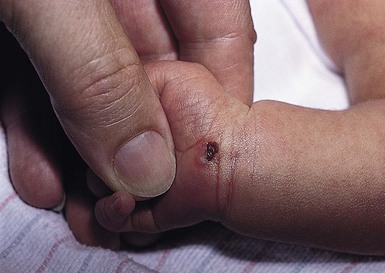
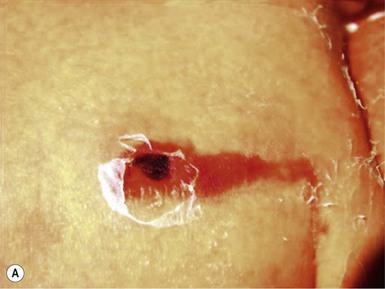
Amniocentesis scars are depressed, dimple-like lesions that usually measure 1–5 mm in diameter, although scars as large as 12 mm in diameter and 8 mm in depth have been documented (Fig. 8.1). They may be solitary or multiple, and are often inconspicuous. Shallow linear lesions have also been described.2 Although sometimes present at birth, they are often not noticed until the infant is several weeks to months old. The most frequent sites of injury are the extremities, followed by the head, neck, and chest. Mid-trimester amniocentesis has the lowest risk of puncture because the fetus occupies only about 50% of the amniotic cavity; in both the first and third trimesters, there is less room to maneuver, and sudden movements of the fetus may make injury unavoidable.
Amniocentesis scars must be differentiated from congenital sinus tracts, aplasia cutis, focal dermal dysplasia, amniotic band syndrome, accessory nipples, and dimples associated with congenital rubella, diastematomyelia, Bloom syndrome, and cerebrohepatorenal syndrome.
Chorionic villus sampling
Chorionic villus sampling (CVS), which can be performed early in the first trimester, is the preferred procedure for patients at risk for certain single gene disorders. The technique yields mitotically active cells suitable for rapid DNA analysis and permits detection of placental mosaicism. Of concern, however, are reports of increased risk of limb and jaw malformations as well as an increased risk of infantile hemangiomas, particularly in fetuses undergoing CVS at <9 weeks’ gestation.1,3 An analysis of 138 996 outcomes in a multicenter study disputes this notion4 but is not universally accepted, and thus the issue remains a controversial area still under study. A distinctive defect of absent distal third fingers with tapering of other digits, was more recently reported to be associated with exposure to CVS in a review by Golden and colleagues.5
Fetal monitoring
Intrauterine electronic monitoring of the fetal heart rate via a spiral electrode attached to the presenting part has become standard obstetric practice. Complications are infrequent and consist mainly of minor lacerations, ulcerations, scalp abscesses, and herpetic infections.6,7 Herpetic infections are extremely rare; however, incidence figures for scalp abscesses in monitored infants due to other agents, range from 0.1% to 5.4%,8,9 with most in the 0.3–0.5% range.
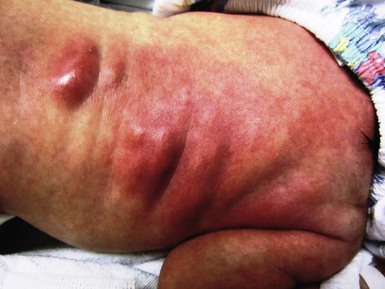
Scalp abscesses are localized collections of suppurative material that present as erythematous, indurated masses with or without fluctuance in the area of electrode application. Usually solitary, they vary in size from one to several centimeters. Onset can be as early as the first day or as late as the third week, but they are most frequently noted on the third or fourth day of life. Enlarged posterior cervical lymph nodes often accompany the abscess. Usually the inflammation remains confined to the skin; however, in a review of neonatal scalp abscesses by Weiner and coworkers, reported complications can include osteomyelitis, cellulitis, seizures, meningitis, brain abscess, bacteremia, and death.10 Contributing factors in some series (but not others) appear to be high-risk pregnancy (prematurity), prolonged rupture of the membranes, and long duration of fetal heart rate monitoring. The presence of amnionitis6,8,11 does not seem to be correlated. Although an infectious cause has been disputed – because cultures obtained from some infants have been sterile – data from large series support the concept of an infectious etiology. Okada and colleagues8 reported on 42 infants with scalp abscess, 100% of whom had positive cultures: 85% were polymicrobial, 58% grew both aerobes and anaerobes, 33% grew aerobes only, and 9% grew anaerobes only. The predominant aerobic organisms were Staphylococcus epidermidis and Streptococcus groups A and B; the predominant anaerobes were Streptococcus and Peptococcus. A confirmatory study by Brook and Frazier12 demonstrated similar findings in 23 infants. Andrews and colleagues evaluated vaginal cultures from 5732 mothers and found the MRSA colonization rate to be 3.5%, with no cases of early-onset neonatal MRSA infection postpartum.13 It is critical to distinguish infants with intrapartum inoculation of herpes simplex virus (HSV) from neonates with a bacterial scalp abscess (see Chapter 13). Although HSV infection as a complication of scalp monitoring is distinctly uncommon, the outcome can be devastating, with permanent neurologic damage,14 or death from systemic disease.15 Both type 1,15 and type 2 infections7 have been documented; unfortunately, this complication may occur with asymptomatic shedding of the virus and in the absence of a history of overt clinical disease.
Scalp abscesses usually heal uneventfully but may leave minor degrees of scarring, hypopigmentation, and alopecia, causing confusion with aplasia cutis, nevus sebaceus, or focal dermal hypoplasia in later years.
Needle marks and scars
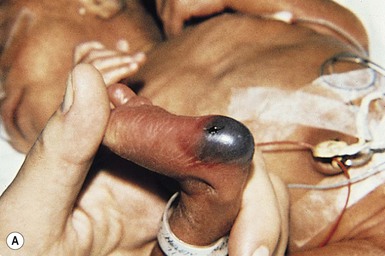
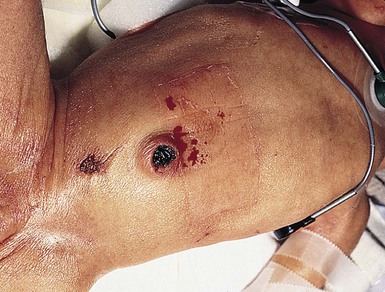
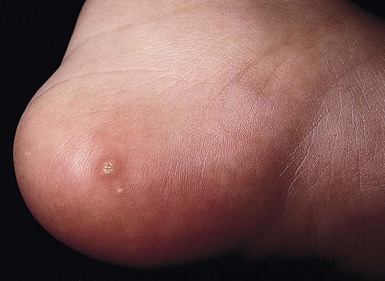
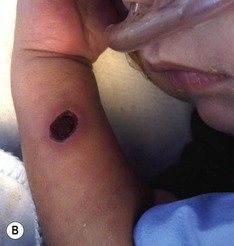
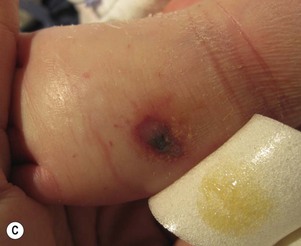
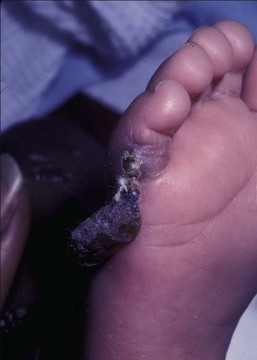
Needle marks consisting of hypopigmented pinhead-size lesions, when presenting in large numbers, may impart a speckled appearance to the skin. These marks are due to venipuncture, arterial punctures, and catheter insertion, and are most commonly seen on the scalp, hands, wrists, feet, ankles, arms, and legs. Fox and Rutter16 reported an improvement in the appearance of needle marks by 9 years of age, in their cohort of 90 patients. Heel-pricks from blood sampling may cause dimpling or, rarely, calcified nodules (see below), hypertrophic scars, or even gangrene (Fig. 8.2).
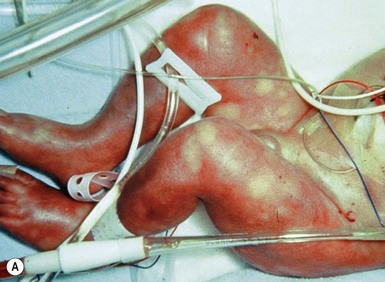
Birth-related trauma to the skin and scalp
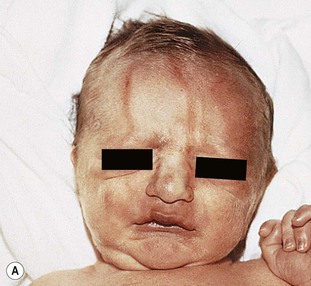
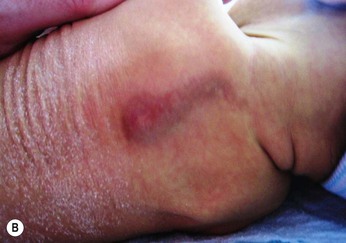
Injury to the soft tissues may occur in the setting of a prolonged labor because of cephalopelvic disproportion (Fig. 8.3), or with forceps delivery. Erythema, abrasions, and forceps marks are most common over the face, but rarely cause significant injury, and usually resolve spontaneously (Fig. 8.4).
Petechiae and ecchymoses
Petechiae on the head, neck, and upper body are likely to be caused by pressure differences that occur during passage of the chest through the birth canal. It is important to exclude the possibility of an underlying infection or hematologic disorder with appropriate laboratory studies. Petechiae caused by trauma are innocuous and usually fade within 2–3 days.
Ecchymoses may be extensive following a traumatic or breech delivery. Large areas of bruising may result in hyperbilirubinemia, requiring phototherapy. Ecchymoses resolve gradually, but may take up to several days to disappear completely.
Caput succedaneum
Diffuse edematous swelling of the scalp, when it is the presenting part, is known as caput succedaneum. Extravasation of blood or serum above the periosteum occurs as a result of venous congestion caused by pressure of the uterus, cervix, and the vaginal wall on the infant’s head during a prolonged or difficult labor and delivery. Because the accumulation of fluid is external to the periosteum, it crosses the midline and is not limited by the suture lines. If labor is prolonged, petechiae, purpura, and ecchymoses, as well as molding of the head and overriding sutures, may be prominent features. Unlike cephalhematoma, with which a caput is occasionally confused, the skin findings resolve within a few days. The molding may take a few weeks to disappear. Occasionally, if severe, tissue necrosis and a localized area of scarring alopecia may ensue.
Cephalhematoma
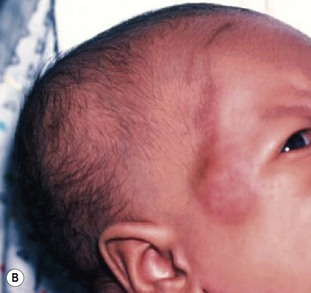
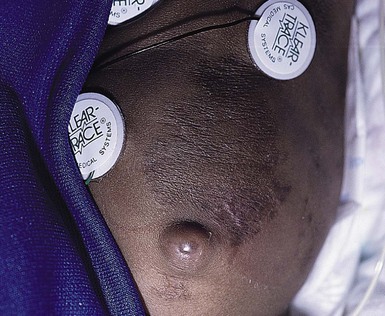
Cephalhematoma is caused by rupture of the emissary or diploic veins of the skull during a prolonged or difficult labor or delivery. The result of subperiosteal hemorrhage, it differs clinically from caput succedaneum in that it is almost always unilateral. The hematoma is localized most often to the area over the parietal bone, and the mass is confined by the periosteum, which adheres to the margin of the bone (Fig. 8.5). Cephalhematoma less frequently involves the occipital bones, and only rarely the frontal bones. If both parietal bones are involved, the hematomas are sharply demarcated and separated by a midline depression corresponding to the intervening suture. The overlying scalp is not discolored.
Cephalhematomas are seen more commonly in vacuum-assisted vaginal deliveries than in forceps or spontaneous births.17,18 The swelling may not become apparent until several hours to days after birth. As the hematoma ages, it develops a calcified rim and is gradually completely overlaid with bone. Estimates of underlying skull fractures have ranged from 5.4% to 25%.19 Differential diagnosis includes caput succedaneum and cranial meningocele.20 Meningoceles can be differentiated by the presence of pulsations, increased pressure when crying, and the presence of a bony defect on X-rays. Infection of the mass and severe hemorrhage resulting in anemia and hyperbilirubinemia are rare complications for which antibiotics, blood transfusions, and phototherapy may be required.21 Treatment is unnecessary for uncomplicated lesions. Most cephalhematomas are resorbed during the first few weeks of life and are of no consequence.17 Occasionally, they calcify and persist for months to years.
Subgaleal hemorrhage
Subgaleal hemorrhage is a rare but potentially life-threatening complication that occurs when emissary veins are ruptured, most commonly by instrumentation at delivery. Like cephalhematomas, vacuum-assisted delivery increases the incidence of subgaleal hemorrhage. Bleeding into the loose connective tissue of the subgaleal (also known as the subaponeurotic) space can be extensive, leading to severe anemia, disseminated intravascular coagulation (DIC) and hypovolemic shock.22 The area of swelling crosses suture lines and can extend from the brow line to the nape, as well as laterally to the temporal fascia located behind the ears.23 Unlike cephalhematomas, which are limited by the periosteum and suture lines, the aponeurotic space may accommodate up to 260 mL of blood, approaching the circulating blood volume of a neonate, which is approximately 80 mL/kg. Physical assessment may reveal dependent swelling on the scalp that varies from firm to fluctuant, and fluid waves may be present when the area is palpated. The neonate may exhibit hypotonia, pallor and tachypnea. As hypovolemia worsens, signs of poor perfusion, tachycardia, oliguria, and eventually hypotension may ensue. The infant must be closely monitored for signs and symptoms of neurologic compromise, shock and bleeding. Treatment is aimed at maintaining normovolemia, and controlling coagulopathy.
Untoward effects of vacuum extraction
The formation of some type of hematoma is a common occurrence with the use of a vacuum extractor, although with the introduction of softer silicone cups, the risk has been reduced.24 A ‘chignon,’ or artificial caput succedaneum, is created by adherence of the cup to the scalp and is most obvious immediately following removal of the cup. However, the swelling usually disperses relatively rapidly after birth. If a chignon is formed in the presence of a natural caput, the scalp may have a boggy sensation suggesting subgaleal hemorrhage (see above).25
Cephalhematomas, a ring of suction blisters, lacerations, and abrasions may also result from the use of the vacuum extractor.26 The latter are usually the result of prolonged traction and sudden detachment of the cup. Subcutaneous emphysema of the scalp has been attributed to vacuum extraction in an infant with a coexistent scalp electrode wound.27
Cases of vesicular eruptions following vacuum extraction with or without the presence of herpes simplex virus (Fig. 8.6) have been reported, presumably from the combination of mechanical trauma and colonization.28
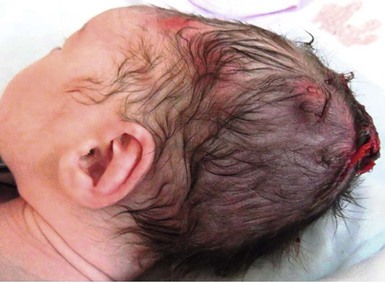
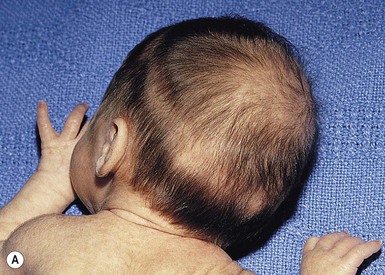
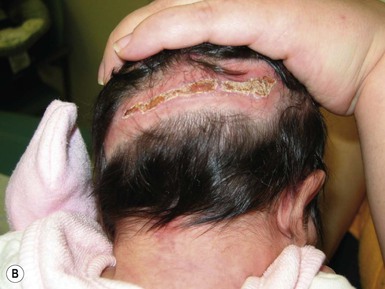
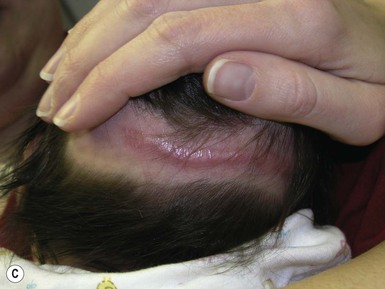
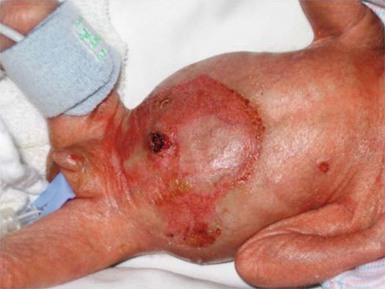
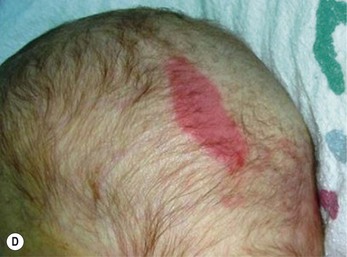
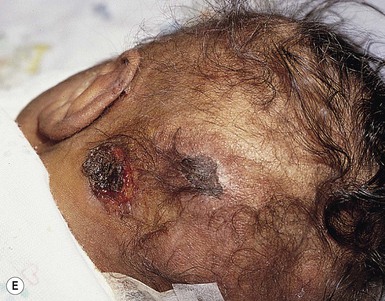
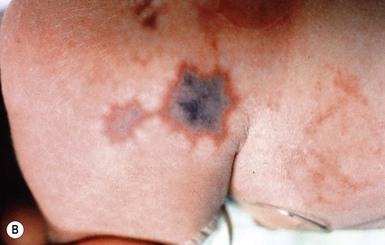
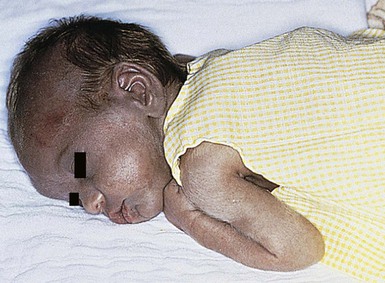
Halo scalp ring
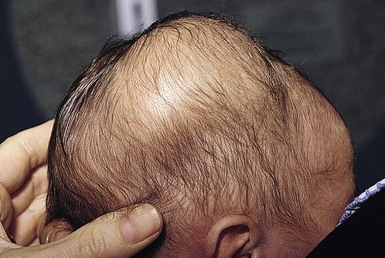
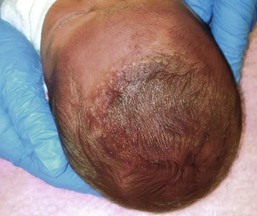
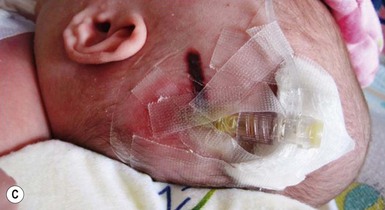
Alopecia in an annular configuration, presumably the consequence of localized injury during the birth process, has been referred to as ‘halo scalp ring’ injury.29 Often – but not always – there is a history of a prolonged labor. The hair loss is manifest at birth, or shortly thereafter, as a band of alopecia ranging in width from 1 to 4 cm, usually located over the vertex (Fig. 8.7A). There is typically an associated caput succedaneum and in some instances, frank tissue necrosis (Fig. 8.7B). If the injury is mild, the alopecia is usually temporary;29,30 however, scarring alopecia may result if the injury is severe (Fig. 8.7C).31,32 The areas of scarring can often be corrected with plastic surgery. The presence of halo scalp ring implies soft-tissue hypoxia and as such, infants with this condition should be monitored for developmental defects, which could have accompanied prolonged labor and associated hypoxic states.
Lacerations
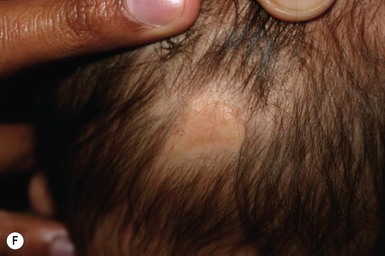
Scalpel lacerations to the infant during cesarean section represent a potential form of injury. Smith and coworkers33 found an incidence of fetal injury of 1.9% in a series of 896 cesarean deliveries. Lacerations were much more common in those deliveries where the indication was nonvertex presentation (breech or transverse lie). In these infants, the injuries were almost always located on the lower portion of the body, whereas infants in a vertex presentation usually sustained their lacerations on the head. Failure to recognize the injury in the delivery room was a common occurrence.
Burns and thermal injury
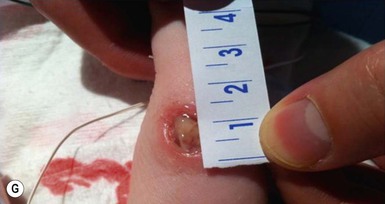
Chemical burns
Very low birthweight (VLBW), preterm infants are particularly predisposed to skin damage from topically applied chemicals and medications.34 This is largely due to an immature epidermal barrier, and their vulnerability may be accentuated by hypoxia and hypothermia.
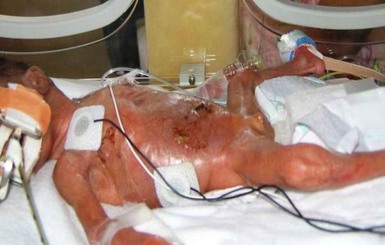
Chemical burns from concentrated disinfectants or other solvents have been reported following their use in the neonatal intensive care nursery (see Chapter 5). Burns are evident as intense erythema associated with blister formation and sloughing of the damaged skin (Fig. 8.8).
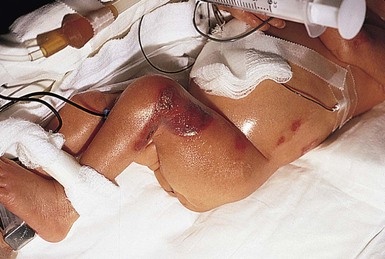
Isopropyl alcohol has caused second- and third-degree burns when substituted for electrode paste beneath electrocardiograph (ECG) leads or used as a preparation for the umbilical area. Alcohol-based skin cleansers, containing chlorhexidine gluconate (CHG), have also been reported to cause extensive burns in neonates (Fig. 8.9).35–37 Tissue damage can be prevented if the alcohol in the preparation is allowed to evaporate before draping for invasive procedures and carefully removed afterwards. Because of the reported incidence of CHG-related skin breakdown, use should be limited or avoided for VLBW infants.
Thermal injury
Scald injury and contact burns must be considered in the differential diagnosis of bullous lesions of unknown etiology. Inadvertent immersion injury was described in one such instance where the temperature of the hospital water supply was raised for purposes of infection control.38 Contact with a disposable warmer causing cicatricial alopecia and a cranial defect requiring bone grafts was reported in another neonate.39 Reports of deep burn injuries with relatively low-temperature (42°C) warming bottles have also been reported in the neonatal period.40,41
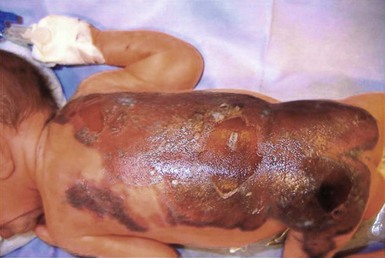
Burns related to attempts to warm hypothermic neonates with hot water-filled gloves or other items heated in a microwave have also been reported (Fig. 8.10).42 Scald burns become a much more common issue in older infants with non-accidental injuries, e.g. being forced to be immersed in very hot bath water.
Transillumination blisters
Thermal burns may occur as a complication of the use of transillumination devices for the detection of hydrocephalus, subdural effusions, cystic hygroma, pneumothorax, pneumomediastinum, or for localization of arteries and veins for blood sampling. Typical lesions are small (<5 mm), round, discrete blisters with a necrotic base that develop at sites of transillumination (Fig. 8.11).43 It is thought that specific wavelengths of the high-intensity fiberoptic light are converted to heat energy in the skin, causing thermal damage. Infrared and ultraviolet filters within the light source, usually a quartz halogen lamp, eliminate wavelengths of <570 nm, reducing the risk of thermal injury. A defect in the transilluminator unit, missing filters,44 prolonged contact with the skin or failure of the filter to function properly have accounted for the occurrence of these blisters in neonates.
Subcutaneous fat necrosis associated with therapeutic hypothermia
Therapeutic hypothermia has become the standard of care for treatment of hypoxic ischemic encephalopathy and has been shown to decrease mortality and reduce the incidence of long-term neurodevelopmental disability at 18–24 months’ follow-up.45 As a result, more tertiary neonatal units are utilizing this treatment modality and subcutaneous fat necrosis (SCFN) has been noted in some infants undergoing whole body cooling.45,46 SCFN can also be seen with perinatal hypoxia without therapeutic cooling,47 so infants cooled have an additional risk factor for this condition. SCFN is discussed in greater detail in Chapter 27.
Physical findings include firm nodules and plaques varying in color from flesh colored to erythematous, blue or purple.45 For the infant on cooling therapy, the areas most affected are those in contact with the cooling blanket, including the back, shoulders, upper arms, thighs, and buttocks (Fig. 8.12).46
Hypercalcemia occasionally complicates the course in these infants.48 Rarely, there is accompanying soft tissue calcification identifiable by biopsy or radiography.49 The presence of soft tissue calcification does not seem to portend a more ominous prognosis and eventually resolves. Preventative care involves frequent assessment of the skin, positional changes and the use of pressure-relieving mattresses and pillows.
Mechanical injury
Dermal stripping
Application and removal of adhesives and dressings can lead to inadvertent removal of the stratum corneum, pain, inflammation, edema, disrupted barrier function and increased transepidermal water loss (TEWL).50,51 For the preterm infant, the bond between adhesives and epidermis may be stronger than the bond between epidermis and dermis, leading to extensive tissue loss with adhesive removal (Figs 8.13, 8.14).51
Pressure ulcers
Pressure ulcers are defined as a localized injury to the skin and/or underlying tissue, usually over a bony prominence, due to pressure or pressure in combination with shear and/or friction (Fig. 8.15).52 The classification for pressure ulcers can be found on the National Pressure Ulcer Advisory Panel’s (NPUAP) website.53 The incidence of pressure ulcers has been reported to be as high as 23% in the neonatal intensive care unit (NICU) and 27% in the pediatric intensive care unit (PICU).54 A more recent multisite study of nine PICUs, including 5346 patients, showed the aggregate incidence of pressure ulcers to be 10.2%. The study found risk factors for development of pressure ulcers to include: age <2 years; PICU stay of >4 days; and treatment including the use of BiPAP, CPAP, conventional mechanical ventilation, high frequency oscillatory ventilation (HFOV) and extracorporeal membrane oxygenation (ECMO) (Fig. 8.15A,B).55 Neonates and infants with limited mobility, neuromuscular immaturity, hemodynamic instability, decreased sensory perception, and dependence on their caregivers for positional changes, are at increased risk of skin breakdown (Fig. 8.15C). Because of their disproportionately large head relative to body size, the most common site for pressure ulcer development in the infant is the occiput (Fig. 8.15D).54,56 Scarring alopecia of the occipital scalp has been documented as a consequence of ischemia and compromised oxygenation, or as a complication of extracorporeal membrane oxygenation (ECMO) therapy in neonates.57 During a 6-month period, five infants in a neonatal ICU were observed to develop erythema and edema that progressed to crusted ulcerations (Fig. 8.15E) and resulted in a patch of scarring alopecia (Fig. 8.15F). The ulcers were believed to be the result of prolonged pressure in a setting of hypoperfusion, acidosis, and hypoxemia. The institution of a protocol requiring frequent repositioning of the head and use of a temperature-stable gel pad as preventive measures, eliminated this problem.
For the VLBW infant, the combination of lengthy hospitalization, immature skin and dependence on medical devices increases the risk for pressure-related injury (Fig. 8.15G,H). Use of nasal continuous positive airway pressure (NCPAP) has been correlated with development of pressure ulcers in the preterm infant (Fig. 8.15I). The greatest risk factors include gestational age <32 weeks; birthweight <1500 g; >5 days on NCPAP, and >14-day stay in the NICU.58 Case reports of forehead pressure necrosis from the CPAP apparatus with resultant scarring and alopecia have also been published (Fig. 8.15J).59
Use of a validated assessment tool such as the Braden Q Scale60 can detect risk early. Use of support surfaces to minimize friction and shear, frequent repositioning and head to toe assessment (especially under medical devices) can aid in prevention of pressure ulcers for neonates and infants.55,61
Umbilical artery catheterization
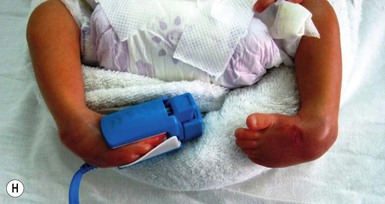
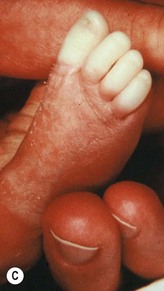
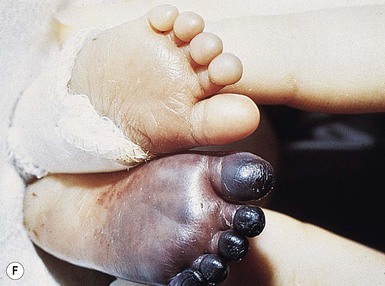
Umbilical arterial catheterization (UAC) is standard practice in the neonatal intensive care unit for monitoring intravascular pressures, chemistries, pH, and blood gases, and for administering fluids and medications to critically ill neonates. However, this procedure is fraught with risk of serious complications, even in experienced hands. Thrombosis is the most frequent problem, as documented by aortography. Other potential complications include vasospasm, blanching of the limbs (Fig. 8.16A), embolism, perforation of the vessels, vascular damage from hypertonic solutions, hemorrhage, infections, and ischemic (Fig. 8.16B) and chemical necrosis.62 These untoward events are usually caused by incorrect placement of the catheter, or to unduly rapid infusion of hazardous drugs or hypertonic solutions, rather than vessel injury.
Vasospasm may occur during placement of the catheter in the umbilical artery. It is manifest by temporary blanching or cyanosis of the leg and foot (Fig. 8.16C,D). Prompt removal of the catheter is indicated, and if this procedure is followed, sequelae are unlikely.
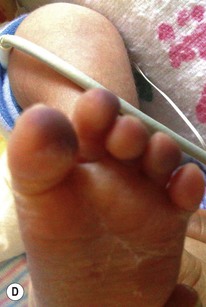
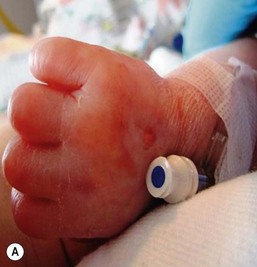
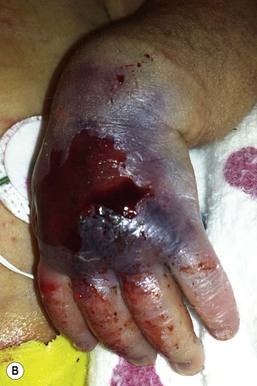
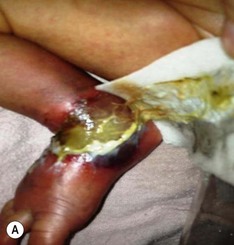
Thromboses and multiple small emboli can cause infarcts of the toes and lower extremities. Depending on the location of the thrombus, unilateral or bilateral gangrene of the feet, lower extremities, or buttocks can occur.62–64 Gangrene of the distal upper extremity with loss of fingers has also been documented secondary to similar events following percutaneous radial artery catheterization.65 Early skin changes include erythema, transient blanching, vesicles, and bulla formation (Fig. 8.16E). These lesions may abate or may progress to extensive skin and subcutaneous tissue necrosis, demarcation, and gangrene (Fig. 8.16F).64
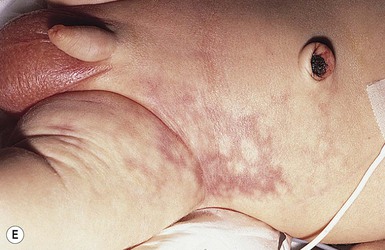
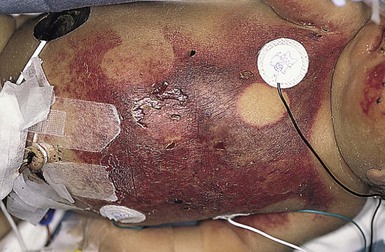
Perinatal gangrene of the buttock
This alarming and fortunately rare occurrence is usually attributed to iatrogenic causes66 but has also been documented as an apparently spontaneous event.67,68 The onset is heralded by the sudden appearance of an erythematous patch involving the buttock, perineum, and genitalia. Within hours, the involved area rapidly becomes edematous and then rock hard and cold to the touch, with well-defined black borders. Bullae may form on the surface. Generally, the lower limbs are spared and remain warm and normal in color, with palpable femoral pulses. Over the subsequent several days, the necrotic tissue demarcates and sloughs, leaving a deep ulcer that heals by secondary intention with scarring.
The diagnosis is made on the basis of the abrupt onset and clinical findings. The differential diagnosis is mainly that of an infectious process, but cultures are invariably negative, as are biochemical and hematologic laboratory studies. Chemical or thermal injury must also be considered.
The presumed cause is an occlusive vascular event involving the internal iliac artery. This artery, which feeds into the umbilical artery, splits into two terminal branches, the inferior gluteal and the internal pudendal arteries; these two vessels supply the buttock, perineum, vulva, and scrotum. Vasospasm followed by thrombus formation resulting from a variety of factors, such as injury to the umbilical cord or obstruction by a misdirected umbilical catheter, may result in this condition. Despite the extent of the gangrene, affected skin usually heals without complications and the sphincters remain intact.66,67
Complications of phototherapy
Visible light phototherapy has become standard therapy in the newborn nursery for infants with significant indirect hyperbilirubinemia. Visible light energy isomerizes unconjugated bilirubin to more polar forms, which are excreted into the bile and ultimately into the stool within minutes of exposure. Bilirubin absorbs light maximally in the blue portion of the spectrum (420–520 nm). Blue, green and turquoise light are considered the most effective at reducing bilirubin. Light emitting diodes (LEDs) are a newer form of therapy that provides high irradiance without concern for heat-related side-effects. The blue hue emitted from this type of unit has been reported to cause visual disturbances and dizziness for staff members, so amber LEDs have been added to avoid this side-effect without affecting irradiance. Other available delivery systems include halogen spotlights which can generate heat, therefore cannot be placed closer to the infant than manufacturer recommendations, and fluorescent tubes in the form of bank lights and fiberoptic blankets.69
Adverse effects of phototherapy are uncommon, but include erythematous, purpuric, and vesicular transient eruptions (see Chapters 5 and 20), and bronze baby syndrome (see below and Fig. 8.20).
Phototherapy-induced drug eruptions
Erythematous and vesiculobullous eruptions may be associated with phototherapy under other circumstances. Drug-induced phototoxicity eruptions have been documented in neonates receiving certain therapeutic agents (e.g., furosemide or fluorescein dye for a radiologic procedure) or exposed to methylene blue dye prenatally.70–72 These eruptions have occurred in infants given a photosensitizing drug and exposed to light of the appropriate wavelength to cause photoactivation of the chemical compound. As with true burns, these bullae develop only on light-exposed skin. Discontinuation of therapy usually results in an uneventful recovery.
Transient porphyrinemia and phototherapy eruptions
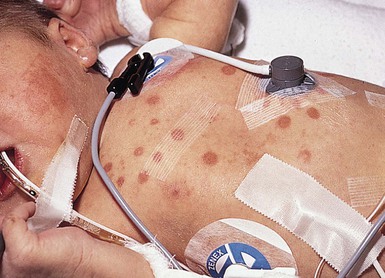
Transient porphyrinemia in combination with phototherapy has also been documented as a cause of blisters and erosions,73 as well as erythematous and purpuric lesions74,75 in several neonates with hemolytic disease (Fig. 8.17). The eruptions in all cases were confined to exposed areas, sparing the sites protected from the lights (e.g. skin under leads, dressings, and temperature probes). Onset is typically between 1 and 4 days after initiation of phototherapy, but occasionally delayed.73 Reactions include violaceous discoloration resembling sunburn,74 purpura,75 and least commonly, erosions and skin fragility.73
Skin biopsy specimens from purpuric lesions show only extravasation of erythrocytes75 without epidermal changes, thus distinguishing the eruption from a burn. The porphyrin levels in affected infants vary but can include elevated free erythrocyte protoporphyrin and zinc protoporphyrin levels,74 and increased levels of both plasma coproporphyrin and protoporphyrins.73,75 The cause of elevated porphyrin levels in these infants is postulated to be due to multiple factors, including cholestasis, altered hepatic function, concomitant administration of photosensitizing drugs, blood products, or renal failure. The differential diagnosis includes true porphyria, infections, epidermolysis bullosa, neonatal lupus erythematosus, metabolic photosensitivity eruptions, and drug eruptions. Both the cutaneous eruption and the transient porphyrinemia clear spontaneously within a few weeks, without significant sequelae.
Bronze baby syndrome
In this rare complication of phototherapy, occurring exclusively in infants with cholestatic jaundice, the infant’s skin, serum, and urine become a gray-brown color after several hours under the phototherapy lamps (Fig. 8.18).76 All affected infants have had prior evidence of hepatic dysfunction, marked by conjugated hyperbilirubinemia and retention of bile acids.77 The serum acquires a dark brown color and shows a nonspecific absorbance from 380 to 520 nm on spectroanalysis.77 The peculiar color has been attributed to the formation of a photo-oxidation product of bilirubin or to copper-bound porphyrins, which yield brown photoproducts in the presence of bilirubin.78 It has also been suggested that biliverdin pigments may contribute to the bronzing effect.79 The odd hue is easily distinguished from that of cyanosis or typical neonatal jaundice. The discoloration fades over time after phototherapy is discontinued, and there are no significant sequelae.
Calcinosis cutis
Calcification of the skin occurs as a consequence of deposition of hydroxyapatite crystals and amorphous calcium phosphate in the soft tissues. Based on the pathophysiologic mechanisms, calcinosis cutis is usually classified as idiopathic (normal tissue and a normal calcium/phosphorus ratio); dystrophic (damaged tissue and a normal calcium/phosphorus ratio); or metastatic (normal tissue and an abnormal calcium/phosphorus ratio). Iatrogenic calcinosis cutis in neonates is usually of the dystrophic type and is most often the result of an intravenous infusion of calcium gluconate or calcium chloride for treatment of neonatal hypocalcemia.80–82 It may also occur following the application of electrode paste containing calcium chloride for electroencephalography, electromyography, or brainstem auditory evoked potentials, particularly if applied to abraded skin,83 in association with subcutaneous fat necrosis, or as a result of repeated trauma from heel-sticks.
Calcinosis cutis from infusion of calcium salts
Visible evidence of soft tissue calcification develops on average 13 days after infusion of the calcium solution, with a range of 2 h to 24 days (Fig. 8.19). There may be marked swelling with an intense inflammatory response, even in the absence of extravasation of fluid, and occasionally, soft tissue necrosis. The calcification may take the form of papules, nodules, an annular plaque, a large subcutaneous plaque, or may have a linear configuration conforming to the vein in which the solution is administered. Lesions are firm, erythematous, and brown, yellow, or white; when extravasation has occurred they may be tender, warm, and fluctuant, resembling an abscess.80
Radiographic changes can be detected as early as 4–5 days following the infusion.81 Three radiologic patterns have been described: (1) a calcified mass localized to or near the site of injection; (2) more diffuse calcification along fascial planes; and (3) a pattern of vascular or perivascular calcification.81,84
The diagnosis of calcinosis cutis can be made clinically, based on the distinctive appearance of the skin lesions and confirmed by skin biopsy and/or radiographs. Differential diagnosis includes cellulitis, osteomyelitis, periostitis, hematoma, abscess, and subcutaneous fat necrosis.
Treatment is generally symptomatic, and spontaneous resolution occurs over several months by transepidermal elimination of the calcified material. An animal study has suggested that intralesional injection of triamcinolone may be effective in reducing inflammation and facilitating the resorption of calcium.85
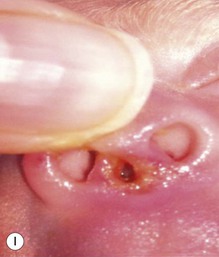
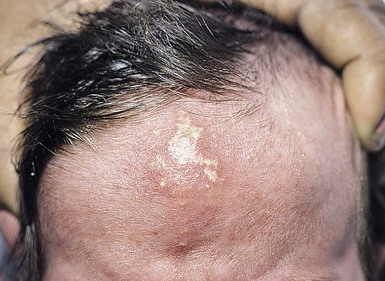
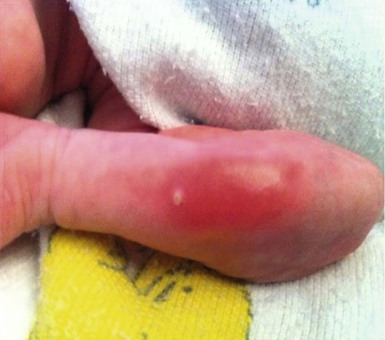
Calcified nodules of the heels
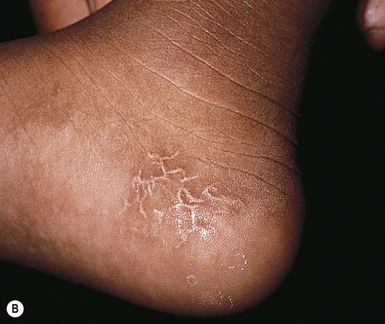
These lesions have been seen in association with blood sampling from the heel, principally in infants of low birthweight and young gestational age, who as neonates received numerous heel-sticks in the nursery.86 Rho and colleagues,87 however, reported a calcified heel nodule after a single heel-stick. Onset is usually between 4 and 12 months after birth and is marked by the appearance of multiple tiny white or yellow specks within depressed areas on the heels (Fig. 8.20). The papules may enlarge and become elevated and firm, but are usually not inflamed or symptomatic.
Infiltration and extravasation injuries
Neonates and infants with peripheral intravascular catheters (PIVs) and peripherally inserted central catheters (PICC lines) are wholly dependent on their caregivers to continuously monitor the sites to prevent complications. This population is at high risk for infiltration and extravasation injury secondary to their inability to verbalize pain and due to the fragility of their veins.88 Infiltration is defined as the inadvertent leakage of a non-vesicant into surrounding tissue, for example, isotonic fluids such as 5% dextrose, normal saline and blood (Fig. 8.21). Extravasation involves leakage of a vesicant into surrounding tissue, for example, amino acid solutions, calcium salts, and vasopressors. Extravasation of intravenous fluids and medications into subcutaneous tissue can lead to tissue necrosis and scar formation, particularly in premature infants who have fragile skin and minimal subcutaneous tissue.
The prevalence of extravasation injuries in neonatal intensive care units in the UK has been estimated to be 38 per 1000 babies, with 70% of injuries occurring in infants of 26 weeks’ gestation or less.89 The majority of extravasation injuries occur with peripheral catheters, with extent of damage dependent on the volume and physicochemical characteristics of the infiltrated solution.90 Extravasation injuries are usually characterized by pain and swelling near the intravenous site, which may progress to blanching, signs of impaired perfusion, blistering, discoloration, ulceration, and eschar formation (Fig. 8.22), or with calcinosis cutis after infiltration of calcium solutions (see above). Scar formation is related to the degree of tissue damage and can result in either aesthetic or functional impact. Over time, the scarring may improve.16
There is no consensus on treatment for extravasation injuries in neonates. Elevation, multiple puncture technique, saline flushing, liposuction, phentolamine and hyaluronidase have been reported to be helpful, although further studies are needed in neonates.90–92 Wound care with hydrocolloid or hydrogel dressings may promote healing and help to minimize scar formation. Prevention of extravasation injury requires frequent monitoring,93 securing IVs to allow visualization and stabilization of the site, and the use of central venous access for administration of potentially irritating fluids and medications.88,92
Phlebitis
Phlebitis, while rare in neonates and infants, can occur with resultant erythema, swelling, pain, warmth and a palpable cord.94 Chemical, mechanical and bacterial factors may contribute to development of phlebitis (Fig. 8.23). Use of central lines for administration of caustic or irritating medications can lower incidence of chemical phlebitis. PICC lines are a common type of vascular access for ill newborns and infants. Phlebitis is one potential complication. Measures to ensure strict hand hygiene, skin antisepsis and aseptic catheter maintenance can minimize bacterial phlebitis. Selection of the smallest catheter relative to vessel size can decrease mechanical irritation to the intima, as can careful securement of IVs in areas of flexion with the use of armboards and securement devices to prevent ‘movement’ of the IV.92
Complications of monitoring
The use of noninvasive techniques for skin surface monitoring of blood gases and temperature has become routine practice in the newborn intensive care nursery.95 Transcutaneous measurements of oxygen and carbon dioxide tension and pulse oximetry for assessment of arterial saturation levels provide accurate, reproducible information facilitating clinical management of premature and sick infants. Although these techniques are widely used, they do pose some risk of damage to the infant’s skin at sites of contact with the sensors and electrodes.
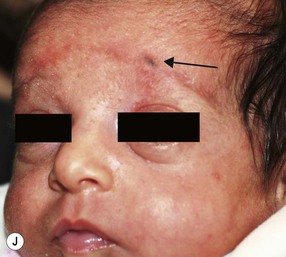
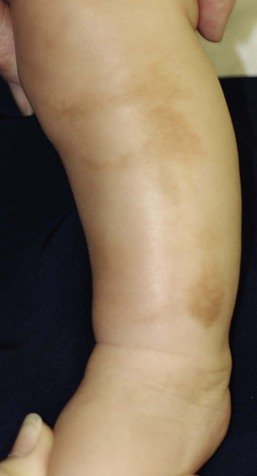
Anetoderma of prematurity
This entity consists of atrophic patches of skin as a sequelae of extreme prematurity (24–29 weeks) or low birthweight and lengthy stays in a neonatal intensive care unit.96,97 Characteristic skin lesions are absent at birth and lack an identifiable antecedent inflammatory phase, typically being noticed between 6 weeks and 10 months of age. The patches are usually confined to the anterior trunk and proximal limbs, are oval or circular, appear depressed, and measure a few millimeters to several centimeters in diameter (Fig. 8.24). They often develop at sites of application of adhesives and placement of monitoring devices. Histopathologic examination of a skin biopsy specimen demonstrates a reduction or absence of dermal elastic tissue.96
It has been postulated that the condition is a result of a subclinical inflammatory reaction to trauma or a transient metabolic derangement in the skin. These scars persist, though they may become less noticeable over time.
Pulse oximetry
Pulse oximetry relies on the spectrophotometric analysis of light to measure the oxygen saturation of hemoglobin. The technique employs a sensor that wraps around a hand, foot, or finger, or an ear probe that clips to the antihelix. It requires no calibration and no skin heating and provides almost continuous measurement of oxygen saturation. Tight application of a probe can result in skin erosion, hyperpigmentation, blister, or pressure necrosis.98 Because pulse oximetry does not require tight contact with the skin, this complication is avoidable by frequent inspection, avoidance of tourniquet type fit and rotation of the probe site.98
Transcutaneous oxygen monitoring
Although also considered a noninvasive procedure, transcutaneous oxygen monitoring poses a greater risk for local skin damage because the electrode must be heated to 42–45°C to promote adequate blood flow.95,99,100 Thermal injury is a relatively frequent problem and is directly related to the temperature of the sensor, the sensitivity of the infant’s skin, and the duration of placement of the monitoring device at a single skin site. First-degree burns are common, although the erythema is likely to fade by 12 h (Fig. 8.25).99 A smaller number of infants, more often those born prematurely, sustain a more severe thermal reaction such as a second or even third degree burn.99 Pulse oximetry is now widely used, decreasing iatrogenic burns from transcutaneous oxygen monitoring.
Transcutaneous carbon dioxide monitoring
Transcutaneous carbon dioxide monitoring (TCO2) is a noninvasive method of measuring carbon dioxide in the NICU. This form of monitoring involves application of a probe that heats the skin to 42–45°C. Isolated cases of burns, especially in the preterm population,101 have been reported. Therefore, careful assessment and rotation of sites is recommended.
Meatal ulceration following circumcision
Mackenzie102 has proposed that meatal ulceration is a frequently unrecognized consequence of neonatal circumcision. He has suggested that removal of the prepuce subjects the epithelium of the glans penis to undue irritation from the diaper, which may result in erosions followed by healing with stenosis. Because the erosions do not necessarily occur in the immediate postoperative period, the cause-and-effect relationship is not appreciated. Other rare complications include direct injury to the glans and urethra, bleeding, and infection.
Cutaneous necrosis following suture ligation of supernumerary digits and accessory tragi
Supernumerary digits and accessory tragi (which may erroneously be referred to as ‘tags’) are sometimes removed with suture ligation rather than surgical excision. This method, by its nature, constricts the blood flow to the skin and leads to skin necrosis. If the appendage is not tiny, the amount of necrosis can be considerable (Fig. 8.26) and can even serve as a potential nidus for infection. Accessory tragi have associated cartilage and for this reason, are not well-suited to this technique.103 Supernumerary digits treated with suture ligation can also result in traumatic neuroma and chronic pain.104
Auriculotemporal nerve syndrome
Auriculotemporal nerve (Frey) syndrome is characterized by unilateral flushing and sweating in the distribution of the auriculotemporal nerve (lateral cheek, medial ear, and frontotemporal scalp) in response to gustatory stimuli. In children, it is usually a consequence of perinatal birth trauma to the parotid region, especially associated with forceps delivery.105 When the auriculotemporal nerve is damaged, regenerating parasympathetic nerve fibers intended for the parotid may be misdirected and anastomose with the sympathetic nerve fibers innervating the sweat glands and small blood vessels. Frey syndrome usually presents around the time of introduction of solid foods as chewing elicits a stronger stimulation of the parotid gland than does sucking of formula or breast milk.106 Erythema and flushing typically begins shortly after mastication, and lasts 15–45 min. Unlike adults, sweating is uncommon in children with Frey syndrome, possibly due to immaturity of the eccrine sweat glands. Bilateral involvement has been reported, but is uncommon.107 Treatment is usually not necessary in children, as spontaneous resolution does occur within a few years.106
Horner syndrome
Horner syndrome is characterized by hemifacial anhidrosis and flushing with ipsilateral miosis, mild ptosis, apparent enophthalmos with slight elevation of the lower eyelid, and iris hypochromia.108 It is secondary to damage to the sympathetic nerves on the ipsilateral side to the defects. In infants and children, Horner syndrome can be caused by birth trauma with brachial plexus injury, neuroblastoma, vertebral abnormalities, thoracic surgery, and carotid artery thrombosis;109 however, George and colleagues reported no identifiable cause in 70% of 23 infants with Horner syndrome.110
Linear bands of infancy
Linear bands of infancy (raised limb bands) is an uncommon entity which was first described in 2002 by Meggitt and coworkers.111 There have been limited cases described since then, although this is likely due to under-reporting.112–116 Most cases present in the first 1–4 months of life with linear, flesh colored to reddish-brown or hyperpigmented patches or plaques, which can be circumferential or near-circumferential on the extremities (Fig. 8.27). They are most commonly found on the lower legs but can be seen on the arms as well. Unfortunately, the lesions are often persistent but can improve with time. The etiology is unclear, although it has been speculated that linear bands are related to constriction bands. They have been associated with both prematurity and amniotic bands in several of the reported cases, although the cases associated with amniotic bands were much more extensive.112 More recently, it has been postulated that the linear bands may be associated with mast cell activation and subsequent tissue fibrosis after local pressure, as there is often a history of the lesions forming after contact with elasticized sock tops.115 There has been one report of complete resolution of the linear bands after application of olopatadine hydrochloride, a topical antihistamine ophthalmic solution.116
Cutaneous vaccination reactions
Cutaneous reactions to vaccinations in infants are not infrequent. Injection site reactions are most common (19%) with rash occurring in 8% of children.117 Injection site reactions can vary from mild erythema and/or swelling at the injection site to extensive swelling along limbs, occasionally involving adjacent joints.118 There are many other cutaneous reactions reported in the literature that can occur in association with vaccinations, including Gianotti Crosti-like eruptions, Henoch Schönlein purpura, serum sickness-like reaction, pseudolymphoma, lichen striatus, psoriasis, lichen planus, granuloma annulare, erythema multiforme, anetoderma, morphea, mastocytomas, and incontinentia pigmenti reactivation. BCG vaccinations have specific associated reactions in addition to the expected typical scar formation at the vaccination site including vaccine granulomas and tuberculid-like reactions.
Kemmeren and colleagues reported 1162 cases of ‘discolored leg syndrome’ in infants after vaccinations.119 They estimated the incidence to be 58 per 100 000 children vaccinated in their European population. The clinical presentation varies from patchy red, blue, or purple discoloration of the leg and/or petechiae with or without swelling. Onset typically occurs within 4 h of vaccination and resolves within 12 h. Bilateral lower extremity involvement is most common, although homolateral and even contralateral involvement can occur. Discolored leg syndrome is often accompanied by fierce crying and, less commonly, pallor and cyanosis. Pathophysiology is unknown, but it is thought to be a vasomotor reaction.
Nicolau syndrome (embolia cutis medicamentosa) is very rare; however, nine cases have been reported in children related to vaccinations.120–122 It is a consequence of accidental intravascular or perivascular drug injection, with subsequent arterial embolism. It is characterized by the sudden onset of painful swelling, livedoid erythema, hemorrhagic patches or bullae, followed by necrosis of the skin, subcutaneous fat, and occasionally muscular tissue. Lipoatrophy secondary to inadvertent administration of diphtheria/tetanus/pertussis (DTP) vaccinations into the subcutaneous tissue has also been reported.123
Non-accidental trauma
Both accidental and non-accidental trauma are unfortunately common in infants and toddlers. It is important to recognize signs of non-accidental trauma early, as approximately 50% of children who have been abused are at risk of subsequent injury and 10% are at risk of death.124,125 Up to 90% of victims of physical abuse present with skin findings.126 Cutaneous manifestations of abuse can be distinctive and distinguishable from accidental trauma, although this distinction can be difficult and other clues are often needed. Red flags in the history include vague explanations of injury, history that changes with time, delay in seeking medical care, repeated emergency room visits or repeated injuries/fractures, history inconsistent with the physical findings, and inappropriate developmental stage of the child for the injury to be plausibly accidental.126
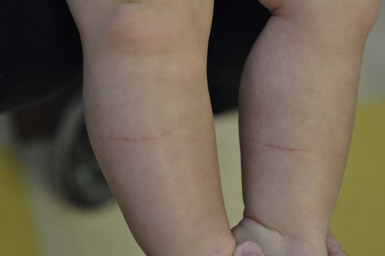
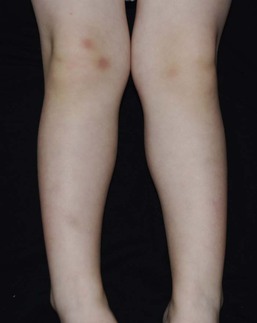
Cutaneous manifestations of physical abuse include bruises, lacerations, abrasions, burns, oral trauma, bite marks, and traumatic alopecia. Accidental bruising is most common over the knees and anterior tibial area (Fig. 8.28), but can occur over any bony prominence. Bruising on relatively protected sites (upper arms, medial and posterior thighs, hands, trunk, cheeks, ears, neck, genitalia, buttocks) should raise suspicion of abuse.126 Bruising is very rare in infants <6 months of age, as they are not yet mobile and should also raise concern for abuse. Pattern bruising, including linear or circumferential bruises, loop marks, finger marks, or bruises in the shape of an object, is a strong indicator of abuse. Human bite marks are usually circular or oval and are often superficial causing only bruising.
Inflicted burns are more common in children under 3 years old, and scalds are the most frequent form of burn abuse.127,128 Forced immersion burns are symmetrical and have clear lines of demarcation with uniform burn depth, whereas accidental immersion burns have irregular borders, nonuniform depth, and splash marks. Inflicted scald burns usually involve the buttocks, perineum, and lower extremities and include stocking and glove distribution, zebra stripes, and donut hole sparing.126,129 Stocking and glove burns occur with forcible immersion of hands and/or feet in hot water, resulting in symmetrical, circumferential, well-demarcated burns. Zebra stripes are caused by sparing of the flexural creases when the body is immersed in hot water in a flexed position. Donut-hole sparing occurs when the buttocks are pressed against the bathtub which is relatively cooler than the water in it. Patterned contact burns in the form of objects are also highly suspicious of abuse. Cigarette burns are common and are characterized by well-demarcated 7–10 mm burns with a deep central ulceration. They are most common on the face, hands, and feet.
Petechiae over the head and neck can be a sign of attempted strangulation or holding an infant’s neck while shaking. In infants, subtle injuries may be the only clue to serious internal injury; therefore, it may be necessary to rule out associated head or abdominal trauma with CT or MRI.130 Skeletal survey is necessary in all cases of suspected abuse in children younger than 2 years old.
Reporting suspected abuse is mandatory, and involving professionals experienced in the maltreatment of children is crucial. The differential diagnosis of suspected child abuse is broad. Mongolian spots, leukemia cutis, neuroblastoma, hemorrhagic edema of infancy, coagulation disorders, lichen sclerosis with purpura, and coining can all be mistaken for bruising. Bullous impetigo, erysipelas, ecthyma, Staphylococcal scalded skin syndrome, contact dermatitis, phytophotodermatitis (Fig. 8.29), epidermolysis bullosa, incontinentia pigmenti, and laxative ingestion can be confused with burns.
Access the full reference list at ExpertConsult.com ![]()