Chapter 1 Hypoxic-Ischemic Brain Damage
The brain is our most essential organ but also the most sensitive to oxygen deprivation. Diffuse hypoxia and ischemia result in global cerebral damage that follows a typical pattern defined by the selective vulnerability of brain regions. Irreversible injury occurs when systemic blood pressure drops below the minimal levels required for sustaining effective brain metabolism and energy production. Physiologically, this occurs when mean arterial pressure falls below the lower limit of cerebral autoregulation. Whereas moderately severe reductions in cerebral blood flow and oxygen supply result in depression or suppression of brain tissue metabolism, critically severe reductions cause irreversible disruption of cellular membranes (responsible for the development of cytotoxic edema) and cell death.
The most characteristic example of hypoxic-ischemic brain damage is produced by cardiac arrest. Attempts to prognosticate outcome accurately after cardiac arrest have generated abundant research. Although clinical examination remains the preeminent tool to predict the chances of recovery after cardiac resuscitation, a number of electrophysiological and neuroimaging techniques provide valuable aid.1,2 This chapter summarizes the most important and useful features of neuroimaging in the diagnosis and prognosis of patients with global hypoxic-ischemic brain damage.
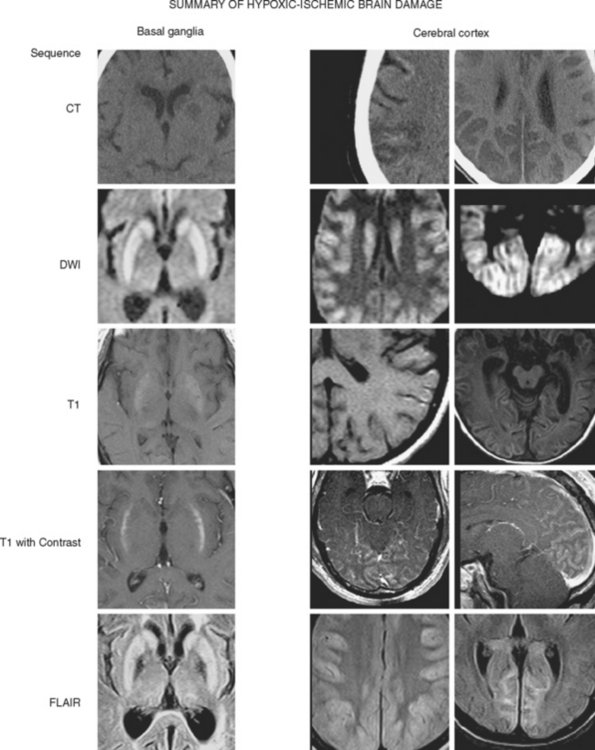
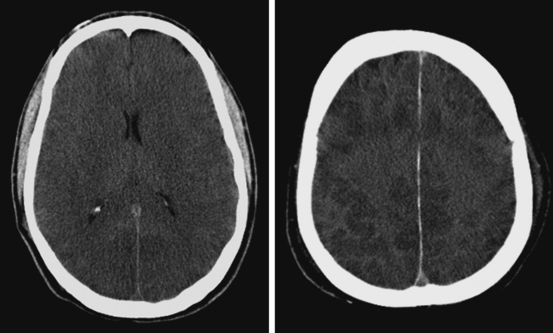
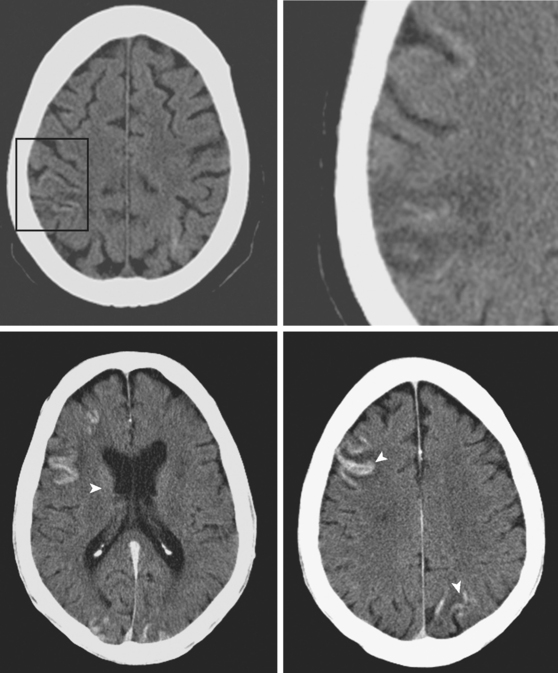
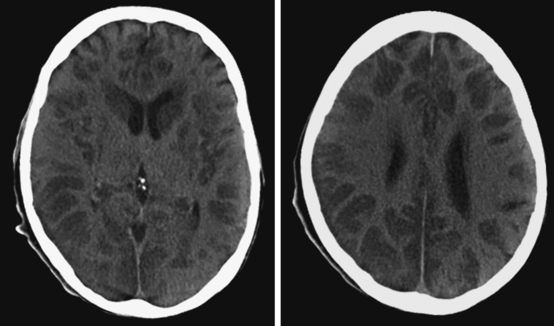
Computed tomography (CT) scan has limited sensitivity to diagnose the extent of brain damage after a diffuse hypoxic insult. Loss of the normal differentiation between cortical gray matter and subcortical white matter and effacement of the delineation of deep gray matter structures are the best known signs of global hypoxia on CT scan. They represent early stages of brain swelling, mostly due to cytotoxic edema. However, these findings may be subtle and difficult to recognize. Additionally, CT scans can be deceiving, showing little change in patients with severe hypoxic damage or presenting signs that may be confused with other conditions (i.e., pseudo-subarachnoid hemorrhage).3–5 In patients who develop areas of infarction, CT scans may fail to reveal any focal hypodensities until 24 to 48 hours after the episode.
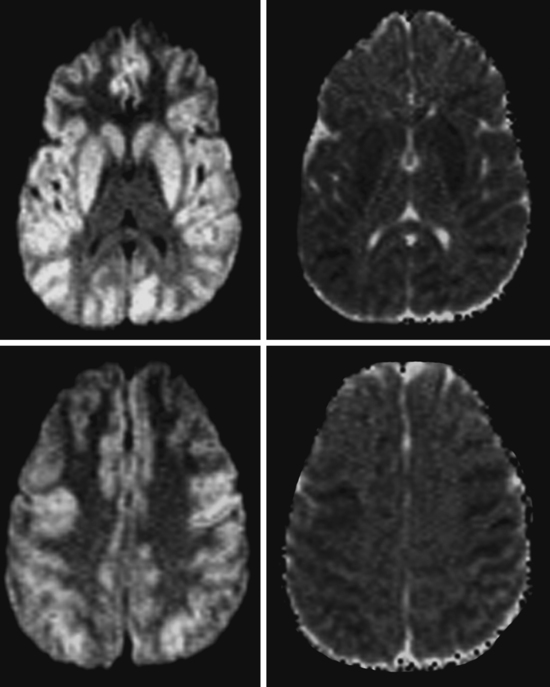
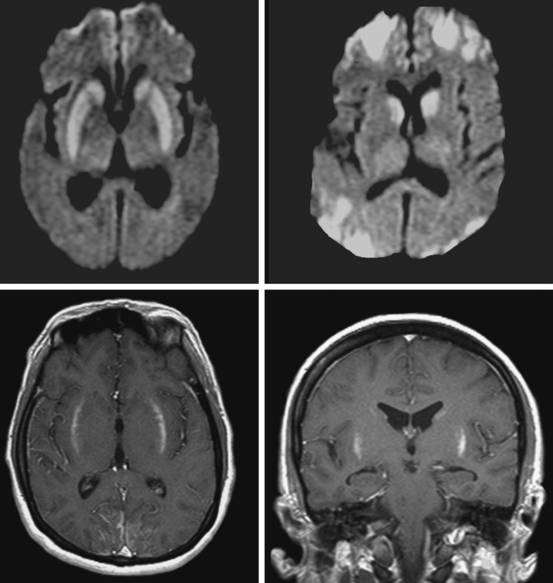
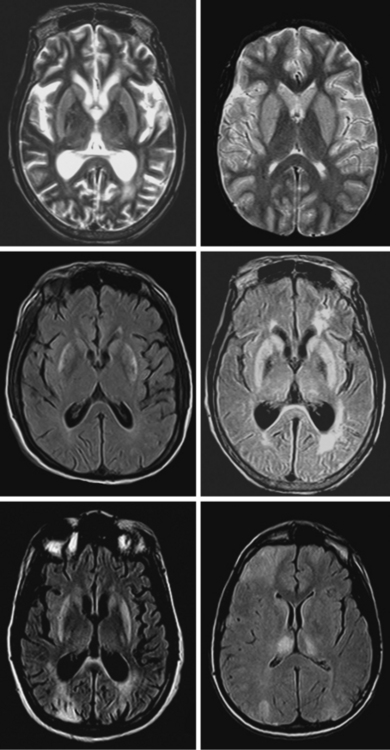
In contrast, magnetic resonance imaging (MRI) scans are extremely useful to recognize the severity of structural damage even very shortly after a hypoxic-ischemic event. The prognostic usefulness of MRI scans is becoming increasingly well established. The advent of diffusion-weighted imaging (DWI) has added a new dimension to the role of MRI in the workup of patients with acute global brain hypoxia-ischemia. This sequence allows good visualization of laminar necrosis and other characteristic signs of hypoxic injury, and it offers reliable information of prognostic importance with unsurpassed promptness.5–11.
Figure 1-1 summarizes the main radiological findings encountered in patients with severe hypoxic-ischemic brain damage.
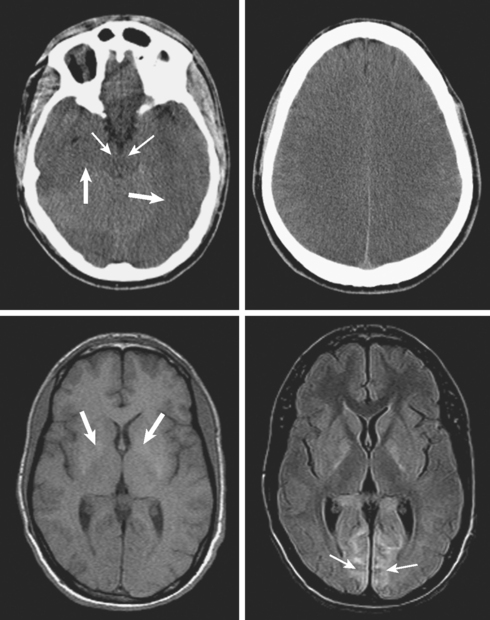
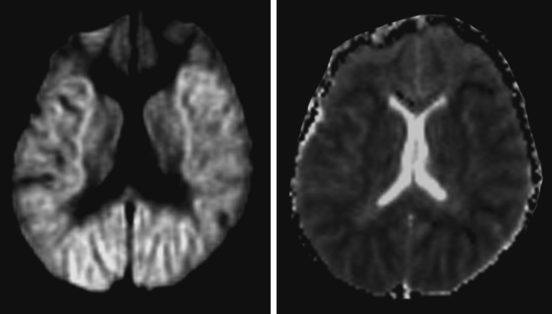
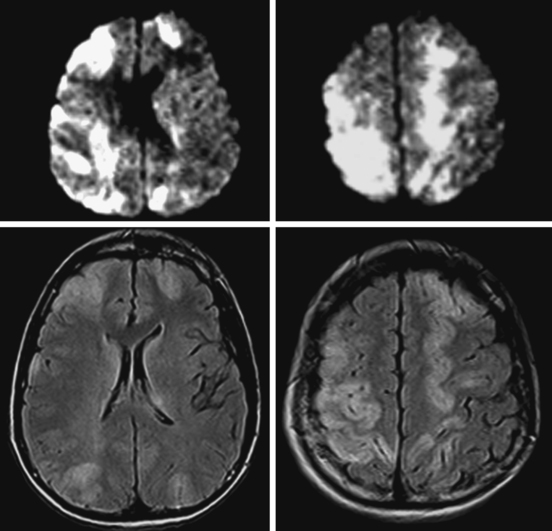
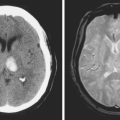
A 29-year-old, previously healthy man collapsed after a lightning strike. A bystander at the scene noted absence of pulse and audible heartbeat and performed basic cardiopulmonary resuscitation for nearly 15 minutes. On arrival, paramedics confirmed the diagnosis of cardiac arrest and initiated full advanced cardiac life support. Electrical defibrillation resulted in return of spontaneous circulation. Initial neurological examination at the hospital revealed that the patient was comatose but with intact brainstem reflexes. He had a Glasgow coma scale sum score of 4 and exhibited frequent myoclonic jerks (myoclonic status). He subsequently failed to regain consciousness. Five days later, he was transferred to a tertiary care center. That day, an electroencephalogram (EEG) showed a very low-amplitude, slow (delta, occasional theta) background. A brain CT scan disclosed severe diffuse edema (Figure 1-2, upper row). A brain MRI performed 13 days after the insult displayed signs of extensive laminar necrosis (Figure 1-2, lower row). A second EEG was essentially unchanged almost 1 month after the arrest. He remained in vegetative state 2 months later.
ADDITIONAL EXAMPLES OF GLOBAL BRAIN EDEMA
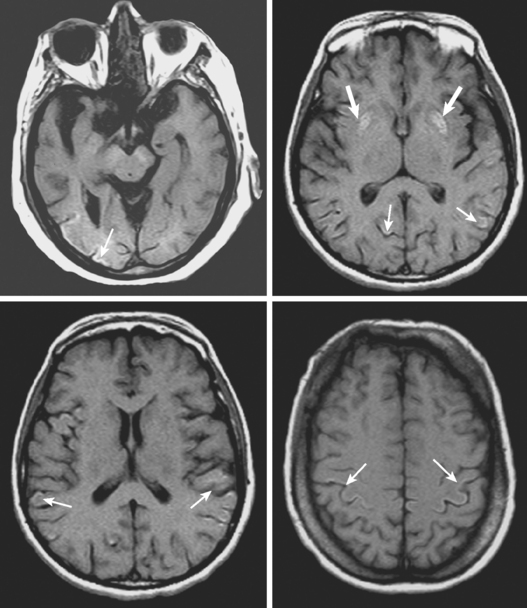
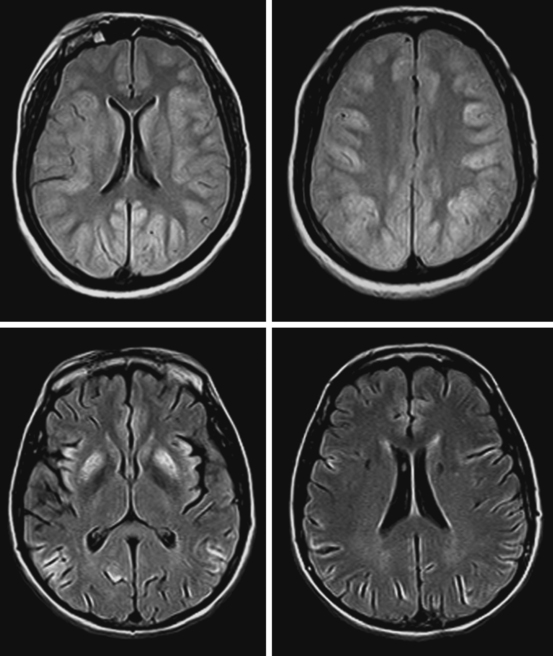
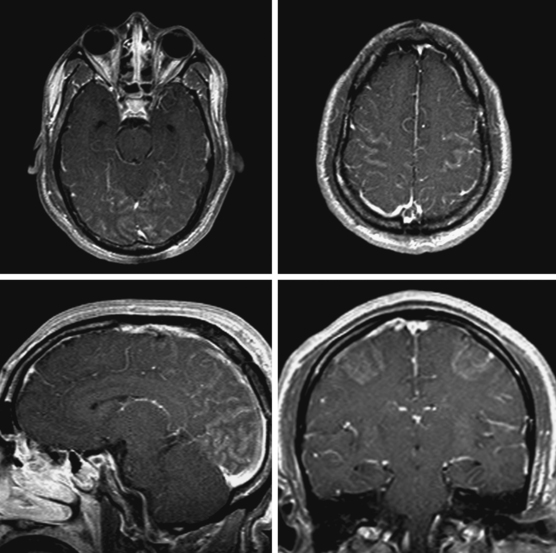
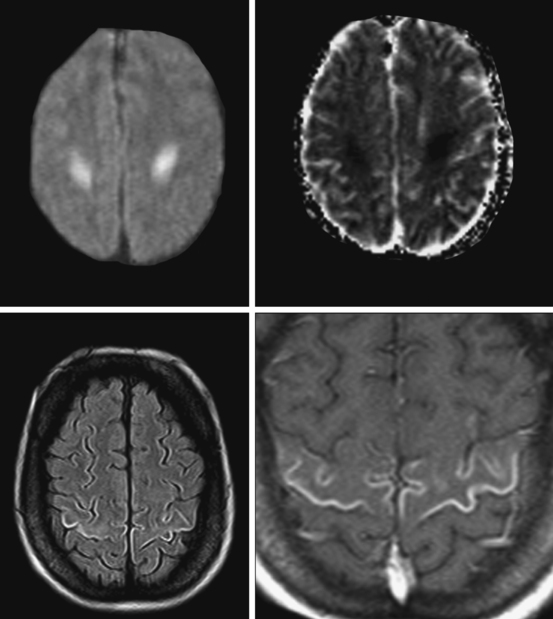
Cortical Laminar Necrosis
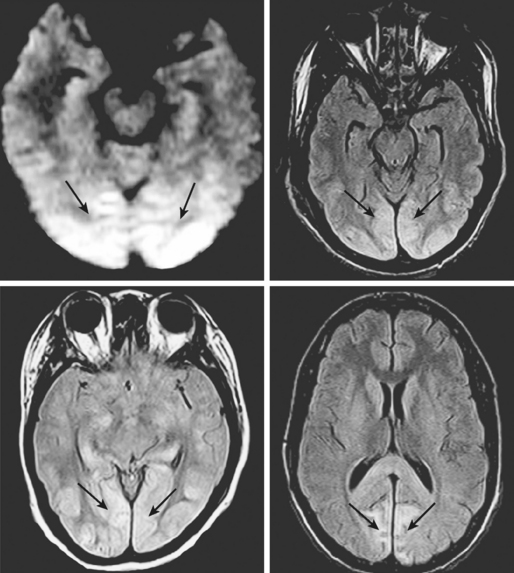
Basal Ganglia Involvement
Watershed Infarctions
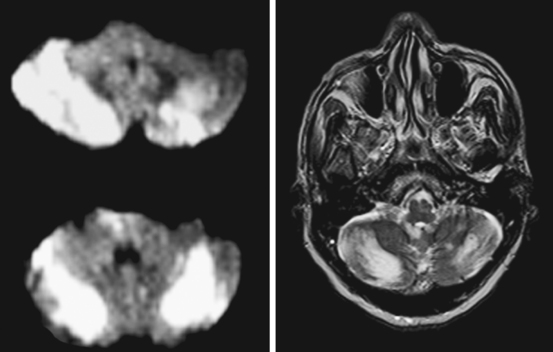
Vulnerable Cortical Areas: Perirolandic and Occipital Cortex
False Radiological Signs: Pseudo-Subarachnoid Hemorrhage and False Middle Cerebral Artery Sign
Early and Delayed White Matter Changes: Anoxic Leukoencephalopathy
1 Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253:1420-1426.
2 Maramattom BV, Wijdicks EF. Postresuscitation encephalopathy. Current views, management, and prognostication. Neurologist. 2005;11:234-243.
3 Given CA, Burdette JH, Elster AD, Williams DWIII. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003;24:254-256.
4 Phan TG, Wijdicks EF, Worrell GA, Fulgham JR. False subarachnoid hemorrhage in anoxic encephalopathy with brain swelling. J Neuroimaging. 2000;10:236-238.
5 Wijdicks EF, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. AJNR Am J Neuroradiol. 2001;22:1561-1565.
6 Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol. 1999;20:999-1007.
7 Els T, Kassubek J, Kubalek R, Klisch J. Diffusion-weighted MRI during early global cerebral hypoxia: a predictor for clinical outcome? Acta Neurol Scand. 2004;110:361-367.
8 Goto Y, Wataya T, Arakawa Y, Hojo M, Chin M, Yamagata S, et al. [Magnetic resonance imaging findings of postresuscitation encephalopathy: sequential change and correlation with clinical outcome]. No To Shinkei. 2001;53:535-540.
9 Komiyama M, Nishikawa M, Yasui T. Cortical laminar necrosis in brain infarcts: chronological changes on MRI. Neuroradiology. 1997;39:474-479.
10 McKinney AM, Teksam M, Felice R, Casey SO, Cranford R, Truwit CL, et al. Diffusion-weighted imaging in the setting of diffuse cortical laminar necrosis and hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol. 2004;25:1659-1665.
11 Lovblad KO, Wetzel SG, Somon T, Wilhelm K, Mehdizade A, Kelekis A, et al. Diffusion-weighted MRI in cortical ischaemia. Neuroradiology. 2004;46:175-182.
12 Han BK, Towbin RB, De Courten-Myers G, McLaurin RL, Ball WSJr. Reversal sign on CT: effect of anoxic/ischemic cerebral injury in children. AJNR Am J Neuroradiol. 1989;10:1191-1198.
13 Tippin J, Adams HPJr, Smoker WR. Early computed tomographic abnormalities following profound cerebral hypoxia. Arch Neurol. 1984;41:1098-1100.
14 Komiyama M, Nakajima H, Nishikawa M, Yasui T. Serial MR observation of cortical laminar necrosis caused by brain infarction. Neuroradiology. 1998;40:771-777.
15 Siskas N, Lefkopoulos A, Ioannidis I, Charitandi A, Dimitriadis AS. Cortical laminar necrosis in brain infarcts: serial MRI. Neuroradiology. 2003;45:283-288.
16 Barrett KM, Freeman WD, Weindling SM, Brott TG, Broderick DF, Heckman MG, et al. Brain injury after cardiopulmonary arrest and its assessment with diffusion-weighted magnetic resonance imaging. Mayo Clin Proc. 2007;82:828-835.
17 Konaka K, Miyashita K, Naritomi H. Changes in diffusion-weighted magnetic resonance imaging findings in the acute and subacute phases of anoxic encephalopathy. J Stroke Cerebrovasc Dis. 2007;16:82-83.
18 Takahashi S, Higano S, Ishii K, Matsumoto K, Sakamoto K, Iwasaki Y, et al. Hypoxic brain damage: cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology. 1993;189:449-456.
19 Ginsberg MD, Hedley-Whyte ET, Richardson EPJr. Hypoxic-ischemic leukoencephalopathy in man. Arch Neurol. 1976;33:5-14.
20 Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology. 2001;56:481-485.