26 Hypothalamus
Gross Anatomy
Boundaries
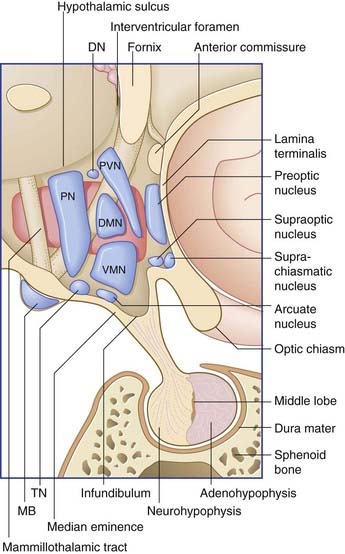
The boundaries of the hypothalamus are as follows (see Figures 26.1 and 26.2):
Subdivisions and nuclei
In the sagittal plane, it is customary to divide the hypothalamus into three regions: anterior (supraoptic), middle (tuberal), and posterior (mammillary). The descriptive use of ‘regions’ has been convenient for animal experiments involving placement of lesions. Named nuclei in the three regions are listed in Table 26.1.
| Posterior | Middle | Anterior |
|---|---|---|
| Posterior | Paraventricular | Preoptic |
| Mammillary | Dorsomedial | Supraoptic |
| Tuberomammillary | Lateral | Suprachiasmatic |
| Dorsal | Ventromedial Arcuate |
Functions
Hypothalamic control of the pituitary gland
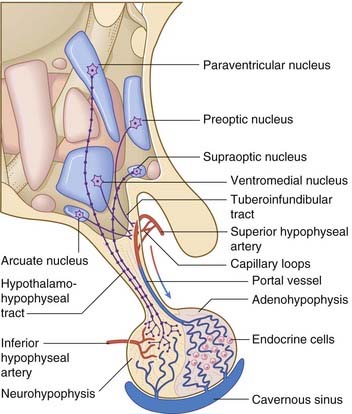
The arterial supply of the pituitary gland comes from hypophyseal branches of the internal carotid artery (Figure 26.3). One set of branches supplies a capillary bed in the wall of the infundibulum. These capillaries drain into portal vessels which pass into the adenohypophysis (anterior lobe). There they break up to form a second capillary bed which bathes the endocrine cells and drains into the cavernous sinus.
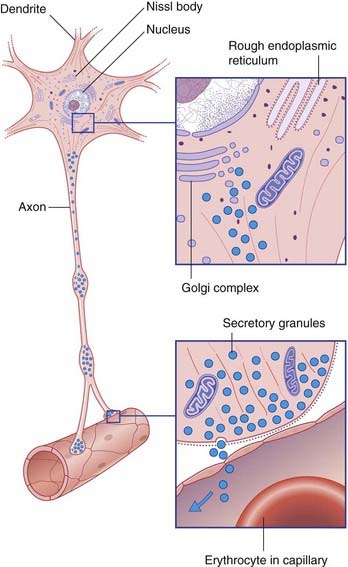
Secretions of the pituitary gland are controlled by two sets of neuroendocrine cells. Neuroendocrine cells are true neurons in having dendrites and axons and in conducting nerve impulses. They are also true endocrine cells because they liberate their secretions into capillary beds (Figure 26.4). With one exception (mentioned below), the secretions are peptides, synthesized in clumps of granular endoplasmic reticulum and packaged in Golgi complexes. The peptides are attached to long-chain polypeptides called neurophysins. The capillaries concerned are outside the blood–brain barrier, and are fenestrated.
The parvocellular neuroendocrine system
Parvocellular neurons of the hypophysiotropic area give rise to the tuberoinfundibular tract, which reaches the infundibular capillary bed. Action potentials traveling along these neurons result in calcium-dependent exocytosis of releasing hormones from some and inhibiting hormones from others, for transport to the adenohypophysis in the portal vessels. The cell types of the adenohypophysis are stimulated/inhibited in accordance with Table 26.2. In the left-hand column, the only non-peptide parvocellular hormone is the prolactin-inhibiting hormone, which is dopamine, secreted from the arcuate (infundibular) nucleus.
Table 26.2 Hypothalamic parvocellular releasing/inhibiting hormones (RH/IH)
| RH/IH | Anterior lobe hormone |
|---|---|
| Corticotropin RH | ACTH |
| Thyrotropin RH | Thyrotropin |
| Growth hormone RH | Growth hormone |
| Growth hormone IH | Growth hormone |
| Prolactin RH | Prolactin |
| Prolactin IH | Prolactin |
| Gonadotropic hormone RH | FSH/LH |
Multiple controls exist for parvocellular neurons of the hypophysiotropic area. The controls include: depolarization by afferents entering from the limbic system and from the reticular formation; hyperpolarization by local-circuit GABA neurons, some of which are sensitive to circulating hormones; and inhibition of transmitter release by opiate-releasing internuncials, which are numerous in the intermediate region of the hypothalamus. The picture is further complicated by the fact that opiates and other modulatory peptides may be released into the portal vessels and activate receptors on the endocrine cells of the adenohypophysis. Stress causes increased secretion of ACTH, which in turn stimulates the adrenal cortex to raise the plasma concentration of glucocorticoids, including cortisol. Normally, cortisol exerts a negative feedback effect by exciting inhibitory hypothalamic neurons having glucocorticoid receptors. In patients suffering from major depression, this feedback system fails (Clinical Panel 26.1).
Clinical Panel 26.1 Major depression
Major depression is characterized by at least several of the following features:
The front line of therapy is dominated by drugs that enhance serotonergic transmission. The range of antidepressants is large and their sites of action vary, e.g. some inhibit reuptake from the synaptic cleft, others inhibit degradation by monoamine oxidase (Ch. 13). They take several weeks to take effect; the latent interval is taken up with desensitizing (inhibitory) autoreceptors on serotonergic cell membranes.
The magnocellular neuroendocrine system
Magnocellular neurons in the supraoptic and paraventricular nuclei give rise to the hypothalamohypophyseal tract, which descends to the neurohypophysis (posterior lobe) (Figure 26.3). Minor contributions to the tract are received from opiatergic and other peptidergic neurons in the periventricular region of the hypothalamus, and from aminergic neurons of the brainstem.
Antidiuretic hormone
Antidiuretic hormone (ADH) continuously stimulates water uptake by the distal convoluted tubules and collecting ducts of the kidneys. The chief regulator of electrical activity in the ADH-secreting neurons is the osmotic pressure of the blood. A rise of as little as 1% in the osmotic pressure causes the plasma to be diluted to normal levels by means of increased water uptake. The neurons are themselves sensitive to osmolar changes, but they are facilitated by inputs from osmolar and volume detectors elsewhere, notably from the vascular and subfornical circumventricular organs (Box 26.1).
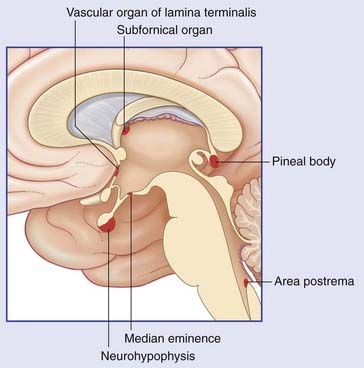
Box 26.1 Circumventricular organs
Six patches of brain tissue close to the ventricular system contain neurons and specialized glial cells abutting fenestrated capillaries. These are the circumventricular organs (CVOs) (Figure Box 26.1.1). The median eminence and neurohypophysis are described in the main text. The vascular organ of the lamina terminalis and the subfornical organ close to the interventricular foramen send axons into the supraoptic and paraventricular nuclei of the hypothalamus and facilitate depolarization of neurons secreting ADH. In conditions of lowered blood volume, the kidney secretes renin, which, on conversion to angiotensin II, stimulates these two CVOs to complete a positive feedback loop.
Withdrawal of ADH secretion results in diabetes insipidus (Clinical Panel 26.2).
Other hypothalamic connections and functions
Autonomic centers
In animals, stimulation of the anterior hypothalamic area produces parasympathetic effects: slowing of the heart, constriction of the pupil, salivary secretion, and intestinal peristalsis. On the other hand, stimulation of the posterior hypothalamic area produces sympathetic effects: increase in heart rate and blood pressure, pupillary dilation, and intestinal stasis. Axons from both areas project to autonomic nuclei in the brainstem and spinal cord. In the midbrain and pons, this projection occupies the posterior longitudinal fasciculus as seen in Chapter 17.
Temperature regulation
The preoptic nucleus in the anterior hypothalamus contains thermosensitive neurons which initiate appropriate responses to changes in the core temperature of the body. Activity of these neurons is reinforced by information received (via the spinoreticular tract) from thermosensitive neurons supplying the skin (Ch. 11).
Drinking
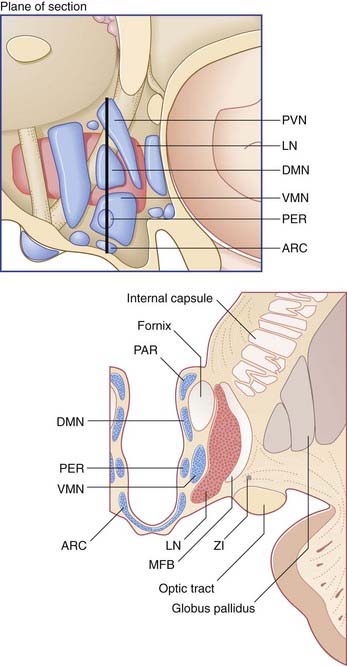
The chief center controlling the intake of water appears to be a ribbon of cells alongside the lateral nucleus known as the zona incerta (Figure 26.2). Stimulation of this region may produce excessive drinking; lesions may result in refusal to drink, with consequent severe dehydration.
Hypothalamic response to psychological stress: gender matters
Historically, it transpires that in laboratory analyses of the stress response, the great majority of human subjects tested have been men. Stress tests carried out on women by Taylor et al. (2000) demonstrated that their response to stressful situations was characterized by ‘tend-and-befriend’. ‘Tend’ translates as protecting offspring. ‘Befriend’ translates as affiliating with social groups with a view to protecting the future of the family group. A significant calming effect in stressed females is achieved by oxytocin released into the capillary bed of the neurohypophysis, in combination with estrogen. Together, they counteract sympathetic overactivity in stressful situations.
Recent fMRI studies in both genders reveals male activation of the lateral prefrontal cortex (a significant decision center in the context of approach or withdrawal, cf. Ch. 29); the predominant female activation was in the cingulate gyrus, the predominant cortical emotional control center (cf. Ch. 34).
Rage and fear
The lateral and ventromedial nuclei are concerned with mood as well as food. Cats that are overweight in consequence of ventromedial lesions tend to be highly aggressive. Conversely, animals rendered underweight by ventromedial stimulation tend to be unduly docile (see also the amygdala in Ch. 34).
Sleeping and waking
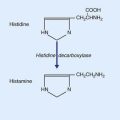
Lesions of the posterior hypothalamic area may cause hypersomnolence or even coma. This area contains the tuberomammillary nucleus (Figure 26.1), housing hundreds of histaminergic neurons, which project widely to the gray matter of the brain and spinal cord. Some of the fibers run rostrally within the medial forebrain bundle, in company with aminergic fibers of brainstem origin. Histaminergic fibers destined for the cerebral cortex fan out below the genu of the corpus callosum. They branch within the superficial layers of the frontal cortex, and run back to supply the cortex of the parietal, occipital, and temporal lobes.
In animals, there is abundant physiological evidence in support of an arousal function for the histaminergic system. The tuberomammillary nucleus is normally activated during the awake state by the peptide orexin liberated by a small group of neurons in the lateral hypothalamus. Failure of orexin production appears to underly the disabling sleep attacks characteristic of narcolepsy (Ch. 30).
Alvarez EO. The role of histamine in cognition. Behav Brain Res. 2008;199:183-189.
Bao A-M, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531-553.
Benarroch E. Thermoregulation: recent concepts and remaining questions. Neurology. 2007;69:1293-1297.
Kousaku O, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Frontiers of neuroendocrinology. 2000;29:70-87.
Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402:435-441.
Swaab DF, Hofman MA. Age, sex and light: variability in the human suprachiasmatic nucleus in relation to its functions. Prog Brain Res. 1994;100:261-265.
Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275-286.
Taylor SE, Klein LC, Lewis BP, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411-429.
Wang J, Korczykowski M, Rao H, et al. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227-239.