16 Hypomagnesemia
Magnesium is an important ion that participates in over 300 enzymatic reactions, especially those involving adenosine triphosphate (ATP) as a cofactor. Hypomagnesemia is common in critically ill patients and associated with increased mortality.1 This chapter provides a brief overview of magnesium physiology and homeostasis, as well as potential etiologies, signs, and symptoms of magnesium deficiency and guidelines for treating hypomagnesemia in critically ill patients.
 Cellular Physiology and Metabolism of Magnesium
Cellular Physiology and Metabolism of Magnesium
Magnesium is a divalent cation (Mg++) that is predominantly localized to the intracellular compartment (99%). It is the second most abundant intracellular cation after potassium and plays an important role in cellular metabolism and homeostasis. At the cellular level, Mg++ influences membrane function by regulating ion transport; Mg++ is required for sodium/potassium–adenosine triphosphatase (Na+/K+-ATPase) activity, which maintains transmembrane gradients for Na+ and K+.2,3 Magnesium also regulates intracellular calcium (Ca++) flux by competing for Ca++ binding sites and influencing intracellular Ca++ transport.2,3 It is an essential cofactor for most ATP-requiring processes. Magnesium acts by neutralizing the negative charge on the phosphate anion of ATP to facilitate enzyme binding and hydrolysis of the phosphate moiety. Intracellular Mg++ is required for numerous critical biochemical processes, including DNA synthesis, activation of gene transcription, initiation of protein synthesis, and regulation of energy metabolism via glycolytic and tricarboxylic acid cycles.2–5
Total body magnesium (21-28 g) is distributed in bone (53%), muscle (27%), soft tissue (19%), and blood (0.8%).2 The normal concentration of total magnesium in serum is 1.5 to 2.3 mg/dL. Approximately 19% of circulating magnesium is bound to protein (predominantly albumin), whereas 14% is complexed to serum anions (citrate, phosphate, and bicarbonate). The majority in serum exists in ionized form (67%), which represents the physiologically active species.2,6 Consequently, measurements of total serum magnesium may not accurately reflect the relative abundance of circulating Mg++.1,2
Magnesium homeostasis is maintained by the small intestine, kidney, and bone.2,7 Average dietary intake is approximately 300 mg per day. Normally, only one-third of dietary Mg++ is absorbed.7,8 However, intestinal Mg++ uptake may increase to compensate for dietary or total body Mg++ deficiency.2,7,8 Unlike calcium, there are no hormonal mechanisms for regulating Mg++. Consequently, normal renal filtration and reabsorption of Mg++ represent important regulatory mechanisms for Mg++ homeostasis.2,7 Non–protein bound Mg++ is filtered by the glomerulus. Under normal conditions, up to 95% of filtered Mg++ is reabsorbed in either the proximal tubule (35%) or in the thick ascending loop of Henle (60%). Mg++ reabsorption in the loop of Henle is linked to sodium chloride (NaCl) transport and inversely related to flow. Consequently, diuretic use and other conditions associated with increased tubular flow result in decreased Mg++ reabsorption.2,7 Under conditions of persistent Mg++ deficiency, mobilization of Mg++ from bone also represents a potential homeostatic mechanism.2
 Prevalence and Etiology of Hypomagnesemia in Patients in the Intensive Care Unit
Prevalence and Etiology of Hypomagnesemia in Patients in the Intensive Care Unit
The reported prevalence of hypomagnesemia in adult intensive care unit (ICU) admissions ranges from 15 to 60, depending on whether total or ionized magnesium is measured.1,9 A recent study identified severe ionized hypomagnesemia most commonly following liver transplantation and in patients with severe sepsis.1 Magnesium deficiency in critically ill patients may be caused by inadequate Mg++ intake, increased renal or gastrointestinal (GI) losses, acute intracellular shifts of Mg++, and other medical conditions (e.g., burn injury, massive blood transfusion, or cardiopulmonary bypass [CPB]). Increased renal losses of Mg++ are associated with alcohol abuse, diabetes, acute tubular necrosis (ATN), diuretics, aminoglycosides, amphotericin, cyclosporin, cisplatin, digoxin, and other medications.1,2,7,10 Vomiting, diarrhea, nasogastric tube losses, and pancreatitis are associated with increased GI losses of Mg++.1,2,7,11 Acute intracellular shifts caused by refeeding with glucose or amino acids, insulin, catecholamines, or metabolic acidosis also may result in hypomagnesemia.1,2,7,11 Hypoalbuminemia is associated with reductions in total Mg++ in plasma, but the ionized fraction may remain normal. The use of continuous renal replacement therapy causes significant loss of Mg++, requiring more replacement than what is commonly prescribed in standard parenteral nutrition formulas.12
Critically ill patients are at increased risk for hypomagnesemia, and its development is associated with an increased risk of mortality.1 Although the cause and effect of this association are unclear, the clinical effects of hypomagnesemia are significant from cardiovascular, metabolic, and neuromuscular standpoints.
 Clinical Signs and Symptoms of Hypomagnesemia
Clinical Signs and Symptoms of Hypomagnesemia
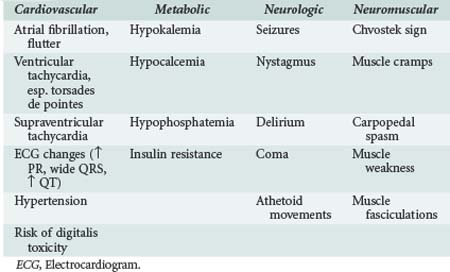
Hypomagnesemia is frequently asymptomatic in critically ill patients and commonly identified through routine laboratory studies or when hypomagnesemia is clinically suspected.7,9,10 However, the relationship between systemic and cytoplasmic hypomagnesemia is unclear, and whether changes in enzymatic function caused by cytoplasmic hypomagnesemia can subsequently lead to clinically significant problems is unknown. Hypomagnesemia is most commonly seen in conjunction with hypokalemia, hypocalcemia, and other electrolyte abnormalities. Consequently, determining the clinical consequences of isolated hypomagnesemia has been difficult. In most instances, symptoms were attributed to Mg++ deficiency only after other electrolyte abnormalities had been corrected.2,7,9,10 As summarized in Table 16-1, the clinical sequelae of Mg++ deficiency are most commonly related to cardiovascular, metabolic, and neuromuscular systems.
Hypomagnesemia is associated with electrocardiogram (ECG) changes similar to those found in hypokalemia: flattened T-waves, U-waves, and prolonged QT interval. Magnesium is a cofactor for Na+/K+-ATPase in cardiac tissue.2,7,9,10 Reductions in intracellular K+ result in cellular depolarization and can lower the threshold for generation of an action potential as well as decrease the time for repolarization. Consequently, hypomagnesemia is associated with both atrial (premature atrial contractions, atrial fibrillation, multifocal atrial tachycardia), digoxin-related, and ventricular (ventricular tachycardia, torsades de pointes) dysryhthmias.7,9,10 Magnesium is recommended as the initial therapy for torsades de pointes and as an adjunctive treatment for refractory ventricular dysrhythmias.2,7,9,10 Magnesium administration during acute myocardial infarction was associated with reduced mortality in the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2).11 Based on that study, there is some evidence that Mg++ may be beneficial if given prior to coronary reperfusion.13
Hypomagnesemia is commonly associated with both hypokalemia and hypocalcemia.7 These associations are related in part to the fact that medications and homeostatic changes that affect magnesium handling often affect K+ handling as well. In addition, renal losses of potassium are increased under hypomagnesemic conditions and are refractory to supplementation unless the magnesium is replaced first.2,7 A somewhat similar condition exists for hypocalcemia in that hypomagnesemia suppresses parathyroid hormone release and activity.14 Consequently, hypocalcemia is refractory to Ca++ replacement unless Mg++ is replaced as well.2,7
Magnesium can have a depressant effect on the nervous system through its ability to cause presynaptic inhibition.2,7,10 It may also depress the seizure threshold by its ability to competitively inhibit N-methyl-D-aspartate receptors.2,7,9,10 The neurologic and neuromuscular manifestations of hypomagnesemia include coma, seizures, weakness, and signs of muscular irritability. Hypomagnesemic patients may have a positive Chvostek sign even when ionized calcium concentration is normal; they may develop nystagmus, tetany, or seizures followed by rhabdomyolysis.2,7,9,10 Serum Mg++ deficit was also found to correlate with the severity of traumatic brain injury.15 Consequently, Mg++ replacement is indicated in this setting and is also commonly used in pregnant patients with preeclampsia (blood pressure >140/90 mm Hg with proteinuria) or eclampsia (associated seizures).9,10
Magnesium replacement has been used to treat bronchospasm in patients with asthma.9,10 The proposed mechanism of action for the therapeutic benefit of Mg++ in bronchospasm involves its relaxant effects on smooth muscle. Several studies have shown improved forced expiratory volume in the first second of expansion (FEV1) following intravenous (IV) magnesium administration or improved peak flow rates with nebulized magnesium, while others have not.10 Consequently, additional studies will be needed to adequately define the role of Mg++ in patients with asthma.
Soliman HM, Mercan D, Lobo SM, Melot C, Vincent JL. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med. 2003;31(4):1082-1087.
A classic study demonstrating increased mortality in ICU patients with ionized hypomagnesemia.
Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002;28(6):667-679.
Woods KI, Fletcher S, Roffe C, et al. Intravenous magnesium sulfate in suspected acute myocardial infarction: results of the second Leicester Intravenous Magnesium Intervention Trial. Lancet. 1992;339(8809):1553-1558.
Klein CJ, Moser-Veillon PB, Schweitzer A, et al. Magnesium, calcium, zinc and nitrogen loss in trauma patients during continuous renal replacement therapy. JPEN J Parenter Enteral Nutr. 2002;26(2):77-92.
1 Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem. 1991;28(pt 1):19-26.
2 Vernon WB. The role of magnesium in nucleic acid and protein metabolism. Magnesium. 1988;7(5-6):234-248.
3 Garfinkel L, Garfinkel D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium. 1985;4(2-3):60-72.
4 Altura BT, Altura BM. A method for distinguishing ionized, complexed, and protein bound Mg in normal and diseased subjects. Scand J Clin Lab Invest. 1994;54(Suppl 17):83-87.
5 Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord. 2003;4(1):195-206.
6 Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88(2):396-402.
7 Fox C, Ramsoomair D, Carter C. Magnesium: its proven and potential clinical significance. South Med J. 2001;94(12):1195-1201.
8 Dacey MJ. Hypomagnesemic disorders. Crit Care Clin. 2001;17(1):155-173.
9 Agus MS, Agus ZS. Cardiovascular actions of magnesium. Crit Care Clin. 2001;17(1):175-185.
10 Anast CS, Winnacker JL, Forte LR, Burns TW. Impaired release of parathyroid hormone in magnesium deficiency. J Clin Endocrinol Metab. 1976;42(4):707-717.
11 Kahraman S, Ozzgurtas T, Kayali H. Monitoring of serum ionized magnesium in neurosurgical intensive care unit: preliminary results. Clin Chim Acta. 2003;334(1-2):211-215.