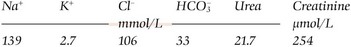Hypokalaemia
The factors affecting potassium balance have been described previously (p. 22). Hypokalaemia may be due to reduced potassium intake, but much more frequently results from increased losses or from redistribution of potassium into cells. As with hyperkalaemia, the clinical effects of hypokalaemia are seen in ‘excitable’ tissues like nerve and muscle. Symptoms include muscle weakness, hyporeflexia and cardiac arrhythmias. Figure 12.1 shows the changes that may be found on ECG in hypokalaemia.
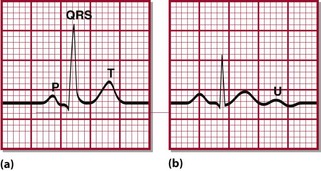
Fig 12.1 Typical ECG changes associated with hypokalaemia. (a) Normal ECG (lead II). (b) Patient with hypokalaemia: note flattened T-wave. U-waves are prominent in all leads.
Diagnosis
Redistribution into cells
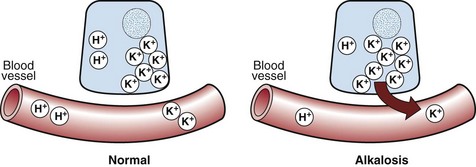
 Metabolic alkalosis. The reciprocal relationship between potassium and hydrogen ions means that in just the same way as metabolic acidosis is associated with hyperkalaemia, so metabolic alkalosis is associated with hypokalaemia. As the concentration of hydrogen ions decreases, so potassium ions move inside cells in order to maintain electrochemical neutrality (Fig 12.2).
Metabolic alkalosis. The reciprocal relationship between potassium and hydrogen ions means that in just the same way as metabolic acidosis is associated with hyperkalaemia, so metabolic alkalosis is associated with hypokalaemia. As the concentration of hydrogen ions decreases, so potassium ions move inside cells in order to maintain electrochemical neutrality (Fig 12.2).
 Treatment with insulin. Insulin stimulates cellular uptake of potassium, and plays a central role in treatment of severe hyperkalaemia (see pp. 22–23). It should come as no surprise therefore that when insulin is given in the treatment of diabetic ketoacidosis (see pp. 66–67), there is a risk of hypokalaemia. This is well recognized, and virtually all treatment protocols for diabetic ketoacidosis take this into account.
Treatment with insulin. Insulin stimulates cellular uptake of potassium, and plays a central role in treatment of severe hyperkalaemia (see pp. 22–23). It should come as no surprise therefore that when insulin is given in the treatment of diabetic ketoacidosis (see pp. 66–67), there is a risk of hypokalaemia. This is well recognized, and virtually all treatment protocols for diabetic ketoacidosis take this into account.
 Refeeding. The so-called ‘refeeding syndrome’ was first described in prisoners of war. It occurs when previously malnourished patients are fed with high carbohydrate loads. The result is a rapid fall in phosphate, magnesium and potassium, mediated by insulin as it moves glucose into cells. Vulnerable groups include those with anorexia nervosa, cancer, alcoholism and postoperative patients. Many of the complications of this result from hypophosphataemia rather than hypokalaemia.
Refeeding. The so-called ‘refeeding syndrome’ was first described in prisoners of war. It occurs when previously malnourished patients are fed with high carbohydrate loads. The result is a rapid fall in phosphate, magnesium and potassium, mediated by insulin as it moves glucose into cells. Vulnerable groups include those with anorexia nervosa, cancer, alcoholism and postoperative patients. Many of the complications of this result from hypophosphataemia rather than hypokalaemia.
 β-Agonism. Acute physiological stress can cause potassium to move into cells, an effect mediated by catecholamines through their actions on β2-receptors. β-agonists like salbutamol (used to treat asthma) or dobutamine (heart failure) predictably induce a similar effect.
β-Agonism. Acute physiological stress can cause potassium to move into cells, an effect mediated by catecholamines through their actions on β2-receptors. β-agonists like salbutamol (used to treat asthma) or dobutamine (heart failure) predictably induce a similar effect.
 Treatment of anaemia. Folic acid or vitamin B12 for megaloblastic anaemia often produce hypokalaemia in the first couple of days of treatment, due to the uptake of potassium by the new blood cells. Treatment of iron deficiency anaemia results in a much slower rate of new blood cell production and is therefore rarely implicated.
Treatment of anaemia. Folic acid or vitamin B12 for megaloblastic anaemia often produce hypokalaemia in the first couple of days of treatment, due to the uptake of potassium by the new blood cells. Treatment of iron deficiency anaemia results in a much slower rate of new blood cell production and is therefore rarely implicated.
 Hypokalaemic periodic paralysis. Like its hyperkalaemic counterpart, hypokalaemic periodic paralysis can be inherited (as an autosomal dominant trait), and be precipitated by rest after exercise. However, it can also be acquired as a result of thyrotoxicosis (possibly due to increased sensitivity to catecholamines), especially in Chinese males. It resembles refeeding in that both can be precipitated by carbohydrate loads and both are associated with low phosphate and magnesium as well.
Hypokalaemic periodic paralysis. Like its hyperkalaemic counterpart, hypokalaemic periodic paralysis can be inherited (as an autosomal dominant trait), and be precipitated by rest after exercise. However, it can also be acquired as a result of thyrotoxicosis (possibly due to increased sensitivity to catecholamines), especially in Chinese males. It resembles refeeding in that both can be precipitated by carbohydrate loads and both are associated with low phosphate and magnesium as well.
Increased losses
Urinary
 Diuretics. Both loop diuretics and thiazide diuretics produce hypokalaemia. Various mechanisms are implicated, including increased flow of water and sodium to the site of distal potassium secretion, and secondary hyperaldosteronism induced by the loss of volume. Loop diuretics also interfere with potassium reabsorption in the loop of Henle.
Diuretics. Both loop diuretics and thiazide diuretics produce hypokalaemia. Various mechanisms are implicated, including increased flow of water and sodium to the site of distal potassium secretion, and secondary hyperaldosteronism induced by the loss of volume. Loop diuretics also interfere with potassium reabsorption in the loop of Henle.
 Mineralocorticoid excess. We have indicated previously (p. 15) that aldosterone increases sodium reabsorption in the renal tubules at the expense of potassium and hydrogen ions. This mineralocorticoid effect is shared by many steroid molecules, and hypokalaemia is a predictable and frequent consequence of mineralocorticoid excess. Overproduction of steroid hormones is dealt with in more detail on pp. 98–99. Less frequently, renal artery stenosis drives the renin–angiotensin–aldosterone axis (Fig 7.4 on p. 15), resulting in hypokalaemia associated with severe, refractory hypertension.
Mineralocorticoid excess. We have indicated previously (p. 15) that aldosterone increases sodium reabsorption in the renal tubules at the expense of potassium and hydrogen ions. This mineralocorticoid effect is shared by many steroid molecules, and hypokalaemia is a predictable and frequent consequence of mineralocorticoid excess. Overproduction of steroid hormones is dealt with in more detail on pp. 98–99. Less frequently, renal artery stenosis drives the renin–angiotensin–aldosterone axis (Fig 7.4 on p. 15), resulting in hypokalaemia associated with severe, refractory hypertension.
 Hypomagnesaemia. Hypomagnesaemia from any cause may lead to hypokalaemia due to impaired renal tubular absorption. This effect is usually not observed unless the magnesium is less than 0.6 mmol/L. The combination of hypomagnesaemia and proton pump inhibitors is a potent and increasingly common cause of hypokalaemia.
Hypomagnesaemia. Hypomagnesaemia from any cause may lead to hypokalaemia due to impaired renal tubular absorption. This effect is usually not observed unless the magnesium is less than 0.6 mmol/L. The combination of hypomagnesaemia and proton pump inhibitors is a potent and increasingly common cause of hypokalaemia.
 Tubulopathies. The most common causes of the tubulopathies are chemotherapeutic agents, especially platinum containing drugs. A small number of inherited defects in tubular function produce hypokalaemia by various mechanisms. They may need to be considered in cases of persistent unexplained hypokalaemia.
Tubulopathies. The most common causes of the tubulopathies are chemotherapeutic agents, especially platinum containing drugs. A small number of inherited defects in tubular function produce hypokalaemia by various mechanisms. They may need to be considered in cases of persistent unexplained hypokalaemia.
Investigation
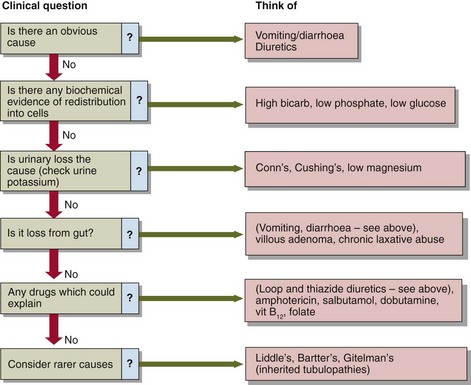
Figure 12.3 provides a framework for investigation of hypokalaemia. Often the cause is obvious e.g. vomiting, diarrhoea, and further investigations are not required. Some of the causes of hypokalaemia listed above are well recognized as such by clinicians and hypokalaemia is not a diagnostic dilemma, e.g. diuretics, gut loss, insulin treatment. Other causes are infrequently implicated (treatment of anaemia), or are simply very rare (hypokalaemic periodic paralysis), and may not be considered. Where the cause is not immediately evident, it may help to go back to first principles by classifying potential causes into the three broad categories outlined above: reduced intake, redistribution, and increased loss. Measurement of urinary potassium excretion may help to identify (or exclude) renal loss as the likely mechanism. Diagnoses which can be difficult to pin down include laxative abuse(because it is sometimes intermittent) and the eponymous tubulopathies (again, because their phenotypic expression can vary over time).