18 Hypoglycemia
The American Diabetes Association Workgroup on Hypoglycemia set the alert level for hypoglycemia at plasma glucose concentrations ≤70 mg/dL (3.9 mmol/L) in patients with diabetes mellitus. When the plasma glucose concentration is less than this threshold value, actions should be undertaken to prevent clinical/symptomatic hypoglycemia.1 Clinical/symptomatic hypoglycemia is characterized by the Whipple triad: (1) symptoms of hypoglycemia, (2) simultaneous low blood glucose concentration, and (3) relief of symptoms with the administration of glucose. These symptoms may be neurogenic/autonomic or neuroglycopenic (Table 18-1). Symptoms of hypoglycemia are similar in type 1 and type 2 diabetes.2 Elderly patients report fewer neurogenic/autonomic symptoms.3 They and all other patients with “hypoglycemia unawareness” have a sevenfold increased risk of severe hypoglycemia. Episodes of hypoglycemia in these patients tend to be recurrent and unpredictable.4 “Hypoglycemia unawareness” is the loss of autonomic warning symptoms of developing hypoglycemia. Likely pathogenic mechanisms for hypoglycemia unawareness include recurrent exposure to hypoglycemia, leading to increases in brain glucose uptake and possibly reduced β-adrenergic sensitivity.5 Fortunately, scrupulous avoidance of hypoglycemia for a period of weeks to months restores hypoglycemia awareness.6,7
TABLE 18-1 Symptoms of Hypoglycemia
| Neurogenic | Neuroglycopenic |
|---|---|
| THE RESULT OF AN AUTONOMIC RESPONSE | THE RESULT OF BRAIN GLUCOSE DEPRIVATION |
| Blood glucose <55 mg/dL (3.7 mmol/L) | Blood glucose <45 mg/dL (2.5 mmol/L) |
| Cholinergic: hunger, sweating, paresthesias | Cognitive impairment |
| Behavioral change | |
| Adrenergic: tremor, palpitations, anxiety | Psychomotor abnormalities |
| Seizure and coma |
In critically ill patients, however, sedation strongly masks symptoms, so one can only rely on frequent and accurate blood glucose measurements to detect hypoglycemia. The most commonly used definition of hypoglycemia during critical illness is a plasma glucose concentration below 40 mg/dL (2.2 mmol/L) in the absence of symptoms.8–10 Most reflectance blood glucose meters in home and hospital use have poor precision at low levels of blood glucose.11 Capillary blood glucose testing may not be sufficiently reliable to guide management of blood glucose levels in critically ill patients.12 The use of arterial blood samples for glucose measurements is recommended. However, anemia in critically ill patients can result in falsely elevated blood glucose measurements and mask hypoglycemia when using these blood glucose meters. Also, the recently developed continuous interstitial glucose monitoring system13 and the noninvasive GlucoWatch Biographer14 are less effective at detecting low blood glucose levels and can have a delayed response to low blood glucose concentrations. Therefore, for diabetes patients, the laboratory measurement of a low plasma glucose concentration in the presence of appropriate symptoms remains the most reliable way to diagnose severe hypoglycemia. In the ICU, measurements of arterial blood glucose concentration using modern blood gas analyzers approach the accuracy of conventional laboratory methods.8,12
Specific characteristics of the patient can also determine whether hypoglycemia will be symptomatic or increase the risk of hypoglycemia. For example, a precipitous fall from hyperglycemia to euglycemia in a patient with diabetes can produce hypoglycemic symptoms.15 In contrast, hypoglycemia with glucose levels as low as 30 mg/dL (1.7 mmol/L) can occur asymptomatically during fasting in normal women and during pregnancy.16 Some ICU patient populations, such as those with liver or renal failure and septic shock, are at higher risk for hypoglycemia.17 The characteristics of the hypoglycemia itself (absolute level, duration) and its treatment (avoiding overcorrection) also play a significant role (Table 18-2).
TABLE 18-2 Risk Factors Involved in Hypoglycemia
| Hypoglycemia | Patient |
|---|---|
| Level of hypoglycemia | Liver failure |
| Duration | Renal failure |
| (Over)correction of hypoglycemia | Sepsis or shock |
| Reperfusion damage | Prior history of diabetes mellitus |
 Incidence of Severe Hypoglycemia
Incidence of Severe Hypoglycemia
A retrospective study of adults requiring hospitalization indicated that 0.4% of acute medical admissions per year are hypoglycemia related.18 Severe hypoglycemia (i.e., with symptoms severe enough to require assistance) occurs commonly in patients with type 1 diabetes.19 In type 2 diabetes, even with intensive therapy, the risk is probably 100-fold less. Over 6 years of observation in the United Kingdom Prospective Diabetes Study, severe hypoglycemia was reported in 2.4% of patients treated with metformin, 3.3% of those treated with a sulfonylurea, and 11.2% of those treated with insulin.20 As insulin usage among patients with type 2 diabetes increases, it is inevitable that severe hypoglycemia will become more common in daily practice.
With the introduction of tight blood glucose control during ICU stay,8 the incidence of blood glucose values below 40 mg/dL (2.2 mmol/L) has been reported to range from 5.1% to 18.7% of patients, depending on the targeted level of blood glucose control and the patient population under study.8,9 With the use of accurate glycemia measurement methodologies and algorithms that advise frequent blood glucose measurements (i.e., every 1–4 hours), the incidence and impact of these brief episodes of hypoglycemia should be minimized.17
 Physiologic Barriers Against Hypoglycemia
Physiologic Barriers Against Hypoglycemia
The central nervous system (CNS) relies primarily on glucose for the generation of cellular energy. Cells in the CNS have endogenous glucose reserves that are sufficient for only minutes if the supply of glucose from the bloodstream is inadequate. In addition, neurons are unable to synthesize glucose. Finally, the brain cannot use fuels other than glucose during acute hypoglycemia.21 Hence, when the brain is acutely deprived of glucose, serious neurologic dysfunction occurs. Accordingly, the body has several mechanisms to maintain the plasma glucose concentration within the narrow range of 60 to 140 mg/dL (3.3–7.7 mmol/L) in both the fed and fasting states. When glucose use exceeds glucose production, the brain senses decreasing blood glucose levels and activates counterregulatory pathways.22 The glucose threshold for activation of these mechanisms is approximately 67 mg/dL (3.6 mmol/L), but this setpoint can be altered by recent hyperglycemia or antecedent hypoglycemia. As glucose levels decline, the first counterregulatory mechanism activated is the suppression of endogenous insulin secretion.23 Next in the hierarchy of responses is the release of two hormones, glucagon and epinephrine, that antagonize the action of insulin. These hormones activate glycogenolysis and gluconeogenesis and stimulate fatty acid oxidation and protein breakdown to provide substrates for gluconeogenesis. With more severe or prolonged hypoglycemia (>3 hours), increases in growth hormone and cortisol release raise blood glucose levels.
The physiologic responses to hypoglycemia and the glucose threshold at which they occur can be modulated. In type 1 diabetes, the glucagon response to hypoglycemia is lost within 3 years after diagnosis, rendering patients dependent on epinephrine-mediated counterregulation and making them more vulnerable to prolonged episodes of severe hypoglycemia. Exposure to antecedent hypoglycemia diminishes the counterregulatory response to a subsequent episode. The brain adapts to antecedent hypoglycemia by increasing glucose uptake so that a more profound hypoglycemic stimulus is required to trigger sympathoadrenal activation and autonomic symptoms.24 The level of glycemic control also affects counterregulatory thresholds. With strict glycemic control, epinephrine release is not triggered until a lower glucose level is reached.25,26 Conversely, diabetic patients with poor glycemic control can experience hypoglycemic symptoms when the blood glucose concentration decreases to lower values within the normal or even hyperglycemic range.27
 Sequelae
Sequelae
Although severe hypoglycemia induces marked cognitive dysfunction, most patients recover rapidly and completely. The effect of repeated severe hypoglycemia on cognitive function in adults is controversial.28,29 Although focal neurologic symptoms secondary to severe hypoglycemia occur occasionally, severe and permanent cognitive impairment is usually the result of protracted hypoglycemia, often in association with excessive alcohol consumption. The neuronal regions that are particularly vulnerable to hypoglycemia are the cerebral cortex, the substantia nigra, the basal ganglia, and the hippocampus.
The long-term neurologic effects of hypoglycemia during critical illness are poorly delineated.17 It appears that brief episodes of hypoglycemia do not cause severe acute brain damage. A recent nested case-control study using more sophisticated neurocognitive tests showed that hypoglycemia mildly aggravated critical illness–induced neurocognitive dysfunction, notably the visuospatial domain.30 This association, however, could not be dissociated from an effect of hyperglycemia or of glucose variability, as the patients who experienced hypoglycemia were also those with more severe hyperglycemia and greater glucose variability.
The overall mortality from severe hypoglycemia is unknown. The mortality rate from alcohol-induced hypoglycemia may be as high as 10% in adults.31 About 2% to 4% of deaths in patients with type 1 diabetes have been attributed to hypoglycemia. Severe hypoglycemia is the cause of unexpected overnight deaths in young diabetic patients.32 It may be explained by the impairment of hormonal responses to hypoglycemia during sleep, resulting in sudden cardiac arrhythmias.
The association of hypoglycemia and mortality during critical illness is very controversial.33–35 Not only is hypoglycemia more frequent in the most severely ill patients (e.g., those with hepatic or renal failure or septic shock), these spontaneous hypoglycemic episodes also more strongly correlate with mortality risk than hypoglycemia induced by intensive insulin therapy. Nevertheless, as a quality-control measure, intensive insulin therapy in the ICU should be implemented with meticulous monitoring of the incidence of hypoglycemic episodes. The importance of careful monitoring of blood glucose concentration is further emphasized by the demonstration of a tight correlation between blood glucose variability and mortality.36
 Differential Diagnosis
Differential Diagnosis
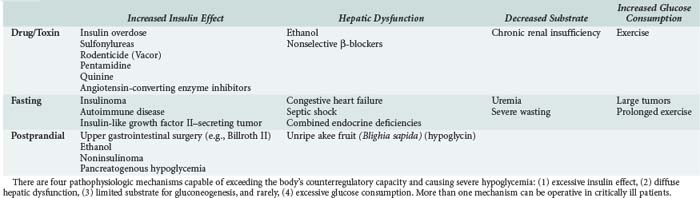
A clinical classification of hypoglycemic disorders separates patients who appear to be healthy (with or without coexistent disease) from those who appear to be ill (including those with a predisposing illness and those who are hospitalized). For otherwise healthy patients, the most important causes of fasting hypoglycemia are accidental or factitious drug ingestion and insulinoma. The differential diagnosis in ill or hospitalized patients includes predisposing illness, drug interactions, and other iatrogenic factors (Table 18-3).37
Insulin treatment of diabetes is the most common cause of hypoglycemia in adults. Risk factors for frequent severe hypoglycemia in type 1 diabetes include lower HbA1C levels, higher daily insulin dose, longer duration of diabetes, absence of residual C peptide, hypoglycemia unawareness, and a prior history of severe hypoglycemia.19 Insulin-treated type 2 diabetics are also vulnerable to severe hypoglycemia, especially if their disease is well controlled and they have been on insulin for many years.2 Whether intensive insulin therapy increases the incidence of severe hypoglycemia with sequelae is disputed.38,39 Newer insulin analogs such as glargine and lispro, as well as continuous insulin delivery systems, may lessen the risk of fasting or postprandial severe hypoglycemia.40,41
Sulfonylureas are a common cause of severe hypoglycemia. The incidence is higher in the elderly, in patients with renal or liver insufficiency, and with the use of long-acting agents like glibenclamide.42 Liver dysfunction prolongs the hypoglycemic activity of gliquidone and repaglinide. Renal insufficiency prolongs the activity of glyburide, chlorpropamide, and nateglinide.43 A crude rate of serious hypoglycemia of 1.23 events per 100 person-years has been reported among elderly users of sulfonylureas.44 Sulfonylurea-induced hypoglycemia can be prolonged (up to 27 days), and recurrences can occur after initial normalization of glucose levels.45 Discovery of inadvertent or factitious sulfonylurea overdose can help avoid an exhaustive search for insulinoma in patients who present with hyperinsulinemic hypoglycemia.46
The metabolism of ethanol depletes hepatocellular levels of nicotinamide adenine dinucleotide, which is a cofactor critical for the entry of substrates into gluconeogenesis pathways.47 Ethanol also inhibits cortisol and growth hormone responses and delays the epinephrine response to hypoglycemia.48 However, ethanol does not inhibit glycogenolysis, so ethanol-induced hypoglycemia does not occur until hepatic glycogen stores have been depleted (after 8–12 hours of fasting).49 There is no correlation between blood ethanol levels (although alcohol is usually detected) and the degree of hypoglycemia. The incidence of alcohol-induced hypoglycemia is generally less than 1% in adults, but hypoglycemic coma is commonly related to ethanol ingestion.50
In the absence of a drug or toxic cause, adults with severe fasting hypoglycemia should be evaluated for insulinoma, insulin-secreting tumor of the islets of Langerhans,51 or unusual causes such as excessive production of insulin-like growth factor II or rapid glucose consumption by tumors, diffuse hepatic dysfunction, septic shock, panhypopituitarism, polyglandular endocrine deficiency syndromes, and autoimmune hypoglycemia. The diagnosis of postprandial (reactive) hypoglycemia remains controversial.52
 Evaluation
Evaluation
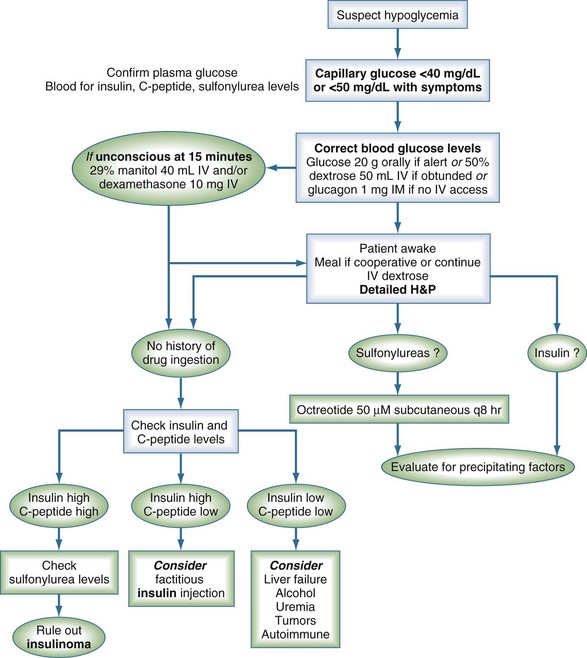
The first step in the evaluation of a patient with suspected hypoglycemia is documentation of low plasma glucose concentration in the presence of neuroglycopenic symptoms (Figure 18-1). Unless there is an obvious medication-related cause for severe hypoglycemia, blood should be drawn for the measurement of glucose, insulin, and C peptide before the administration of glucose and, when indicated, for the workup of thyroid hormone and cortisol deficiency or uremia. In cases of fasting hypoglycemia, intentional, accidental, or surreptitious ingestion of glucose-lowering medications should be investigated to avoid the lengthy workup for insulinoma.51 Sulfonylurea ingestion causes elevated insulin and C peptide levels, which mimics the findings associated with an insulinoma. Confirmation of the diagnosis of sulfonylurea ingestion can be made using high-pressure liquid chromatography or radioimmunoassay to detect sulfonylureas in blood or urine. The results of these tests are extremely important for further management.
 Management
Management
When patients are unwilling or unable to take oral carbohydrates, IV dextrose (glucose) should be given. The recommended initial dose of 50 mL of 50% dextrose provides 25 g dextrose; within 5 minutes, it produces a mean rise in blood glucose to 220 mg/dL (12.5 mmol/L) from nadir values as low as 20 mg/dL (1.1 mmol/L).53 In ICU patients receiving insulin by continuous IV infusion and also receiving a baseline enteral or intravenous glucose load, a 10-g glucose bolus is usually sufficient to correct hypoglycemia, and the smaller glucose load avoids the need to greatly modify the insulin dosing regimen.54 For prolonged hypoglycemia (e.g., caused by sulfonylurea overdose), prolonged dextrose infusion plus octreotide may be required.55
In cases of sulfonylurea overdose, octreotide reverses hyperinsulinemia, reduces dextrose requirements, and prevents recurrent hypoglycemia.55 The recommended dose of octreotide as an antidote for sulfonylurea overdose is 50 µg subcutaneously, repeated every 8 hours if necessary. Activated charcoal binds sulfonylureas and can be administered in cases of suspected overdose.
Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169-3176.
Diabetes Control and Complications Trial research group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271-286.
Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356(18):1842-1852.
Marks V, Teale JD. Drug-induced hypoglycemia. Endocrinol Metab Clin North Am. 1999;28(3):555-577.
Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type 1 diabetes. Lancet. 1993;341(8856):1306-1309.
1 American Diabetes Association Workgroup on Hypoglycemia Reference. Defining and reporting hypoglycemia in diabetes. Diabetes Care. 2005;28:1245-1249.
2 Hepburn DA, MacLeod KM, Pell AC, et al. Frequency and symptoms of hypoglycemia experienced by patients with type 2 diabetes treated with insulin. Diabet Med. 1993;10:231-237.
3 Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
4 Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type 1 diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697-703.
5 Fritsche AF, Stefan N, Haring H, et al. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing β-adrenergic sensitivity in type 1 diabetes. Ann Intern Med. 2001;134:729-736.
6 Cranston I, Lomas J, Maran A, et al. Restoration of hypoglycemia unawareness in patients with long-duration insulin-dependent diabetes mellitus. Lancet. 1994;344:283-287.
7 Mitrakou A, Fanelli C, Veneman T, et al. Reversibility of hypoglycemia unawareness in patients with insulinomas. N Engl J Med. 1993;329:834-839.
8 Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
9 Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461.
10 Finfer S, Chittock DR, Su SY, Blair D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297.
11 Brunner GA, Ellmerer M, Sendlhofer G, et al. Validation of home blood glucose meters with respect to clinical and analytical approaches. Diabetes Care. 1998;21:585-590.
12 Kanji S, Buffie J, Hutton B, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778-2785.
13 Tamada JA, Garg SK, Jovanovic L, et al. Non-invasive glucose monitoring: Comprehensive clinical results. JAMA. 1999;282:1839-1844.
14 Garg SK, Potts RO, Ackerman NR, et al. Correlation of fingerstick blood glucose measurements with GlucoWatch Biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708-1714.
15 Boyle PJ, Schwartz NS, Shah SD, et al. Plasma glucose concentration at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in non-diabetics. N Engl J Med. 1988;318:1487-1492.
16 Merimee TJ, Tyson JE. Stabilization of plasma glucose during fasting: Normal variation in two separate studies. N Engl J Med. 1974;291:1275-1278.
17 Vriesendorp TM, DeVries JH, Hoekstra JB. Hypoglycemia and strict glycemic control in critically ill patients. Curr Opin Crit Care. 2008 Aug;14(4):397-402.
18 Hart SP, Frier BM. Causes, management and morbidity of acute hypoglycemia in adults requiring hospital admission. Q J Med. 1998;91:505-510.
19 Diabetes Control and Complications Trial research group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271-286.
20 UKPDS research group. Overview of 6 years of therapy of type II diabetes: A progressive disease. Diabetes. 1995;44:1249-1258.
21 Wahren J, Ekberg K, Fernquist-Forbes E, Nair S. Brain substrate utilisation during acute hypoglycemia. Diabetologia. 1999;42:812-818.
22 Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169-3176.
23 Gerich JE, Cryer P, Rizza RA. Hormonal mechanisms in acute glucose counterregulation: The relative roles of glucagon, epinephrine, norepinephrine, growth hormone and cortisol. Metabolism. 1980;29:1165.
24 Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:1726-1731.
25 Davis M, Mellman M, Friedman S, et al. Recovery of epinephrine response but not hypoglycemic symptoms threshold after intensive insulin therapy in type 1 diabetes. Am J Med. 1994;97:535-542.
26 Burge MR, Sobhy TA, Qualls CR, Schade DS. Effect of short-term glucose control on glycemic thresholds for epinephrine and hypoglycemic symptoms. J Cin Endocrinol Metab. 2001;86:5471-5478.
27 Burge MR, Schmitz-Fiorentino K, Fischette C, et al. A prospective trial of risk factors for sulfonlyurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA. 1998;279:137-143.
28 Austin EJ, Dreary IJ. Effects of repeated hypoglycemia on cognitive function: A pyschometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care. 1999;22:1273-1277.
29 Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842-1852.
30 Duning T, Van den Heuvel I, Dickmann A, et al. Hypoglycemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care. 2010;33:639-644.
31 Sporer KA, Ernst A, Conte R. The incidence of ethanol-induced hypoglycemia. Am J Emerg Med. 1992;10:403-405.
32 Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care. 1999;Suppl 2:B40-B42.
33 Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430-1434.
34 Vriesendorp TM, DeVries JH, van Santen S, et al. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med. 2006;34:2714-2718.
35 Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151-3159.
36 Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244-252.
37 Service FJ. Classification of hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28:501-517.
38 Diabetes Control and Complications Trial research group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271-286.
39 Hepburn DA, MacLeod KM, Pell AC, et al. Frequency and symptoms of hypoglycemia experienced by patients with type 2 diabetes treated with insulin. Diabet Med. 1993;10:231-237.
40 Bott S, Bott U, Berger M, Mulhauser I. Intensified insulin therapy and the risk of severe hypoglycemia. Diabetologia. 1997;40:926-932.
41 Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type 1 diabetes. Lancet. 1993;341:1306-1309.
42 Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130-1136.
43 Ferguson SC, Strachan MW, Janes JM, Frier BM. Severe hypoglycemia in patients with type 1 diabetes and impaired awareness of hypoglycemia: A comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev. 2001;17:285-291.
44 Stahl M, Berger W. Higher incidence of severe hypoglycemia leading to hospital admission in type 2 diabetic patients treated with long-acting versus short-acting sulphonylureas. Diabet Med. 1999:586-590.
45 Lubowsky ND, Siegel R, Pittas AG. Management of glycemia in patients with diabetes mellitus and CKD. Am J Kidney Dis. 2007;50:865-879.
46 Shorr RI, Ray WA, Daugherty JR, Griffin MR. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. JAMA. 1997;278:40-43.
47 Ciechanowski K, Borowiak KS, Potocka BA, et al. Chlorpropamide toxicity with survival despite 27-day hypoglycemia. J Toxicol Clin Toxicol. 1999;37:869-871.
48 Klonoff DC, Barrett BJ, Nolte MS, et al. Hypoglycemia following inadvertent and factitious sulfonylurea overdoses. Diabetes Care. 1995;18:563-567.
49 Wilson NM, Brown PM, Juul SM, et al. Glucose turnover and metabolic and hormonal changes in ethanol-induced hypoglycemia. BMJ. 1981;282:849-853.
50 Sood V, Sobhy T, Schade DS, Burge MR. Low dose ethanol alters epinephrine responses and decreases glucose production during hypoglycemia in patients with type 2 diabetes. Diabetes. 2001;50(Suppl 1):A139.
51 Marks V, Teale JD. Drug-induced hypoglycemia. Endocrinol Metab Clin North Am. 1999;28:555-577.
52 Sporer KA, Ernst A, Conte R. The incidence of ethanol-induced hypoglycemia. Am J Emerg Med. 1992;10:403-405.
53 Service FJ. Diagnostic approach to adults with hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28:519-532.
54 Service FJ, Natt N, Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: A novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kirb. 2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84:1582.
55 Frier BM. Hypoglycemia and cognitive function in diabetes. Int J Clin Pract. 2001;Suppl 123:30-37.
56 Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359-366.
57 Strachan MW, Dreary IJ, Ewing FM, Frier BM. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23:305-312.