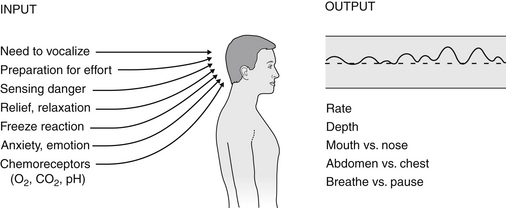
Chapter 55 Hyperventilation Syndrome/Breathing Pattern Disorders
 Introduction
Introduction
Hyperventilation Syndrome/Breathing Pattern Disorders Defined
Hyperventilation syndrome/breathing pattern disorders (HVS/BPDs) are described as follows:
• Hyperventilation is a pattern of overbreathing in which the depth and rate exceed the metabolic needs of the body at that time. This is usually seen at 30 breaths/min or more.
• Breathlessness usually occurs at rest or with only mild exercise.
• Physical, environmental, or psychological stimuli override the automatic activity of the respiratory centers, which are tuned to maintain arterial carbon dioxide (PaCO2) levels within a narrow range.
• Although at any given time, the body’s carbon dioxide (CO2) production is set at a certain level, the exaggerated breathing depth and rate associated with HVS/ BPDs eliminates CO2 at a faster pace, resulting in arterial hypocapnia (low CO2 in blood).
• The arterial hydrogen ion (pH) (acid/alkaline balance) rises into the alkaline region, thus inducing respiratory alkalosis.1
As a direct result of HVS/BPDs, many patients present with multiple symptoms, some of which mimic serious disease. However, blood tests, electrocardiograms (ECGs), and thorough physical examinations may reveal nothing out of the ordinary. Up to 10% of patients in general internal medicine practice reportedly experience HVS/BPDs as their primary diagnosis.2 Many individuals with HVS/BPDs experience severe and genuinely distressing symptoms, and considerable medical expenses are incurred in excluding more serious pathology.
Gender
More females than males have HVS/BPDs, ranging from a ratio of 2:1 to 7:1. The peak age of incidence is 15 to 55 years, although other ages can be affected.2 Women may be more at risk because of hormonal influences, because progesterone stimulates respiratory rate, and in the luteal (postovulation/premenstrual) phase, CO2 levels drop on average 25%. Additional stress can then “increase ventilation at a time when CO2 levels are already low.”3 A case report linked progesterone (medroxyprogesterone) therapy as a cause of hyperventilation in a 52-year-old menopausal woman.4
Normal Breathing Pattern
To recognize HVS/BPDs, one must be aware of the characteristics of a normal breathing pattern.
• The breathing rate should be 10 to 14 breaths/min, moving 3 to 5 L of air per minute through the airways of the chest.
• During the active inhalation phase, air flows in through the nose where it is warmed, filtered, and humidified before being drawn into the lungs by the downward movement of the diaphragm and the outward movement of the abdominal wall and lower thoracic structures.
• The upper chest and accessory breathing muscles should remain relaxed.
• The expiratory phase is ideally effortless as the abdominal wall and lower intercostals relax downward and the diaphragm ascends back to its original domed position, aided by the elastic recoil of the lung.
• A relaxed pause at the end of exhalation releases the diaphragm briefly from the negative and positive pressures exerted across it during breathing.
• Under normal circumstances individuals are quite unaware of their breathing.
• Breathing rates and volumes increase or fluctuate in response to physical or emotional demands, but in normal subjects they return to relaxed low chest patterns after the stimuli ceases.
Benefits of Normal Respiratory Function
• Normal performance of the brain, organs, and tissues of the body
• Normal speech and human nonverbal expression (e.g., sighing)
• Fluid movement (lymph, blood)
• Spinal mobility through regular, mobilizing, thoracic cage movement
• Digestive function via rhythmic positive and negative pressure fluctuations, via normal diaphragmatic function
Any chronic alteration in breathing function automatically modifies these functions negatively.
The Carbon Dioxide–Oxygen Balancing Act
Chaitow et al10 addressed the misconception that oxygen is “good” and carbon dioxide is “bad” by stating:
Respiratory Homeostasis
Jennett5 described the delicate homeostatic balancing act in which pH and CO2 are key features:
Pathophysiology
Physiologic and Pathophysiologic Causes of Altered Patterns of Breathing
Hyperventilation can be an appropriate physiologic response to the body’s metabolic needs; for example, tachypnea (rapid breathing) or hyperpnea (increase in respiratory rate proportional to increase in metabolism) may result as the respiratory centers respond automatically and appropriately to rising CO2 production due to exercise or organic disease that may be creating acidosis. It is therefore important to exclude organic causes that diminish PaO2 or elevate PaCO2 levels.6
• Respiratory: asthma, chronic obstructive respiratory disease, pneumonia, pulmonary embolus, pneumothorax, and pleural effusion. A case of carbon monoxide poisoning presenting as HVS has been reported.7
• Cardiovascular: acute and chronic left heart failure, right heart failure, tachyarrhythmias
• Renal: nephrotic syndrome, acute and chronic kidney failure
• Endocrine: Diabetes with ketoacidosis, pregnancy
• Pharmaceutic: aspirin, caffeine, amphetamine, nicotine, progesterone therapy
BPDs may also emerge from a background of established pathology (e.g., asthma, cardiovascular disease, kidney failure, chronic pain). Even tumor infiltrates into brain respiratory centers and central chemoreceptors have caused hyperventilation.8 Where this is the case, the aim of this chapter is not to explore these states, since they are discussed elsewhere in this textbook.
Fluctuating blood glucose levels may trigger HVS/BPD symptoms in patients with high carbohydrate diets, which produce rapid rises followed by sharp falls to fasting levels or below.6,9
Chaitow et al10 noted that the following factors could lead to altered breathing patterns through pH shifts:
• Ketoacidosis promotes deeper, faster breathing because the breathing centers respond to the higher CO2 content.
• Diarrhea results in the loss of alkaline plasma bicarbonate ions, which if prolonged, leads to acidosis. This stimulates corrective overbreathing to remove CO2 (as carbonic acid [H2CO3]) and normalizes the pH.
• Excessive vomiting causes loss of hydrochloric acid, shifting the body toward alkalosis, slowing breathing to allow CO2 to build up and restore pH. Hypoventilation is the result.
Categorization of Causes
The reasons for an individual breathing inappropriately can derive directly from structural, biomechanical causes, such as a restricted thoracic spine, rib immobility, or shortness of key primary and accessory respiratory muscles.
Other catalysts that may affect breathing function include environmental factors (e.g., altitude, humidity).11
The etiology of HVS/BPD may involve combinations of the factors listed previously; however, in most instances, altered breathing patterns, whatever their origins, seem to be maintained by nothing more sinister than pure habit.6,12
The Carbon Dioxide–Hydrogen Ion Connection
• The acidity of the blood is determined mainly by CO2.
• CO2 is the end-product of aerobic metabolism, deriving mainly from the mitochondria. CO2 is odorless, heavier than air and, if inhaled in its pure form, causes suffocation.
• CO2 is present in the atmosphere at a concentration of around two-hundredths of 1%, and is harmless to humans but adequate to sustain plant life.
• Transportation of CO2 occurs from the tissues into the blood and then to the lungs for exhalation. The body converts CO2 to H2CO3, of which there is a perpetual surplus.
• The lungs exhale around 12,000 mEq of H2CO3 per day, compared with less than 100 mEq of fixed acids excreted by the kidneys. Normal range of end-tidal CO2 pressure is 35 to 45 mm Hg.13
• Any increase in bodily activity produces CO2, acidifying the blood, unless more CO2 is excreted and/or exhaled.
• It is therefore obvious that changes in breathing volume relative to CO2 production regulate the moment-to-moment concentration of pH of the bloodstream (longer term regulation of pH is shared with the kidneys).
• It is the concentration of CO2 in the blood, not oxygen, that is the major regulator of breathing drive.
• Higher CO2 levels immediately stimulate more breathing, apparently on the assumption that abundance of CO2 means oxygen-poor air is being breathed, breathing has stopped, or something else is happening that is an antecedent to suffocation.
Oxygen Delivery and Smooth Muscle Constriction
An exercising muscle is acid, hypercarbic, and hot, and it benefits from increased unloading of oxygen from its capillaries.11
The effect of pH on oxyhemoglobin dissociation is called the Bohr effect.
Psychology and Hyperventilation Syndrome/Breathing Pattern Disorders
On a psychological level, Bradley14 described a “cascade of symptoms” (see Figure 55-1) in which an original cause (emotional or physical) leads to tension and anxiety that results in hyperventilation, possibly an acute hyperventilation attack, which (with repetition) over time, results in anticipation, anxiety, and avoidance behaviors or phobias, or both.
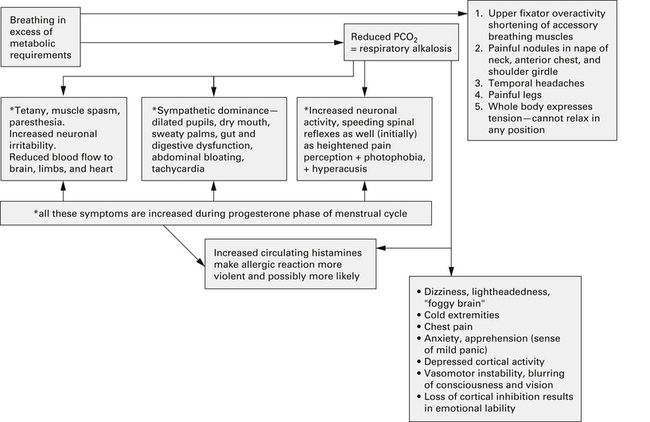
FIGURE 55-1 Negative health influences of a dysfunctional breathing pattern such as hyperventilation.
(From Chaitow L, Bradley D, Gilbert C. Multidisciplinary approaches to breathing pattern disorders. London: Churchill Livingstone, 2002:90.)
Chaitow et al10 described aspects of the influence of emotion on breathing15–17:
Conway et al18 used hypnosis to investigate the sources of hyperventilation episodes and found emotional events such as loss, separation, and impotent anger were common precipitating factors that began the hyperventilation trend. They concluded that hypnosis might be helpful in discovering the underlying cause of hyperventilation.
Freeman et al19 also showed that individuals who reported several symptoms indicating hyperventilation (including chest pain/palpitations and dizziness—not exclusively respiratory symptoms) displayed rather strong hyperventilation in response to recalling emotionally disturbing events, whereas the control subjects did not. Bereavement, loss of control, grief, and anger were common topics associated with the symptoms.
Chaitow et al10 concluded (Figure 55-2):
 Diagnostic Considerations
Diagnostic Considerations
Symptoms
Acute hyperventilation represents approximately 1% of all cases of hyperventilation, which is well outnumbered by chronic hyperventilation.20 The symptoms and signs of HVS are extremely variable, and none are absolutely diagnostic. The following symptoms are indications of possible breathing pattern dysfunction:
• A feeling of constriction in the chest
• Accelerated or deepened breathing
• Feeling tense (the Nijmegen questionnaire avoids the use of the word anxiety)
Table 55-1 is not fully comprehensive, but does represent the most common symptoms and signs of HVS/BPD. For greater depth, see Timmons and Ley,6 Gardner,21 Nixon,22 and Chaitow et al.10
TABLE 55-1 Most Common Symptoms and Signs of Hyperventilation Syndrome/Breathing Pattern Disorders
| SYSTEM | SYMPTOMS | SUGGESTED CAUSES |
|---|---|---|
| Cardiovascular | Chest pain and angina, palpitation and arrhythmias, tachycardia, lightheadedness and syncope, altered ECG features | Reduced coronary blood flow, altered excitability of SA and AV nodes of cardiac muscle, reduced cardiac output, peripheral vasodilatation |
| Gastrointestinal | Discomfort in lower chest and epigastric area, esophageal reflux and heartburn, bloating/distension, exacerbation of hiatal hernia symptoms, dry mouth, air swallowing and belching | Aerophagia, increased swallowing rate, mouth breathing |
| Neurologic | Headache; numbness and tingling (mainly involving extremities and perioral); positive Trousseau’s and Chvostek’s signs; dizziness/giddiness; ataxia and tremor; blurred and tunnel vision; anxiety and panic; phobias; irritability; depersonalization; detachment from reality; impaired concentration, cognition, performance; easy fatigue; insomnia; hallucinations | Cerebrovascular constriction (see notes on smooth muscle contraction below); vasoconstriction of vertebral or carotid arteries, or both; reduced oxygen delivery, neuronal excitability resulting from alkalosis; hypocalcemia |
| Respiratory | Breathlessness; restricted sensation around thorax; sighing/yawning; obvious use of upper chest, accessory breathing muscles (e.g., scalenes) on inhalation; chest tenderness | Overuse of accessory breathing muscles and fatigue of primary respiratory muscles |
| Muscular | Stiffness and aching, weakness in limbs, cramping, carpopedal spasm, tetany | Hyperexcitability of motor nerves, muscle fatigue, calcium/magnesium imbalance |
AV, atrioventricular; ECG, electrocardiogram; SA, sinoatrial.
Metabolic Disturbances and Hyperventilation Syndrome
• Two tests of nerve hyperexcitability produced by hypocapnia-induced hypocalcemia are Trousseau’s and Chvostek’s signs.
• Chest pain associated with HVS/BPDs requires that heart disease is excluded as a diagnosis. Adrenaline-induced electrocardiographic changes can occur in hyperventilation, uncomplicated by coronary heart disease. One study suggested that up to 90% of noncardiac chest pain is thought to be induced by HVS/BPDs.23
Alternatively, hyperventilation can trigger spasms of normal caliber coronary arteries.
• Rapid breathing or mouth breathing instigates aerophagia from air gulping, causing bloating, burping, and extreme epigastric discomfort. Irritable bowel syndrome is listed as a common symptom of chronic overbreathing. Fear and anxiety may induce abdominal cramps and diarrhea.1 The median swallowing rate in healthy, nondyspeptic controls is 3 or 4 swallows per 15 minutes. In the absence of food, up to 5 mL of air accompanies saliva into the gastrointestinal tract with each swallow.24
• Hyperventilation leading to hypocapnia causes cerebral arterioles to constrict, increasing vascular resistance and reducing cerebral blood flow. This is a natural response to changes in CO2 to regulate oxygen delivery to the brain.25
Acute Hyperventilation Progression
• The patient presenting with an acute hyperventilation episode appears distressed.
• The pattern of respiration is of deep, rapid breaths, using the accessory muscles visible in the neck and the upper chest.
• Wheezing may be heard as a result of bronchospasm triggered by hypocapnia.
• A stressful precipitating event is usually reported.
• Hypocapnia reduces blood flow to the brain (2% decrease in flow per 1 mm Hg reduction in arterial CO2), causing frightening central nervous system symptoms. The reduced oxygenation of brain and tissues of the body results from contraction of smooth muscle surrounding blood vessels and a reluctance of the hemoglobin carrier molecule to release oxygen in the increasingly alkaline environment caused by excessive loss of CO2.
• Poor concentration and memory lapses may occur as a result, with tunnel vision and onset in those susceptible to migraine-type headaches or tinnitus.
• Sympathetic dominance brings on tremors, sweating, clammy hands, palpitations, and autonomic instability of blood vessels causing labile blood pressures.26
• Bilateral perioral and upper extremity paresthesia and numbness may be reported. Unilateral tingling is most often confined to the left side.
• Dizziness, weakness, visual disturbances, tremor, and confusion—sometimes fainting or even seizures—are typical symptoms.
• Spinal reflexes become exaggerated through increased neuronal activity caused by loss of CO2 ions from the neurons.
Chronic Hyperventilation
Careful inquiries as to the precipitating causes of attacks helps both with the diagnosis and focusing on choice of treatment. Nixon22 suggested that there are often attacks where there is no preceding stressful event. For example, in chronic hyperventilators, the respiratory center may have been reset to tolerate a lower than normal partial pressure of PaCO2 levels in the blood. In such patients, a single sigh or one deep breath may reduce the PaCO2 enough to trigger symptoms.
Laboratory and Office Tests for Hyperventilation Syndrome/Breathing Pattern Disorders
Many possible tests for respiratory function exist. Some are difficult to perform (e.g., airway resistance), and some are invasive (e.g., blood gases).14 A selection of tests are listed as follows:
• Preliminary tests to exclude respiratory and cardiac disease including peak expiratory flow rate, chest radiograph, ECG, and an exercise ECG if chest pain is present.
• Palpation and observation can demonstrate a paradoxic breathing pattern in which the abdomen retracts and the upper chest expands on inhalation (as opposed to normal abdominal protrusion and lower thorax expansion).
• The breath-holding time test does not require additional measurements or equipment. The time a hyperventilating patient can hold his or her breath is usually greatly reduced, often not beyond 10 to 12 seconds. Thirty seconds has been used as the approximate dividing line between hyperventilators and normals by some clinicians. It is worth noting that breathless patients without hyperventilation may have equal difficulty in breath holding.21
• A peak expiratory flow rate measurement, compared with age, sex, and height tables, provides a simply done, quick exclusion of significant respiratory restriction in the clinic room.
• If a hyperventilation provocation test (HVPT) is performed (during which the patient is asked to voluntarily overbreathe to bring on symptoms), ECG should be monitored (see “Caution” later).
• Elevated erythrocyte carbonic anhydrase (ECA) was recently (2009) suggested as a clinical marker for hyperventilation. The values of ECA were significantly elevated (~31 U/g hemoglobin) in patients who hyperventilated compared with controls (24.7 U/g hemoglobin). However, hyperventilation sensitivity and specificity were only 52.1% and 76.7%, respectively. There are other conditions that might elevate ECA, such as glucose-6-phosphate dehydrogenase deficiency and different anemias (aplastic, iron deficiency, autoimmune hemolytic, β-thalassemia).28
• Arterial blood gas determination is invasive and painful (arterial puncture) but appropriate in the emergency department, where the diagnosis of acute hyperventilation is required. For patients in whom chronic hyperventilation is suspected, the pressure of end-tidal carbon dioxide (PET CO2) can be measured noninvasively from a continuous sampling through nasal prongs or cannula with the mouth occluded, or the tube can be sited in an oral airway for those with nasal obstruction to monitor CO2 deficits. The PET CO2 is the level of CO2 released at the end of expiration.
• Capnography: PET CO2 can be evaluated after a 4-minute quiet breathing rest period, followed by exercise and recovery, or a HVPT can be conducted in the recovery period. Most patients with chronic hyperventilation have a PET CO2 at or below 30 mm Hg and a markedly delayed recovery from hypocapnia after overbreathing, sometimes lasting 30 minutes after testing.29,30 By measuring PET CO2 or transcutaneous CO2 levels while a hyperventilation provoking activity is performed, a potential link can be made between symptoms and CO2 levels.31
• The think test32 may be initiated 3 to 4 minutes into the recovery period. The patient is asked to recall a painful emotional experience during which symptoms developed. If the PET CO2 drops 10 mm Hg, the test supports hyperventilation. Bradley14 noted: “In some patients with hyperventilation the PaCO2 and the PET CO2 may be in the normal range. In those who are asymptomatic at the time of testing, this finding could be accepted. However, a normal level while experiencing symptoms negates hypocapnia as the cause of symptoms. It prompts a search for an alternative explanation.”
• Buteyko performed comparative studies with a simple breath-holding technique to test CO2 levels and found that a simple technique of breath holding after expiration could predict the percent of alveolar CO2 and therefore the degree of hyperventilation to a high degree of accuracy. According to his calculations, optimal levels of alveolar PCO2 correlated with postexpiratory breath-holding time of 40 to 60 seconds. Many asthmatics and hyperventilators are found to be able to hold the breath out for less than 10 seconds.33–35
The Nijmegen Questionnaire
Bradley14 pointed out that no “gold standard” exists for chronic HVS, but the Nijmegen Questionnaire is noninvasive with a high level of sensitivity (up to 91%)36 and specificity (up to 95%).37 It is also a way to monitor the progress of treatment by re-evaluating symptoms. Bradley noted that the results of this simple test also helped indicate whether the initiating trigger causing the HVS/BPDs resolved, suggesting that the patient had to deal with only the “bad breathing” habit and musculoskeletal and motor pattern changes, or whether the initiating triggers were ongoing or unresolved and might need further cognitive help (Figure 55-3).
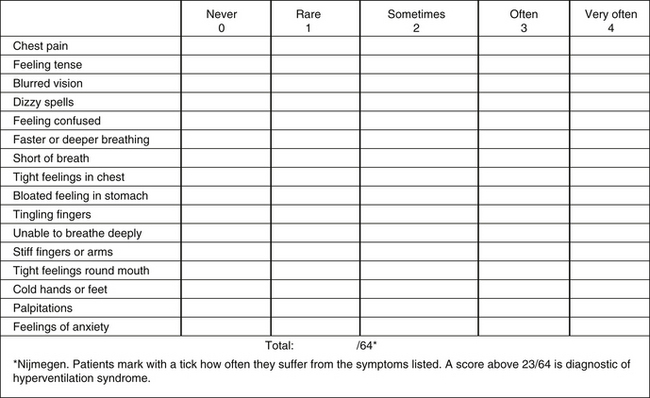
FIGURE 55-3 Nijmegen questionnaire.
(From Chaitow L, Bradley D, Gilbert C. Multidisciplinary approaches to breathing pattern disorders. London: Churchill Livingstone; 2002:176.)
Warburton and Jack30 stated the Nijmegen Questionnaire was neither sensitive nor specific for chronic idiopathic hyperventilation without physiologic testing because many of the symptoms on the questionnaire were common to an organic respiratory disease.
 Biomechanical (Structural) Considerations
Biomechanical (Structural) Considerations
The Structure–Function Continuum
• Structural adaptations can prevent normal breathing function.
• Abnormal breathing function ensures continued structural adaptational stresses.38
Individually, neither approach is as useful as a combination of restoration of structural integrity, combined with functional improvement. It should be obvious that other underlying etiologic features, whether these relate to psychosocial, biochemical, or biomechanical factors, should be addressed as far as possible as a prerequisite of rehabilitation.
The Muscles of Breathing
Space does not permit in-depth discussion of the muscles of respiration, further details of which can be found in Chaitow et al.10
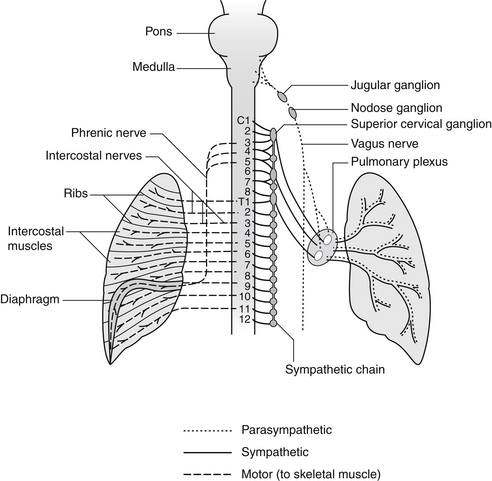
Neural Regulation of Breathing
Respiratory centers in the brainstem unconsciously influence and adjust alveolar ventilation to maintain arterial blood oxygen and CO2 pressures at relatively constant levels, to sustain life under varying conditions and requirements.10
The three main groups are as follows:
• The dorsal respiratory group, located in the distal portion of the medulla, receives input from peripheral chemoreceptors and other types of receptors via the vagus and glossopharyngeal nerves. These impulses generate inspiratory movements and are responsible for the basic rhythm of breathing.
• The pneumotaxic center in the superior part of the pons transmits inhibitory signals to the dorsal respiratory center, controlling the filling phase of breathing.
• The ventral respiratory group, located in the medulla, causes either inspiration or expiration. It is inactive in quiet breathing but is important in stimulating abdominal expiratory muscles during levels of high respiratory demand.
The autonomic nervous system enables the automatic unconscious maintenance of the internal environment of the body in ideal efficiency and adjusts to the various demands of the external environment, be it sleep with repair and growth, quiet or extreme physical activity, or stress (Figure 55-4).
A “third” nervous system regulating the airways has been recognized, called the nonadrenergic noncholinergic (NANC) system. Containing inhibitory and stimulatory fibers, nitric oxide has been identified as the NANC neurotransmitter.39
• NANC inhibitory nerves cause calcium ions to enter the neuron, mediating smooth muscle relaxation and bronchodilation.
• NANC stimulatory fibers—also called C-fibers—are found in the lung supporting tissue, airways, and pulmonary blood vessels, and appear to be involved in bronchoconstriction after exercise-induced asthma.40
Voluntary Control of Breathing
Automatic breathing can be overridden by higher cortical conscious input (directly via the spinal neurons, which drive the respiratory muscles) in response to, for instance, fear or sudden surprise. Speaking requires voluntary control to interrupt the normal rhythmicity of breathing, as do singing and wind instrument playing. Evidence indicates that the cerebral cortex and thalamus also supply part of the drive for normal respiratory rhythm during wakefulness. (Cerebral influences on the medullary centers are withdrawn during sleep.) Habitual BPDs and HVS probably originate from some of these higher centers.6
 Therapeutic Considerations and Therapeutic Approach
Therapeutic Considerations and Therapeutic Approach
Treatment and Rehabilitation of Hyperventilation Syndrome/Breathing Pattern Disorders
Different models of care in managing HVS/BPDs are available.
Bradley’s14,41 physical therapy rehabilitation follows stages that are summarized in the acronym BETTER: Breathing retraining, Esteem/self-image, Total body relaxation, Talk/breath control, Exercise prescription, Rest/sleep.
An Osteopathic/Naturopathic Protocol for Care of Hyperventilation Syndrome/Breathing Pattern Disorders
• Initial (and continual or periodic) assessment of breathing function based on functional evidence and palpation determines what needs to be done to improve breathing function.
• Education and information are vital for creating motivation and awareness as to why homework is essential in normalizing BPDs.
• The patient must understand clearly that the practitioner or therapist can do no more than create an environment, a possibility, for restoration of more normal function, but the breathing work itself is up to the patient.
• Treatment of muscles and joints alone, no matter how appropriate, can never restore normal breathing patterns without cooperative effort.
• Conversely, breathing retraining without the freeing of restricted structures is far more difficult to achieve.
• Psychotherapy and counseling are also unlikely to be successful unless retraining is introduced, and structural factors are dealt with.
• Manual attention to the upper fixators and/or accessory breathing muscles (upper trapezii, levator scapulae, scalenes, sternocleidomastoid, pectorals, and latissimus dorsi) is usually required.
• The diaphragm area also requires direct attention as a rule (lower anterior intercostals, sternum, costal margin, beneath costal margin, abdominal attachments, quadratus lumborum, and psoas).
• Active trigger points in these muscles may need deactivating manually or via acupuncture.
• Acupuncture being administered for 30 minutes, twice weekly, for 4 weeks showed reduction in Nijmegen score from 31 to 24. The focus was on reducing anxiety, thereby reducing hyperventilation. The points used were colon 4, liver 3, and stomach 36 bilaterally.42
• The thoracic spine and ribs may require mobilization (osteopathic or chiropractic adjustments).
• Osteopathic lymphatic pump methods may be required if there is evidence of stasis.
• Retraining: various breathing exercises should be introduced, individualized to the specific needs of the patient, commonly on the basis of pursed lip breathing and pranayama yoga methods (see Box 55-1).43–47
• Relaxation methods, including autogenic training or progressive muscular relaxation, or both, might usefully be introduced.
• Sleep pattern disturbances might require attention.
• Exercise of aerobic nature should be carefully introduced.
• Dietary advice and counseling should be introduced as appropriate.
BOX 55-1 Breathing Rehabilitation Exercises
Pursed lip breathing, combined with diaphragmatic breathing, enhances pulmonary efficiency.
3. Recitation of mantra or prayer
Breathing Rehabilitation Exercises
Box 55-1 describes three breathing rehabilitation exercises.
Lum1 reported that more than 1000 anxious and phobic patients were treated using breathing retraining, physical therapy, and relaxation. Results indicated the following:
• Symptoms were usually abolished in 1 to 6 months, with some younger patients requiring only a few weeks.
• At 12 months, 75% were free of all symptoms, 20% had only mild symptoms, and about 1 in 20 patients had intractable symptoms.
Breathing rehabilitation therapy was evaluated in patients with HVS; the diagnosis was based on the presence of several stress-related complaints and reproduced by voluntary hyperventilation.48 Patients with organic diseases were excluded, and most patients met the criteria for an anxiety disorder.
Therapy was conducted in the following sequence:
• Brief, voluntary hyperventilation to reproduce the reported complaints
• Reattribution of the cause of the symptoms to hyperventilation
• Explaining the rationale of therapy involving reduction of hyperventilation by acquiring an abdominal breathing pattern, with slowing down of expiration
• Breathing retraining for 2 to 3 months working with a physiotherapist
 Summary
Summary
HVS/BPDs are a common disorder affecting up to 10% of patients in a general internal medicine practice. However, it is rarely recognized or appropriately treated. Breathing retraining is effective clinically and economically, as long as enough patience is exercised to actively engage the patient in a program requiring weeks to months of behavioral change. Acupuncture was also shown to improve symptoms of HVS, especially with anxiety as the etiology.
1. Lum L.C. Hyperventilation syndromes in medicine and psychiatry: a review. J R Soc Med. 1987;80:229–231.
2. Kern B., Rosh A.J. Hyperventilation syndrome. Emedicine, 2009. http://emedicine.medscape.com/article/807277-overview. Accessed 9/23/10
3. Damas-Mora J., Davies L., Taylor W., et al. Menstrual respiratory changes and symptoms. Br J Psychiatry. 1980;136:492–497.
4. Farrukh A., Ramsden G.H. Hyperventilation with medroxyprogesterone therapy in a postmenopausal woman. J Obstet Gynaecol. 2007;27:323.
5. Jennett S. Control of breathing and its disorders. In: Timmons B.H., Ley R. Behavioral and psychological approaches to breathing disorders. New York: Plenum Press, 1994.
6. Timmons B.H., Ley R. Behavioral and psychological approaches to breathing disorders. New York: Plenum Press; 1994.
7. Ong J.R., Hou S.W., Shu H.T., et al. Diagnostic pitfall: carbon monoxide poisoning mimicking hyperventilation syndrome. Am J Emerg Med. 2005;23:903–904.
8. Tarulli A.W., Lim C., Bui J.D., et al. Central neurogenic hyperventilation: a case report and discussion of pathophysiology. Arch Neurol. 2005;62:1632–1634.
9. Brostoff J. Complete guide to food allergy. London: Bloomsbury; 1993.
10. Chaitow L., Bradley D., Gilbert C. Multidisciplinary approaches to breathing pattern disorders. Edinburgh: Churchill Livingstone; 2002.
11. West J. Respiratory physiology. Philadelphia: Lippincott Williams & Wilkins; 2000.
12. Lum L.C. Hyperventilation and anxiety state. J R Soc Med. 1981;74:1–4.
13. Farmand M. Blood gas analysis and the fundamentals of acid-base balance. Neonatal Netw. 2009;28:125–128.
14. Bradley D. Physiotherapy breathing rehabilitation strategies. In: Chaitow L., Bradley D., Gilbert C. Multidisciplinary approaches to breathing pattern disorders. Edinburgh: Churchill Livingstone, 2002.
15. Han J.N., Stegen K., Simkens K., et al. Unsteadiness of breathing in patients with hyperventilation syndrome and anxiety disorders. Eur Respir J. 1997;10:167–176.
16. Beck J.G., Shipherd J.C., Ohtake P. Do panic symptom profiles influence response to a hypoxic challenge in patients with panic disorder? A preliminary report. Psychosom Med. 2000;62:678–683.
17. Wilhelm F.H., Trabert W., Roth W.T. Characteristics of sighing in panic disorder. Biol Psychiatry. 2001;49:606–614.
18. Conway A.V., Freeman L.J., Nixon P.G. Hypnotic examination of trigger factors in the hyperventilation syndrome. Am J Clin Hyper. 1988;30:296–304.
19. Freeman L.J., Conway A., Nixon P.G. Physiological responses to psychological challenge under hypnosis in patients considered to have the hyperventilation syndrome: implications for diagnosis and therapy. J R Soc Med. 1986;79:76–83.
20. Lum L.C. Hyperventilation: the tip and the iceberg. J Psychosom Res. 1975;19:375–383.
21. Gardner W.N. The pathophysiology of hyperventilation disorders. Chest. 1996;109:516–534.
22. Nixon P.G. The grey area of effort syndrome and hyperventilation: from Thomas Lewis to today. J R Coll Physicians Lond. 1993;27:377–383.
23. DeGuire S., Gevirtz R., Kawahara Y., et al. Hyperventilation syndrome and the assessment of treatment for functional cardiac symptoms. Am J Cardiol. 1992;70:673–677.
24. Calloway S.P., Fonagy P. Aerophagia and irritable bowel syndrome. Lancet. 1985;2:1368.
25. Kucewicz J.C., Dunmire B., Giardino N.D., et al. Tissue pulsatility imaging of cerebral vasoreactivity during hyperventilation. Ultrasound Med Biol. 2008;34:1200–1208.
26. Magarian G.J. Hyperventilation syndromes: infrequently recognized common expressions of anxiety and stress. Medicine (Baltimore). 1982;61:219–236.
27. Fried R., Grimaldi J. The psychology and physiology of breathing. New York: Plenum Press; 1993.
28. Teng Y.H., Tsai H.T., Hsieh Y.S., et al. Elevated erythrocyte carbonic anhydrase activity is a novel clinical marker in hyperventilation syndrome. Clin Chem Lab Med. 2009;47:441–445.
29. Chambers J.B., Kiff P.J., Gardner W.N., et al. Value of measuring end tidal partial pressure of carbon dioxide as an adjunct to treadmill exercise testing. Br Med J (Clin Res Ed). 1988;296:1281–1285.
30. Warburton C.J., Jack S. Can you diagnose hyperventilation? Chron Respir Dis. 2006;3:113–115.
31. White K.M., Quinn J.M., Hagan L.L., et al. Exercise-induced hyperventilation. Ann Allergy Asthma Immunol. 2008;100:171–172.
32. Nixon P.G., Freeman L.J. The ‘think test’: a further technique to elicit hyperventilation. J R Soc Med. 1988;81:277–279.
33. Courteney R. The Buteyko Method – an osteopathic approach to asthma? Osteopathy Today. August 2002:16–19.
34. Bowler S.D., Green A., Mitchell C.A. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Med J Aust. 1998;169:575–578.
35. Buteyko K. Buteyko method; experience of application in medical practice. Moscow: Patriot; 1990.
36. Vansteenkiste J., Rochette F., Demedts M. Diagnostic tests of hyperventilation syndrome. Eur Respir J. 1991;4:393–399.
37. van Dixhoorn J., Duivenvoorden H.J. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29:199–206.
38. Garland W. Somatic changes in hyperventilating subject. Saint Flour, France: International Society for the Advancement of Respiratory Psychophysiology Congress; 1994. September 25-28, 1994
39. Snyder S.H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992;257:494–496.
40. Beachey W. Respiratory care anatomy & physiology. St. Louis: Mosby; 1998.
41. Bradley D. Hyperventilation syndrome/breathing pattern disorders. Auckland, NZ: Tandem Press; 1998.
42. Gibson D., Bruton A., Lewith G.T., et al. Effects of acupuncture as a treatment for hyperventilation syndrome: a pilot, randomized crossover trial. J Altern Complement Med. 2007;13:39–46.
43. Cappo B.M., Holmes D.S. The utility of prolonged respiratory exhalation for reducing physiological and psychological arousal in non-threatening and threatening situations. J Psychosom Res. 1984;28:265–273.
44. Faling L. Controlled breathing techniques and chest physical therapy in chronic obstructive pulmonary disease. In: Casaburi R., ed. Principles and practices of pulmonary therapy. Philadelphia: WB Saunders, 1993.
45. Tiep B.L., Burns M., Kao D., et al. Pursed lips breathing training using ear oximetry. Chest. 1986;90:218–221.
46. Grossman P., de Swart J.C., Defares P.B. A controlled study of a breathing therapy for treatment of hyperventilation syndrome. J Psychosom Res. 1985;29:49–58.
47. Bernardi L., Sleight P., Bandinelli G., et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ. 2001;323:1446–1449.
48. Han J.N., Stegen K., De Valck C., et al. Influence of breathing therapy on complaints, anxiety and breathing pattern in patients with hyperventilation syndrome and anxiety disorders. J Psychosom Res. 1996;41:481–493.