CHAPTER 64 Hypertrophic Cardiomyopathy
PREVALENCE AND EPIDEMIOLOGY
The incidence of HCM is reported to be as high as 1/500 individuals, making it the most common genetically inherited cardiovascular disease.1 Nevertheless, HCM is thought to represent no more than 1% of cardiac outpatients,1 suggesting that many genetically affected individuals remain asymptomatic for most or all of their lives.
ETIOLOGY AND PATHOPHYSIOLOGY
HCM is inherited as an autosomal dominant trait with variable penetrance. The distribution of hypertrophy in HCM is variable, with septal, right ventricular, left ventricular, septal, apical, midventricular, or concentric patterns. Asymmetric hypertrophy of the ventricular septum is by far the most common.2 Histologically, HCM is characterized by myofibrillar disarray.1
MANIFESTATIONS OF DISEASE
Clinical Presentation
The clinical presentation of HCM varies from asymptomatic to sudden cardiac death, which is the leading cause of mortality in affected patients. HCM is responsible for approximately one third of cases of sudden cardiac death in young athletes.3 The mechanism for sudden cardiac death is ventricular fibrillation.4 The 1-year mortality rate for clinically evident HCM is approximately 1% to 4%.2 Risk stratification has been advocated to help define those patients who may benefit most from antiarrhythmic therapy or an implantable cardiac defibrillator.4 Ventricular wall thickness more than 30 mm has been shown to be an independent risk factor for sudden cardiac death.5 The positive predictive value of hypertrophy more than 30 mm is greatly increased when other risk factors are taken into account.6 More than 90% of patients with HCM have asymmetric septal hypertrophy. Other distributions of hypertrophy include apical, midventricular, and concentric.
In addition to sudden cardiac death, atrial fibrillation and heart failure represent the major morbidities associated with HCM. Atrial fibrillation is encountered in up to 25% of patients.1 Heart failure in HCM is most often caused by diastolic dysfunction. In patients with significant septal hypertrophy, heart failure may also be attributed to left ventricular outflow tract obstruction.2
Imaging Techniques and Findings
Ultrasound
Patients clinically suspected to have hypertrophic cardiomyopathy are initially referred for echocardiography.2 Asymmetric septal hypertrophy is the most common finding. Septal thickening of 13 to 15 mm or more and a septal-to-posterior wall ratio of 1.5 : 1 or higher have been the usual definitions of septal hypertrophy.7 In general, however, any abnormal hypertrophy noted on echocardiography that is not otherwise explained raises the suspicion of HCM.
Computed Tomography
Computed tomography (CT) has been shown to demonstrate morphologic and functional abnormalities in HCM (Fig. 64-1). Multidetector helical CT allows the acquisition of multiphase imaging relatively free of motion, as well as three-dimensional reformations. Reports applying electrocardiographically gated helical CT to the assessment of HCM are encouraging.8 However, CT has disadvantages, including the use of ionizing radiation and iodinated contrast agent, as well as limited contrast resolution.
Magnetic Resonance Imaging
MRI with electrocardiographic gating provides excellent depictions of cardiac anatomy in hypertrophic cardiomyopathy. MRI can be used for accurate identification of the site and extent of ventricular hypertrophy and subaortic stenosis, and for quantification of myocardial mass.9 MRI also differentiates septal hypertrophy from septal neoplasm, an important distinction, because the two entities may present with similar symptoms.10
MRI can be used for precise and reproducible quantification of left ventricular function, including the measurement of stroke volume, ejection fraction, and mass. MRI can also be used to assess the presence and functional significance of mitral regurgitation and left ventricular outflow tract obstruction.11 The response of left ventricular outflow tract obstruction to therapy, including septal myectomy and percutaneous transluminal septal wall ablation, has also been demonstrated with MRI.12 Abnormal systolic wall thickening, a sign of myocardial dysfunction, may be seen in patients with HCM using MRI.11 In addition, MRI is able to identify associated perfusion abnormalities, such as impaired global perfusion, diminished coronary flow reserve, and isolated perfusion deficits.13 MRI also may be used to detect abnormal myocardial architecture caused by fibrosis or necrosis.14
In patients with suspected HCM not fully assessed by echocardiography, MRI offers improved detection of disease. Specifically, echocardiography is often of limited value in the assessment of the anterior and lateral left ventricular wall and of the ventricular apex.15 Without the limits of acoustic windows inherent in echocardiography, MRI demonstrates myocardial thickening regardless of location. In addition, the high reproducibility of MRI is an advantage compared with echocardiography in monitoring regression or progression of left ventricular mass in response to therapy.
Morphology
MRI readily shows the location and extent of ventricular hypertrophy. Patients are referred for MRI when echocardiography is nondiagnostic because of a poor acoustic window or when echocardiographic evaluation of myocardial segments is incomplete. End-diastolic ventricular thickness of 1.5 cm or more on MRI scans is a commonly used indicator of HCM in patients without hypertension, aortic stenosis, or other explanations for hypertrophy.16 Asymmetric hypertrophy is demonstrated by calculating a ratio of wall thickness, such as septal to posterolateral thickness.16 Electrocardiographically gated black blood and white blood steady-state free precession MRI sequences in the short- and long-axis planes ensure that all myocardial segments are clearly visualized (Table 64-1; Fig. 64-2).17
TABLE 64-1 Selected Magnetic Resonance Imaging Sequences Used for Hypertrophic Cardiomyopathy
| Sequence | Indications and Use | Imaging Plane |
|---|---|---|
| Black blood (double-inversion recovery) |
LVOT, left ventricular outflow tract.
Asymmetric septal hypertrophy is the most common distribution of hypertrophy in HCM.1 MRI readily demonstrates septal hypertrophy in patients with HCM (Fig. 64-3).11 Other patterns, such as apical hypertrophy (Fig. 64-4), midventricular hypertrophy, concentric hypertrophy (Fig. 64-5), and right ventricular hypertrophy (Fig. 64-6) are also well visualized by MRI.15 In particular, the usefulness of MRI for apical HCM has been emphasized.16 Defining the location of hypertrophy is of clinical importance because some forms of HCM, such as apical HCM, have a more favorable prognosis and because treatment may depend on the distribution of hypertrophy.2
Ventricular mass can be used as a predictor of prognosis in patients with HCM.13 The myocardial mass can be calculated accurately with MRI (Fig. 64-7).17 Echocardiographically derived masses are the product of several measurements entered into a formula that relies on geometric assumptions regarding cardiac morphology. Given the variable nature of hypertrophy encountered with HCM, these assumptions are often incorrect and lead to erroneous mass values.14 Importantly, the interstudy reproducibility of left ventricular mass measurement with MRI is less than 5%,18 so MRI is the procedure of choice for monitoring left ventricular mass over time and in response to therapy.
Cardiac Neoplasms
Focal hypertrophic cardiomyopathy can be difficult to distinguish from an intramural cardiac mass on echocardiography. MRI can be used to differentiate HCM from a neoplasm.10,19 Images obtained using MR tagging can identify active myocardial contraction; whereas focal HCM may exhibit decreased systolic thickening or abnormal wall motion, the absence of identifiable contraction suggests a neoplasm.19 Gadolinium-enhanced MRI can also distinguish a cardiac mass from HCM (Fig. 64-8).10
Cardiac Function
MRI permits the quantitative analysis of cardiac function in patients with HCM. Compared with echocardiography, MRI assessment of cardiac function has the advantages of high reproducibility and operator independence. MRI is therefore ideal for patient monitoring. Stroke volume, ejection fraction, and cardiac output may all be calculated (Fig. 64-9). Although often clinically insignificant, mild diastolic dysfunction is the most common functional abnormality seen in HCM, often with an increased ejection fraction in the setting of decreased stroke volume and cardiac output.1 Patients with end-stage HCM may exhibit systolic dysfunction caused by progressive myocardial fibrosis, which can cause ventricular wall thinning and dilation.2
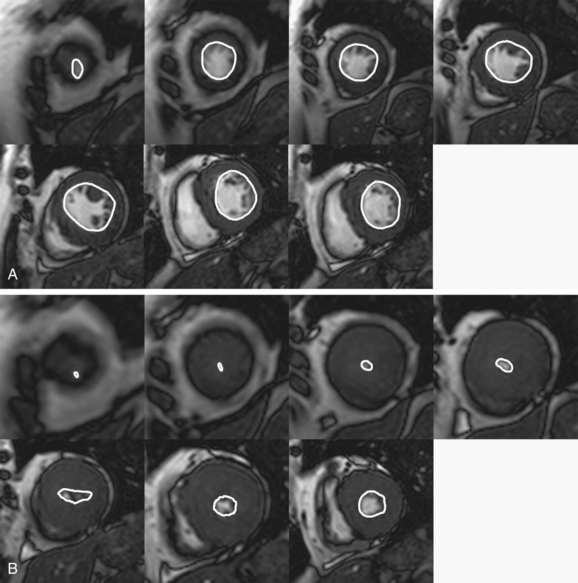
 FIGURE 64-9 Calculation of ejection fraction, stroke volume, and cardiac output. Shown are end-diastolic (A) and end-systolic (B) images from the cardiac apex to the base. These are cine MR images obtained in the short-axis plane. The endocardium is outlined on each image. The area that is outlined is multiplied by the slice thickness and summated across all the slice locations; the sum, from apex to base, yields the total ventricle chamber volume at end-systole and end-diastole. From these values, one can determine the stroke volume (end-diastolic volume minus end-systolic volume), ejection fraction (stroke volume divided by end-diastolic volume), and cardiac output (stroke volume multiplied by heart rate). In this patient, end-diastolic volume = 89 mL and end-systolic volume = 13 mL, stroke volume = 76 mL, ejection fraction = 86%, and cardiac output = 4.6 L/min. Normal ejection fraction for men = 69% and for women = 70%.29
FIGURE 64-9 Calculation of ejection fraction, stroke volume, and cardiac output. Shown are end-diastolic (A) and end-systolic (B) images from the cardiac apex to the base. These are cine MR images obtained in the short-axis plane. The endocardium is outlined on each image. The area that is outlined is multiplied by the slice thickness and summated across all the slice locations; the sum, from apex to base, yields the total ventricle chamber volume at end-systole and end-diastole. From these values, one can determine the stroke volume (end-diastolic volume minus end-systolic volume), ejection fraction (stroke volume divided by end-diastolic volume), and cardiac output (stroke volume multiplied by heart rate). In this patient, end-diastolic volume = 89 mL and end-systolic volume = 13 mL, stroke volume = 76 mL, ejection fraction = 86%, and cardiac output = 4.6 L/min. Normal ejection fraction for men = 69% and for women = 70%.29
Abnormal wall motion may be noted in some patients with HCM. MRI scans obtained using myocardial tagging can demonstrate reduced wall motion in areas of hypertrophy and impaired systolic thickening.20 Cine MR images can also be used to show impaired systolic thickening (Fig. 64-10).21
Myocardial Perfusion
MRI can be used to demonstrate impaired myocardial perfusion and perfusion reserve in patients with HCM.13 First-pass gadolinium MRI allows the direct visualization of impaired perfusion. First-pass images both before and after administration of coronary vasodilator agent are analyzed to determine perfusion reserve. First-pass gadolinium imaging has correlated decreased perfusion reserve to both the site and extent of ventricular hypertrophy.13
Viability
Delayed hyperenhancement MRI can be used to differentiate viable myocardial tissue from areas of fibrosis that presumably result from previous ischemia and infarction.14 Because gadolinium chelate contrast agent cannot cross cell membranes, it accumulates in regions of fibrosis because of slow distribution kinetics and expansion of extracellular space.22,23 MRI performed 10 to 15 minutes following contrast agent administration may demonstrate this accumulation of gadolinium–diethylenetriamine penta-acetic acid (Gd-DTPA; Fig. 64-11). In HCM, delayed hyperenhancement has been shown in the most hypertrophied areas.14 Areas of delayed hyperenhancement exhibit reduced systolic wall thickening, an indicator of decreased myocardial function.24 The presence and severity of delayed hyperenhancement in HCM may be related to a worse prognosis.
Coronary Flow Reserve
Abnormal coronary vasculature and a mismatch between myocardial mass and coronary circulation are thought to be responsible for impaired coronary flow reserve and intermittent myocardial ischemia.1 The result of these processes is myocyte death, with subsequent fibrosis. The areas of scarring in the setting of acute ischemia, hypotension, or intense physical exertion may be the arrythmogenic substrate responsible for sudden cardiac death by ventricular tachycardia or ventricular fibrillation.1
Quantification of coronary flow reserve is possible with MRI.24 Velocity-encoded cine phase contrast MRI through the coronary sinus permits measurement of coronary flow. Patients with HCM have impairment of coronary flow reserve.24
Left Ventricular Outflow Tract Obstruction
In patients with asymmetric septal HCM, left ventricular outflow tract obstruction can result from severe septal hypertrophy or systolic anterior motion of the anterior mitral leaflet. Patients with left ventricular outflow tract obstruction may suffer from angina, dyspnea, or syncope, even on minimal exertion. Identification of left ventricular outflow tract obstruction is an important clinical goal, because patients may benefit from invasive therapy such as septal myectomy or percutaneous septal wall ablation. The severity of outflow tract obstruction is reflected in the pressure gradient across the outflow tract. Surgical or transcathether septal myomectomy is often advocated when the pressure gradient is more than 30 mm Hg.6
Because of its high reproducibility and interobserver agreement, MRI is superior to echocardiography for patient monitoring in left ventricular outflow tract obstruction. This is especially true when the feasibility and subsequent efficacy of medical or surgical management must be evaluated. MRI can identify left ventricular outflow tract obstruction in a number of ways. Obstruction is frequently visualized on steady-state free precession cine MR images as a signal void, representing a high-velocity jet in the outflow tract (Fig. 64-12). MRI can directly depict the outflow tract region, and decreased outflow tract area in patients can be quantified.12
Systolic anterior motion of the anterior mitral valve leaflet is the most common cause of left ventricular outflow tract obstruction in patients with septal HCM; it also leads to mitral regurgitation. Cine MR images in the horizontal long-axis plane can depict the anterior mitral leaflet adjacent to or near the septum in systole.21 Mitral regurgitation is visualized with MRI as a systolic signal void emanating from the mitral valve into the left atrium (Fig. 64-13).17 MRI can be used for qualitative or quantitative characterization of the severity of regurgitation.25
Therapy
Invasive therapy for left ventricular outflow tract obstruction is considered when medical management has failed to control symptoms in patients with severe heart failure (NYHA Class III or IV) and a significant outflow tract pressure gradient.26 Current options include surgical myotomy or myectomy of the septal wall and alcohol septal myocardial ablation.1,26
Surgical myotomy or myectomy is a proven technique, with subjective improvement of symptoms in 70% of patients and a mortality of less than 2%. However, there are few centers skilled in these techniques, and many patients are not candidates for surgery given the advanced nature of their disease.1 Following surgical myotomy or myectomy, MRI can verify improvement of outflow tract obstruction by demonstrating reduction of the signal void in the outflow tract and decrease of the systolic anterior motion of the mitral valve. Moreover, left ventricular function and mass can be measured and compared with presurgical values.
Alcohol septal myocardial ablation is a technique whereby a septal perforator branch of the left anterior descending artery is selectively catheterized and injected with ethanol, causing limited septal infarction.27 Preliminary studies have shown that this technique has been successful, as judged by a reduction in the pressure gradient across the outflow tract and by symptomatic improvement.28 MRI offers a useful method to evaluate patients selected for alcohol septal myocardial ablation (Fig. 64-14). A perfusion defect and delayed hyperenhancement are noted after treatment, clearly demonstrating the location and extent of infarcted myocardium. Hypokinesis of the septal wall is readily displayed on cine MR images following intervention.
Angiography
Cardiac catheterization is also performed in cases of HCM, most often to address a particular clinical concern. Catheterization may be performed to determine the magnitude of outflow tract obstruction caused by septal hypertrophy by measuring pressures proximal and distal to the obstruction.12 Also, catheterization is performed when invasive therapy, such as myectomy or percutaneous transluminal septal wall ablation, is being considered.13 Angiographic ventriculograms may reveal a characteristic deformity of the left ventricle at end-systole in some forms of HCM, such as the apical form.1
SYNOPSIS OF TREATMENT OPTIONS
Surgical and Interventional
In patients with hypertrophic cardiomyopathy, surgical treatment via septal myectomy has traditionally been used.5 Percutaneous transluminal septal myocardial ablation with ethanol has shown promising success in the treatment of significant outflow obstruction.9,10
Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. AJR Am J Roentgenol. 2007;189:1335-1343.
Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part 2, Differential diagnosis, risk stratification, and posttreatment MRI appearances. AJR Am J Roentgenol. 2007;189:1344-1352.
1 Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308-1320.
2 Wigle ED. Cardiomyopathy: the diagnosis of hypertrophic cardiomyopathy. Heart. 2001;86:709-714.
3 Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075.
4 McKenna WJ, Behr ER. Hypertrophic cardiomyopathy: management, risk stratification, and prevention of sudden death. Heart. 2002;87:169-176.
5 Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778-1785.
6 Elliott PM, Gimeno Blanes JR, Mahon NG, et al. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420-424.
7 Tam JW, Shaikh N, Sutherland E. Echocardiographic assessment of patients with hypertrophic and restrictive cardiomyopathy: imaging and echocardiography. Curr Opin Cardiol. 2002;17:470-477.
8 Funabashi N, Yoshida K, Komuro I. Thinned myocardial fibrosis with thrombus in the dilated form of hypertrophic cardiomyopathy demonstrated by multislice computed tomography. Heart. 2003;89:858.
9 Higgins CB, Byrd BF3rd, Stark D, et al. Magnetic resonance imaging in hypertrophic cardiomyopathy. Am J Cardiol. 1985;55:1121-1126.
10 Funari M, Fujita N, Peck WW, Higgins CB. Cardiac tumors: assessment with Gd-DTPA enhanced MRI. J Comput Assist Tomogr. 1991;15:953-958.
11 Park JH, Kim YM. MRI of cardiomyopathy. Magn Reson Imaging Clin North Am. 1996;4:269-286.
12 Schulz-Menger J, Strohm O, Waigand J, et al. The value of magnetic resonance imaging of the left ventricular outflow tract in patients with hypertrophic obstructive cardiomyopathy after septal artery embolization. Circulation. 2000;101:1764-1766.
13 Sipola P, Lauerma K, Husso-Saastamoinen M, et al. First-pass MRI in the assessment of perfusion impairment in patients with hypertrophic cardiomyopathy and the Asp175Asn mutation of the alpha-tropomyosin gene. Radiology. 2003;226:129-137.
14 Bogaert J, Goldstein M, Tannouri F, et al. Late myocardial enhancement in hypertrophic cardiomyopathy with contrast-enhanced MRI. AJR Am J Roentgenol. 2003;180:981-985.
15 Devlin AM, Moore NR, Ostman-Smith I. A comparison of MRI and echocardiography in hypertrophic cardiomyopathy. Br J Radiol. 1999;72:258-264.
16 Soler R, Rodriguez E, Rodriguez JA, et al. Magnetic resonance imaging of apical hypertrophic cardiomyopathy. J Thorac Imaging. 1997;12:221-225.
17 Katz J, Milliken MC, Stray-Gundersen J, et al. Estimation of human myocardial mass with MRI. Radiology. 1988;169:495-498.
18 Semelka RC, Tomei E, Wagner S, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367-1373.
19 Bergey PD, Axel L. Focal hypertrophic cardiomyopathy simulating a mass: MR tagging for correct diagnosis. AJR Am J Roentgenol. 2000;174:242-244.
20 Dong SJ, MacGregor JH, Crawley AP, et al. Left ventricular wall thickness and regional systolic function in patients with hypertrophic cardiomyopathy. A three-dimensional tagged magnetic resonance imaging study. Circulation. 1994;90:1200-1209.
21 Arrive L, Assayag P, Russ G, et al. MRI and cine MRI of asymmetric septal hypertrophic cardiomyopathy. J Comput Assist Tomogr. 1994;18:376-382.
22 Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318-3326.
23 Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703-710.
24 Kawada N, Sakuma H, Yamakado T, et al. Hypertrophic cardiomyopathy: MR measurement of coronary blood flow and vasodilator flow reserve in patients and healthy subjects. Radiology. 1999;211:129-135.
25 Didier D, Ratib O, Lerch R, Friedli B. Detection and quantification of valvular heart disease with dynamic cardiac MRI. Radiographics. 2000;20:1279-1299.
26 Braunwald E, Seidman CE, Sigwart U. Contemporary evaluation and management of hypertrophic cardiomyopathy. Circulation. 2002;106:1312-1316.
27 Seggewiss H, Gleichmann U, Faber L, et al. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998;31:252-258.
28 Faber L, Meissner A, Ziemssen P, Seggewiss H. Percutaneous transluminal septal myocardial ablation for hypertrophic obstructive cardiomyopathy: long-term follow-up of the first series of 25 patients. Heart. 2000;83:326-331.
29 Marcus JT, De Waal LK, Gotte MJ, et al. MRI-derived left ventricular function parameters and mass in healthy young adults: relation with gender and body size. Int J Cardiac Imaging. 1999;15:411-419.

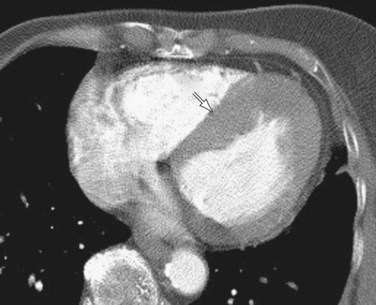
 FIGURE 64-1
FIGURE 64-1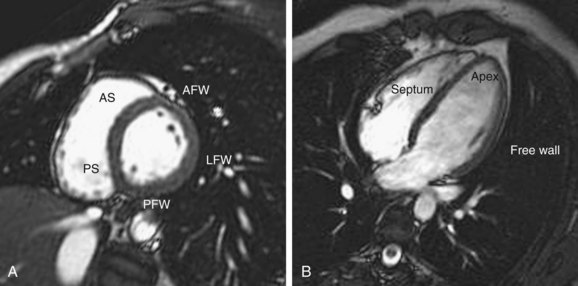
 FIGURE 64-2
FIGURE 64-2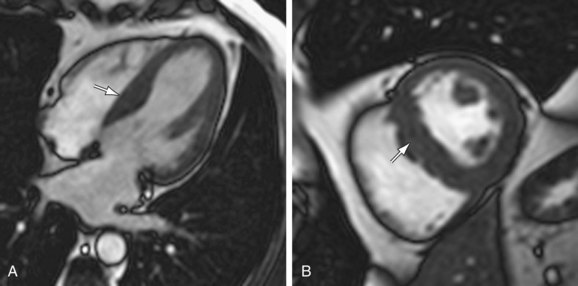
 FIGURE 64-3
FIGURE 64-3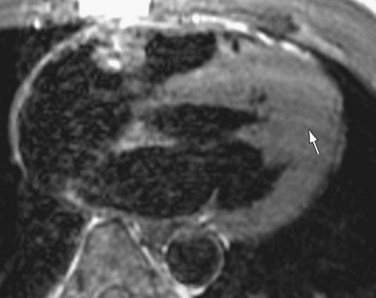
 FIGURE 64-4
FIGURE 64-4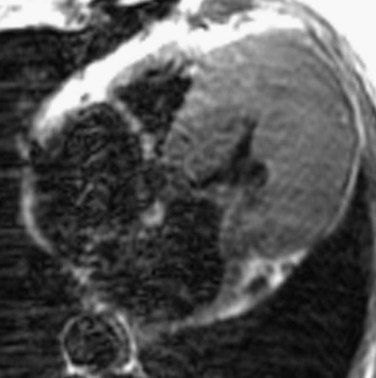
 FIGURE 64-5
FIGURE 64-5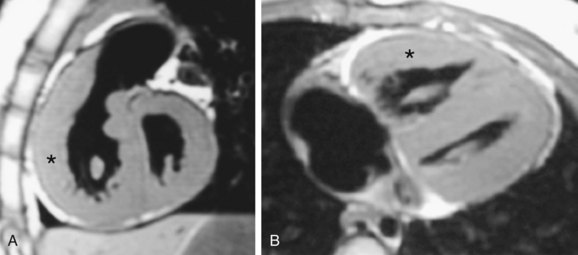
 FIGURE 64-6
FIGURE 64-6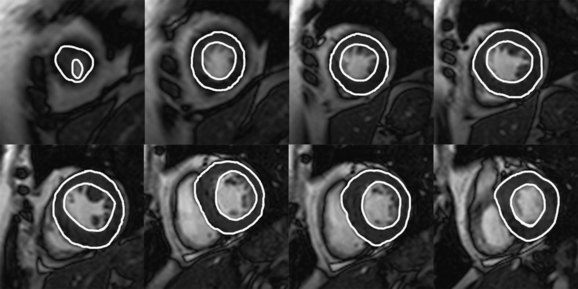
 FIGURE 64-7
FIGURE 64-7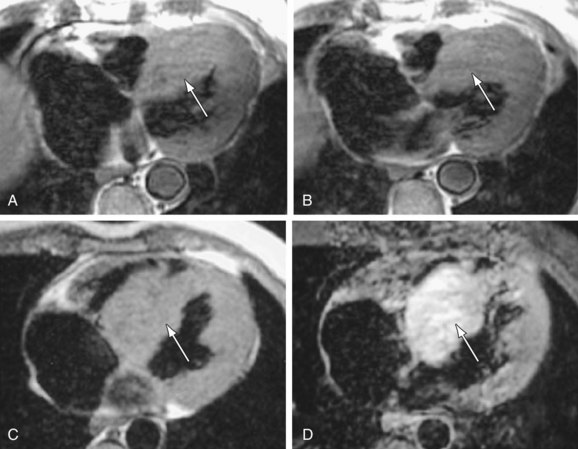
 FIGURE 64-8
FIGURE 64-8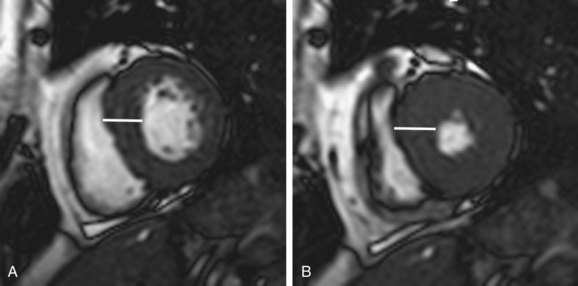
 FIGURE 64-10
FIGURE 64-10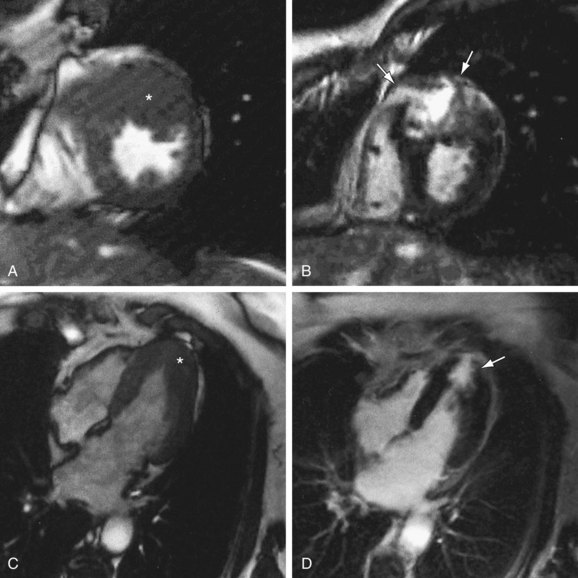
 FIGURE 64-11
FIGURE 64-11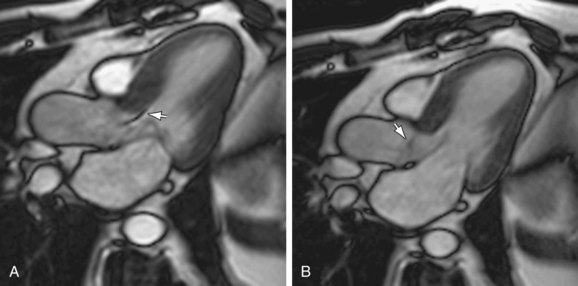
 FIGURE 64-12
FIGURE 64-12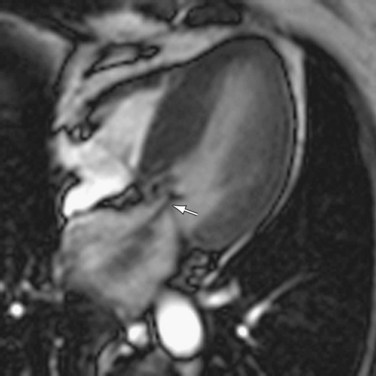
 FIGURE 64-13
FIGURE 64-13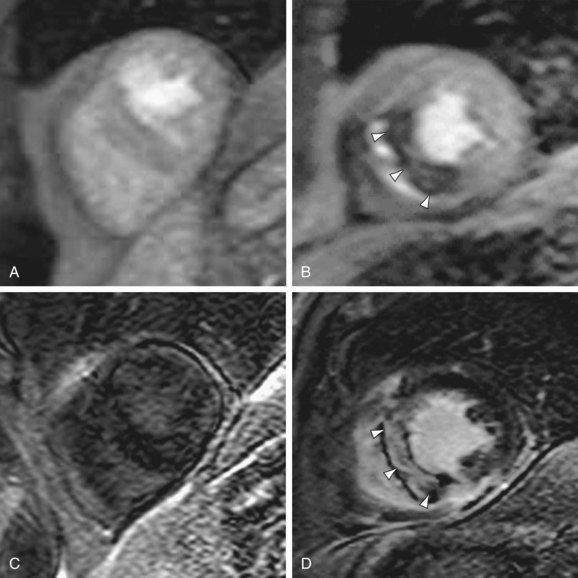
 FIGURE 64-14
FIGURE 64-14



