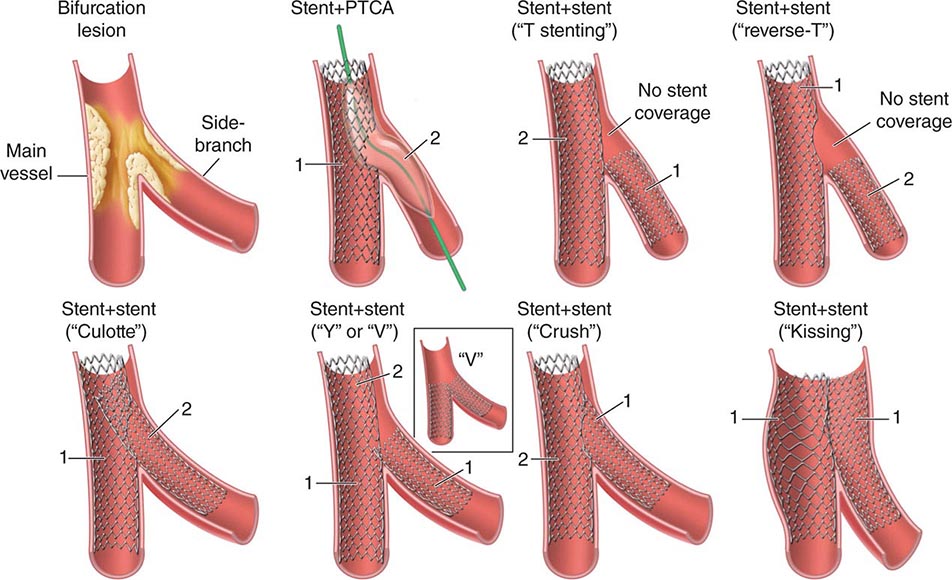
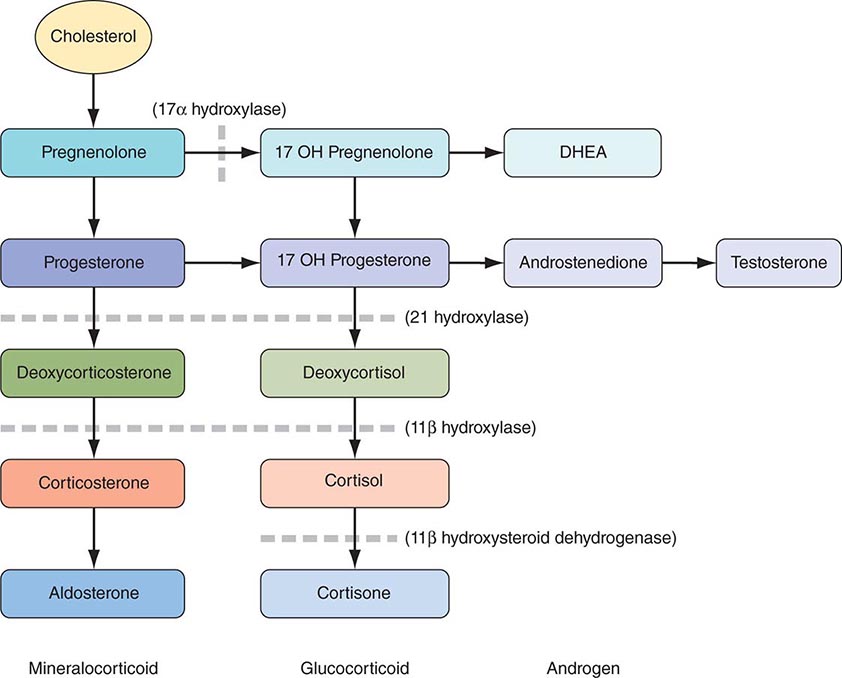
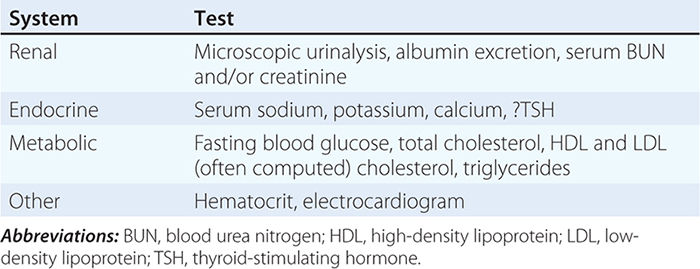
Criteria for Acute Myocardial Infarction
The term acute myocardial infarction (MI) should be used when there is evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. Under these conditions, any one of the following criteria meets the diagnosis for MI:
• Detection of a rise and/or fall of cardiac biomarker values (preferably cardiac troponin [cTn]) with at least one value above the 99th percentile upper reference limit (URL) and with at least one of the following:
• Symptoms of ischemia
• New or presumed new significant ST-segment T-wave (ST-T) changes or new left bundle branch block (LBBB)
• Development of pathologic Q waves in the electrocardiogram (ECG)
• Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
• Identification of an intracoronary thrombus by angiography or autopsy
• Cardiac death with symptoms suggestive of myocardial ischemia and presumed new ischemic ECG changes of new LBBB, but death occurred before cardiac biomarkers were obtained or before cardiac biomarker values would be increased.
• Percutaneous coronary intervention (PCI)–related MI is arbitrarily defined by elevation of cTn values (>5 × 99th percentile URL) in patients with normal baseline values (≤99th percentile URL) or a rise of cTn values >20% if the baseline values are elevated and are stable or falling. In addition, either (i) symptoms suggestive of myocardial ischemia, or (ii) new ischemic ECG changes, or (iii) angiographic findings consistent with a procedural complication, or (iv) imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality are required.
• Stent thrombosis associated with MI when detected by coronary angiography or autopsy in the setting of myocardial ischemia and with a rise and/or fall of cardiac biomarker values with at least one value above the 99th percentile URL.
• Coronary artery bypass grafting (CABG)–related MI is arbitrarily defined by elevation of cardiac biomarker values (>10 × 99th percentile URL) in patients with normal baseline cTn values (≤99th percentile URL). In addition, either (i) new pathologic Q waves or new LBBB, or (ii) angiographic documented new graft or new native coronary artery occlusion, or (iii) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.
Source: K Thygesen: Eur Heart J 33:2551, 2012.
|
CLASSIFICATION OF MYOCARDIAL INFARCTION |
Source: K Thygesen: Eur Heart J 33:2551, 2012.
INITIAL MANAGEMENT
PREHOSPITAL CARE
The prognosis in STEMI is largely related to the occurrence of two general classes of complications: (1) electrical complications (arrhythmias) and (2) mechanical complications (“pump failure”). Most out-of-hospital deaths from STEMI are due to the sudden development of ventricular fibrillation. The vast majority of deaths due to ventricular fibrillation occur within the first 24 h of the onset of symptoms, and of these, over half occur in the first hour. Therefore, the major elements of prehospital care of patients with suspected STEMI include (1) recognition of symptoms by the patient and prompt seeking of medical attention; (2) rapid deployment of an emergency medical team capable of performing resuscitative maneuvers, including defibrillation; (3) expeditious transportation of the patient to a hospital facility that is continuously staffed by physicians and nurses skilled in managing arrhythmias and providing advanced cardiac life support; and (4) expeditious implementation of reperfusion therapy (Fig. 295-3). The greatest delay usually occurs not during transportation to the hospital but, rather, between the onset of pain and the patient’s decision to call for help. This delay can best be reduced by health care professionals educating the public concerning the significance of chest discomfort and the importance of seeking early medical attention. Regular office visits with patients having a history of or who are at risk for ischemic heart disease are important “teachable moments” for clinicians to review the symptoms of STEMI and the appropriate action plan.
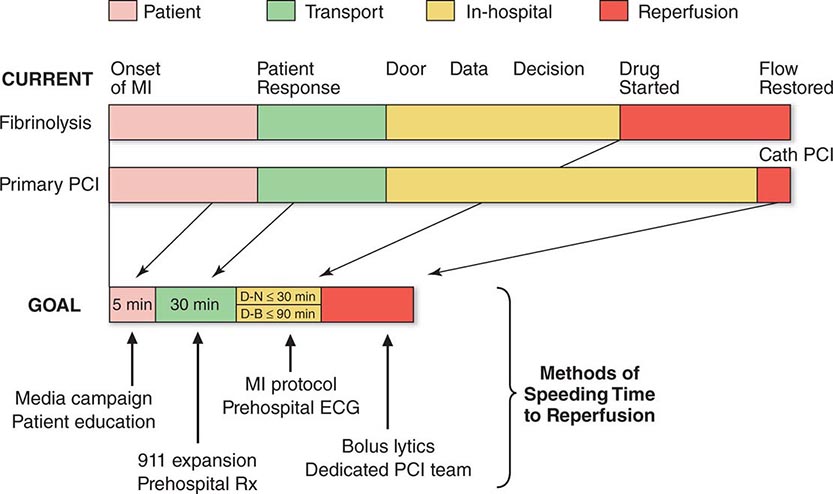
FIGURE 295-3 Major components of time delay between onset of symptoms from ST-segment elevation myocardial infarction and restoration of flow in the infarct-related artery. Plotted sequentially from left to right are the times for patients to recognize symptoms and seek medical attention, transportation to the hospital, in-hospital decision making, implementation of reperfusion strategy, and restoration of flow once the reperfusion strategy has been initiated. The time to initiate fibrinolytic therapy is the “door-to-needle” (D-N) time; this is followed by the period of time required for pharmacologic restoration of flow. More time is required to move the patient to the catheterization laboratory for a percutaneous coronary interventional (PCI) procedure, referred to as the “door-to-balloon” (D-B) time, but restoration of flow in the epicardial infarct–related artery occurs promptly after PCI. At the bottom is a variety of methods for speeding the time to reperfusion along with the goals for the time intervals for the various components of the time delay. (Adapted from CP Cannon et al: J Thromb Thrombol 1:27, 1994.)
Increasingly, monitoring and treatment are carried out by trained personnel in the ambulance, further shortening the time between the onset of the infarction and appropriate treatment. General guidelines for initiation of fibrinolysis in the prehospital setting include the ability to transmit 12-lead ECGs to confirm the diagnosis, the presence of paramedics in the ambulance, training of paramedics in the interpretation of ECGs and management of STEMI, and online medical command and control that can authorize the initiation of treatment in the field.
MANAGEMENT IN THE EMERGENCY DEPARTMENT
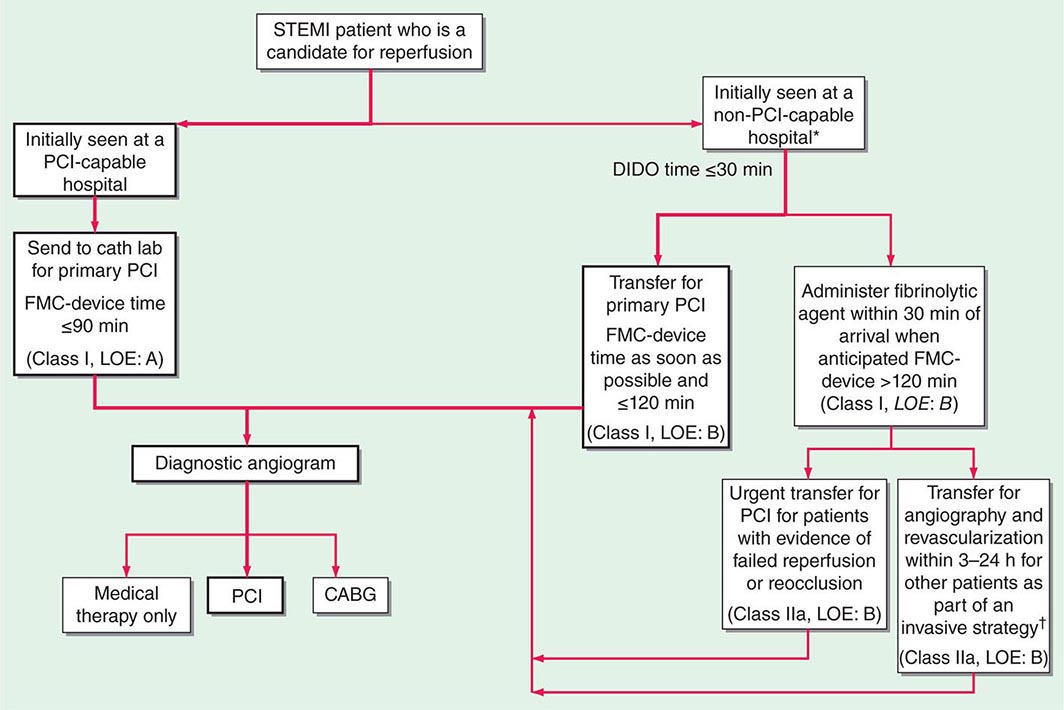
In the Emergency Department, the goals for the management of patients with suspected STEMI include control of cardiac discomfort, rapid identification of patients who are candidates for urgent reperfusion therapy, triage of lower-risk patients to the appropriate location in the hospital, and avoidance of inappropriate discharge of patients with STEMI. Many aspects of the treatment of STEMI are initiated in the Emergency Department and then continued during the in-hospital phase of management (Fig. 295-4). The overarching goal is to minimize the time from first medical contact to initiation of reperfusion therapy. This may involve transfer from a non-PCI hospital to one that is PCI capable, with a goal of initiating PCI within 120 min of first medical contact (Fig. 295-4).
FIGURE 295-4 Reperfusion therapy for patients with ST-segment elevation myocardial infarction (STEMI). The bold arrows and boxes are the preferred strategies. Performance of percutaneous coronary intervention (PCI) is dictated by an anatomically appropriate culprit stenosis. *Patients with cardiogenic shock or severe heart failure initially seen at a non–PCI-capable hospital should be transferred for cardiac catheterization and revascularization as soon as possible, irrespective of time delay from myocardial infarction (MI) onset (Class I, LOE: B). †Angiography and revascularization should not be performed within the first 2 to 3 hours after administration of fibrinolytic therapy. CABG, coronary artery bypass graft; DIDO, door-in–door-out; FMC, first medical contact; LOE, level of evidence; STEMI, ST-elevation myocardial infarction. (Adapted with permission from P O’Gara et al: Circulation 127:e362, 2013.)
Aspirin is essential in the management of patients with suspected STEMI and is effective across the entire spectrum of acute coronary syndromes (Fig. 295-1). Rapid inhibition of cyclooxygenase-1 in platelets followed by a reduction of thromboxane A2 levels is achieved by buccal absorption of a chewed 160–325-mg tablet in the Emergency Department. This measure should be followed by daily oral administration of aspirin in a dose of 75–162 mg.
In patients whose arterial O2 saturation is normal, supplemental O2 is of limited if any clinical benefit and therefore is not cost-effective. However, when hypoxemia is present, O2 should be administered by nasal prongs or face mask (2–4 L/min) for the first 6–12 h after infarction; the patient should then be reassessed to determine if there is a continued need for such treatment.
CONTROL OF DISCOMFORT
Sublingual nitroglycerin can be given safely to most patients with STEMI. Up to three doses of 0.4 mg should be administered at about 5-min intervals. In addition to diminishing or abolishing chest discomfort, nitroglycerin may be capable of both decreasing myocardial oxygen demand (by lowering preload) and increasing myocardial oxygen supply (by dilating infarct-related coronary vessels or collateral vessels). In patients whose initially favorable response to sublingual nitroglycerin is followed by the return of chest discomfort, particularly if accompanied by other evidence of ongoing ischemia such as further ST-segment or T-wave shifts, the use of intravenous nitroglycerin should be considered. Therapy with nitrates should be avoided in patients who present with low systolic arterial pressure (<90 mmHg) or in whom there is clinical suspicion of RV infarction (inferior infarction on ECG, elevated jugular venous pressure, clear lungs, and hypotension). Nitrates should not be administered to patients who have taken a phosphodiesterase-5 inhibitor for erectile dysfunction within the preceding 24 h, because it may potentiate the hypotensive effects of nitrates. An idiosyncratic reaction to nitrates, consisting of sudden marked hypotension, sometimes occurs but can usually be reversed promptly by the rapid administration of intravenous atropine.
Morphine is a very effective analgesic for the pain associated with STEMI. However, it may reduce sympathetically mediated arteriolar and venous constriction, and the resulting venous pooling may reduce cardiac output and arterial pressure. These hemodynamic disturbances usually respond promptly to elevation of the legs, but in some patients, volume expansion with intravenous saline is required. The patient may experience diaphoresis and nausea, but these events usually pass and are replaced by a feeling of well-being associated with the relief of pain. Morphine also has a vagotonic effect and may cause bradycardia or advanced degrees of heart block, particularly in patients with inferior infarction. These side effects usually respond to atropine (0.5 mg intravenously). Morphine is routinely administered by repetitive (every 5 min) intravenous injection of small doses (2–4 mg), rather than by the subcutaneous administration of a larger quantity, because absorption may be unpredictable by the latter route.
Intravenous beta blockers are also useful in the control of the pain of STEMI. These drugs control pain effectively in some patients, presumably by diminishing myocardial O2 demand and hence ischemia. More important, there is evidence that intravenous beta blockers reduce the risks of reinfarction and ventricular fibrillation (see “Beta-Adrenoceptor Blockers” below). However, patient selection is important when considering beta blockers for STEMI. Oral beta blocker therapy should be initiated in the first 24 h for patients who do not have any of the following: (1) signs of heart failure, (2) evidence of a low-output state, (3) increased risk for cardiogenic shock, or (4) other relative contraindications to beta blockade (PR interval greater than 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease). A commonly employed regimen is metoprolol, 5 mg every 2–5 min for a total of three doses, provided the patient has a heart rate >60 beats/min, systolic pressure >100 mmHg, a PR interval <0.24 s, and rales that are no higher than 10 cm up from the diaphragm. Fifteen minutes after the last intravenous dose, an oral regimen is initiated of 50 mg every 6 h for 48 h, followed by 100 mg every 12 h.
Unlike beta blockers, calcium antagonists are of little value in the acute setting, and there is evidence that short-acting dihydropyridines may be associated with an increased mortality risk.
MANAGEMENT STRATEGIES
The primary tool for screening patients and making triage decisions is the initial 12-lead ECG. When ST-segment elevation of at least 2 mm in two contiguous precordial leads and 1 mm in two adjacent limb leads is present, a patient should be considered a candidate for reperfusion therapy (Fig. 295-4). The process of selecting patients for fibrinolysis versus primary PCI (angioplasty or stenting; Chap. 296e) is discussed below. In the absence of ST-segment elevation, fibrinolysis is not helpful, and evidence exists suggesting that it may be harmful.
LIMITATION OF INFARCT SIZE
The quantity of myocardium that becomes necrotic as a consequence of a coronary artery occlusion is determined by factors other than just the site of occlusion. While the central zone of the infarct contains necrotic tissue that is irretrievably lost, the fate of the surrounding ischemic myocardium (ischemic penumbra) may be improved by timely restoration of coronary perfusion, reduction of myocardial O2 demands, prevention of the accumulation of noxious metabolites, and blunting of the impact of mediators of reperfusion injury (e.g., calcium overload and oxygen-derived free radicals). Up to one-third of patients with STEMI may achieve spontaneous reperfusion of the infarct-related coronary artery within 24 h and experience improved healing of infarcted tissue. Reperfusion, either pharmacologically (by fibrinolysis) or by PCI, accelerates the opening of infarct-related arteries in those patients in whom spontaneous fibrinolysis ultimately would have occurred and also greatly increases the number of patients in whom restoration of flow in the infarct-related artery is accomplished. Timely restoration of flow in the epicardial infarct–related artery combined with improved perfusion of the downstream zone of infarcted myocardium results in a limitation of infarct size. Protection of the ischemic myocardium by the maintenance of an optimal balance between myocardial O2 supply and demand through pain control, treatment of congestive heart failure (CHF), and minimization of tachycardia and hypertension extends the “window” of time for the salvage of myocardium by reperfusion strategies.
Glucocorticoids and nonsteroidal anti-inflammatory agents, with the exception of aspirin, should be avoided in patients with STEMI. They can impair infarct healing and increase the risk of myocardial rupture, and their use may result in a larger infarct scar. In addition, they can increase coronary vascular resistance, thereby potentially reducing flow to ischemic myocardium.
PRIMARY PERCUTANEOUS CORONARY INTERVENTION
(See also Chap. 296e) PCI, usually angioplasty and/or stenting without preceding fibrinolysis, referred to as primary PCI, is effective in restoring perfusion in STEMI when carried out on an emergency basis in the first few hours of MI. It has the advantage of being applicable to patients who have contraindications to fibrinolytic therapy (see below) but otherwise are considered appropriate candidates for reperfusion. It appears to be more effective than fibrinolysis in opening occluded coronary arteries and, when performed by experienced operators in dedicated medical centers, is associated with better short-term and long-term clinical outcomes. Compared with fibrinolysis, primary PCI is generally preferred when the diagnosis is in doubt, cardiogenic shock is present, bleeding risk is increased, or symptoms have been present for at least 2–3 h when the clot is more mature and less easily lysed by fibrinolytic drugs. However, PCI is expensive in terms of personnel and facilities, and its applicability is limited by its availability, around the clock, in only a minority of hospitals (Fig. 295-4).
FIBRINOLYSIS
If no contraindications are present (see below), fibrinolytic therapy should ideally be initiated within 30 min of presentation (i.e., door-to-needle time ≤30 min). The principal goal of fibrinolysis is prompt restoration of full coronary arterial patency. The fibrinolytic agents tissue plasminogen activator (tPA), streptokinase, tenecteplase (TNK), and reteplase (rPA) have been approved by the U.S. Food and Drug Administration for intravenous use in patients with STEMI. These drugs all act by promoting the conversion of plasminogen to plasmin, which subsequently lyses fibrin thrombi. Although considerable emphasis was first placed on a distinction between more fibrin-specific agents, such as tPA, and non-fibrin-specific agents, such as streptokinase, it is now recognized that these differences are only relative, as some degree of systemic fibrinolysis occurs with the former agents. TNK and rPA are referred to as bolus fibrinolytics since their administration does not require a prolonged intravenous infusion.
When assessed angiographically, flow in the culprit coronary artery is described by a simple qualitative scale called the Thrombolysis in Myocardial Infarction (TIMI) grading system: grade 0 indicates complete occlusion of the infarct-related artery; grade 1 indicates some penetration of the contrast material beyond the point of obstruction but without perfusion of the distal coronary bed; grade 2 indicates perfusion of the entire infarct vessel into the distal bed, but with flow that is delayed compared with that of a normal artery; and grade 3 indicates full perfusion of the infarct vessel with normal flow. The latter is the goal of reperfusion therapy, because full perfusion of the infarct-related coronary artery yields far better results in terms of limiting infarct size, maintenance of LV function, and reduction of both short- and long-term mortality rates. Additional methods of angiographic assessment of the efficacy of fibrinolysis include counting the number of frames on the cine film required for dye to flow from the origin of the infarct-related artery to a landmark in the distal vascular bed (TIMI frame count) and determining the rate of entry and exit of contrast dye from the microvasculature in the myocardial infarct zone (TIMI myocardial perfusion grade). These methods have an even tighter correlation with outcomes after STEMI than the more commonly employed TIMI flow grade.
tPA and the other relatively fibrin-specific plasminogen activators, rPA and TNK, are more effective than streptokinase at restoring full perfusion—i.e., TIMI grade 3 coronary flow—and have a small edge in improving survival as well. The current recommended regimen of tPA consists of a 15-mg bolus followed by 50 mg intravenously over the first 30 min, followed by 35 mg over the next 60 min. Streptokinase is administered as 1.5 million units (MU) intravenously over 1 h. rPA is administered in a double-bolus regimen consisting of a 10-MU bolus given over 2–3 min, followed by a second 10-MU bolus 30 min later. TNK is given as a single weight-based intravenous bolus of 0.53 mg/kg over 10 s. In addition to the fibrinolytic agents discussed earlier, pharmacologic reperfusion typically involves adjunctive antiplatelet and antithrombotic drugs, as discussed subsequently.
Clear contraindications to the use of fibrinolytic agents include a history of cerebrovascular hemorrhage at any time, a nonhemorrhagic stroke or other cerebrovascular event within the past year, marked hypertension (a reliably determined systolic arterial pressure >180 mmHg and/or a diastolic pressure >110 mmHg) at any time during the acute presentation, suspicion of aortic dissection, and active internal bleeding (excluding menses). While advanced age is associated with an increase in hemorrhagic complications, the benefit of fibrinolytic therapy in the elderly appears to justify its use if no other contraindications are present and the amount of myocardium in jeopardy appears to be substantial.
Relative contraindications to fibrinolytic therapy, which require assessment of the risk-to-benefit ratio, include current use of anticoagulants (international normalized ratio ≥2), a recent (<2 weeks) invasive or surgical procedure or prolonged (>10 min) cardiopulmonary resuscitation, known bleeding diathesis, pregnancy, a hemorrhagic ophthalmic condition (e.g., hemorrhagic diabetic retinopathy), active peptic ulcer disease, and a history of severe hypertension that is currently adequately controlled. Because of the risk of an allergic reaction, patients should not receive streptokinase if that agent had been received within the preceding 5 days to 2 years.
Allergic reactions to streptokinase occur in ∼2% of patients who receive it. While a minor degree of hypotension occurs in 4–10% of patients given this agent, marked hypotension occurs, although rarely, in association with severe allergic reactions.
Hemorrhage is the most frequent and potentially the most serious complication. Because bleeding episodes that require transfusion are more common when patients require invasive procedures, unnecessary venous or arterial interventions should be avoided in patients receiving fibrinolytic agents. Hemorrhagic stroke is the most serious complication and occurs in ∼0.5–0.9% of patients being treated with these agents. This rate increases with advancing age, with patients >70 years experiencing roughly twice the rate of intracranial hemorrhage as those <65 years. Large-scale trials have suggested that the rate of intracranial hemorrhage with tPA or rPA is slightly higher than with streptokinase.
INTEGRATED REPERFUSION STRATEGY
Evidence has emerged that suggests PCI plays an increasingly important role in the management of STEMI. Prior approaches that segregated the pharmacologic and catheter-based approaches to reperfusion have now been replaced with an integrated approach to triage and transfer of STEMI patients to receive PCI (Fig. 295-4). To achieve the degree of integration required to care for a patient with STEMI, all communities should create and maintain a regional system of STEMI care that includes assessment and continuous quality improvement of emergency medical services and hospital-based activities.
Cardiac catheterization and coronary angiography should be carried out after fibrinolytic therapy if there is evidence of either (1) failure of reperfusion (persistent chest pain and ST-segment elevation >90 min), in which case a rescue PCI should be considered; or (2) coronary artery reocclusion (re-elevation of ST segments and/or recurrent chest pain) or the development of recurrent ischemia (such as recurrent angina in the early hospital course or a positive exercise stress test before discharge), in which case an urgent PCI should be considered. Routine angiography and elective PCI even in asymptomatic patients following administration of fibrinolytic therapy are used with less frequency, given the numerous technologic advances that have occurred in the catheterization laboratory and the increasing number of skilled interventionalists. Coronary artery bypass surgery should be reserved for patients whose coronary anatomy is unsuited to PCI but in whom revascularization appears to be advisable because of extensive jeopardized myocardium or recurrent ischemia.
HOSPITAL PHASE MANAGEMENT
CORONARY CARE UNITS
These units are routinely equipped with a system that permits continuous monitoring of the cardiac rhythm of each patient and hemodynamic monitoring in selected patients. Defibrillators, respirators, noninvasive transthoracic pacemakers, and facilities for introducing pacing catheters and flow-directed balloon-tipped catheters are also usually available. Equally important is the organization of a highly trained team of nurses who can recognize arrhythmias; adjust the dosage of antiarrhythmic, vasoactive, and anticoagulant drugs; and perform cardiac resuscitation, including electroshock, when necessary.
Patients should be admitted to a coronary care unit early in their illness when it is expected that they will derive benefit from the sophisticated and expensive care provided. The availability of electrocardiographic monitoring and trained personnel outside the coronary care unit has made it possible to admit lower-risk patients (e.g., those not hemodynamically compromised and without active arrhythmias) to “intermediate care units.”
The duration of stay in the coronary care unit is dictated by the ongoing need for intensive care. If symptoms are controlled with oral therapy, patients may be transferred out of the coronary care unit. Also, patients who have a confirmed STEMI but who are considered to be at low risk (no prior infarction and no persistent chest discomfort, CHF, hypotension, or cardiac arrhythmias) may be safely transferred out of the coronary care unit within 24 h.
Activity Factors that increase the work of the heart during the initial hours of infarction may increase the size of the infarct. Therefore, patients with STEMI should be kept at bed rest for the first 6–12 h. However, in the absence of complications, patients should be encouraged, under supervision, to resume an upright posture by dangling their feet over the side of the bed and sitting in a chair within the first 24 h. This practice is psychologically beneficial and usually results in a reduction in the pulmonary capillary wedge pressure. In the absence of hypotension and other complications, by the second or third day, patients typically are ambulating in their room with increasing duration and frequency, and they may shower or stand at the sink to bathe. By day 3 after infarction, patients should be increasing their ambulation progressively to a goal of 185 m (600 ft) at least three times a day.
Diet Because of the risk of emesis and aspiration soon after STEMI, patients should receive either nothing or only clear liquids by mouth for the first 4–12 h. The typical coronary care unit diet should provide ≤30% of total calories as fat and have a cholesterol content of ≤300 mg/d. Complex carbohydrates should make up 50–55% of total calories. Portions should not be unusually large, and the menu should be enriched with foods that are high in potassium, magnesium, and fiber, but low in sodium. Diabetes mellitus and hypertriglyceridemia are managed by restriction of concentrated sweets in the diet.
Bowel Management Bed rest and the effect of the narcotics used for the relief of pain often lead to constipation. A bedside commode rather than a bedpan, a diet rich in bulk, and the routine use of a stool softener such as dioctyl sodium sulfosuccinate (200 mg/d) are recommended. If the patient remains constipated despite these measures, a laxative can be prescribed. Contrary to prior belief, it is safe to perform a gentle rectal examination on patients with STEMI.
Sedation Many patients require sedation during hospitalization to withstand the period of enforced inactivity with tranquility. Diazepam (5 mg), oxazepam (15–30 mg), or lorazepam (0.5–2 mg), given three to four times daily, is usually effective. An additional dose of any of the above medications may be given at night to ensure adequate sleep. Attention to this problem is especially important during the first few days in the coronary care unit, where the atmosphere of 24-h vigilance may interfere with the patient’s sleep. However, sedation is no substitute for reassuring, quiet surroundings. Many drugs used in the coronary care unit, such as atropine, H2 blockers, and narcotics, can produce delirium, particularly in the elderly. This effect should not be confused with agitation, and it is wise to conduct a thorough review of the patient’s medications before arbitrarily prescribing additional doses of anxiolytics.
PHARMACOTHERAPY
ANTITHROMBOTIC AGENTS
The use of antiplatelet and anticoagulant therapy during the initial phase of STEMI is based on extensive laboratory and clinical evidence that thrombosis plays an important role in the pathogenesis of this condition. The primary goal of treatment with antiplatelet and anticoagulant agents is to maintain patency of the infarct-related artery, in conjunction with reperfusion strategies. A secondary goal is to reduce the patient’s tendency to thrombosis and, thus, the likelihood of mural thrombus formation or deep venous thrombosis, either of which could result in pulmonary embolization. The degree to which antiplatelet and anticoagulant therapy achieves these goals partly determines how effectively it reduces the risk of mortality from STEMI.
As noted previously (see “Management in the Emergency Department” earlier), aspirin is the standard antiplatelet agent for patients with STEMI. The most compelling evidence for the benefits of antiplatelet therapy (mainly with aspirin) in STEMI is found in the comprehensive overview by the Antiplatelet Trialists’ Collaboration. Data from nearly 20,000 patients with MI enrolled in 15 randomized trials were pooled and revealed a relative reduction of 27% in the mortality rate, from 14.2% in control patients to 10.4% in patients receiving antiplatelet agents.
Inhibitors of the P2Y12 ADP receptor prevent activation and aggregation of platelets. The addition of the P2Y12 inhibitor clopidogrel to background treatment with aspirin to STEMI patients reduces the risk of clinical events (death, reinfarction, stroke) and, in patients receiving fibrinolytic therapy, has been shown to prevent reocclusion of a successfully reperfused infarct artery. New P2Y12 ADP receptor antagonists, such as prasugrel and ticagrelor, are more effective than clopidogrel in preventing ischemic complications in STEMI patients undergoing PCI, but are associated with an increased risk of bleeding. Glycoprotein IIb/IIIa receptor inhibitors appear useful for preventing thrombotic complications in patients with STEMI undergoing PCI.
The standard anticoagulant agent used in clinical practice is unfractionated heparin (UFH). The available data suggest that when UFH is added to a regimen of aspirin and a non-fibrin-specific thrombolytic agent such as streptokinase, additional mortality benefit occurs (about 5 lives saved per 1000 patients treated). It appears that the immediate administration of intravenous UFH, in addition to a regimen of aspirin and relatively fibrin-specific fibrinolytic agents (tPA, rPA, or TNK), helps to maintain patency of the infarct-related artery. This effect is achieved at the cost of a small increased risk of bleeding. The recommended dose of UFH is an initial bolus of 60 U/kg (maximum 4000 U) followed by an initial infusion of 12 U/kg per hour (maximum 1000 U/h). The activated partial thromboplastin time during maintenance therapy should be 1.5–2 times the control value.
Alternatives to UFH for anticoagulation of patients with STEMI are the low-molecular-weight heparin (LMWH) preparations, a synthetic version of the critical pentasaccharide sequence (fondaparinux), and the direct antithrombin bivalirudin. Advantages of LMWHs include high bioavailability permitting administration subcutaneously, reliable anticoagulation without monitoring, and greater antiXa:IIa activity. Enoxaparin has been shown to reduce significantly the composite endpoints of death/nonfatal reinfarction and death/nonfatal reinfarction/urgent revascularization compared with UFH in STEMI patients who receive fibrinolysis. Treatment with enoxaparin is associated with higher rates of serious bleeding, but net clinical benefit—a composite endpoint that combines efficacy and safety—still favors enoxaparin over UFH. Interpretation of the data on fondaparinux is difficult because of the complex nature of the pivotal clinical trial evaluating it in STEMI (OASIS-6). Fondaparinux appears superior to placebo in STEMI patients not receiving reperfusion therapy, but its relative efficacy and safety compared with UFH is less certain. Due to the risk of catheter thrombosis, fondaparinux should not be used alone at the time of coronary angiography and PCI but should be combined with another anticoagulant with antithrombin activity such as UFH or bivalirudin. Contemporary trials of bivalirudin used an open-label design to evaluate its efficacy and safety compared with UFH plus a glycoprotein IIb/IIIa inhibitor. Bivalirudin was associated with a lower rate of bleeding, largely driven by reductions in vascular access site hematomas ≥5 cm or the administration of blood transfusions.
Patients with an anterior location of the infarction, severe LV dysfunction, heart failure, a history of embolism, two-dimensional echocardiographic evidence of mural thrombus, or atrial fibrillation are at increased risk of systemic or pulmonary thromboembolism. Such individuals should receive full therapeutic levels of anticoagulant therapy (LMWH or UFH) while hospitalized, followed by at least 3 months of warfarin therapy.
BETA-ADRENOCEPTOR BLOCKERS
The benefits of beta blockers in patients with STEMI can be divided into those that occur immediately when the drug is given acutely and those that accrue over the long term when the drug is given for secondary prevention after an infarction. Acute intravenous beta blockade improves the myocardial O2 supply-demand relationship, decreases pain, reduces infarct size, and decreases the incidence of serious ventricular arrhythmias. In patients who undergo fibrinolysis soon after the onset of chest pain, no incremental reduction in mortality rate is seen with beta blockers, but recurrent ischemia and reinfarction are reduced.
Thus, beta-blocker therapy after STEMI is useful for most patients (including those treated with an angiotensin-converting enzyme [ACE] inhibitor) except those in whom it is specifically contraindicated (patients with heart failure or severely compromised LV function, heart block, orthostatic hypotension, or a history of asthma) and perhaps those whose excellent long-term prognosis (defined as an expected mortality rate of <1% per year, patients <55 years, no previous MI, with normal ventricular function, no complex ventricular ectopy, and no angina) markedly diminishes any potential benefit.
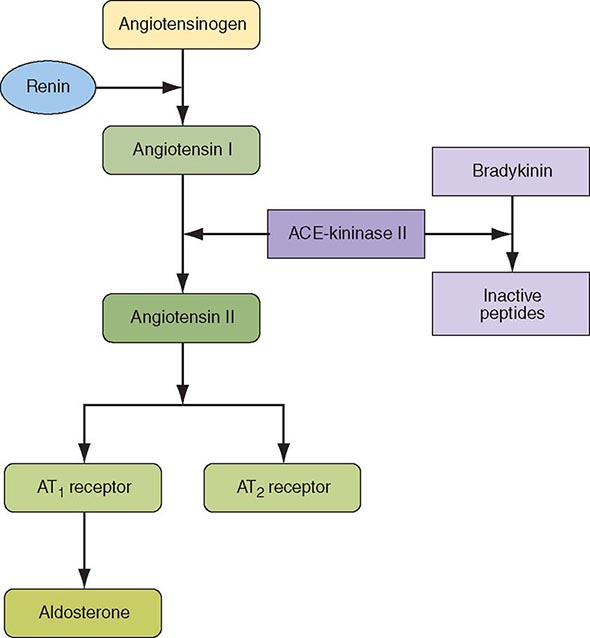
INHIBITION OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
ACE inhibitors reduce the mortality rate after STEMI, and the mortality benefits are additive to those achieved with aspirin and beta blockers. The maximum benefit is seen in high-risk patients (those who are elderly or who have an anterior infarction, a prior infarction, and/or globally depressed LV function), but evidence suggests that a short-term benefit occurs when ACE inhibitors are prescribed unselectively to all hemodynamically stable patients with STEMI (i.e., those with a systolic pressure >100 mmHg). The mechanism involves a reduction in ventricular remodeling after infarction (see “Ventricular Dysfunction” later) with a subsequent reduction in the risk of CHF. The rate of recurrent infarction may also be lower in patients treated chronically with ACE inhibitors after infarction.
Before hospital discharge, LV function should be assessed with an imaging study. ACE inhibitors should be continued indefinitely in patients who have clinically evident CHF, in patients in whom an imaging study shows a reduction in global LV function or a large regional wall motion abnormality, or in those who are hypertensive.
Angiotensin receptor blockers (ARBs) should be administered to STEMI patients who are intolerant of ACE inhibitors and who have either clinical or radiologic signs of heart failure. Long-term aldosterone blockade should be prescribed for STEMI patients without significant renal dysfunction (creatinine ≥2.5 mg/dL in men and ≥2.0 mg/dL in women) or hyperkalemia (potassium ≥5.0 mEq/L) who are already receiving therapeutic doses of an ACE inhibitor, have an LV ejection fraction ≤40%, and have either symptomatic heart failure or diabetes mellitus. A multidrug regimen for inhibiting the renin-angiotensin-aldosterone system has been shown to reduce both heart failure–related and sudden cardiac death–related cardiovascular mortality after STEMI, but has not been as thoroughly explored as ACE inhibitors in STEMI patients.
OTHER AGENTS
Favorable effects on the ischemic process and ventricular remodeling (see below) previously led many physicians to routinely use intravenous nitroglycerin (5–10 μg/min initial dose and up to 200 μg/min as long as hemodynamic stability is maintained) for the first 24–48 h after the onset of infarction. However, the benefits of routine use of intravenous nitroglycerin are less in the contemporary era where beta-adrenoceptor blockers and ACE inhibitors are routinely prescribed for patients with STEMI.
Results of multiple trials of different calcium antagonists have failed to establish a role for these agents in the treatment of most patients with STEMI. Therefore, the routine use of calcium antagonists cannot be recommended. Strict control of blood glucose in diabetic patients with STEMI has been shown to reduce the mortality rate. Serum magnesium should be measured in all patients on admission, and any demonstrated deficits should be corrected to minimize the risk of arrhythmias.
COMPLICATIONS AND THEIR MANAGEMENT
VENTRICULAR DYSFUNCTION
After STEMI, the left ventricle undergoes a series of changes in shape, size, and thickness in both the infarcted and noninfarcted segments. This process is referred to as ventricular remodeling and generally precedes the development of clinically evident CHF in the months to years after infarction. Soon after STEMI, the left ventricle begins to dilate. Acutely, this results from expansion of the infarct, i.e., slippage of muscle bundles, disruption of normal myocardial cells, and tissue loss within the necrotic zone, resulting in disproportionate thinning and elongation of the infarct zone. Later, lengthening of the noninfarcted segments occurs as well. The overall chamber enlargement that occurs is related to the size and location of the infarct, with greater dilation following infarction of the anterior wall and apex of the left ventricle and causing more marked hemodynamic impairment, more frequent heart failure, and a poorer prognosis. Progressive dilation and its clinical consequences may be ameliorated by therapy with ACE inhibitors and other vasodilators (e.g., nitrates). In patients with an ejection fraction <40%, regardless of whether or not heart failure is present, ACE inhibitors or ARBs should be prescribed (see “Inhibition of the Renin-Angiotensin-Aldosterone System” earlier).
HEMODYNAMIC ASSESSMENT
Pump failure is now the primary cause of in-hospital death from STEMI. The extent of infarction correlates well with the degree of pump failure and with mortality, both early (within 10 days of infarction) and later. The most common clinical signs are pulmonary rales and S3 and S4 gallop sounds. Pulmonary congestion is also frequently seen on the chest roentgenogram. Elevated LV filling pressure and elevated pulmonary artery pressure are the characteristic hemodynamic findings, but these findings may result from a reduction of ventricular compliance (diastolic failure) and/or a reduction of stroke volume with secondary cardiac dilation (systolic failure) (Chap. 279).
A classification originally proposed by Killip divides patients into four groups: class I, no signs of pulmonary or venous congestion; class II, moderate heart failure as evidenced by rales at the lung bases, S3 gallop, tachypnea, or signs of failure of the right side of the heart, including venous and hepatic congestion; class III, severe heart failure, pulmonary edema; and class IV, shock with systolic pressure <90 mmHg and evidence of peripheral vasoconstriction, peripheral cyanosis, mental confusion, and oliguria. When this classification was established in 1967, the expected hospital mortality rate of patients in these classes was as follows: class I, 0–5%; class II, 10–20%; class III, 35–45%; and class IV, 85–95%. With advances in management, the mortality rate in each class has fallen, perhaps by as much as one-third to one-half.
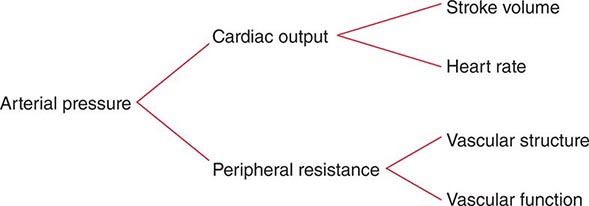
Hemodynamic evidence of abnormal global LV function appears when contraction is seriously impaired in 20–25% of the left ventricle. Infarction of ≥40% of the left ventricle usually results in cardiogenic shock (Chap. 326). Positioning of a balloon flotation (Swan-Ganz) catheter in the pulmonary artery permits monitoring of LV filling pressure; this technique is useful in patients who exhibit hypotension and/or clinical evidence of CHF. Cardiac output can also be determined with a pulmonary artery catheter. With the addition of intra-arterial pressure monitoring, systemic vascular resistance can be calculated as a guide to adjusting vasopressor and vasodilator therapy. Some patients with STEMI have markedly elevated LV filling pressures (>22 mmHg) and normal cardiac indices (2.6–3.6 L/[min/m2]), while others have relatively low LV filling pressures (<15 mmHg) and reduced cardiac indices. The former patients usually benefit from diuresis, while the latter may respond to volume expansion.
HYPOVOLEMIA
This is an easily corrected condition that may contribute to the hypotension and vascular collapse associated with STEMI in some patients. It may be secondary to previous diuretic use, to reduced fluid intake during the early stages of the illness, and/or to vomiting associated with pain or medications. Consequently, hypovolemia should be identified and corrected in patients with STEMI and hypotension before more vigorous forms of therapy are begun. Central venous pressure reflects RV rather than LV filling pressure and is an inadequate guide for adjustment of blood volume, because LV function is almost always affected much more adversely than RV function in patients with STEMI. The optimal LV filling or pulmonary artery wedge pressure may vary considerably among patients. Each patient’s ideal level (generally ∼20 mmHg) is reached by cautious fluid administration during careful monitoring of oxygenation and cardiac output. Eventually, the cardiac output level plateaus, and further increases in LV filling pressure only increase congestive symptoms and decrease systemic oxygenation without raising arterial pressure.
CARDIOGENIC SHOCK
Prompt reperfusion, efforts to reduce infarct size and treatment of ongoing ischemia and other complications of MI appear to have reduced the incidence of cardiogenic shock from 20% to about 7%. Only 10% of patients with this condition present with it on admission, while 90% develop it during hospitalization. Typically, patients who develop cardiogenic shock have severe multivessel coronary artery disease with evidence of “piecemeal” necrosis extending outward from the original infarct zone. The evaluation and management of cardiogenic shock and severe power failure after STEMI are discussed in detail in Chap. 326.
RIGHT VENTRICULAR INFARCTION
Approximately one-third of patients with inferior infarction demonstrate at least a minor degree of RV necrosis. An occasional patient with inferoposterior LV infarction also has extensive RV infarction, and rare patients present with infarction limited primarily to the RV. Clinically significant RV infarction causes signs of severe RV failure (jugular venous distention, Kussmaul’s sign, hepatomegaly [Chap. 267]) with or without hypotension. ST-segment elevations of right-sided precordial ECG leads, particularly lead V4R, are frequently present in the first 24 h in patients with RV infarction. Two-dimensional echocardiography is helpful in determining the degree of RV dysfunction. Catheterization of the right side of the heart often reveals a distinctive hemodynamic pattern resembling constrictive pericarditis (steep right atrial “y” descent and an early diastolic dip and plateau in RV waveforms) (Chap. 288). Therapy consists of volume expansion to maintain adequate RV preload and efforts to improve LV performance with attendant reduction in pulmonary capillary wedge and pulmonary arterial pressures.
ARRHYTHMIAS
(See also Chaps. 274 and 276) The incidence of arrhythmias after STEMI is higher in patients seen early after the onset of symptoms. The mechanisms responsible for infarction-related arrhythmias include autonomic nervous system imbalance, electrolyte disturbances, ischemia, and slowed conduction in zones of ischemic myocardium. An arrhythmia can usually be managed successfully if trained personnel and appropriate equipment are available when it develops. Since most deaths from arrhythmia occur during the first few hours after infarction, the effectiveness of treatment relates directly to the speed with which patients come under medical observation. The prompt management of arrhythmias constitutes a significant advance in the treatment of STEMI.
Ventricular Premature Beats Infrequent, sporadic ventricular premature depolarizations occur in almost all patients with STEMI and do not require therapy. Whereas in the past, frequent, multifocal, or early diastolic ventricular extrasystoles (so-called warning arrhythmias) were routinely treated with antiarrhythmic drugs to reduce the risk of development of ventricular tachycardia and ventricular fibrillation, pharmacologic therapy is now reserved for patients with sustained ventricular arrhythmias. Prophylactic antiarrhythmic therapy (either intravenous lidocaine early or oral agents later) is contraindicated for ventricular premature beats in the absence of clinically important ventricular tachyarrhythmias, because such therapy may actually increase the mortality rate. Beta-adrenoceptor blocking agents are effective in abolishing ventricular ectopic activity in patients with STEMI and in the prevention of ventricular fibrillation. As described earlier (see “Beta-Adrenoceptor Blockers”), they should be used routinely in patients without contraindications. In addition, hypokalemia and hypomagnesemia are risk factors for ventricular fibrillation in patients with STEMI; to reduce the risk, the serum potassium concentration should be adjusted to approximately 4.5 mmol/L and magnesium to about 2.0 mmol/L.
Ventricular Tachycardia and Fibrillation Within the first 24 h of STEMI, ventricular tachycardia and fibrillation can occur without prior warning arrhythmias. The occurrence of ventricular fibrillation can be reduced by prophylactic administration of intravenous lidocaine. However, prophylactic use of lidocaine has not been shown to reduce overall mortality from STEMI. In fact, in addition to causing possible noncardiac complications, lidocaine may predispose to an excess risk of bradycardia and asystole. For these reasons, and with earlier treatment of active ischemia, more frequent use of beta-blocking agents, and the nearly universal success of electrical cardioversion or defibrillation, routine prophylactic antiarrhythmic drug therapy is no longer recommended.
Sustained ventricular tachycardia that is well tolerated hemodynamically should be treated with an intravenous regimen of amiodarone (bolus of 150 mg over 10 min, followed by infusion of 1.0 mg/min for 6 h and then 0.5 mg/min) or procainamide (bolus of 15 mg/kg over 20–30 min; infusion of 1–4 mg/min); if it does not stop promptly, electroversion should be used (Chap. 276). An unsynchronized discharge of 200–300 J (monophasic waveform; approximately 50% of these energies with biphasic waveforms) is used immediately in patients with ventricular fibrillation or when ventricular tachycardia causes hemodynamic deterioration. Ventricular tachycardia or fibrillation that is refractory to electroshock may be more responsive after the patient is treated with epinephrine (1 mg intravenously or 10 mL of a 1:10,000 solution via the intracardiac route) or amiodarone (a 75–150-mg bolus).
Ventricular arrhythmias, including the unusual form of ventricular tachycardia known as torsades des pointes (Chaps. 276 and 277), may occur in patients with STEMI as a consequence of other concurrent problems (such as hypoxia, hypokalemia, or other electrolyte disturbances) or of the toxic effects of an agent being administered to the patient (such as digoxin or quinidine). A search for such secondary causes should always be undertaken.
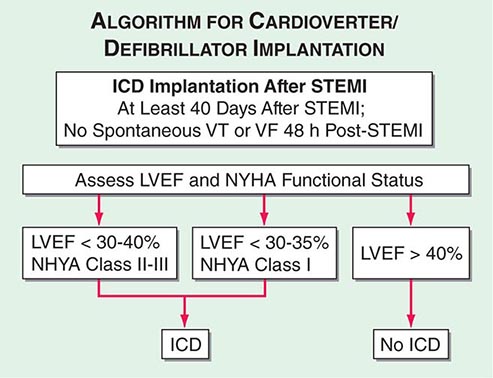
Although the in-hospital mortality rate is increased, the long-term survival is excellent in patients who survive to hospital discharge after primary ventricular fibrillation; i.e., ventricular fibrillation that is a primary response to acute ischemia that occurs during the first 48 h and is not associated with predisposing factors such as CHF, shock, bundle branch block, or ventricular aneurysm. This result is in sharp contrast to the poor prognosis for patients who develop ventricular fibrillation secondary to severe pump failure. For patients who develop ventricular tachycardia or ventricular fibrillation late in their hospital course (i.e., after the first 48 h), the mortality rate is increased both in-hospital and during long-term follow-up. Such patients should be considered for electrophysiologic study and implantation of a cardioverter-defibrillator (ICD) (Chap. 276). A more challenging issue is the prevention of sudden cardiac death from ventricular fibrillation late after STEMI in patients who have not exhibited sustained ventricular tachyarrhythmias during their index hospitalization. An algorithm for selection of patients who warrant prophylactic implantation of an ICD is shown in Fig. 295-5.
FIGURE 295-5 Algorithm for assessment of need for implantation of a cardioverter-defibrillator. The appropriate management is selected based on measurement of left ventricular ejection fraction and assessment of the New York Heart Association (NYHA) functional class. Patients with depressed left ventricular function at least 40 days after ST-segment elevation myocardial infarction (STEMI) are referred for insertion of an implantable cardioverter-defibrillator (ICD) if the left ventricular ejection fraction (LVEF) is <30–40% and they are in NYHA class II–III or if the LVEF is <30–35% and they are in NYHA class I functional status. Patients with preserved left ventricular function (LVEF >40%) do not receive an ICD regardless of NYHA functional class. All patients are treated with medical therapy after STEMI. VF, ventricular fibrillation; VT, ventricular tachycardia. (Adapted from data contained in DP Zipes et al: ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death; a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines [Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death]. J Am Coll Cardiol 48:1064, 2006.)
Accelerated Idioventricular Rhythm Accelerated idioventricular rhythm (AIVR, “slow ventricular tachycardia”), a ventricular rhythm with a rate of 60–100 beats/min, often occurs transiently during fibrinolytic therapy at the time of reperfusion. For the most part, AIVR, whether it occurs in association with fibrinolytic therapy or spontaneously, is benign and does not presage the development of classic ventricular tachycardia. Most episodes of AIVR do not require treatment if the patient is monitored carefully, as degeneration into a more serious arrhythmia is rare.
Supraventricular Arrhythmias Sinus tachycardia is the most common supraventricular arrhythmia. If it occurs secondary to another cause (such as anemia, fever, heart failure, or a metabolic derangement), the primary problem should be treated first. However, if it appears to be due to sympathetic overstimulation (e.g., as part of a hyperdynamic state), then treatment with a beta blocker is indicated. Other common arrhythmias in this group are atrial flutter and atrial fibrillation, which are often secondary to LV failure. Digoxin is usually the treatment of choice for supraventricular arrhythmias if heart failure is present. If heart failure is absent, beta blockers, verapamil, or diltiazem are suitable alternatives for controlling the ventricular rate, as they may also help to control ischemia. If the abnormal rhythm persists for >2 h with a ventricular rate >120 beats/min, or if tachycardia induces heart failure, shock, or ischemia (as manifested by recurrent pain or ECG changes), a synchronized electroshock (100–200 J monophasic waveform) should be used.
Accelerated junctional rhythms have diverse causes but may occur in patients with inferoposterior infarction. Digitalis excess must be ruled out. In some patients with severely compromised LV function, the loss of appropriately timed atrial systole results in a marked reduction of cardiac output. Right atrial or coronary sinus pacing is indicated in such instances.
Sinus Bradycardia Treatment of sinus bradycardia is indicated if hemodynamic compromise results from the slow heart rate. Atropine is the most useful drug for increasing heart rate and should be given intravenously in doses of 0.5 mg initially. If the rate remains <50–60 beats/min, additional doses of 0.2 mg, up to a total of 2.0 mg, may be given. Persistent bradycardia (<40 beats/min) despite atropine may be treated with electrical pacing. Isoproterenol should be avoided.
Atrioventricular and Intraventricular Conduction Disturbances (See also Chap. 274) Both the in-hospital mortality rate and the postdischarge mortality rate of patients who have complete atrioventricular (AV) block in association with anterior infarction are markedly higher than those of patients who develop AV block with inferior infarction. This difference is related to the fact that heart block in inferior infarction is commonly a result of increased vagal tone and/or the release of adenosine and therefore is transient. In anterior wall infarction, however, heart block is usually related to ischemic malfunction of the conduction system, which is commonly associated with extensive myocardial necrosis.
Temporary electrical pacing provides an effective means of increasing the heart rate of patients with bradycardia due to AV block. However, acceleration of the heart rate may have only a limited impact on prognosis in patients with anterior wall infarction and complete heart block in whom the large size of the infarct is the major factor determining outcome. It should be carried out if it improves hemodynamics. Pacing does appear to be beneficial in patients with inferoposterior infarction who have complete heart block associated with heart failure, hypotension, marked bradycardia, or significant ventricular ectopic activity. A subgroup of these patients, those with RV infarction, often respond poorly to ventricular pacing because of the loss of the atrial contribution to ventricular filling. In such patients, dual-chamber AV sequential pacing may be required.
External noninvasive pacing electrodes should be positioned in a “demand” mode for patients with sinus bradycardia (rate <50 beats/min) that is unresponsive to drug therapy, Mobitz II second-degree AV block, third-degree heart block, or bilateral bundle branch block (e.g., right bundle branch block plus left anterior fascicular block). Retrospective studies suggest that permanent pacing may reduce the long-term risk of sudden death due to bradyarrhythmias in the rare patient who develops combined persistent bifascicular and transient third-degree heart block during the acute phase of MI.
OTHER COMPLICATIONS
Recurrent Chest Discomfort Because recurrent or persistent ischemia often heralds extension of the original infarct or reinfarction in a new myocardial zone and is associated with a near tripling of mortality after STEMI, patients with these symptoms should be referred for prompt coronary arteriography and mechanical revascularization. Administration of a fibrinolytic agent is an alternative to early mechanical revascularization.
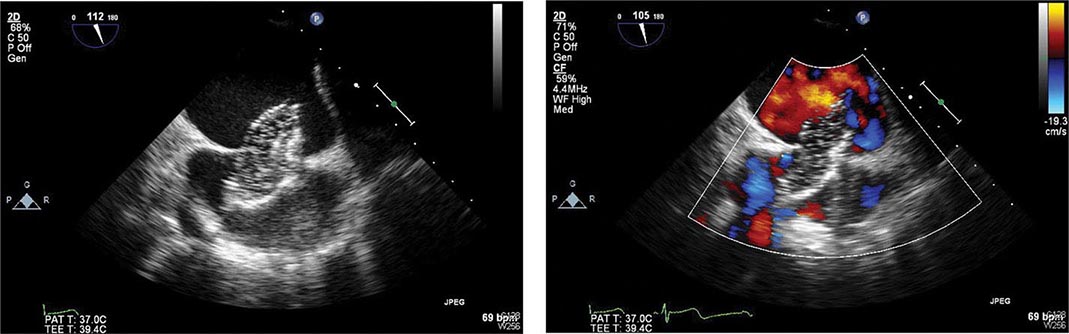
Pericarditis (See also Chap. 288) Pericardial friction rubs and/or pericardial pain are frequently encountered in patients with STEMI involving the epicardium. This complication can usually be managed with aspirin (650 mg four times daily). It is important to diagnose the chest pain of pericarditis accurately, because failure to recognize it may lead to the erroneous diagnosis of recurrent ischemic pain and/or infarct extension, with resulting inappropriate use of anticoagulants, nitrates, beta blockers, or coronary arteriography. When it occurs, complaints of pain radiating to either trapezius muscle is helpful, because such a pattern of discomfort is typical of pericarditis but rarely occurs with ischemic discomfort. Anticoagulants potentially could cause tamponade in the presence of acute pericarditis (as manifested by either pain or persistent rub) and therefore should not be used unless there is a compelling indication.
Thromboembolism Clinically apparent thromboembolism complicates STEMI in ∼10% of cases, but embolic lesions are found in 20% of patients in necropsy series, suggesting that thromboembolism is often clinically silent. Thromboembolism is considered to be an important contributing cause of death in 25% of patients with STEMI who die after admission to the hospital. Arterial emboli originate from LV mural thrombi, while most pulmonary emboli arise in the leg veins.
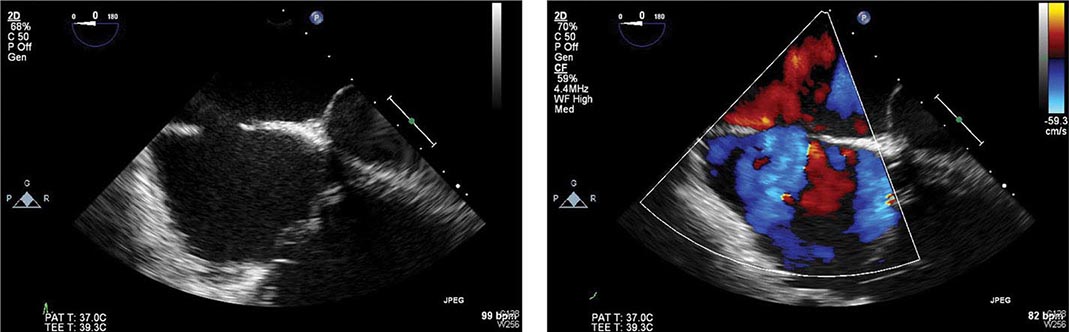
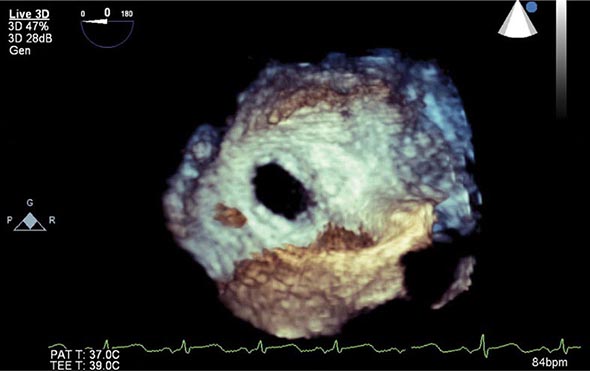
Thromboembolism typically occurs in association with large infarcts (especially anterior), CHF, and an LV thrombus detected by echocardiography. The incidence of arterial embolism from a clot originating in the ventricle at the site of an infarction is small but real. Two-dimensional echocardiography reveals LV thrombi in about one-third of patients with anterior wall infarction but in few patients with inferior or posterior infarction. Arterial embolism often presents as a major complication, such as hemiparesis when the cerebral circulation is involved or hypertension if the renal circulation is compromised. When a thrombus has been clearly demonstrated by echocardiographic or other techniques or when a large area of regional wall motion abnormality is seen even in the absence of a detectable mural thrombus, systemic anticoagulation should be undertaken (in the absence of contraindications), as the incidence of embolic complications appears to be markedly lowered by such therapy. The appropriate duration of therapy is unknown, but 3–6 months is probably prudent.
Left Ventricular Aneurysm The term ventricular aneurysm is usually used to describe dyskinesis or local expansile paradoxical wall motion. Normally functioning myocardial fibers must shorten more if stroke volume and cardiac output are to be maintained in patients with ventricular aneurysm; if they cannot, overall ventricular function is impaired. True aneurysms are composed of scar tissue and neither predispose to nor are associated with cardiac rupture.
The complications of LV aneurysm do not usually occur for weeks to months after STEMI; they include CHF, arterial embolism, and ventricular arrhythmias. Apical aneurysms are the most common and the most easily detected by clinical examination. The physical finding of greatest value is a double, diffuse, or displaced apical impulse. Ventricular aneurysms are readily detected by two-dimensional echocardiography, which may also reveal a mural thrombus in an aneurysm.
Rarely, myocardial rupture may be contained by a local area of pericardium, along with organizing thrombus and hematoma. Over time, this pseudoaneurysm enlarges, maintaining communication with the LV cavity through a narrow neck. Because a pseudoaneurysm often ruptures spontaneously, it should be surgically repaired if recognized.
POSTINFARCTION RISK STRATIFICATION AND MANAGEMENT
Many clinical and laboratory factors have been identified that are associated with an increase in cardiovascular risk after initial recovery from STEMI. Some of the most important factors include persistent ischemia (spontaneous or provoked), depressed LV ejection fraction (<40%), rales above the lung bases on physical examination or congestion on chest radiograph, and symptomatic ventricular arrhythmias. Other features associated with increased risk include a history of previous MI, age >75, diabetes mellitus, prolonged sinus tachycardia, hypotension, ST-segment changes at rest without angina (“silent ischemia”), an abnormal signal-averaged ECG, nonpatency of the infarct-related coronary artery (if angiography is undertaken), and persistent advanced heart block or a new intraventricular conduction abnormality on the ECG. Therapy must be individualized on the basis of the relative importance of the risk(s) present.
The goal of preventing reinfarction and death after recovery from STEMI has led to strategies to evaluate risk after infarction. In stable patients, submaximal exercise stress testing may be carried out before hospital discharge to detect residual ischemia and ventricular ectopy and to provide the patient with a guideline for exercise in the early recovery period. Alternatively, or in addition, a maximal (symptom-limited) exercise stress test may be carried out 4–6 weeks after infarction. Evaluation of LV function is usually warranted as well. Recognition of a depressed LV ejection fraction by echocardiography or radionuclide ventriculography identifies patients who should receive medications to inhibit the renin-angiotensin-aldosterone system. Patients in whom angina is induced at relatively low workloads, those who have a large reversible defect on perfusion imaging or a depressed ejection fraction, those with demonstrable ischemia, and those in whom exercise provokes symptomatic ventricular arrhythmias should be considered at high risk for recurrent MI or death from arrhythmia (Fig. 295-5). Cardiac catheterization with coronary angiography and/or invasive electrophysiologic evaluation is advised.
Exercise tests also aid in formulating an individualized exercise prescription, which can be much more vigorous in patients who tolerate exercise without any of the previously mentioned adverse signs. In addition, predischarge stress testing may provide an important psychological benefit, building the patient’s confidence by demonstrating a reasonable exercise tolerance.
In many hospitals, a cardiac rehabilitation program with progressive exercise is initiated in the hospital and continued after discharge. Ideally, such programs should include an educational component that informs patients about their disease and its risk factors.
The usual duration of hospitalization for an uncomplicated STEMI is about 5 days. The remainder of the convalescent phase may be accomplished at home. During the first 1–2 weeks, the patient should be encouraged to increase activity by walking about the house and outdoors in good weather. Normal sexual activity may be resumed during this period. After 2 weeks, the physician must regulate the patient’s activity on the basis of exercise tolerance. Most patients will be able to return to work within 2–4 weeks.
SECONDARY PREVENTION
Various secondary preventive measures are at least partly responsible for the improvement in the long-term mortality and morbidity rates after STEMI. Long-term treatment with an antiplatelet agent (usually aspirin) after STEMI is associated with a 25% reduction in the risk of recurrent infarction, stroke, or cardiovascular mortality (36 fewer events for every 1000 patients treated). An alternative antiplatelet agent that may be used for secondary prevention in patients intolerant of aspirin is clopidogrel (75 mg orally daily). ACE inhibitors or ARBs and, in appropriate patients, aldosterone antagonists should be used indefinitely by patients with clinically evident heart failure, a moderate decrease in global ejection fraction, or a large regional wall motion abnormality to prevent late ventricular remodeling and recurrent ischemic events.
The chronic routine use of oral beta-adrenoceptor blockers for at least 2 years after STEMI is supported by well-conducted, placebo-controlled trials.
Evidence suggests that warfarin lowers the risk of late mortality and the incidence of reinfarction after STEMI. Most physicians prescribe aspirin routinely for all patients without contraindications and add warfarin for patients at increased risk of embolism (see “Thromboembolism” earlier). Several studies suggest that in patients <75 years old a low dose of aspirin (75–81 mg/d) in combination with warfarin administered to achieve an international normalized ratio >2.0 is more effective than aspirin alone for preventing recurrent MI and embolic cerebrovascular accident. However, there is an increased risk of bleeding and a high rate of discontinuation of warfarin that has limited clinical acceptance of combination antithrombotic therapy. There is increased risk of bleeding when warfarin is added to dual antiplatelet therapy (aspirin and clopidogrel). However, patients who have had a stent implanted and have an indication for anticoagulation should receive dual antiplatelet therapies in combination with warfarin. Such patients should also receive a proton pump inhibitor to minimize the risk of gastrointestinal bleeding and should have regular monitoring of their hemoglobin levels and stool hematest while on combination antithrombotic therapy.
Finally, risk factors for atherosclerosis (Chap. 265e) should be discussed with the patient and, when possible, favorably modified.
296e |
Percutaneous Coronary Interventions and Other Interventional Procedures |
Percutaneous transluminal coronary angioplasty (PTCA) was first introduced by Andreas Gruentzig in 1977 as an alternative to coronary bypass surgery. The concept was initially demonstrated by Charles Dotter in 1964 in peripheral vessels. The development of a small inelastic balloon catheter by Gruentzig allowed expansion of the technique into smaller peripheral and coronary vessels. Initial coronary experience was limited to single-vessel coronary disease and discrete proximal lesions due to the technical limitations of the equipment. Advances in technology and greater operator experience allowed the procedure to grow rapidly with expanded use in patients with more complex lesions and multivessel disease. The introduction of coronary stents in 1994 was one of the major advances in the field. These devices reduced acute complications and reduced by half the significant problem of restenosis (or recurrence of the stenosis). Further reductions in restenosis were achieved by the introduction of drug-eluting stents in 2003. These stents slowly release antiproliferative drugs directly into the plaque over a few months. Percutaneous coronary intervention (PCI) is the most common revascularization procedure in the United States and is performed more than twice as often as coronary artery bypass surgery: nearly 600,000 patients a year.
Interventional cardiology is a separate discipline in cardiology that requires a dedicated 1-year interventional cardiology fellowship following a 3-year general cardiology fellowship in order to obtain a separate board certification. The discipline has also expanded to include interventions for structural heart disease including treatment of congenital heart disease and valvular heart disease; it also includes interventions to treat peripheral vascular disease, including atherosclerotic and nonatherosclerotic lesions in the carotid, renal, aortic, and peripheral circulations.
TECHNIQUE
The initial procedure is performed in a similar manner as a diagnostic cardiac catheterization (Chap. 272). Arterial access is obtained via the femoral or radial artery. To prevent thrombotic complications during the procedure, patients who are anticipated to need an angioplasty are given aspirin (325 mg) and may be given clopidogrel (loading dose of 300–600 mg), prasugrel (loading dose of 60 mg), or ticagrelor (loading dose of 180 mg) before the procedure. During the procedure, anticoagulation is achieved by administration of unfractionated heparin, enoxaparin (a low-molecular-weight heparin), or bivalirudin (a direct thrombin inhibitor). The latter has gained popularity due to the significant reduction in bleeding complications. In patients with ST-elevation myocardial infarction, high-risk acute coronary syndrome, or a large thrombus in the coronary artery, an intravenous glycoprotein IIb/IIIa inhibitor (abciximab, tirofiban, or eptifibatide) may also be given.
Following placement of an introducing sheath, preformed guiding catheters are used to cannulate selectively the origins of the coronary arteries. Through the guiding catheter, a flexible, steerable guidewire is negotiated down the coronary artery lumen using fluoroscopic guidance; it is then advanced through the stenosis and into the vessel beyond. This guidewire then serves as a “rail” over which angioplasty balloons, stents, or other therapeutic devices can be advanced to enlarge the narrowed segment of coronary artery. The artery is usually dilated with a balloon catheter followed by placement of a stent. The catheters and introducing sheath are removed and the artery manually held or closed using one of several femoral arterial closure devices to achieve hemostasis. Because PCI is performed under local anesthesia and mild sedation, it requires only a short (1-day) hospitalization or less.
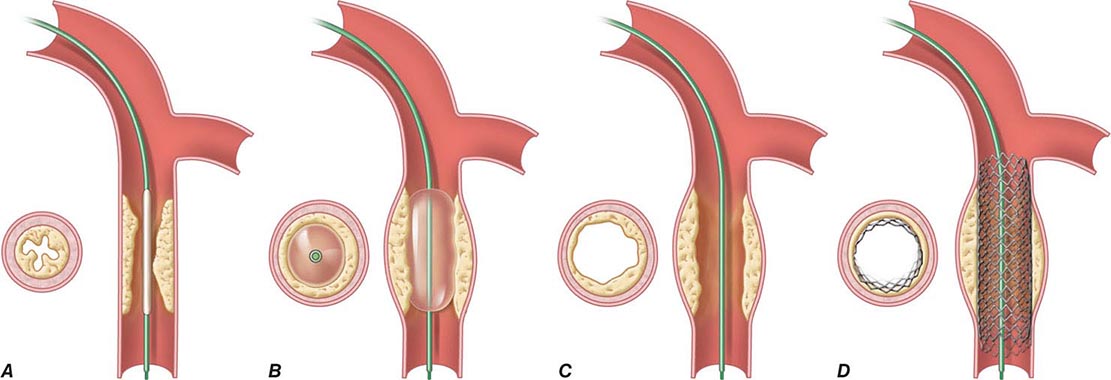
Angioplasty works by stretching the artery and displacing the plaque away from the lumen, enlarging the entire vessel (Figs. 296e-1 and 296e-2). The procedure rarely results in embolization of atherosclerotic material. Owing to inelastic elements in the plaque, the stretching of the vessel by the balloon results in small localized dissections that can protrude into the lumen and be a nidus for acute thrombus formation. If the dissections are severe, then they can obstruct the lumen or induce a thrombotic occlusion of the artery (acute closure). Stents have largely prevented this complication by holding the dissection flaps up against the vessel wall (Fig. 296e-1).
FIGURE 296e-1 Schematic diagram of the primary mechanisms of balloon angioplasty and stenting. A. A balloon angioplasty catheter is positioned into the stenosis over a guidewire under fluoroscopic guidance. B. The balloon is inflated temporarily occluding the vessel. C. The lumen is enlarged primarily by stretching the vessel, often resulting in small dissections in the neointima. D. A stent mounted on a deflated balloon is placed into the lesion and pressed against the vessel wall with balloon inflation (not shown). The balloon is deflated and removed, leaving the stent permanently against the wall acting as a scaffold to hold the dissections against the wall and prevent vessel recoil. (Adapted from EJ Topol: Textbook of Cardiovascular Medicine, 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 2002.)
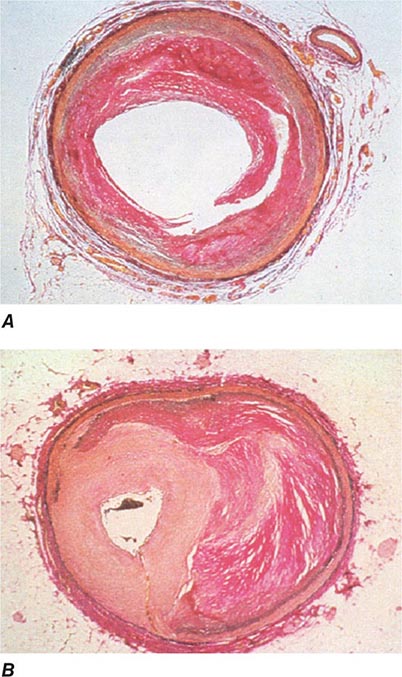
FIGURE 296e-2 Pathology of acute effects of balloon angioplasty with intimal dissection and vessel stretching (A) and an example of neointimal hyperplasia and restenosis showing renarrowing of the vessel (B). (Panel A from M Ueda et al: Eur Heart J 12:937, 1991; with permission. Panel B from CE Essed et al: Br Heart J 49:393, 1983; with permission.)
Stents are currently used in more than 90% of coronary angioplasty procedures. Stents are wire meshes (usually made of stainless steel) that are compressed over a deflated angioplasty balloon. When the balloon is inflated, the stent is enlarged to approximate the “normal” vessel lumen. The balloon is then deflated and removed, leaving the stent behind to provide a permanent scaffold in the artery. Owing to the design of the struts, these devices are flexible, allowing their passage through diseased and tortuous coronary vessels. Stents are rigid enough to prevent elastic recoil of the vessel and have dramatically improved the success and safety of the procedure as a result.
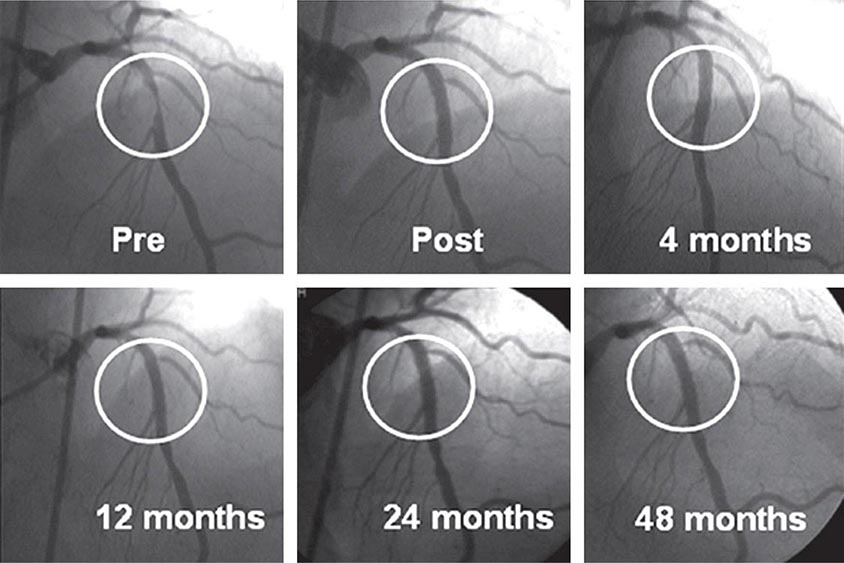
Drug-eluting stents further enhanced the efficacy of PCI. An antiproliferative agent is attached to the metal stent by use of a thin polymer coating. The antiproliferative drug elutes from the stent over a 1- to 3-month period after implantation. Drug-eluting stents have been shown to reduce clinical restenosis by 50%, so that in uncomplicated lesions symptomatic restenosis occurs in 5–10% of patients. Not surprisingly, this led to the rapid acceptance of these devices; currently 80–90% of all stents implanted are drug-eluting. The first-generation devices were coated with either sirolimus or paclitaxel. Second-generation drug-eluting stents use newer agents such as everolimus, biolimus, and zotarolimus. These second-generation drug-eluting stents appear to be more effective with fewer complications, such as early or late stent thrombosis, than the first-generation devices and, therefore, have replaced the first-generation stents. Biodegradable polymers that are used to attach the drugs to the stents may be superior to permanent polymers in preventing late stent thrombosis and are under investigation. In addition, the everolimus-eluting biodegradable vascular scaffold (BVS) stent has been shown to be safe and effective with gradual degradation over several years with return of normal vessel function. It is currently approved in Europe. Additional stents are under investigation. Other interventional devices include atherectomy devices and thrombectomy catheters. These devices are designed to remove atherosclerotic plaque or thrombus and are used in conjunction with balloon dilatation and stent placement. Rotational atherectomy is the most commonly used adjunctive device and is modeled after a dentist’s drill, with small round burrs of 1.25–2.5 mm at the tip of a flexible wire shaft. They are passed over the guidewire up to the stenosis and drill away atherosclerotic material. Because the atherosclerotic particles are ≤25 μm, they pass through the coronary microcirculation and rarely cause problems. The device is particularly useful in heavily calcified plaques that are resistant to balloon dilatation. Given the current advances in stents, rotational atherectomy is infrequently used. Directional atherectomy catheters are not used in the coronaries any longer but are used in peripheral arterial disease. In acute ST-elevation myocardial infarction, specialized catheters without a balloon are used to aspirate thrombus in order to prevent embolization down the coronary vessel and to improve blood flow before angioplasty and stent placement. Some data suggest that manual catheter thrombus aspiration may reduce mortality in addition to improving blood flow in primary PCI.
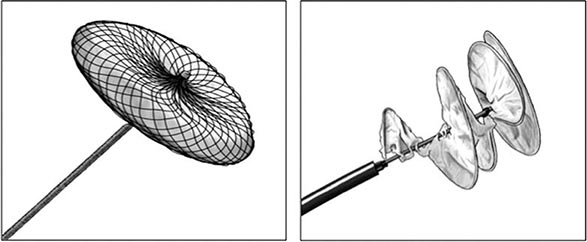
PCI of degenerated saphenous vein graft lesions has been associated with a significant incidence of distal embolization of atherosclerotic material, unlike PCI of native vessel disease. A number of distal protection devices have been shown to significantly reduce embolization and myocardial infarction in this setting. Most devices work by using a collapsible wire filter at the end of a guidewire that is expanded in the distal vessel before PCI. If atherosclerotic debris is dislodged, the basket captures the material, and at the end of the PCI, the basket is pulled into a delivery catheter and the debris safely removed from the patient.
SUCCESS AND COMPLICATIONS
A successful procedure (angiographic success), defined as a reduction of the stenosis to less than a 20% diameter narrowing, occurs in 95–99% of patients. Lower success rates are seen in patients with tortuous, small, or calcified vessels or chronic total occlusions. Chronic total occlusions have the lowest success rates (60–70%), and their recanalization is usually not attempted unless the occlusion is recent (within 3 months) or there are favorable anatomic features. Improvements in equipment and technique have increased the success rates of recanalization of chronic total occlusions.
Serious complications are rare but include a mortality rate of 0.1–0.3% for elective cases, a large myocardial infarction in less than 3%, and stroke in less than 0.1%. Patients who are elderly (>65 years), undergoing an emergent or urgent procedure, have chronic kidney disease, present with an ST-segment elevation myocardial infarction (STEMI), or are in shock have significantly higher risk. Scoring systems can help to estimate the risk of the procedure. Myocardial infarction during PCI can occur for multiple reasons including an acute occluding thrombus, severe coronary dissection, embolization of thrombus or atherosclerotic material, or closure of a side branch vessel at the site of angioplasty. Most myocardial infarctions are small and only detected by a rise in the creatine phosphokinase (CPK) or troponin level after the procedure. Only those with significant enzyme elevations (more than three to five times the upper limit of normal) are associated with a less favorable long-term outcome. Coronary stents have largely prevented coronary dissections due to the scaffolding effect of the stent.
Metallic stents are also prone to stent thrombosis (1–3%), either acute (<24 h) or subacute (1–30 days), which can be ameliorated by greater attention to full initial stent deployment and the use of dual antiplatelet therapy (DAPT) (aspirin, plus a platelet P2Y12 receptor blocker [clopidogrel, prasugrel, or ticagrelor]). Late (30 days–1 year) and very late stent thromboses (>1 year) occur very infrequently with stents but are slightly more common with first-generation drug-eluting stents, necessitating DAPT for up to 1 year or longer. Use of the second-generation stents is associated with lower rates of late and very late stent thromboses, and shorter durations of DAPT may be possible. Premature discontinuation of DAPT, particularly in the first month after implantation, is associated with a significantly increased risk for stent thrombosis (three- to ninefold greater). Stent thrombosis results in death in 10–20% and myocardial infarction in 30–70% of patients. Elective surgery that requires discontinuation of antiplatelet therapy after drug-eluting stent implantation should be postponed until after 6 months and preferably after 1 year, if at all possible.
Restenosis, or renarrowing of the dilated coronary stenosis, is the most common complication of angioplasty and occurs in 20–50% of patients with balloon angioplasty alone, 10–30% of patients with bare metal stents, and 5–15% of patients with drug-eluting stents within the first year. The fact that stent placement provides a larger acute luminal area than balloon angioplasty alone reduces the incidence of subsequent restenosis. Drug-eluting stents further reduce restenosis through a reduction in excessive neointimal growth over the stent. If restenosis does not occur, the long-term outcome is excellent (Fig. 296e-3). Clinical restenosis is recognized by recurrence of angina or symptoms within 9 months of the procedure. Less frequently, patients with restenosis can present with non-ST-segment elevation myocardial infarction (NSTEMI) (10%) or STEMI (2%) as well. Clinical restenosis requires confirmation of a significant stenosis at the site of the prior PCI. Target lesion revascularization (TLR) or target vessel revascularization (TVR) is defined as angiographic restenosis with repeat PCI or coronary artery bypass grafting (CABG). By angiography, the incidence of restenosis is significantly higher than clinical restenosis (TLR or TVR) because many patients have mild restenosis that does not result in a recurrence of symptoms. The management of clinical restenosis is usually to repeat the PCI with balloon dilatation and placement of a drug-eluting stent. Once a patient has had restenosis, the risk of a second restenosis is further increased. The risk factors for restenosis are diabetes, myocardial infarction, long lesions, small-diameter vessels, and suboptimal initial PCI result.
FIGURE 296e-3 Long-term results from one of the first patients to receive a sirolimus-eluting stent from early Sao Paulo experience. (From: GW Stone, in D Baim [ed]: Cardiac Catheterization, Angiography and Intervention, 7th ed. Philadelphia, Lippincott Williams & Wilkins, 2006; with permission.)
INDICATIONS
The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines extensively review the indications for PCI in patients with stable angina, unstable angina, NSTEMI, and STEMI and should be referred to for a comprehensive discussion of the indications. Briefly, the two principal indications for coronary revascularization in patients with chronic stable angina (Chap. 293) are (1) to improve anginal symptoms in patients who remain symptomatic despite adequate medical therapy and (2) to reduce mortality rates in patients with severe coronary disease. In patients with stable angina who are well controlled on medical therapy, studies such as the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trials have shown that initial revascularization does not lead to better outcomes and can be safely delayed until symptoms worsen or evidence of severe ischemia on noninvasive testing occurs. When revascularization is indicated, the choice of PCI or CABG depends on a number of clinical and anatomic factors (Fig. 296e-4). The Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial compared PCI with the paclitaxel drug-eluting stent to CABG in 1800 patients with three-vessel coronary disease or left main disease. The study found no difference in death or myocardial infarction at 1 year, but repeat revascularization was significantly higher in the stent-treated group (13.5 vs. 5.9%), while stroke was significantly higher in the surgical group (2.2 vs. 0.6%). The primary endpoint of death, myocardial infarction, stroke, or revascularization was significantly better with CABG, particularly in those with the most extensive coronary artery disease. The 3-year results confirm these findings. The Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial randomized 1900 patients with diabetes and multivessel disease and showed a significantly lower primary endpoint of death, myocardial infarction, or stroke with CABG than PCI. These studies support CABG for those with the most severe left main and three-vessel disease or those with diabetes. Lesser degrees of multivessel disease in patients with or without diabetes have an equal outcome with PCI.
FIGURE 296e-4 In patients requiring revascularization, several factors need to be considered in choosing between bare metal stents, drug-eluting stents, or coronary artery bypass surgery. ACS, acute coronary syndrome; BMS, bare metal stent; CABG, coronary artery bypass grafting; DES, drug-eluting stent; IVUS, intravascular ultrasound; STEMI, ST-segment elevation myocardial infarction. (From AA Bavry, DL Bhatt: Circulation 116:696, 2007; with permission.)
The choice of PCI versus CABG is also related to the anticipated procedural success and complications of PCI and the risks of CABG. For PCI, the characteristics of the coronary anatomy are critically important. The location of the lesion in the vessel (proximal or distal), the degree of tortuosity, and the size of the vessel are considered. In addition, the lesion characteristics, including the degree of the stenosis, the presence of calcium, lesion length, and presence of thrombus, are assessed. The most common reason to decide not to do PCI is that the lesion felt to be responsible for the patient’s symptoms is not treatable. This is most commonly due to the presence of a chronic total occlusion (>3 months in duration). In this setting, the historical success rate has been low (30–70%) and complications are more common. A lesion classification to characterize the likelihood of success or failure of PCI has been developed by the ACC/AHA. Lesions with the highest success are called type A lesions (such as proximal noncalcified subtotal lesions), and those with the lowest success or highest complication rate are type C lesions (such as chronic total occlusions). Intermediate lesions are classified as type B1 or B2 depending on the number of unfavorable characteristics. Approximately 25–30% of patients will not be candidates for PCI due to unfavorable anatomy, whereas only 5% of CABG patients will not be candidates for surgery due to coronary anatomy. The primary reason for being considered inoperable with CABG is the presence of severe comorbidities such as advanced age, frailty, severe chronic obstructive pulmonary disease (COPD), or poor left ventricular function.
Another consideration in choosing a revascularization strategy is the degree of revascularization. In patients with multivessel disease, bypass grafts can usually be placed to all vessels with significant stenosis, whereas PCI may be able to treat only some of the lesions due to the presence of unfavorable anatomy. Assessment of the significance of intermediate lesions using fractional flow reserve (FFR) (Chap. 272) can assist in determining which lesions should be revascularized. The Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) trial showed a 30% reduction in adverse events when revascularization by PCI was restricted to those lesions that were hemodynamically significant (FFR ≤0.80) rather than when guided by angiography alone. Thus, complete revascularization of all functionally significant lesions should be favored and considered when choosing the optimal revascularization strategy. Given the multiple factors that need to be considered in choosing the best revascularization for an individual patient with multivessel disease, it is optimal to have a discussion among the cardiac surgeon, interventional cardiologist, and the physicians caring for the patient (so-called Heart Team) to properly weigh the choices.
Patients with acute coronary syndrome are at excess risk of short- and long-term mortality. Randomized clinical trials have shown that PCI is superior to intensive medical therapy in reducing mortality and myocardial infarction, with the benefit largely confined to those patients who are high risk. High-risk patients are defined as those with any one of the following: refractory ischemia, recurrent angina, positive cardiac-specific enzymes, new ST-segment depression, low ejection fraction, severe arrhythmias, or a recent PCI or CABG. PCI is preferred over surgical therapy in most high-risk patients with acute coronary syndromes unless they have severe multivessel disease or the culprit lesion responsible for the unstable presentation cannot be adequately treated. In STEMI, thrombolysis or PCI (primary PCI) are effective methods to restore coronary blood flow and salvage myocardium within the first 12 h after onset of chest pain. Because PCI is more effective in restoring flow than thrombolysis, it is preferred if readily available. PCI is also performed following thrombolysis to facilitate adequate reperfusion or as a rescue procedure in those who do not achieve reperfusion from thrombolysis or in those who develop cardiogenic shock.
OTHER INTERVENTIONAL TECHNIQUES
STRUCTURAL HEART DISEASE
Interventional treatment for structural heart disease (adult congenital heart disease and valvular heart disease) is a significant and growing component of the field of interventional cardiology.
The most common adult congenital lesion to be treated with percutaneous techniques is closure of atrial septal defects (Chap. 282). The procedure is done as in a diagnostic right heart catheterization with the passage of a catheter up the femoral vein into the right atrium. With echo and fluoroscopic guidance, the size and location of the defect can be accurately defined, and closure is accomplished using one of several approved devices. All devices use a left atrial and right atrial wire mesh or covered disk that are pulled together to capture the atrial septum around the defect and seal it off. The Amplatzer Septal Occluder device (AGA Medical, Minneapolis, Minnesota) is the most commonly used in the United States. The success rate in selected patients is 85–95%, and the device complications are rare and include device embolization, infection, or erosion. Closure of patent foramen ovale (PFO) is done in a similar way. PFO closure may be considered in patients who have had recurrent paradoxical stroke or transient ischemic attack (TIA) despite adequate medical therapy including anticoagulation or antiplatelet therapy. The benefit, however, has not been proven. The CLOSURE I trial randomized 909 patients with cryptogenic stroke or TIA who had a PFO. Closure did not reduce the primary endpoint of death within 30 days or death following a neurologic cause during 2 years of follow-up or stroke/TIA within 2 years. Other trials have confirmed these findings. The use in the treatment of migraine is under clinical investigation and is not an approved indication.
Similar devices can also be used to close patent ductus arteriosus and ventricular septal defects. Other congenital diseases that can be treated percutaneously include coarctation of the aorta, pulmonic stenosis, peripheral pulmonary stenosis, and other abnormal communications between the cardiac chambers or vessels.
The treatment of valvular heart disease is the most rapidly growing area in interventional cardiology. Until recently, the only available techniques were balloon valvuloplasty for the treatment of aortic, mitral, or pulmonic stenosis (Chap. 283). Mitral valvuloplasty is the preferred treatment for symptomatic patients with rheumatic mitral stenosis who have favorable anatomy. The outcome in these patients is equal to that of surgical commissurotomy. The success is highly related to the echocardiographic appearance of the valve. The most favorable setting is commissural fusion without calcification or subchordal fusion and the absence of significant mitral regurgitation. Access is obtained from the femoral vein using a transseptal technique in which a long metal catheter with a needle tip is advanced from the femoral vein through the right atrium and atrial septum at the level of the foramen ovale into the left atrium. A guidewire is advanced into the left ventricle, and a balloon-dilatation catheter is negotiated across the mitral valve and inflated to a predetermined size to enlarge the valve. The most commonly used dilatation catheter is the Inoue balloon. The technique splits the commissural fusion and commonly results in a doubling of the mitral valve area. The success of the procedure in favorable anatomy is 95% and severe complications are rare (1–2%). The most common complications are tamponade due to puncture into the pericardium or the creation of severe mitral regurgitation.
For regurgitant valvular lesions, only severe mitral regurgitation can be effectively treated percutaneously using the MitraClip (Abbott, Abbott Park, Ill) device. The procedure involves the passage of a catheter into the left atrium using the transseptal technique. A special catheter with a metallic clip on the end is passed through the mitral valve and retracted to catch and clip together the mid portion of the anterior and posterior mitral valve leaflet. The clip creates a double opening in the mitral valve and thereby reduces mitral regurgitation similar to the surgical Alfieri repair. In the Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) trial, the device was less effective than surgical repair or replacement but was shown to be safe. It is currently used for patients who are not good candidates for surgical repair, particularly when the regurgitation is due to functional causes.
Severe aortic stenosis can be treated with balloon valvuloplasty as well. In this setting, the valvuloplasty balloon catheter is placed retrograde across the aortic valve from the femoral artery and briefly inflated to stretch open the valve. The success is much less favorable, with only 50% achieving an aortic valve area of >1 cm2 and a restenosis rate of 25–50% after 6–12 months. This poor success rate has limited its use to patients who are not surgical candidates or as a bridge to surgery or transcatheter aortic valve replacement (TAVR). In this setting, the intermediate-term mortality rate of the procedure is high (10%). Repeat aortic valvuloplasty as a treatment for aortic valve restenosis has been reported.
Percutaneous aortic valve replacement (TAVR) has been shown to be an effective treatment for high-risk and inoperable patients with aortic stenosis. Currently, two valve models, the Edwards SAPIEN valve (Edwards Lifescience, Irvine, California) and the CoreValve ReValving system (Medtronic, Minneapolis, Minnesota) are available. In more than 10,000 cases worldwide, follow-up shows no evidence of restenosis or severe prosthetic valve dysfunction in the midterm. The CoreValve is self-expanding, while the Edwards valve is balloon expanded. The cannulas are large (14–22 French), and retrograde access via the femoral artery is most commonly chosen, if possible. In patients with peripheral artery disease, access via the subclavian artery or transapically through a surgical incision can be used. Following balloon valvuloplasty, the valve is positioned across the valve and deployed with postdeployment balloon inflation to ensure full contact with the aortic annulus. The success rate is 80–90%, and the 30-day mortality rate is 10–15%, not unexpectedly as only high-risk patients are undergoing the procedure currently. The Placement of Aortic Transcatheter Valve (PARTNER) randomized trial of the Edwards valve showed a 55% reduction in 1-year mortality and major adverse events in the extreme-risk group randomized to TAVR compared to medical therapy. In a separate randomized trial, high-risk patients had a similar outcome to surgical valve replacement at 1 year. As a result, this valve is approved for both high-risk and extreme-risk patients with severe aortic stenosis.
Pulmonic stenosis can also be effectively treated with balloon valvuloplasty and percutaneously replaced with the Melody stent (Medtronic). Tricuspid valve interventions remain experimental.
PERIPHERAL ARTERY INTERVENTIONS
The use of percutaneous interventions to treat symptomatic patients with arterial obstruction in the carotid, renal, aortic, and peripheral vessels is also part of the field of interventional cardiology. Randomized clinical trial data already support the use of carotid stenting in patients at high risk of complications from carotid endarterectomy (Fig. 296e-5). Recent trials suggest similar outcomes with carotid stenting and carotid endarterectomy in patients at average risk, although depending on the patient’s risk for periprocedural stroke or myocardial infarction, one procedure may be preferred over the other. The success rate of peripheral artery interventional procedures has been improving, including for long segments of occlusive disease historically treated by peripheral bypass surgery (Fig. 296e-6). Peripheral intervention is increasingly part of the training of an interventional cardiologist, and most programs now require an additional year of training after the interventional cardiology training year. The techniques and outcomes are described in detail in the chapter on peripheral vascular disease (Chap. 302).
FIGURE 296e-5 An example of a high-risk patient who requires carotid revascularization, but who is not a candidate for carotid endarterectomy. Carotid artery stenting resulted in an excellent angiographic result. (From M Belkin, DL Bhatt: Circulation 119:2302, 2009; with permission.)
FIGURE 296e-6 Peripheral interventional procedures have become highly effective at treating anatomic lesions previously amenable only to bypass surgery. A. Complete occlusion of the left superficial femoral artery. B. Wire and catheter advanced into subintimal space. C. Intravascular ultrasound positioned in the subintimal space to guide retrograde wire placement through the occluded vessel. D. Balloon dilation of the occlusion. E. Stent placement with excellent angiographic result. (From A Al Mahameed, DL Bhatt: Cleve Clin J Med 73:S45, 2006; with permission.)
CIRCULATORY SUPPORT TECHNIQUES
The use of circulatory support techniques is occasionally needed in order to safely perform PCI on hemodynamically unstable patients. It also can be useful in helping to stabilize patients before surgical interventions. The most commonly used device is the percutaneous intraaortic balloon pump developed in the early 1960s. A 7- to 10-French, 25- to 50-mL balloon catheter is placed retrograde from the femoral artery into the descending aorta between the aortic arch and the abdominal aortic bifurcation. It is connected to a helium gas inflation system that synchronizes the inflation to coincide with early diastole with deflation by mid-diastole. As a result, it increases early diastolic pressure, lowers systolic pressure, and lowers late diastolic pressure through displacement of blood from the descending aorta (counterpulsation). This results in an increase in coronary blood flow and a decrease in afterload. It is contraindicated in patients with aortic regurgitation, aortic dissection, or severe peripheral artery disease. The major complications are vascular and thrombotic. Intravenous heparin is given in order to reduce thrombotic complications.
Another potentially useful tool is the Impella device (Abiomed, Danvers, Massachusetts). The catheter is placed percutaneously from the femoral artery into the left ventricle. The catheter has a small microaxial pump at its tip that can pump up to 2.5–5 L/min from the left ventricle to the aorta. Other support devices include TandemHeart (CardiacAssist, Pittsburgh, Pennsylvania), which involves placement of a large 21-French catheter from the femoral vein through the right atrium into the left atrium using the transseptal technique and a catheter in the femoral artery. A centrifugal pump can deliver 5 L of blood per minute. It may be useful in patients in shock or with STEMI or very-high-risk PCI. Patients can also be placed on peripheral extracorporeal membrane oxygenation using large cannulas placed in the femoral artery and vein.
INTERVENTIONS FOR PULMONARY EMBOLISM
The treatment of deep vein thrombosis is intravenous anticoagulation, with placement of an inferior vena cava filter if recurrent pulmonary emboli occur. Postphlebitic syndrome is a serious condition due to chronic venous obstruction that can lead to chronic leg edema and venous ulcers. Preliminary studies suggest that mechanical treatments may have a role in treatment, and a large trial is ongoing.
Pulmonary emboli (PE) should be treated with fibrinolytic agents if massive and in some cases if submassive. Surgical pulmonary embolectomy is an option for the treatment of massive PE with hemodynamic instability in patients who have contraindications for systemic fibrinolysis or those in whom it has failed. Catheter-based therapies for submassive and massive PEs are still evolving, but studies have shown promise. The techniques employed include the use of aspiration of the clot with a large catheter (10 French), intraclot infusion of a thrombolytic agent followed by aspiration, ultrasound-assisted catheter-directed thrombolysis, and use of rheolytic thrombectomy. Success for these techniques has been reported to be 80–90%, with major complications occurring in 2–4% of patients.
INTERVENTIONS FOR REFRACTORY HYPERTENSION
The recent recognition of the importance of the renal sympathetic nerves in modulating blood pressure has led to a technique to selectively denervate renal sympathetic nerves in patients with refractory hypertension. The procedure involves applying low-power radiofrequency treatment via a catheter along the length of both renal arteries. In the randomized Symplicity HTN-2 trial, renal denervation significantly reduced blood pressure compared with medical therapy. The Symplicity device (Medtronic) is approved in Europe, though the randomized and blinded U.S. Symplicity HTN-3 trial showed no effect.
CONCLUSION
Interventional cardiology continues to expand its borders. Treatment for coronary artery disease, including complex anatomic subsets, continues to advance, encroaching on what has traditionally been treated by CABG. Technological advances such as drug-eluting stents, now already in their second generation, and manual thrombus aspiration devices are improving the results of PCI. In particular, PCI has been shown to prevent future ischemic events in acute coronary syndromes. For patients with stable coronary disease, PCI has an important role in symptom alleviation. Treatment of peripheral and cerebrovascular disease has also benefited from the application of percutaneous techniques. Structural heart disease is increasingly being treated with percutaneous options, with a high likelihood that interventional approaches will compete with open-heart surgery in a significant proportion of cases in years to come.
297e |
Atlas of Percutaneous Revascularization |
Percutaneous coronary intervention (PCI) is the most widely employed coronary revascularization procedure worldwide (Chap. 296e). It is now applied to patients with stable angina; patients with acute coronary syndromes, including unstable angina and non-ST-segment elevation myocardial infarction (NSTEMI); and as a primary treatment strategy in patients with ST-segment elevation myocardial infarction (STEMI). PCI is also applicable to patients with either single-vessel or multivessel disease.
In this chapter, the use of PCI will be illustrated in a variety of commonly encountered clinical and anatomic situations, such as chronic total occlusion of a coronary artery, bifurcation disease, acute STEMI, saphenous vein graft disease, left main coronary artery disease, multivessel disease, and stent thrombosis. In addition, the use of interventional techniques to treat structural heart disease will be shown, including closure of an atrial septal defect (ASD) and transcatheter aortic valve replacement (TAVR).
CASE 1: CHRONIC TOTAL OCCLUSION
(Videos 297e-1 to 297e-7)
• An 81-year-old male with angina, New York Heart Association (NYHA) class IV congestive heart failure and inferoapicoposterior ischemia on an exercise technetium-99m scan.
• Diagnostic cardiac catheterization revealed a left dominant system with a totally occluded left circumflex (LCx) artery. The distal LCx filled via collaterals from the left anterior descending (LAD) artery, indicating chronicity of the total occlusion.
VIDEO 297e-1 Baseline left coronary angiogram shows an occluded LCx with left-to-left collaterals originating from LAD septal vessels.
VIDEO 297e-2 Attempts to cross the total occlusion in the LCx using a hydrophilic wire and an antegrade approach were not successful with the wire tracking to the right of the trajectory.
VIDEO 297e-3 The LAD septal collateral is accessed with a guidewire that is directed toward the distal LCx to cross the total occlusion retrograde.
VIDEO 297e-4 The total occlusion is crossed retrograde. The wire is snared in the guide, exteriorized, and used to provide antegrade access to the LCx.
VIDEO 297e-5 Antegrade flow in the LCx is restored after balloon inflation.
VIDEO 297e-6 Following stenting of the total occlusion, blood flow in the distal vessel is improved and a second significant stenosis is seen.
VIDEO 297e-7 Final result after LCx stenting.
SUMMARY
• Approximately 15–30% of all patients referred for cardiac catheterization will have a chronic total occlusion (CTO) of a coronary artery.
• CTO often leads to a surgical referral for complete revascularization.
• Incomplete revascularization due to an untreated CTO is associated with an increased mortality rate (hazard ratio = 1.36; 95% confidence interval [CI], 1.12–1.66, p <.05).
• Successful PCI of a CTO leads to a 3.8–8.4% absolute reduction in mortality, symptom relief, and improved left ventricular function.
• Newer techniques, such as the retrograde approach to crossing total occlusions, are useful when the antegrade approach fails or is not feasible and there are well-developed collateral vessels.
(Case contributed with permission by Dr. Frederick G.P. Welt.)
CASE 2: BIFURCATION STENTING
(Fig. 297e-1; Videos 297e-8 to 297e-16)
• A 52-year-old male with an acute coronary syndrome and a troponin I = 0.18 (upper limit of normal, <0.04).
• Diagnostic cardiac catheterization showed single-vessel coronary artery disease with a significant stenosis in the mid-LAD and a bifurcation lesion involving a large diagonal branch.
FIGURE 297e-1 Schematic representation of one-stent and two-stent techniques to treat bifurcation lesions. PTCA, percutaneous transluminal coronary angioplasty
VIDEO 297e-8 Baseline angiogram of the left coronary circulation shows the significant stenosis in the mid-LAD and the bifurcation lesion involving a large diagonal branch.
VIDEO 297e-9 Both vessels are accessed with guidewires and pretreated with balloon angioplasty.
VIDEO 297e-10 Result after balloon angioplasty.
VIDEO 297e-11 Stent being positioned in the LAD.
VIDEO 297e-12 LAD poststent result.
VIDEO 297e-13 Stent deployed in the diagonal branch through the stent struts in the LAD using the “culotte” technique.
VIDEO 297e-14 Diagonal branch poststent result.
VIDEO 297e-15 Simultaneous inflation of two 2.5-mm “kissing” balloons.
VIDEO 297e-16 Final postbifurcation stenting result.
SUMMARY
• Approximately 15–20% of PCIs will involve the treatment of bifurcation lesions.
• Bifurcation lesions require consideration of PCI strategies that protect side-branch patency.
• There are both one-stent and two-stent techniques to treat bifurcation lesions; the selection of technique depends on anatomic considerations, including plaque burden, angle of side-branch take-off, plaque shift during angioplasty, and side-branch distribution.
• Rates of target lesion revascularization and stent thrombosis are similar between one-stent and two-stent procedures.
CASE 3: INFERIOR MYOCARDIAL INFARCTION—THROMBUS AND MANUAL THROMBECTOMY
(Figs. 297e-2 to 297e-4; Videos 297e-17 to 297e-22)
• A 59-year-old male presented to the emergency room with 2 h of severe midsternal chest pressure.
• His systolic blood pressure was 100 mmHg, and he was tachycardic in sinus rhythm with a heart rate of 90–100 beats/min.
• His initial electrocardiogram (ECG) showed inferior ST-segment elevations with lateral ST-segment depressions.
• He was referred emergently to the cardiac catheterization laboratory for primary PCI.
FIGURE 297e-2 Preprocedure ECG showing inferior ST-segment elevations and lateral ST-segment depressions.
FIGURE 297e-3 Example of an organized red thrombus retrieved by manual thrombectomy.
FIGURE 297e-4 Postprocedure ECG showing resolution of ST-segment elevations.
VIDEO 297e-17 The right coronary artery (RCA) is totally occluded with filling defects in the vessel after contrast injection, indicating thrombus is present in the vessel.
VIDEO 297e-18 An angioplasty wire is threaded through the thrombotic lesion, but this does not restore blood flow to the distal vessel.
VIDEO 297e-19 Result after manual thrombectomy and thrombus extraction. The “culprit” ruptured plaque and residual thrombus are now apparent in the vessel.
VIDEO 297e-20 After balloon angioplasty and stenting, thrombus is still present.
VIDEO 297e-21 After repeat manual thrombectomy and expansion of the stent, the thrombus is no longer present.
VIDEO 297e-22 Final result.
SUMMARY
• An acute STEMI occurs following plaque rupture that promotes thrombotic occlusion of a coronary artery.
• Despite successful revascularization of the epicardial coronary artery, microemboli liberated during balloon angioplasty and stenting may lead to persistent microvascular dysfunction. When present, microvascular dysfunction is associated with a larger infarct size, heart failure, malignant ventricular arrhythmias, and death.
• Manual thrombectomy is used to aspirate or remove thrombus in the vessel and limit distal embolization during angioplasty and stenting.
• Manual thrombectomy in primary PCI is associated with improved myocardial perfusion and a reduction in mortality.
• Adjunctive antiplatelet and antithrombin agents are important to aid in the resolution of intracoronary thrombus.
CASE 4: SAPHENOUS VEIN GRAFT INTERVENTION WITH DISTAL PROTECTION
(Fig. 297e-5; Videos 297e-23 to 297e-26)
• A 62-year-old male with a history of chronic stable angina.
• A four-vessel coronary artery bypass grafting (CABG) surgery was performed 17 years earlier with a left internal mammary artery graft to the LAD, a right internal mammary artery graft to the right coronary artery (RCA), a saphenous vein graft to the first obtuse marginal branch, and a saphenous vein graft to the first diagonal branch.
• The patient had a recent increase in angina with exertion and was found to have lateral ischemia on an exercise technetium-99m scan.
• Diagnostic cardiac catheterization revealed a significant stenosis in the body of the saphenous vein graft to the first obtuse marginal branch.
FIGURE 297e-5 Distal protection device showing captured atherosclerotic debris liberated by initial balloon dilation.
VIDEO 297e-23 Saphenous vein graft to a first obtuse marginal branch with an 80% eccentric stenosis in the midgraft.
VIDEO 297e-24 A distal protection device is deployed past the lesion.
VIDEO 297e-25 Angioplasty balloon inflation with the distal protection device in place.
VIDEO 297e-26 Final result after stent placement.
SUMMARY
• Saphenous vein grafts have a failure rate of up to 20% after 1 year and as high as 50% by 5 years.
• Graft failure (after >1 month) results from intimal hyperplasia and atherosclerosis.
• Saphenous vein graft PCI is associated with distal embolization of atherosclerotic debris and microthrombi leading to microvascular occlusion, reduced antegrade blood flow (the “no-reflow” phenomenon), and myocardial infarction.
• Embolic distal protection devices decrease the risk of distal embolization, as well as the incidence of no-reflow and myocardial infarction associated with saphenous vein graft interventions.
CASE 5: UNPROTECTED LEFT MAIN PCI IN A HIGH-RISK PATIENT
(Figs. 297e-6 and 297e-7; Videos 297e-27 to 297e-34)
• An 89-year-old woman presented with a NSTEMI associated with 5-mm ST-segment depression in the apical leads occurring 2 weeks after hospitalization for a NSTEMI that was treated conservatively.
• Chronic obstructive lung disease, elderly age, and the patient’s refusal to consider cardiac surgery restricted the choice of therapeutic options to medical and/or percutaneous interventions.
• Diagnostic catheterization revealed a left dominant circulation with a heavily calcified 80% distal left main coronary artery stenosis extending into the LAD and into the proximal LCx coronary arteries. A 70% proximal LAD lesion was also present.
• After consultation with the patient, family, and a cardiac surgeon, PCI was performed with intraaortic balloon pump support and a temporary pacemaker in the right ventricle.
FIGURE 297e-6 During chest pain, the ECG showed diffuse ST-segment depression of up to 5 mm in the inferior and lateral leads.
FIGURE 297e-7 Following resolution of the chest pain, the ST-segment depression is less marked.
VIDEO 297e-27 Baseline left coronary artery injection in right anterior oblique (RAO) cranial projection shows a high-grade calcified stenosis in the left main coronary artery and a significant stenosis in the proximal LAD.
VIDEO 297e-28 In the left anterior oblique (LAO) caudal view, the left main coronary artery lesion can be seen to extend into the ostia of both the LCx and the LAD.
VIDEO 297e-29 Guidewires were placed into both the LCx and LAD. After the left main coronary artery and LCx are dilated with balloon angioplasty, the proximal LAD is dilated, and a long drug-eluting stent is placed to cover a lesion dissection that occurred with wiring of the vessel.
VIDEO 297e-30 The bifurcation lesion in the left main coronary artery extending into the LCx and LAD ostia is treated using a “culotte” technique. First, a drug-eluting stent is placed in the left main coronary artery and into the proximal LCx.
VIDEO 297e-31 Next, the LAD wire is removed and passed through the stent into the distal LAD. A second drug-eluting stent is deployed through the struts of the left main coronary artery/LCx stent.
VIDEO 297e-32 Following rewiring of the LCx, both stents are re-dilated simultaneously (“kissing” balloons).
VIDEO 297e-33 The final result in the LAO caudal view.
VIDEO 297e-34 The final result in the RAO cranial view showing patent left main, LCx, and LAD coronary arteries.
SUMMARY
• Left main coronary artery disease occurs in 5–10% of patients with symptomatic coronary artery disease.
• In patients with left main coronary artery disease, revascularization with CABG has been shown to decrease mortality significantly over 5–10 years of follow-up.
• PCI with drug-eluting stents in selected cases has been shown to have equal in-hospital and 1-year death and myocardial infarction rates compared with CABG in the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial. Long-term outcome differences between the two treatment strategies are not known.
• Indications for PCI of left main coronary artery lesions are high-risk surgical patients and patients with protected left main coronary artery disease (i.e., prior CABG with patent bypass grafts). Patients who are good candidates for bypass surgery may also undergo a stenting procedure, but discussion with the patient, the interventional cardiologist, and the cardiac surgeon should be undertaken to determine the best treatment option in an individual case.
• Outcomes are better for patients with an isolated lesion in the ostium and body of the left main coronary artery where a single stent can be placed compared to bifurcation lesions that involve the ostium of the LAD and LCx.
CASE 6: MULTIVESSEL PCI IN A DIABETIC PATIENT
(Videos 297e-35 to 297e-42)
• A 58-year-old man presented with a NSTEMI.
• The patient has hyperlipidemia and type 2 diabetes mellitus treated with oral hypoglycemic agents.
• Diagnostic catheterization revealed two-vessel disease with a total occlusion of the second obtuse marginal branch that was felt to be responsible for the patient’s symptoms (culprit lesion). In addition, there was a high-grade stenosis in a large ramus intermedius branch, and the RCA had a significant stenosis in the midsegment of the vessel.
VIDEO 297e-35 Baseline angiogram of the left coronary circulation in the RAO view shows the total occlusion of the second obtuse marginal branch with delayed retrograde filling via collateral vessels and a high-grade stenosis in the ramus intermedius.
VIDEO 297e-36 A guidewire is passed through the total occlusion, and the lesion is pretreated with balloon angioplasty.
VIDEO 297e-37 Following placement of a drug-eluting stent in the lesion, the vessel is widely patent. A third obtuse marginal vessel, not previously seen, now fills faintly (Thrombolysis in Myocardial Infarction [TIMI] grade 1 flow) with contrast but was not treated.
VIDEO 297e-38 The ramus intermedius lesion was crossed with a guidewire and pretreated with balloon angioplasty.
VIDEO 297e-39 A drug-eluting stent is placed across the ramus lesion and deployed. The final result shows no residual stenosis in either the ramus or second obtuse marginal vessels.
VIDEO 297e-40 Baseline angiogram of the RCA shows a high-grade lesion in the midsegment of the vessel.
VIDEO 297e-41 The lesion was pretreated with balloon dilation followed by stent deployment.
VIDEO 297e-42 The final result shows no residual stenosis in the mid-RCA.
SUMMARY
• Multivessel PCI is performed commonly and may be done in one setting or staged with two or more procedures.
• Acute and long-term studies of multivessel PCI have shown comparable rates of death and myocardial infarction when compared to CABG, but a higher incidence of repeat revascularization as a result of restenosis is associated with PCI.
• In the randomized Bypass Angioplasty Revascularization Investigation (BARI) trial, diabetic patients treated with PCI had a worse long-term mortality than diabetic patients treated with CABG. However, the BARI registry found that in selected diabetic patients with favorable anatomy, PCI can result in outcomes equal to those observed with CABG.
CASE 7: VERY LATE STENT THROMBOSIS OF A PROXIMAL LAD DRUG-ELUTING STENT
(Figs. 297e-8 and 297e-9; Videos 297e-43 to 297e-46)
• A 62-year-old male had a drug-eluting stent placed in a proximal LAD lesion to treat severe angina. He received dual antiplatelet therapy with aspirin and clopidogrel for 1 year and then discontinued clopidogrel per protocol.
• He remained asymptomatic until 15 months after the initial stent placement when he presented with severe chest pain due to an acute anterior STEMI.
• He was taken to the catheterization laboratory within 70 min of presentation, and his initial angiogram showed a total occlusion of the proximal LAD stent.
FIGURE 297e-8 Optical coherence tomography image following initial balloon dilation. Residual thrombus that is adherent to the stent struts is seen.
FIGURE 297e-9 Pathologic specimen of late stent thrombosis obtained at autopsy. Thrombus is seen filling the LAD vessel lumen and extending into a diagonal branch (LD). Stent struts occupied the space denoted by the asterisk (*) (left). A magnified view of the vessel reveals thrombus around the stent strut and neointima formation (arrow) (right).
VIDEO 297e-43 Baseline angiogram showing a total occlusion of the proximal LAD within the drug-eluting stent and a significant stenosis at the origin of the LCx.
VIDEO 297e-44 The LAO view shows the LCx stenosis with a filling defect indicating that thrombus is present in the vessel lumen.
VIDEO 297e-45 The LAD lesion was crossed with a guidewire, which resulted in slow filling of the mid-LAD (TIMI 2 flow) and revealed thrombus filling the stent.
VIDEO 297e-46 The final result after LAD and LCx stenting. The LAD lesion was pretreated with balloon angioplasty, and a bare metal stent was deployed to cover the proximal lesion. The LCx ostial lesion was dilated with balloon angioplasty, and a bare metal stent was placed using a “V stenting” technique.
SUMMARY
• Stent thrombosis is an infrequent (1–2%) but serious complication of stent placement. It occurs most commonly within the first month, but rarely can occur as late as 1 year (0.2–0.6%) with bare metal stents. Very late stent thrombosis (VLST), which occurs after 1 year, is very rare with bare metal stents but can occur with drug-eluting stents.
• Premature discontinuation of dual antiplatelet therapy is the most common cause of early and late stent thrombosis; however, the etiology of VLST is not clear.
• The majority of patients with stent thrombosis present with acute coronary syndromes or STEMI; this presentation is associated with a high mortality rate (10%).
• Treatment is immediate PCI with balloon angioplasty or re-stenting.
CASE 8: TRANSCATHETER AORTIC VALVE REPLACEMENT
(Figs. 297e-10 to 297e-14; Videos 297e-47 to 297e-50)
• A 75-year-old female with symptomatic aortic stenosis and a valve area of 0.58 cm2 by transthoracic echocardiogram.
• Chronic obstructive pulmonary disease (forced expiratory volume in 1 s [FEV1] = 0.54) and other comorbidities contributed to an unacceptably high cardiac surgical risk (calculated logistic Euroscore = 29.57%) for aortic valve replacement.
• She was referred for transcatheter aortic valve replacement (TAVR) as part of a clinical trial.
FIGURE 297e-10 Transesophageal echocardiogram shows a calcified trileaflet aortic valve (left) with reduced leaflet excursion and a narrowed orifice in peak systole (right).
FIGURE 297e-11 Hemodynamically significant aortic (AO) stenosis. Simultaneous recording of AO and left ventricle (LV) pressures shows an 82 mmHg peak-to-peak gradient and a 63.3 mmHg mean gradient between the LV (154/9 mmHg) and AO (72/29 mmHg) pressures. This is consistent with an aortic valve area of 0.58 cm2.
FIGURE 297e-12 After balloon valvuloplasty, the LV-AO mean pressure gradient decreased to 37.3 mmHg, indicating that the aortic valve area increased to 0.95 cm2.
FIGURE 297e-13 The Edwards SAPIEN transcatheter heart valve. (Reprinted with permission from A Zajarias, AG Cribier: J Am Coll Cardiol 53:1829, 2009.)
FIGURE 297e-14 Once the valve was deployed, the pressure gradient between the LV and AO decreased to 11.6 mmHg, and the functional valve area is 1.34 cm2.
VIDEO 297e-47 Aortogram shows patent coronary arteries and minimal aortic insufficiency.
VIDEO 297e-48 Balloon valvuloplasty is performed with rapid ventricular pacing at 180 beats/min.
VIDEO 297e-49 A 26-mm Edwards SAPIEN valve is positioned using fluoroscopic and transesophageal echo guidance and deployed.
VIDEO 297e-50 Aortogram after valve deployment shows a functional valve with mild aortic insufficiency and without impingement of the coronary ostia.
SUMMARY
• The prevalence of calcific aortic stenosis is 2–3% in individuals age ≥75 years.
• Symptomatic aortic stenosis is associated with an average survival of 2–3 years and an increased risk of sudden death; aortic valve replacement improves both symptoms and survival.
• In high-risk patients with severe aortic stenosis who are not surgical candidates, 1- and 5-year survival rates are ~62% and 38%, respectively.
• TAVR is approved in Europe and was recently approved in the United States as an alternative to surgical aortic valve replacement in high-risk patients.
(Case contributed with permission by Dr. Andrew C. Eisenhauer.)
CASE 9: ATRIAL SEPTAL DEFECT CLOSURE
(Figs. 297e-15 to 297e-21; Videos 297e-51 to 297e-53)
• A 48-year-old female with increased shortness of breath, exercise intolerance, and an 18-mm secundum ASD.
• Echocardiogram showed a dilated right atrium (RA) and right ventricle (RV) with evidence of right ventricular volume overload.
• A shunt ratio (Qp/Qs) of 2.3:1 was determined at cardiac catheterization.
• Based on her symptoms, evidence of right-side chamber dilation, and a moderately sized ASD, the patient was referred for percutaneous closure of the ASD.
FIGURE 297e-15 Anatomic location of common atrial septal defects (ASDs). The most common ASDs are the sinus venosus, ostium secundum, and ostium primum ASDs. The sinus venosus ASD is located at the junction of the superior vena cava (SVC) and the right atrium (RA). This ASD is often associated with anomalous drainage of the right-side pulmonary veins into the RA instead of the left atrium. The secundum ASD is located at the foramen ovale and allows blood to flow between the RA and the left atrium. The primum ASD, also known as an atrioventricular septal defect, connects the RA and right ventricle with the left atrium and ventricle. (Illustration by Justin E. Tribuna.)
FIGURE 297e-16 Transesophageal echocardiogram of a secundum ASD. The ASD is seen as “dropout” in the interatrial septum between the left atrium (LA) and RA (left). Doppler color flow imaging shows blue in the RA consistent with left-to-right flow (right).
FIGURE 297e-17 Three-dimensional echocardiographic reconstruction of the secundum ASD. The ASD is round and has an acceptable margin of tissue to seat a septal occluder device.
FIGURE 297e-18 ASD percutaneous closure devices. The Amplatzer® septal occluder (left) and the HELEX® septal occluder (right) are among the devices used for percutaneous closure of ASDs. The Amplatzer® septal occluder is a plug between two disks that are positioned on each side of the ASD to obstruct blood flow. The HELEX® Septal Occluder is positioned on each side of the ASD to allow circular rings covered with rapidly stretching polytetrafluoroethylene device to limit blood flow. The delivery catheters are detached and the devices are left in place. (Illustration by Justin E. Tribuna.)
FIGURE 297e-19 Transesophageal echocardiogram showing sizing balloon (left) and no flow (right) across the atrial septal defect.
FIGURE 297e-20 Amplatzer® septal occluder in place (left). There is no blood flow across the device (right).
FIGURE 297e-21 Postprocedure lateral chest x-ray showing the Amplatzer® septal occluder in place.
VIDEO 297e-51 A sizing balloon is placed across the ASD.
VIDEO 297e-52 An Amplatzer® septal occluder is being positioned across the ASD.
VIDEO 297e-53 The two disks of the device in place across the ASD.
SUMMARY
• Unrepaired ASDs lead to signs and symptoms of increased pulmonary blood flow and right heart failure, dyspnea, exercise intolerance, fatigue, palpitations and atrial arrhythmias, and pulmonary infections.
• Percutaneous closure of an ASD may be recommended for individuals with a secundum ASD and evidence of RA and RV enlargement with or without symptoms.
• Percutaneous closure is contraindicated in patients with irreversible pulmonary arterial hypertension and no left-to-right shunt. It is not recommended for closure of sinus venosus, coronary sinus, or primum ASDs.
• After the atrial septal occluder device is placed, patients are treated with antiplatelet agents and use antibiotic prophylaxis for certain procedures for 6 months. Follow-up echocardiograms to assess for device migration or erosion, residual shunting, thrombus, or pericardial effusion are recommended at 1 day, 1 month, 6 months, 1 year, and periodically thereafter.
(Case contributed with permission by Dr. Andrew C. Eisenhauer.)
298 |
Hypertensive Vascular Disease |
Hypertension is one of the leading causes of the global burden of disease. Approximately 7.6 million deaths (13–15% of the total) and 92 million disability-adjusted life years worldwide were attributable to high blood pressure in 2001. Hypertension doubles the risk of cardiovascular diseases, including coronary heart disease (CHD), congestive heart failure (CHF), ischemic and hemorrhagic stroke, renal failure, and peripheral arterial disease. It often is associated with additional cardiovascular disease risk factors, and the risk of cardiovascular disease increases with the total burden of risk factors. Although antihypertensive therapy reduces the risks of cardiovascular and renal disease, large segments of the hypertensive population are either untreated or inadequately treated.
EPIDEMIOLOGY
Blood pressure levels, the rate of age-related increases in blood pressure, and the prevalence of hypertension vary among countries and among subpopulations within a country. Hypertension is present in all populations except for a small number of individuals living in developing countries. In industrialized societies, blood pressure increases steadily during the first two decades of life. In children and adolescents, blood pressure is associated with growth and maturation. Blood pressure “tracks” over time in children and between adolescence and young adulthood. In the United States, average systolic blood pressure is higher for men than for women during early adulthood, although among older individuals the age-related rate of rise is steeper for women. Consequently, among individuals age 60 and older, systolic blood pressures of women are higher than those of men. Among adults, diastolic blood pressure also increases progressively with age until ∼55 years, after which it tends to decrease. The consequence is a widening of pulse pressure (the difference between systolic and diastolic blood pressure) beyond age 60.
In the United States, based on results of the National Health and Nutrition Examination Survey (NHANES), approximately 30% (age-adjusted prevalence) of adults, or at least 65 million individuals, have hypertension (defined as any one of the following: systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, taking antihypertensive medications). Hypertension prevalence is 33.5% in non-Hispanic blacks, 28.9% in non-Hispanic whites, and 20.7% in Mexican Americans. The likelihood of hypertension increases with age, and among individuals age ≥60, the prevalence is 65.4%. Recent evidence suggests that the prevalence of hypertension in the United States may be increasing, possibly as a consequence of increasing obesity. The prevalence of hypertension and stroke mortality rates are higher in the southeastern United States than in other regions. In African Americans, hypertension appears earlier, is generally more severe, and results in higher rates of morbidity and mortality from stroke, left ventricular hypertrophy, CHF, and end-stage renal disease (ESRD) than in white Americans.
Both environmental and genetic factors may contribute to regional and racial variations in hypertension prevalence. Studies of societies undergoing “acculturation” and studies of migrants from a less to a more urbanized setting indicate a profound environmental contribution to blood pressure. Obesity and weight gain are strong, independent risk factors for hypertension. It has been estimated that 60% of hypertensives are >20% overweight. Among populations, hypertension prevalence is related to dietary NaCl intake, and the age-related increase in blood pressure may be augmented by a high NaCl intake. Low dietary intakes of calcium and potassium also may contribute to the risk of hypertension. The urine sodium-to-potassium ratio (an index of both sodium and potassium intakes) is a stronger correlate of blood pressure than is either sodium or potassium alone. Alcohol consumption, psychosocial stress, and low levels of physical activity also may contribute to hypertension.
Adoption, twin, and family studies document a significant heritable component to blood pressure levels and hypertension. Family studies controlling for a common environment indicate that blood pressure heritabilities are in the range 15–35%. In twin studies, heritability estimates of blood pressure are ∼60% for males and 30–40% for females. High blood pressure before age 55 occurs 3.8 times more frequently among persons with a positive family history of hypertension. However, to date, only a fraction of high heritability estimates are accounted for by specific genetic determinants.
GENETIC CONSIDERATIONS
![]() Although specific genetic variants have been identified in rare Mendelian forms of hypertension (Table 298–5), these variants are not applicable to the vast majority (>98%) of patients with hypertension. For most individuals, it is likely that hypertension represents a polygenic disorder in which a combination of genes acts in concert with environmental exposures to make only a modest contribution to blood pressure. Further, different subsets of genes may lead to different phenotypes associated with hypertension, e.g., obesity, dyslipidemia, insulin resistance.
Although specific genetic variants have been identified in rare Mendelian forms of hypertension (Table 298–5), these variants are not applicable to the vast majority (>98%) of patients with hypertension. For most individuals, it is likely that hypertension represents a polygenic disorder in which a combination of genes acts in concert with environmental exposures to make only a modest contribution to blood pressure. Further, different subsets of genes may lead to different phenotypes associated with hypertension, e.g., obesity, dyslipidemia, insulin resistance.
Several strategies are being used in the search for specific hypertension-related genes. Animal models (including selectively bred rats and congenic rat strains) provide a powerful approach for evaluating genetic loci and genes associated with hypertension. Comparative mapping strategies allow for the identification of syntenic genomic regions between the rat and human genomes that may be involved in blood pressure regulation. In association studies, different alleles (or combinations of alleles at different loci) of specific candidate genes or chromosomal regions are compared in hypertensive patients and normotensive control subjects. Current evidence suggests that genes that encode components of the renin-angiotensin-aldosterone system, along with angiotensinogen and angiotensin-converting enzyme (ACE) polymorphisms, may be related to hypertension and to blood pressure sensitivity to dietary NaCl. The alpha-adducin gene is thought to be associated with increased renal tubular absorption of sodium, and variants of this gene may be associated with hypertension and salt sensitivity of blood pressure. Other genes possibly related to hypertension include genes encoding the AT1 receptor, aldosterone synthase, atrial natriuretic peptide, and the β2 adrenoreceptor. Genomewide association studies involve rapidly scanning markers across the entire genome to identify loci (not specific genes) associated with an observable trait (e.g., blood pressure) or a particular disease. This strategy has been facilitated by the availability of dense genotyping chips and the International HapMap. Results of candidate gene studies often have not been replicated, and in contrast to several other polygenic disorders, genomewide association studies have had limited success in identifying genetic determinants of hypertension.
Preliminary evidence suggests that there may also be genetic determinants of target organ damage attributed to hypertension. Family studies indicate significant heritability of left ventricular mass, and there is considerable individual variation in the responses of the heart to hypertension. Family studies and variations in candidate genes associated with renal damage suggest that genetic factors also may contribute to hypertensive nephropathy. Specific genetic variants have been linked to CHD and stroke.
In the future, it is possible that DNA analysis will predict individual risk for hypertension and target organ damage and will identify responders to specific classes of antihypertensive agents. However, with the exception of the rare, monogenic hypertensive diseases, the genetic variants associated with hypertension remain to be confirmed, and the intermediate steps by which these variants affect blood pressure remain to be determined.
MECHANISMS OF HYPERTENSION
To provide a framework for understanding the pathogenesis of and treatment options for hypertensive disorders, it is useful to understand factors involved in the regulation of both normal and elevated arterial pressure. Cardiac output and peripheral resistance are the two determinants of arterial pressure (Fig. 298-1). Cardiac output is determined by stroke volume and heart rate; stroke volume is related to myocardial contractility and to the size of the vascular compartment. Peripheral resistance is determined by functional and anatomic changes in small arteries (lumen diameter 100–400 μm) and arterioles.
FIGURE 298-1 Determinants of arterial pressure.
INTRAVASCULAR VOLUME
Sodium is predominantly an extracellular ion and is a primary determinant of the extracellular fluid volume. When NaCl intake exceeds the capacity of the kidney to excrete sodium, vascular volume may initially expand and cardiac output may increase. However, many vascular beds have the capacity to autoregulate blood flow, and if constant blood flow is to be maintained in the face of increased arterial pressure, resistance within that bed must increase, since
The initial elevation of blood pressure in response to vascular volume expansion may be related to an increase of cardiac output; however, over time, peripheral resistance increases and cardiac output reverts toward normal. Whether this hypothesized sequence of events occurs in the pathogenesis of hypertension is not clear. What is clear is that salt can activate a number of neural, endocrine/paracrine, and vascular mechanisms, all of which have the potential to increase arterial pressure. The effect of sodium on blood pressure is related to the provision of sodium with chloride; nonchloride salts of sodium have little or no effect on blood pressure. As arterial pressure increases in response to a high NaCl intake, urinary sodium excretion increases and sodium balance is maintained at the expense of an increase in arterial pressure. The mechanism for this “pressure-natriuresis” phenomenon may involve a subtle increase in the glomerular filtration rate, decreased absorbing capacity of the renal tubules, and possibly hormonal factors such as atrial natriuretic factor. In individuals with an impaired capacity to excrete sodium, greater increases in arterial pressure are required to achieve natriuresis and sodium balance.
NaCl-dependent hypertension may be a consequence of a decreased capacity of the kidney to excrete sodium, due either to intrinsic renal disease or to increased production of a salt-retaining hormone (mineralocorticoid) resulting in increased renal tubular reabsorption of sodium. Renal tubular sodium reabsorption also may be augmented by increased neural activity to the kidney. In each of these situations, a higher arterial pressure may be required to achieve sodium balance. Conversely, salt-wasting disorders are associated with low blood pressure levels. ESRD is an extreme example of volume-dependent hypertension. In ∼80% of these patients, vascular volume and hypertension can be controlled with adequate dialysis; in the other 20%, the mechanism of hypertension is related to increased activity of the renin-angiotensin system and is likely to be responsive to pharmacologic blockade of renin-angiotensin.
AUTONOMIC NERVOUS SYSTEM
Adrenergic reflexes modulate blood pressure over the short term, and adrenergic function, in concert with hormonal and volume-related factors, contributes to the long-term regulation of arterial pressure. Norepinephrine, epinephrine, and dopamine all play important roles in tonic and phasic cardiovascular regulation.
The activities of the adrenergic receptors are mediated by guanosine nucleotide-binding regulatory proteins (G proteins) and by intracellular concentrations of downstream second messengers. In addition to receptor affinity and density, physiologic responsiveness to catecholamines may be altered by the efficiency of receptor-effector coupling at a site “distal” to receptor binding. The receptor sites are relatively specific both for the transmitter substance and for the response that occupancy of the receptor site elicits. Based on their physiology and pharmacology, adrenergic receptors have been divided into two principal types: α and β. These types have been differentiated further into α1, α2, β1, and β2 receptors. Recent molecular cloning studies have identified several additional subtypes. α Receptors are occupied and activated more avidly by norepinephrine than by epinephrine, and the reverse is true for β receptors. α1 Receptors are located on postsynaptic cells in smooth muscle and elicit vasoconstriction. α2 Receptors are localized on presynaptic membranes of postganglionic nerve terminals that synthesize norepinephrine. When activated by catecholamines, α2 receptors act as negative feedback controllers, inhibiting further norepinephrine release. In the kidney, activation of α1-adrenergic receptors increases renal tubular reabsorption of sodium. Different classes of antihypertensive agents either inhibit α1 receptors or act as agonists of α2 receptors and reduce systemic sympathetic outflow. Activation of myocardial β1 receptors stimulates the rate and strength of cardiac contraction and consequently increases cardiac output. β1 Receptor activation also stimulates renin release from the kidney. Another class of antihypertensive agents acts by inhibiting β1 receptors. Activation of β2 receptors by epinephrine relaxes vascular smooth muscle and results in vasodilation.
Circulating catecholamine concentrations may affect the number of adrenoreceptors in various tissues. Downregulation of receptors may be a consequence of sustained high levels of catecholamines and provides an explanation for decreasing responsiveness, or tachyphylaxis, to catecholamines. For example, orthostatic hypotension frequently is observed in patients with pheochromocytoma, possibly due to the lack of norepinephrine-induced vasoconstriction with assumption of the upright posture. Conversely, with chronic reduction of neurotransmitter substances, adrenoreceptors may increase in number or be upregulated, resulting in increased responsiveness to the neurotransmitter. Chronic administration of agents that block adrenergic receptors may result in upregulation, and abrupt withdrawal of those agents may produce a condition of temporary hypersensitivity to sympathetic stimuli. For example, clonidine is an antihypertensive agent that is a centrally acting α2 agonist that inhibits sympathetic outflow. Rebound hypertension may occur with the abrupt cessation of clonidine therapy, probably as a consequence of upregulation of α1 receptors.
Several reflexes modulate blood pressure on a minute-to-minute basis. One arterial baroreflex is mediated by stretch-sensitive sensory nerve endings in the carotid sinuses and the aortic arch. The rate of firing of these baroreceptors increases with arterial pressure, and the net effect is a decrease in sympathetic outflow, resulting in decreases in arterial pressure and heart rate. This is a primary mechanism for rapid buffering of acute fluctuations of arterial pressure that may occur during postural changes, behavioral or physiologic stress, and changes in blood volume. However, the activity of the baroreflex declines or adapts to sustained increases in arterial pressure such that the baroreceptors are reset to higher pressures. Patients with autonomic neuropathy and impaired baroreflex function may have extremely labile blood pressures with difficult-to-control episodic blood pressure spikes associated with tachycardia.
In both normal-weight and obese individuals, hypertension often is associated with increased sympathetic outflow. Based on recordings of postganglionic muscle nerve activity (detected by a microelectrode inserted in a peroneal nerve in the leg), sympathetic outflow tends to be higher in hypertensive than in normotensive individuals. Sympathetic outflow is increased in obesity-related hypertension and in hypertension associated with obstructive sleep apnea. Baroreceptor activation via electrical stimulation of carotid sinus afferent nerves lowers blood pressure in patients with “resistant” hypertension. Drugs that block the sympathetic nervous system are potent antihypertensive agents, indicating that the sympathetic nervous system plays a permissive, although not necessarily a causative, role in the maintenance of increased arterial pressure.
Pheochromocytoma is the most blatant example of hypertension related to increased catecholamine production, in this instance by a tumor. Blood pressure can be reduced by surgical excision of the tumor or by pharmacologic treatment with an α1 receptor antagonist or with an inhibitor of tyrosine hydroxylase, the rate-limiting step in catecholamine biosynthesis.
RENIN-ANGIOTENSIN-ALDOSTERONE
The renin-angiotensin-aldosterone system contributes to the regulation of arterial pressure primarily via the vasoconstrictor properties of angiotensin II and the sodium-retaining properties of aldosterone. Renin is an aspartyl protease that is synthesized as an enzymatically inactive precursor, prorenin. Most renin in the circulation is synthesized in the renal afferent renal arteriole. Prorenin may be secreted directly into the circulation or may be activated within secretory cells and released as active renin. Although human plasma contains two to five times more prorenin than renin, there is no evidence that prorenin contributes to the physiologic activity of this system. There are three primary stimuli for renin secretion: (1) decreased NaCl transport in the distal portion of the thick ascending limb of the loop of Henle that abuts the corresponding afferent arteriole (macula densa), (2) decreased pressure or stretch within the renal afferent arteriole (baroreceptor mechanism), and (3) sympathetic nervous system stimulation of renin-secreting cells via β1 adrenoreceptors. Conversely, renin secretion is inhibited by increased NaCl transport in the thick ascending limb of the loop of Henle, by increased stretch within the renal afferent arteriole, and by β1 receptor blockade. In addition, angiotensin II directly inhibits renin secretion due to angiotensin II type 1 receptors on juxtaglomerular cells, and renin secretion increases in response to pharmacologic blockade of either ACE or angiotensin II receptors.
Once released into the circulation, active renin cleaves a substrate, angiotensinogen, to form an inactive decapeptide, angiotensin I (Fig. 298-2). A converting enzyme, located primarily but not exclusively in the pulmonary circulation, converts angiotensin I to the active octapeptide, angiotensin II, by releasing the C-terminal histidyl-leucine dipeptide. The same converting enzyme cleaves a number of other peptides, including and thereby inactivating the vasodilator bradykinin. Acting primarily through angiotensin II type 1 (AT1) receptors on cell membranes, angiotensin II is a potent pressor substance, the primary tropic factor for the secretion of aldosterone by the adrenal zona glomerulosa, and a potent mitogen that stimulates vascular smooth muscle cell and myocyte growth. Independent of its hemodynamic effects, angiotensin II may play a role in the pathogenesis of atherosclerosis through a direct cellular action on the vessel wall. The angiotensin II type 2 (AT2) receptor has the opposite functional effects of the AT1 receptor. The AT2 receptor induces vasodilation, sodium excretion, and inhibition of cell growth and matrix formation. Experimental evidence suggests that the AT2 receptor improves vascular remodeling by stimulating smooth muscle cell apoptosis and contributes to the regulation of glomerular filtration rate. AT1 receptor blockade induces an increase in AT2 receptor activity.
FIGURE 298-2 Renin-angiotensin-aldosterone axis. ACE, angiotensin-converting enzyme.
Renin-secreting tumors are clear examples of renin-dependent hypertension. In the kidney, these tumors include benign hemangiopericytomas of the juxtaglomerular apparatus and, infrequently, renal carcinomas, including Wilms’ tumors. Renin-producing carcinomas also have been described in lung, liver, pancreas, colon, and adrenals. In these instances, in addition to excision and/or ablation of the tumor, treatment of hypertension includes pharmacologic therapies targeted to inhibit angiotensin II production or action. Renovascular hypertension is another renin-mediated form of hypertension. Obstruction of the renal artery leads to decreased renal perfusion pressure, thereby stimulating renin secretion. Over time, possibly as a consequence of secondary renal damage, this form of hypertension may become less renin dependent.
Angiotensinogen, renin, and angiotensin II are also synthesized locally in many tissues, including the brain, pituitary, aorta, arteries, heart, adrenal glands, kidneys, adipocytes, leukocytes, ovaries, testes, uterus, spleen, and skin. Angiotensin II in tissues may be formed by the enzymatic activity of renin or by other proteases, e.g., tonin, chymase, and cathepsins. In addition to regulating local blood flow, tissue angiotensin II is a mitogen that stimulates growth and contributes to modeling and repair. Excess tissue angiotensin II may contribute to atherosclerosis, cardiac hypertrophy, and renal failure and consequently may be a target for pharmacologic therapy to prevent target organ damage.
Angiotensin II is the primary tropic factor regulating the synthesis and secretion of aldosterone by the zona glomerulosa of the adrenal cortex. Aldosterone synthesis is also dependent on potassium, and aldosterone secretion may be decreased in potassium-depleted individuals. Although acute elevations of adrenocorticotropic hormone (ACTH) levels also increase aldosterone secretion, ACTH is not an important tropic factor for the chronic regulation of aldosterone.
Aldosterone is a potent mineralocorticoid that increases sodium reabsorption by amiloride-sensitive epithelial sodium channels (ENaC) on the apical surface of the principal cells of the renal cortical collecting duct (Chap. 332e). Electric neutrality is maintained by exchanging sodium for potassium and hydrogen ions. Consequently, increased aldosterone secretion may result in hypokalemia and alkalosis.
Cortisol also binds to the mineralocorticoid receptor but normally functions as a less potent mineralocorticoid than aldosterone because cortisol is converted to cortisone by the enzyme 11 β-hydroxysteroid dehydrogenase type 2. Cortisone has no affinity for the mineralocorticoid receptor. Primary aldosteronism is a compelling example of mineralocorticoid-mediated hypertension. In this disorder, adrenal aldosterone synthesis and release are independent of renin-angiotensin, and renin release is suppressed by the resulting volume expansion.
Mineralocorticoid receptors are expressed in a number of tissues in addition to the kidney, and mineralocorticoid receptor activation induces structural and functional alterations in the heart, kidney, and blood vessels, leading to myocardial fibrosis, nephrosclerosis, and vascular inflammation and remodeling, perhaps as a consequence of oxidative stress. These effects are amplified by a high salt intake. In animal models, high circulating aldosterone levels stimulate cardiac fibrosis and left ventricular hypertrophy, and spironolactone (an aldosterone antagonist) prevents aldosterone-induced myocardial fibrosis. Pathologic patterns of left ventricular geometry also have been associated with elevations of plasma aldosterone concentration in hypertensive patients. In patients with CHF, low-dose spironolactone reduces the risk of progressive heart failure and sudden death from cardiac causes by 30%. Due to a renal hemodynamic effect, in patients with primary aldosteronism, high circulating levels of aldosterone also may cause glomerular hyperfiltration and albuminuria. These renal effects are reversible after removal of the effects of excess aldosterone by adrenalectomy or spironolactone.
Increased activity of the renin-angiotensin-aldosterone axis is not invariably associated with hypertension. In response to a low-NaCl diet or to volume contraction, arterial pressure and volume homeostasis may be maintained by increased activity of the renin-angiotensin-aldosterone axis. Secondary aldosteronism (i.e., increased aldosterone secondary to increased renin-angiotensin), but not hypertension, also is observed in edematous states such as CHF and liver disease.
VASCULAR MECHANISMS
Vascular radius and compliance of resistance arteries are important determinants of arterial pressure. Resistance to flow varies inversely with the fourth power of the radius, and consequently, small decreases in lumen size significantly increase resistance. In hypertensive patients, structural, mechanical, or functional changes may reduce the lumen diameter of small arteries and arterioles. Remodeling refers to geometric alterations in the vessel wall without a change in vessel volume. Hypertrophic (increased cell size, and increased deposition of intercellular matrix) or eutrophic vascular remodeling results in decreased lumen size and, hence, increased peripheral resistance. Apoptosis, low-grade inflammation, and vascular fibrosis also contribute to remodeling. Lumen diameter also is related to elasticity of the vessel. Vessels with a high degree of elasticity can accommodate an increase of volume with relatively little change in pressure, whereas in a semirigid vascular system, a small increment in volume induces a relatively large increment of pressure.
Hypertensive patients may have stiffer arteries due to arteriosclerosis, and high systolic blood pressures and wide pulse pressures are a consequence of decreased vascular compliance. Due to arterial stiffness, central blood pressures (aortic, carotid) may not correspond to brachial artery pressures. Ejection of blood into the aorta elicits a pressure wave that is propagated at a given velocity. The forward travelling wave generates a reflected wave that travels backward toward the ascending aorta. Although mean arterial pressure is determined by cardiac output and peripheral resistance, pulse pressure is related to the functional properties of large arteries and the amplitude and timing of the incident and reflected waves. Increased arterial stiffness results in increased pulse wave velocity of both incident and reflected waves. Due to the timing of these waves, the consequence is augmentation of aortic systolic pressure and a reduction of aortic diastolic pressure, i.e., an increase in pulse pressure. The aortic augmentation index, an index of arterial stiffening, is calculated as the ratio of central arterial pressure-to-pulse pressure. Central blood pressure may be measured directly by placing a sensor in the aorta or noninvasively by radial tonometry using commercially available devices. Central blood pressure and the aortic augmentation index are strong, independent predictors of cardiovascular disease and all-cause mortality.
Ion transport by vascular smooth muscle cells may contribute to hypertension-associated abnormalities of vascular tone and vascular growth, both of which are modulated by intracellular pH (pHi). Three ion transport mechanisms participate in the regulation of pHi: (1) Na+-H+ exchange, (2) Na+-dependent HCO3–-Cl– exchange, and (3) cation-independent HCO3–-Cl– exchange. Based on measurements in cell types that are more accessible than vascular smooth muscle (e.g., leukocytes, erythrocytes, platelets, skeletal muscle), activity of the Na+-H+ exchanger is increased in hypertension, and this may result in increased vascular tone by two mechanisms. First, increased sodium entry may lead to increased vascular tone by activating Na+-Ca2+ exchange and thereby increasing intracellular calcium. Second, increased pHi enhances calcium sensitivity of the contractile apparatus, leading to an increase in contractility for a given intracellular calcium concentration. Additionally, increased Na+-H+ exchange may stimulate growth of vascular smooth muscle cells by enhancing sensitivity to mitogens.
Vascular endothelial function also modulates vascular tone. The vascular endothelium synthesizes and releases several vasoactive substances, including nitric oxide, a potent vasodilator. Endothelium-dependent vasodilation is impaired in hypertensive patients. This impairment often is assessed with high-resolution ultrasonography before and after the hyperemic phase of reperfusion that follows 5 minutes of forearm ischemia. Alternatively, endothelium-dependent vasodilation may be assessed in response to an intra-arterially infused endothelium-dependent vasodilator, e.g., acetylcholine. Endothelin is a vasoconstrictor peptide produced by the endothelium, and orally active endothelin antagonists may lower blood pressure in patients with resistant hypertension.
Currently, it is not known if the hypertension-related vascular abnormalities of ion transport and endothelial function are primary alterations or secondary consequences of elevated arterial pressure. Limited evidence suggests that vascular compliance and endothelium-dependent vasodilation may be improved by aerobic exercise, weight loss, and antihypertensive agents. It remains to be determined whether these interventions affect arterial structure and stiffness via a blood pressure–independent mechanism and whether different classes of antihypertensive agents preferentially affect vascular structure and function.
PATHOLOGIC CONSEQUENCES OF HYPERTENSION
Hypertension is an independent predisposing factor for heart failure, coronary artery disease, stroke, renal disease, and peripheral arterial disease (PAD).
HEART
Heart disease is the most common cause of death in hypertensive patients. Hypertensive heart disease is the result of structural and functional adaptations leading to left ventricular hypertrophy, CHF, abnormalities of blood flow due to atherosclerotic coronary artery disease and microvascular disease, and cardiac arrhythmias.
Individuals with left ventricular hypertrophy are at increased risk for CHD, stroke, CHF, and sudden death. Aggressive control of hypertension can regress or reverse left ventricular hypertrophy and reduce the risk of cardiovascular disease. It is not clear whether different classes of antihypertensive agents have an added impact on reducing left ventricular mass, independent of their blood pressure–lowering effect.
CHF may be related to systolic dysfunction, diastolic dysfunction, or a combination of the two. Abnormalities of diastolic function that range from asymptomatic heart disease to overt heart failure are common in hypertensive patients. Approximately one-third of patients with CHF have normal systolic function but abnormal diastolic function. Diastolic dysfunction is an early consequence of hypertension-related heart disease and is exacerbated by left ventricular hypertrophy and ischemia. Cardiac catheterization provides the most accurate assessment of diastolic function. Alternatively, diastolic function can be evaluated by several noninvasive methods, including echocardiography and radionuclide angiography.
BRAIN
Stroke is the second most frequent cause of death in the world; it accounts for 5 million deaths each year, with an additional 15 million persons having nonfatal strokes. Elevated blood pressure is the strongest risk factor for stroke. Approximately 85% of strokes are due to infarction, and the remainder are due to either intracerebral or subarachnoid hemorrhage. The incidence of stroke rises progressively with increasing blood pressure levels, particularly systolic blood pressure in individuals >65 years. Treatment of hypertension decreases the incidence of both ischemic and hemorrhagic strokes.
Hypertension also is associated with impaired cognition in an aging population, and longitudinal studies support an association between midlife hypertension and late-life cognitive decline. Hypertension-related cognitive impairment and dementia may be a consequence of a single infarct due to occlusion of a “strategic” larger vessel or multiple lacunar infarcts due to occlusive small vessel disease resulting in subcortical white matter ischemia. Several clinical trials suggest that antihypertensive therapy has a beneficial effect on cognitive function, although this remains an active area of investigation.
Cerebral blood flow remains unchanged over a wide range of arterial pressures (mean arterial pressure of 50–150 mmHg) through a process termed autoregulation of blood flow. In patients with the clinical syndrome of malignant hypertension, encephalopathy is related to failure of autoregulation of cerebral blood flow at the upper pressure limit, resulting in vasodilation and hyperperfusion. Signs and symptoms of hypertensive encephalopathy may include severe headache, nausea and vomiting (often of a projectile nature), focal neurologic signs, and alterations in mental status. Untreated, hypertensive encephalopathy may progress to stupor, coma, seizures, and death within hours. It is important to distinguish hypertensive encephalopathy from other neurologic syndromes that may be associated with hypertension, e.g., cerebral ischemia, hemorrhagic or thrombotic stroke, seizure disorder, mass lesions, pseudotumor cerebri, delirium tremens, meningitis, acute intermittent porphyria, traumatic or chemical injury to the brain, and uremic encephalopathy.
KIDNEY
The kidney is both a target and a cause of hypertension. Primary renal disease is the most common etiology of secondary hypertension. Mechanisms of kidney-related hypertension include a diminished capacity to excrete sodium, excessive renin secretion in relation to volume status, and sympathetic nervous system overactivity. Conversely, hypertension is a risk factor for renal injury and ESRD. The increased risk associated with high blood pressure is graded, continuous, and present throughout the distribution of blood pressure above optimal pressure. Renal risk appears to be more closely related to systolic than to diastolic blood pressure, and black men are at greater risk than white men for developing ESRD at every level of blood pressure.
Atherosclerotic, hypertension-related vascular lesions in the kidney primarily affect preglomerular arterioles, resulting in ischemic changes in the glomeruli and postglomerular structures. Glomerular injury also may be a consequence of direct damage to the glomerular capillaries due to glomerular hyperperfusion. Studies of hypertension-related renal damage, primarily in experimental animals, suggest that loss of autoregulation of renal blood flow at the afferent arteriole results in transmission of elevated pressures to an unprotected glomerulus with ensuing hyperfiltration, hypertrophy, and eventual focal segmental glomerular sclerosis. With progressive renal injury there is a loss of autoregulation of renal blood flow and glomerular filtration rate, resulting in a lower blood pressure threshold for renal damage and a steeper slope between blood pressure and renal damage. The result may be a vicious cycle of renal damage and nephron loss leading to more severe hypertension, glomerular hyperfiltration, and further renal damage. Glomerular pathology progresses to glomerulosclerosis, and eventually the renal tubules may also become ischemic and gradually atrophic. The renal lesion associated with malignant hypertension consists of fibrinoid necrosis of the afferent arterioles, sometimes extending into the glomerulus, and may result in focal necrosis of the glomerular tuft.
Clinically, macroalbuminuria (a random urine albumin/creatinine ratio >300 mg/g) or microalbuminuria (a random urine albumin/creatinine ratio 30–300 mg/g) are early markers of renal injury. These are also risk factors for renal disease progression and cardiovascular disease.
PERIPHERAL ARTERIES
In addition to contributing to the pathogenesis of hypertension, blood vessels are a target organ for atherosclerotic disease secondary to long-standing elevated blood pressure. In hypertensive patients, vascular disease is a major contributor to stroke, heart disease, and renal failure. Further, hypertensive patients with arterial disease of the lower extremities are at increased risk for future cardiovascular disease. Although patients with stenotic lesions of the lower extremities may be asymptomatic, intermittent claudication is the classic symptom of PAD. The ankle-brachial index is a useful approach for evaluating PAD and is defined as the ratio of noninvasively assessed ankle to brachial (arm) systolic blood pressure. An ankle-brachial index <0.90 is considered diagnostic of PAD and is associated with >50% stenosis in at least one major lower limb vessel. An ankle-brachial index <0.80 is associated with elevated blood pressure, particularly systolic blood pressure.
DEFINING HYPERTENSION
From an epidemiologic perspective, there is no obvious level of blood pressure that defines hypertension. In adults, there is a continuous, incremental risk of cardiovascular disease, stroke, and renal disease across levels of both systolic and diastolic blood pressure. The Multiple Risk Factor Intervention Trial (MRFIT), which included >350,000 male participants, demonstrated a continuous and graded influence of both systolic and diastolic blood pressure on CHD mortality, extending down to systolic blood pressures of 120 mmHg. Similarly, results of a meta-analysis involving almost 1 million participants indicate that ischemic heart disease mortality, stroke mortality, and mortality from other vascular causes are directly related to the height of the blood pressure, beginning at 115/75 mmHg, without evidence of a threshold. Cardiovascular disease risk doubles for every 20-mmHg increase in systolic and 10-mmHg increase in diastolic pressure. Among older individuals, systolic blood pressure and pulse pressure are more powerful predictors of cardiovascular disease than is diastolic blood pressure.
Clinically, hypertension may be defined as that level of blood pressure at which the institution of therapy reduces blood pressure–related morbidity and mortality. Current clinical criteria for defining hypertension generally are based on the average of two or more seated blood pressure readings during each of two or more outpatient visits. A recent classification recommends blood pressure criteria for defining normal blood pressure, prehypertension, hypertension (stages I and II), and isolated systolic hypertension, which is frequent among the elderly (Table 298-1). In children and adolescents, hypertension generally is defined as systolic and/or diastolic blood pressure consistently >95th percentile for age, sex, and height. Blood pressures between the 90th and 95th percentiles are considered prehypertensive and are an indication for lifestyle interventions.
|
BLOOD PRESSURE CLASSIFICATION |
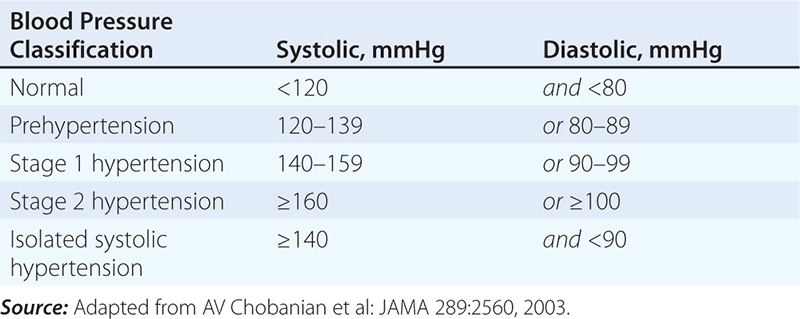
Home blood pressure and average 24-h ambulatory blood pressure measurements are generally lower than clinic blood pressures. Because ambulatory blood pressure recordings yield multiple readings throughout the day and night, they provide a more comprehensive assessment of the vascular burden of hypertension than do a limited number of office readings. Increasing evidence suggests that home blood pressures, including 24-h blood pressure recordings, more reliably predict target organ damage than do office blood pressures. Blood pressure tends to be higher in the early morning hours, soon after waking, than at other times of day. Myocardial infarction and stroke are more common in the early morning hours. Nighttime blood pressures are generally 10–20% lower than daytime blood pressures, and an attenuated nighttime blood pressure “dip” may be associated with increased cardiovascular disease risk. Recommended criteria for a diagnosis of hypertension, based on 24-h blood pressure monitoring, are average awake blood pressure ≥135/85 mmHg and asleep blood pressure ≥120/75 mmHg. These levels approximate a clinic blood pressure of 140/90 mmHg.
Approximately 15–20% of patients with stage 1 hypertension (as defined in Table 298-1) based on office blood pressures have average ambulatory readings <135/85 mmHg. This phenomenon, so-called white coat hypertension, also may be associated with an increased risk of target organ damage, although to a lesser extent than in individuals with elevated office and ambulatory readings. Individuals with white coat hypertension are also at increased risk for developing sustained hypertension.
CLINICAL DISORDERS OF HYPERTENSION
Depending on methods of patient ascertainment, ~80–95% of hypertensive patients are diagnosed as having primary, or “essential,” hypertension. In the remaining 5–20% of hypertensive patients, a specific underlying disorder causing the elevation of blood pressure can be identified (Tables 298-2 and 298-3). In individuals with “secondary” hypertension, a specific mechanism for the blood pressure elevation is often more apparent.
|
SYSTOLIC HYPERTENSION WITH WIDE PULSE PRESSURE |
|
SECONDARY CAUSES OF SYSTOLIC AND DIASTOLIC HYPERTE NSION |
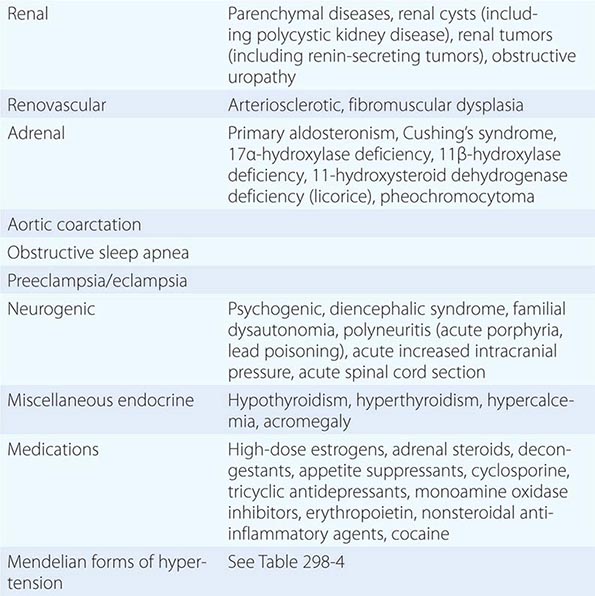
PRIMARY HYPERTENSION
Primary hypertension tends to be familial and is likely to be the consequence of an interaction between environmental and genetic factors. The prevalence of primary hypertension increases with age, and individuals with relatively high blood pressures at younger ages are at increased risk for the subsequent development of hypertension. It is likely that primary hypertension represents a spectrum of disorders with different underlying pathophysiologies. In the majority of patients with established hypertension, peripheral resistance is increased and cardiac output is normal or decreased; however, in younger patients with mild or labile hypertension, cardiac output may be increased and peripheral resistance may be normal.
When plasma renin activity (PRA) is plotted against 24-h sodium excretion, ~10–15% of hypertensive patients have high PRA and 25% have low PRA. High-renin patients may have a vasoconstrictor form of hypertension, whereas low-renin patients may have volume-dependent hypertension. Inconsistent associations between plasma aldosterone and blood pressure have been described in patients with primary hypertension. The association between aldosterone and blood pressure is more striking in African Americans, and PRA tends to be low in hypertensive African Americans. This raises the possibility that subtle increases in aldosterone may contribute to hypertension in at least some groups of patients who do not have overt primary aldosteronism. Furthermore, spironolactone, an aldosterone antagonist, may be a particularly effective antihypertensive agent for some patients with primary hypertension, including some patients with “drug-resistant” hypertension.
OBESITY AND THE METABOLIC SYNDROME
(See also Chap. 422) There is a well-documented association between obesity (body mass index >30 kg/m2) and hypertension. Further, cross-sectional studies indicate a direct linear correlation between body weight (or body mass index) and blood pressure. Centrally located body fat is a more important determinant of blood pressure elevation than is peripheral body fat. In longitudinal studies, a direct correlation exists between change in weight and change in blood pressure over time. Sixty percent of hypertensive adults are more than 20% overweight. It has been established that 60–70% of hypertension in adults may be directly attributable to adiposity.
Hypertension and dyslipidemia frequently occur together and in association with resistance to insulin-stimulated glucose uptake. This clustering of risk factors is often, but not invariably, associated with obesity, particularly abdominal obesity. Insulin resistance also is associated with an unfavorable imbalance in the endothelial production of mediators that regulate platelet aggregation, coagulation, fibrinolysis, and vessel tone. When these risk factors cluster, the risks for CHD, stroke, diabetes, and cardiovascular disease mortality are increased further.
Depending on the populations studied and the methodologies for defining insulin resistance, ~25–50% of nonobese, nondiabetic hypertensive persons are insulin resistant. The constellation of insulin resistance, abdominal obesity, hypertension, and dyslipidemia has been designated as the metabolic syndrome. As a group, first-degree relatives of patients with primary hypertension are also insulin resistant, and hyperinsulinemia (a surrogate marker of insulin resistance) may predict the eventual development of hypertension and cardiovascular disease. Although the metabolic syndrome may in part be heritable as a polygenic condition, the expression of the syndrome is modified by environmental factors, such as degree of physical activity and diet. Insulin sensitivity increases and blood pressure decreases in response to weight loss. The recognition that cardiovascular disease risk factors tend to cluster within individuals has important implications for the evaluation and treatment of hypertension. Evaluation of both hypertensive patients and individuals at risk for developing hypertension should include assessment of overall cardiovascular disease risk. Similarly, introduction of lifestyle modification strategies and drug therapies should address overall risk and not focus exclusively on hypertension.
RENAL PARENCHYMAL DISEASES
Virtually all disorders of the kidney may cause hypertension (Table 298-3), and renal disease is the most common cause of secondary hypertension. Hypertension is present in >80% of patients with chronic renal failure. In general, hypertension is more severe in glomerular diseases than in interstitial diseases such as chronic pyelonephritis. Conversely, hypertension may cause nephrosclerosis, and in some instances it may be difficult to determine whether hypertension or renal disease was the initial disorder. Proteinuria >1000 mg/d and an active urine sediment are indicative of primary renal disease. In either instance, the goals are to control blood pressure and retard the rate of progression of renal dysfunction.
RENOVASCULAR HYPERTENSION
Hypertension due to an occlusive lesion of a renal artery, renovascular hypertension, is a potentially curable form of hypertension. In the initial stages, the mechanism of hypertension generally is related to activation of the renin-angiotensin system. However, renin activity and other components of the renin-angiotensin system may be elevated only transiently; over time, recruitment of other pressure mechanisms may contribute to elevated arterial pressure. Two groups of patients are at risk for this disorder: older arteriosclerotic patients who have a plaque obstructing the renal artery, frequently at its origin, and patients with fibromuscular dysplasia. Atherosclerosis accounts for the large majority of patients with renovascular hypertension. Although fibromuscular dysplasia may occur at any age, it has a strong predilection for young white women. The prevalence in females is eightfold that in males. There are several histologic variants of fibromuscular dysplasia, including medial fibroplasia, perimedial fibroplasia, medial hyperplasia, and intimal fibroplasia. Medial fibroplasia is the most common variant and accounts for approximately two-thirds of patients. The lesions of fibromuscular dysplasia are frequently bilateral and, in contrast to atherosclerotic renovascular disease, tend to affect more distal portions of the renal artery.
Several clues from the history and physical examination may suggest renovascular hypertension. The diagnosis should be considered in patients with other evidence of atherosclerotic vascular disease. Although response to antihypertensive therapy does not exclude the diagnosis, severe or refractory hypertension, recent loss of hypertension control or recent onset of moderately severe hypertension, and unexplained deterioration of renal function or deterioration of renal function associated with an ACE inhibitor should raise the possibility of renovascular hypertension. Approximately 50% of patients with renovascular hypertension have an abdominal or flank bruit, and the bruit is more likely to be hemodynamically significant if it lateralizes or extends throughout systole into diastole.
If blood pressure is adequately controlled with a simple antihypertensive regimen and renal function remains stable, there may be little impetus to pursue an evaluation for renal artery stenosis, particularly in an older patient with atherosclerotic disease and comorbid conditions. Patients with long-standing hypertension, advanced renal insufficiency, or diabetes mellitus are less likely to benefit from renal vascular repair. The most effective medical therapies include an ACE inhibitor or an angiotensin II receptor blocker; however, these agents decrease glomerular filtration rate in a stenotic kidney owing to efferent renal arteriolar dilation. In the presence of bilateral renal artery stenosis or renal artery stenosis to a solitary kidney, progressive renal insufficiency may result from the use of these agents. Importantly, the renal insufficiency is generally reversible after discontinuation of the offending drug.
If renal artery stenosis is suspected and if the clinical condition warrants an intervention such as percutaneous transluminal renal angioplasty (PTRA), placement of a vascular endoprosthesis (stent), or surgical renal revascularization, imaging studies should be the next step in the evaluation. As a screening test, renal blood flow may be evaluated with a radionuclide [131I]-orthoiodohippurate (OIH) scan, or glomerular filtration rate may be evaluated with a [99mTc]-diethylenetriamine pentaacetic acid (DTPA) scan before and after a single dose of captopril (or another ACE inhibitor). The following are consistent with a positive study: (1) decreased relative uptake by the involved kidney, which contributes <40% of total renal function, (2) delayed uptake on the affected side, and (3) delayed washout on the affected side. In patients with normal, or nearly normal, renal function, a normal captopril renogram essentially excludes functionally significant renal artery stenosis; however, its usefulness is limited in patients with renal insufficiency (creatinine clearance <20 mL/min) or bilateral renal artery stenosis. Additional imaging studies are indicated if the scan is positive. Doppler ultrasound of the renal arteries produces reliable estimates of renal blood flow velocity and offers the opportunity to track a lesion over time. Positive studies usually are confirmed at angiography, whereas false-negative results occur frequently, particularly in obese patients. Gadolinium-contrast magnetic resonance angiography offers clear images of the proximal renal artery but may miss distal lesions. An advantage is the opportunity to image the renal arteries with an agent that is not nephrotoxic. Contrast arteriography remains the “gold standard” for evaluation and identification of renal artery lesions. Potential risks include nephrotoxicity, particularly in patients with diabetes mellitus or preexisting renal insufficiency.
Some degree of renal artery obstruction may be observed in almost 50% of patients with atherosclerotic disease, and there are several approaches for evaluating the functional significance of such a lesion to predict the effect of vascular repair on blood pressure control and renal function. Each approach has varying degrees of sensitivity and specificity, and no single test is sufficiently reliable to determine a causal relationship between a renal artery lesion and hypertension. Functionally significant lesions generally occlude more than 70% of the lumen of the affected renal artery. On angiography, the presence of collateral vessels to the ischemic kidney suggests a functionally significant lesion. A lateralizing renal vein renin ratio (ratio >1.5 of affected side/contralateral side) has a 90% predictive value for a lesion that would respond to vascular repair; however, the false-negative rate for blood pressure control is 50–60%. Measurement of the pressure gradient across a renal artery lesion does not reliably predict the response to vascular repair.
In the final analysis, a decision concerning vascular repair vs. medical therapy and the type of repair procedure should be individualized. Patients with fibromuscular disease have more favorable outcomes than do patients with atherosclerotic lesions, presumably owing to their younger age, shorter duration of hypertension, and less systemic disease. Because of its low risk-versus-benefit ratio and high success rate (improvement or cure of hypertension in 90% of patients and restenosis rate of 10%), PTRA is the initial treatment of choice for these patients. Surgical revascularization may be undertaken if PTRA is unsuccessful or if a branch lesion is present. In atherosclerotic patients, vascular repair should be considered if blood pressure cannot be controlled adequately despite optimal medical therapy or if renal function deteriorates. Surgery may be the preferred initial approach for younger atherosclerotic patients without comorbid conditions; however, for most atherosclerotic patients, depending on the location of the lesion, the initial approach may be PTRA and/or stenting. Surgical revascularization may be indicated if these approaches are unsuccessful, the vascular lesion is not amenable to PTRA or stenting, or concomitant aortic surgery is required, e.g., to repair an aneurysm. A National Institutes of Health–sponsored prospective, randomized clinical trial is in progress comparing medical therapy alone with medical therapy plus renal artery stenting regarding Cardiovascular Outcomes for Renal Atherosclerotic Lesions (CORAL).
PRIMARY ALDOSTERONISM
Excess aldosterone production due to primary aldosteronism is a potentially curable form of hypertension. In patients with primary aldosteronism, increased aldosterone production is independent of the renin-angiotensin system, and the consequences are sodium retention, hypertension, hypokalemia, and low PRA. The reported prevalence of this disorder varies from <2% to ~15% of hypertensive individuals. In part, this variation is related to the intensity of screening and the criteria for establishing the diagnosis.
History and physical examination provide little information about the diagnosis. The age at the time of diagnosis is generally the third through fifth decade. Hypertension is usually mild to moderate but occasionally may be severe; primary aldosteronism should be considered in all patients with refractory hypertension. Hypertension in these patients may be associated with glucose intolerance. Most patients are asymptomatic; however, infrequently, polyuria, polydipsia, paresthesias, or muscle weakness may be present as a consequence of hypokalemic alkalosis. Although aldosterone is a salt-retaining hormone, patients with primary aldosteronism rarely have edema. Renal dysfunction and cardiovascular disease are strikingly increased in patients with primary aldosteronism compared to those with primary hypertension.
In a hypertensive patient with unprovoked hypokalemia (i.e., unrelated to diuretics, vomiting, or diarrhea), the prevalence of primary aldosteronism approaches 40–50%. In patients on diuretics, serum potassium <3.1 mmol/L (<3.1 meq/L) also raises the possibility of primary aldosteronism; however, serum potassium is an insensitive and nonspecific screening test. Serum potassium is normal in ~25% of patients subsequently found to have an aldosterone-producing adenoma, and higher percentages of patients with other etiologies of primary aldosteronism are not hypokalemic. Additionally, hypokalemic hypertension may be a consequence of secondary aldosteronism, other mineralocorticoid- and glucocorticoid-induced hypertensive disorders, and pheochromocytoma.
The ratio of plasma aldosterone to plasma renin activity (PA/PRA) is a useful screening test. These measurements preferably are obtained in ambulatory patients in the morning. A ratio >30:1 in conjunction with a plasma aldosterone concentration >555 pmol/L (>20 ng/dL) reportedly has a sensitivity of 90% and a specificity of 91% for an aldosterone-producing adenoma. In a Mayo Clinic series, an aldosterone-producing adenoma subsequently was confirmed surgically in >90% of hypertensive patients with a PA/PRA ratio ≥20 and a plasma aldosterone concentration ≥415 pmol/L (≥15 ng/dL). There are, however, several caveats to interpreting the ratio. The cutoff for a “high” ratio is laboratory- and assay-dependent. Some antihypertensive agents may affect the ratio (e.g., aldosterone antagonists, angiotensin receptor antagonists, and ACE inhibitors may increase renin; aldosterone antagonists may increase aldosterone). Current recommendations are to withdraw aldosterone antagonists for at least 4–6 weeks before obtaining these measurements. Because aldosterone biosynthesis is potassium-dependent, hypokalemia should be corrected with oral potassium supplements prior to screening. With these caveats, the ratio has been reported to be useful as a screening test in measurements obtained with patients taking their usual antihypertensive medications. A high ratio in the absence of an elevated plasma aldosterone level is considerably less specific for primary aldosteronism since many patients with primary hypertension have low renin levels in this setting, particularly African Americans and elderly patients. In patients with renal insufficiency, the ratio may also be elevated because of decreased aldosterone clearance. In patients with an elevated PA/PRA ratio, the diagnosis of primary aldosteronism can be confirmed by demonstrating failure to suppress plasma aldosterone to <277 pmol/L (<10 ng/dL) after IV infusion of 2 L of isotonic saline over 4 h; post-saline infusion plasma aldosterone values between 138 and 277 pmol/L (5–10 ng/dL) are not determinant. Alternative confirmatory tests include failure to suppress aldosterone (based on test specific criteria) in response to an oral NaCl load, fludrocortisone, or captopril.
Several sporadic and familial adrenal abnormalities may culminate in the syndrome of primary aldosteronism, and appropriate therapy depends on the specific etiology. The two most common causes of sporadic primary aldosteronism are an aldosterone-producing adenoma and bilateral adrenal hyperplasia. Together, they account for >90% of all patients with primary aldosteronism. The tumor is almost always unilateral, and most often measures <3 cm in diameter. Most of the remainder of these patients have bilateral adrenocortical hyperplasia (idiopathic hyperaldosteronism). Rarely, primary aldosteronism may be caused by an adrenal carcinoma or an ectopic malignancy, e.g., ovarian arrhenoblastoma. Most aldosterone-producing carcinomas, in contrast to adrenal adenomas and hyperplasia, produce excessive amounts of other adrenal steroids in addition to aldosterone. Functional differences in hormone secretion may assist in the diagnosis of adenoma vs. hyperplasia. Aldosterone biosynthesis is more responsive to ACTH in patients with adenoma and more responsive to angiotensin in patients with hyperplasia. Consequently, patients with adenoma tend to have higher plasma aldosterone in the early morning that decreases during the day, reflecting the diurnal rhythm of ACTH, whereas plasma aldosterone tends to increase with upright posture in patients with hyperplasia, reflecting the normal postural response of the renin-angiotensin-aldosterone axis. However, there is overlap in the ability of these measurements to discriminate between adenoma and hyperplasia. Rare familial forms of primary aldosteronism include glucocorticoid-remediable primary aldosteronism and familial aldosteronism types II and III. Genetic testing may assist in the diagnosis of these familial disorders.
Adrenal computed tomography (CT) should be carried out in all patients diagnosed with primary aldosteronism. High-resolution CT may identify tumors as small as 0.3 cm and is positive for an adrenal tumor 90% of the time. If the CT is not diagnostic, an adenoma may be detected by adrenal scintigraphy with 6 β-[I131] iodomethyl-19-norcholesterol after dexamethasone suppression (0.5 mg every 6 h for 7 days); however, this technique has decreased sensitivity for adenomas <1.5 cm.
When carried out by an experienced radiologist, bilateral adrenal venous sampling for measurement of plasma aldosterone is the most accurate means of differentiating unilateral from bilateral forms of primary aldosteronism. The sensitivity and specificity of adrenal venous sampling (95% and 100%, respectively) for detecting unilateral aldosterone hypersecretion are superior to those of adrenal CT; success rates are 90–96%, and complication rates are <2.5%. One frequently used protocol involves sampling for aldosterone and cortisol levels in response to ACTH stimulation. An ipsilateral/contralateral aldosterone ratio >4, with symmetric ACTH-stimulated cortisol levels, is indicative of unilateral aldosterone production.
Hypertension generally is responsive to surgery in patients with adenoma but not in patients with bilateral adrenal hyperplasia. Unilateral adrenalectomy, often done via a laparoscopic approach, is curative in 40–70% of patients with an adenoma. Transient hypoaldosteronism may occur up to 3 months postoperatively, resulting in hyperkalemia. Potassium should be monitored during this time, and hyperkalemia should be treated with potassium-wasting diuretics and with fludrocortisone, if needed. Patients with bilateral hyperplasia should be treated medically. The drug regimen for these patients, as well as for patients with an adenoma who are poor surgical candidates, should include an aldosterone antagonist and, if necessary, other potassium-sparing diuretics.
Glucocorticoid-remediable hyperaldosteronism is a rare, monogenic autosomal dominant disorder characterized by moderate to severe hypertension, often occurring at an early age. These patients may have a family history of hemorrhagic stroke at a young age. Hypokalemia is usually mild or absent. Normally, angiotensin II stimulates aldosterone production by the adrenal zona glomerulosa, whereas ACTH stimulates cortisol production in the zona fasciculata. Owing to a chimeric gene on chromosome 8, ACTH also regulates aldosterone secretion by the zona fasciculata in patients with glucocorticoid-remediable hyperaldosteronism. The consequence is overproduction in the zona fasciculata of both aldosterone and hybrid steroids (18-hydroxycortisol and 18-oxocortisol) due to oxidation of cortisol. The diagnosis may be established by urine excretion rates of these hybrid steroids that are 20 to 30 times normal or by direct genetic testing. Therapeutically, suppression of ACTH with low-dose glucocorticoids corrects the hyperaldosteronism, hypertension, and hypokalemia. Aldosterone antagonists are also therapeutic options. Patients with familial aldosteronism types II and III are treated with aldosterone antagonists or adrenalectomy.
CUSHING’S SYNDROME
(See also Chap. 406) Cushing’s syndrome is related to excess cortisol production due either to excess ACTH secretion (from a pituitary tumor or an ectopic tumor) or to ACTH-independent adrenal production of cortisol. Hypertension occurs in 75–80% of patients with Cushing’s syndrome. The mechanism of hypertension may be related to stimulation of mineralocorticoid receptors by cortisol and increased secretion of other adrenal steroids. If clinically suspected based on phenotypic characteristics, in patients not taking exogenous glucocorticoids, laboratory screening may be carried out with measurement of 24-h excretion rates of urine free cortisol or an overnight dexamethasone-suppression test. Late night salivary cortisol is also a sensitive and convenient screening test. Further evaluation is required to confirm the diagnosis and identify the specific etiology of Cushing’s syndrome. Appropriate therapy depends on the etiology.
PHEOCHROMOCYTOMA
(See also Chap. 407) Catecholamine-secreting tumors are located in the adrenal medulla (pheochromocytoma) or in extra-adrenal paraganglion tissue (paraganglioma) and account for hypertension in ~0.05% of patients. If unrecognized, pheochromocytoma may result in lethal cardiovascular consequences. Clinical manifestations, including hypertension, are primarily related to increased circulating catecholamines, although some of these tumors may secrete a number of other vasoactive substances. In a small percentage of patients, epinephrine is the predominant catecholamine secreted by the tumor, and these patients may present with hypotension rather than hypertension. The initial suspicion of the diagnosis is based on symptoms and/or the association of pheochromocytoma with other disorders (Table 298-4). Approximately 20% of pheochromocytomas are familial with autosomal dominant inheritance. Inherited pheochromocytomas may be associated with multiple endocrine neoplasia (MEN) type 2A and type 2B, von Hippel-Lindau disease, and neurofibromatosis (Table 298-4). Each of these syndromes is related to specific, identifiable germ-line mutations. Additionally, mutations of succinate dehydrogenase genes are associated with paraganglioma syndromes, generally characterized by head and neck paragangliomas. Laboratory testing consists of measuring catecholamines in either urine or plasma, e.g., 24-h urine metanephrine excretion or fractionated plasma free metanephrines. The urine measurement is less sensitive but more specific. Genetic screening is available for evaluating patients and relatives suspected of harboring a pheochromocytoma associated with a familial syndrome. Surgical excision is the definitive treatment of pheochromocytoma and results in cure in ~90% of patients.
|
RARE MENDELIAN FORMS OF HYPERTENSION |
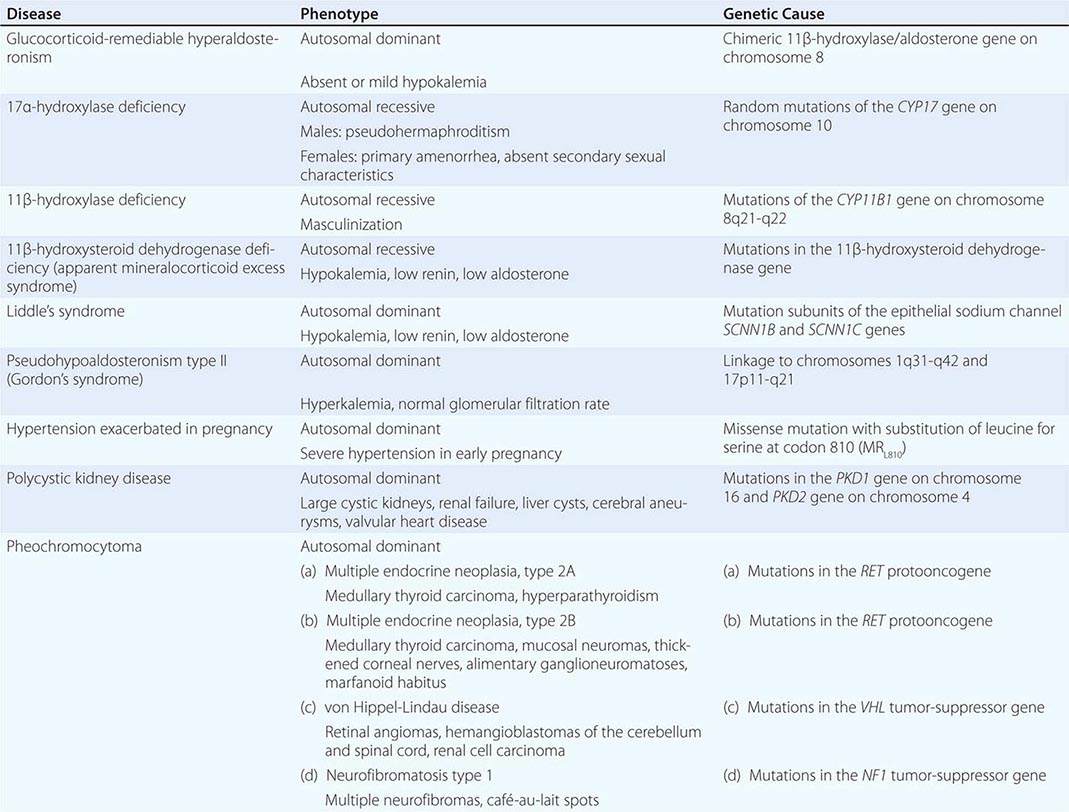
MISCELLANEOUS CAUSES OF HYPERTENSION
Independent of obesity, hypertension occurs in >50% of individuals with obstructive sleep apnea. The severity of hypertension correlates with the severity of sleep apnea. Approximately 70% of patients with obstructive sleep apnea are obese. Hypertension related to obstructive sleep apnea also should be considered in patients with drug-resistant hypertension and patients with a history of snoring. The diagnosis can be confirmed by polysomnography. In obese patients, weight loss may alleviate or cure sleep apnea and related hypertension. Continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) administered during sleep is an effective therapy for obstructive sleep apnea. With CPAP or BiPAP, patients with apparently drug-resistant hypertension may be more responsive to antihypertensive agents.
Coarctation of the aorta is the most common congenital cardiovascular cause of hypertension (Chap. 282). The incidence is 1–8 per 1000 live births. It is usually sporadic but occurs in 35% of children with Turner’s syndrome. Even when the anatomic lesion is surgically corrected in infancy, up to 30% of patients develop subsequent hypertension and are at risk of accelerated coronary artery disease and cerebrovascular events. Patients with less severe lesions may not be diagnosed until young adulthood. Physical findings include diminished and delayed femoral pulses and a systolic pressure gradient between the right arm and the legs and, depending on the location of the coarctation, between the right and left arms. A blowing systolic murmur may be heard in the posterior left interscapular areas. The diagnosis may be confirmed by chest x-ray and transesophageal echocardiography. Therapeutic options include surgical repair and balloon angioplasty, with or without placement of an intravascular stent. Subsequently, many patients do not have a normal life expectancy but may have persistent hypertension, with death due to ischemic heart disease, cerebral hemorrhage, or aortic aneurysm.
Several additional endocrine disorders, including thyroid diseases and acromegaly, cause hypertension. Mild diastolic hypertension may be a consequence of hypothyroidism, whereas hyperthyroidism may result in systolic hypertension. Hypercalcemia of any etiology, the most common being primary hyperparathyroidism, may result in hypertension. Hypertension also may be related to a number of prescribed or over-the-counter medications.
MONOGENIC HYPERTENSION
In addition to glucocorticoid-remediable primary aldosteronism, a number of rare forms of monogenic hypertension have been identified (Table 298–4). These disorders may be recognized by their characteristic phenotypes, and in many instances the diagnosis may be confirmed by genetic analysis. Several inherited defects in adrenal steroid biosynthesis and metabolism result in mineralocorticoid-induced hypertension and hypokalemia. In patients with a 17α-hydroxylase deficiency, synthesis of sex hormones and cortisol is decreased (Fig. 298-3). Consequently, these individuals do not mature sexually; males may present with pseudohermaphroditism and females with primary amenorrhea and absent secondary sexual characteristics. Because cortisol-induced negative feedback on pituitary ACTH production is diminished, ACTH-stimulated adrenal steroid synthesis proximal to the enzymatic block is increased. Hypertension and hypokalemia are consequences of increased synthesis of mineralocorticoids proximal to the enzymatic block, particularly desoxycorticosterone. Increased steroid production and, hence, hypertension may be treated with low-dose glucocorticoids. An 11β-hydroxylase deficiency results in a salt-retaining adrenogenital syndrome that occurs in 1 in 100,000 live births. This enzymatic defect results in decreased cortisol synthesis, increased synthesis of mineralocorticoids (e.g., desoxycorticosterone), and shunting of steroid biosynthesis into the androgen pathway. In the severe form, the syndrome may present early in life, including the newborn period, with virilization and ambiguous genitalia in females and penile enlargement in males, or in older children as precocious puberty and short stature. Acne, hirsutism, and menstrual irregularities may be the presenting features when the disorder is first recognized in adolescence or early adulthood. Hypertension is less common in the late-onset forms. Patients with an 11β-hydroxysteroid dehydrogenase deficiency have an impaired capacity to metabolize cortisol to its inactive metabolite, cortisone, and hypertension is related to activation of mineralocorticoid receptors by cortisol. This defect may be inherited or acquired, due to licorice-containing glycyrrhizin acid. The same substance is present in the paste of several brands of chewing tobacco. The defect in Liddle’s syndrome (Chaps. 63 and 406) results from constitutive activation of amiloride-sensitive epithelial sodium channels on the distal renal tubule, resulting in excess sodium reabsorption; the syndrome is ameliorated by amiloride. Hypertension exacerbated in pregnancy (Chap. 8) may be due to activation of the mineralocorticoid receptor by progesterone.
FIGURE 298-3 Adrenal enzymatic defects. DHEA, dehydroepiandrosterone.
|
PATIENT’S RELEVANT HISTORY |
Abbreviation: TIA, transient ischemic attack.
|
BASIC LABORATORY TESTS FOR INITIAL EVALUATION |
|
LIFESTYLE MODIFICATIONS TO MANAGE HYPERTENSION |
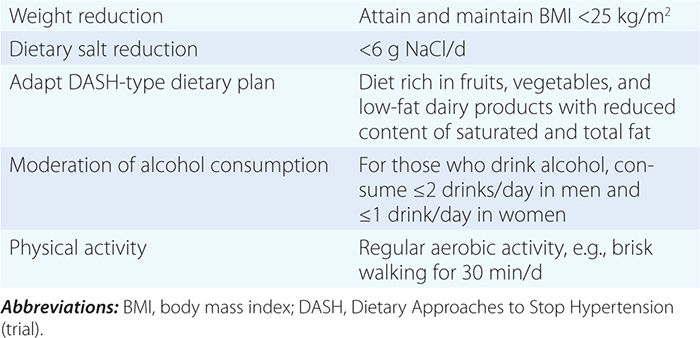
Prevention and treatment of obesity are important for reducing blood pressure and cardiovascular disease risk. In short-term trials, even modest weight loss can lead to a reduction of blood pressure and an increase in insulin sensitivity. Average blood pressure reductions of 6.3/3.1 mmHg have been observed with a reduction in mean body weight of 9.2 kg. Regular physical activity facilitates weight loss, decreases blood pressure, and reduces the overall risk of cardiovascular disease. Blood pressure may be lowered by 30 min of moderately intense physical activity, such as brisk walking, 6–7 days a week, or by more intense, less frequent workouts.
There is individual variability in the sensitivity of blood pressure to NaCl, and this variability may have a genetic basis. Based on results of meta-analyses, lowering of blood pressure by limiting daily NaCl intake to 4.4–7.4 g (75–125 meq) results in blood pressure reductions of 3.7–4.9/0.9–2.9 mmHg in hypertensive individuals and lesser reductions in normotensive individuals. Several long-term, prospective, randomized clinical trials have reported that a reduced salt intake results in a decreased incidence of cardiovascular events. Although reduced salt intakes are generally recommended for both the prevention and treatment of hypertension, overly rigorous salt restriction may have adverse cardiovascular outcomes in diabetic patients and in patients with CHF aggressively treated with diuretics. Potassium and calcium supplementation have inconsistent, modest antihypertensive effects, and, independent of blood pressure, potassium supplementation may be associated with reduced stroke mortality. Consuming three or more alcoholic drinks per day (a standard drink contains ~14 g ethanol) is associated with higher blood pressures, and a reduction of alcohol consumption is associated with a reduction of blood pressure. In patients with advanced renal disease, dietary protein restriction may have a modest effect in mitigating renal damage by reducing the intrarenal transmission of systemic arterial pressure.
The DASH (Dietary Approaches to Stop Hypertension) trial convincingly demonstrated that over an 8-week period a diet high in fruits, vegetables, and low-fat dairy products lowers blood pressure in individuals with high-normal blood pressures or mild hypertension. Reduction of daily NaCl intake to <6 g (100 meq) augmented the effect of this diet on blood pressure. Fruits and vegetables are enriched sources of potassium, magnesium, and fiber, and dairy products are an important source of calcium.
PHARMACOLOGIC THERAPY
Drug therapy is recommended for individuals with blood pressures ≥140/90 mmHg. The degree of benefit derived from antihypertensive agents is related to the magnitude of the blood pressure reduction. Lowering systolic blood pressure by 10–12 mmHg and diastolic blood pressure by 5–6 mmHg confers relative risk reductions of 35–40% for stroke and 12–16% for CHD within 5 years of the initiation of treatment. Risk of heart failure is reduced by >50%. Hypertension control is the single most effective intervention for slowing the rate of progression of hypertension-related kidney disease.
There is considerable variation in individual responses to different classes of antihypertensive agents, and the magnitude of response to any single agent may be limited by activation of counter-regulatory mechanisms. Most available agents reduce systolic blood pressure by 7–13 mmHg and diastolic blood pressure by 4–8 mmHg when corrected for placebo effect. More often than not, combinations of agents, with complementary antihypertensive mechanisms, are required to achieve goal blood pressure reductions. Selection of antihypertensive agents and combinations of agents should be individualized, taking into account age, severity of hypertension, other cardiovascular disease risk factors, comorbid conditions, and practical considerations related to cost, side effects, and frequency of dosing (Table 298-8).
|
EXAMPLES OF ORAL DRUGS USED IN TREATMENT OF HYPERTENSION |
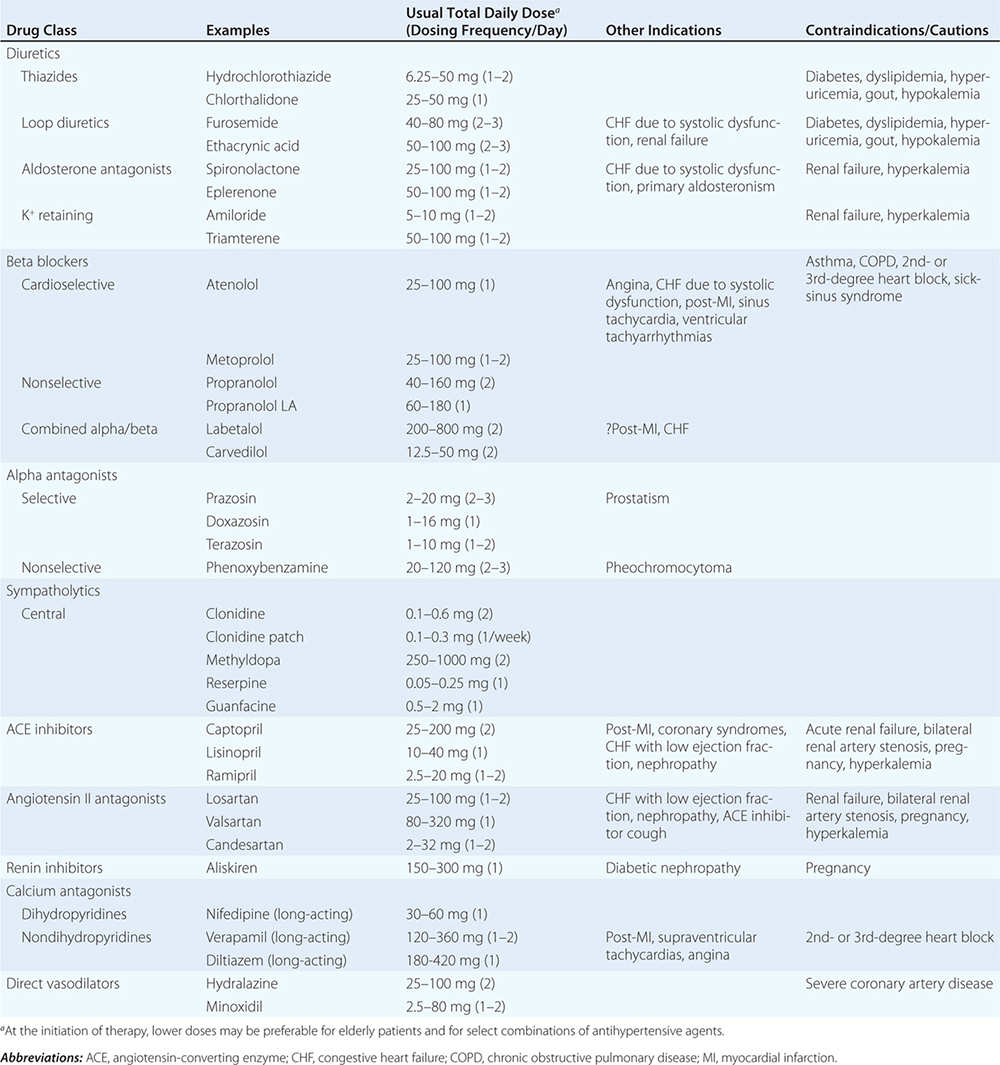
Diuretics Low-dose thiazide diuretics may be used alone or in combination with other antihypertensive drugs. Thiazides inhibit the Na+/Cl– pump in the distal convoluted tubule and hence increase sodium excretion. In the long term, they also may act as vasodilators. Thiazides are safe, efficacious, inexpensive, and reduce clinical events. They provide additive blood pressure–lowering effects when combined with beta blockers, angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin receptor blockers (ARBs). In contrast, addition of a diuretic to a calcium channel blocker is less effective. Usual doses of hydrochlorothiazide range from 6.25–50 mg/d. Owing to an increased incidence of metabolic side effects (hypokalemia, insulin resistance, increased cholesterol), higher doses generally are not recommended. Chlorthalidone is a diuretic structurally similar to hydrochlorothiazide, and like hydrochlorothiazide, it blocks sodium-chloride cotransport in the early distal tubule. However, chlorthalidone has a longer half-life (40–60 h vs. 9–15 h) and an antihypertensive potency ~1.5–2.0 times that of hydrochlorothiazide. Potassium loss is also greater with chlorthalidone. Two potassium-sparing diuretics, amiloride and triamterene, act by inhibiting epithelial sodium channels in the distal nephron. These agents are weak antihypertensive agents but may be used in combination with a thiazide to protect against hypokalemia. The main pharmacologic target for loop diuretics is the Na+-K+-2Cl– cotransporter in the thick ascending limb of the loop of Henle. Loop diuretics generally are reserved for hypertensive patients with reduced glomerular filtration rates (reflected in serum creatinine >220 μmol/L [>2.5 mg/dL]), CHF, or sodium retention and edema for some other reason, such as treatment with a potent vasodilator, e.g., minoxidil.