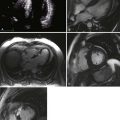
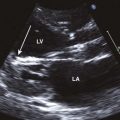
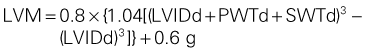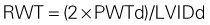5 Hypertensive Heart Failure
Left Ventricular Hypertrophy
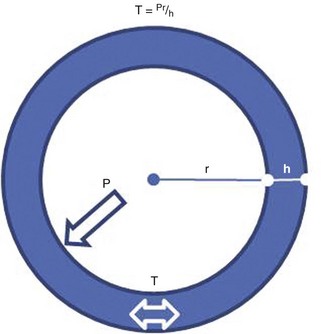
How to Evaluate LV Mass
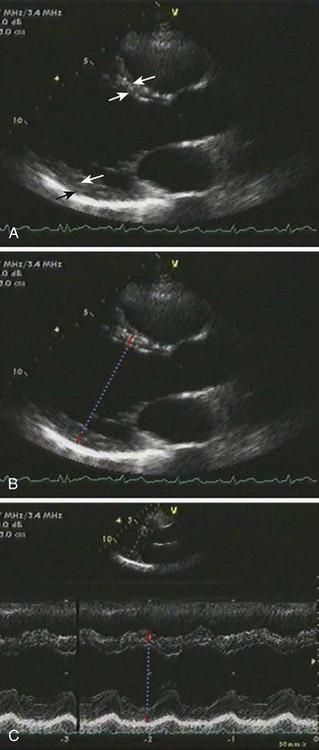
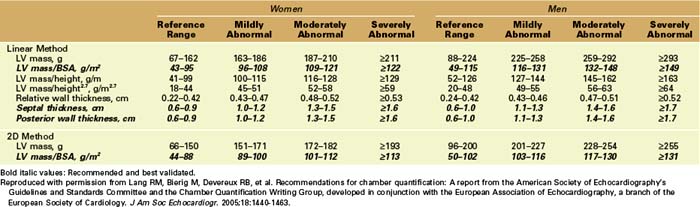
Linear Measurements
Key Points
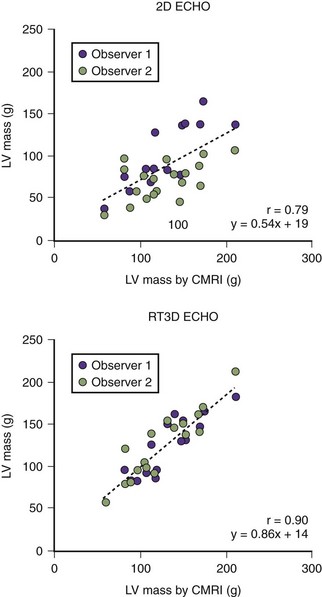
2D Measurement of LVM
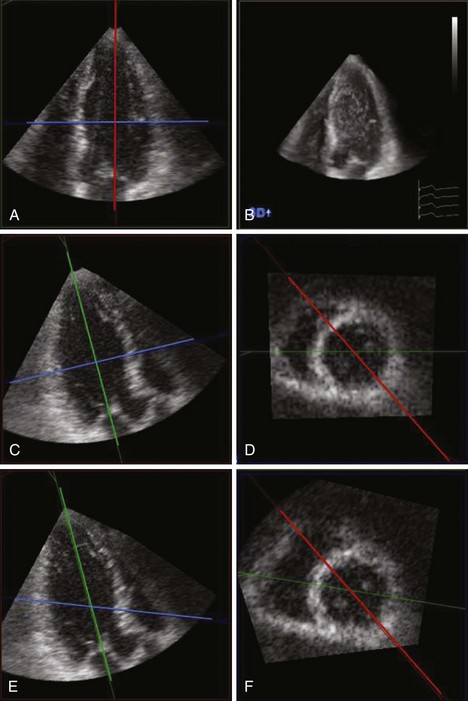
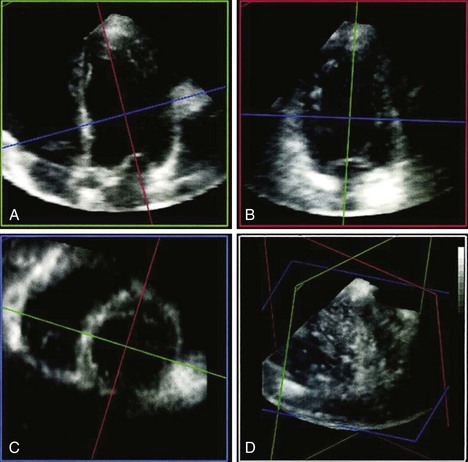
3D Measurement of LVM
Key Points
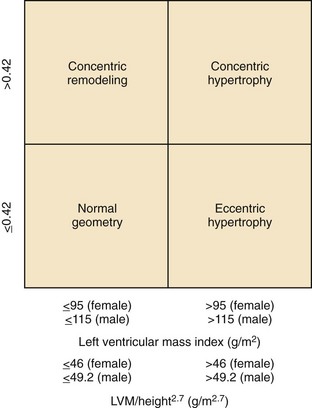
Definition of LVH and Abnormal LV Geometry
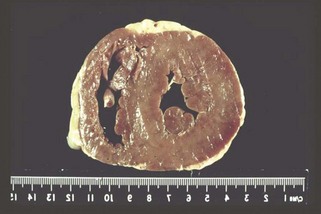
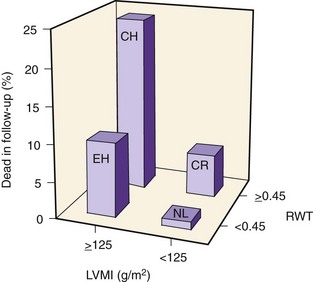
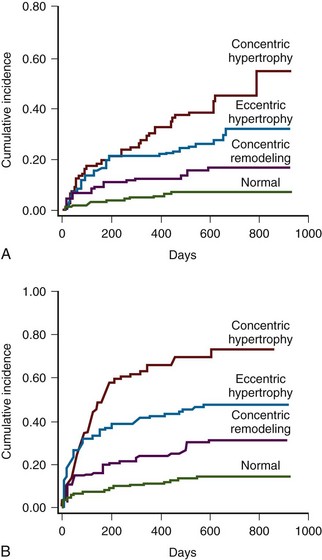
LV Hypertrophy
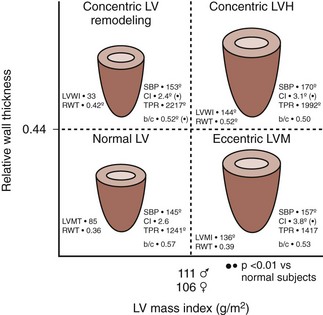
LV Geometry (Figure 5-8)
Key Points
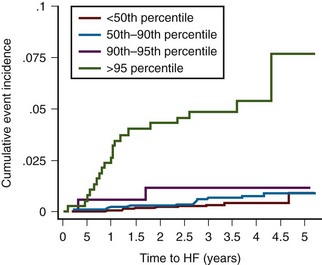
Figure 5-11 LVM and LVH raise the risk of incident heart failure events in the Multi-Ethnic Study of Atherosclerosis.
(Reprinted with permission from Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.)
Clinical Vignettes
Case 1
A 48-year-old diabetic, hypertensive man with unlimited exercise tolerance was referred to echocardiography to evaluate atypical chest pain. His blood pressure was 160/100 mm Hg on valsartan and hydrochlorothiazide. He was mildly overweight with a normal exam. The LV ejection fraction (LVEF) was normal with normal valvular function. On echocardiographic exam, LV geometry was normal (Figure 5-13A):
Case 2
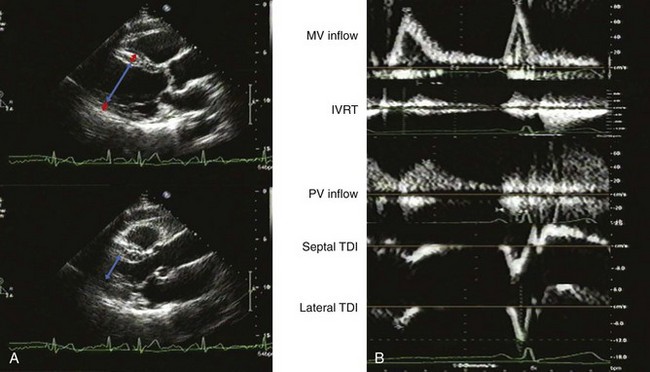
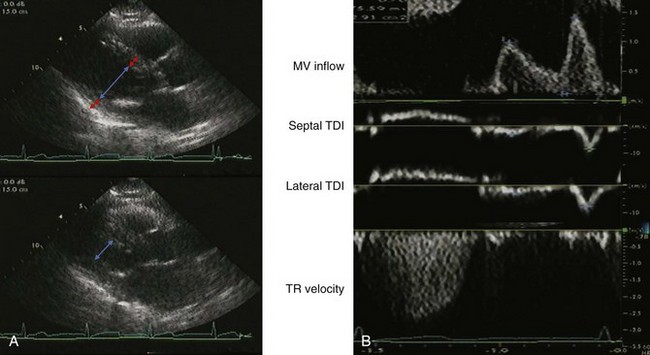
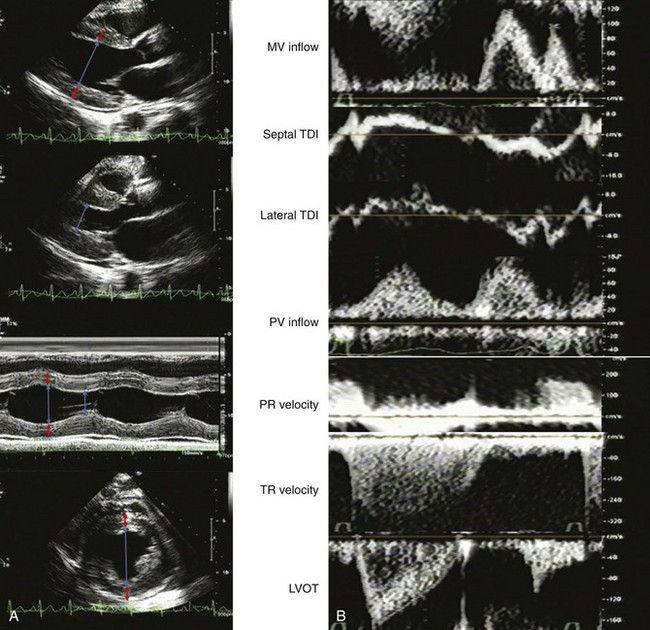
A 60-year-old man with hypertension, myelofibrosis, and multiple falls underwent echocardiography during evaluation of presyncope. His blood pressure was 131/77 mm Hg on amlodipine and captopril. He was normal weight with a normal exam. His hemoglobin was 8.0 gm/dL. The LVEF was normal with normal valvular function (Figure 5-14A). On echocardiographic exam:
The mitral filling pattern was normal for age (Figure 5-14B):
Case 3
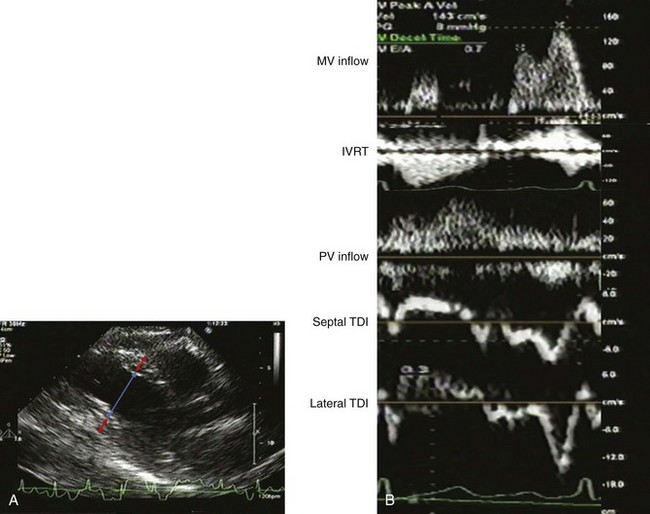
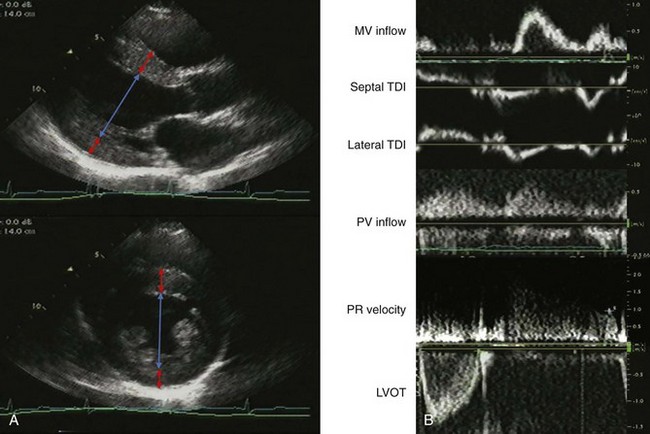
A 75-year-old woman with hypertension underwent echocardiography during evaluation of palpitations. She had unlimited exercise tolerance with no other symptoms. Her blood pressure was 135/70 mm Hg on atenolol. She was normal weight with a I/VI systolic murmur at the left upper sternal border and trace lower extremity edema. The LVEF was normal with normal valvular function. There was mild mitral and aortic annular calcification. Estimated glomerular filtration rate (GFR) by MDRD was 57 mL/min (Figure 5-15A). On echocardiographic exam, LV geometry was consistent with borderline eccentric hypertrophy:
The filling pattern was normal for age (Figure 5-15B):
The IVC was normal in size with normal respiratory collapse. She had an abnormal relaxation pattern with E/A reversal and prolonged deceleration time and IVRT, and S-dominant pulmonary venous flow, which may be consistent with normal aging (Figure 5-16).
Case 4
A 94-year-old woman with diabetes was referred to evaluate dyspnea on exertion. She had a  block exercise tolerance without other signs or symptoms of heart failure. Her blood pressure was 112/76 mm Hg on no antihypertensives. Her brain natriuretic peptide (BNP) was 23. An ECG demonstrated sinus tachycardia. The LVEF was normal with mild aortic stenosis. LA size was normal for age and body size (Figure 5-17A). On echocardiographic exam, the LV geometry was consistent with concentric remodeling:
block exercise tolerance without other signs or symptoms of heart failure. Her blood pressure was 112/76 mm Hg on no antihypertensives. Her brain natriuretic peptide (BNP) was 23. An ECG demonstrated sinus tachycardia. The LVEF was normal with mild aortic stenosis. LA size was normal for age and body size (Figure 5-17A). On echocardiographic exam, the LV geometry was consistent with concentric remodeling:
There was evidence of elevated LV filling pressures (Figure 5-17B):
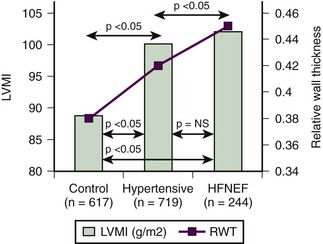
LV filling parameters were suggestive of heart failure with a normal EF7 (Figure 5-18).
Case 5
A 93-year-old woman with hypertension and a history of non-sustained ventricular tachycardia was referred to echocardiography for syncope. Her blood pressure was 94/56 mm Hg before and 115/70 mm Hg after hydration. The LVEF was hyperdynamic with moderate mitral and tricuspid regurgitation (Figure 5-19A). On echocardiographic exam, LV geometry was consistent with concentric remodeling:
Filling parameters were consistent with abnormal relaxation and elevated LV filling pressures (Figure 5-19B):
Case 6
A 41-year-old man with stage V chronic kidney disease secondary to hypertension was referred to echocardiography for an abnormal ECG. He had an unlimited exercise tolerance with no symptoms of heart failure. His blood pressure was 140/93 mm Hg on amlodipine, labetalol, furosemide, hydralazine, and isosorbide dinitrate. On exam, he had trace lower extremity edema without jugular venous distention or crackles. The LVEF was normal with normal valvular function. The LA was severely dilated by volume measurement (Figure 5-20A). On echocardiographic exam, LV geometry was consistent with concentric hypertrophy:
LV filling parameters were consistent with pseudonormalization and mildly elevated LV filling pressures (Figure 5-20B):
Case 7
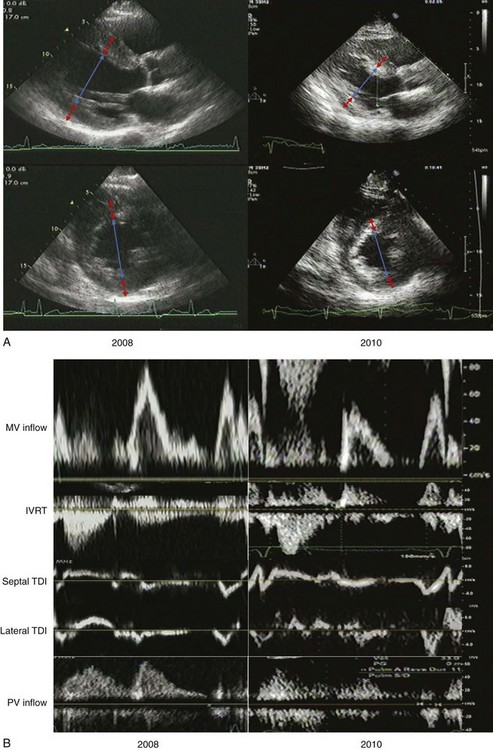
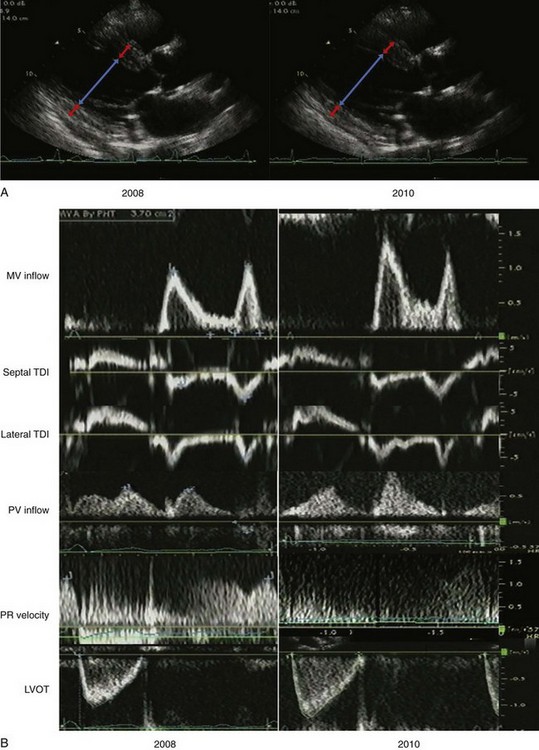
A 64-year-old man was referred for echocardiography to evaluate LV function after discharge from hospitalization for a hypertensive emergency. He had an unlimited exercise tolerance and no symptoms. His blood pressure was 144/66 mm Hg on carvedilol, losartan, amlodipine, and furosemide. His estimated GFR was 73 mL/min. LV function was normal with normal valvular function. The LA was dilated, with an LA volume index of 47 mL/m2. Two years later, he had progression of renal insufficiency and was referred to echocardiography for lower extremity edema. Exercise tolerance was unlimited. The blood pressure was 160/88 mm Hg on carvedilol, amlodipine, furosemide, isosorbide dinitrate and hydralazine in lieu of losartan due to hyperkalemia (Figure 5-21A).
LV geometry in 2008 was consistent with concentric LVH:
LV geometry in 2010 was consistent with less severe concentric LVH:
LV filling in 2008 was consistent with pseudonormalization and possibly elevated LV filling pressures (Figure 5-21B):
LV filling in 2010 was consistent with abnormal relaxation and normal LV filling pressures:
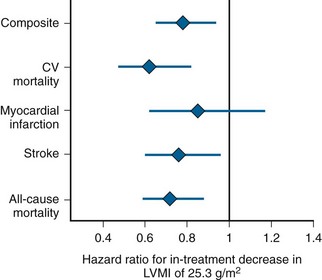
Figure 5-22 shows the association of in-treatment LV mass with risk of cardiovascular events.
Case 8
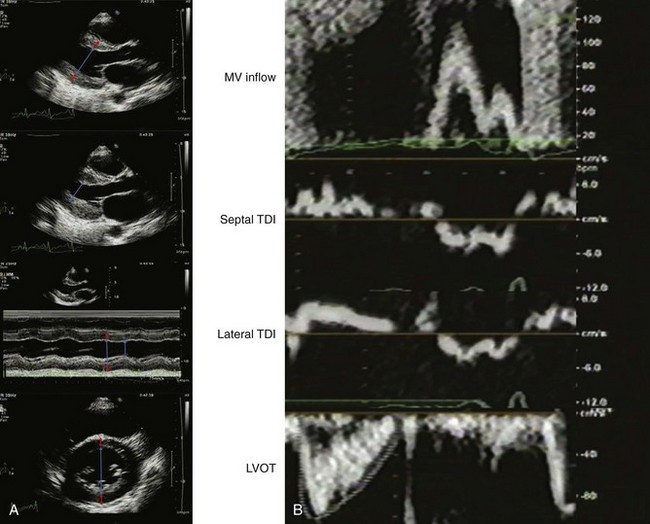
A 41-year-old man with stage III chronic kidney disease secondary to hypertension underwent echocardiography for dyspnea with exertion. His blood pressure was 134/75 mm Hg on amlodipine, labetalol, furosemide, hydralazine, and isosorbide dinitrate. On exam, he had jugular venous distention to the jaw and bibasilar crackles with mild pedal edema. His LVEF was normal with normal valvular function. The left atrium was mildly dilated (Figure 5-23A). On echocardiographic exam, LV geometry was consistent with concentric hypertrophy:
Doppler parameters were consistent with pseudonormalized filling, indeterminate LV filling pressures and high cardiac output (Figure 5-23B).
Case 9
Two years later, he was referred for echocardiography to evaluate LV function in the setting of worsening renal insufficiency. His blood pressure was 156/62 mm Hg on higher doses of the same medications, with no edema or evidence of volume overload. The follow-up echocardiogram demonstrated normal LV function without wall motion abnormalities. The patient was diagnosed with chronic allograft rejection (Figure 5-24A). LV geometry in 2010 was consistent with eccentric LVH:
Filling parameters were consistent with abnormal relaxation and elevated LV filling pressures (Figure 5-24B):
Case 10
A 36-year-old woman with poorly controlled type 1 diabetes and stage III chronic kidney disease was referred to echocardiography for evaluation of dyspnea with exertion and lower extremity edema. Her blood pressure was 176/90 mm Hg on labetalol and enalapril. Her exam was notable for a 2/6 holosystolic murmur, an S4, and no evidence of volume overload. Her echocardiogram demonstrated diffuse mild hypokinesis with mildly reduced LV function (LVEF 53%), moderate to severe mitral regurgitation with a regurgitant fraction of 50%, and severe TR. She underwent cardiac catheterization and was diagnosed with three-vessel CAD. The LV end-diastolic pressure was 22 mm Hg (Figure 5-25A). On echocardiographic exam, LV geometry was consistent with concentric LVH:
The LA size was normal by volumetric measurement (not shown in Figure 5-25).
Filling parameters were consistent with restrictive physiology and elevated LV filling pressures confirmed by cardiac catheterization (Figure 5-25B):
Case 11
Case 12
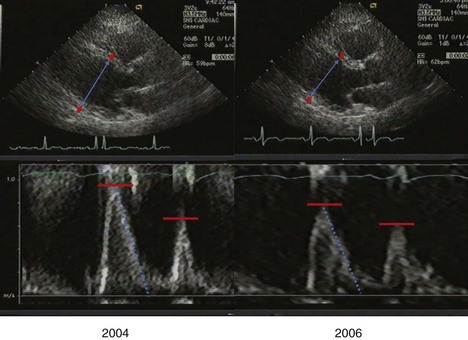
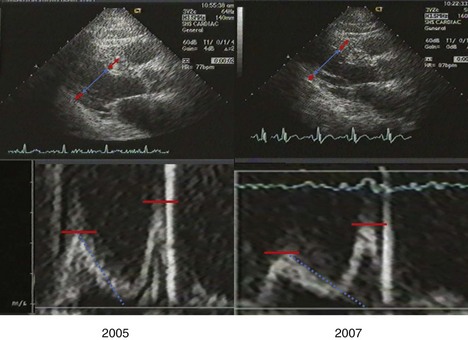
A 78-year-old Native American diabetic man with a prior history of myocardial infarction had severely depressed LVEF (33%) with inferior and inferolateral akinesis at baseline (2005), and unchanged left ventricular function at follow-up in 2007 after reduction of blood pressure from 163/82 to 132/73 mm Hg (Figure 5-27):
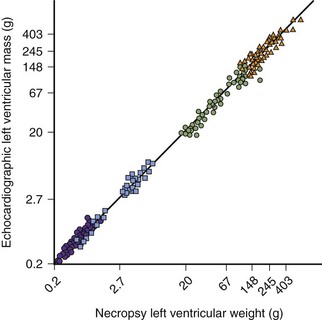
1 Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450-458.
This paper established the echocardiographic validation of left ventricular mass and hypertrophy.
2 Mor-Avi V, Sugeng L, Weinert L, et al. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: Comparison with magnetic resonance imaging. Circulation. 2004;110:1814-1818.
3 Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550-1558.
4 Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345-352.
5 Verma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: The VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582-591.
6 Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107-133.
7 Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539-2550.
8 Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350-2356.
9 Mishra RK, Galloway JM, Lee ET, et al. The ratio of mitral deceleration time to E-wave velocity and mitral deceleration slope outperform deceleration time alone in predicting cardiovascular outcomes: The Strong Heart Study. J Am Soc Echocardiogr. 2007;20:1300-1306.
10 Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207-2215.
This paper demonstrates the prognostic value of LVH for systolic dysfunction.
11 Fox ER, Taylor J, Taylor H, et al. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: Clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238-244.
12 From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300-305.
13 Lonn E, Shaikholeslami R, Yi Q, et al. Effects of ramipril on left ventricular mass and function in cardiovascular patients with controlled blood pressure and with preserved left ventricular ejection fraction: A substudy of the Heart Outcomes Prevention Evaluation (HOPE) Trial. J Am Coll Cardiol. 2004;43:2200-2206.
14 Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: The SANDS randomized trial. JAMA. 2008;299:1678-1689.
15 Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: The Strong Heart Study. Am J Cardiol. 2000;86:1090-1096.
16 Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592-1597.
17 Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29:641-647.
18 Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
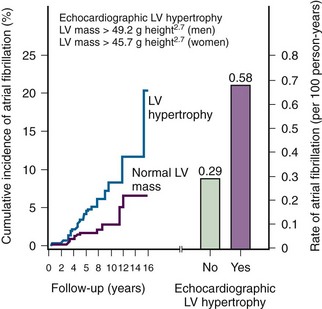
19 Verdecchia P, Reboldi G, Gattobigio R, et al. Atrial fibrillation in hypertension: Predictors and outcome. Hypertension. 2003;41:218-223.
20 Borlaug BA, Melenovsky V, Redfield MM, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570-1577.
21 Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463.