Chapter 45 Hypertension in pregnancy
Cardiovascular changes in pregnancy
There are significant physiological adaptations of the cardiovascular system to pregnancy. Blood volume rises from an average non-pregnant 2600 mL to 3800 mL at about 32 weeks gestation. The total red cell volume grows constantly until term from 1400 mL to 1700 mL, so there is a fall in haemoglobin concentration as gestation progresses. Cardiac output rises from 5 L/minute to 7.5 L/minute, mostly during the first trimester, and the heart rate rises by 10% with an average resting rate of 88 beats/minute. The peripheral resistance is lowered by a combination of increased vasodilatory substances during pregnancy and decreased sensitivity to vasopressor substances.
Uterine blood flow increases steeply from 24 weeks gestation.
Definitions. Hypertension in pregnancy is defined as:
Pre-eclampsia
Pathophysiology
Maternal contribution to pre-eclampsia occurs when endothelial activation results in acceleration of the normal systemic inflammatory response, which is present in all pregnancies. Activation of leucocytes and the coagulation process, and subsequent metabolic changes, result in the clinical features which are typically seen in pre-eclampsia: hypertension, oedema, proteinuria, platelet dysfunction, clotting derangements and possibly eclampsia.
Risk factors associated with pre-eclampsia
Risk factors are listed in Table 45.1. Other factors associated with pre-eclampsia include chronic hypertension, preexisting renal disease, autoimmune disease, more than 10 years since a previous pregnancy, a short sexual relationship prior to conception, and other thrombophilias (e.g. Factor V Leiden and possibly periodontal disease).
| Risk factor | Relative risk |
|---|---|
| Previous history of pre-eclampsia | 7.19 |
| Antiphospholipid antibodies | 9.72 |
| Preexisting diabetes | 3.56 |
| Multiple pregnancy | 2.91 |
| Nulliparity | 2.90 |
| Family history of pre-eclampsia | 2.90 |
| Elevated body mass index (BMI) >25 | 2.47 |
| Maternal age >40 | 1.96 |
| Diastolic blood pressure >80 mmHg at first antenatal visit | 1.38 |
Clinical spectrum
Pre-eclampsia is a multisystem disorder with both maternal and fetal consequences.
Hypertension
Hypertension may be labile, often with flattened or the reverse of normal diurnal rhythm. This is thought to be due to decreased responsiveness to angiotensin II. Many of the complications of pre-eclampsia are due to arterial damage and loss of vascular autoregulation.
Hepatic changes
Central nervous system involvement
Management of pre-eclampsia
Prevention
Investigations
Indications for delivery in pre-eclampsia or gestational hypertension
For indications for delivery in pre-eclampsia or gestational hypertension, see Table 45.2.
Table 45.2 Indications for delivery in pre-eclampsia or gestational hypertension
| Maternal | Fetal |
|---|---|
| Gestational age >37 weeks | Severe intrauterine growth restriction |
| Inability to control hypertension | Non-reassuring fetal status |
| Deteriorating platelet count | |
| Deteriorating liver function tests | |
| Deteriorating renal function tests | |
| Placental abruption | |
| Persistent neurological symptoms | |
| Eclampsia | |
| Persistent epigastric pain, nausea or vomiting with abnormal liver function tests | |
| Acute pulmonary oedema |
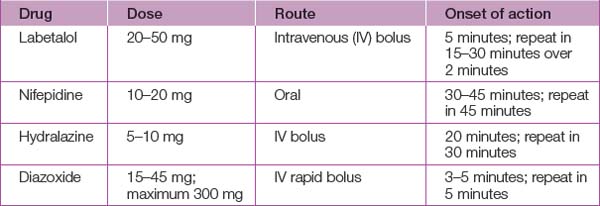
Antihypertensive therapy
Severe hypertension
Antihypertensive treatment (see Table 45.3) should be started in all women with a systolic blood pressure >170 mmHg or a diastolic blood pressure >110 mmHg because of the risk of intracerebral haemorrhage and eclampsia. A Cochrane review has concluded that there is no good evidence to support the use of any short-acting agent over any other and practice should therefore be guided by local experience and familiarity.
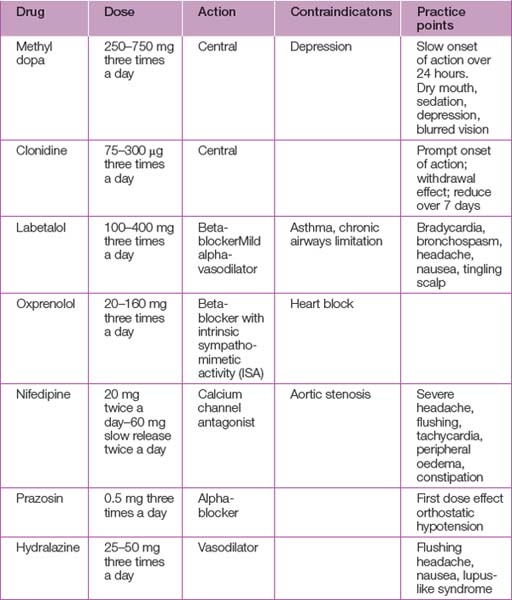
Mild to moderate hypertension
In the absence of compelling evidence, treatment of mild to moderate hypertension in the range 140–160/90–100 mmHg should be considered an option and will reflect local practice (see Table 45.4). Above these levels, treatment should be considered mandatory.
Management of eclampsia
Resuscitation
Delivery
When delivery is indicated, the mode of delivery depends on favourability of the cervix, the speed required for delivery and the fetal condition. In many cases, induction of labour and vaginal delivery is appropriate. In severe pre-eclampsia, prophylactic antihypertensive and anticonvulsant therapy are continued. Lumbar epidural is favoured for analgesia due to its ability to lower blood pressure and possibly increase uterine blood flow. Caution with epidural with strict investigation of platelet levels, coagulation profile and clotting times is important to avoid complications of bleeding and spinal haematoma. The use of general anaesthesia for caesarean section is associated with a marked hypertensive response to laryngoscopy and intubation.
Chronic hypertension in pregnancy
Unusual causes of hypertension in pregnancy
Phaeochromocytoma
This is a tumour of the adrenal medulla associated with significant maternal and fetal mortality.
Long-term consequences
It is recommended all women with hypertensive disease in pregnancy have an annual review for blood pressure and other cardiovascular risk factors.
Askie L.M., Duley L., Henderson-Smart D.J., Stewart L.A. PARIS Collaborative Group. Antiplatelet agents for the prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
Australasian Society for the Study of Hypertension in Pregnancy. Management of hypertension in pregnancy: executive summary. Medical Journal of Australia. 1993;158:700-702.
Brown M. Pre-eclampsia: a lifelong disorder. Medical Journal of Australia. 2003;179:182-184.
CLASP Collaborative Group. CLASP: a randomised trial of low dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619-629.
Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455-1463.
Khong T.Y., Mott C. Immunohistologic demonstration of endothelial disruption in acute atherosis in pre-eclampsia. European Journal of Obstetrics and Gynecology and Reproductive Biology. 1993;51:193-197.
Levine R.J., Maynard S.E., Qian C., et al. Circulating angiogenic factors and the risk of preeclampsia. New England Journal of Medicine. 2004;350:672-683.
Magpie Collaborative Group. Do women with pre-eclampsia and their babies benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
Maynard S.E., Min J.Y., Merchan J., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649-658.
Redman C.W.G. The placenta and preeclampsia. Placenta. 1991;2:301-308.
Redman C.W.G., Sacks G.P., Sargent I.L. Preeclampsia: an excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics and Gynecology. 1999;180:499-506.
Roberts J.M., Redman C.W.G. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447-1451.
Scott J.S. Pregnancy toxaemia associated with hydrops foetalis, hydatidiform mole and hydramnios. Journal of Obstetrics and Gynaecology of the British Empire. 1958;65:689-701.
SOMANZ Society of Obstetric Medicine of Australia and New Zealand. Guidelines for the management of hypertensive disorders of pregnancy. www.somanz.org, 2008. Available at:
Therapeutic Guidelines. Therapeutic guidelines: cardiovascular 2008. In Version 5. Melbourne: Therapeutic Guidelines; 2008.