Chapter 19 Hypertension
Introduction
Hypertension (HT) is a major risk factor for cardiovascular diseases (CVDs).
Estimates from the World Health Organization show that CVD accounted for approximately 17 million deaths in 2001, this being approximately 30% of the total 57 million deaths due to chronic diseases.1 This in fact represents approximately the total current population of the Australian continent. It is now a disease that is not restricted to the affluent Western world alone but the developing countries as well.2 Hence, as of the mid-1990s, CVD is also the leading cause of death in developing countries. In fact, a global CVD epidemic has been predicted based on the epidemiologic transition in which control of infectious, parasitic, and nutritional diseases allows most of the population to reach the ages in which CVD manifests itself. Moreover, diet and lifestyle changes contribute to an increase in overweight and obesity and in the incidence of type 2 diabetes in Western countries, both of which are risk factors for CVD.3 It then becomes possible to predict that disability from CVD will be a world leader by the year 2010.1
The medical profession must implement comprehensive preventative programs that address lifestyle and nutritional issues if it is to achieve a significant reversal of this adverse trend.1 The major emphasis currently is on treatment of CVD however, there is increasing interest in dealing with the factors responsible for this disease.
The cornerstone to the management of essential HT is lifestyle advice, including diet, smoking avoidance, reduced salt and caffeine intake, exercising, reducing stress and correcting sleep problems.4 Nutraceuticals have been reported to be beneficial in the prevention and risk management of CVD and may be broadly classified as those used in prevention or treatment of congestive heart failure, arrhythmias, hypertension, angina and hyperlipidemias.5 This chapter will explore these areas and look at the scientific evidence for non-drug approaches to help prevent and treat HT.
Lifestyle interventions
Numerous lifestyle and nutritional factors can prevent, delay the onset of, reduce the severity of, assist to treat, and control HT in many patients from diverse population groups.6 Lifestyle protective factors against HT are:
Recently updated National Heart Foundation guidelines recommend that advice on reducing and ceasing smoking, nutrition, reducing alcohol use, promoting regular physical activity and achieving a healthy body weight are effective methods in lowering blood pressure (BP) and should be part of routine management of HT for all patients, regardless of drug therapy. Smoking cessation is recommended to reduce overall cardiovascular risk.7
Mind–body medicine
Stress reduction and management
A population-based, prospective, observational study using participant data from the Coronary Artery Risk Development in Young Adults (CARDIA) study (3308 young adults aged 18–30) showed that those who rushed, were impatient and hostile had nearly double the risk of developing HT over 15 years, compared with their peers.8 Lifestyle stressors are important markers for the development of HT. Recently the results of a further study suggest that cumulative stressful life events have a negative effect on mental health and quality of life in young black men with high BP.9
A US study has reported that people who felt they had no control over an unpleasant stimulus had significantly higher BP and peripheral artery resistance than those who believed they were in control.10
A number of studies have demonstrated techniques used to lower stress levels can assist to lower BP, such as progressive muscle relaxation, psycho-education, biofeedback and self-hypnosis.11–14
A small trial of stress reduction demonstrated that 70% of the participants who had mild to moderate HT and who were taught stress reduction techniques, were able to reduce their medication after 6 weeks and, after 1 year, 55% required no medication.15
A meta-analysis including 29 randomised control trials (RCTs) indicated that relaxation resulted in small but statistically significant reductions in systolic and diastolic BP compared to control although most trials were of poor quality. Consequently the authors concluded ‘the evidence in favour of causal association between relaxation and BP reduction is weak’.16
Individual psychological therapy, including anger management and stress management techniques, reduced BP by more than 5mmHg in half of the hypertensive patients with BP greater than 140/90.17 The men and women were randomised to 10 1-hour individual sessions of therapy or a waiting list for 3 months and then therapy. However, as only half of all treated patients showed major improvement, the study recommendations were to ‘consider patients for psychological treatment when they report a great deal of subjective stress and/or find psychological interventions appealing’.17
Given that psychosocial stress has been implicated in disproportionately higher rates of HT among African Americans, a recent RCT of stress reduction in African Americans treated for HT for over 1 year showed that a selected stress reduction approach, through a transcendental meditation program, may be useful as an adjunct in the long-term treatment of HT in African Americans.18
Also note that studies have been undertaken to determine the extent of the white-coat phenomenon in patients with resistant hypertension. It is estimated that about 25% of patients with HT due to white-coat HT actually have normal BP.19, 20
Transcendental meditation
A review on the effectiveness of the transcendental meditation program in treatment and prevention of CVD has concluded that this technique reduces BP, carotid artery intima-media thickness, myocardial ischemia, left ventricular hypertrophy, mortality, and other relevant outcomes.21 The magnitudes of these effects compare favourably with those of conventional interventions for secondary prevention.
Sleep
Clinical research demonstrates that BP increases in hypertensive patients results from insufficient sleep.22 Researchers suggested this may be due to increased sympathetic nervous activity at night. A recent review has concluded that a healthy amount of sleep is paramount to leading a healthy and productive lifestyle. Further, that under strict experimental conditions, short-term restriction of sleep results in a variety of adverse physiologic effects, including hypertension, activation of the sympathetic nervous system, impairment of glucose control, and increased inflammation.23
A recent US cross-sectional study of 1741 adults found chronic insomnia and shortened duration of sleep significantly increased the risk of HT by 2.4 times compared with those of normal sleep.24 This risk increased by fivefold when the sleep duration was less than 5 hours.
Environment
An innovative study demonstrated that outdoor temperature and seasonal variations strongly correlated with BP fluctuations, particularly in elderly over 80 years of age. Systolic BP decreased with increasing temperature, with an 8.0mmHg decrease between the lowest (<7.9°C) and the highest (21.2°C) temperature quintile.25 Consequently, the elderly should be cautious with extreme temperatures and monitor their BP and medication dosage carefully to avoid complications.
Physical activity
Epidemiologic studies demonstrate that men who lead a physically active life can reduce their risk of developing HT by approximately 35% to 70%, compared to sedentary individuals.5 A review of the literature shows that, on average, 75% of hypertensive patients can decrease their systolic blood pressure (SBP) and diastolic blood pressure (DBP) by 11mmHg and 8mmHg respectively within 1 to 10 weeks of starting physical activity regimens (i.e. exercise training).26 A recent epidemiological study has reported that regular physical activity was negatively associated with HT in women.27
Tai chi
A systematic review of 47 studies that looked at the effects of tai chi on cardiovascular and BP found overall beneficial effects for healthy people, including increased muscle strength, flexibility, less falls, and improved mood.28 The reviewers concluded that more well-designed studies were necessary.
Qigong
A recent review suggests that there is encouraging evidence of qigong having efficacy in lowering SBP.29 A recent study of self-practiced qigong for less than 1 year demonstrated that it was better in decreasing BP in patients with essential HT than in no-treatment controls, but is not superior to that in active controls. More methodologically strict studies are needed to prove real clinical benefits of qigong, and to explore its potential mechanism.30
Nutritional influences
Diets, weight loss and weight management
The scientific evidence is strong for dietary changes that promote weight reduction in overweight hypertensive individuals, irrespective of age.31–35 This is significant because recently it was emphasised how important the prevention of obesity was in order to prevent future related problems such as HT in children and adolescents.36
DASH diet
Large epidemiological studies investigating dietary intake, such as that from the Dietary Approaches to Stop HT (DASH),37 and also the ATTICA study carried out in the Greek region of Attica,38, 39 report significant health benefits. The DASH trial was a landmark, multi-centre, randomised study (n>400) that investigated the effects of a diet rich in fruits, vegetables, and low-fat dairy on people with and without HT.40 This study reported that adherence to the DASH-style diet is associated with a lower risk of chronic heart disease (CHD) and stroke among middle-aged women, during 24 years of follow-up. Further, the researchers found that either a significantly reduced sodium intake (below 2.4g/day) or the DASH diet substantially lowered BP. Combining the 2 interventions had an even greater effect, comparable to first-line antihypertensive medications. The DASH diet that is rich in fruit and vegetables assists with reducing BP.41
Similarly, a Mediterranean style diet is reported to be protective. It was reported that older people, with low education, abdominal obesity, lower adherence to the Mediterranean diet, and increased inflammation, constitute a model of pre-hypertensive individuals that are prone to develop HT.38, 39
Oats
A US study of 88 people being treated for HT were randomised to a daily serving of wholegrain oat-based cereal (equivalent 3gm soluble fibre) or refined grain wheat-based cereal (less than 1gm soluble fibre) for 12 weeks.42 Participants receiving the oats had a significant positive change in BP with 73% needing to stop or reduce their medication by half during the study, compared to 42% of the participants in the wheat group. The participants who were unable to reduce their medication still had substantial improvement in BP. Furthermore, those in the oats group also had improved glucose levels, a 15% decrease in total cholesterol, and 16% decrease in LDL-cholesterol.
Weight loss
A further US study of 1000 hypertensive patients without cardiovascular disease demonstrated that with weight loss and salt reduction, up to 80% of patients could have their BP controlled without drugs a year after withdrawal of their antihypertensives.43 Although the lifestyle changes were challenging for the patients, the goals included weight loss of at least 4.5kg and maintaining a 24-hour urinary sodium excretion of 80mmol or less.
A recent Cochrane review of 18 trials concluded that weight-reducing diets in overweight hypertensive persons with a weight loss in the range of 3–9% of body weight caused only modest BP decreases of roughly 3mmHg systolic and diastolic but it was noted that weight-reducing diets ‘may decrease dosage requirements for persons taking antihypertensive medications’.44
A combination of dietary changes that promotes weight loss with physical activity may have significant additive effects in reducing BP in both men and women with mild HT.45
Salt reduction
A recent Cochrane review that included 20 RCTs with 822 otherwise untreated hypertensive adult patients compared the effect on BP of a modest restricted intake of dietary salt with that of usual salt intake.46 Modest dietary salt restriction was equal to 2.4gm/day decrease in salt intake measured by net change in 24-hour urinary sodium — by definition. Median reduction of salt intake across the trials was 4.6g/day. This regimen was maintained for 4 weeks (median, 5 weeks; range, 4 weeks–1 year). This dietary intervention produced an average decrease of 5.06 mmHg in SBP (95% confidence interval [CI], 4.31–5.81mmHg) and 2.76mmHg in DBP (95% CI, 1.97–3.55mmHg).46
There was also demonstrated a significant dose-response relationship between dietary salt restriction and BP decreases. Namely, a 6g/day decrease in salt intake resulted in a reduction of 7.2mmHg SBP (95% CI, 5.6–8.8mmHg) and 3.8mmHg DBP (95% CI, 2.8-4.7mmHg).46 The study results showed that a modest decrease in dietary salt intake in adults with diagnosed HT could prevent approximately 14% of deaths due to stroke and 9% of deaths due to ischemic heart disease.46
A more recent meta-analysis of 7 RCTs with 491 hypertensive adult patients compared an intervention that advised dietary salt reduction of 4–6g/day with that of usual salt intake. This intervention was associated with a documented reduction in SBP and DBPs of 4.7mmHg (95% CI, 2.2–7.2mmHg) and 2.5mmHg (95% CI, 1.8– l3.3mmHg), respectively, at a follow-up of 8 weeks or more.47
A further recent meta-analysis of 10 RCTs with 966 normotensive and hypertensive children (median age, 13 years; range, 8–16 years) reported that a 42% decrease in salt intake was associated with a 1.17mmHg (95% CI, 0.56–1.78mmHg) reduction in SBP and a 1.29mmHg (95% CI, 0.65–1.94 mmHg) reduction in DBP.48 This important finding suggests that significantly reducing the intake of dietary salt may also be an effective approach for lowering BP among children with HT.
Nutritional effects of Hypertension
There are numerous foods and nutraceuticals that have been shown to have angiotensin enzyme inhibitor activity and hence influence and better regulate BP.49
Macronutrients
Proteins
Observational and epidemiological studies demonstrate a consistent association between a high protein intake and a reduction in BP in Japanese rural farmers, Japanese–American men in Hawaii, American men in 2 cohort studies, British men and women, Chinese men and women, and American children as well as children in other countries where the degree of reduction is dependent on the protein source.50–53 The protein source is an important factor in the BP effect, animal protein (e.g. red meat and chicken) being less effective than non-animal protein (e.g. soy, legumes, nuts and seeds).54 However, it has been reported that lean or wild animal protein, such as fish, rabbit, kangaroo and turkey, with less saturated fat and more essential n-3 and n-6 fatty acids (FAs) may reduce BP, lipids, and CHD risk.53, 54, 55
The Intermap Study, a large international observational study, showed an inverse correlation of BP with total protein intake and with protein intake from non-animal sources.54 The INTERSALT Study supported the hypothesis that higher dietary protein intake has favourable influences on BP.51 The study evaluated 10 020 men and women in 32 countries worldwide and found that the average SBP and DBP were 3.0 and 2.5mmHg lower, respectively, for those whose dietary protein was 30% above the overall mean than for those 30% below the overall mean (81gm/day versus 44gm/day).
A study of 41 men and women with SBP between 130–160 on 1 antihypertensive medication were randomised to diets of low-protein (12.5% of energy from protein), low-fibre (15gm/day), then high-fibre diet or both high-protein (25% energy as protein) and high-fibre (30gm/day) diet. The results showed that there was a significant reduction in BP of nearly 6mmHg in the high protein/fibre diet in 2 months.56
Milk and soy protein
Fermented milk supplemented with whey protein concentrate significantly reduced BP in animal models (rats) and human studies.56 Kawase et. al.57 studied 20 healthy men given 200 mL of fermented milk supplemented with 4.4% of whey protein twice daily for 8 weeks. The SBP was reduced (P<.05), HDL-C increased and triglycerides fell in the treated group compared with the control group. Natural bioactive substances in milk and colostrum including minerals, vitamins, and peptides have been demonstrated to reduce BP.58,59 Milk ingestion increases protein, vitamins A, D, and B12, riboflavin, pantothenate, Ca++, phosphorous, Mg++, Zn++, and K+.55 These findings are consistent with the combined diet of fruits, vegetables, grains, and low-fat dairy in the DASH-I and DASH-II studies in reducing BP.40, 60 Soy protein at intakes of 25–30g/day lowers BP and increases arterial compliance61, 62 and reduces LDL-cholesterol and total cholesterol by 6% to 7% and LDL-cholesterol oxidation.61, 62 Soy contains many active compounds that produce these antihypertensive and hypolipidemic effects including isoflavones, amino acids, saponins, phytic acid, trypsin inhibitors, fibre, and globulins.61, 62 Numerous foods are abundant in genistein and daidzein such as currants, raisins, hazelnuts, peanuts, coconuts, passion fruit, prunes, as well as many other fruits and nuts.63
Whey protein
In a study by Pins and Keenan,64 who administered 20g of hydrolysed whey protein to 30 hypertensive participants, noted a BP reduction of 11/7mmHg compared with control participants at 1 week, that was sustained throughout the study. The antihypertensive effect was thought to be mediated by an angiotensin converting enzyme inhibitor (ACEI) mechanism. These data indicate that the whey protein must be hydrolysed to exhibit an antihypertensive effect and that the maximum BP response is dose dependent. Bovine casein-derived peptides and whey protein-derived peptides exhibit ACEI active-B-caseins, B-Ig fractions, B2-microglobulin, and serum albumin. Whey protein hydrolysates exhibit both in vitro and in vivo ACEI and antihypertensive activity in in vivo animal and human studies.57, 58, 59, 64, 65
Fish protein
Sardine muscle protein, which only contains valyl-tyrosine (VAL-TYR), significantly lowers BP in hypertensive participants.66 Kawasaki et. al. treated 29 hypertensive participants with 3mg of VAL-TYR sardine muscle-concentrated extract for 4 weeks and lowered BP by 9.7/5.3mmHg.66
In addition to ACEI effects, protein intake may also alter catecholamine responses and induce natriuresis.67 The optimal protein intake, depending on level of activity, renal function, stress, and other factors, is about 1.0–1.5g per kg per day.68, 69
Fats
Epidemiological studies that include observational, biochemical, cross-sectional studies and clinical trials on the effect of fats on BP have been inconsistent.70–73 However, many of these studies have suffered from inaccurate measurements of dietary components through recall or recording and most probably have missed small associations. Some also had inadequate or incorrect BP measurements, and did not correct for numerous dietary or non dietary confounding factors.70
A comprehensive meta-analysis and review of these studies is reported by Morris.70 In the National Diet Heart Study, there was no change in BP with a polyunsaturated to saturated fat ratio (P/S ratio) in the range of 0.3 to 4.5 in 1218 participants over a 52-week study period.71 The Multiple Risk Factor Intervention Trial demonstrated that consumption of an extra 6g of trans-fatty acids (TFAs) per day increased SBP 1.4mmHg and DBP 1.0mmHg.68 However, the addition of 2g/day of linolenic acid reduced mean BP by 1.0mmHg. Two large prospective clinical studies, the Nurses Health Study69 and the US Male Study (USMS),72 showed a neutral effect on BP by all the fats (total fat, saturated fat, and polyunsaturated fat) or TFAs studied.
A recent randomised control 8-week study of 80 obesity-related hypertensive patients on Ramipril found that supplementation with dietary conjugated linoleic acid (CLA) significantly enhanced the antihypertensive effect on BP.74
See Table 19.1 for a list of common essential fatty acids.
| Common name | Lipid name | Chemical name |
|---|---|---|
| Omega-3 | ||
| CC–Linolenic acid (ALA) | 18:3 (n−3) | all−cis−9,12,15−octadecatrienoic acid |
| Eicosapentaenoic acid (EPA) | 20:5 (n−3) | all−cis−5,8,11,14,17−eicosapentaenoic acid |
| Docosahexaenoic acid (DHA) | 22:6 (n−3) | all−cis−4,7,10,13,16,19−docosahexaenoic acid |
| Omega-6 | ||
| Linoleic acid (LA) | 18:2 (n−6) | 9,12−octadecadienoic acid |
| Gamma−linolenic acid (GLA) | 18:3 (n−6) | 6,9,12−octadecatrienoic acid |
| Dihomo−gamma−linolenic acid (DGLA) | 20:3 (n−6) | 8,11,14−eicosatrienoic acid |
| Arachidonic acid (AA) | 20:4 (n−6) | 5,8,11,14−eicosatetraenoic acid |
| Omega-9 | ||
| Oleic acid (OA) | 18:1 (n−9) | 9−octadecenoic acid |
n-3 PUFAs α-Linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are primary members of the n-3 PUFA family.75, 76 n-3 fatty acids are found in coldwater fish (herring, haddock, Atlantic salmon, trout, tuna, cod, and mackerel) and the contamination with mercury, which is always a concern, varies based on where the fish are caught. Fish oils, flax, flax seed, flax oil, and nuts, with flax seed and walnuts having the highest content72, 73 n-3 PUFAs, significantly lower BP in observational, epidemiological and some small prospective clinical trials.72, 75–83
Two meta-analyses of controlled trials concluded that approximately 3g/day of n-3 fatty acids of fish oil (containing on average 160mg DHA, 90mg EPA per 1000mg capsule) can significantly lower BP in hypertensive, but not normotensive, individuals.84, 85 It is possible to consume this amount of fish oil by eating fish daily, but this would depend on the amount and type of fish consumed.75
The meta-analysis by Appel et. al.84 was of 17 controlled clinical trials of n-3 supplementation with an average dose > 3g/day. Significant reductions in systolic BP and diastolic BP were observed in normotensive individuals and untreated hypertensives. Side-effects included nausea and a fishy taste. The researchers concluded that a diet supplementation with a relatively high dose of n-3FAs, generally more than 3g/day, can lead to clinically relevant BP reductions in individuals with untreated HT.
A meta-analysis of 31 studies of the effects of fish oil on BP has shown that there is a dose-related response in HT as well as a relationship to the specific concomitant diseases associated with HT.86 At fish oil doses of 4g/day, there was no change in BP in the mildly hypertensive participants. At 4–7g/day, BP fell 1.6 to 2.9mmHg; at 15g (2.04 gm EPA and 1.4 gm DHA) of fish oil from salmon per day and greater, BP decreased 5.8 to 8.1mmHg.86 There was no change in BP in the normotensive participants. There are no known major studies that indicate that too much fish oil supplementation adversely affect the BP of some people except some trials indicating a possible tendency to bleed with dose >10gm/day.
Small trials, such as that by Knapp and FitzGerald,83 have demonstrated a significant reduction in BP in a group of hypertensive participants given 15g/day of fish oil. There was inadequate data relating to side-effects. Bao et. al.87 studied 69 obese hypertensive participants for 16 weeks randomised to 3 groups. The treatments included fish oils only (3.65g n-3 FAs per day), a combination with a weight loss regimen and a weight loss regimen only. Group I participants taking 3.65g/day of n-3 FAs alone reduced BP by 6/3mmHg. Group II participants who lost an average of 5.6kg of weight, but received no fish oil, had a 5.5/2.2mmHg reduction in BP. The best BP results were seen in group III participants, with combined fish oil n-3 FAs and weight loss, whose BP and HR decreased by 13.0/9.3mmHg and an average of 6 beats/min respectively.
Mori et. al.88 studied 63 hypertensive and hyperlipidemic participants treated with n-3 FAs (3.65gg/day for 16 weeks) and found significant reductions in BP, increase in HDL2-C, decrease in HDL3-C, decrease in triglycerides (29%), but no change in LDL-C, TC, or total HDL-C. Serum glucose and insulin levels also declined. Recent studies indicate that DHA is very effective in reducing BP and HR.88, 89
Reports also indicate that eating coldwater fish 3 times per week (150g fish weight) is as effective as high-dose fish oil by reducing BP in hypertensive patients, and the protein in the fish may also have antihypertensive effects.84 The BP is usually unaffected in healthy non-hypertensive patients.84, 86 Formation of EPA and ultimately DHA from ALA is decreased in the presence of increased linoleic acid (LA) in the diet (n-6 FAs), increased dietary saturated fats and TFAs, alcohol, and ageing through inhibitory effects or reduced activity of delta-6-desaturase, delta-5-desaturase, or delta-4-desaturase.88, 89
A recent randomised controlled study.90 demonstrated that in dyslipaedemic patients supplementation with ALA (flaxseed oil at a dose of 8g/day) resulted in significantly lower systolic and DBP levels compared with LA (P = 0.016 and P = 0.011, respectively.
Dosage
The reported dosage of n-3 fatty acids for a significant reduction in BP is at least 4g/day.6 There is no concern in relation to high doses of fish oil except possible bleeding tendency with dose >10gm/day.75
Toxicity
Fish oil capsules available in Australia have zero or near zero methyl-mercury content. Fish oil capsules in Australia contain very low levels of dioxins (polychlorinated biphenyl.75
For more information on fish oil supplementation refer to The Australian Heart Foundation website: http://www.heartfoundation.org.au/document/NHF/HW_FS_FishOils_PS_FINAL.pdf
n-6 FAs (sunflower, safflower oils and margarines)
The n-6 FAs family, which includes LA, GLA, DGLA, and AA (Table 19.1), have been reported to not significantly lower BP directly,71 but that may prevent increases in BP induced by saturated fats.91 The ideal ratio of n-3 FAs to n-6 FAs is between 1:1 and 1:2, with a polysaturated fat ratio greater than 1:5 to 2:0.92 Hydrogenated or partially hydrogenated vegetable oils, which all contain variable amounts of TFAs, should be avoided because they will increase BP and CHD risk.93
n-9 FAs (olive oil)
Olive oil is rich in monounsaturated FAs (MUFAs) (∼72% oleic acid) which have been associated with BP and lipid reduction in Mediterranean and other diets.92 Ferrara and colleagues93 studied 23 hypertensive participants in a double-blind, randomised, cross-over study for 6 months, comparing MUFAs with PUFAs. Extra virgin olive oil (MUFAs) — using 40g in males (about 4 spoonfuls) and 30g in females (about 3 spoonfuls) — was compared with sunflower oil (PUFAs) rich in LA (n-6 FAs). The SBP fell 8mmHg and the DBP fell 6mmHg in the MUFA-treated participants compared with the PUFA-treated participants. In addition, the need for antihypertensive medications was reduced by 48% in the MUFA group versus 4% in the PUFA (n-6 FAs) group.
Strazzullo and colleagues94 found an increase in SBP and DBP in patients when olive oil was replaced with saturated FAs. Thomsen et. al.95 compared hypertensive type II diabetics in a cross-over study comparing MUFAs (olive oil) with PUFAs. There was a significant reduction in clinic BP and 24-hour ambulatory blood pressure measurement (ABM). However, in normotensive healthy participants given an olive oil-rich diet versus a carbohydrate-rich diet, no change in BP was observed.96
Extra virgin olive oil is a rich source of polyphenolic compounds and has 5mg of phenols per 10g, which equates to a dose of 4 tsp of olive oil.89, 97 Approximately 4 teaspoons of extra virgin olive oil is equal to 40g.88The MUFAs tend to increase HDL-cholesterol more than PUFAs,94 and the oleate-rich LDL-cholesterol is more resistant to oxidation than to oxidised LDL-cholesterol (oxLDL-C).98
Recent studies on Mediterranean diet have further confirmed the additive value of olive oil in reducing HT.99, 100 The data suggested a significant sub-additive effect of the combined consumption of wine, fruit and vegetables and the anti-lipid effect of MUFAs from olive oil.
Other foods
Caffeine and alcohol
Patients with HT or cardiovascular disease should be advised to limit their intake of caffeine as even 2 cups of caffeine or 250mg caffeine can result in prolonged deterioration in aortic elasticity and increase in SBP and DBP.101
However, a recent review in contrast to early studies concludes that habitual moderate coffee intake does not represent a health hazard and may even be associated with beneficial effects on cardiovascular health.102
However, moderate restriction of alcohol and caffeine in addition to other lifestyle modifications can prevent, delay the onset of, reduce the severity of, treat, and control HT in many patients.51
Chocolate
Chocolate has been shown to have beneficial effects on lowering BP103 in a trial of 13 hypertensive elderly people. Two weeks of eating 100g of dark chocolate daily resulted in a 5.1mmHg drop in SBP and a 1.8mmHg drop in DBP. The BP returned to pre-trial levels 2 days after stopping the chocolate. The researchers reported that the polyphenols present in cocoa solids were responsible for the BP drop. Moreover, a small sample study of otherwise healthy individuals with above-optimal BP indicated that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved formation of vasodilative nitric oxide.104
Breastfeeding
A UK study assessed 7276 7-year old children and found mean SBP was 1.2mmHg lower and mean DBP 0.9mmHg lower in children who had been breastfed, compared with children who had not.105 According to the researchers the significance of a 1% reduction in population SBP levels is associated with an approximate 1.5% reduction in all-cause mortality. A recent systematic review and meta-analysis concluded that the small reduction in BP associated with breastfeeding could confer important benefits on cardiovascular health at a population level.106
Nutritional supplements
Vitamins
Vitamin C
Numerous epidemiological, observational, and clinical studies have demonstrated that the dietary intake of vitamin C or plasma ascorbate concentration in human beings is inversely correlated with SBP, DBP, and HR.107–112
Controlled intervention trials have been inconclusive though as to the relationship between vitamin C administration and BP.113–116 The systematic review by Ness et. al. on HT and vitamin C concluded that if vitamin C has any effect on BP, it is small.116 In the 18 studies that were reviewed worldwide, 10 of 14 showed a significant BP reduction with increased plasma ascorbate levels and 3 of 5 demonstrated a decreased BP with increased dietary vitamin C.116 Moreover, in 4 small RCTs of 20–57 participants, 1 had significant BP reduction, 1 had no significant BP reduction, and 2 were not interpretable. In 2 uncontrolled trials, there was a significant reduction in BP. The conclusion was that further studies were required. Duffy et. al. evaluated 39 hypertensive participants (DBP, 90-110mmHg) in a placebo-controlled 4-week study.108 A 2000g loading dose of vitamin C was given initially, followed by 500g/day. The SBP was reduced 11mmHg, DBP decreased by 6mmHg and mean arterial pressure (MAP) fell 10mmHg.
Ceriello and colleagues107 administered intravenous vitamin C to hypertensive patients with DM and reduced BP significantly. Further a randomised placebo-controlled trial demonstrated that 500mg of daily vitamin C significantly reduced SBP by 13mmHg in 45 patients with mild or moderate HT compared with placebo after 1 month of treatment.108 Mean SBP reduced from 155 to 142mmHg. Vitamin C also reduced DBP but this was not different to the placebo group.
The variation in the published data can be explained by numerous deficits in methodological design that included: lack of a control group; no baseline BP; small study population; short trial duration; variable vitamin C doses; variable demographics and study population; unknown premorbid vitamin C status or pre-morbid general vitamin or antioxidant status; concomitant or unknown multivitamin intake; and unknown nutritional status. In addition, existing concomitant diseases — confounding factors such as stress, smoking, alcohol, weight changes, and fibre, among others — were not stated or evaluated, plasma ascorbic acid levels were not measured, the P value and CIs were not reported, variable BP measurement techniques were used (clinic or office, home, 24-hour ABM), unknown genetic polymorphisms exist, or there was publication bias.111
Vitamin E
There are several human clinical studies that have investigated the relationship between vitamin E intake and BP.117–120 The results report that α-tocopherol has an antihypertensive effect, and that it is probably small and may be limited to untreated hypertensive patients or those with known vascular disease or other concomitant problems such as diabetes or hyperlipidemia.120
Vitamin D
Epidemiological and clinical investigations demonstrate a relationship between plasma levels of 1,25 (OH)2 D3 (1,25-dihydroxycholecalciferol), the active form of vitamin D and BP,121–125, 130–134 including vitamin D-mediated reduction in BP in hypertensive patients.
It has been difficult to dissociate the effects of calcium from vitamin D on BP in humans.127,128 Numerous studies have verified the finding of an inverse relationship between dietary calcium intake and BP.127, 128, 129 In The Tromso Study there was a negative association between calcium intake from dairy products and BP.129 Calcium and vitamin D intake was assessed in 7542 men and 8053 women and the study found a significant linear decrease in SBP and DBP with increasing dietary calcium intake in both sexes; however, vitamin D intake had no significant effect on BP. It should be noted that this group did not measure blood vitamin D levels. The relationship between vitamin D, calcium and HT showed that intakes of low-fat dairy products, calcium, and vitamin D were each inversely associated with risk of HT in middle-aged and older women. This study suggested the potential roles vitamin D and calcium may have in the primary prevention of HT.130
It has been documented that higher calcium levels can decrease vitamin D levels.136
Vitamin D may have an independent and direct role in the regulation of BP and insulin metabolism.131,135 A study of 34 middle-aged men demonstrated that serum levels of 1,125 (OH)2 D3 were inversely correlated with BP (P<.02), very LDL cholesterol (P<.005) and triglyceride removal after intravenous fat tolerance test (P<.05).134 Serum levels of 25 (OH)2 D3 were correlated with fasting insulin (P<.05), insulin sensitivity during clamp (P<.001), and lipoprotein lipase activity in adipose tissue (P<.005) and skeletal muscle (P<.03). Scragg and colleagues, found no difference in serum 25 (OH)2 D3 levels in a group of hypertensive versus normotensive participants.133 Further, a recent study reported that calcium plus vitamin D3 supplementation did not reduce the risk of developing diabetes over 7 years of follow-up in this randomised placebo-controlled trial.135 Higher doses of vitamin D may be required it was suggested to affect diabetes risk, and/or associations of calcium and vitamin D intake with improved glucose metabolism observed in non-randomised studies. Earlier reported positive outcomes may be the result of confounding or of other components of foods containing these nutrients.135
B Group vitamins
Animal and human studies have demonstrated that low serum vitamin B6 levels were associated with HT.137–143 However, the effect of homocysteine-lowering vitamins on BP has not been well studied.
A recent clinical trial measured blood pressure in older people with elevated baseline homocysteine and reported that SBP and DBP as well as pulse pressure in the vitamin-supplemented group did not differ from the placebo group. However, over the duration of the trial plasma homocysteine decreased. The mean differences in BPs, adjusted for baseline values, did not exceed 1mmHg.143 B-vitamins supplemented group lowered plasma homocysteine but had no effect on blood pressure.144
Minerals
Sodium (Na+)
Epidemiological, observational, and controlled clinical trials demonstrate that an increased Na+ intake is associated with higher BP.145 A reduction in Na+ intake in hypertensive patients, especially the salt-sensitive patients, will significantly lower BP by 4 to 6/2 to 3mmHg.146–149 The BP reduction is proportional to the severity of Na+ restriction.150, 151 In the TOHP-I Trial,151 a 100mmol Na+ intake per day (2400mg) reduced the incidence of HT by 20% in a group of high-risk participants, improved HT control in elderly participants taking medication in the TONE Study,152 reduced cardiovascular disease in obese participants,153, 154, 155 and reduced proteinuria and progression of renal disease.150, 156, 157 The TOHP-II Trial had a mean BP reduction of 2.9 F 1.6mmHG with moderate Na+ restriction.156 A Cochrane review concludes that evidence from large and small trials show that a low sodium diet helps in maintenance of lower BP following withdrawal of antihypertensives.158 Recent reports on observational follow-up of the trials of HT prevention (TOHP) and others strongly suggest that Na+ reduction, previously shown to lower BP, also reduce long-term risk of cardiovascular events.159, 160 The TOHP study found that the low-salt group 10–15 years later had 25% lower heart disease and stroke.160
Potassium (K+)
Numerous epidemiological, observational, and clinical trials have demonstrated a significant reduction in BP with increased dietary K+ intake.161, 162, 163 The magnitude of BP reduction with a K+ supplementation of 60–120mEq/d is approximately 4.4/2.5mmHg in hypertensive patients and 1.8/1.0mmHg in normotensive patients.164, 165 A meta-analysis of all K+ supplementation clinical trials in the treatment of HT demonstrated a racial difference, with black participants having a more substantial reduction in BP compared with white participants.163 A high K+ intake is most effective in reducing BP in patients with diuretic-induced hypokalemia, in those with a high Na+ intake,163–166 in patients with salt-sensitive HT, severe HT, or a positive family history,164 as well as in non-white populations such as Chinese,165 and African Americans.166
It has been suggested that alteration of the K+/Na+ ratio to a higher level is important for both antihypertensives as well as cardiovascular and cerebrovascular effects.168 High K+ intake reduces the incidence of cardiovascular and CVAs independent of BP reduction.168
Proposed mechanisms include improvements in vascular smooth muscle function and structure, natriuresis, modulation of baroreflex sensitivity, direct vasodilation, reduced vasoconstrictive sensitivity to norepinephrine (NE) and A-II, increased serum and urinary kallikrein, increased Na+/K+ adenosine triphosphatase activity, and DNA synthesis and proliferation in VSMCs and SNS cells.167, 168
Gu and colleagues recently demonstrated for the first time that K+ supplementation at 60mmol of KCI per day for 12 weeks significantly reduced SBP<5.0mmHg in 150 Chinese men and women aged 35 to 64 years.165 This study confirmed that the higher the initial BP, the greater the response. In addition, K+ may have a Ca++ conserving effect that would further minimise the effects of a high Na+ intake.170 The interactions of Na+, Ca++, K+, and Mg++ are more important in BP control than isolated changes in 1 mineral.167, 168, 169
Calcium (Ca++)
Epidemiological studies show a link between HT and Ca++,49, 170 but clinical trials that administer Ca++ supplements to patients have shown inconsistent effects on BP.171–180 Higher dietary Ca++ is not only associated with a lower BP, but also with a decreased risk of developing HT.165 A 23% reduction in the risk of developing HT was noted in those individuals taking a dose higher than 800mg/day compared with those taking 1 lower than 400mg/day.165 A meta-analysis of the effect of Ca++ supplementation in hypertensive patients found a reduction in the SBP of 4.3/1.5mmHg.176 Foods high in Ca++ were more effective than supplements in reducing BP.176
Karanja and colleagues177 assessed the effects of CaCO3 versus Ca++ contained in the diet and found significant increases in magnesium (Mg++), riboflavin, and vitamin D in the dietary group that correlated with Ca++ intake. There is an additive or synergistic effect on BP reduction with a combination of minerals and vitamins as compared with Ca++ alone. Also, the heterogeneous responses to Ca++ supplementation have been explained, by Resnick,178 as being dependant on the population or hypertensive subtype. Those patients with the greatest reduction in BP with Ca++ supplements include black patients, those with low-renin HT, ageing adults and pregnant women (pregnancy-induced HT).179
In a Cochrane review of 12 trials the risk of pre-eclampsia was reduced with calcium supplementation rather than placebo (11 trials, n = 14 946 women).180 This review supports the potential role of Ca++ supplementation for gestational HT, especially high-risk women and in communities with low-dietary calcium intake.
Of interest, a 2-year RCT that assessed the long-term effect of calcium and vitamin D3 fortified milk on BP and serum lipid concentrations in healthy older men found no added benefit on BP and lipid concentrations compared with the control group (ingesting non-fortified milk).181
A dose of calcium carbonate or citrate 1000–3000mg/day is recommended.
Magnesium (Mg++)
Several studies have indicated there may be a role for Mg++ supplementation for its relaxation effects on smooth muscles of blood vessels causing vasodilation and therefore lowering BP. According to a double-blind, cross-over study,182 365mg of Mg++ per day with beta-blockers significantly reduced BP compared with beta-blockers alone.
According to Japanese researchers, Mg++ supplements may have a valid role in BP control.183 The study randomised 60 hypertensive volunteers with daily small doses of magnesium oxide (20mmol) for 8 weeks. Office, home and ambulatory BP measurements all showed small but significant reductions in BP. There appears to be a positive correlation between low levels of serum and erythrocyte Mg++ levels and HT, only if there is a family history.184
In another double-blind controlled trial,185 91 middle-aged and elderly women with mild to moderate HT who were not on antihypertensive medication were randomly assigned to treatment with magnesium aspartate-HCl (20mmol Mg++/day) or placebo for 6 months. Magnesium was well tolerated and not associated with an increased frequency of diarrhoea compared with placebo at this dose. At the end of the study, SBP had fallen by 2.7mmHg and DBP by 3.4mmHg more in the magnesium group than in the placebo group. BP response was not associated with baseline Mg++ status, as measured by dietary magnesium intake and urinary magnesium excretion.
Another double-blind, placebo-controlled study186 involved 33 participants who were allocated to undergo either a 4-week treatment with oral Mg++ supplementation (Mg[OH]2; 411–548mg/day) or a placebo. The SBP and DBP values decreased significantly in the magnesium group, but not in the placebo group. The results suggested that Mg++ supplementation may lower BP through the suppression of the adrenergic activity and possible natriuresis.
A recent review summarises the evidence for benefits of Mg++ on metabolic abnormalities, inflammatory parameters, and cardiovascular risk factors.187 In conclusion, the review states that there is strong biological plausibility for the direct impact of Mg++ intake on metabolic and cardiovascular risk factors, but in vivo Mg++ deficiency might play only a modest role.
A dose of magnesium orotate 400–800mg/day is recommended.
Dosages for magnesium and potassium
A high dietary intake of Mg++ of at least 500–1000mg/day reduces BP in most of the reported epidemiological, observational, and clinical trials.6
The recommended intake of K+ is 650 mEq/day with a K+/Na+ ratio of more than 5:1. Numerous epidemiological, observational, and clinical trials have demonstrated a significant reduction in BP with increased dietary K+ intake. The magnitude of BP reduction with a K+ supplementation of 60–120 mEq/day is 4.4/2.5mmHg in hypertensive patients and 1.8/1.0mmHg in normotensive patients.6
Zinc (Zn++)
Low serum Zn++ levels in observational studies correlate with HT as well as CHD, type II diabetes mellitus, hyperlipidemia (especially hypertriglyceridemia, low-density lipoprotein cholesterol [LDL-cholesterol] and elevated lipoprotein a), 2-hour postprandial increased plasma insulin levels, and insulin resistance.188 In elderly hypertensive patients with very low plasma renin activity there is a high urinary excretion of Zn++ and low serum levels that are partially corrected by the administration of oral Ca++ in a dose greater than 800mg/day.189 There is a close relationship between Zn++, Ca++, Na+, Mg++, and K+ in various hormonal systems (renin–angiotensin–aldosterone system) that modulate BP.190
Antioxidants
Galley and colleagues191 administered antioxidants to 40 hypertensive and normotensive adult participants in a randomised, double-blind, cross-over design placebo-controlled study for 8 weeks. The antioxidants administered were zinc sulfate 200mg/day, ascorbic acid 500mg/day, α-tocopherol 600mg/day, and β carotene 30mg/day. The SBP decreased significantly in the hypertensive participants but not in the normotensive participants. Increases in plasma levels of antioxidants and increased urine nitrate excretion occurred in the hypertensive participants, suggesting an increased bioavailability of nitrous oxide.191
Bergomi and colleagues evaluated Zn++ and Ca++ status in 60 hypertensive compared with 60 normotensive control participants.192 An inverse correlation of BP and serum Zn++ was observed, but there was a direct correlation with serum Ca++.
Other nutritional supplements
Coenzyme Q10 (CoQ10)
Despite several studies193–200 demonstrating that CoQ10 may be of benefit for HT, the mechanism of how it works at this stage and its potential use in HT is not clear and larger controlled trials are required to confirm its clinical efficacy.
In 1 study, 80 type 2 diabetics with dyslipidaemia were entered into a randomised double-blind, placebo-controlled interventional trial over 12 weeks.201 The groups received 200mg CoQ10 and 200mg fenofibrate (FF) per day. The other 3 groups received: CoQ10 plus FF placebo; CoQ10 placebo plus FF; or all placebo capsules. The CoQ10 had no effect on lipid levels but a significant effect on SBP and DBP.
In a further study, 109 patients with essential HT were supplemented with an average oral dose of 225mg/day CoQ10 in addition to their existing antihypertensive drug regimen.202 Patients were able to reduce their antihypertensive drug therapy gradually during the first 1–6 months. Fifty-one percent of patients completely discontinued between 1 and 3 antihypertensive drugs on average over 4.4 months after starting CoQ10.
A meta-analysis of 12 clinical trials concluded that CoQ10 had the potential in hypertensive patients to lower SBP by up to 17mmHg and DBP by up to 10mmHg without significant side-effects.203
Lycopene
Lycopene has recently been shown to produce a significant reduction in BP, and serum lipids.204, 205 Paran and Engelhard evaluated 30 patients with grade 1 HT and raised lipids, aged 40–65 years, taking no antihypertensive, or anti-lipid medication treated with a tomato lycopene extract, for 8 weeks.204 The mean SBP was reduced from 144 to 135mmHg (9mmHg reduction) and DBP fell from 91 to 84mmHg (7mmHg reduction) in supplemented group. A similar study with 35 patients with grade 1 HT showed similar results with reduction of SBP but not on DBP.205 Serum lipids were significantly improved in both studies without any change in serum homocysteine.
Herbal medicines
Garlic (Allium sativum)
Good clinical trials using the correct type and dose of garlic have shown consistent reductions in BP in hypertensive patients.206–222 However, not all garlic preparations are processed similarly and are not comparable in antihypertensive potency.215, 216 Moreover, cultivated garlic (Allium sativum), wild uncultivated garlic or bear garlic (Allium urisinum),49, 215, 216 and aged216 or fresh garlic will have variable effects.207, 208 Cooking garlic, for instance, will reduce allicin compounds in the garlic and thus reduce its hypotensive properties. Raw garlic is preferred but usually causes garlic breath and odour. Hence, garlic preparations in capsules improve compliance and are usually preferred as they reduce these unwanted side-effects. According to a recent meta-analysis of garlic on HT, most studies used garlic powder dosages of 600–900mg/day, providing potentially 3.6–5.4mg of allicin, the active compound in garlic.218 This compares to fresh garlic cloves (∼2g) yielding about 5–9mg allicin. The authors concluded the ‘meta-analysis suggests that garlic preparations are superior to placebo in reducing BP in individuals with HT’.217 A further meta-analysis also confirms this conclusion.219
Most positive studies have used quantities of garlic beyond typical dietary levels (equivalent to 5–20 average-sized cloves/day or dried garlic powder preparation, average 900mg/day). An older meta-analysis of 8 randomised trials (n = 415) suggested some effect in mildly hypertensive patients, but not enough to recommend its routine clinical use and more rigorous trials were encouraged.212 Of the 7 trials that compared the effect with that of placebo, 3 showed a significant reduction in SBP and 4 in DBP.
Hawthorn berries
A review reports that hawthorn may reduce systemic vascular resistance and BP, decrease the pressure-rate product in the myocardium, improve ejection fraction and congestive heart failure (CHF), improve arrhythmias, lower cholesterol, dilate coronary arteries, and improve myocardial perfusion and angina.220 The mechanism of some of these effects is the ACE inhibitor effect of hawthorne. Doses of about 160–900mg/day of a standardised extract of hawthorn have been used to achieve these cardiovascular effects, which appear to be safe. The first RCT investigated the effects of hawthorn for HT in patients with type 2 diabetes and demonstrated a hypotensive effect.221
Indian snakeroot (Rauwolfia serpentine)
Rauwolfia serpentine is a Hindu Ayurvedic herb known since ancient times and is the natural source of the alkaloid reserpine, one of the first commercially available antihypertensive drugs. It works by depleting catecholamines in the central and peripheral nervous system. However, adverse effects, including depression, sedation and peptic ulceration were common.220
Rauwolfia serpentine has been studied in a 10-year intervention trial.222 It was concluded that the overall effectiveness of lowering BP and in reducing complications and treatment failure was 60%. Further, given the low level of excess risk in mild uncomplicated HT, the use of Rauwolfia serpentine may be a reasonable alternative in lowering BP.
Conclusion
There is a role for the selected use of single and component nutraceuticals, such as vitamins, antioxidants, minerals, fish oils and herbs in the treatment of HT based on scientifically controlled studies as a complement to optimal nutritional, dietary intake from food and other lifestyle modifications.
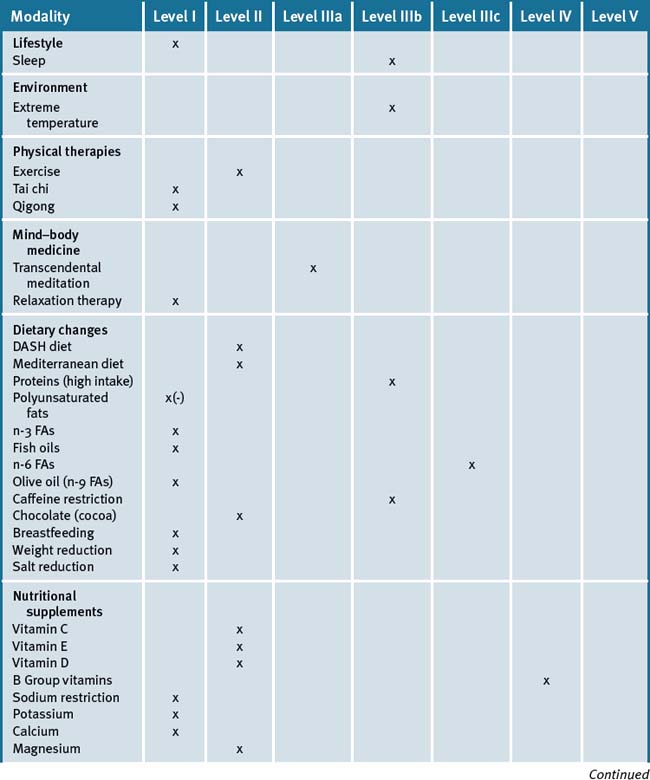
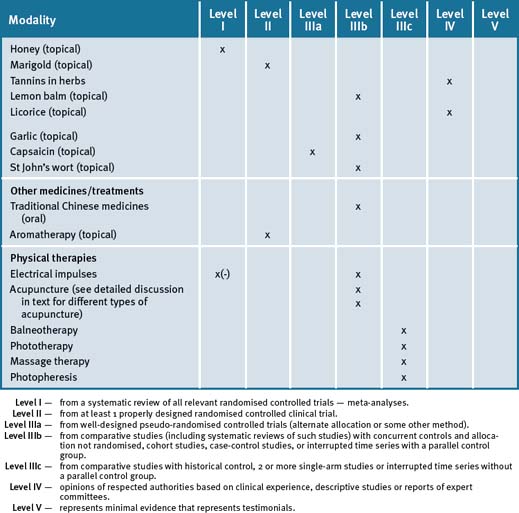
A recent review has concluded that there is a significant amount of evidence to support the use of various evidence based CAM modalities in the treatment of HT (Table 19.2).223
Clinical tips handout for patients — hypertension
1 Lifestyle advice
Sleep
Sunshine
2 Physical activity/exercise
3 Mind-body medicine
4 Environment
5 Dietary changes
7 Supplements
Fish oils
Vitamins and minerals
Vitamin C
Magnesium and calcium (best provided together)
Herbal medicines
Garlic (Allium sativum)
1 World Health Organization – Cardiovascular disease: prevention and control 2003. Online. Available: http://www.who.int/dietphysicalactivity/media/en/gsfs_cvd.pdf (accessed April 2008)
2 Flegal K.M., Carroll M.D., Ogden C.L., et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723-1727.
3 Bonow R.O., Eckel R.H. Diet, obesity, and cardiovascular risk. N Engl J Med. 2003;348(21):2057-2058.
4 Kokkinos P.F., Papademetriou V. Exercise and hypertension. Coronary Artery Dis. 2000;11:99-102.
5 Sali A., Vitetta L. Nutritional supplements and cardiovascular disease. Heart Lung Circ. 2004;13(4):363-366.
6 Chockalingam A. Healthy weight, healthy blood pressure. Canadian J Cardiology. 2010;26(5):259-260.
7 Huang N., Duggan K., Harman J. Lifestyle management of hypertension. Australian Prescriber. 2008;31:150-153.
8 Yan L.L., Liu K., Matthews K.A., et al. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2003;290(16):2138-2148.
9 Han H.R., Kim M.T., Rose L., et al. Effects of stressful life events in young black men with high blood pressure. Ethn Dis. 2006 Winter;16(1):64-70.
10 Weinstein S.E., Quigley K.S., Mordkoff J.T. Influence of control and physical effort on cardiovascular reactivity to a video game task. Psychophysiology. 2002;39:591-598.
11 Nakao M., Nomura S., Shimosawa T., et al. Clinical Effects of Blood Pressure Biofeedback Treatment on Hypertension by Auto Shaping. Psych Med. 1997;59:331-338.
12 Raskin R., Raps C., Luskin F., et al. Pilot study of the effect of self-hypnosis on the medical management of essential hypertension. Stress Med. 1999;15:243-247.
13 Shufan Z. Effects of patient education and biofeedback: interim results. J of Human Hypertension. 1995;9(1):51.
14 Yung P.M., Keltner A.A. A controlled comparison on the effects of muscle and cognitive relaxation procedures on blood pressure: implications for the behavioral treatment of borderline hypertensives. Behav Res Ther. 1996;34:821-826.
15 Shapiro D, Hui KK, Oakley ME et al. Reductions in drug requirements for hypertension by means of a cognitive-behavioural intervention. Am J Hypertens1997;10:9-17
16 Dickinson H.O., Campbell F., Beyer F.R., et al. Relaxation therapies for the management of primary hypertension in adults. Cochrane Database Syst Rev. (1):2008 Jan 23. CD004935
17 Linden W., Lenz J.W., Con A.H. Individualised Stress Management for Primary Hypertension: A Randomised Trial. Arch Internal Med. 2001;161:1071-1080.
18 Schneider R.H., Alexander C.N., Staggers F., et al. A randomised controlled trial of stress reduction in African Americans treated for hypertension for over one year. Am J Hypertens. 2005;18(1):88-98.
19 Brown M.A., Buddle M.L., Martin A. Is resistant hypertension really resistant? American Journal of Hypertension. 2001;14:1263-1269.
20 Björklund K., Lind L., Vessby B., et al. Different metabolic predictors of white-coat and sustained hypertension over a 20-year follow-up period: a population-based study of elderly men. Circulation. 2002;106:63-68.
21 Walton K.G., Schneider R.H., Nidich S.I., et al. Psychosocial stress and cardiovascular disease Part 2: effectiveness of the Transcendental Meditation program in treatment and prevention. Behav Med. 2002 Fall;28(3):106-123.
22 Lisard P., et al. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24 hour study. Am J Hypertension. 1999;12:63-68.
23 Alvarez G.G., Ayas N.T. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs. 2004 Spring;19(2):56-59.
24 Vgontzas A.N., Liao D., Bixler E.D., et al. Insomnia with objective short sleep duration is associated with high risk of hypertension. Sleep. 2009;32:491-497.
25 Alpérovitch A., Lacombe J., Hanon O., et al. Relationship Between Blood Pressure and Outdoor Temperature in a Large Sample of Elderly Individuals. The Three-City Study. Arch Intern Med. 2009;169(1):75-80.
26 Hagberg J.M., Park J.J., Brown M.D. The role of exercise training in the treatment of hypertension: an update. Sports Med. 2000;30:193-206.
27 Carlsson A.C., Wändell P.E., de Faire U., et al. Risk Factors Associated With Newly Diagnosed High Blood Pressure in Men and Women. Am J Hypertens. 2008 Apr 17. [Epub ahead of print]
28 Wang C., Collet J.P., Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493-501.
29 Guo X., Zhou B., Nishimura T., et al. Clinical effect of qigong practice on essential hypertension: a meta-analysis of randomised controlled trials. J Altern Complement Med. 2008;14(1):27-37.
30 Lee M.S., Pittler M.H., Guo R., et al. Qigong for hypertension: a systematic review of randomised clinical trials. J Hypertens. 2007;25(8):1525-1532.
31 Appel L.J., et al. A clinical trial of the effects of dietary patterns on blood pressure. NEJM. 1997;336:1117-1124.
32 Ascherio A., Hennekens C., Willet W.C., et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension. 1996;27:1065-1072.
33 Heyka R. Lifestyle management and prevention of hypertension. In: Rippe J., editor. Lifestyle Medicine. 1st edn. Malden, Mass: Blackwell Science; 1999:109-119.
34 Yong L.C., Kuller L.H., Rutan G., et al. Longitudinal study of blood pressure: changes and determinants from adolescence to middle age. The Dormont High School follow-up study, 1957-1963 and 1989-1990. Am J Epidemiol. 1993;138:973-983.
35 Lopes H.L., Martin K.L., Nashar K., et al. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertens. 2003;41:422-430.
36 Gundogdu Z. Relationship between BMI and blood pressure in girls and boys. Public Health Nutr. 2008;April 22:1-4.
37 Fung T.T., Chiuve S.E., McCullough M.L., et al. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch Intern Med. 2008;168(7):713-720.
38 Pitsavos C., Chrysohoou C., Panagiotakos D.B., et al. Abdominal obesity and inflammation predicts hypertension among prehypertensive men and women: the ATTICA Study. Heart Vessels. 2008;23(2):96-103.
39 Pitsavos C., Panagiotakos D.B., Tzima N., et al. Diet, exercise, and C-reactive protein levels in people with abdominal obesity: the ATTICA epidemiological study. Angiology. 2007;58(2):225-233.
40 Sacks F.M., Svetkey L.P., Volmer W.M., et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N Engl J Med. 2001;344:3-10.
41 Conlin P.R., Chow D., Miller E.R.III, et al. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens. 2000;13:949-955.
42 Pins J.J., Geleva D., Keenan J.M., et al. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? Fam Pract. 2002;51(4):353-359.
43 Espeland M.A., Whelton P.K., Kostis J.B., et al. Predictors and mediators of successful long-term withdrawal from antihypertensive medications. TONE Cooperative Research Group. Trial of Nonpharmacologic Interventions in the Elderly. Arch Fam Med. 1999;8(3):228-236.
44 Mulrow C.D., Chiquette E., Angel L., et al. Dieting to reduce body weight for controlling hypertension in adults. The Cochrane Library. (1):2004.
45 Blumenthal J.A., Sherwood A., Gullette E.C., et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160(13):1947-1958.
46 He F.J., MacGregor G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 3, 2004. CD004937
47 Dickinson H.O., Mason J.M., Nicolson D.J., et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomised controlled trials. J Hypertens. 2006;24:215-233.
48 He F.J., MacGregor G.A. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
49 Houston M.C. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiovasc Dis. 2005;47(6):396-449.
50 Obarzanek E., Velletri P.A., Cutler J.A. Dietary protein and blood pressure. JAMA. 1996;274:1598-1603.
51 Stamler J., Elliott P., Kesteloot H., et al. Inverse relation of dietary protein markers with blood pressure. Findings for 10,020 men and women in the Intersalt Study. Intersalt Cooperative Research Group. International study of salt and blood pressure. Circulation. 1996;94:1629-1634.
52 He J., Welton P.K. Effect of dietary fiber and protein intake on blood pressure: A review of epidemiologic evidence. Clin Exp Hypertens. 1999;21:785-796.
53 Zhou B. The relationship of dietary animal protein and electrolytes to blood pressure. A study on three Chinese populations. Int J Epidem. 1994;23:716-722.
54 Elliott P., Dennis B., Dyer A.R., et al. Relation of dietary protein (total, vegetable, animal) to blood pressure: INTERMAP epidemiologic study. Chicago, IL: Presented at the 18th Scientific Meeting of the International Society of Hypertension; August 20-24, 2000.
55 Wolfe B.M. Potential role of raising dietary protein intake for reducing risk of atherosclerosis. Can J Cardiol. 1995;11:127G-131G.
56 Burke V., Hodgson J.M., Beilin L.J., et al. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension. 2001;38:821-826.
57 Kawase M., Hashimoto H., Hosoda M., et al. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255-263.
58 Groziak S.M., Miller G.D. Natural bioactive substances in milk and colostrum: Effects on the arterial blood pressure system. Br J Nutr. 2000;84:S119-S125.
59 Barr S.I., McCarron D.A., Heaney R.P., et al. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J Am Diet Assoc. 2000;100:810-817.
60 Appel L.J., Moore T.J., Obarzanek E., et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117-1124.
61 Hasler C.M., Kundrat S., Wool D. Functional foods and cardiovascular disease. Curr Atheroscler Rep. 2000;2:467-475.
62 Tikkanen M.J., Adlercreutz H. Dietary soy–derived isoflavone phytoestrogens: Could they have a role in coronary heart disease prevention? Biochem Pharmacol. 2000;60:1-5.
63 Liggins J., Bluck L.J.C., Runswick S., et al. Daidzein and genistein content of fruits and nuts. J Nutr Biochem. 2000;11:326-331.
64 Pins J., Keenan J. The antihypertensive effects of a hydrolyzed whey protein supplement. Cardiovasc Drugs Ther. 2002;16(Suppl):68.
65 Yamamoto N., Akino A., Takano T. Antihypertensive effect of different kinds of fermented milk in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 1994;58:776-778.
66 Kawasaki T., Seki E., Osajima K., et al. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolysate, on mild hypertensive subjects. J Hum Hypertens. 2000;14:519-523.
67 Kuchel O. Differential catecholamine responses to protein intake in healthy and hypertensive patients. Am J Physiol. 1998;275:R1164-R1173.
68 Millward D.J. Optimal intakes of protein in the human diet. Proc Nutr Soc. 1999;58:403-413.
69 Lemon P.W.R. Is increased dietary protein necessary or beneficial for individuals with a physically active lifestyle? Nutr Rev. 1996;54:S169-S175.
70 Morris M.C. Dietary fats and blood pressure. J Cardiov Risk. 1994;1:21-30.
71 Ueshima H., Stamler J., Elliott P., et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50(2):313-319.
72 Witteman J., Willett W., Stampfer M., et al. A prospective study of nutritional factors and hypertension among US women. Circulation. 1989;80:1320-1327.
73 Ascherio A., Rimm E.B., Giovannucci E.L., et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475-1484.
74 Zhao W.S., Zhai J.J., Wang Y.H., et al. Conjugated Linoleic Acid Supplementation Enhances Antihypertensive Effect of Ramipril in Chinese Patients With Obesity-Related Hypertension. Am J Hypertens. 2009 Mar 19. Epub ahead of print
75 Vitetta L., Sali A. Omega–3 Fatty Acids PUFA – A Review PART I. Journal of Complementary Medicine. 2006;5(6):52-59.
76 Vitetta L., Sali A. Omega–3 Fatty Acids PUFA – A Review PART II. Journal of Complementary Medicine. 2007;6(1):48-52.
77 Bao D.Q., Mori T.A., Burke V., et al. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710-717.
78 Mori T.A., Bao D.Q., Burke V., et al. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253-260.
79 DeBusk R.M. Dietary supplements and cardiovascular disease. Curr Atheroscler Rep. 2000;2:508-514.
80 Alexander J.W. Immunonutrition: The role of omega-3 fatty acids. Nutrition. 1998;14:627-633.
81 Toff I., Bønaa K.H., Ingebretsen O.C., et al. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension: A randomised, controlled trial. Ann Intern Med. 1995;123:911-918.
82 Bønaa K.H., Bjerve K.S., Straume B., et al. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension: A population-based intervention trial from the Tromso study. N Engl J Med. 1990;322:795-801.
83 Knapp H.R., FitzGerald G.A. The antihypertensive effects of fish oil: A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N Engl J Med. 1989;320:1037-1043.
84 Appel L.J., Miller E.R., Seidler A.J., et al. Does supplementation of diet with fish oil reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429-1438.
85 Morris M., Sacks F., Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523-533.
86 Prisco D., Paniccia R., Bandinelli B., et al. Effect of medium term supplementation with a moderate dose of n-3 polyunsaturated fatty acid on blood pressure in mild hypertensive patients. Thromb Res. 1998;91:105-112.
87 Bao D.Q., Mori T.A., Burke V., et al. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710-717.
88 Mori T.A., Bao D.Q., Burke V., et al. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253-260.
89 Mori T.A., Woodman R.J. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95-104.
90 Paschos G.K., Magkos F., Panagiotakos D.B., et al. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr. 2007;61(10):1201-1206.
91 Eaton S.B., Eaton S.B.III, Konner M.J. Paleolithic nutrition revisited: A twelve-year retrospective on its nature and implications. A review. Eur J Clin Nutr. 1997;51:207-216.
92 Mozaffarian D., Willett W.C. Trans fatty acids and cardiovascular risk: a unique cardiometabolic imprint? Curr Atheroscler Rep. 2007;9(6):486-493.
93 Ferrara L.A., Raimondi S., d’Episcopa I., et al. Olive oil and reduced need for antihypertensive medications. Arch Intern Med. 2000;160:837-842.
94 Strazzullo P., Ferro-Luzzi A., Siani A., et al. Changing the Mediterranean diet: Effects on blood pressure. J Hypertens. 1986;4:407-412.
95 Thomsen C., Rasmussen O.W., Hansen K.W., et al. Comparison of the effects on the diurnal blood pressure, glucose, and lipid levels of a diet rich in monounsaturated fatty acids with a diet rich in polyunsaturated fatty acids in type 2 diabetic subjects. Diabet Med. 1995;12:600-606.
96 Mensink R.P., Janssen M.C., Katan M.B. Effect on blood pressure of two diets differing in total fat but not in saturated and polyunsaturated fatty acids in healthy volunteers. Am J Clin Nutr. 1988;47:976-980.
97 Papadopoulos G., Boskou D. Antioxidant effect of natural phenols in olive oil. J Am Oil Chem Soc. 1991;68:669-671.
98 Mensink R.P., Katan M.B. Effect of dietary fatty acids on serum lipids and lipoproteins: A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911-919.
99 Nuñez-Cordoba J.M., Alonso A., Beunza J.J., et al. Role of vegetables and fruits in Mediterranean diets to prevent hypertension. Eur J Clin Nutr. 2008 Feb 27. Epub ahead of print
100 Papamichael C.M., Karatzi K.N., Papaioannou T.G., et al. Acute combined effects of olive oil and wine on pressure wave reflections: another beneficial influence of the Mediterranean diet antioxidants? J Hypertens. 2008;26(2):223-229.
101 James J.E. Critical review of dietary caffeine and blood pressure: a relationship that should be taken more seriously. Psychosom Med. 2004;66(1):63-71.
102 Sudano I., Binggeli C., Spieker L. Cardiovascular effects of coffee: is it a risk factor? Prog Cardiovasc Nurs. 2005 Spring;20(2):65-69.
103 Taubert D., Berkels R., Roesen R., et al. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029-1030.
104 Taubert D., Roesen R., Lehmann C., et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomised controlled trial. JAMA. 2007;298(1):49-60.
105 Martin R.M., Ness A.R., Gunnell D., et al. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266.
106 Martin R.M., Gunnell D., Smith G.D. Breastfeeding in infancy and blood pressure in later life: systematic review and meta-analysis. Am J Epidemiol. 2005;161(1):15-26.
107 Ceriello A., Giugliano D., Quatraro A., et al. Antioxidants show an antihypertensive effect in diabetic and hypertensive subjects. Clin Sci. 1991;81:739-742.
108 Duffy S.J., Gokce N., Holbrook M., et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048-2049.
109 Plantiga Y., Ghiadoni L., Magagna A., et al. Supplementation with vitamins C and E improved arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypert. 2007;20(4):392-397.
110 Bates C.J., Walmsley C.M., Prentice A., et al. Does vitamin C reduce blood pressure? Results of a large study of people aged 65 or older. J Hypertens. 1998;16:925-932.
111 Ness A.R., Khaw K.-T., Bingham S., et al. Vitamin C status and blood pressure. J Hypertens. 1996;14:503-508.
112 Osilesi O., Trout D.L., Ogunwole J., et al. Blood pressure and plasma lipids during ascorbic acid supplementation in borderline hypertensive and normotensive adults. Nutr Res. 1991;11:405-412.
113 Simon J.A. Vitamin C and cardiovascular disease: A review. J Am Coll Nutr. 1992;11:107-125.
114 Lovat L.B., Lu Y., Palmer A.J., et al. Double-blind trial of vitamin C in elderly hypertensives. J Hum Hypertens. 1993;7:403-405.
115 Duffy S.J., Gokce N., Holbrook M., et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048-2049.
116 Ness A.R., Chee D., Elliot P. Vitamin C and blood pressure—an overview. J Hum Hypertens. 1997;11:343-350.
117 Palumbo G., Avanzini F., Alli C., et al. Effects of vitamin E on clinic and ambulatory blood pressure in treated hypertensive patients. Am J Hypertens. 2000;13:564-567.
118 Mottram P., Shige H., Nestel P. Vitamin E improves arterial compliance in middle-aged men and women. Atherosclerosis. 1999;145:399-404.
119 Skyrme-Jones R.A., O’Brien R.C., Berry K.L., et al. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: A randomised, placebo-controlled study. J Am Coll Card. 2000;36:94-102.
120 Lino K., Abe K., Kariya S., et al. A controlled, double blind study of DL-alpha-tocopheryl nicotinate for treatment of symptoms in hypertension and cerebral arteriosclerosis. Jpn Heart J. 1977;18:277-286.
121 Dakshinamurti K., Lal K.J.. Vitamins and hypertension. Simopoulos A.P., editor. Nutrients in the Control of Metabolic Diseases. World Rev Nutr Diet, vol. 69.. Karger, Basal, 1996;40-73.
122 Lind L., Wengle B.O., Junghall S. Blood pressure is lowered by vitamin D (alphacalcidol) during long-term treatment of patients with intermittent hypercalcemia. Acta Med Scand. 1987;222:423-427.
123 Lind L., Lithell H., Skarfos E., et al. Reduction of blood pressure by treatment with alphacalcidol. Acta Med Scand. 1988;223:211-217.
124 Lind L., Wengle B.O., Wide L., et al. Hypertension in primary hyperparathyroidism—reduction of blood pressure by long-term treatment with vitamin D (alphacalcidol). A double-blind, placebo-controlled study. Am J Hypertens. 1988;1:397-402.
125 Pfeifer M., Begerow B., Minne H.W., et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Cli Endocr Metab. 2001;86:1633-1637.
126 McCarron D.A. Calcium metabolism and hypertension. Kidney Int. 1989;35:717-736.
127 McCarron D.A. Low serum concentrations of ionised calcium in patients with hypertension. N Engl J Med. 1982;307:226-228.
128 Resnick L.M. Dietary calcium and hypertension. J Nutr. 1987;117:1806-1808.
129 Jorde R., Bonaa K.H. Calcium from dairy products, vitamin D intake and blood pressure: The Tromso Study. Am J Clin Nutr. 2000;71:1530-1535.
130 Wang L., Manson J.E., Buring J.E., et al. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51(4):1073-1079.
131 Boucher B.J. Inadequate vitamin D status. Does it contribute to the disorders comprising syndrome X? Br J Nutr. 1998;79:315-327.
132 Scragg R., Holdaway S.R., Singh V., et al. Serum 25-hydroxycholecalciferol concentration in newly detected hypertension. Am J Hypertens. 1995;4:429-432.
133 Scragg R., Sowers M., Bell C. Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813-2818.
134 Lind L., Hanni L.L., Huarfner L.H., et al. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:894-901.
135 de Boer I.H., Tinker L.F., Connelly S., et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31(4):701-707.
136 Bai S., Favus M.J. Vitamin D and calcium receptors: links to hypercalciuria. Curr Opin Nephrol Hypertens. 2006;15(4):381-385.
137 Fregly M.J., Cade J.R. Effect of pyridoxine and tryptophan, alone and in combined, on the development of deoxycorticosterone acetate–induced hypertension in rats. Pharmacology. 1995;50:298-306.
138 Lal K.J., Krishnamurti D., Thliverv J. The effect of vitamin B6 on the systolic blood pressure of rats in various animal models of hypertension. J Hypertens. 1996;14:355-363.
139 Keniston R., Enriquez J.I.Sr. Relationship between blood pressure and plasma vitamin B6 levels in healthy middle-aged adults. Ann N Y Acad Sci. 1990;585:499-501.
140 Dakshinamurti K., Lal K.J. Vitamins and hypertension. World Rev Nutr Diet. 1992;69:40-73.
141 Bender D.A. Non-nutritional uses of vitamin B-6. Br J Nutr. 1999;81:7-20.
142 Dakshinamurti K., Paulose C.S., Viswanathan M. Vitamin B6 and hypertension. Ann N Y Acad Sci. 1990;575:241-249.
143 Sali A., Vitetta L. Nutritional supplements and cardiovascular disease. Heart Lung Circ. 2004;13(4):363-366.
144 McMahon J.A., Skeaff C.M., Williams S.M., et al. Lowering homocysteine with B vitamins has no effect on blood pressure in older adults. J Nutr. 2007;137(5):1183-1187.
145 Kotchen T.A., McCarron D.A. AHA Science Advisory. Dietary electrolytes and blood pressure. Circulation. 1998;98:613-617.
146 Midgley J.P., Matthew A.G., Greenwood C.M., et al. Effect of reduced dietary sodium on blood pressure: A meta-analysis of randomised controlled trials. JAMA. 1996;275:1590-1597.
147 Cutler J.A., Follman D., Allender P.S. Randomised trials of sodium reduction: An overview. Am J Clin Nutr. 1997;65:643S-651S.
148 Graudal N.A., Galloe A.M., Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: A meta-analysis. JAMA. 1998;279:1383-1391.
149 Svetkey L.P., Sacks F.M., Obarzanek E., et al. The DASH diet, sodium intake and blood pressure (the DASH-Sodium Study): Rationale and design. J Am Diet Assoc. 1999;99:S96-S104.
150 Egan B.M., Lackland D.T. Biochemical and metabolic effects of very-low-salt diets. Am J Med Sci. 2000;320:233-239.
151 The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high normal blood pressure: The Trials of Hypertension Prevention,. Phase II. Arch Intern Med. 1997;157:657-667.
152 Whelton P.K., Appel L.J., Espeland M.A., et al. Efficacy of sodium reduction and weight loss in the treatment of hypertension in older persons: Main results of the randomised, controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA. 1998;279:839-846.
153 He J., Ogden L.G., Vupputuri S., et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027-2034.
154 Steven V.J., Obarzanek E., Cook N.R., et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001;134:1-11.
155 Kostis J.B., Shindler D.M., Lacy C.R. Non-drug therapy for hypertension: Do effects on weight and sodium intake persist after discontinuation of intervention. Am J Med. 2000;109:734-735.
156 Lasser V.I., Raczynski J.M., Stevens V.J., et al. Trials of Hypertension Prevention, phase II. Structure and content of the weight loss and dietary sodium reduction interventions. Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann Epidemiol. 1995;5(2):156-164.
157 Cianciaruso B., Belliszi V., Minutolo R., et al. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab. 1998;24:296-301.
158 Hooper L., Bartlett C., Davey S.G., et al. Advice to reduce dietary salt for prevention of cardiovascular disease. Cochrane Database Syst Rev. (1):2004. CD003656
159 Penner S.B., Campbell N.R., Chockalingam A., et al. Dietary sodium and cardiovascular outcomes: a rational approach. Can J Cardiol. 2007;23(7):567-572. Review
160 Cook N.R., Cutler J.A., Obarzanek E., et al. Long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885.
161 Whelton P.K., He J. Potassium in preventing and treating high blood pressure. Semin Nephrol. 1999;19:494-499.
162 Siani A., Strazzullo P., Giacco A., et al. Increasing the dietary potassium intake reduces the need for antihypertensive medication. Ann Intern Med. 1991;115:753-759.
163 Whelton P.K., He J., Cutler J.A., et al. Effects of oral potassium on blood pressure: Meta-analysis of randomised controlled clinical trials. JAMA. 1997;227:1624-1632.
164 McCarron D.A., Reusser M.E. Nonpharmacologic therapy in hypertension: From single components to overall dietary management. Prog Cardiovasc Dis. 1999;41:451-460.
165 Gu D., He J., Xigui W., et al. Effect of potassium supplementation on blood pressure in Chinese: A randomised, placebo-controlled trial. J Hypertens. 2001;19:1325-1331.
166 Barri Y.M., Wingo C.S. The effects of potassium depletion and supplementation on blood pressure: A clinical review. Am J Med Sci. 1997;3:37-40.
167 Ma G., Mamaril J.L.C., Young D.B., et al. Increased potassium concentration inhibits stimulation of vascular smooth muscle proliferation by PDGF-BB and bFGF. Am J Hypertens. 2000;13:1055-1060.
168 Karanja N., Roullet J.B., Aickin M., et al. Mineral metabolism, blood pressure, and dietary patterns: Findings from the DASH trial. J Am Soc Nephrol. 1998;9:151A.
169 Hamet P., Daignault-Gelinas M., Lambert J., et al. Epidemiological evidence of an interaction between calcium and sodium intake impacting on blood pressure: A Montreal study. Am J Hypertens. 1992;5:378-385.
170 Saito K., Sano H., Furuta Y., et al. Effect of oral calcium on blood pressure response in salt-loaded borderline hypertensive patients. Hypertension. 1989;13:219-226.
171 Cappuccio F.P., Elliott P., Allender P.S., et al. Epidemiologic association between dietary calcium intake and blood pressure: A meta-analysis of published data. Am J Epidemiol. 1995;142:935-945.
172 Allender P.S., Cutler J.A., Follmann D., et al. Dietary calcium and blood pressure: A meta-analysis of randomised clinical trials. Ann Intern Med. 1996;124:825-831.
173 McCarron D.A. Calcium metabolism in hypertension. Keio J Med. 1995;44:105-114.
174 Witteman J.C.M., Willett W.C., Stampfer M.J., et al. A prospective study of nutritional factors and hypertension among US women. Circulation. 1989;80:1320-1327.
175 Bucher H.C., Cook R.J., Guyatt G.H., et al. Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomised controlled trials. JAMA. 1996;275:1016-1022.
176 Griffith L., Guyatt G.H., Cook R.J., et al. The influence of dietary and non-dietary calcium supplementation on blood pressure: An updated meta-analysis of randomised clinical trials. Am J Hypertens. 1999;12:84-92.
177 Karanja N, Morris CD, Rufolo P et al. The impact of increasing dietary calcium intake on nutrient consumption, plasma lipids and lipoproteins in humans. Am J Hypertens 994;59:900-7
178 Resnick L.M. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14:505-520.
179 Hofmeyr G.J., Roodt A., Atallah A.N., et al. Calcium supplementation to prevent pre-eclampsia–a systematic review. S Afr Med J. 2003;93:224-228.
180 Atallah A.N., Hofmeyr G.J., Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 3, 2006. CD001059
181 Daly R.M., Nowson C.A. Long-term effect of calcium-vitamin D3 fortified milk on blood pressure and serum lipid concentrations in healthy older men. European Journal of Clinical Nutrition advance online publication 21. January 2009. doi: 10.1038/ejcn.2008.79
182 Wirell M.P., Wester P.O., Stegmayr B.G. Nutritional dose of magnesium in hypertensive patients on beta blockers lowers systolic blood pressure: a double blind, cross-over study. J Intern Med. 1994;236:189-195.
183 Kawano Y., Matsuoka H., Takishita S., et al. Effects of magnesium supplementation in hypertensive patients: assessment by office, home, and ambulatory blood pressures. Hypertension. 1998;32:260-265.
184 Shibutani Y., Sakamoto K., Katsuno S., et al. Relation of serum and erythrocyte magnesium levels to blood pressure and a family history of Hypertension. A follow-up study in Japanese children, 12-14 years old. Acta Paediatr Scand. 1990;79(3):316-321.
185 Witteman J.C., Grobbee D.E., Derkx F.H., et al. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr. 1994;60(1):129-135.
186 Itoh K., Kawasaka T., Nakamura M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br J Nutr. 1997;78(5):737-750.
187 Bo S., Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19(1):50-56.
188 Singh R.B., Niaz M.A., Rastogi S.S., et al. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr. 1998;17:564-570.
189 Zozaya J.L. Nutritional factors in high blood pressure. J Hum Hypertens. 2000;1:S100-S104.
190 Garcia Zozaya J.L., Padilla Viloria M. Alterations of calcium, magnesium, and zinc in essential hypertension: Their relation to the renin-angiotensinaldosterone system. Invest Clin. 1997;38:27-40.
191 Galley H.F., Thornton J., Howdle P.D., et al. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci. 1997;92:361-365.
192 Bergomi M., Rovesti S., Vinceti M., et al. Zinc and copper status and blood pressure. J Trace Elem Med Biol. 1997;11:166-169.
193 Langsjoen P.H., Langsjoen A.M. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9:273-284.
194 Langsjoen H., Langsjoen P., Langsjoen P., et al. Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Mol Aspects Med. 1994;15:S165-S175.
195 Langsjoen P.H., Langsjoen P.H., Folkers K. Isolated diastolic dysfunction of the myocardium and its response to CoQ10 treatment. Clin Investig. 1993;71:S140-S144.
196 Folkers K., Drzewoski J., Richardson P.C., et al. Bioenergetics in clinical medicine. XVI. Reduction of hypertension in patients by therapy with coenzyme Q10. Res Commun Chem Pathol Pharmacol. 1981;31:129-140.
197 Yamagami T., Shibata N., Folkers K. Bioenergetics in clinical medicine. VIII. Adminstration of coenzyme Q10 to patients with essential hypertension. Res Commun Chem Pathol Pharmacol. 1976;14:721-727.
198 Langsjoen P., Langsjoen P., Willis R., et al. Treatment of Essential Hypertension with Coenzyme Q10. Mol Aspects Med. 1994;15:s265-s272.
199 McCarty M.F. Coenzyme Q versus hypertension: does CoQ decrease endothelial superoxide generation? Med Hypoth. 1999;53:300-304.
200 Digiesi V., Cantini F., Oradei A., et al. Coenzyme Q10 in essential hypertension. Mol Aspects Med. 1994;15:S257-S263.
201 Hodgson J.M., Watts G.F., Playford D.A., et al. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with Type 2 diabetes. European Journal of Clinical Nutrition. 2002;56:1137-1142.
202 Shah S.A., Sander S., Cios D., et alAnn Pharmacother. Electrocardiographic and hemodynamic effects of coenzyme Q10 in healthy individuals: a double-blind,randomised controlled trial. 2007;41(3):420-425.
203 Rosenfeldt F.L., Haas S.J., Krum H., et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21(4):297-306.
204 Paran E., Engelhard Y.N. Effect of lycopene, an oral natural antioxidant on blood pressure. J Hypertens. 2001;19:S74.
205 Paran E., Engelhard Y. Effect of tomato’s lycopene on blood pressure, serum lipoproteins, plasma homocysteine and oxidative stress markers in grade I hypertensive patients. Am J Hypertens. 2001;14:141A.
206 DeBusk R.M. Dietary supplements and cardiovascular disease. Curr Atheroscler Rep. 2000;2:508-514.
207 Auer W., Eiber A., Hertkorn E., et al. Hypertension and hyperlipidaemia: Garlic helps in mild cases. Br J Clin Pract. 1990;69:3-6.
208 McMahon F.G., Vargas R. Can garlic lower blood pressure? A pilot study. Pharmacotherapy. 1993;13:406-407.
209 Pedraza-Chaverri J, Tapia E, Medina-Campos ON et al. Garlic prevents hypertension induced by chronic inhibition of nitric oxide synthesis. Life Sci1999;62:71-77
210 Orekhov A.N., Grunwald J. Effects of garlic on atherosclerosis. Nutrition. 1997;13:656-663.
211 Ernst E. Cardiovascular effects of garlic (Allium savitum): A review. Pharmatherapeutica. 1987;5:83-89.
212 Silagy C.A., Neil A.W. A meta-analysis of the effect of garlic on blood pressure. J Hypertens. 1994;12:463-468.
213 Silagy C., Neil A. Garlic as a lipid lowering agent: A meta analysis. J R Coll Physicians Lond. 1994;28:39-45.
214 Kleinjnen J., Knipschild P. Ter Riet G. Garlic, onions and cardiovascular risk factors: A review of the evidence from human experiments with emphasis on commercially available preparations. Br J Clin Pharmacol. 1989;28:535-544.
215 Reuter H.D., Sendl A. Allium sativum and Allium ursinum: Chemistry, pharmacology and medicinal applications. Econ Med Plant Res. 1994;6:55-113.
216 Mohamadi A., Jarrell S.T., Shi S.J., et al. Effects of wild versus cultivated garlic on blood pressure and other parameters in hypertensive rats. Heart Dis. 2001;2:3-9.
217 Budoff M. Aged garlic extract retards progression of coronary artery calcification. J Nutr. 2006;136(3 Suppl):741S-744S.
218 Ried K., Frank O.R., Stocks N.P., et al. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13.
219 Reinhart K.M., Coleman C.I., Teevan C., et al. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother. 2008;42(12):1766-1771.
220 Mashour N.H., Lin G.I., Frishman W.H. Herbal Medicine for the Treatment of Cardiovascular Disease. Arch Intern Med. 1998;158:2225-2234.
221 Walker A.F., Marakis G., Simpson E., et al. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: a randomised controlled trial. Br J Gen Pract. 2006;56(527):437-443.
222 Smith W.M. Treatment of mild hypertension: results of a ten-year intervention trial. Circ Res. 1977;40:198-205.
223 Nahas R. Complementary and alternative medicine approaches to blood pressure reduction: An evidence-based review. Can Fam Physician. 2008;54(11):1529-1533.