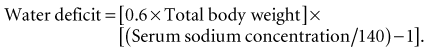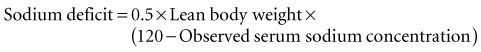13 Hypernatremia and Hyponatremia
 Hypernatremia
Hypernatremia
Hypernatremia is a common clinical problem, observed in up to 2% of the general hospital population and 15% of patients admitted to the intensive care unit.1–4 In the outpatient setting, hypernatremia is most prevalent in the geriatric patient population; in hospitalized patients, it is observed in all age groups.1,5 Mortality rates in patients with hypernatremia can range as high as 70%.1–6 Although the high mortality rate no doubt reflects the severity of underlying disease in these patients, there is significant morbidity related to hypernatremia itself. Neurologic sequelae from hypernatremia are common, particularly in the pediatric population.6
The brain is particularly susceptible to the effects of hypernatremia. When the sodium concentration in plasma is higher than normal, water moves across cytosolic membranes (from the inside of cells to the outside of cells) to preserve osmotic equilibrium. As a consequence of intracellular dehydration, there is a net loss of brain volume, which in turn places mechanical stress on cerebral vessels, possibly resulting in bleeding.6 With chronic hypernatremia, however, cellular adaptation occurs. Under these circumstances, so-called idiogenic osmoles accumulate in brain cells, minimizing cellular dehydration. Importantly, the presence of these idiogenic osmoles presents a risk for the development of cerebral edema during the treatment of hypernatremia.
The treatment of hypernatremia is water repletion (Box 13-1). Assuming total body water is 60%, the water deficit may be estimated as follows:
 Hyponatremia
Hyponatremia
Hyponatremia is one of the most common electrolyte abnormalities seen in hospitalized patients. It occurs in 2% to 4% of hospitalized patients and up to 30% of patients in intensive care units.7–10 Mortality for patients with acute hyponatremia is reportedly as high as 50%, whereas mortality for those with chronic hyponatremia is 10% to 20%.7–11
In acute hyponatremia, nausea, vomiting, lethargy, and confusion can progress to coma, seizures, eventual cerebral herniation, and death.11,12 The elderly and the young are more likely to be symptomatic from hyponatremia.9 Menstruating women also tend to be more symptomatic and are at greater risk for neurologic complications from acute hyponatremia.11 Early in the development of hyponatremia, the symptoms are difficult to separate from those related to the underlying disease process. Hyponatremic patients who have clinically significant space-occupying lesions in the CNS should be aggressively treated. Meanwhile, efforts should be made to determine the cause of hyponatremia by assessing intravascular volume status, measuring urine output, seeking the presence of exogenous sugars or sugar alcohols (e.g., mannitol), and determining urine sodium concentration and osmolarity.
Treatment of hyponatremia is dependent on the acuteness of the hyponatremia and the presence and severity of symptoms (Box 13-2). Acute (<48 hours) or chronic (>48 hours) symptomatic hyponatremia (e.g., seizures) requires immediate therapy. However, the optimal approach for the treatment of these patients is controversial.12–14 The controversy results from reports of the occurrence of a central demyelination syndrome associated with the correction of hyponatremia in some patients.15–22 This syndrome appears to be more common with chronic hyponatremia (>48 hours), overcorrection of hyponatremia, large corrections (>12 to 25 mEq/L per 24 hours), and rapid correction (>1 to 2 mEq/L per hour).19–22
Box 13-2
Treatment of Hyponatremia
Two new Food and Drug Administration (FDA)-approved vasopressin receptor antagonists are now available in the United States. One of these agents, tolvaptan, is selective for the vasopressin 2 (V2) receptor. The other agent, conivaptan, is less selective and binds to both V1A and V2 receptors. Both are indicated for treating euvolemic hyponatremia. Tolvaptan also is indicated for the treatment of hypervolemic hyponatremia. Neither drug has been extensively studied, and the effect of treatment with these agents on hard endpoints such as mortality have not been assessed. Both drugs, however, have been investigated for the adjunctive treatment of congestive heart failure, and neither has been shown to improve mortality or morbidity.23,24 Given the paucity of clinically meaningful outcomes, we do not recommend the use of either of these antagonists for routine therapy of hyponatremia.
Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117(11):891-897.
Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124(2):197-203.
Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients: a heterogeneous, morbid, and iatrogenic entity. Ann Intern Med. 1987;107(3):309-319.
Sterns RH, Cappuccio JD, Silver SM, et al. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4(8):1522-1530.
1 Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124:197-203.
2 Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients: a heterogeneous, morbid, and iatrogenic entity. Ann Intern Med. 1987;107:309-319.
3 Molaschi M, et al. Hypernatremic dehydration in the elderly on admission to hospital. J Nutr Health Aging. 1997;1:156-160.
4 Polderman KH, et al. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med. 1999;27:1105-1108.
5 Daggett P, et al. Severe hypernatraemia in adults. BMJ. 1979;1:1177-1180.
6 Simmons MA, et al. Hypernatremia and intracranial hemorrhage in neonates. N Engl J Med. 1974;291:6-10.
7 Natkunam A, Shek CC, Swaminathan R. Hyponatremia in a hospital population. J Med. 1991;22:83-96.
8 Madiba TE, Haffejee AA, Mokoena TR. Hyponatraemia—a prospective analysis of surgical patients. S Afr J Surg. 1998;36:78-81.
9 Kennedy PG, Mitchell DM, Hoffbrand BI. Severe hyponatraemia in hospital inpatients. BMJ. 1978;2:1251-1253.
10 DeVita MV, Gardenswartz MH, Konechy A, et al. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34:163-166.
11 Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117:891-897.
12 Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102:67-77.
13 Ayus JC, Arieff AI. Chronic hyponatremic encephalopathy in post-menopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281:2299-2304.
14 Sterns RH. Severe hyponatremia: the case for conservative management. Crit Care Med. 1992;20:534-539.
15 Cohen BJ, Jordan MH, Chapin SD, et al. Pontine myelinolysis after correction of hyponatremia during burn resuscitation. J Burn Care Rehabil. 1991;12:153-156.
16 Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983;13:232-242.
17 Laureno R, Karp BI. Myelinolysis after correction of hyponatremia. Ann Intern Med. 1997;126:57-62.
18 Laureno R, Karp BI. Pontine and extrapontine myelinolysis following rapid correction of hyponatraemia. Lancet. 1988;1:1439-1441.
19 Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72:359-373.
20 Sterns RH. Neurological deterioration following treatment for hyponatremia. Am J Kidney Dis. 1989;13:434-437.
21 Sterns RH, Cappuccio JD, Silver SM, et al. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4:1522-1530.
22 Sterns RH, Riggs JE, Schochet SSJr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535-1542.
23 Gheorghiade M, Gottlieb SS, et al. Vasopressin V2 receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol. 2006;97(7):1064-1067.
24 Murphy T, Dhar R, et al. Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit. Neurocrit Care. 2009;11(1):14-19.