163 Hyperglycemic Comas
Diabetic ketoacidosis (DKA) and hyperosmolar nonketotic hyperglycemia syndrome (HNHS) are life-threatening syndromes caused by metabolic derangement associated with diabetes mellitus, both insulin dependent (type 1) and non–insulin dependent (type 2). Although a distinction is made in the definitions of the two syndromes, there is much commonality between them, with up to 30% of presentations having features of both syndromes. DKA is approximately three times as common as HNHS in patients presenting with hyperglycemic syndromes.1 Although the metabolic derangement seen in DKA and HNHS is extreme, the death rate associated with these syndromes is low with appropriate and meticulous therapy. Surveys of patients presenting with hyperglycemic syndromes have found an overall mortality rate of less than 5% associated with DKA and 15% associated with HNHS.1,2 Most deaths are not caused by the metabolic derangement but occur as a result of coexisting disease (e.g., myocardial infarction), sepsis (particularly pneumonia), or less frequently, the management methods employed.2
 Hyperglycemic Syndromes
Hyperglycemic Syndromes
Diabetic Ketoacidosis
DKA has an incidence of approximately 8.6% in diabetics2 and occurs in a younger age group (mean age, 33 years) compared with DKA-HNHS (44 years) or HNHS (69 years).1 Precipitating factors associated with the development of DKA include3–5:
Laboratory tests supporting the diagnosis of DKA commonly reveal the following:
Hyperosmolar Nonketotic Hyperglycemia Syndrome
Laboratory test results are similar to those listed for DKA but differ somewhat in degree, in that:
Metabolic Derangements in Hyperglycemic Syndromes
The main metabolic derangements that result in morbidity and must be urgently addressed in the management of both DKA and HNHS are severe dehydration, insulin deficit, electrolyte depletion, and metabolic acidosis. These are discussed in detail in Chapters 12 and 18.
Severe dehydration is estimated to be a water deficit in the range of 100 to 200 mL/kg.4 Although there is no consensus on the ideal approach to fluid management in these patients, prompt restoration of the circulation with isotonic fluid (e.g., normal saline or preferably compound sodium lactate solution), followed by more moderate replacement of the water deficit using hypotonic fluid, are the underlying principles.
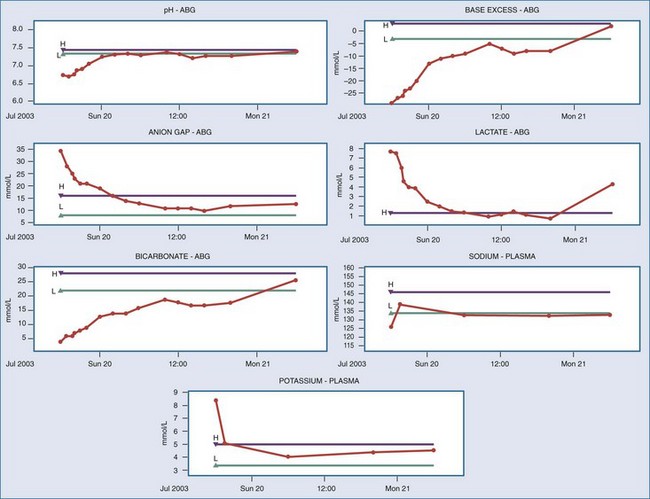
Figure 163-1 shows serial measurements taken from a typical patient with DKA on presentation and during his treatment in the ICU.
 Neurologic Sequelae of the Hyperglycemic Syndromes
Neurologic Sequelae of the Hyperglycemic Syndromes
Cerebral Edema
Rapid correction of hyperglycemia and hyperosmolality is associated with the development of cerebral edema in patients with hyperglycemic syndromes. The mechanism of how the cerebral edema arises is unclear.6–8 The edema might be due to an effect of pH on the Na+/K+ exchange pump causing entry of sodium and water into brain cells, osmotic or inflammatory disruption of the blood-brain barrier, or accumulation of osmotically active solutes (“pseudo-osmoles”) such as amino acids, polyols, and trimethylamines as an adaptation to the hyperosmolar environment. Other theories of the mechanism of cerebral edema include paradoxical central nervous system acidosis or a left shift in the oxygen-hemoglobin dissociation curve that reduces tissue oxygenation.
Cerebral edema after treatment for a hyperglycemic syndrome usually manifests as prolongation of the altered mental state seen on presentation or new development of an altered mental state with features as described previously. In adults, the signs and symptoms may be very subtle and abate over the course of a few days. Usually no specific therapy is required besides good supportive care. Rarely, cerebral edema can produce focal and permanent neurologic damage.9 Cerebral edema associated with DKA in children is a much more serious condition with a considerable mortality.6,8 Urgent treatment of severe cerebral edema relies on intravenous osmotherapy (e.g., mannitol) in the first instance, followed by steroids and loop diuretics as second-line therapy.
Focal Neurologic Deficits Associated With Hyperglycemic Syndromes
There are isolated reports in the literature describing focal neurologic damage in patients with hyperglycemic syndromes. Most commonly, cerebrovascular accidents (CVA), particularly hemorrhagic and thrombotic types, have been associated with HNHS. This is not surprising, because CVA may be the precipitating factor for the development of HNHS in diabetic patients, and the hyperosmolar state in both DKA and HNHS may predispose to thrombotic CVA. Intracerebral venous thrombosis has also been reported10 and has a poor outlook.
CVA may result in neurologic deficit evident on presentation, but often the final clinical picture is obscured by the altered mental state and only becomes clear after treatment of the hyperglycemic syndrome. The high incidence of neurologic signs and symptoms in diabetics may make the detection of new neurodeficits difficult. Many of the focal neurologic signs seen in these patients, particularly those with HNHS, disappear after treatment of the hyperglycemic syndrome. This may represent unmasking of focal areas of cerebrovascular insufficiency by the dehydration.5
Focal neurologic damage may also occur as a result of fluid and electrolyte shifts produced during treatment of the hyperglycemic syndromes (e.g., putaminal hemorrhage,9 lateral pontine and extrapontine myelinolysis11). In patients who are treated for prolonged periods in the ICU for complications related to their episode of hyperglycemic syndrome, critical illness polyneuropathy is also a possibility.
Cognitive Impairment after Hyperglycemic Syndromes
Cognitive impairment may occur after hyperglycemic syndrome. This impairment may be gross and clinically apparent (more common in elderly patients) or very subtle (e.g., poor concentration, loss of memory). It may be associated with focal or global neurologic deficit, as described previously, or it may be apparent in the presence of a structurally normal brain. Most cognitive impairment that is not caused by structural brain damage improves with time. Sensory evoked potentials have shown promise as a sensitive test to detect subclinical brain dysfunction in patients with severe DKA.12
Seizures Associated With Hyperglycemic Syndromes
Focal and generalized seizures are common in patients with hyperglycemic syndromes and may be resistant to treatment with the usual anticonvulsant agents.5 Epilepsia partialis continua, an unusual form of seizure typified by abnormal MRI signal intensity in the precentral gyrus, can occur in DKA or HNHS.13
 Clinical Approach to the Obtunded Hyperglycemic Patient in the Intensive Care Unit
Clinical Approach to the Obtunded Hyperglycemic Patient in the Intensive Care Unit
Management Principles
Complications of Treatment
Complications of the treatment itself must also be dealt with and may include:
Ongoing Care
Once the patient is stable and has been adequately resuscitated and metabolic control has been reestablished, arrangements should be made for the smooth transition of care to an endocrinologist familiar with the chronic care of diabetic patients. This may be facilitated by the institution of enteral feeding and conversion from short-acting intravenous insulin to longer-acting subcutaneous insulin before handover. Ongoing care of the patient must address preventable precipitating factors (e.g., prompt treatment of septic foci, compliance with diabetic treatment regimens).3
Key Points
Kearney T, Dang C. Diabetic and endocrine emergencies. Postgrad Med J. 2007;83:79-86.
Chiasson J, Aris-Jilwan N, Belanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168:859-866.
Kitabachi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crisis in patients with diabetes. Diabetes Care. 2001;24:131-153.
MacIsaac RJ, Lee LY, McNeil KJ, et al. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32:379-385.
1 MacIsaac RJ, Lee LY, McNeil KJ, et al. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32:379-385.
2 Kearney T, Dang C. Diabetic and endocrine emergencies. Postgrad Med J. 2007;83:79-86.
3 Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Endocrinol Metab Clin North Am. 2000;29:683-705.
4 Chiasson J, Aris-Jilwan N, Belanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168:859-866.
5 Trence DL, Hirsch IB. Hyperglycemic crisis in diabetes mellitus type 2. Endocrinol Metab Clin North Am. 2001;30:817-831.
6 Brown TB. Cerebral oedema in childhood diabetic ketoacidosis: is treatment a factor? Emerg Med J. 2004;21:141-144.
7 Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138-148.
8 Glaser N. New perspectives on the pathogenesis of cerebral edema complicating diabetic ketoacidosis in children. Pediatr Endocrinol Rev. 2006;3:379-386.
9 Cho S, Won TK, Hwang S, et al. Bilateral putaminal hemorrhage with cerebral edema in hyperglycemic hyperosmolar syndrome. Yonsei Med J. 2002;43:533-535.
10 Keane S, Gallagher A, Ackroyd S, et al. Cerebral venous thrombosis during diabetic ketoacidosis. Arch Dis Child. 2002;86:204-205.
11 McComb RD, Pfeiffer RF, Casey JH, et al. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol. 1989;8:284-288.
12 Eisenhuber E, Madl C, Kramer L, et al. Detection of subclinical brain dysfunction by sensory evoked potentials in patients with severe diabetic ketoacidosis. Intensive Care Med. 1997;23:587-589.
13 Placidi F, Floris R, Bozzao A, et al. Ketotic hyperglycemia and epilepsia partialis continua. Neurology. 2001;57:534-537.