164 Hyperglycemia and Blood Glucose Control
 Altered Glucose Regulation in Stress
Altered Glucose Regulation in Stress
At the end of the 19th century, Claude Bernard described the link between acute trauma and the development of hyperglycemia irrespective of underlying diabetes. It was considered to be an adaptive stress response ensuring adequate glucose supply to the obligatory glucose-consuming neurons, phagocytes, and reparative cells.1,2 Stress-induced hyperglycemia is evoked by integrated hormonal, cytokine, and nervous “counter-regulatory” signals on glucose metabolic pathways. Essentially, the hyperglycemia is due to insulin resistance in the liver and skeletal muscle. Hepatic insulin resistance leads to increased hepatic gluconeogenesis and glucose output.3 Decreased glycogen synthesis and a shift from insulin-dependent to non–insulin-dependent glucose uptake characterize skeletal muscle insulin resistance.4
In the acute phase of critical illness, it is assumed that increased levels of glucagon, cortisol, and growth hormone jointly increase hepatic gluconeogenesis. In addition, the catecholamines epinephrine and norepinephrine, released in response to acute injury, promote hepatic glycogenolysis. The cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF) may directly or indirectly enhance both of these hyperglycemic responses.5
The important exercise-stimulated glucose uptake in skeletal muscle totally disappears because of the immobilization of the critically ill patient. Insulin-dependent glucose uptake is hampered also through a combined inhibition of glucose transporter-4 (GLUT-4) and glycogen synthase activity.6,7 Although some studies have shown decreased glucose oxidation8 through pyruvate produced by glycolysis, others have demonstrated an opposite effect during critical illness.9 The decrease in insulin-dependent glucose uptake in skeletal muscle is completely offset by a strong increase in total body glucose uptake, of which the mononuclear phagocyte system in liver, spleen, and ileum are the main receivers.10 However, in skeletal muscle, non–insulin-dependent glucose uptake is also increased by increased expression of GLUT-1.11,12 The overall increased peripheral glucose uptake13 in light of hyperglycemia underscores the pivotal role of increased hepatic glucose production during critical illness, which cannot be suppressed by exogenous glucose.14
The position of adipose tissue in the regulation of glucose metabolism during critical illness has been neglected. Nevertheless, in diabetes mellitus, adipose tissue strongly modulates insulin resistance, as it is regarded as an insulin-dependent glucose uptake organ. Recent studies have now revealed that during critical illness, adipose tissue undergoes major changes.15 Possibly stimulated by illness-induced macrophage infiltration, adipocytes become more numerous and smaller and have an increased expression of the non–insulin-dependent glucose transporters, GLUT-1 and GLUT-3. The levels of GLUT-4 remain unaltered. As such, adipose tissue seems reprogrammed during critical illness to facilitate glucose uptake independent of circulating insulin levels.
 Hyperglycemia in Critically Ill Patients
Hyperglycemia in Critically Ill Patients
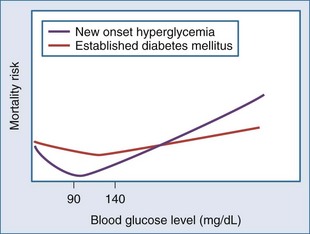
However, stress hyperglycemia is also associated with adverse outcome in several critically ill patient populations. More precisely, a large cohort study of over 66,000 critically ill patients revealed a J-curved relationship between on-admission blood glucose level and the risk of mortality, with the nadir between 100 and 150 mg/dL (5.6-8.3 mmol/L).16 In patients with an acute coronary syndrome, a similar association has been observed, with the lowest risk of mortality at blood glucose levels between 80 and 100 mg/dL (4.4-5.5 mmol/L).17–20 Importantly, in patients with established diabetes mellitus prior to critical illness or an acute coronary syndrome, the relationship between hyperglycemia and mortality is significantly blunted and somewhat shifted to the higher blood glucose17 (Figure 164-1).
Until recently, it was considered state of the art to tolerate blood glucose levels up to 220 mg/dL (12 mmol/L) in fed critically ill patients. It was even suggested that this moderate hyperglycemia in critically ill patients was beneficial for organs such as the brain and the blood cells which rely solely on glucose for their energy supply and do not require insulin for glucose uptake. Motivation for treatment of blood glucose levels higher than 12 mmol/L was primarily the occurrence of hyperglycemia-induced osmotic diuresis and fluid shifts. Also, from the diabetes literature it was known that uncontrolled and pronounced hyperglycemia predisposes to infectious complications.21 In patients with known diabetes mellitus, usually more attention was paid to blood glucose levels and consequently more strictly controlled. This approach contrasts—in hindsight—with the blunting of the J-shaped relation between glycemia and mortality risk. Observational studies have also revealed that hyperglycemia in patients with established diabetes mellitus gives an at least threefold higher risk of mortality compared to patients with known diabetes.22
 Maintenance of Normoglycemia in the Intensive Care Unit
Maintenance of Normoglycemia in the Intensive Care Unit
The Leuven Studies
In 2001, a large prospective, randomized, controlled trial (RCT) was the first to challenge the classic dogma of beneficial stress hyperglycemia.23 It examined the effect of tight glycemic control (TGC) with intensive insulin therapy on mortality and morbidity of critically ill patients. Over a 1-year period, 1548 mechanically ventilated patients admitted to the intensive care unit (ICU), predominantly after extensive or complicated surgery or trauma, were randomly allocated to either intensive insulin therapy with blood glucose levels kept tightly between 80 and 110 mg/dL (4.5-6.1 mmol/L) or the conventional approach, which recommended insulin therapy only if blood glucose levels exceeded 12 mmol/L. The intervention of TGC comprised accurate arterial blood glucose measurements by a blood gas analyzer and a reliable continuous infusion of insulin exclusively via a central venous line, using an accurate syringe-driven infusion pump. The fine insulin dose adaptations were performed by trained bedside nurses and based on a guideline which requires a high level of intuitive and anticipating decision making. In this study, patients were kept in a nonfasting state at all times. Dextrose 20% was administered on the first day (192 g glucose over 24 hours or 768 kcal/d). Thereafter, enteral nutrition was started, with the daily amount progressively increased as tolerated. When enteral nutrition was insufficient, early supplemental parenteral nutrition was given, resulting in administration on average of 1100 nonprotein kcal/d.
Intensive insulin therapy, resulting in the administration of on average 1100 nonprotein kcal/d, lowered ICU mortality from 8% to 4.6% (absolute risk reduction [ARR] 3.4%) and in-hospital mortality from 10.9% to 7.2% (ARR 3.7%). The effect occurred particularly in the population with prolonged critical illness, among whom mortality was reduced from 20.2% to 10.6%. Even patients in the conventional insulin treatment schedule with only moderate hyperglycemia (110-150 mg/dL) showed higher mortality compared with patients in the strict glycemic control schedule.24 Intensive insulin therapy also had a major effect on morbidity. It decreased the duration of ventilatory support and ICU stay, reduced the need for blood transfusions, and lowered the incidence of bloodstream infections and excessive inflammation. Even more striking, intensive insulin therapy caused a highly significant decrease in the development of critical illness polyneuropathy and acute kidney failure.
Subsequently, the effect of TGC was tested in a medical ICU setting by the same group.25 The difference in in-hospital mortality, 40.0% in the control group and 37.3% in the intervention group, was not statistically significant in an intention-to-treat analysis of the 1200 included patients. However, in patients who stayed in the ICU for 3 or more days, in-hospital mortality was reduced from 52.5% to 43.0% by TGC. Intensive insulin therapy also reduced morbidity (incidence of acute kidney failure, weaning of the ventilator, ICU/hospital stay) but not as strikingly as in the surgical study. This was in part explained by a larger fraction of patients in medical ICUs who were admitted with established organ damage, possibly reducing the opportunity of prevention by glucose lowering.26 The fact that intensive insulin therapy to normal-for-age blood glucose targets in mainly postoperative pediatric critically ill patients did reduce mortality by an ARR of 3% may further corroborate this finding.27
The Initial Repeat Studies
Two European multicenter studies designed to assess whether intensive insulin therapy exerts benefit, with mortality as the primary endpoint, failed to reproduce the Leuven findings. The VISEP (Volume substitution and Insulin therapy in severe SEPsis) (N = 537) trial was designed as a four-arm study to assess the difference between two choices of fluid resuscitation (10% pentastarch versus modified Ringer’s lactate) and the efficacy and safety of intensive insulin therapy in patients with severe sepsis and septic shock.28 In this study, blood glucose targets comparable to the Leuven studies were set out for the intervention (80-110 mg/L) and control (180-200 mg/dL) groups. Likewise, the insulin administration and blood glucose measurements had been standardized. Nevertheless, the insulin arm of the study was stopped early after 488 patients had been included, because the rate of hypoglycemia (12.1%) in the intensive insulin therapy group was considered unacceptably high and may be associated with higher mortality. Then at the first planned interim analysis, the fluid resuscitation arm of the study was also suspended because of increased risk of organ failure in the 10% pentastarch arm. The primary endpoint, 90-day mortality, was 39.7% in the intensive versus 35.4% in the conventional treatment arm.
The GLUCONTROL multicenter RCT (N=1101) investigated whether tight glycemic control (80 and 110 mg/dL) with intensive insulin therapy versus an intermediate target for blood glucose (140-180 mg/dL [7.8-10.0 mmol/L]) improves survival in a mixed population of critically ill patients.29 This study was also stopped early because the target glycemic control was not reached and the incidence of hypoglycemia was 9.8%. ICU mortality did not differ between the intensive insulin therapy group (17.2%) and the control group (15.3%).
Two single-center studies in a mixed medical/surgical ICU population, both smaller than the Leuven studies, followed and were unable to reproduce a significant mortality benefit.30,31 In contrast, a number of small RCTs in selected subpopulations, mostly focusing on morbidity as primary endpoint, as well as several larger implementation studies revealed improved outcome as did the Leuven studies.32–35
Nice-Sugar
All the described studies were in fact statistically underpowered to detect a reasonable mortality difference. To address this issue, the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) included 6100 patients over 41 participating centers.36 This study compared a blood glucose target of below 108 mg/dL (<6.0 mmol/L) versus “usual care,” which meant an intermediate blood glucose target of 140 to 180 mg/dL (8 to 10 mmol/L). Owing to the results from the Leuven studies, clinicians had become aware of the negative impact of hyperglycemia, so tolerating higher glucose levels was considered unacceptable or even unethical by clinicians and investigators. The aim of NICE-SUGAR, therefore, was to assess whether further lowering blood glucose levels to less than 108 mg/dL (<6.0 mmol/L) in a broad context of clinical practice in ICUs, predominantly located in Australia and New-Zealand, and using the normal daily clinical practice tools available would exert additional benefit. Contrary to expectations, NICE-SUGAR revealed that targeting 108 mg/dL with insulin increased 90-day mortality from 24.9% to 27.5% as compared with the 140 to 180 mg/dL (8-10 mmol/L) glucose target. Excess deaths were attributed to cardiovascular causes.
Coiitss
Patients with septic shock requiring administration of glucocorticoids are faced with a high mortality risk; the severity of illness and glucocorticoid treatment make hyperglycemia common. Therefore, this would be an optimal population in whom to study whether TGC could reduce mortality. In the Corticosteroids and Intensive Insulin Therapy for Septic Shock (COIITSS) multicenter study, 509 patients were randomized to either intensive insulin therapy aiming for blood glucose levels between 80 and 110 mg/dL or to conventional insulin therapy.37 In the latter group, an intermediate target was used, as the physicians were recommended to follow the 2004 Surviving Sepsis Campaign Guidelines (blood glucose levels < 150 mg/dL [8.3 mmol/L]). Hospital mortality in the intensive insulin therapy group (45.9%) did not differ from the conventional group (42.9%). Poor separation of the blood glucose levels between the study groups and the small size of the study may have made it hard to detect any treatment effect of TGC.
Meta-Analyses
Nowadays, practice guidelines ideally are based on systematic reviews and meta-analyses. The two most recent meta-analyses showed that in critically ill adult patients, TGC did not significantly reduce hospital mortality but is associated with an increased risk of hypoglycemia.38,39 However, TGC may be beneficial to patients admitted to a surgical ICU.
 Critical Appraisal of the Evidence for Tight Glycemic Control in the ICU
Critical Appraisal of the Evidence for Tight Glycemic Control in the ICU
Given that the effect of controlling blood glucose levels during critical illness ranges from benefit, to no effect, to potentially harmful, most clinicians are now in agreement that blood glucose levels do in fact play a role in patient outcome. The pre-2001 era where blood glucose levels were hardly measured in critically ill patients has passed forever. However, discrepancies in the study results have made it difficult to make strong recommendations. Likewise, consensus statements on glycemic management of hospitalized patients by the American Association of Clinical Endocrinologists and the American Diabetes Association have changed significantly over the last years.40 While the 2004 and 2006 statements recommended stricter targets for glycemic management in the ICU, in 2009 it was advised that the starting threshold for intravenous insulin therapy in the ICU should be 180 mg/dL (10 mmol/L). And once started, blood glucose levels should be maintained between 140 and 180 mg/dL (7.8-10 mmol/L). Somewhat lower levels may be appropriate in selected patient populations. Targets below 110 mg/dL (<6.1 mmol/L) are not recommended.
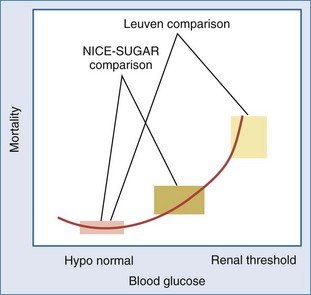
Still, more can be learned from the differences between the Leuven proof-of-concept studies and the subsequent repeat trials.41 First, “normoglycemia” was compared with distinct “control” targets (140-180 mg/dL or 8-10 mmol/L in NICE-SUGAR and GLUCONTROL; 180-215 mg/dL or 10-12 mmol/L in Leuven), making the studies fundamentally different. The control group in the Leuven studies reflected the assumption of hyperglycemia as a potentially beneficial adaptation. Hence, a “do-not-touch” approach unless glucose exceeded the renal threshold of 215 mg/dL was used in this group. In contrast, the NICE-SUGAR trial was executed in the “flatter” part of the observational glycemia-mortality risk curve, with 70% of the patients in the control group receiving insulin treatment to target an intermediate blood glucose level of 140 to 180 mg/dL (8-10 mmol/L) (Figure 164-2).16–19 The control group in NICE-SUGAR, as a result of the changed usual care, already could have benefited from reducing blood glucose as compared with the control group in Leuven. The lower observed mortality than the carefully documented expected mortality (24.9% versus 30%, respectively) in the NICE-SUGAR control group may indeed suggest that there was already such a benefit in the control group.
Second, the level of therapy compliance, in this case the degree of success in reaching and maintaining the preset target range for glucose in the intervention group, as well as the degree of overlap with the control group, varied greatly between the studies. The methodological aspects of glucose measurement and the level of expertise of the nursing team with blood glucose control in the Leuven studies may have played a key role. In the Leuven studies, 70% of the patients in the intervention group were on average in target,42 whereas this was much less than 50% in NICE-SUGAR and in several of the other repeat studies. This could be important, as a recent meta-analysis suggested that studies that actually managed to adequately achieve the blood glucose target showed a reduced mortality, whereas studies that did not succeed in reaching the target reported no benefit or even increased mortality.38,43 Maintaining normoglycemia may be more feasible in patients after surgical critical illness than in those with medical illnesses.
Third, a requirement for safe insulin dose adjusting to reach and maintain normoglycemia is a standardized, accurate glucose measurement technology. In NICE-SUGAR, a variety of glucose meters were allowed, whereas most of them have recently been shown to be unsuitable for this purpose.44 Accuracy of certain glucometers has been shown to be extremely poor in the ICU setting, and the wide error goes in the opposite direction for the low and high glucose ranges, making it impossible to use them for targeting a very narrow glucose range.45,46 In addition, varying sampling sites (arterial, venous, and capillary) were accepted in the context of routine clinical practice, and these too have led to erroneous results for blood glucose.47 Inaccuracy of glucose measurement may have misguided the insulin titration and thereby induced (undetected) hypoglycemia and large blood glucose fluctuations. Avoiding highly variable blood glucose levels requires experience and thus has a learning curve, which is inherent with complex interventions.
Fifth, in a setting where hyperglycemia is triggered by surgery or trauma, the equivalent of acute ischemia/reperfusion, the delay between onset of hyperglycemia and the start of glycemic control is short. In contrast, when ICU patients already suffered from chronic illness prior to ICU admission and hyperglycemia was present for a longer time, adaptive changes to protect the cells against elevated extracellular glucose may have been induced such that acute lowering of blood glucose may be harmful. Alternatively, the time window for prevention of toxicity may have passed and irreversible damage done.48 Such a mechanism was suggested by the pooled analysis of the two Leuven trials42 and by the different results of RCTs on glucose control in patients with type 2 diabetes.49–54
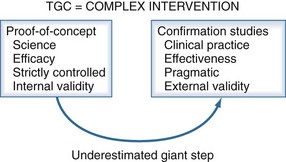
All these differences may have contributed to the different outcomes in different studies. It has become clear that results from single-center, proof-of-concept studies cannot simply be repeated in large multicenter effectiveness trials, certainly when studying the effects of a complex intervention which is too often incompletely implemented in the repeat studies.55 Hence, in reality, such studies did not investigate the same intervention as the proof-of-concept study.
 Biological Rationale for Tight Glycemic Control
Biological Rationale for Tight Glycemic Control
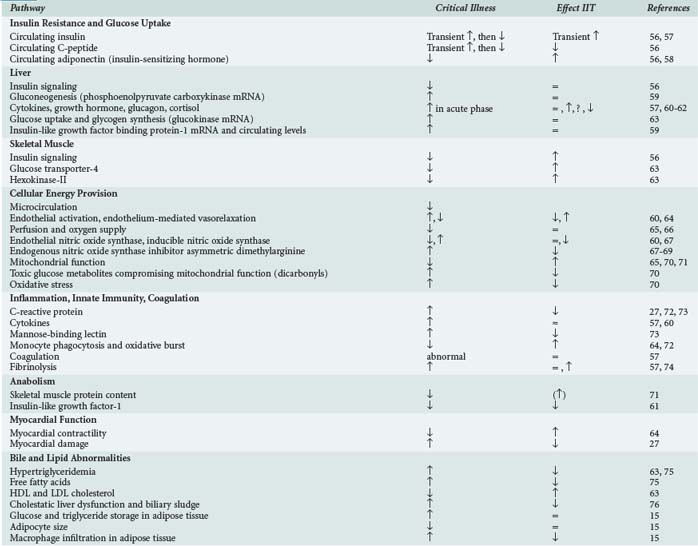
Research using human material, animal models, and in vitro systems has unraveled potential mechanistic explanations for the beneficial effects of TGC (Table 164-1). As in diabetes mellitus, insulinization to lower blood glucose levels exerts its effects on a wide array of biological pathways. Striving for metabolic control and inhibiting excess inflammation and mitochondrial damage seem of chief importance. Further molecular biology research will not only be essential to fine-tune TGC with other metabolic treatment strategies, it will also contribute to the quest to explain the potential harm of glucose lowering in critical illness.
TABLE 164-1 Studies of Biological Effects of Tight Glycemic Control Also Point to Its Potential Benefit
Implications for Daily Practice
The failure to repeat the results from well-controlled, meticulously executed, proof-of-concept studies in large pragmatic confirmation trials has indicated that the TGC is not yet ready to be broadly implemented in every ICU across the globe (Figure 164-3). This does not undermine the scientific validity of the benefits of TGC in critically ill patients. Blood glucose levels should be normalized as much as safely possible without causing a too-rapid lowering of blood glucose, without an increase in the incidence of hypoglycemia, and without large blood glucose fluctuations. Therefore it is advisable to gradually tighten glycemic control under diligent monitoring of the safety aspects. Nevertheless, three conditions should always be met:
Key Points
Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533-551.
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
NICE-SUGAR Study InvestigatorsFinfer S, Chittock DR, Su SY, Blair D, Foster DA, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297.
Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, et al. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94:3163-3170.
1 Van Cromphaut SJ. Hyperglycaemia as part of the stress response: The underlying mechanisms. Best Pract Res Clin Anaesthesiol. 2009;23:375-386.
2 Mizock BA. Alterations in fuel metabolism in critical illness: Hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533-551.
3 Siegel JH, Cerra FB, Coleman B, Giovannini I, Shetye M, Border JR, et al. Physiological and metabolic correlations in human sepsis. Invited commentary. Surgery. 1979;86:163-193.
4 Vary TC, Drnevich D, Jurasinski C, Brennan WAJr. Mechanisms regulating skeletal muscle glucose metabolism in sepsis. Shock. 1995;3:403-410.
5 Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748-756.
6 Stephens JM, Bagby GJ, Pekala PH, Shepherd RE, Spitzer JJ, Lang CH. Differential regulation of glucose transporter gene expression in adipose tissue or septic rats. Biochem Biophys Res Commun. 1992;183:417-422.
7 Virkamaki A, Yki-Jarvinen H. Mechanisms of insulin resistance during acute endotoxemia. Endocrinology. 1994;134:2072-2078.
8 Stoner HB, Little RA, Frayn KN, Elebute AE, Tresadern J, Gross E. The effect of sepsis on the oxidation of carbohydrate and fat. Br J Surg. 1983;70:32-35.
9 Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg. 1996;224:97-102.
10 Meszaros K, Lang CH, Bagby GJ, Spitzer JJ. In vivo glucose utilization by individual tissues during nonlethal hypermetabolic sepsis. FASEB J. 1988;2:3083-3086.
11 Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock. 2000;13:379-385.
12 Bird TA, Davies A, Baldwin SA, Saklatvala J. Interleukin 1 stimulates hexose transport in fibroblasts by increasing the expression of glucose transporters. J Biol Chem. 1990;265:13578-13583.
13 Meszaros K, Lang CH, Bagby GJ, Spitzer JJ. Contribution of different organs to increased glucose consumption after endotoxin administration. J Biol Chem. 1987;262:10965-10970.
14 Long CL, Schiller WR, Geiger JW, Blakemore WS. Gluconeogenic response during glucose infusions in patients following skeletal trauma or during sepsis. JPEN J Parenter Enteral Nutr. 1978;2:619-626.
15 Langouche L, Vander Perre S, Thiessen S, Gunst J, Hermans G, D’Hoore A, et al. Alterations in adipose tissue during critical illness: An adaptive and protective response? Am J Respir Crit Care Med. 2010;182:507-516.
16 Bagshaw SM, Egi M, George C, Bellomo R. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37:463-470.
17 Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: A scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610-1619.
18 Kosiborod M, Inzucchi SE, Krumholz HM, Masoudi FA, Goyal A, et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009;169:438-446.
19 Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation. 2005;111:3078-3086.
20 Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: The global registry of acute coronary events. Arch Intern Med. 2009;169:402-409.
21 McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107-124.
22 Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982.
23 Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
24 Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359-366.
25 Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461.
26 Schetz M, Vanhorebeek I, Wouters PJ, Wilmer A, Van den Berghe G. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol. 2008;19:571-578.
27 Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: A prospective, randomised controlled study. Lancet. 2009;373:547-556.
28 Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139.
29 Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The glucontrol study. Intensive Care Med. 2009;35:1738-1748.
30 Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, et al. Intensive versus conventional insulin therapy: A randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190-3197.
31 De La Rosa Gdel C, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: A randomised clinical trial. Crit Care. 2008;12:R120.
32 Furnary AP, Cheek DB, Holmes SC, Howell WL, Kelly SP. Achieving tight glycemic control in the operating room: Lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care. Semin Thorac Cardiovasc Surg. 2006;18:339-345.
33 Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992-1000.
34 Lecomte P, Foubert L, Nobels F, Coddens J, Nollet G, Casselman F, et al. Dynamic tight glycemic control during and after cardiac surgery is effective, feasible, and safe. Anesth Analg. 2008;107:51-58.
35 Lecomte P, Van Vlem B, Coddens J, Cammu G, Nollet G, Nobels F, et al. Tight perioperative glucose control is associated with a reduction in renal impairment and renal failure in non-diabetic cardiac surgical patients. Crit Care. 2008;12:R154.
36 Finfer S, Chittock D, Su S, Blair D, Foster DA, Dhingra V, et al. A comparison of intensive and conventional insulin therapy in critically ill patients: An international multicenter randomized controlled trial. N Engl J Med. 2009.
37 Annane D, Cariou A, Maxime V, Azoulay E, D’Honneur G, Timsit JF, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: A randomized controlled trial. JAMA. 2010;303:341-348.
38 Griesdale DEG, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821-827.
39 Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933-944.
40 Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119-1131.
41 Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, et al. Clinical review: Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94:3163-3170.
42 Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: Benefit versus harm. Diabetes. 2006;55:3151-3159.
43 Van den Berghe G, Mesotten D, Vanhorebeek I. Intensive insulin therapy in the intensive care unit. CMAJ. 2009;180:799.
44 Scott MG, Bruns DE, Boyd JC, Sacks DB. Tight glucose control in the intensive care unit: Are glucose meters up to the task? Clin Chem. 2009;55:18-20.
45 Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778-2785.
46 Vlasselaers D, Van Herpe T, Milants I, Eerdekens M, Wouters PJ, De Moor B, et al. Blood glucose measurements in arterial blood of intensive care unit patients submitted to tight glycemic control: Agreement between bedside tests. J Diabetes Sci Technol. 2008;2:932-938.
47 Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33:2079-2084.
48 Ceriello A, Ihnat MA, Thorpe JE. Clinical review 2: The “Metabolic memory”: Is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab. 2009;94:410-415.
49 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
50 Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865.
51 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853.
52 Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
53 Gerstein HC, Miller ME, Byington RP, Goff DCJr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
54 Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
55 Padkin A. How to weigh the current evidence for clinical practice. Best Pract Res Clin Anaesthesiol. 2009;23:487-496.
56 Langouche L, Vander Perre S, Wouters PJ, D’Hoore A, Hansen TK, Van den Berghe G. Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J Clin Endocrinol Metab. 2007;92:3890-3897.
57 Langouche L, Meersseman W, Vander Perre S, Milants I, Wouters PJ, Hermans G, et al. Effect of insulin therapy on coagulation and fibrinolysis in medical intensive care patients. Crit Care Med. 2008;36:1475-1480.
58 Langouche L, Vander Perre S, Frystyk J, Flyvbjerg A, Hansen TK, Van den Berghe G. Adiponectin, retinol-binding protein 4, and leptin in protracted critical illness of pulmonary origin. Crit Care. 2009;13:R112.
59 Mesotten D, Delhanty PJ, Vanderhoydonc F, Hardman KV, Weekers F, Baxter RC, et al. Regulation of insulin-like growth factor binding protein-1 during protracted critical illness. J Clin Endocrinol Metab. 2002;87:5516-5523.
60 Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277-2286.
61 Mesotten D, Wouters PJ, Peeters RP, Hardman KV, Holly JM, Baxter RC, et al. Regulation of the somatotropic axis by intensive insulin therapy during protracted critical illness. J Clin Endocrinol Metab. 2004;89:3105-3113.
62 Vanhorebeek I, Peeters RP, Vander Perre S, Jans I, Wouters PJ, Skogstrand K, et al. Cortisol response to critical illness: Effect of intensive insulin therapy. J Clin Endocrinol Metab. 2006;91:3803-3813.
63 Mesotten D, Swinnen JV, Vanderhoydonc F, Wouters PJ, Van den Berghe G. Contribution of circulating lipids to the improved outcome of critical illness by glycemic control with intensive insulin therapy. J Clin Endocrinol Metab. 2004;89:219-226.
64 Ellger B, Debaveye Y, Vanhorebeek I, Langouche L, Giulietti A, Van Etten E, et al. Survival benefits of intensive insulin therapy in critical illness: Impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes. 2006;55:1096-1105.
65 Vanhorebeek I, Gunst J, Ellger B, Boussemaere M, Lerut E, Debaveye Y, et al. Hyperglycemic kidney damage in an animal model of prolonged critical illness. Kidney Int. 2009;76:512-520.
66 Vanhorebeek I, Ellger B, De Vos R, Boussemaere M, Debaveye Y, Perre SV, et al. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit Care Med. 2009;37:1355-1364.
67 Ellger B, Langouche L, Richir M, Debaveye Y, Vanhorebeek I, Teerlink T, et al. Modulation of regional nitric oxide metabolism: Blood glucose control or insulin? Intensive Care Med. 2008;34:1525-1533.
68 Ellger B, Richir MC, van Leeuwen PA, Debaveye Y, Langouche L, Vanhorebeek I, et al. Glycemic control modulates arginine and asymmetrical-dimethylarginine levels during critical illness by preserving dimethylarginine-dimethylaminohydrolase activity. Endocrinology. 2008;149:3148-3157.
69 Siroen MP, van Leeuwen PA, Nijveldt RJ, Teerlink T, Wouters PJ, Van den Berghe G. Modulation of asymmetric dimethylarginine in critically ill patients receiving intensive insulin treatment: A possible explanation of reduced morbidity and mortality? Crit Care Med. 2005;33:504-510.
70 Vanhorebeek I, Ellger B, De Vos R, Boussemaere M, Debaveye Y, Vander Perre S, et al. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit Care Med. 2009.
71 Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53-59.
72 Weekers F, Giulietti AP, Michalaki M, Coopmans W, Van Herck E, Mathieu C, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144:5329-5338.
73 Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082-1088.
74 Savioli M, Cugno M, Polli F, Taccone P, Bellani G, Spanu P, et al. Tight glycemic control may favor fibrinolysis in patients with sepsis. Crit Care Med. 2009;37:424-431.
75 Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553-560.
76 Mesotten D, Wauters J, Van den Berghe G, Wouters PJ, Milants I, Wilmer A. The effect of strict blood glucose control on biliary sludge and cholestasis in critically ill patients. J Clin Endocrinol Metab. 2009;94:2345-2352.