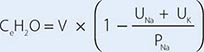POLYURIA
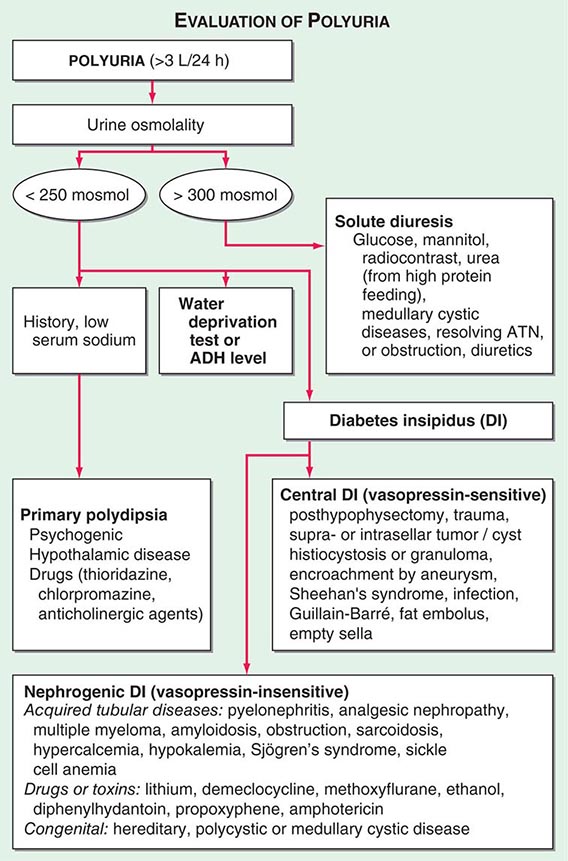
By history, it is often difficult for patients to distinguish urinary frequency (often of small volumes) from true polyuria (>3 L/d), and a quantification of volume by 24-h urine collection may be needed (Fig. 61-4). Polyuria results from two potential mechanisms: (1) excretion of nonabsorbable solutes (such as glucose) or (2) excretion of water (usually from a defect in ADH production or renal responsiveness). To distinguish a solute diuresis from a water diuresis and to determine whether the diuresis is appropriate for the clinical circumstances, urine osmolality is measured. The average person excretes between 600 and 800 mosmol of solutes per day, primarily as urea and electrolytes. If the urine output is >3 L/d and the urine is dilute (<250 mosmol/L), total mosmol excretion is normal and a water diuresis is present. This circumstance could arise from polydipsia, inadequate secretion of vasopressin (central diabetes insipidus), or failure of renal tubules to respond to vasopressin (nephrogenic diabetes insipidus). If the urine volume is >3 L/d and urine osmolality is >300 mosmol/L, a solute diuresis is clearly present and a search for the responsible solute(s) is mandatory.
FIGURE 61-4 Approach to the patient with polyuria. ADH, antidiuretic hormone; ATN, acute tubular necrosis.
Excessive filtration of a poorly reabsorbed solute such as glucose or mannitol can depress reabsorption of NaCl and water in the proximal tubule and lead to enhanced excretion in the urine. Poorly controlled diabetes mellitus with glucosuria is the most common cause of a solute diuresis, leading to volume depletion and serum hypertonicity. Since the urine sodium concentration is less than that of blood, more water than sodium is lost, causing hypernatremia and hypertonicity. Common iatrogenic solute diuresis occurs in association with mannitol administration, radiocontrast media, and high-protein feedings (enteral or parenteral), leading to increased urea production and excretion. Less commonly, excessive sodium loss may result from cystic renal diseases or Bartter’s syndrome or may develop during a tubulointerstitial process (such as resolving ATN). In these so-called salt-wasting disorders, the tubule damage results in direct impairment of sodium reabsorption and indirectly reduces the responsiveness of the tubule to aldosterone. Usually, the sodium losses are mild, and the obligatory urine output is <2 L/d; resolving ATN and postobstructive diuresis are exceptions and may be associated with significant natriuresis and polyuria.
Formation of large volumes of dilute urine is usually due to polydipsic states or diabetes insipidus. Primary polydipsia can result from habit, psychiatric disorders, neurologic lesions, or medications. During deliberate polydipsia, extracellular fluid volume is normal or expanded and plasma vasopressin levels are reduced because serum osmolality tends to be near the lower limits of normal. Urine osmolality is also maximally dilute at 50 mosmol/L.
Central diabetes insipidus may be idiopathic in origin or secondary to a variety of conditions, including hypophysectomy, trauma, neoplastic, inflammatory, vascular, or infectious hypothalamic diseases. Idiopathic central diabetes insipidus is associated with selective destruction of the vasopressin-secreting neurons in the supraoptic and paraventricular nuclei and can either be inherited as an autosomal dominant trait or occur spontaneously. Nephrogenic diabetes insipidus can occur in a variety of clinical situations, as summarized in Fig. 61-4.
A plasma vasopressin level is recommended as the best method for distinguishing between central and nephrogenic diabetes insipidus. Alternatively, a water deprivation test plus exogenous vasopressin may distinguish primary polydipsia from central and nephrogenic diabetes insipidus. For a detailed discussion, see Chap. 404.
62e |
Atlas of Urinary Sediments and Renal Biopsies |
Key diagnostic features of selected diseases in renal biopsy are illustrated, with light, immunofluorescence, and electron microscopic images. Common urinalysis findings are also documented.
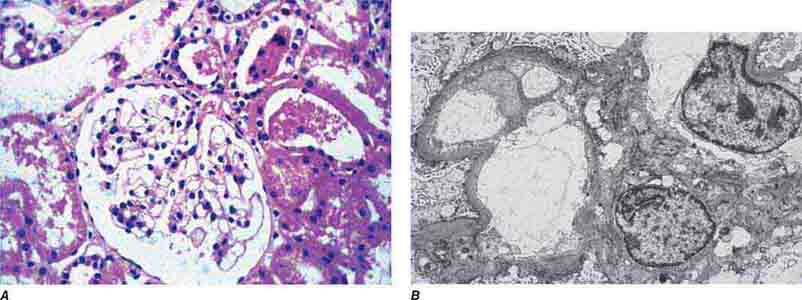
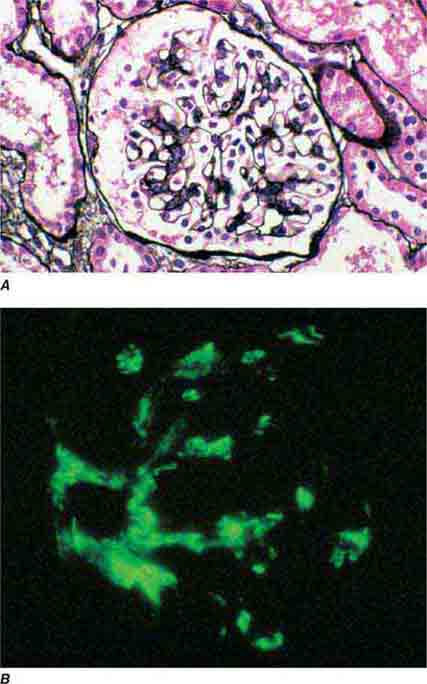
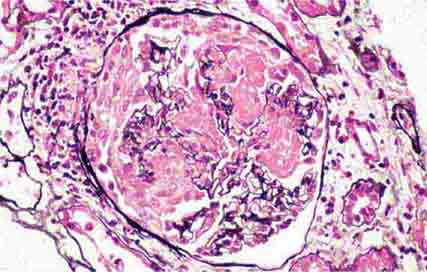
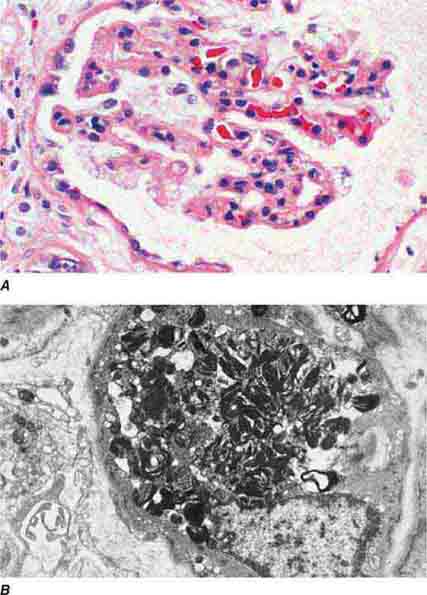
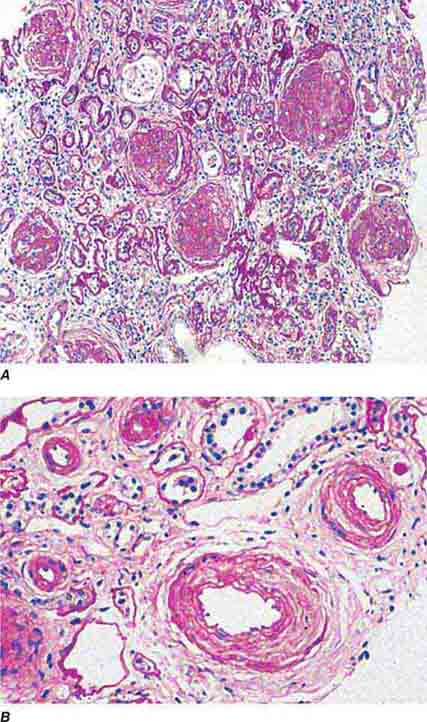
FIGURE 62e-1 Minimal-change disease. In minimal-change disease, light microscopy is unremarkable (A), whereas electron microscopy (B) reveals podocyte injury evidenced by complete foot process effacement. (ABF/Vanderbilt Collection.)
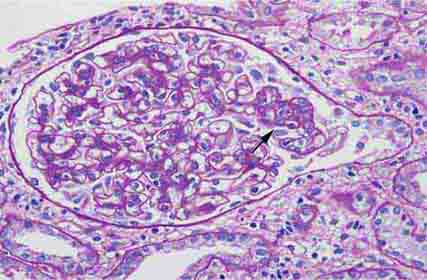
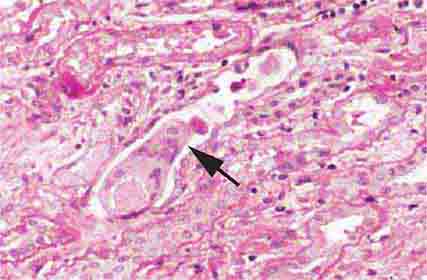
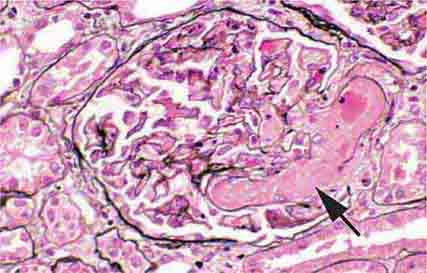
FIGURE 62e-2 Focal segmental glomerulosclerosis (FSGS). There is a well-defined segmental increase in matrix and obliteration of capillary loops (arrow), the sine qua non of segmental sclerosis not otherwise specified (NOS) type. (EGN/UPenn Collection.)
FIGURE 62e-3 Collapsing glomerulopathy. There is segmental collapse (arrow) of the glomerular capillary loops and overlying podocyte hyperplasia. This lesion may be idiopathic or associated with HIV infection and has a particularly poor prognosis. (ABF/Vanderbilt Collection.)
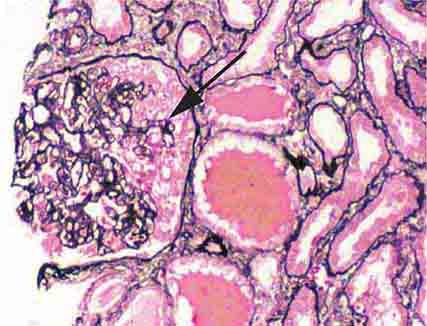
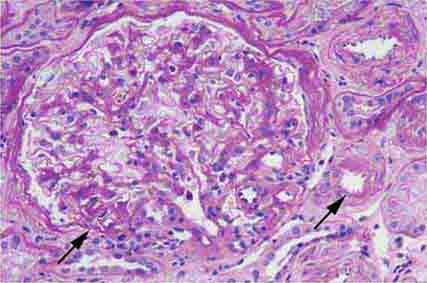
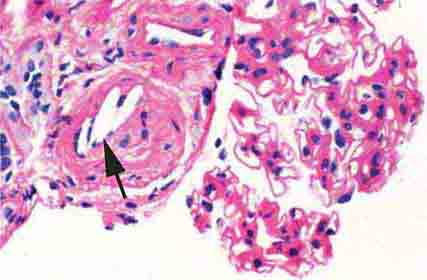
FIGURE 62e-4 Hilar variant of FSGS. There is segmental sclerosis of the glomerular tuft at the vascular pole with associated hyalinosis, also present in the afferent arteriole (arrows). This lesion often occurs as a secondary response when nephron mass is lost due to, e.g., scarring from other conditions. Patients usually have less proteinuria and less steroid response than FSGS, NOS type. (ABF/Vanderbilt Collection.)
FIGURE 62e-5 Tip lesion variant of FSGS. There is segmental sclerosis of the glomerular capillary loops at the proximal tubular outlet (arrow). This lesion has a better prognosis than other types of FSGS. (ABF/Vanderbilt Collection.)
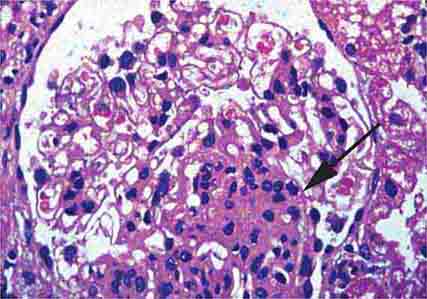
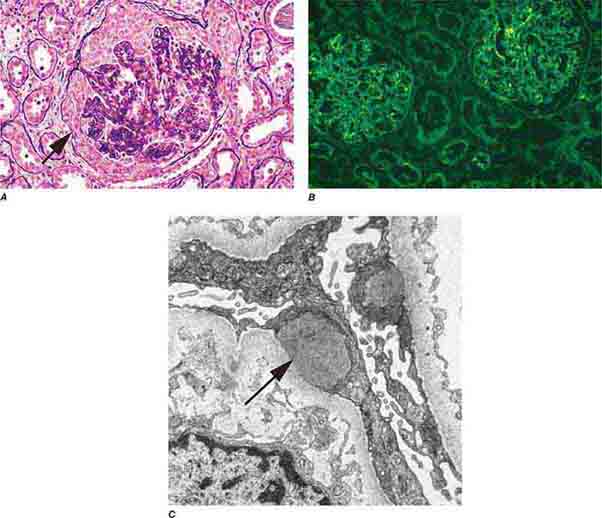
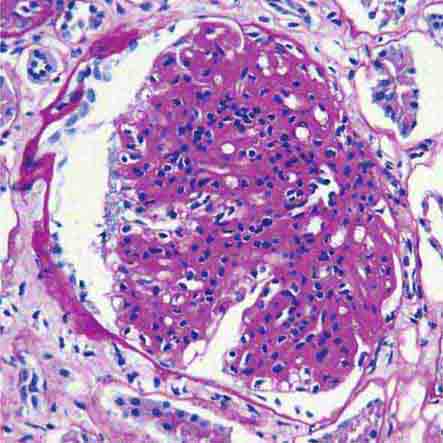
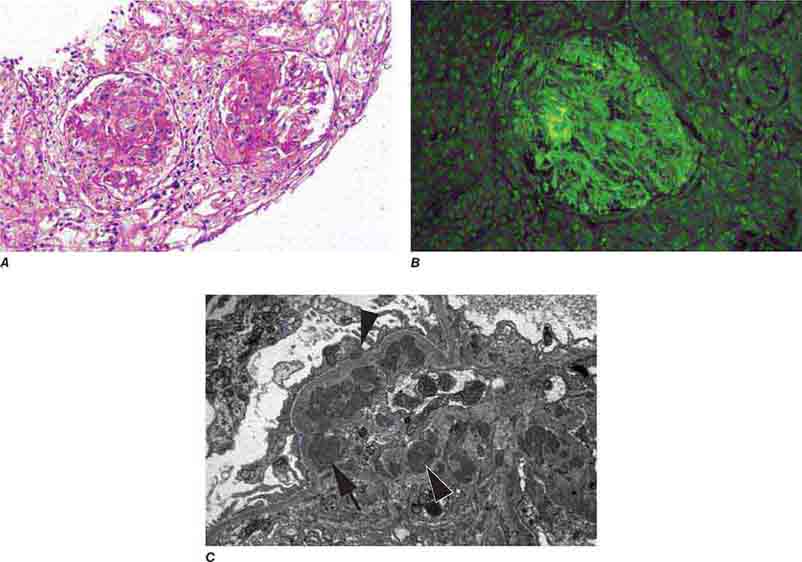
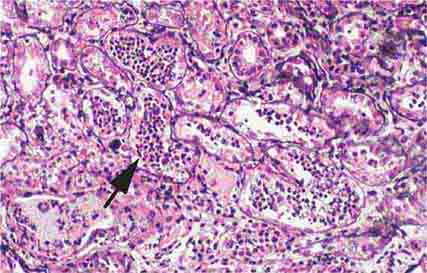
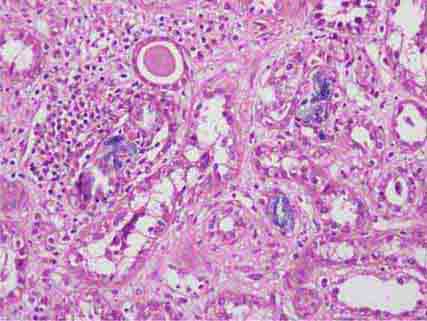
FIGURE 62e-6 Postinfectious (poststreptococcal) glomerulonephritis. The glomerular tuft shows proliferative changes with numerous polymorphonuclear leukocytes (PMNs), with a crescentic reaction (arrow) in severe cases (A). These deposits localize in the mesangium and along the capillary wall in a subepithelial pattern and stain dominantly for C3 and to a lesser extent for IgG (B). Subepithelial hump-shaped deposits are seen by electron microscopy (arrow) (C). (ABF/Vanderbilt Collection.)
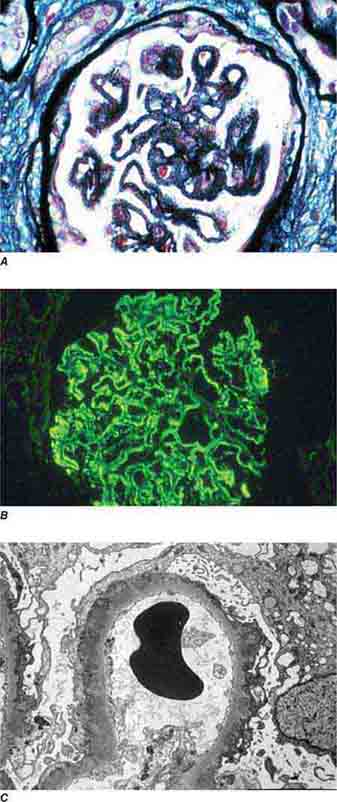
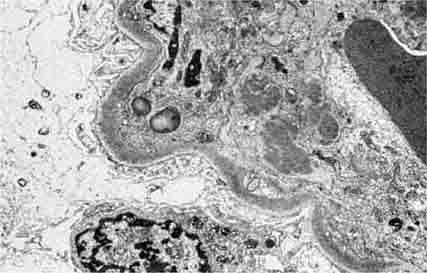
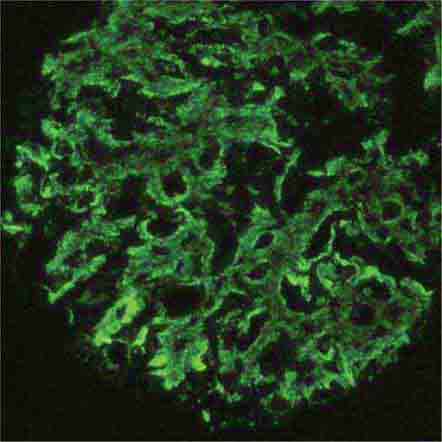
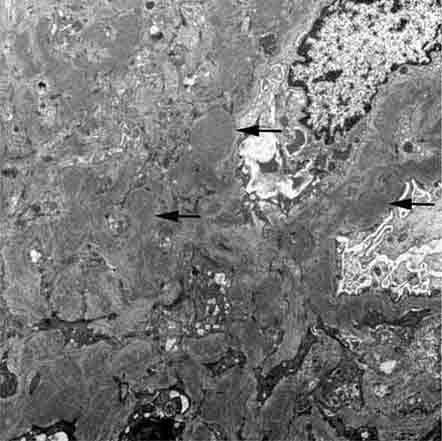
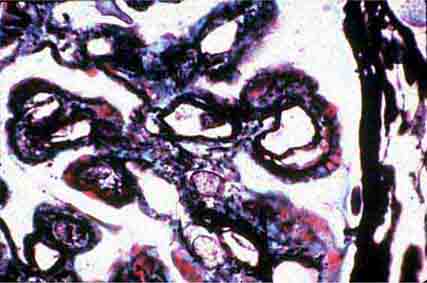
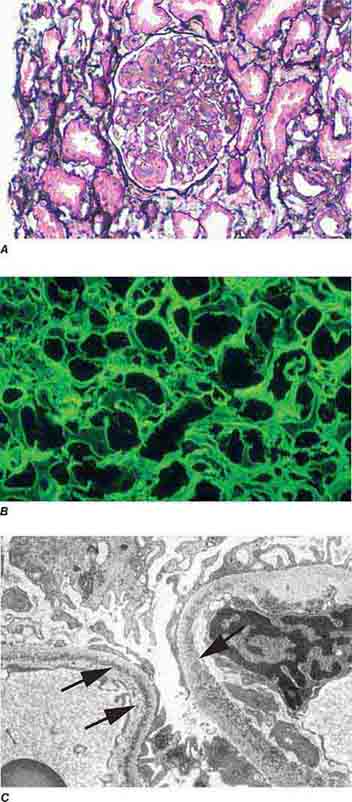
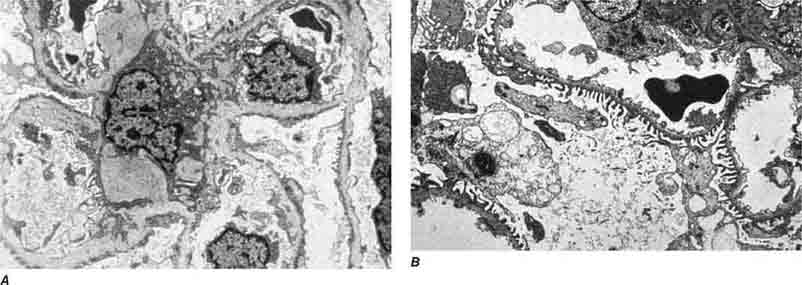
FIGURE 62e-7 Membranous glomerulopathy. Membranous glomerulopathy is due to subepithelial deposits, with resulting basement membrane reaction, resulting in the appearance of spike-like projections on silver stain (A). The deposits are directly visualized by fluorescent anti-IgG, revealing diffuse granular capillary loop staining (B). By electron microscopy, the subepithelial location of the deposits and early surrounding basement membrane reaction is evident, with overlying foot process effacement (C). (ABF/Vanderbilt Collection.)
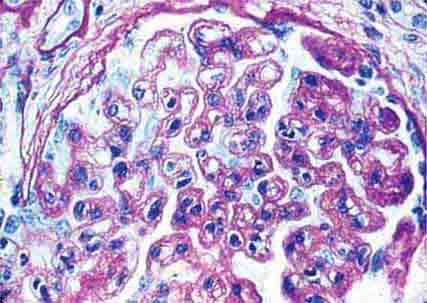
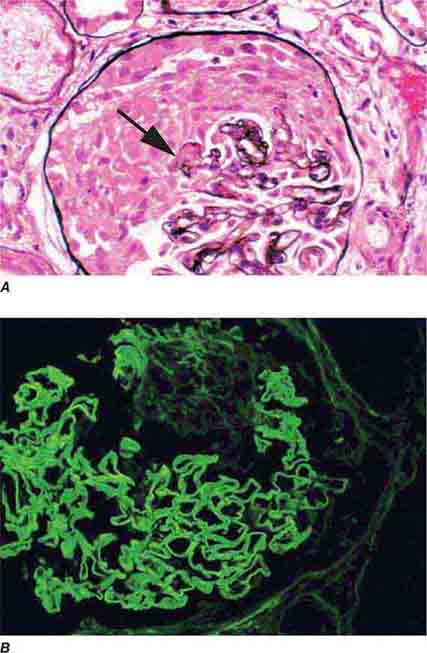
FIGURE 62e-8 IgA nephropathy. There is variable mesangial expansion due to mesangial deposits, with some cases also showing endocapillary proliferation or segmental sclerosis (A). By immunofluorescence, mesangial IgA deposits are evident (B). (ABF/Vanderbilt Collection.)
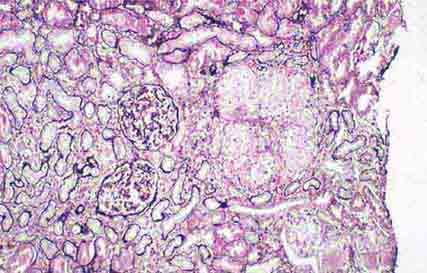
FIGURE 62e-9 Membranoproliferative glomerulonephritis. There is mesangial expansion and endocapillary proliferation with cellular interposition in response to subendothelial deposits, resulting in the “tram-track” of duplication of glomerular basement membrane. (EGN/UPenn Collection.)
FIGURE 62e-10 Dense deposit disease (membranoproliferative glomerulonephritis type II). By light microscopy, there is a membranoproliferative pattern. By electron microscopy, there is a dense transformation of the glomerular basement membrane with round, globular deposits within the mesangium. By immunofluorescence, only C3 staining is usually present. Dense deposit disease is part of the group of renal diseases called C3 glomerulopathy, related to underlying complement dysregulation. (ABF/Vanderbilt Collection.)
FIGURE 62e-11 C3 glomerulonephritis. By light microscopy, there is a membranoproliferative pattern. C3 glomerulonephritis is part of the group of renal diseases called C3 glomerulopathy, related to underlying complement dysregulation. (ABF/Vanderbilt Collection.)
FIGURE 62e-12 C3 glomerulonephritis. By immunofluorescence, only C3 staining is usually present, with occasional minimal immunoglobulin, in an irregular capillary wall and mesangial distribution. (ABF/Vanderbilt Collection.)
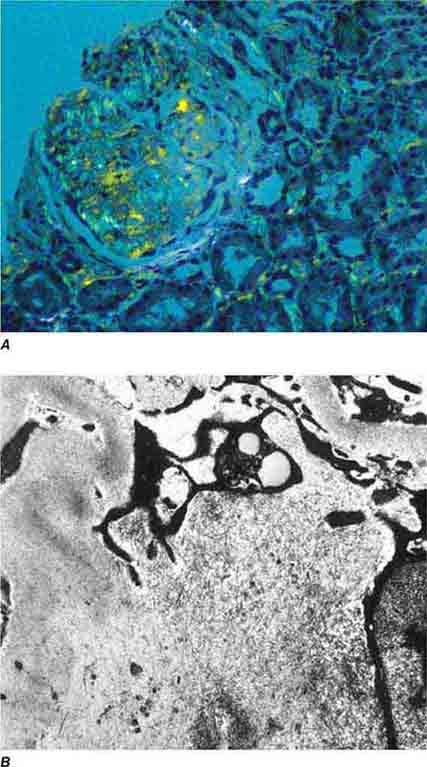
FIGURE 62e-13 C3 glomerulonephritis. By electron microscopy, usual density deposits are present (arrows), including mesangial, subendothelial, and occasional hump-type subepithelial deposits. (ABF/Vanderbilt Collection.)
FIGURE 62e-14 Mixed proliferative and membranous glomerulonephritis. This specimen shows pink subepithelial deposits with spike reaction, and the “tram-track” sign of reduplication of glomerular basement membrane, resulting from subendothelial deposits, as may be seen in mixed membranous and proliferative lupus nephritis (International Society of Nephrology [ISN]/Renal Pathology Society [RPS] class V and IV). (EGN/UPenn Collection.)
FIGURE 62e-15 Lupus nephritis. Proliferative lupus nephritis, ISN/RPS class III (focal) or IV (diffuse), manifests as endocapillary proliferation, which may result in segmental necrosis due to deposits, particularly in the subendothelial area (A). By immunofluorescence, chunky irregular mesangial and capillary loop deposits are evident, with some of the peripheral loop deposits having a smooth, molded outer contour due to their subendothelial location. These deposits typically stain for all three immunoglobulins, IgG, IgA, IgM, and both C3 and C1q (B). By electron microscopy, subendothelial (arrow), mesangial (white rim arrowhead), and rare subepithelial (black arrowhead) dense immune complex deposits are evident, along with extensive foot process effacement (C). (ABF/Vanderbilt Collection.)
FIGURE 62e-16 Granulomatosis with polyangiitis (Wegener’s). This pauci-immune necrotizing crescentic glomerulonephritis shows numerous breaks in the glomerular basement membrane with associated segmental fibrinoid necrosis and a crescent formed by proliferation of the parietal epithelium. Note that the uninvolved segment of the glomerulus (at ∼5 o’clock) shows no evidence of proliferation or immune complexes. (ABF/Vanderbilt Collection.)
FIGURE 62e-17 Anti–glomerular basement membrane antibody-mediated glomerulonephritis. There is segmental necrosis with a break of the glomerular basement membrane (arrow) and a cellular crescent (A), and immunofluorescence for IgG shows linear staining of the glomerular basement membrane with a small crescent at ∼1 o’clock (B). (ABF/Vanderbilt Collection.)
FIGURE 62e-18 Amyloidosis. Amyloidosis shows amorphous, acellular expansion of the mesangium, with material often also infiltrating glomerular basement membranes, vessels, and the interstitium, with apple-green birefringence by polarized Congo red stain (A). The deposits are composed of randomly organized 9- to 11-nm fibrils by electron microscopy (B). (ABF/Vanderbilt Collection.)
FIGURE 62e-19 Light chain deposition disease. There is mesangial expansion, often nodular by light microscopy (A), with immunofluorescence showing monoclonal staining, more commonly with kappa than lambda light chain, of tubules (B) and glomerular tufts. By electron microscopy (C), the deposits show an amorphous granular appearance and line the inside of the glomerular basement membrane (arrows) and are also found along the tubular basement membranes. (ABF/Vanderbilt Collection.)
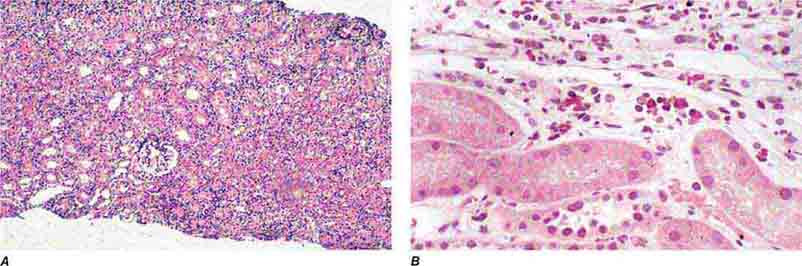
FIGURE 62e-20 Light chain cast nephropathy (myeloma kidney). Monoclonal light chains precipitate in tubules and result in a syncytial giant cell reaction (arrow) surrounding the casts and a surrounding chronic interstitial nephritis with tubulointerstitial fibrosis. (ABF/Vanderbilt Collection.)
FIGURE 62e-21 Fabry’s disease. Due to deficiency of α-galactosidase, there is abnormal accumulation of glycolipids, resulting in foamy podocytes by light microscopy (A). These deposits can be directly visualized by electron microscopy (B), where the glycosphingolipid appears as whorled so-called myeloid bodies, particularly in the podocytes. (ABF/Vanderbilt Collection.)
FIGURE 62e-22 Alport’s syndrome and thin glomerular basement membrane lesion. In Alport’s syndrome, there is irregular thinning alternating with thickened so-called basket-weaving abnormal organization of the glomerular basement membrane (A). In benign familial hematuria, or in early cases of Alport’s syndrome or female carriers, only extensive thinning of the glomerular basement membrane is seen by electron microscopy (B). (ABF/Vanderbilt Collection.)
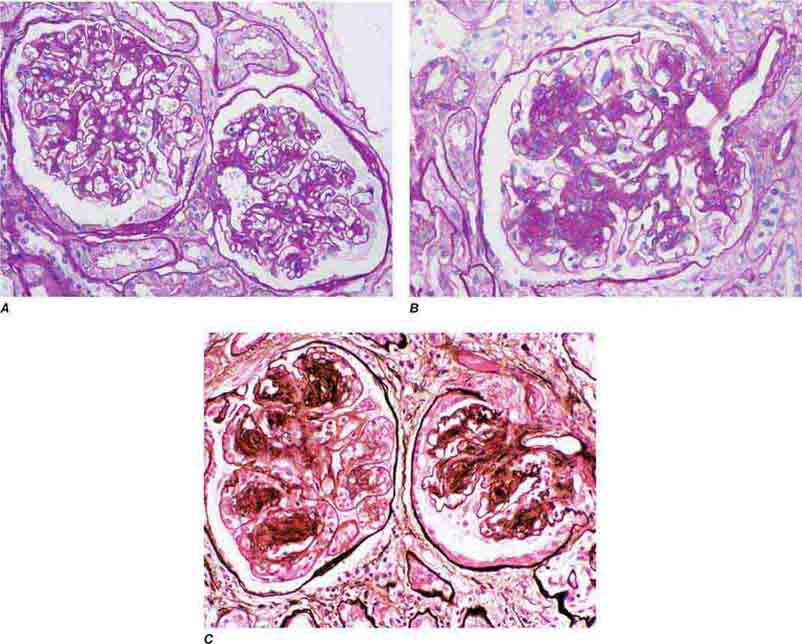
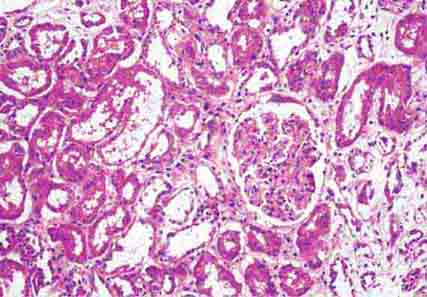
FIGURE 62e-23 Diabetic nephropathy. In the earliest stage of diabetic nephropathy, only mild mesangial increase and prominent glomerular basement membranes (confirmed to be thickened by electron microscopy) are present (A). In slightly more advanced stages, more marked mesangial expansion with early nodule formation develops, with evident arteriolar hyaline (B). In established diabetic nephropathy, there is nodular mesangial expansion, so-called Kimmelstiel-Wilson nodules, with increased mesangial matrix and cellularity, microaneurysm formation in the glomerulus on the left, and prominent glomerular basement membranes without evidence of immune deposits and arteriolar hyalinosis of both afferent and efferent arterioles (C). (ABF/Vanderbilt Collection.)
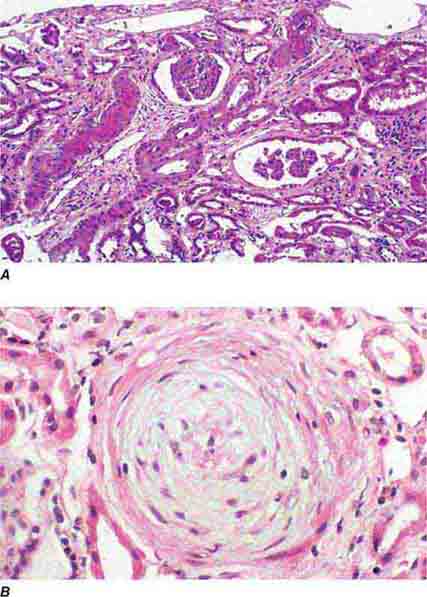
FIGURE 62e-24 Arterionephrosclerosis. Hypertension-associated injury often manifests extensive global sclerosis of glomeruli, with accompanying and proportional tubulointerstitial fibrosis and pericapsular fibrosis, and there may be segmental sclerosis (A). The vessels show disproportionately severe changes of intimal fibrosis, medial hypertrophy, and arteriolar hyaline deposits (B). (ABF/Vanderbilt Collection.)
FIGURE 62e-25 Cholesterol emboli. Cholesterol emboli cause cleft-like spaces (arrow) where the lipid has been extracted during processing, with smooth outer contours and surrounding fibrotic and mononuclear cell reaction in these arterioles. (ABF/Vanderbilt Collection.)
FIGURE 62e-26 Hemolytic-uremic syndrome. There are characteristic intraglomerular fibrin thrombi, with a chunky pink appearance (thrombotic microangiopathy) (arrow). The remaining portion of the capillary tuft shows corrugation of the glomerular basement membrane due to ischemia. (ABF/Vanderbilt Collection.)
FIGURE 62e-27 Progressive systemic sclerosis. Acutely, there is fibrinoid necrosis of interlobular and larger vessels, with intervening normal vessels and ischemic change in the glomeruli (A). Chronically, this injury leads to intimal proliferation, the so-called onion-skinning appearance (B). (ABF/Vanderbilt Collection.)
FIGURE 62e-28 Acute pyelonephritis. There are characteristic intratubular plugs and casts of PMNs (arrow) with inflammation extending into the surrounding interstitium and accompanying tubular injury. (ABF/Vanderbilt Collection.)
FIGURE 62e-29 Acute tubular injury. There is extensive flattening of the tubular epithelium and loss of the brush border, with mild interstitial edema, characteristic of acute tubular injury due to ischemia. (ABF/Vanderbilt Collection.)
FIGURE 62e-30 Acute interstitial nephritis. There is extensive interstitial lymphoplasmocytic infiltrate with mild edema and associated tubular injury (A), which is frequently associated with interstitial eosinophils (B) when caused by a drug hypersensitivity reaction. (ABF/Vanderbilt Collection.)
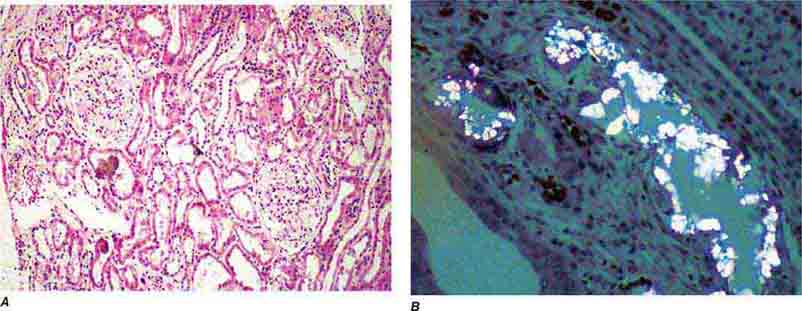
FIGURE 62e-31 Oxalosis. Calcium oxalate crystals have caused extensive tubular injury, with flattening and regeneration of tubular epithelium (A). Crystals are well visualized as sheaves when viewed under polarized light (B). (ABF/Vanderbilt Collection.)
FIGURE 62e-32 Acute phosphate nephropathy. There is extensive acute tubular injury with intratubular nonpolarizable calcium phosphate crystals. (ABF/Vanderbilt Collection.)
FIGURE 62e-33 Sarcoidosis. There is chronic interstitial nephritis with numerous, confluent, nonnecrotizing granulomas. The glomeruli are unremarkable, but there is moderate tubular atrophy and interstitial fibrosis. (ABF/Vanderbilt Collection.)
FIGURE 62e-34 Hyaline cast. (ABF/Vanderbilt Collection.)
FIGURE 62e-35 Coarse granular cast. (ABF/Vanderbilt Collection.)
FIGURE 62e-36 Fine granular casts. (ABF/Vanderbilt Collection.)
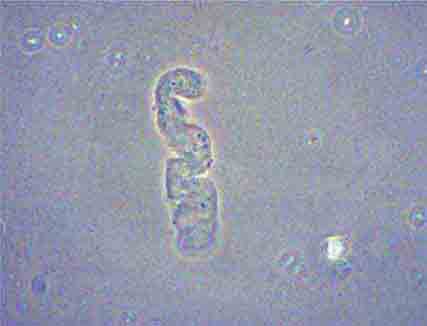
FIGURE 62e-37 Red blood cell cast. (ABF/Vanderbilt Collection.)
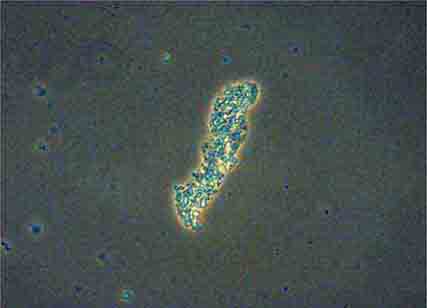
FIGURE 62e-38 White blood cell cast. (ABF/Vanderbilt Collection.)
FIGURE 62e-39 Triple phosphate crystals. (ABF/Vanderbilt Collection.)
FIGURE 62e-40 “Maltese cross” formation in an oval fat body. (ABF/Vanderbilt Collection.)
FIGURE 62e-41 Uric acid crystals. (ABF/Vanderbilt Collection.)
63 |
Fluid and Electrolyte Disturbances |
SODIUM AND WATER
COMPOSITION OF BODY FLUIDS
Water is the most abundant constituent in the body, comprising approximately 50% of body weight in women and 60% in men. Total-body water is distributed in two major compartments: 55–75% is intracellular (intracellular fluid [ICF]), and 25–45% is extracellular (extracellular fluid [ECF]). The ECF is further subdivided into intravascular (plasma water) and extravascular (interstitial) spaces in a ratio of 1:3. Fluid movement between the intravascular and interstitial spaces occurs across the capillary wall and is determined by Starling forces, i.e., capillary hydraulic pressure and colloid osmotic pressure. The transcapillary hydraulic pressure gradient exceeds the corresponding oncotic pressure gradient, thereby favoring the movement of plasma ultrafiltrate into the extravascular space. The return of fluid into the intravascular compartment occurs via lymphatic flow.
The solute or particle concentration of a fluid is known as its osmolality, expressed as milliosmoles per kilogram of water (mOsm/kg). Water easily diffuses across most cell membranes to achieve osmotic equilibrium (ECF osmolality = ICF osmolality). Notably, the extracellular and intracellular solute compositions differ considerably owing to the activity of various transporters, channels, and ATP-driven membrane pumps. The major ECF particles are Na+ and its accompanying anions Cl– and HCO3–, whereas K+ and organic phosphate esters (ATP, creatine phosphate, and phospholipids) are the predominant ICF osmoles. Solutes that are restricted to the ECF or the ICF determine the “tonicity” or effective osmolality of that compartment. Certain solutes, particularly urea, do not contribute to water shifts across most membranes and are thus known as ineffective osmoles.
Water Balance Vasopressin secretion, water ingestion, and renal water transport collaborate to maintain human body fluid osmolality between 280 and 295 mOsm/kg. Vasopressin (AVP) is synthesized in magnocellular neurons within the hypothalamus; the distal axons of these neurons project to the posterior pituitary or neurohypophysis, from which AVP is released into the circulation. A network of central “osmoreceptor” neurons, which includes the AVP-expressing magnocellular neurons themselves, sense circulating osmolality via nonselective, stretch-activated cation channels. These osmoreceptor neurons are activated or inhibited by modest increases and decreases in circulating osmolality, respectively; activation leads to AVP release and thirst.
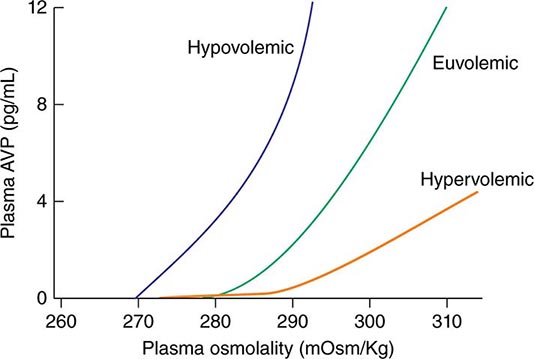
AVP secretion is stimulated as systemic osmolality increases above a threshold level of ~285 mOsm/kg, above which there is a linear relationship between osmolality and circulating AVP (Fig. 63-1). Thirst and thus water ingestion are also activated at ~285 mOsm/kg, beyond which there is an equivalent linear increase in the perceived intensity of thirst as a function of circulating osmolality. Changes in blood volume and blood pressure are also direct stimuli for AVP release and thirst, albeit with a less sensitive response profile. Of perhaps greater clinical relevance to the pathophysiology of water homeostasis, ECF volume strongly modulates the relationship between circulating osmolality and AVP release, such that hypovolemia reduces the osmotic threshold and increases the slope of the response curve to osmolality; hypervolemia has an opposite effect, increasing the osmotic threshold and reducing the slope of the response curve (Fig. 63-1). Notably, AVP has a half-life in the circulation of only 10–20 minutes; thus, changes in ECF volume and/or circulating osmolality can rapidly affect water homeostasis. In addition to volume status, a number of other “nonosmotic” stimuli have potent activating effects on osmosensitive neurons and AVP release, including nausea, intracerebral angiotensin II, serotonin, and multiple drugs.
FIGURE 63-1 Circulating levels of vasopressin (AVP) in response to changes in osmolality. Plasma AVP becomes detectable in euvolemic, healthy individuals at a threshold of ~285 mOsm/kg, above which there is a linear relationship between osmolality and circulating AVP. The vasopressin response to osmolality is modulated strongly by volume status. The osmotic threshold is thus slightly lower in hypovolemia, with a steeper response curve; hypervolemia reduces the sensitivity of circulating AVP levels to osmolality.
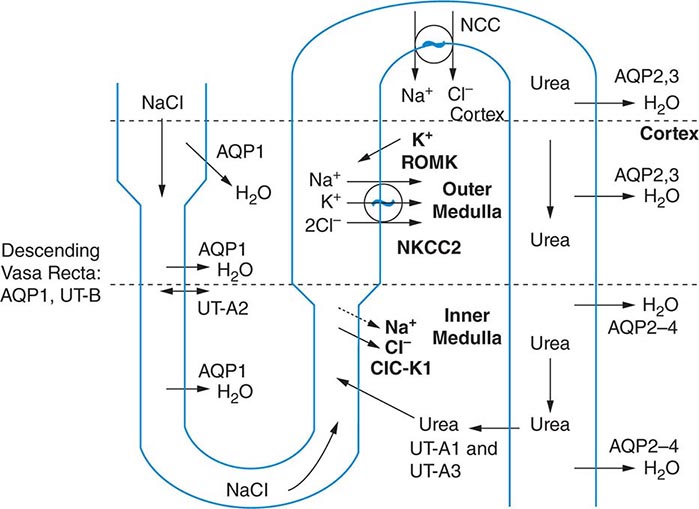
The excretion or retention of electrolyte-free water by the kidney is modulated by circulating AVP. AVP acts on renal, V2-type receptors in the thick ascending limb of Henle and principal cells of the collecting duct (CD), increasing intracellular levels of cyclic AMP and activating protein kinase A (PKA)–dependent phosphorylation of multiple transport proteins. The AVP- and PKA-dependent activation of Na+-Cl– and K+ transport by the thick ascending limb of the loop of Henle (TALH) is a key participant in the countercurrent mechanism (Fig. 63-2). The countercurrent mechanism ultimately increases the interstitial osmolality in the inner medulla of the kidney, driving water absorption across the renal CD. However, water, salt, and solute transport by both proximal and distal nephron segments participates in the renal concentrating mechanism (Fig. 63-2). Water transport across apical and basolateral aquaporin-1 water channels in the descending thin limb of the loop of Henle is thus involved, as is passive absorption of Na+-Cl– by the thin ascending limb, via apical and basolateral CLC-K1 chloride channels and paracellular Na+ transport. Renal urea transport in turn plays important roles in the generation of the medullary osmotic gradient and the ability to excrete solute-free water under conditions of both high and low protein intake (Fig. 63-2).
FIGURE 63-2 The renal concentrating mechanism. Water, salt, and solute transport by both proximal and distal nephron segments participates in the renal concentrating mechanism (see text for details). Diagram showing the location of the major transport proteins involved; a loop of Henle is depicted on the left, collecting duct on the right. AQP, aquaporin; CLC-K1, chloride channel; NKCC2, Na-K-2Cl cotransporter; ROMK, renal outer medullary K+ channel; UT, urea transporter. (Used with permission from JM Sands: Molecular approaches to urea transporters. J Am Soc Nephrol 13:2795, 2002.)
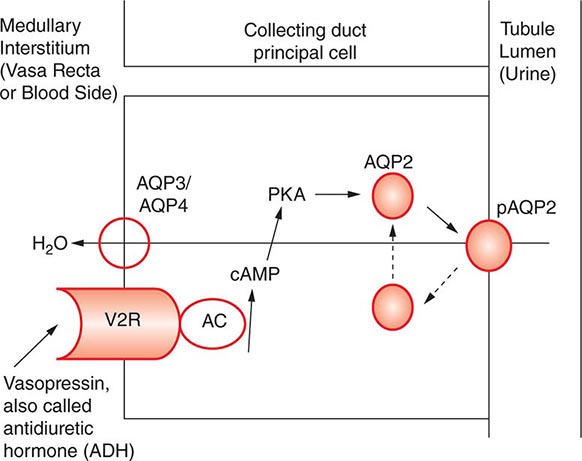
AVP-induced, PKA-dependent phosphorylation of the aquaporin-2 water channel in principal cells stimulates the insertion of active water channels into the lumen of the CD, resulting in transepithelial water absorption down the medullary osmotic gradient (Fig. 63-3). Under “antidiuretic” conditions, with increased circulating AVP, the kidney reabsorbs water filtered by the glomerulus, equilibrating the osmolality across the CD epithelium to excrete a hypertonic, “concentrated” urine (osmolality of up to 1200 mOsm/kg). In the absence of circulating AVP, insertion of aquaporin-2 channels and water absorption across the CD is essentially abolished, resulting in secretion of a hypotonic, dilute urine (osmolality as low as 30–50 mOsm/kg). Abnormalities in this “final common pathway” are involved in most disorders of water homeostasis, e.g., a reduced or absent insertion of active aquaporin-2 water channels into the membrane of principal cells in diabetes insipidus.
FIGURE 63-3 Vasopressin and the regulation of water permeability in the renal collecting duct. Vasopressin binds to the type 2 vasopressin receptor (V2R) on the basolateral membrane of principal cells, activates adenylyl cyclase (AC), increases intracellular cyclic adenosine monophosphatase (cAMP), and stimulates protein kinase A (PKA) activity. Cytoplasmic vesicles carrying aquaporin-2 (AQP) water channel proteins are inserted into the luminal membrane in response to vasopressin, thereby increasing the water permeability of this membrane. When vasopressin stimulation ends, water channels are retrieved by an endocytic process and water permeability returns to its low basal rate. The AQP3 and AQP4 water channels are expressed on the basolateral membrane and complete the transcellular pathway for water reabsorption. pAQP2, phosphorylated aquaporin-2. (From JM Sands, DG Bichet: Nephrogenic diabetes insipidus. Ann Intern Med 144:186, 2006, with permission.)
Maintenance of Arterial Circulatory Integrity Sodium is actively pumped out of cells by the Na+/K+-ATPase membrane pump. In consequence, 85–90% of body Na+ is extracellular, and the ECF volume (ECFV) is a function of total-body Na+ content. Arterial perfusion and circulatory integrity are, in turn, determined by renal Na+ retention or excretion, in addition to the modulation of systemic arterial resistance. Within the kidney, Na+ is filtered by the glomeruli and then sequentially reabsorbed by the renal tubules. The Na+ cation is typically reabsorbed with the chloride anion (Cl–), and, thus, chloride homeostasis also affects the ECFV. On a quantitative level, at a glomerular filtration rate (GFR) of 180 L/d and serum Na+ of ~140 mM, the kidney filters some 25,200 mmol/d of Na+. This is equivalent to ~1.5 kg of salt, which would occupy roughly 10 times the extracellular space; 99.6% of filtered Na+-Cl– must be reabsorbed to excrete 100 mM per day. Minute changes in renal Na+-Cl– excretion will thus have significant effects on the ECFV, leading to edema syndromes or hypovolemia.
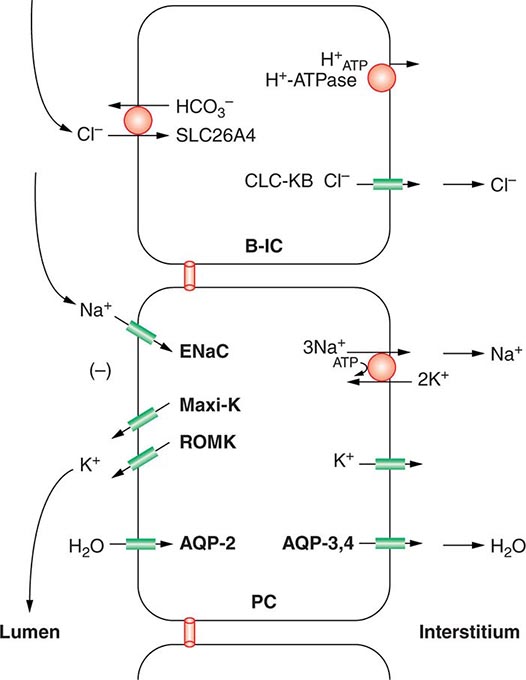
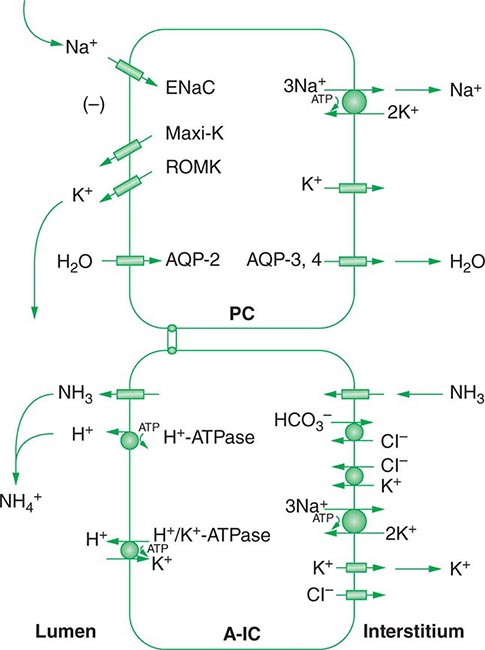
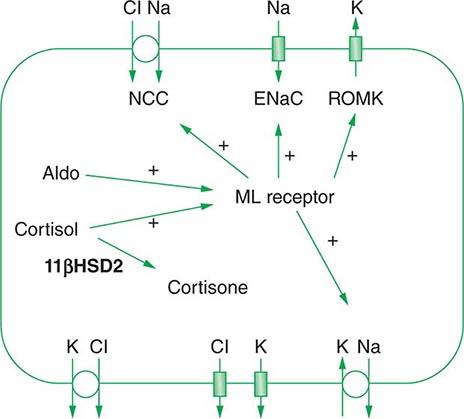
Approximately two-thirds of filtered Na+-Cl– is reabsorbed by the renal proximal tubule, via both paracellular and transcellular mechanisms. The TALH subsequently reabsorbs another 25–30% of filtered Na+-Cl– via the apical, furosemide-sensitive Na+-K+-2Cl– cotransporter. The adjacent aldosterone-sensitive distal nephron, comprising the distal convoluted tubule (DCT), connecting tubule (CNT), and CD, accomplishes the “fine-tuning” of renal Na+-Cl– excretion. The thiazide-sensitive apical Na+-Cl– cotransporter (NCC) reabsorbs 5–10% of filtered Na+-Cl– in the DCT. Principal cells in the CNT and CD reabsorb Na+ via electrogenic, amiloride-sensitive epithelial Na+ channels (ENaC); Cl– ions are primarily reabsorbed by adjacent intercalated cells, via apical Cl– exchange (Cl–-OH– and Cl–-HCO3– exchange, mediated by the SLC26A4 anion exchanger) (Fig. 63-4).
FIGURE 63-4 Sodium, water, and potassium transport in principal cells (PC) and adjacent β-intercalated cells (B-IC). The absorption of Na+ via the amiloride-sensitive epithelial sodium channel (ENaC) generates a lumen-negative potential difference, which drives K+ excretion through the apical secretory K+ channel ROMK (renal outer medullary K+ channel) and/or the flow-dependent BK channel. Transepithelial Cl– transport occurs in adjacent β-intercalated cells, via apical Cl–-HCO3– and Cl–-OH– exchange (SLC26A4 anion exchanger, also known as pendrin) basolateral CLC chloride channels. Water is absorbed down the osmotic gradient by principal cells, through the apical aquaporin-2 (AQP-2) and basolateral aquaporin-3 and aquaporin-4 (Fig. 63-3).
Renal tubular reabsorption of filtered Na+-Cl– is regulated by multiple circulating and paracrine hormones, in addition to the activity of renal nerves. Angiotensin II activates proximal Na+-Cl– reabsorption, as do adrenergic receptors under the influence of renal sympathetic innervation; locally generated dopamine, in contrast, has a natriuretic effect. Aldosterone primarily activates Na+-Cl– reabsorption within the aldosterone-sensitive distal nephron. In particular, aldosterone activates the ENaC channel in principal cells, inducing Na+ absorption and promoting K+ excretion (Fig. 63-4).
Circulatory integrity is critical for the perfusion and function of vital organs. “Underfilling” of the arterial circulation is sensed by ventricular and vascular pressure receptors, resulting in a neurohumoral activation (increased sympathetic tone, activation of the renin-angiotensin-aldosterone axis, and increased circulating AVP) that synergistically increases renal Na+-Cl– reabsorption, vascular resistance, and renal water reabsorption. This occurs in the context of decreased cardiac output, as occurs in hypovolemic states, low-output cardiac failure, decreased oncotic pressure, and/or increased capillary permeability. Alternatively, excessive arterial vasodilation results in relative arterial underfilling, leading to neurohumoral activation in the defense of tissue perfusion. These physiologic responses play important roles in many of the disorders discussed in this chapter. In particular, it is important to appreciate that AVP functions in the defense of circulatory integrity, inducing vasoconstriction, increasing sympathetic nervous system tone, increasing renal retention of both water and Na+-Cl–, and modulating the arterial baroreceptor reflex. Most of these responses involve activation of systemic V1A AVP receptors, but concomitant activation of V2 receptors in the kidney can result in renal water retention and hyponatremia.
HYPOVOLEMIA
Etiology True volume depletion, or hypovolemia, generally refers to a state of combined salt and water loss, leading to contraction of the ECFV. The loss of salt and water may be renal or nonrenal in origin.
RENAL CAUSES Excessive urinary Na+-Cl– and water loss is a feature of several conditions. A high filtered load of endogenous solutes, such as glucose and urea, can impair tubular reabsorption of Na+-Cl– and water, leading to an osmotic diuresis. Exogenous mannitol, often used to decrease intracerebral pressure, is filtered by glomeruli but not reabsorbed by the proximal tubule, thus causing an osmotic diuresis. Pharmacologic diuretics selectively impair Na+-Cl– reabsorption at specific sites along the nephron, leading to increased urinary Na+-Cl– excretion. Other drugs can induce natriuresis as a side effect. For example, acetazolamide can inhibit proximal tubular Na+-Cl– absorption via its inhibition of carbonic anhydrase; other drugs, such as the antibiotics trimethoprim and pentamidine, inhibit distal tubular Na+ reabsorption through the amiloride-sensitive ENaC channel, leading to urinary Na+-Cl– loss. Hereditary defects in renal transport proteins are also associated with reduced reabsorption of filtered Na+-Cl– and/or water. Alternatively, mineralocorticoid deficiency, mineralocorticoid resistance, or inhibition of the mineralocorticoid receptor (MLR) can reduce Na+-Cl– reabsorption by the aldosterone-sensitive distal nephron. Finally, tubulointerstitial injury, as occurs in interstitial nephritis, acute tubular injury, or obstructive uropathy, can reduce distal tubular Na+-Cl– and/or water absorption.
Excessive excretion of free water, i.e., water without electrolytes, can also lead to hypovolemia. However, the effect on ECFV is usually less marked, given that two-thirds of the water volume is lost from the ICF. Excessive renal water excretion occurs in the setting of decreased circulating AVP or renal resistance to AVP (central and nephrogenic diabetes insipidus, respectively).
EXTRARENAL CAUSES Nonrenal causes of hypovolemia include fluid loss from the gastrointestinal tract, skin, and respiratory system. Accumulations of fluid within specific tissue compartments, typically the interstitium, peritoneum, or gastrointestinal tract, can also cause hypovolemia.
Approximately 9 L of fluid enter the gastrointestinal tract daily, 2 L by ingestion and 7 L by secretion; almost 98% of this volume is absorbed, such that daily fecal fluid loss is only 100–200 mL. Impaired gastrointestinal reabsorption or enhanced secretion of fluid can cause hypovolemia. Because gastric secretions have a low pH (high H+ concentration), whereas biliary, pancreatic, and intestinal secretions are alkaline (high HCO3– concentration), vomiting and diarrhea are often accompanied by metabolic alkalosis and acidosis, respectively.
Evaporation of water from the skin and respiratory tract (so-called “insensible losses”) constitutes the major route for loss of solute-free water, which is typically 500–650 mL/d in healthy adults. This evaporative loss can increase during febrile illness or prolonged heat exposure. Hyperventilation can also increase insensible losses via the respiratory tract, particularly in ventilated patients; the humidity of inspired air is another determining factor. In addition, increased exertion and/or ambient temperature will increase insensible losses via sweat, which is hypotonic to plasma. Profuse sweating without adequate repletion of water and Na+-Cl– can thus lead to both hypovolemia and hypertonicity. Alternatively, replacement of these insensible losses with a surfeit of free water, without adequate replacement of electrolytes, may lead to hypovolemic hyponatremia.
Excessive fluid accumulation in interstitial and/or peritoneal spaces can also cause intravascular hypovolemia. Increases in vascular permeability and/or a reduction in oncotic pressure (hypoalbuminemia) alter Starling forces, resulting in excessive “third spacing” of the ECFV. This occurs in sepsis syndrome, burns, pancreatitis, nutritional hypoalbuminemia, and peritonitis. Alternatively, distributive hypovolemia can occur due to accumulation of fluid within specific compartments, for example within the bowel lumen in gastrointestinal obstruction or ileus. Hypovolemia can also occur after extracorporeal hemorrhage or after significant hemorrhage into an expandable space, for example, the retroperitoneum.
Diagnostic Evaluation A careful history will usually determine the etiologic cause of hypovolemia. Symptoms of hypovolemia are nonspecific and include fatigue, weakness, thirst, and postural dizziness; more severe symptoms and signs include oliguria, cyanosis, abdominal and chest pain, and confusion or obtundation. Associated electrolyte disorders may cause additional symptoms, for example, muscle weakness in patients with hypokalemia. On examination, diminished skin turgor and dry oral mucous membranes are less than ideal markers of a decreased ECFV in adult patients; more reliable signs of hypovolemia include a decreased jugular venous pressure (JVP), orthostatic tachycardia (an increase of >15–20 beats/min upon standing), and orthostatic hypotension (a >10–20 mmHg drop in blood pressure on standing). More severe fluid loss leads to hypovolemic shock, with hypotension, tachycardia, peripheral vasoconstriction, and peripheral hypoperfusion; these patients may exhibit peripheral cyanosis, cold extremities, oliguria, and altered mental status.
Routine chemistries may reveal an increase in blood urea nitrogen (BUN) and creatinine, reflective of a decrease in GFR. Creatinine is the more dependable measure of GFR, because BUN levels may be influenced by an increase in tubular reabsorption (“prerenal azotemia”), an increase in urea generation in catabolic states, hyperalimentation, or gastrointestinal bleeding, and/or a decreased urea generation in decreased protein intake. In hypovolemic shock, liver function tests and cardiac biomarkers may show evidence of hepatic and cardiac ischemia, respectively. Routine chemistries and/or blood gases may reveal evidence of acid-base disorders. For example, bicarbonate loss due to diarrheal illness is a very common cause of metabolic acidosis; alternatively, patients with severe hypovolemic shock may develop lactic acidosis with an elevated anion gap.
The neurohumoral response to hypovolemia stimulates an increase in renal tubular Na+ and water reabsorption. Therefore, the urine Na+ concentration is typically <20 mM in nonrenal causes of hypovolemia, with a urine osmolality of >450 mOsm/kg. The reduction in both GFR and distal tubular Na+ delivery may cause a defect in renal potassium excretion, with an increase in plasma K+ concentration. Of note, patients with hypovolemia and a hypochloremic alkalosis due to vomiting, diarrhea, or diuretics will typically have a urine Na+ concentration >20 mM and urine pH of >7.0, due to the increase in filtered HCO3–; the urine Cl– concentration in this setting is a more accurate indicator of volume status, with a level <25 mM suggestive of hypovolemia. The urine Na+ concentration is often >20 mM in patients with renal causes of hypovolemia, such as acute tubular necrosis; similarly, patients with diabetes insipidus will have an inappropriately dilute urine.
SODIUM DISORDERS
Disorders of serum Na+ concentration are caused by abnormalities in water homeostasis, leading to changes in the relative ratio of Na+ to body water. Water intake and circulating AVP constitute the two key effectors in the defense of serum osmolality; defects in one or both of these two defense mechanisms cause most cases of hyponatremia and hypernatremia. In contrast, abnormalities in sodium homeostasis per se lead to a deficit or surplus of whole-body Na+-Cl– content, a key determinant of the ECFV and circulatory integrity. Notably, volume status also modulates the release of AVP by the posterior pituitary, such that hypovolemia is associated with higher circulating levels of the hormone at each level of serum osmolality. Similarly, in “hypervolemic” causes of arterial underfilling, e.g., heart failure and cirrhosis, the associated neurohumoral activation is associated with an increase in circulating AVP, leading to water retention and hyponatremia. Therefore, a key concept in sodium disorders is that the absolute plasma Na+ concentration tells one nothing about the volume status of a given patient, which furthermore must be taken into account in the diagnostic and therapeutic approach.
HYPONATREMIA
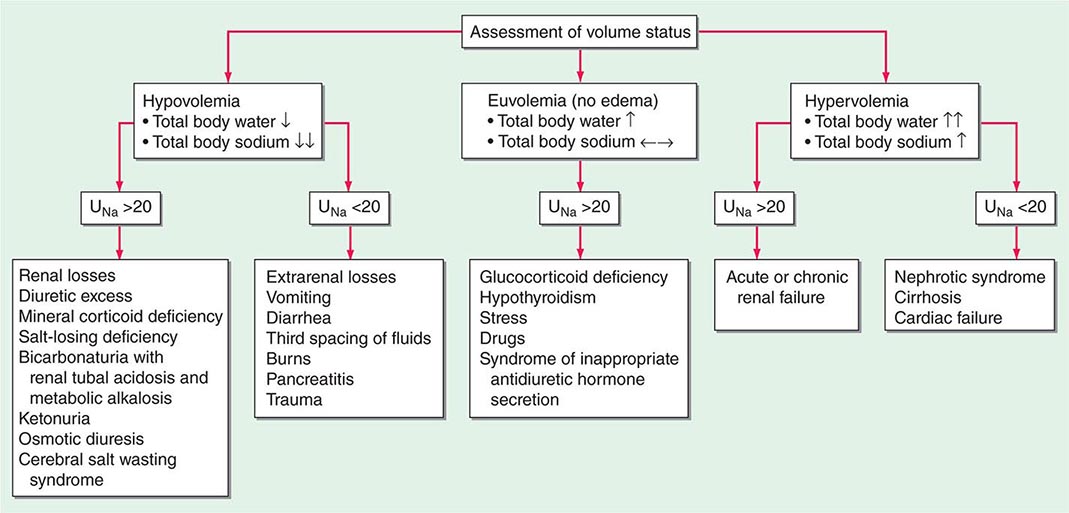
Hyponatremia, which is defined as a plasma Na+ concentration <135 mM, is a very common disorder, occurring in up to 22% of hospitalized patients. This disorder is almost always the result of an increase in circulating AVP and/or increased renal sensitivity to AVP, combined with an intake of free water; a notable exception is hyponatremia due to low solute intake (see below). The underlying pathophysiology for the exaggerated or “inappropriate” AVP response differs in patients with hyponatremia as a function of their ECFV. Hyponatremia is thus subdivided diagnostically into three groups, depending on clinical history and volume status, i.e., “hypovolemic,” “euvolemic,” and “hypervolemic” (Fig. 63-5).
FIGURE 63-5 The diagnostic approach to hyponatremia. (From S Kumar, T Berl: Diseases of water metabolism, in Atlas of Diseases of the Kidney, RW Schrier [ed]. Philadelphia, Current Medicine, Inc, 1999; with permission.)
Hypovolemic Hyponatremia Hypovolemia causes a marked neurohumoral activation, increasing circulating levels of AVP. The increase in circulating AVP helps preserve blood pressure via vascular and baroreceptor V1A receptors and increases water reabsorption via renal V2 receptors; activation of V2 receptors can lead to hyponatremia in the setting of increased free water intake. Nonrenal causes of hypovolemic hyponatremia include GI loss (e.g., vomiting, diarrhea, tube drainage) and insensible loss (sweating, burns) of Na+-Cl– and water, in the absence of adequate oral replacement; urine Na+ concentration is typically <20 mM. Notably, these patients may be clinically classified as euvolemic, with only the reduced urinary Na+ concentration to indicate the cause of their hyponatremia. Indeed, a urine Na+ concentration <20 mM, in the absence of a cause of hypervolemic hyponatremia, predicts a rapid increase in plasma Na+ concentration in response to intravenous normal saline; saline therapy thus induces a water diuresis in this setting, as circulating AVP levels plummet.
The renal causes of hypovolemic hyponatremia share an inappropriate loss of Na+-Cl– in the urine, leading to volume depletion and an increase in circulating AVP; urine Na+ concentration is typically >20 mM (Fig. 63-5). A deficiency in circulating aldosterone and/or its renal effects can lead to hyponatremia in primary adrenal insufficiency and other causes of hypoaldosteronism; hyperkalemia and hyponatremia in a hypotensive and/or hypovolemic patient with high urine Na+ concentration (much greater than 20 mM) should strongly suggest this diagnosis. Salt-losing nephropathies may lead to hyponatremia when sodium intake is reduced, due to impaired renal tubular function; typical causes include reflux nephropathy, interstitial nephropathies, postobstructive uropathy, medullary cystic disease, and the recovery phase of acute tubular necrosis. Thiazide diuretics cause hyponatremia via a number of mechanisms, including polydipsia and diuretic-induced volume depletion. Notably, thiazides do not inhibit the renal concentrating mechanism, such that circulating AVP retains a full effect on renal water retention. In contrast, loop diuretics, which are less frequently associated with hyponatremia, inhibit Na+-Cl– and K+ absorption by the TALH, blunting the countercurrent mechanism and reducing the ability to concentrate the urine. Increased excretion of an osmotically active nonreabsorbable or poorly reabsorbable solute can also lead to volume depletion and hyponatremia; important causes include glycosuria, ketonuria (e.g., in starvation or in diabetic or alcoholic ketoacidosis), and bicarbonaturia (e.g., in renal tubular acidosis or metabolic alkalosis, where the associated bicarbonaturia leads to loss of Na+).
Finally, the syndrome of “cerebral salt wasting” is a rare cause of hypovolemic hyponatremia, encompassing hyponatremia with clinical hypovolemia and inappropriate natriuresis in association with intracranial disease; associated disorders include subarachnoid hemorrhage, traumatic brain injury, craniotomy, encephalitis, and meningitis. Distinction from the more common syndrome of inappropriate antidiuresis is critical because cerebral salt wasting will typically respond to aggressive Na+-Cl– repletion.
Hypervolemic Hyponatremia Patients with hypervolemic hyponatremia develop an increase in total-body Na+-Cl– that is accompanied by a proportionately greater increase in total-body water, leading to a reduced plasma Na+ concentration. As in hypovolemic hyponatremia, the causative disorders can be separated by the effect on urine Na+ concentration, with acute or chronic renal failure uniquely associated with an increase in urine Na+ concentration (Fig. 63-5). The pathophysiology of hyponatremia in the sodium-avid edematous disorders (congestive heart failure [CHF], cirrhosis, and nephrotic syndrome) is similar to that in hypovolemic hyponatremia, except that arterial filling and circulatory integrity is decreased due to the specific etiologic factors (e.g., cardiac dysfunction in CHF, peripheral vasodilation in cirrhosis). Urine Na+ concentration is typically very low, i.e., <10 mM, even after hydration with normal saline; this Na+-avid state may be obscured by diuretic therapy. The degree of hyponatremia provides an indirect index of the associated neurohumoral activation and is an important prognostic indicator in hypervolemic hyponatremia.
Euvolemic Hyponatremia Euvolemic hyponatremia can occur in moderate to severe hypothyroidism, with correction after achieving a euthyroid state. Severe hyponatremia can also be a consequence of secondary adrenal insufficiency due to pituitary disease; whereas the deficit in circulating aldosterone in primary adrenal insufficiency causes hypovolemic hyponatremia, the predominant glucocorticoid deficiency in secondary adrenal failure is associated with euvolemic hyponatremia. Glucocorticoids exert a negative feedback on AVP release by the posterior pituitary such that hydrocortisone replacement in these patients will rapidly normalize the AVP response to osmolality, reducing circulating AVP.
The syndrome of inappropriate antidiuresis (SIAD) is the most frequent cause of euvolemic hyponatremia (Table 63-1). The generation of hyponatremia in SIAD requires an intake of free water, with persistent intake at serum osmolalities that are lower than the usual threshold for thirst; as one would expect, the osmotic threshold and osmotic response curves for the sensation of thirst are shifted downward in patients with SIAD. Four distinct patterns of AVP secretion have been recognized in patients with SIAD, independent for the most part of the underlying cause. Unregulated, erratic AVP secretion is seen in about a third of patients, with no obvious correlation between serum osmolality and circulating AVP levels. Other patients fail to suppress AVP secretion at lower serum osmolalities, with a normal response curve to hyperosmolar conditions; others have a “reset osmostat,” with a lower threshold osmolality and a left-shifted osmotic response curve. Finally, the fourth subset of patients have essentially no detectable circulating AVP, suggesting either a gain in function in renal water reabsorption or a circulating antidiuretic substance that is distinct from AVP. Gain-in-function mutations of a single specific residue in the V2 AVP receptor have been described in some of these patients, leading to constitutive activation of the receptor in the absence of AVP and “nephrogenic” SIAD.
|
CAUSES OF THE SYNDROME OF INAPPROPRIATE ANTIDIURESIS (SIAD) |
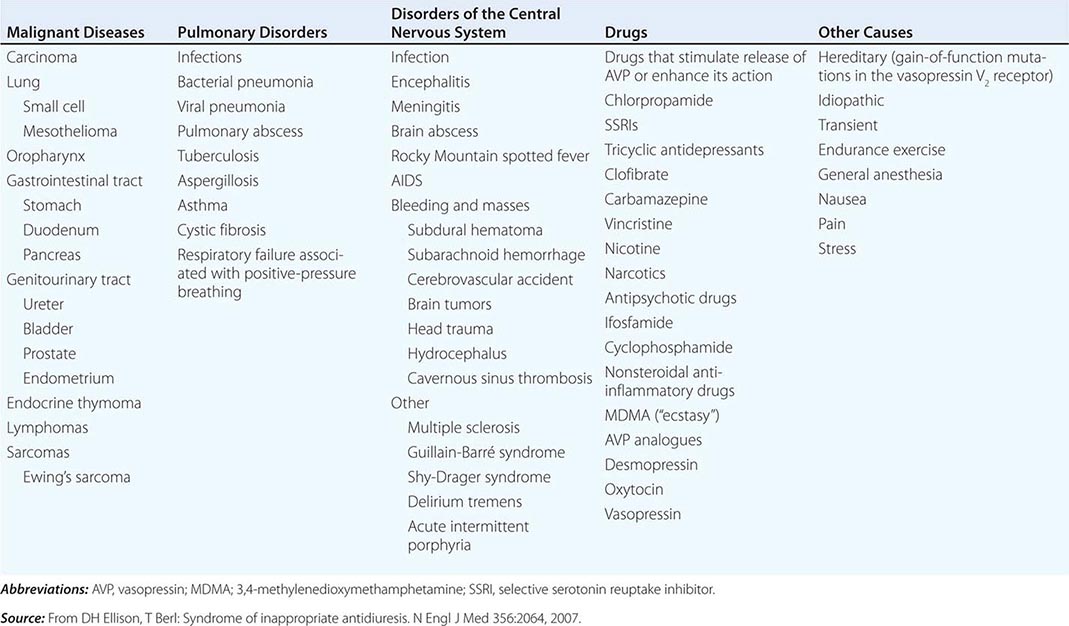
Strictly speaking, patients with SIAD are not euvolemic but are subclinically volume-expanded, due to AVP-induced water and Na+-Cl– retention; “AVP escape” mechanisms invoked by sustained increases in AVP serve to limit distal renal tubular transport, preserving a modestly hypervolemic steady state. Serum uric acid is often low (<4 mg/dL) in patients with SIAD, consistent with suppressed proximal tubular transport in the setting of increased distal tubular Na+-Cl– and water transport; in contrast, patients with hypovolemic hyponatremia will often be hyperuricemic, due to a shared activation of proximal tubular Na+-Cl– and urate transport.
Common causes of SIAD include pulmonary disease (e.g., pneumonia, tuberculosis, pleural effusion) and central nervous system (CNS) diseases (e.g., tumor, subarachnoid hemorrhage, meningitis). SIAD also occurs with malignancies, most commonly with small-cell lung carcinoma (75% of malignancy-associated SIAD); ~10% of patients with this tumor will have a plasma Na+ concentration of <130 mM at presentation. SIAD is also a frequent complication of certain drugs, most commonly the selective serotonin reuptake inhibitors (SSRIs). Other drugs can potentiate the renal effect of AVP, without exerting direct effects on circulating AVP levels (Table 63-1).
Low Solute Intake and Hyponatremia Hyponatremia can occasionally occur in patients with a very low intake of dietary solutes. Classically, this occurs in alcoholics whose sole nutrient is beer, hence the diagnostic label of beer potomania; beer is very low in protein and salt content, containing only 1–2 mM of Na+. The syndrome has also been described in nonalcoholic patients with highly restricted solute intake due to nutrient-restricted diets, e.g., extreme vegetarian diets. Patients with hyponatremia due to low solute intake typically present with a very low urine osmolality (<100–200 mOsm/kg) with a urine Na+ concentration that is <10–20 mM. The fundamental abnormality is the inadequate dietary intake of solutes; the reduced urinary solute excretion limits water excretion such that hyponatremia ensues after relatively modest polydipsia. AVP levels have not been reported in patients with beer potomania but are expected to be suppressed or rapidly suppressible with saline hydration; this fits with the overly rapid correction in plasma Na+ concentration that can be seen with saline hydration. Resumption of a normal diet and/or saline hydration will also correct the causative deficit in urinary solute excretion, such that patients with beer potomania typically correct their plasma Na+ concentration promptly after admission to the hospital.
Clinical Features of Hyponatremia Hyponatremia induces generalized cellular swelling, a consequence of water movement down the osmotic gradient from the hypotonic ECF to the ICF. The symptoms of hyponatremia are primarily neurologic, reflecting the development of cerebral edema within a rigid skull. The initial CNS response to acute hyponatremia is an increase in interstitial pressure, leading to shunting of ECF and solutes from the interstitial space into the cerebrospinal fluid and then on into the systemic circulation. This is accompanied by an efflux of the major intracellular ions, Na+, K+, and Cl–, from brain cells. Acute hyponatremic encephalopathy ensues when these volume regulatory mechanisms are overwhelmed by a rapid decrease in tonicity, resulting in acute cerebral edema. Early symptoms can include nausea, headache, and vomiting. However, severe complications can rapidly evolve, including seizure activity, brainstem herniation, coma, and death. A key complication of acute hyponatremia is normocapneic or hypercapneic respiratory failure; the associated hypoxia may amplify the neurologic injury. Normocapneic respiratory failure in this setting is typically due to noncardiogenic, “neurogenic” pulmonary edema, with a normal pulmonary capillary wedge pressure.
Acute symptomatic hyponatremia is a medical emergency, occurring in a number of specific settings (Table 63-2). Women, particularly before menopause, are much more likely than men to develop encephalopathy and severe neurologic sequelae. Acute hyponatremia often has an iatrogenic component, e.g., when hypotonic intravenous fluids are given to postoperative patients with an increase in circulating AVP. Exercise-associated hyponatremia, an important clinical issue at marathons and other endurance events, has similarly been linked to both a “nonosmotic” increase in circulating AVP and excessive free water intake. The recreational drugs Molly and ecstasy, which share an active ingredient (MDMA, 3,4-methylenedioxymethamphetamine), cause a rapid and potent induction of both thirst and AVP, leading to severe acute hyponatremia.
|
CAUSES OF ACUTE HYPONATREMIA |
Abbreviations: MDMA, 3,4-methylenedioxymethamphetamine; TURP, transurethral resection of the prostate.
Persistent, chronic hyponatremia results in an efflux of organic osmolytes (creatine, betaine, glutamate, myoinositol, and taurine) from brain cells; this response reduces intracellular osmolality and the osmotic gradient favoring water entry. This reduction in intracellular osmolytes is largely complete within 48 h, the time period that clinically defines chronic hyponatremia; this temporal definition has considerable relevance for the treatment of hyponatremia (see below). The cellular response to chronic hyponatremia does not fully protect patients from symptoms, which can include vomiting, nausea, confusion, and seizures, usually at plasma Na+ concentration <125 mM. Even patients who are judged “asymptomatic” can manifest subtle gait and cognitive defects that reverse with correction of hyponatremia; notably, chronic “asymptomatic” hyponatremia increases the risk of falls. Chronic hyponatremia also increases the risk of bony fractures owing to the associated neurologic dysfunction and to a hyponatremia-associated reduction in bone density. Therefore, every attempt should be made to correct safely the plasma Na+ concentration in patients with chronic hyponatremia, even in the absence of overt symptoms (see the section on treatment of hyponatremia below).
The management of chronic hyponatremia is complicated significantly by the asymmetry of the cellular response to correction of plasma Na+ concentration. Specifically, the reaccumulation of organic osmolytes by brain cells is attenuated and delayed as osmolality increases after correction of hyponatremia, sometimes resulting in degenerative loss of oligodendrocytes and an osmotic demyelination syndrome (ODS). Overly rapid correction of hyponatremia (>8–10 mM in 24 h or 18 mM in 48 h) is also associated with a disruption in integrity of the blood-brain barrier, allowing the entry of immune mediators that may contribute to demyelination. The lesions of ODS classically affect the pons, a structure wherein the delay in the reaccumulation of osmotic osmolytes is particularly pronounced; clinically, patients with central pontine myelinolysis can present 1 or more days after overcorrection of hyponatremia with paraparesis or quadriparesis, dysphagia, dysarthria, diplopia, a “locked-in syndrome,” and/or loss of consciousness. Other regions of the brain can also be involved in ODS, most commonly in association with lesions of the pons but occasionally in isolation; in order of frequency, the lesions of extrapontine myelinolysis can occur in the cerebellum, lateral geniculate body, thalamus, putamen, and cerebral cortex or subcortex. Clinical presentation of ODS can, therefore, vary as a function of the extent and localization of extrapontine myelinolysis, with the reported development of ataxia, mutism, parkinsonism, dystonia, and catatonia. Relowering of plasma Na+ concentration after overly rapid correction can prevent or attenuate ODS (see the section on treatment of hyponatremia below). However, even appropriately slow correction can be associated with ODS, particularly in patients with additional risk factors; these include alcoholism, malnutrition, hypokalemia, and liver transplantation.
Diagnostic Evaluation of Hyponatremia Clinical assessment of hyponatremic patients should focus on the underlying cause; a detailed drug history is particularly crucial (Table 63-1). A careful clinical assessment of volume status is obligatory for the classical diagnostic approach to hyponatremia (Fig. 63-5). Hyponatremia is frequently multifactorial, particularly when severe; clinical evaluation should consider all the possible causes for excessive circulating AVP, including volume status, drugs, and the presence of nausea and/or pain. Radiologic imaging may also be appropriate to assess whether patients have a pulmonary or CNS cause for hyponatremia. A screening chest x-ray may fail to detect a small-cell carcinoma of the lung; computed tomography (CT) scanning of the thorax should be considered in patients at high risk for this tumor (e.g., patients with a smoking history).
Laboratory investigation should include a measurement of serum osmolality to exclude pseudohyponatremia, which is defined as the coexistence of hyponatremia with a normal or increased plasma tonicity. Most clinical laboratories measure plasma Na+ concentration by testing diluted samples with automated ion-sensitive electrodes, correcting for this dilution by assuming that plasma is 93% water. This correction factor can be inaccurate in patients with pseudohyponatremia due to extreme hyperlipidemia and/or hyperproteinemia, in whom serum lipid or protein makes up a greater percentage of plasma volume. The measured osmolality should also be converted to the effective osmolality (tonicity) by subtracting the measured concentration of urea (divided by 2.8, if in mg/dL); patients with hyponatremia have an effective osmolality of <275 mOsm/kg.
Elevated BUN and creatinine in routine chemistries can also indicate renal dysfunction as a potential cause of hyponatremia, whereas hyperkalemia may suggest adrenal insufficiency or hypoaldosteronism. Serum glucose should also be measured; plasma Na+ concentration falls by ~1.6–2.4 mM for every 100-mg/dL increase in glucose, due to glucose-induced water efflux from cells; this “true” hyponatremia resolves after correction of hyperglycemia. Measurement of serum uric acid should also be performed; whereas patients with SIAD-type physiology will typically be hypouricemic (serum uric acid <4 mg/dL), volume-depleted patients will often be hyperuricemic. In the appropriate clinical setting, thyroid, adrenal, and pituitary function should also be tested; hypothyroidism and secondary adrenal failure due to pituitary insufficiency are important causes of euvolemic hyponatremia, whereas primary adrenal failure causes hypovolemic hyponatremia. A cosyntropin stimulation test is necessary to assess for primary adrenal insufficiency.
Urine electrolytes and osmolality are crucial tests in the initial evaluation of hyponatremia. A urine Na+ concentration <20–30 mM is consistent with hypovolemic hyponatremia, in the clinical absence of a hypervolemic, Na+-avid syndrome such as CHF (Fig. 63-5). In contrast, patients with SIAD will typically excrete urine with an Na+ concentration that is >30 mM. However, there can be substantial overlap in urine Na+ concentration values in patients with SIAD and hypovolemic hyponatremia, particularly in the elderly; the ultimate “gold standard” for the diagnosis of hypovolemic hyponatremia is the demonstration that plasma Na+ concentration corrects after hydration with normal saline. Patients with thiazide-associated hyponatremia may also present with higher than expected urine Na+ concentration and other findings suggestive of SIAD; one should defer making a diagnosis of SIAD in these patients until 1–2 weeks after discontinuing the thiazide. A urine osmolality <100 mOsm/kg is suggestive of polydipsia; urine osmolality >400 mOsm/kg indicates that AVP excess is playing a more dominant role, whereas intermediate values are more consistent with multifactorial pathophysiology (e.g., AVP excess with a significant component of polydipsia). Patients with hyponatremia due to decreased solute intake (beer potomania) typically have urine Na+ concentration <20 mM and urine osmolality in the range of <100 to the low 200s. Finally, the measurement of urine K+ concentration is required to calculate the urine-to-plasma electrolyte ratio, which is useful to predict the response to fluid restriction (see the section on treatment of hyponatremia below).
HYPERNATREMIA
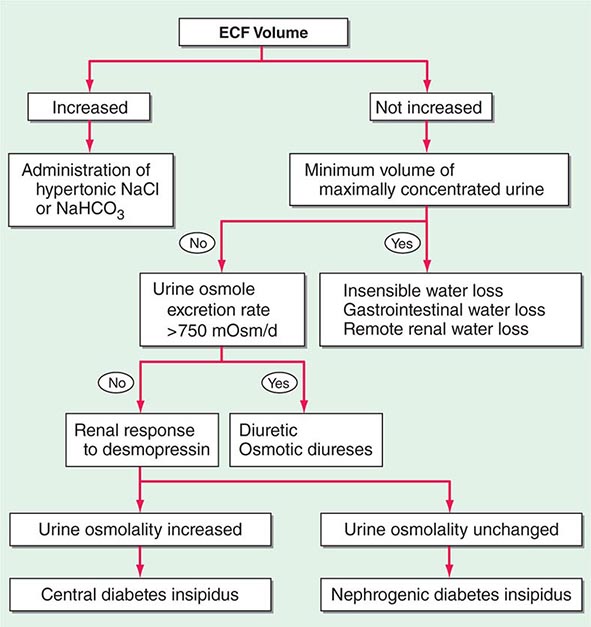
Etiology Hypernatremia is defined as an increase in the plasma Na+ concentration to >145 mM. Considerably less common than hyponatremia, hypernatremia is nonetheless associated with mortality rates of as high as 40–60%, mostly due to the severity of the associated underlying disease processes. Hypernatremia is usually the result of a combined water and electrolyte deficit, with losses of H2O in excess of Na+. Less frequently, the ingestion or iatrogenic administration of excess Na+ can be causative, for example after IV administration of excessive hypertonic Na+-Cl– or Na+-HCO3– (Fig. 63-6).
FIGURE 63-6 The diagnostic approach to hypernatremia. ECF, extracellular fluid.
Elderly individuals with reduced thirst and/or diminished access to fluids are at the highest risk of developing hypernatremia. Patients with hypernatremia may rarely have a central defect in hypothalamic osmoreceptor function, with a mixture of both decreased thirst and reduced AVP secretion. Causes of this adipsic diabetes insipidus include primary or metastatic tumor, occlusion or ligation of the anterior communicating artery, trauma, hydrocephalus, and inflammation.
Hypernatremia can develop following the loss of water via both renal and nonrenal routes. Insensible losses of water may increase in the setting of fever, exercise, heat exposure, severe burns, or mechanical ventilation. Diarrhea is, in turn, the most common gastrointestinal cause of hypernatremia. Notably, osmotic diarrhea and viral gastroenteritides typically generate stools with Na+ and K+ <100 mM, thus leading to water loss and hypernatremia; in contrast, secretory diarrhea typically results in isotonic stool and thus hypovolemia with or without hypovolemic hyponatremia.
Common causes of renal water loss include osmotic diuresis secondary to hyperglycemia, excess urea, postobstructive diuresis, or mannitol; these disorders share an increase in urinary solute excretion and urinary osmolality (see “Diagnostic Approach,” below). Hypernatremia due to a water diuresis occurs in central or nephrogenic diabetes insipidus (DI).
Nephrogenic DI (NDI) is characterized by renal resistance to AVP, which can be partial or complete (see “Diagnostic Approach,” below). Genetic causes include loss-of-function mutations in the X-linked V2 receptor; mutations in the AVP-responsive aquaporin-2 water channel can cause autosomal recessive and autosomal dominant NDI, whereas recessive deficiency of the aquaporin-1 water channel causes a more modest concentrating defect (Fig. 63-2). Hypercalcemia can also cause polyuria and NDI; calcium signals directly through the calcium-sensing receptor to downregulate Na+, K+, and Cl– transport by the TALH and water transport in principal cells, thus reducing renal concentrating ability in hypercalcemia. Another common acquired cause of NDI is hypokalemia, which inhibits the renal response to AVP and downregulates aquaporin-2 expression. Several drugs can cause acquired NDI, in particular lithium, ifosfamide, and several antiviral agents. Lithium causes NDI by multiple mechanisms, including direct inhibition of renal glycogen synthase kinase-3 (GSK3), a kinase thought to be the pharmacologic target of lithium in bipolar disease; GSK3 is required for the response of principal cells to AVP. The entry of lithium through the amiloride-sensitive Na+ channel ENaC (Fig. 63-4) is required for the effect of the drug on principal cells, such that combined therapy within lithium and amiloride can mitigate lithium-associated NDI. However, lithium causes chronic tubulointerstitial scarring and chronic kidney disease after prolonged therapy, such that patients may have a persistent NDI long after stopping the drug, with a reduced therapeutic benefit from amiloride.
Finally, gestational DI is a rare complication of late-term pregnancy wherein increased activity of a circulating placental protease with “vasopressinase” activity leads to reduced circulating AVP and polyuria, often accompanied by hypernatremia. DDAVP is an effective therapy for this syndrome, given its resistance to the vasopressinase enzyme.
Clinical Features Hypernatremia increases osmolality of the ECF, generating an osmotic gradient between the ECF and ICF, an efflux of intracellular water, and cellular shrinkage. As in hyponatremia, the symptoms of hypernatremia are predominantly neurologic. Altered mental status is the most frequent manifestation, ranging from mild confusion and lethargy to deep coma. The sudden shrinkage of brain cells in acute hypernatremia may lead to parenchymal or subarachnoid hemorrhages and/or subdural hematomas; however, these vascular complications are primarily encountered in pediatric and neonatal patients. Osmotic damage to muscle membranes can also lead to hypernatremic rhabdomyolysis. Brain cells accommodate to a chronic increase in ECF osmolality (>48 h) by activating membrane transporters that mediate influx and intracellular accumulation of organic osmolytes (creatine, betaine, glutamate, myoinositol, and taurine); this results in an increase in ICF water and normalization of brain parenchymal volume. In consequence, patients with chronic hypernatremia are less likely to develop severe neurologic compromise. However, the cellular response to chronic hypernatremia predisposes these patients to the development of cerebral edema and seizures during overly rapid hydration (overcorrection of plasma Na+ concentration by >10 mM/d).
Diagnostic Approach The history should focus on the presence or absence of thirst, polyuria, and/or an extrarenal source for water loss, such as diarrhea. The physical examination should include a detailed neurologic exam and an assessment of the ECFV; patients with a particularly large water deficit and/or a combined deficit in electrolytes and water may be hypovolemic, with reduced JVP and orthostasis. Accurate documentation of daily fluid intake and daily urine output is also critical for the diagnosis and management of hypernatremia.
Laboratory investigation should include a measurement of serum and urine osmolality, in addition to urine electrolytes. The appropriate response to hypernatremia and a serum osmolality >295 mOsm/kg is an increase in circulating AVP and the excretion of low volumes (<500 mL/d) of maximally concentrated urine, i.e., urine with osmolality >800 mOsm/kg; should this be the case, then an extrarenal source of water loss is primarily responsible for the generation of hypernatremia. Many patients with hypernatremia are polyuric; should an osmotic diuresis be responsible, with excessive excretion of Na+-Cl–, glucose, and/or urea, then daily solute excretion will be >750–1000 mOsm/d (>15 mOsm/kg body water per day) (Fig. 63-6). More commonly, patients with hypernatremia and polyuria will have a predominant water diuresis, with excessive excretion of hypotonic, dilute urine.
Adequate differentiation between nephrogenic and central causes of DI requires the measurement of the response in urinary osmolality to DDAVP, combined with measurement of circulating AVP in the setting of hypertonicity. By definition, patients with baseline hypernatremia are hypertonic, with an adequate stimulus for AVP by the posterior pituitary. Therefore, in contrast to polyuric patients with a normal or reduced baseline plasma Na+ concentration and osmolality, a water deprivation test (Chap. 61) is unnecessary in hypernatremia; indeed, water deprivation is absolutely contraindicated in this setting, given the risk for worsening the hypernatremia. Patients with NDI will fail to respond to DDAVP, with a urine osmolality that increases by <50% or <150 mOsm/kg from baseline, in combination with a normal or high circulating AVP level; patients with central DI will respond to DDAVP, with a reduced circulating AVP. Patients may exhibit a partial response to DDAVP, with a >50% rise in urine osmolality that nonetheless fails to reach 800 mOsm/kg; the level of circulating AVP will help differentiate the underlying cause, i.e., NDI versus central DI. In pregnant patients, AVP assays should be drawn in tubes containing the protease inhibitor 1,10-phenanthroline, to prevent in vitro degradation of AVP by placental vasopressinase.
For patients with hypernatremia due to renal loss of water, it is critical to quantify ongoing daily losses using the calculated electrolyte-free water clearance, in addition to calculation of the baseline water deficit (the relevant formulas are discussed in Table 63-3). This requires daily measurement of urine electrolytes, combined with accurate measurement of daily urine volume.
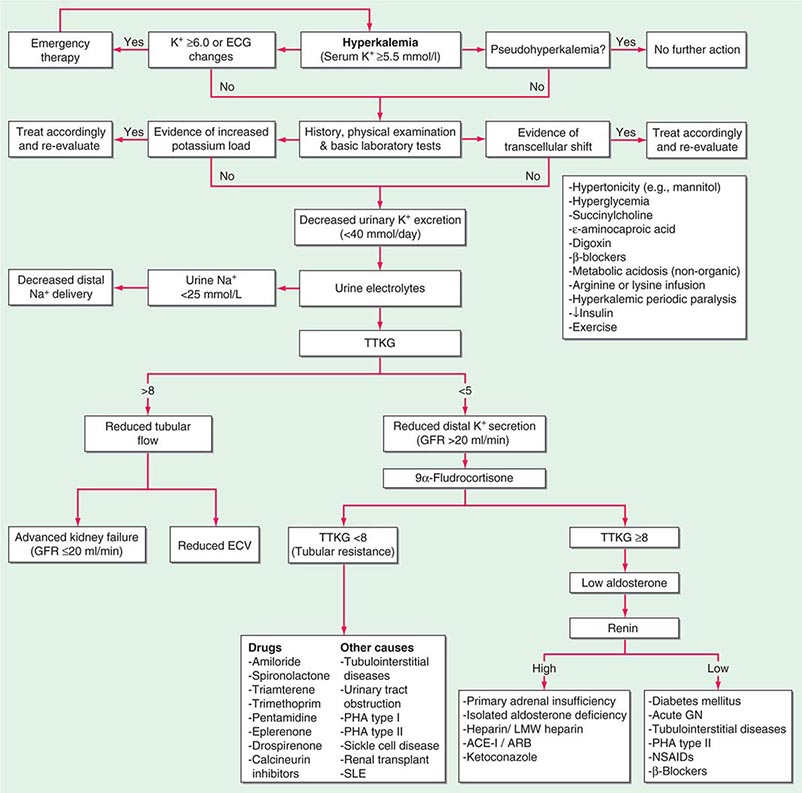
POTASSIUM DISORDERS
Homeostatic mechanisms maintain plasma K+ concentration between 3.5 and 5.0 mM, despite marked variation in dietary K+ intake. In a healthy individual at steady state, the entire daily intake of potassium is excreted, approximately 90% in the urine and 10% in the stool; thus, the kidney plays a dominant role in potassium homeostasis. However, more than 98% of total-body potassium is intracellular, chiefly in muscle; buffering of extracellular K+ by this large intracellular pool plays a crucial role in the regulation of plasma K+ concentration. Changes in the exchange and distribution of intra- and extracellular K+ can thus lead to marked hypo- or hyperkalemia. A corollary is that massive necrosis and the attendant release of tissue K+ can cause severe hyperkalemia, particularly in the setting of acute kidney injury and reduced excretion of K+.
Changes in whole-body K+ content are primarily mediated by the kidney, which reabsorbs filtered K+ in hypokalemic, K+-deficient states and secretes K+ in hyperkalemic, K+-replete states. Although K+ is transported along the entire nephron, it is the principal cells of the connecting segment (CNT) and cortical CD that play a dominant role in renal K+ secretion, whereas alpha-intercalated cells of the outer medullary CD function in renal tubular reabsorption of filtered K+ in K+-deficient states. In principal cells, apical Na+ entry via the amiloride-sensitive ENaC generates a lumen-negative potential difference, which drives passive K+ exit through apical K+ channels (Fig. 63-4). Two major K+ channels mediate distal tubular K+ secretion: the secretory K+ channel ROMK (renal outer medullary K+ channel; also known as Kir1.1 or KcnJ1) and the flow-sensitive big potassium (BK) or maxi-K K+ channel. ROMK is thought to mediate the bulk of constitutive K+ secretion, whereas increases in distal flow rate and/or genetic absence of ROMK activate K+ secretion via the BK channel.
An appreciation of the relationship between ENaC-dependent Na+ entry and distal K+ secretion (Fig. 63-4) is required for the bedside interpretation of potassium disorders. For example, decreased distal delivery of Na+, as occurs in hypovolemic, prerenal states, tends to blunt the ability to excrete K+, leading to hyperkalemia; on the other hand, an increase in distal delivery of Na+ and distal flow rate, as occurs after treatment with thiazide and loop diuretics, can enhance K+ secretion and lead to hypokalemia. Hyperkalemia is also a predictable consequence of drugs that directly inhibit ENaC, due to the role of this Na+ channel in generating a lumen-negative potential difference. Aldosterone in turn has a major influence on potassium excretion, increasing the activity of ENaC channels and thus amplifying the driving force for K+ secretion across the luminal membrane of principal cells. Abnormalities in the renin-angiotensin-aldosterone system can thus cause both hypokalemia and hyperkalemia. Notably, however, potassium excess and potassium restriction have opposing, aldosterone-independent effects on the density and activity of apical K+ channels in the distal nephron, i.e., factors other than aldosterone modulate the renal capacity to secrete K+. In addition, potassium restriction and hypokalemia activates aldosterone-independent distal reabsorption of filtered K+, activating apical H+/K+-ATPase activity in intercalated cells within the outer medullary CD. Reflective perhaps of this physiology, changes in plasma K+ concentration are not universal in disorders associated with changes in aldosterone activity.
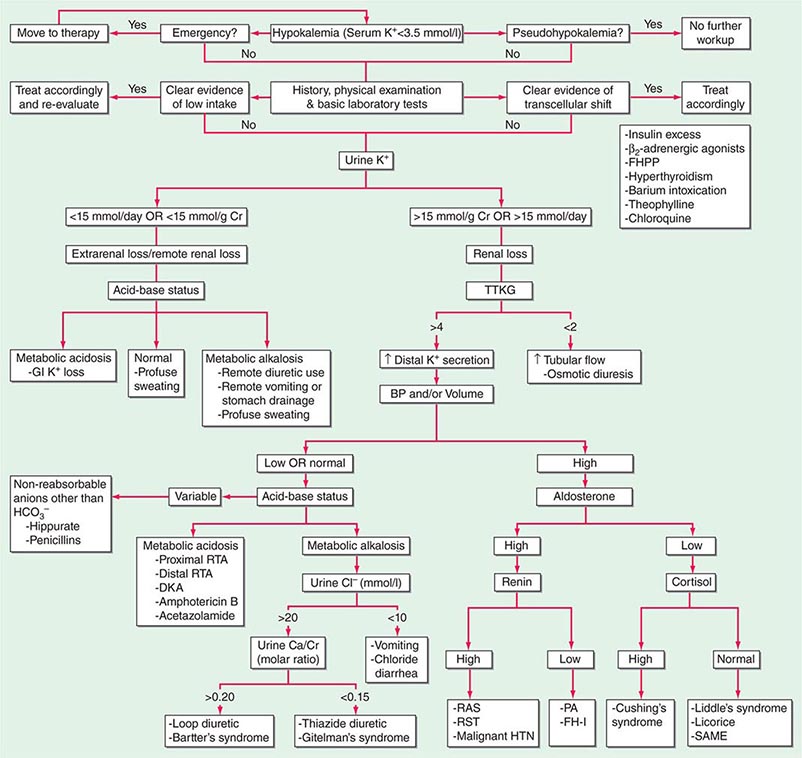
HYPOKALEMIA
Hypokalemia, defined as a plasma K+ concentration of <3.5 mM, occurs in up to 20% of hospitalized patients. Hypokalemia is associated with a 10-fold increase in in-hospital mortality, due to adverse effects on cardiac rhythm, blood pressure, and cardiovascular morbidity. Mechanistically, hypokalemia can be caused by redistribution of K+ between tissues and the ECF or by renal and nonrenal loss of K+ (Table 63-4). Systemic hypomagnesemia can also cause treatment-resistant hypokalemia, due to a combination of reduced cellular uptake of K+ and exaggerated renal secretion. Spurious hypokalemia or “pseudohypokalemia” can occasionally result from in vitro cellular uptake of K+ after venipuncture, for example, due to profound leukocytosis in acute leukemia.
|
CAUSES OF HYPOKALEMIA |
Redistribution and Hypokalemia Insulin, β2-adrenergic activity, thyroid hormone, and alkalosis promote Na+/K+-ATPase-mediated cellular uptake of K+, leading to hypokalemia. Inhibition of the passive efflux of K+ can also cause hypokalemia, albeit rarely; this typically occurs in the setting of systemic inhibition of K+ channels by toxic barium ions. Exogenous insulin can cause iatrogenic hypokalemia, particularly during the management of K+-deficient states such as diabetic ketoacidosis. Alternatively, the stimulation of endogenous insulin can provoke hypokalemia, hypomagnesemia, and/or hypophosphatemia in malnourished patients given a carbohydrate load. Alterations in the activity of the endogenous sympathetic nervous system can cause hypokalemia in several settings, including alcohol withdrawal, hyperthyroidism, acute myocardial infarction, and severe head injury. β2 agonists, including both bronchodilators and tocolytics (ritodrine), are powerful activators of cellular K+ uptake; “hidden” sympathomimetics, such as pseudoephedrine and ephedrine in cough syrup or dieting agents, may also cause unexpected hypokalemia. Finally, xanthine-dependent activation of cAMP-dependent signaling, downstream of the β2 receptor, can lead to hypokalemia, usually in the setting of overdose (theophylline) or marked overingestion (dietary caffeine).
Redistributive hypokalemia can also occur in the setting of hyperthyroidism, with periodic attacks of hypokalemic paralysis (thyrotoxic periodic paralysis [TPP]). Similar episodes of hypokalemic weakness in the absence of thyroid abnormalities occur in familial hypokalemic periodic paralysis, usually caused by missense mutations of voltage sensor domains within the α1 subunit of L-type calcium channels or the skeletal Na+ channel; these mutations generate an abnormal gating pore current activated by hyperpolarization. TPP develops more frequently in patients of Asian or Hispanic origin; this shared predisposition has been linked to genetic variation in Kir2.6, a muscle-specific, thyroid hormone–responsive K+ channel. Patients with TPP typically present with weakness of the extremities and limb girdles, with paralytic episodes that occur most frequently between 1 and 6 AM. Signs and symptoms of hyperthyroidism are not invariably present. Hypokalemia is usually profound and almost invariably accompanied by hypophosphatemia and hypomagnesemia. The hypokalemia in TPP is attributed to both direct and indirect activation of the Na+/K+-ATPase, resulting in increased uptake of K+ by muscle and other tissues. Increases in β-adrenergic activity play an important role in that high-dose propranolol (3 mg/kg) rapidly reverses the associated hypokalemia, hypophosphatemia, and paralysis.
Nonrenal Loss of Potassium The loss of K+ in sweat is typically low, except under extremes of physical exertion. Direct gastric losses of K+ due to vomiting or nasogastric suctioning are also minimal; however, the ensuing hypochloremic alkalosis results in persistent kaliuresis due to secondary hyperaldosteronism and bicarbonaturia, i.e., a renal loss of K+. Diarrhea is a globally important cause of hypokalemia, given the worldwide prevalence of infectious diarrheal disease. Noninfectious gastrointestinal processes such as celiac disease, ileostomy, villous adenomas, inflammatory bowel disease, colonic pseudo-obstruction (Ogilvie’s syndrome), VIPomas, and chronic laxative abuse can also cause significant hypokalemia; an exaggerated intestinal secretion of potassium by upregulated colonic BK channels has been directly implicated in the pathogenesis of hypokalemia in many of these disorders.
Renal Loss of Potassium Drugs can increase renal K+ excretion by a variety of different mechanisms. Diuretics are a particularly common cause, due to associated increases in distal tubular Na+ delivery and distal tubular flow rate, in addition to secondary hyperaldosteronism. Thiazides have a greater effect on plasma K+ concentration than loop diuretics, despite their lesser natriuretic effect. The diuretic effect of thiazides is largely due to inhibition of the Na+-Cl– cotransporter NCC in DCT cells. This leads to a direct increase in the delivery of luminal Na+ to the principal cells immediately downstream in the CNT and cortical CD, which augments Na+ entry via ENaC, increases the lumen-negative potential difference, and amplifies K+ secretion. The higher propensity of thiazides to cause hypokalemia may also be secondary to thiazide-associated hypocalciuria, versus the hypercalciuria seen with loop diuretics; the increases in downstream luminal calcium in response to loop diuretics inhibit ENaC in principal cells, thus reducing the lumen-negative potential difference and attenuating distal K+ excretion. High doses of penicillin-related antibiotics (nafcillin, dicloxacillin, ticarcillin, oxacillin, and carbenicillin) can increase obligatory K+ excretion by acting as nonreabsorbable anions in the distal nephron. Finally, several renal tubular toxins cause renal K+ and magnesium wasting, leading to hypokalemia and hypomagnesemia; these drugs include aminoglycosides, amphotericin, foscarnet, cisplatin, and ifosfamide (see also “Magnesium Deficiency and Hypokalemia,” below).
Aldosterone activates the ENaC channel in principal cells via multiple synergistic mechanisms, thus increasing the driving force for K+ excretion. In consequence, increases in aldosterone bioactivity and/or gains in function of aldosterone-dependent signaling pathways are associated with hypokalemia. Increases in circulating aldosterone (hyperaldosteronism) may be primary or secondary. Increased levels of circulating renin in secondary forms of hyperaldosteronism lead to increased angiotensin II and thus aldosterone; renal artery stenosis is perhaps the most frequent cause (Table 63-4). Primary hyperaldosteronism may be genetic or acquired. Hypertension and hypokalemia, due to increases in circulating 11-deoxycorticosterone, occur in patients with congenital adrenal hyperplasia caused by defects in either steroid 11β-hydroxylase or steroid 17α-hydroxylase; deficient 11β-hydroxylase results in associated virilization and other signs of androgen excess, whereas reduced sex steroids in 17α-hydroxylase deficiency lead to hypogonadism.
The major forms of isolated primary genetic hyperaldosteronism are familial hyperaldosteronism type I (FH-I, also known as glucocorticoid-remediable hyperaldosteronism [GRA]) and familial hyperaldosteronism types II and III (FH-II and FH-III), in which aldosterone production is not repressible by exogenous glucocorticoids. FH-I is caused by a chimeric gene duplication between the homologous 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) genes, fusing the adrenocorticotropic hormone (ACTH)–responsive 11β-hydroxylase promoter to the coding region of aldosterone synthase; this chimeric gene is under the control of ACTH and thus repressible by glucocorticoids. FH-III is caused by mutations in the KCNJ5 gene, which encodes the G-protein-activated inward rectifier K+ channel 4 (GIRK4); these mutations lead to the acquisition of sodium permeability in the mutant GIRK4 channels, causing an exaggerated membrane depolarization in adrenal glomerulosa cells and the activation of voltage-gated calcium channels. The resulting calcium influx is sufficient to produce aldosterone secretion and cell proliferation, leading to adrenal adenomas and hyperaldosteronism.
Acquired causes of primary hyperaldosteronism include aldosterone-producing adenomas (APAs), primary or unilateral adrenal hyperplasia (PAH), idiopathic hyperaldosteronism (IHA) due to bilateral adrenal hyperplasia, and adrenal carcinoma; APA and IHA account for close to 60% and 40%, respectively, of diagnosed hyperaldosteronism. Acquired somatic mutations in KCNJ5 or less frequently in the ATP1A1 (an Na+/K+ ATPase α subunit) and ATP2B3 (a Ca2+ ATPase) genes can be detected in APAs; as in FH-III (see above), the exaggerated depolarization of adrenal glomerulosa cells caused by these mutations is implicated in the excessive adrenal proliferation and the exaggerated release of aldosterone.
Random testing of plasma renin activity (PRA) and aldosterone is a helpful screening tool in hypokalemic and/or hypertensive patients, with an aldosterone:PRA ratio of >50 suggestive of primary hyperaldosteronism. Hypokalemia and multiple antihypertensive drugs may alter the aldosterone:PRA ratio by suppressing aldosterone or increasing PRA, leading to a ratio of <50 in patients who do in fact have primary hyperaldosteronism; therefore, the clinical context should always be considered when interpreting these results.
The glucocorticoid cortisol has equal affinity for the mineralocorticoid receptor (MLR) to that of aldosterone, with resultant “mineralocorticoid-like” activity. However, cells in the aldosterone-sensitive distal nephron are protected from this “illicit” activation by the enzyme 11β-hydroxysteroid dehydrogenase-2 (11βHSD-2), which converts cortisol to cortisone; cortisone has minimal affinity for the MLR. Recessive loss-of-function mutations in the 11βHSD-2 gene are thus associated with cortisol-dependent activation of the MLR and the syndrome of apparent mineralocorticoid excess (SAME), encompassing hypertension, hypokalemia, hypercalciuria, and metabolic alkalosis, with suppressed PRA and suppressed aldosterone. A similar syndrome is caused by biochemical inhibition of 11βHSD-2 by glycyrrhetinic/glycyrrhizinic acid and/or carbenoxolone. Glycyrrhizinic acid is a natural sweetener found in licorice root, typically encountered in licorice and its many guises or as a flavoring agent in tobacco and food products.
Finally, hypokalemia may also occur with systemic increases in glucocorticoids. In Cushing’s syndrome caused by increases in pituitary ACTH (Chap. 406), the incidence of hypokalemia is only 10%, whereas it is 60–100% in patients with ectopic secretion of ACTH, despite a similar incidence of hypertension. Indirect evidence suggests that the activity of renal 11βHSD-2 is reduced in patients with ectopic ACTH compared with Cushing’s syndrome, resulting in SAME.
Finally, defects in multiple renal tubular transport pathways are associated with hypokalemia. For example, loss-of-function mutations in subunits of the acidifying H+-ATPase in alpha-intercalated cells cause hypokalemic distal renal tubular acidosis, as do many acquired disorders of the distal nephron. Liddle’s syndrome is caused by autosomal dominant gain-in-function mutations of ENaC subunits. Disease-associated mutations either activate the channel directly or abrogate aldosterone-inhibited retrieval of ENaC subunits from the plasma membrane; the end result is increased expression of activated ENaC channels at the plasma membrane of principal cells. Patients with Liddle’s syndrome classically manifest severe hypertension with hypokalemia, unresponsive to spironolactone yet sensitive to amiloride. Hypertension and hypokalemia are, however, variable aspects of the Liddle’s phenotype; more consistent features include a blunted aldosterone response to ACTH and reduced urinary aldosterone excretion.
Loss of the transport functions of the TALH and DCT nephron segments causes hereditary hypokalemic alkalosis, Bartter’s syndrome (BS) and Gitelman’s syndrome (GS), respectively. Patients with classic BS typically suffer from polyuria and polydipsia, due to the reduction in renal concentrating ability. They may have an increase in urinary calcium excretion, and 20% are hypomagnesemic. Other features include marked activation of the renin-angiotensin-aldosterone axis. Patients with antenatal BS suffer from a severe systemic disorder characterized by marked electrolyte wasting, polyhydramnios, and hypercalciuria with nephrocalcinosis; renal prostaglandin synthesis and excretion are significantly increased, accounting for much of the systemic symptoms. There are five disease genes for BS, all of them functioning in some aspect of regulated Na+, K+, and Cl– transport by the TALH. In contrast, GS is genetically homogeneous, caused almost exclusively by loss-of-function mutations in the thiazide-sensitive Na+-Cl– cotransporter of the DCT. Patients with GS are uniformly hypomagnesemic and exhibit marked hypocalciuria, rather than the hypercalciuria typically seen in BS; urinary calcium excretion is thus a critical diagnostic test in GS. GS is a milder phenotype than BS; however, patients with GS may suffer from chondrocalcinosis, an abnormal deposition of calcium pyrophosphate dihydrate (CPPD) in joint cartilage (Chap. 339).
Magnesium Deficiency and Hypokalemia Magnesium depletion has inhibitory effects on muscle Na+/K+-ATPase activity, reducing influx into muscle cells and causing a secondary kaliuresis. In addition, magnesium depletion causes exaggerated K+ secretion by the distal nephron; this effect is attributed to a reduction in the magnesium-dependent, intracellular block of K+ efflux through the secretory K+ channel of principal cells (ROMK; Fig. 63-4). In consequence, hypomagnesemic patients are clinically refractory to K+ replacement in the absence of Mg2+ repletion. Notably, magnesium deficiency is also a common concomitant of hypokalemia because many disorders of the distal nephron may cause both potassium and magnesium wasting (Chap. 339).
Clinical Features Hypokalemia has prominent effects on cardiac, skeletal, and intestinal muscle cells. In particular, hypokalemia is a major risk factor for both ventricular and atrial arrhythmias. Hypokalemia predisposes to digoxin toxicity by a number of mechanisms, including reduced competition between K+ and digoxin for shared binding sites on cardiac Na+/K+-ATPase subunits. Electrocardiographic changes in hypokalemia include broad flat T waves, ST depression, and QT prolongation; these are most marked when serum K+ is <2.7 mmol/L. Hypokalemia can thus be an important precipitant of arrhythmia in patients with additional genetic or acquired causes of QT prolongation. Hypokalemia also results in hyperpolarization of skeletal muscle, thus impairing the capacity to depolarize and contract; weakness and even paralysis may ensue. It also causes a skeletal myopathy and predisposes to rhabdomyolysis. Finally, the paralytic effects of hypokalemia on intestinal smooth muscle may cause intestinal ileus.
The functional effects of hypokalemia on the kidney can include Na+-Cl– and HCO3– retention, polyuria, phosphaturia, hypocitraturia, and an activation of renal ammoniagenesis. Bicarbonate retention and other acid-base effects of hypokalemia can contribute to the generation of metabolic alkalosis. Hypokalemic polyuria is due to a combination of central polydipsia and an AVP-resistant renal concentrating defect. Structural changes in the kidney due to hypokalemia include a relatively specific vacuolizing injury to proximal tubular cells, interstitial nephritis, and renal cysts. Hypokalemia also predisposes to acute kidney injury and can lead to end-stage renal disease in patients with long-standing hypokalemia due to eating disorders and/or laxative abuse.
Hypokalemia and/or reduced dietary K+ are implicated in the pathophysiology and progression of hypertension, heart failure, and stroke. For example, short-term K+ restriction in healthy humans and patients with essential hypertension induces Na+-Cl– retention and hypertension. Correction of hypokalemia is particularly important in hypertensive patients treated with diuretics, in whom blood pressure improves with the establishment of normokalemia.
Diagnostic Approach The cause of hypokalemia is usually evident from history, physical examination, and/or basic laboratory tests. The history should focus on medications (e.g., laxatives, diuretics, antibiotics), diet and dietary habits (e.g., licorice), and/or symptoms that suggest a particular cause (e.g., periodic weakness, diarrhea). The physical examination should pay particular attention to blood pressure, volume status, and signs suggestive of specific hypokalemic disorders, e.g., hyperthyroidism and Cushing’s syndrome. Initial laboratory evaluation should include electrolytes, BUN, creatinine, serum osmolality, Mg2+, Ca2+, a complete blood count, and urinary pH, osmolality, creatinine, and electrolytes (Fig. 63-7). The presence of a non–anion gap acidosis suggests a distal, hypokalemic renal tubular acidosis or diarrhea; calculation of the urinary anion gap can help differentiate these two diagnoses. Renal K+ excretion can be assessed with a 24-h urine collection; a 24-h K+ excretion of <15 mmol is indicative of an extrarenal cause of hypokalemia (Fig. 63-7). If only a random, spot urine sample is available, serum and urine osmolality can be used to calculate the transtubular K+ gradient (TTKG), which should be <3 in the presence of hypokalemia (see also “Hyperkalemia”). Alternatively, a urinary K+-to-creatinine ratio of >13 mmol/g creatinine (>1.5 mmol/mmol creatinine) is compatible with excessive renal K+ excretion. Urine Cl– is usually decreased in patients with hypokalemia from a nonreabsorbable anion, such as antibiotics or HCO3–. The most common causes of chronic hypokalemic alkalosis are surreptitious vomiting, diuretic abuse, and GS; these can be distinguished by the pattern of urinary electrolytes. Hypokalemic patients with vomiting due to bulimia will thus have a urinary Cl– <10 mmol/L; urine Na+, K+, and Cl– are persistently elevated in GS, due to loss of function in the thiazide-sensitive Na+-Cl– cotransporter, but less elevated in diuretic abuse and with greater variability. Urine diuretic screens for loop diuretics and thiazides may be necessary to further exclude diuretic abuse.
FIGURE 63-7 The diagnostic approach to hypokalemia. See text for details. AME, apparent mineralocorticoid excess; BP, blood pressure; CCD, cortical collecting duct; DKA, diabetic ketoacidosis; FH-I, familial hyperaldosteronism type I; FHPP, familial hypokalemic periodic paralysis; GI, gastrointestinal; GRA, glucocorticoid remediable aldosteronism; HTN, hypertension; PA, primary aldosteronism; RAS, renal artery stenosis; RST, renin-secreting tumor; RTA, renal tubular acidosis; SAME, syndrome of apparent mineralocorticoid excess; TTKG, transtubular potassium gradient. (Used with permission from DB Mount, K Zandi-Nejad K: Disorders of potassium balance, in Brenner and Rector’s The Kidney, 8th ed, BM Brenner [ed]. Philadelphia, W.B. Saunders & Company, 2008, pp 547-587.)
Other tests, such as urinary Ca2+, thyroid function tests, and/or PRA and aldosterone levels, may also be appropriate in specific cases. A plasma aldosterone:PRA ratio of >50, due to suppression of circulating renin and an elevation of circulating aldosterone, is suggestive of hyperaldosteronism. Patients with hyperaldosteronism or apparent mineralocorticoid excess may require further testing, for example adrenal vein sampling (Chap. 406) or the clinically available testing for specific genetic causes (e.g., FH-I, SAME, Liddle’s syndrome). Patients with primary aldosteronism should thus be tested for the chimeric FH-I/GRA gene (see above) if they are younger than 20 years of age or have a family history of primary aldosteronism or stroke at a young age (<40 years). Preliminary differentiation of Liddle’s syndrome due to mutant ENaC channels from SAME due to mutant 11βHSD-2 (see above), both of which cause hypokalemia and hypertension with aldosterone suppression, can be made on a clinical basis and then confirmed by genetic analysis; patients with Liddle’s syndrome should respond to amiloride (ENaC inhibition) but not spironolactone, whereas patients with SAME will respond to spironolactone.
HYPERKALEMIA
Hyperkalemia is defined as a plasma potassium level of 5.5 mM, occurring in up to 10% of hospitalized patients; severe hyperkalemia (>6.0 mM) occurs in approximately 1%, with a significantly increased risk of mortality. Although redistribution and reduced tissue uptake can acutely cause hyperkalemia, a decrease in renal K+ excretion is the most frequent underlying cause (Table 63-5). Excessive intake of K+ is a rare cause, given the adaptive capacity to increase renal secretion; however, dietary intake can have a major effect in susceptible patients, e.g., diabetics with hyporeninemic hypoaldosteronism and chronic kidney disease. Drugs that impact on the renin-angiotensin-aldosterone axis are also a major cause of hyperkalemia.
|
CAUSES OF HYPERKALEMIA |
Pseudohyperkalemia Hyperkalemia should be distinguished from factitious hyperkalemia or “pseudohyperkalemia,” an artifactual increase in serum K+ due to the release of K+ during or after venipuncture. Pseudohyperkalemia can occur in the setting of excessive muscle activity during venipuncture (e.g., fist clenching), a marked increase in cellular elements (thrombocytosis, leukocytosis, and/or erythrocytosis) with in vitro efflux of K+, and acute anxiety during venipuncture with respiratory alkalosis and redistributive hyperkalemia. Cooling of blood following venipuncture is another cause, due to reduced cellular uptake; the converse is the increased uptake of K+ by cells at high ambient temperatures, leading to normal values for hyperkalemic patients and/or to spurious hypokalemia in normokalemic patients. Finally, there are multiple genetic subtypes of hereditary pseudohyperkalemia, caused by increases in the passive K+ permeability of erythrocytes. For example, causative mutations have been described in the red cell anion exchanger (AE1, encoded by the SLC4A1 gene), leading to reduced red cell anion transport, hemolytic anemia, the acquisition of a novel AE1-mediated K+ leak, and pseudohyperkalemia.
Redistribution and Hyperkalemia Several different mechanisms can induce an efflux of intracellular K+ and hyperkalemia. Acidemia is associated with cellular uptake of H+ and an associated efflux of K+; it is thought that this effective K+-H+ exchange serves to help maintain extracellular pH. Notably, this effect of acidosis is limited to non–anion gap causes of metabolic acidosis and, to a lesser extent, respiratory causes of acidosis; hyperkalemia due to an acidosis-induced shift of potassium from the cells into the ECF does not occur in the anion gap acidoses lactic acidosis and ketoacidosis. Hyperkalemia due to hypertonic mannitol, hypertonic saline, and intravenous immune globulin is generally attributed to a “solvent drag” effect, as water moves out of cells along the osmotic gradient. Diabetics are also prone to osmotic hyperkalemia in response to intravenous hypertonic glucose, when given without adequate insulin. Cationic amino acids, specifically lysine, arginine, and the structurally related drug epsilon-aminocaproic acid, cause efflux of K+ and hyperkalemia, through an effective cation-K+ exchange of unknown identity and mechanism. Digoxin inhibits Na+/K+-ATPase and impairs the uptake of K+ by skeletal muscle, such that digoxin overdose predictably results in hyperkalemia. Structurally related glycosides are found in specific plants (e.g., yellow oleander, foxglove) and in the cane toad, Bufo marinus (bufadienolide); ingestion of these substances and extracts thereof can also cause hyperkalemia. Finally, fluoride ions also inhibit Na+/K+-ATPase, such that fluoride poisoning is typically associated with hyperkalemia.
Succinylcholine depolarizes muscle cells, causing an efflux of K+ through acetylcholine receptors (AChRs). The use of this agent is contraindicated in patients who have sustained thermal trauma, neuromuscular injury, disuse atrophy, mucositis, or prolonged immobilization. These disorders share a marked increase and redistribution of AChRs at the plasma membrane of muscle cells; depolarization of these upregulated AChRs by succinylcholine leads to an exaggerated efflux of K+ through the receptor-associated cation channels, resulting in acute hyperkalemia.
Hyperkalemia Caused by Excess Intake or Tissue Necrosis Increased intake of even small amounts of K+ may provoke severe hyperkalemia in patients with predisposing factors; hence, an assessment of dietary intake is crucial. Foods rich in potassium include tomatoes, bananas, and citrus fruits; occult sources of K+, particularly K+-containing salt substitutes, may also contribute significantly. Iatrogenic causes include simple overreplacement with K+-Cl– or the administration of a potassium-containing medication (e.g., K+-penicillin) to a susceptible patient. Red cell transfusion is a well-described cause of hyperkalemia, typically in the setting of massive transfusions. Finally, severe tissue necrosis, as in acute tumor lysis syndrome and rhabdomyolysis, will predictably cause hyperkalemia from the release of intracellular K+.
Hypoaldosteronism and Hyperkalemia Aldosterone release from the adrenal gland may be reduced by hyporeninemic hypoaldosteronism, medications, primary hypoaldosteronism, or isolated deficiency of ACTH (secondary hypoaldosteronism). Primary hypoaldosteronism may be genetic or acquired (Chap. 406) but is commonly caused by autoimmunity, either in Addison’s disease or in the context of a polyglandular endocrinopathy. HIV has surpassed tuberculosis as the most important infectious cause of adrenal insufficiency. The adrenal involvement in HIV disease is usually subclinical; however, adrenal insufficiency may be precipitated by stress, drugs such as ketoconazole that inhibit steroidogenesis, or the acute withdrawal of steroid agents such as megestrol.
Hyporeninemic hypoaldosteronism is a very common predisposing factor in several overlapping subsets of hyperkalemic patients: diabetics, the elderly, and patients with renal insufficiency. Classically, patients should have suppressed PRA and aldosterone; approximately 50% have an associated acidosis, with a reduced renal excretion of NH4+, a positive urinary anion gap, and urine pH <5.5. Most patients are volume expanded, with secondary increases in circulating atrial natriuretic peptide (ANP) that inhibit both renal renin release and adrenal aldosterone release.
Renal Disease and Hyperkalemia Chronic kidney disease and end-stage kidney disease are very common causes of hyperkalemia, due to the associated deficit or absence of functioning nephrons. Hyperkalemia is more common in oliguric acute kidney injury; distal tubular flow rate and Na+ delivery are less limiting factors in nonoliguric patients. Hyperkalemia out of proportion to GFR can also be seen in the context of tubulointerstitial disease that affects the distal nephron, such as amyloidosis, sickle cell anemia, interstitial nephritis, and obstructive uropathy.
Hereditary renal causes of hyperkalemia have overlapping clinical features with hypoaldosteronism, hence the diagnostic label pseudohypoaldosteronism (PHA). PHA type I (PHA-I) has both an autosomal recessive and an autosomal dominant form. The autosomal dominant form is due to loss-of-function mutations in the MLR; the recessive form is caused by various combinations of mutations in the three subunits of ENaC, resulting in impaired Na+ channel activity in principal cells and other tissues. Patients with recessive PHA-I suffer from lifelong salt wasting, hypotension, and hyperkalemia, whereas the phenotype of autosomal dominant PHA-I due to MLR dysfunction improves in adulthood. PHA type II (PHA-II; also known as hereditary hypertension with hyperkalemia) is in every respect the mirror image of GS caused by loss of function in NCC, the thiazide-sensitive Na+-Cl– cotransporter (see above); the clinical phenotype includes hypertension, hyperkalemia, hyperchloremic metabolic acidosis, suppressed PRA and aldosterone, hypercalciuria, and reduced bone density. PHA-II thus behaves like a gain of function in NCC, and treatment with thiazides results in resolution of the entire clinical phenotype. However, the NCC gene is not directly involved in PHA-II, which is caused by mutations in the WNK1 and WNK4 serine-threonine kinases or the upstream Kelch-like 3 (KLHL3) and Cullin 3 (CUL3), two components of an E3 ubiquitin ligase complex that regulates these kinases; these proteins collectively regulate NCC activity, with PHA-II-associated activation of the transporter.
Medication-Associated Hyperkalemia Most medications associated with hyperkalemia cause inhibition of some component of the renin-angiotensin-aldosterone axis. ACE inhibitors, angiotensin receptor blockers, renin inhibitors, and MLRs are predictable and common causes of hyperkalemia, particularly when prescribed in combination. The oral contraceptive agent Yasmin-28 contains the progestin drospirenone, which inhibits the MLR and can cause hyperkalemia in susceptible patients. Cyclosporine, tacrolimus, NSAIDs, and cyclooxygenase 2 (COX2) inhibitors cause hyperkalemia by multiple mechanisms, but share the ability to cause hyporeninemic hypoaldosteronism. Notably, most drugs that affect the renin-angiotensin-aldosterone axis also block the local adrenal response to hyperkalemia, thus attenuating the direct stimulation of aldosterone release by increased plasma K+ concentration.
Inhibition of apical ENaC activity in the distal nephron by amiloride and other K+-sparing diuretics results in hyperkalemia, often with a voltage-dependent hyperchloremic acidosis and/or hypovolemic hyponatremia. Amiloride is structurally similar to the antibiotics trimethoprim (TMP) and pentamidine, which also block ENaC; risk factors for TMP-associated hyperkalemia include the administered dose, renal insufficiency, and hyporeninemic hypoaldosteronism. Indirect inhibition of ENaC at the plasma membrane is also a cause of drug-associated hyperkalemia; nafamostat, a protease inhibitor used in some countries for the management of pancreatitis, inhibits aldosterone-induced renal proteases that activate ENaC by proteolytic cleavage.
Clinical Features Hyperkalemia is a medical emergency due to its effects on the heart. Cardiac arrhythmias associated with hyperkalemia include sinus bradycardia, sinus arrest, slow idioventricular rhythms, ventricular tachycardia, ventricular fibrillation, and asystole. Mild increases in extracellular K+ affect the repolarization phase of the cardiac action potential, resulting in changes in T-wave morphology; further increase in plasma K+ concentration depresses intracardiac conduction, with progressive prolongation of the PR and QRS intervals. Severe hyperkalemia results in loss of the P wave and a progressive widening of the QRS complex; development of a sine-wave sinoventricular rhythm suggests impending ventricular fibrillation or asystole. Hyperkalemia can also cause a type I Brugada pattern in the electrocardiogram (ECG), with a pseudo–right bundle branch block and persistent coved ST segment elevation in at least two precordial leads. This hyperkalemic Brugada’s sign occurs in critically ill patients with severe hyperkalemia and can be differentiated from genetic Brugada’s syndrome by an absence of P waves, marked QRS widening, and an abnormal QRS axis. Classically, the electrocardiographic manifestations in hyperkalemia progress from tall peaked T waves (5.5–6.5 mM), to a loss of P waves (6.5–7.5 mM) to a widened QRS complex (7.0–8.0 mM), and, ultimately, a to a sine wave pattern (>8.0 mM). However, these changes are notoriously insensitive, particularly in patients with chronic kidney disease or end-stage renal disease.
Hyperkalemia from a variety of causes can also present with ascending paralysis, denoted secondary hyperkalemic paralysis to differentiate it from familial hyperkalemic periodic paralysis (HYPP). The presentation may include diaphragmatic paralysis and respiratory failure. Patients with familial HYPP develop myopathic weakness during hyperkalemia induced by increased K+ intake or rest after heavy exercise. Depolarization of skeletal muscle by hyperkalemia unmasks an inactivation defect in skeletal Na+ channel; autosomal dominant mutations in the SCN4A gene encoding this channel are the predominant cause.
Within the kidney, hyperkalemia has negative effects on the ability to excrete an acid load, such that hyperkalemia per se can contribute to metabolic acidosis. This defect appears to be due in part to competition between K+ and NH4+ for reabsorption by the TALH and subsequent countercurrent multiplication, ultimately reducing the medullary gradient for NH3/NH4 excretion by the distal nephron. Regardless of the underlying mechanism, restoration of normokalemia can, in many instances, correct hyperkalemic metabolic acidosis.
Diagnostic Approach The first priority in the management of hyperkalemia is to assess the need for emergency treatment, followed by a comprehensive workup to determine the cause (Fig. 63-8). History and physical examination should focus on medications, diet and dietary supplements, risk factors for kidney failure, reduction in urine output, blood pressure, and volume status. Initial laboratory tests should include electrolytes, BUN, creatinine, serum osmolality, Mg2+ and Ca2+, a complete blood count, and urinary pH, osmolality, creatinine, and electrolytes. A urine Na+ concentration of <20 mM indicates that distal Na+ delivery is a limiting factor in K+ excretion; volume repletion with 0.9% saline or treatment with furosemide may be effective in reducing plasma K+ concentration. Serum and urine osmolality are required for calculation of the transtubular K+ gradient (TTKG) (Fig. 63-8). The expected values of the TTKG are largely based on historical data, and are <3 in the presence of hypokalemia and >7–8 in the presence of hyperkalemia.
FIGURE 63-8 The diagnostic approach to hyperkalemia. See text for details. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCD, cortical collecting duct; ECG, electrocardiogram; ECV, effective circulatory volume; GFR, glomerular filtration rate; GN, glomerulonephritis; HIV, human immunodeficiency virus; LMW heparin, low-molecular-weight heparin; NSAIDs, nonsteroidal anti-inflammatory drugs; PHA, pseudohypoaldosteronism; SLE, systemic lupus erythematosus; TTKG, transtubular potassium gradient. (Used with permission from DB Mount, K Zandi-Nejad K: Disorders of potassium balance, in Brenner and Rector’s The Kidney, 8th ed, BM Brenner [ed]. Philadelphia, W.B. Saunders & Company, 2008, pp 547-587.)

64e |
Fluid and Electrolyte Imbalances and Acid-Base Disturbances: Case Examples |
CASE 1
A 23-year-old woman was admitted with a 3-day history of fever, cough productive of blood-tinged sputum, confusion, and orthostasis. Past medical history included type 1 diabetes mellitus. A physical examination in the emergency department indicated postural hypotension, tachycardia, and Kussmaul respiration. The breath was noted to smell of “acetone.” Examination of the thorax suggested consolidation in the right lower lobe.
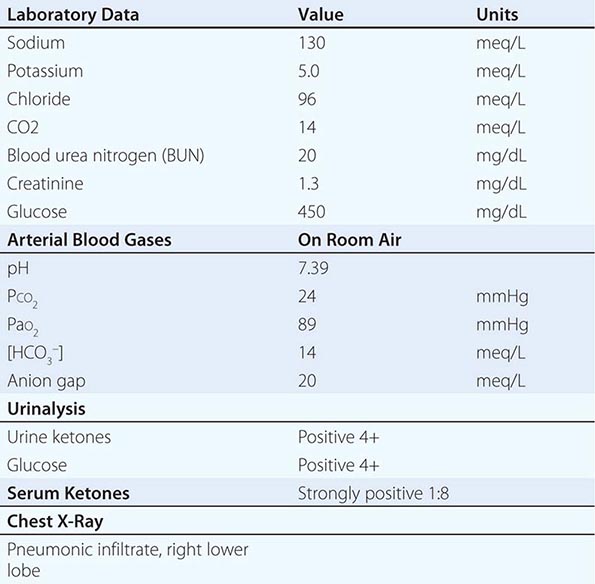
APPROACH TO DIAGNOSIS
The diagnosis of the acid-base disorder should proceed in a stepwise fashion:
1. The normal anion gap (AG) is 8–10 meq/L, but in this case, the AG is elevated (20 meq/L). Therefore, the change in AG (ΔAG) = ~10 meq/L.
2. Compare the ΔAG and the Δ[HCO3–]. In this case, the ΔAG, as noted above, is 10, and the Δ[HCO3–] (25 – 14) is 11. Therefore, the increment in the AG is approximately equal to the decrement in bicarbonate.
3. Estimate the respiratory compensatory response. In this case, the predicted PaCO2 for an [HCO3–] of 14 should be approximately 29 mmHg. This value is obtained by adding 15 to the measured [HCO3–] (15 + 14 = 29) or by calculating the predicted PaCO2 from the Winter equation: 1.5 × [HCO3–] + 8. In either case, the predicted value for PaCO2 of 29 is significantly higher than the measured value of 24. Therefore, the prevailing PaCO2 exceeds the range for compensation alone and is too low, indicating a superimposed respiratory alkalosis.
4. Therefore, this patient has a mixed acid-base disturbance with two components: (a) high AG acidosis secondary to ketoacidosis and (b) respiratory alkalosis (which was secondary to community-acquired pneumonia in this case). The latter resulted in an additional component of hyperventilation that exceeded the compensatory response driven by metabolic acidosis, explaining the normal pH. The finding of respiratory alkalosis in the setting of a high AG acidosis suggests another cause of the respiratory component. Respiratory alkalosis frequently accompanies community-acquired pneumonia.
The clinical features in this case include hyperglycemia, hypovolemia, ketoacidosis, central nervous system (CNS) signs of confusion, and superimposed pneumonia. This clinical scenario is consistent with diabetic ketoacidosis (DKA) developing in a patient with known type 1 diabetes mellitus. Infections in DKA are common and may be a precipitating feature in the development of ketoacidosis.
The diagnosis of DKA is usually not challenging but should be considered in all patients with an elevated AG and metabolic acidosis. Hyperglycemia and ketonemia (positive acetoacetate at a dilution of 1:8 or greater) are sufficient criteria for diagnosis in patients with type 1 diabetes mellitus. The Δ[HCO3–] should approximate the increase in the plasma AG (ΔAG), but this equality can be modified by several factors. For example, the ΔAG will often decrease with IV hydration, as glomerular filtration increases and ketones are excreted into the urine. The decrement in plasma sodium is the result of hyperglycemia, which induces the movement of water into the extracellular compartment from the intracellular compartment of cells that require insulin for the transport of glucose. Additionally, a natriuresis occurs in response to an osmotic diuresis associated with hyperglycemia. Moreover, in patients with DKA, thirst is very common and water ingestion often continues. The plasma potassium concentration is usually mildly elevated, but in the face of acidosis, and as a result of the ongoing osmotic diuresis, a significant total-body deficit of potassium is almost always present. Recognition of the total-body deficit of potassium is critically important. The inclusion of potassium replacement in the therapeutic regimen at the appropriate time and with the appropriate indications (see below) is essential. Volume depletion is a very common finding in DKA and is a pivotal component in the pathogenesis of the disorder.
APPROACH TO MANAGEMENT
Patients with DKA often have a sustained and significant deficit of sodium, potassium, water, bicarbonate, and phosphate. The general approach to treatment requires attention to all of these abnormalities. Successful treatment of DKA involves a stepwise approach, as follows:
1. Replace extracellular fluid (ECF) volume deficits. Because most patients present with actual or relative hypotension and, at times, impending shock, the initial fluid administered should be 0.9% NaCl infused rapidly until the systolic blood pressure is >100 mmHg or until 2–3 L cumulatively have been administered. During the initial 2–3 h of infusion of saline, the decline in blood glucose can be accounted for by dilution and increased renal excretion. Glucose should be added to the infusion as D5 normal saline (NS) or D5 0.45% NS once the plasma glucose declines to 230 mg/dL or below.
2. Abate the production of ketoacids. Regular insulin is required during DKA as an initial bolus of 0.1 U/kg body weight (BW) IV, followed immediately by a continuous infusion of 0.1 U/kg BW per hour in NS. The effectiveness of IV insulin (not subcutaneous) can be tracked by observing the decline in plasma ketones. Because the increment in the AG above the normal value of 10 meq/L represents accumulated ketoacids in DKA, the disappearance of ketoacid anions is reflected by the narrowing and eventual correction of the AG. Typically, the plasma AG returns to normal within 8–12 h.
3. Replace potassium deficits. Although patients with DKA often have hyperkalemia due to insulin deficiency, they are usually severely K+ depleted. KCl (20 meq/L) should be added to each liter of IV fluids when urine output is established and insulin has been administered.
4. Correct the metabolic acidosis. The plasma bicarbonate concentration will usually not increase for several hours because of dilution from administered IV NaCl. The plasma [HCO–] approaches 18 meq/L once ketoacidosis disappears. Sodium bicarbonate therapy is often not recommended or necessary and is contraindicated for children. Bicarbonate is administered to adults with DKA for extreme acidemia (pH <7.1); for elderly patients (>70 years old), a threshold pH of 7.20 is recommended. Sodium bicarbonate, if administered, should only be given in small amounts. Because ketoacids are metabolized in response to insulin therapy, bicarbonate will be added to the ECF as ketoacids are converted. Overshoot alkalosis may occur from the combination of exogenously administered sodium bicarbonate plus metabolic production of bicarbonate.
5. Phosphate. In the first 6–8 h of therapy, it may be necessary to infuse potassium with phosphate because of the unmasking of phosphate depletion during combined insulin and glucose therapy. The latter drives phosphate into the cell. Therefore, in patients with DKA, the plasma phosphate level should be followed closely, but phosphate should never be replaced empirically. Phosphate should be administered to patients with a declining plasma phosphate once the phosphate level declines into the low-normal level. Therapy is advisable in the form of potassium phosphate at a rate of 6 mmol/h.
6. Always seek underlying factors, such as infection, myocardial infarction, pancreatitis, cessation of insulin therapy, or other events, responsible for initiating DKA. The case presented here is illustrative of this common scenario.
7. Volume overexpansion with IV fluid administration is not uncommon and contributes to the development of hyperchloremic acidosis during the later stages of treatment of DKA. Volume overexpansion should be avoided.
CASE 2
A 25-year-old man with a 6-year history of HIV-AIDS complicated recently by Pneumocystis jiroveci pneumonia (PCP) was treated with intravenous trimethoprim-sulfamethoxazole (20 mg trimethoprim/kg per day). On day 4 of treatment, the following laboratory data were obtained:
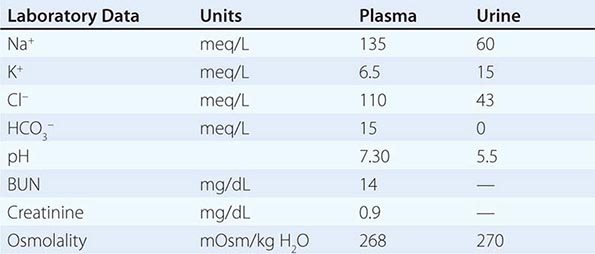
APPROACH TO DIAGNOSIS
What caused the hyperkalemia and metabolic acidosis in this patient? What other medications may be associated with a similar presentation? How does one use the urine electrolyte data to determine if the hyperkalemia is of renal origin or due to a shift from the cell to the extracellular compartment?
Hyperkalemia occurs in 15–20% of hospitalized patients with HIV/AIDS. The usual causes are either adrenal insufficiency, the syndrome of hyporeninemic hypoaldosteronism, or one of several drugs, including trimethoprim, pentamidine, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, spironolactone, and eplerenone. Trimethoprim is usually given in combination with sulfamethoxazole or dapsone for PCP and, on average, increases the plasma K+ concentration by about 1 meq/L; however, the hyperkalemia may be severe. Trimethoprim is structurally and chemically related to amiloride and triamterene and, in this way, may function as a potassium-sparing diuretic. This effect results in inhibition of the epithelial sodium channel (ENaC) in the principal cell of the collecting duct. By blocking the Na+ channel, K+ secretion is also inhibited; K+ secretion is dependent on the lumen-negative potential difference generated by Na+ entry through the ENaC (Fig. 64e-1).
FIGURE 64e-1 Water, sodium, potassium, ammonia, and proton transport in principal cells (PC) and adjacent type A intercalated cells (A-IC). Water is absorbed down the osmotic gradient by principal cells, through the apical aquaporin-2 (AQP-2) and basolateral aquaporin-3 (AQP-3) and aquaporin-4 (AQP-4) channels. The absorption of Na+ via the amiloride-sensitive epithelial sodium channel (ENaC) generates a lumen-negative potential difference, which drives K+ excretion through the apical secretory K+ channel, ROMK (renal outer medullary K+ channel), and/or the flow-dependent maxi-K channel. Transepithelial ammonia (NH3) transport and proton transport occur in adjacent type A intercalated cells, via apical and basolateral ammonia channels and apical H+-ATPase pumps, respectively; NH4+ is ultimately excreted in the urine, in the defense of systemic pH. Electrogenic proton secretion by type A intercalated cells is also affected by the lumen-negative potential difference generated by the adjacent principal cells, such that reduction of this lumen-negative electrical gradient can reduce H+ excretion. Type A intercalated cells also reabsorb filtered K+ in potassium-deficient states, via apical H+/K+-ATPase.
Trimethoprim is associated with a non-AG acidosis that parallels development of hyperkalemia such that the co-occurrence of hyperkalemia and metabolic acidosis is not uncommon in this setting. H+ secretion via apical H+-ATPase pumps in adjacent type A intercalated cells (Fig. 64e-1) is also electrogenic, such that the reduction in the lumen-negative potential difference due to trimethoprim inhibits distal H+ secretion; this is often referred to as a “voltage defect” form of dRTA. Systemic hyperkalemia also suppresses renal ammoniagenesis, ammonium excretion, and, thus, acid excretion; i.e., hyperkalemia per se has multiple effects on urinary acidification.
The inhibitory effect of trimethoprim on K+ and H+ secretion in the cortical collecting tubule follows a dose-response relationship, and therefore, the higher doses of this agent used in HIV/AIDS patients with PCP or in deep tissue infections with methicillin-resistant Staphylococcus aureus (MRSA) result in a higher prevalence of hyperkalemia and acidosis. Conventional does of trimethoprim can also induce hyperkalemia and/or acidosis in predisposed patients, in particular the elderly, patients with renal insufficiency, and/or those with baseline hyporeninemic hypoaldosteronism.
One means by which to assess the role of the kidney in the development of hyperkalemia is to calculate, from a spot urine and coincident plasma sample, the transtubular potassium gradient (TTKG). The TTKG is calculated as (Posmol × UPotassium)/(PPotassium × Uosmol). The expected values of the TTKG are <3 in the presence of hypokalemia (see also Case 7 and Case 8) and >7–8 in the presence of hyperkalemia. In this case, the value for the TTKG of approximately 2 indicates that renal excretion of potassium is abnormally low for the prevailing hyperkalemia. Therefore, the inappropriately low TTKG indicates that the hyperkalemia is of renal tubular origin.
APPROACH TO MANAGEMENT
Knowledge of the factors controlling potassium secretion by the cortical collecting tubule principal cell can be helpful in understanding the basis for treatment of the hyperkalemia, especially if discontinuing the offending agent is not a reasonable clinical option. Potassium secretion is encouraged by a higher urine flow rate, increased distal delivery of sodium, distal delivery of a poorly reabsorbed anion (such as bicarbonate), and/or administration of a loop diuretic. Therefore, the approach to treatment in this patient should include intravenous 0.9% NaCl to expand the ECF and deliver more Na+ and Cl– to the cortical collecting tubule. In addition, because the trimethoprim molecule must be protonated to inhibit ENaC, alkalinization of the renal tubule fluid enhances distal tubular K+ secretion. As an alternative to inducing bicarbonaturia to assist in potassium secretion, a carbonic anhydrase inhibitor may be administered to induce a kaliuresis. However, in the case presented here, for acetazolamide to be effective, the non-AG metabolic acidosis in this patient would first need to be corrected; Acetazolamide would, thus, require the coadministration of intravenous sodium bicarbonate for maximal benefit. Finally, systemic hyperkalemia directly suppresses renal ammoniagenesis, ammonium excretion, and, thus, acid excretion. Correcting the hyperkalemia with a potassium-binding resin (Kayexalate) is sometimes appropriate in these patients; the subsequent decline in the plasma K+ concentration will also increase urinary ammonium excretion, helping correct the acidosis.
CASE 3
A 63-year-old man was admitted to the intensive care unit (ICU) with a severe aspiration pneumonia. Past medical history included schizophrenia, for which he required institutional care; treatment had included neuroleptics and intermittent lithium, the latter restarted 6 months before admission. The patient was treated with antibiotics and intubated for several days, with the development of polyuria (3–5 L/d), hypernatremia, and acute renal insufficiency; the peak plasma Na+ concentration was 156 meq/L, and peak creatinine was 2.6 mg/dL. Urine osmolality was measured once and reported as 157 mOsm/kg, with a coincident plasma osmolality of 318 mOsm/kg. Lithium was stopped on admission to the ICU.
On physical examination, the patient was alert, extubated, and thirsty. Weight was 97.5 kg. Urine output for the previous 24 h had been 3.4 L, with an IV intake of 2 L/d of D5W.
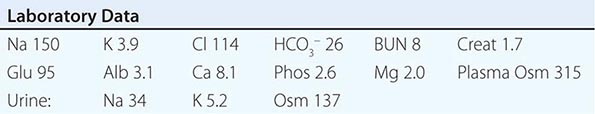
After 3 days of intravenous hydration, a water deprivation test was performed. A single dose of 2 μg IV desmopressin (DDAVP) was given at 9 h (+9):
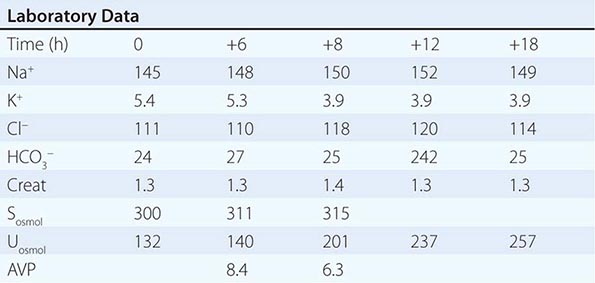
APPROACH TO DIAGNOSIS
Why did the patient develop hypernatremia, polyuria, and acute renal insufficiency? What does the water deprivation test demonstrate? What is the underlying pathophysiology of this patient’s hypernatremic syndrome?
This patient became polyuric after admission to the ICU with severe pneumonia, developing significant hypernatremia and acute renal insufficiency. Polyuria can result from either an osmotic diuresis or a water diuresis. An osmotic diuresis can be caused by excessive excretion of Na+-Cl–, mannitol, glucose, and/or urea, with a daily solute excretion of >750–1000 mOsm/d (>15 mOsm/kg body water per day). In this case, however, the patient was excreting large volumes of very hypotonic urine, with a urine osmolality that was substantially lower than that of plasma; this, by definition, was a water diuresis, resulting in inappropriate excretion of free water and hypernatremia. The appropriate response to hypernatremia and a plasma osmolality >295 mOsm/kg is an increase in circulating vasopressin (AVP) and the excretion of low volumes (<500 mL/d) of maximally concentrated urine, i.e., urine with osmolality >800 mOsm/kg. This patient’s response to hypernatremia was clearly inappropriate, due to either a loss of circulating AVP (central diabetes insipidus [CDI]) or renal resistance to AVP (nephrogenic diabetes insipidus [NDI]). Ongoing loss of free water was sufficiently severe in this patient that absolute hypovolemia ensued, despite the fact that approximately two-thirds of the excreted water was derived from the intracellular fluid compartment rather than the ECF compartment. Hypovolemia led to an acute decrease in the glomerular filtration rate (GFR), i.e., acute renal insufficiency, with gradual improvement following hydration (see below).
Following the correction of hypernatremia and acute renal insufficiency with appropriate hydration (see below), the patient was subjected to a water deprivation test followed by administration of DDAVP. This test helps determine whether an inappropriate water diuresis is caused by CDI or NDI. The patient was water restricted beginning in the early morning, with careful monitoring of vital signs and urine output; overnight water deprivation of patients with diabetes insipidus is unsafe and clinically inappropriate, given the potential for severe hypernatremia. The plasma Na+ concentration, which is more accurate and more immediately available than plasma osmolality, was monitored hourly during water deprivation. A baseline AVP sample was drawn at the beginning of the test, with a second sample drawn once the plasma Na+ reached 148–150 meq/L. At this point, a single 2-μg dose of the V2 AVP receptor agonist DDAVP was administered. An alternative approach would have been to measure AVP and administer DDAVP when the patient was initially hypernatremic; however, it would have been less safe to administer DDAVP in the setting of renal impairment because clearance of DDAVP is renal dependent.
The patient’s water deprivation test was consistent with NDI, with an AVP level within the normal range in the setting of hypernatremia (i.e., no evidence of CDI) and an inappropriately low urine osmolality that failed to increase by >50% or >150 mOsm/kg after both water deprivation and the administration of DDAVP. This defect would be considered compatible with complete NDI; patients with partial NDI can achieve urine osmolalities of 500–600 mOsm/kg after DDAVP treatment but will not maximally concentrate their urine to 800 mOsm/kg or higher.
NDI has a number of genetic and acquired causes, which all share interference with some aspect of the renal concentrating mechanism. For example, loss-of-function mutations in the V2 AVP receptor cause X-linked NDI. This patient suffered from NDI due to lithium therapy, perhaps the most common cause of NDI in adult medicine. Lithium causes NDI via direct inhibition of renal glycogen synthase kinase-3 (GSK3), a kinase thought to be the pharmacologic target of lithium in psychiatric disease; renal GSK3 is required for the response of principal cells to AVP. Lithium also induces the expression of cyclooxygenase-2 (COX2) in the renal medulla; COX2-derived prostaglandins inhibit AVP-stimulated salt transport by the thick ascending limb and AVP-stimulated water transport by the collecting duct, thereby exacerbating lithium-associated polyuria. The entry of lithium through the amiloride-sensitive Na+ channel ENaC (Fig. 64e-1) is required for the effect of the drug on principal cells, such that combined therapy with lithium and amiloride can mitigate lithium-associated NDI. However, lithium causes chronic tubulointerstitial scarring and chronic kidney disease after prolonged therapy, such that patients may have a persistent NDI long after stopping the drug, with a reduced therapeutic benefit from amiloride. Notably, this particular patient had been treated intermittently for several years with lithium, with the development of chronic kidney disease (baseline creatinine of 1.3–1.4) and NDI that persisted after stopping the drug.
APPROACH TO MANAGEMENT
How should this patient be treated? What are the major pitfalls of therapy?
This patient developed severe hypernatremia due to a water diuresis from lithium-associated NDI. Treatment of hypernatremia must include both replacement of the existing free water deficit and daily replacement of ongoing free water loss. The first step is to estimate total-body water (TBW), typically estimated as 50% of the body weight in women and 60% in men. The free water deficit is then calculated as ([Na+ – 140]/140) × TBW. In this patient, the free water deficit was 4.2 L at a weight of 97.5 kg and plasma Na+ concentration of 150 meq/L. This free water deficit should be replaced slowly over 48–72 h to avoid increasing the plasma Na+ concentration by >10 meq/L per 24 h. A common mistake is to replace this deficit while neglecting to replace ongoing losses of free water, such that plasma Na+ concentration either fails to correct or, in fact, increases.
Ongoing losses of free water can be estimated using the equation for electrolyte-free water clearance:
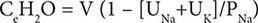
where V is urinary volume, UNa is urinary [Na+], UK is urinary [K+], and PNa is plasma [Na+].
For this patient, the CeH2O was 2.5 L/d when initially evaluated, i.e., with urine Na+ and K+ concentrations of 34 and 5.2 meq/L, plasma Na+ concentration of 150 meq/L, and a urinary volume of 3.4 L. Therefore, the patient was given 2.5 L of D5W over the first 24 h to replace ongoing free water losses, along with 2.1 L of D5W to replace half his free water deficit. Daily random urine electrolytes and urinary volume measurement can be used to monitor CeH2O and adjust daily fluid administration in this manner, while following plasma Na+ concentration. Physicians often calculate the free water deficit to guide therapy of hypernatremia, providing half the deficit in the first 24 h. This approach can be adequate in patients who do not have significant ongoing losses of free water, e.g., with hypernatremia due to decreased free water intake. This case illustrates how free water requirements can be grossly underestimated in hypernatremic patients if ongoing, daily free water losses are not taken into account.
CASE 4
A 78-year-old man was admitted with pneumonia and hyponatremia. Plasma Na+ concentration was initially 129 meq/L, decreasing within 3 days to 118–120 meq/L despite fluid restriction to 1 L/d. A chest computed tomography (CT) revealed a right 2.8 × 1.6 cm infrahilar mass and postobstructive pneumonia. The patient was an active smoker. Past medical history was notable for laryngeal carcinoma treated 15 years prior with radiation therapy, renal cell carcinoma, peripheral vascular disease, and hypothyroidism. On review of systems, he denied headache, nausea, and vomiting. He had chronic hip pain, managed with acetaminophen with codeine. Other medications included cilostazol, amoxicillin/clavulanate, digoxin, diltiazem, and thyroxine. He was euvolemic on examination, with no lymphadenopathy and a normal chest examination.
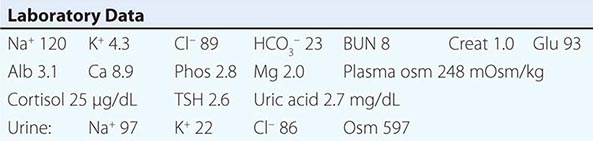
The patient was treated with furosemide, 20 mg PO bid, and salt tablets. The plasma Na+ concentration increased to 129 meq/L with this therapy; however, the patient developed orthostatic hypotension and dizziness. He was started on demeclocycline, 600 mg PO in the morning and 300 mg in the evening, just before discharge from hospital. Plasma Na+ concentration increased to 140 meq/L with a BUN of 23 and creatinine of 1.4, at which point demeclocycline was reduced to 300 mg PO bid. Bronchoscopic biopsy eventually showed small-cell lung cancer; the patient declined chemotherapy and was admitted to hospice.
APPROACH TO DIAGNOSIS AND MANAGEMENT
What factors contributed to this patient’s hyponatremia? What are the therapeutic options?
This patient developed hyponatremia in the context of a central lung mass and postobstructive pneumonia. He was clinically euvolemic, with a generous urine Na+ concentration and low plasma uric acid concentration. He was euthyroid, with no evidence of pituitary dysfunction or secondary adrenal insufficiency. The clinical presentation is consistent with the syndrome of inappropriate antidiuresis (SIAD). Although pneumonia was a potential contributor to the SIAD, it was notable that the plasma Na+ concentration decreased despite a clinical response to antibiotics. It was suspected that this patient had SIAD due to small-cell lung cancer, with a central lung mass on chest CT and a significant smoking history. There was a history of laryngeal cancer and renal cancer but with no evidence of recurrent disease; these malignancies were not considered contributory to his SIAD. Biopsy of the lung mass ultimately confirmed the diagnosis of small-cell lung cancer, which is responsible for ~75% of malignancy-associated SIAD; ~10% of patients with this neuroendocrine tumor will have a plasma Na+ concentration of <130 meq/L at presentation. The patient had no other “nonosmotic” stimuli for an increase in AVP, with no medications associated with SIAD and minimal pain or nausea.
The patient had no symptoms attributable to hyponatremia but was judged at risk for worsening hyponatremia from severe SIAD. Persistent, chronic hyponatremia (duration >48 h) results in an efflux of organic osmolytes (creatine, betaine, glutamate, myoinositol, and taurine) from brain cells; this response reduces intracellular osmolality and the osmotic gradient favoring water entry. This cellular response does not fully protect patients from symptoms, which can include vomiting, nausea, confusion, and seizures, usually at plasma Na+ concentration <125 meq/L. Even patients who are judged “asymptomatic” can manifest subtle gait and cognitive defects that reverse with correction of hyponatremia. Chronic hyponatremia also increases the risk of bony fractures due to an increased risk of falls and to a hyponatremia-associated reduction in bone density. Therefore, every attempt should be made to correct plasma Na+ concentration safely in patients with chronic hyponatremia. This is particularly true in malignancy-associated SIAD, where it can take weeks to months for a tissue diagnosis and the subsequent reduction in AVP following initiation of chemotherapy, radiotherapy, and/or surgery.
What are the therapeutic options in SIAD? Water deprivation, a cornerstone of therapy for SIAD, had little effect on the plasma Na+ concentration in this patient. The urine:plasma electrolyte ratio (urinary [Na+] + [K+]/plasma [Na+]) can be used to estimate electrolyte-free water excretion and the required degree of water restriction; patients with a ratio of >1 should be more aggressively restricted (<500 mL/d), those with a ratio of ~1 should be restricted to 500–700 mL/d, and those with a ratio <1 should be restricted to <1 L/d. This patient had a urine:plasma electrolyte ratio of 1 and predictably did not respond to a moderate water restriction of ~1 L/d. A more aggressive water restriction would have theoretically been successful; however, this can be very difficult for patients with SIAD to tolerate, given that their thirst is also inappropriately stimulated.
Combined therapy with furosemide and salt tablets can often increase the plasma Na+ concentration in SIAD; furosemide reduces maximal urinary concentrating ability by inhibiting the countercurrent mechanism, whereas the salt tablets mitigate diuretic-associated NaCl loss and amplify the ability to excrete free water by increasing urinary solute excretion. This regimen is not always successful and requires careful titration of salt tablets to avoid volume depletion; indeed, in this particular patient, the plasma Na+ concentration remained <130 meq/L and the patient became orthostatic. The principal cell toxin, demeclocycline, is an alternative oral agent in SIAD. Treatment with demeclocycline was very successful in this patient, with an increase in plasma Na+ concentration to 140 meq/L. However, demeclocycline can be natriuretic, leading to a prerenal decrease in GFR. Demeclocycline has also been implicated in nephrotoxic injury, particularly in patients with cirrhosis and chronic liver disease, in whom the drug accumulates. Notably, this particular patient developed a significant but stable decrease in GFR while on demeclocycline, necessitating a reduction in the administered dose.
A major advance in the management of hyponatremia was the clinical development of AVP antagonists (vaptans). These agents inhibit the effect of AVP on renal V2 receptors, resulting in the excretion of electrolyte-free water and correction of hyponatremia. The specific indications for these agents are not as yet clear, despite U.S. Food and Drug Administration (FDA) approval for the management of both euvolemic and hypervolemic hyponatremia. It is, however, anticipated that the vaptans will have an increasing role in the management of SIAD and other causes of hyponatremia. Indeed, if this particular patient had continued with active therapy for his cancer, substitution of demeclocycline with oral tolvaptan (a V2-specific oral vaptan) would have been the next appropriate step, given the development of renal insufficiency with demeclocycline. As with other measures to correct hyponatremia (e.g., hypertonic saline, demeclocycline), the vaptans have the potential to “overcorrect” plasma Na+ concentration (a rise of >8–10 meq/L per 24 h or 18 meq/L per 18 h), thus increasing the risk for osmotic demyelination (see Case 5). Therefore, the plasma Na+ concentration should be monitored closely during the initiation of therapy with these agents. In addition, long-term use of tolvaptan has been associated with abnormalities in liver function tests; hence, use of this agent should be restricted to only 1–2 months.
CASE 5
A 76-year-old woman presented with a several-month history of diarrhea, with marked worsening over the 2–3 weeks before admission (up to 12 stools a day). Review of systems was negative for fever, orthostatic dizziness, nausea and vomiting, or headache. Past medical history included hypertension, kidney stones, and hypercholesterolemia; medications included atenolol, spironolactone, and lovastatin. She also reliably consumed >2 L of liquid per day in management of the nephrolithiasis.
The patient received 1 L of saline over the first 5 h of her hospital admission. On examination at hour 6, the heart rate was 72 sitting and 90 standing, and blood pressure was 105/50 mmHg lying and standing. Her jugular venous pressure (JVP) was indistinct with no peripheral edema. On abdominal examination, the patient had a slight increase in bowel sounds but a nontender abdomen and no organomegaly.
The plasma Na+ concentration on admission was 113 meq/L, with a creatinine of 2.35 (Table 64e-1). At hospital hour 7, the plasma Na+ concentration was 120 meq/L, potassium 5.4 meq/L, chloride 90 meq/L, bicarbonate 22 meq/L, BUN 32 mg/dL, creatinine 2.02 mg/dL, glucose 89 mg/dL, total protein 5.0, and albumin 1.9. The hematocrit was 33.9, white count 7.6, and platelets 405. A morning cortisol was 19.5, with thyroid-stimulating hormone (TSH) of 1.7. The patient was treated with 1 μg of intravenous DDAVP, along with 75 mL/h of intravenous half-normal saline. After the plasma Na+ concentration dropped to 116 meq/L, intravenous fluid was switched to normal saline at the same infusion rate. The subsequent results are shown in Table 64e-1.
|
SERIAL LABORATORY DATA FOR CASE 5 |
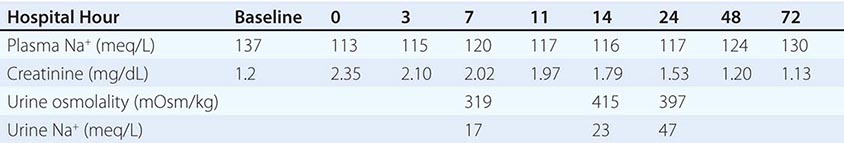
APPROACH TO DIAGNOSIS
This patient presented with hypovolemic hyponatremia and a “prerenal” reduction in GFR, with an increase in serum creatinine. She had experienced diarrhea for some time and manifested an orthostatic tachycardia after a liter of normal saline. As expected for hypovolemic hyponatremia, the urine Na+ concentration was <20 meq/L in the absence of congestive heart failure or other causes of hypervolemic hyponatremia, and she responded to saline hydration with an increase in plasma Na+ concentration and a decrease in creatinine.
The initial hypovolemia increased the sensitivity of this patient’s AVP response to osmolality, both decreasing the osmotic threshold for AVP release and increasing the slope of the osmolality response curve. AVP has a half-life of only 10–20 min; therefore, the acute increase in intravascular volume after a liter of intravenous saline led to a rapid reduction in circulating AVP. The ensuing water diuresis is the primary explanation for the rapid increase in plasma Na+ concentration in the first 7 h of her hospitalization.
APPROACH TO MANAGEMENT
The key concern in this case was the evident chronicity of the patient’s hyponatremia, with several weeks of diarrhea followed by 2–3 days of acute exacerbation. This patient was judged to have chronic hyponatremia, i.e., with a suspected duration of >48 h; as such, she would be predisposed to osmotic demyelination were she to undergo too rapid a correction in her plasma Na+ concentration, i.e., by >8–10 meq/L in 24 h or 18 meq/L in 48 h. At presentation, she had no symptoms that one would typically attribute to acute hyponatremia, and the plasma Na+ concentration had already increased by a sufficient amount to protect from cerebral edema; however, she had corrected by 1 meq/L per hour within the first 7 h of admission, consistent with impending overcorrection. To reduce or halt the increase in plasma Na+ concentration, the patient received 1 μg of intravenous DDAVP along with intravenous free water. Given the hypovolemia and resolving acute renal insufficiency, a decision was made to administer half-normal saline as a source of free water, rather than D5W; this was switched to normal saline when plasma Na+ concentration acutely dropped to 117 meq/L (Table 64e-1).
Overcorrection of chronic hyponatremia is a major risk factor for the development of osmotic demyelination syndrome (ODS). Animal studies show a neurologic and survival benefit in ODS of “re-lowering” plasma Na+ concentration with DDAVP and free water administration; this approach is demonstrably safe in patients with hyponatremia, with no evident risk of seizure or other sequelae. This combination can be used to prevent an overcorrection or to re-lower plasma Na+ concentration in patients who have already overcorrected. DDAVP is required because in most of these patients endogenous AVP levels have plummeted, resulting in a free water diuresis; the administration of free water alone has minimal effect in this setting, given the relative absence of circulating AVP. An alternative approach in patients who present with severe hyponatremia is to treat them prospectively with twice-daily DDAVP to prevent changes in AVP bioactivity, coadministering hypertonic saline to increase slowly the plasma Na+ concentration in a more controlled fashion.
This patient’s plasma Na+ concentration remained depressed for several days after DDAVP administration. It is conceivable that residual hypovolemic hyponatremia attenuated the recovery of the plasma Na+ concentration. Alternatively, attenuated recovery was due to persistent effects of the single dose of DDAVP. Of note, although the plasma half-life of DDAVP is only 1–2 h, pharmacodynamic studies indicate a much more prolonged effect on urine output and/or urine osmolality. One final consideration is the effect of the patient’s initial renal dysfunction on the pharmacokinetics and pharmacodynamics of the administered DDAVP, which is renally excreted; DDAVP should be administered with caution for the reinduction of hyponatremia in patients with chronic kidney disease or acute renal dysfunction.
CASE 6
A 44-year-old woman was referred from a local hospital after presenting with flaccid paralysis. Severe hypokalemia was documented (2.0 meq/L), and an infusion containing KCl was initiated.
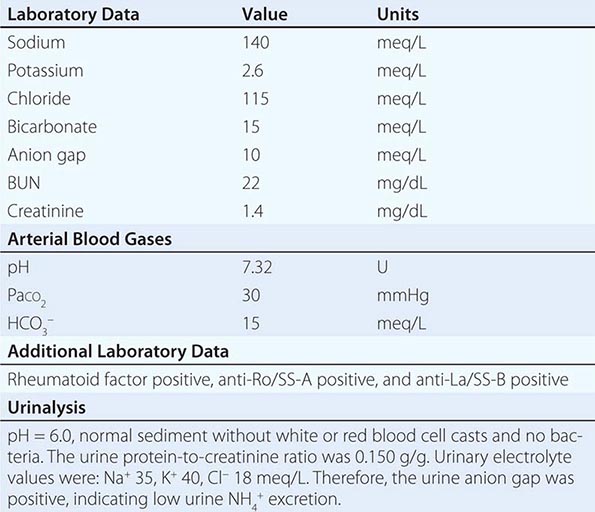
APPROACH TO DIAGNOSIS
The diagnosis in this case is classic hypokalemic dRTA from Sjögren’s syndrome. This patient presented with a non-AG metabolic acidosis. The urine AG was positive, indicating an abnormally low excretion of ammonium in the face of systemic acidosis. The urine pH was inappropriately alkaline, yet there was no evidence of hypercalciuria, nephrocalcinosis, or bone disease. The patient was subsequently shown to exhibit hyperglobulinemia. These findings, taken together, indicate that the cause of this patient’s hypokalemia and non-AG metabolic acidosis was a renal tubular abnormality. The hypokalemia and abnormally low excretion of ammonium, as estimated by the urine AG, in the absence of glycosuria, phosphaturia, or aminoaciduria (Fanconi’s syndrome), defines the entity of classic distal renal tubular acidosis (dRTA), also known as type 1 RTA. Because of the hyperglobulinemia, additional serology was obtained, providing evidence for the diagnosis of primary Sjögren’s syndrome. Furthermore, additional history indicated a 5-year history of xerostomia and keratoconjunctivitis sicca but without synovitis, arthritis, or rash.
Classic dRTA occurs frequently in patients with Sjögren’s syndrome and is a result of an immunologic attack on the collecting tubule, causing failure of the H+-ATPase to be inserted into the apical membrane of type A intercalated cells. Sjögren’s syndrome is one of the best-documented acquired causes of classic dRTA. The loss of H+-ATPase function also occurs with certain inherited forms of classic dRTA. There was no family history in the present case, and other family members were not affected. A number of autoantibodies have been associated with Sjögren’s syndrome; it is likely that these autoantibodies prevent trafficking or function of the H+-ATPase in the type A intercalated cell of the collecting tubule. Although proximal RTA has also been reported in patients with Sjögren’s syndrome, it is much less frequent, and there were no features of proximal tubule dysfunction (Fanconi’s syndrome) in this patient. The hypokalemia is due to secondary hyperaldosteronism from volume depletion.
APPROACH TO MANAGEMENT
The long-term renal prognosis for patients with classic dRTA due to Sjögren’s syndrome has not been established. Nevertheless, the metabolic acidosis and the hypokalemia respond to alkali replacement with either sodium citrate solution (Shohl’s solution) or sodium bicarbonate tablets. Obviously, potassium deficits must be replaced initially, but potassium replacement is usually not required in dRTA patients long term because sodium bicarbonate (or citrate) therapy expands volume and corrects the secondary hyperaldosteronism. A consequence of the interstitial infiltrate seen in patients with Sjögren’s syndrome and classic dRTA is progression of chronic kidney disease. Cytotoxic therapy plus glucocorticoids has been the mainstay of therapy in Sjögren’s syndrome for many years, although B lymphocyte infiltration in salivary gland tissue subsides and urinary acidification improves after treatment with rituximab.
CASE 7
A 32-year-old man was admitted to the hospital with weakness and hypokalemia. The patient had been very healthy until 2 months previously when he developed intermittent leg weakness. His review of systems was otherwise negative. He denied drug or laxative abuse and was on no medications. Past medical history was unremarkable, with no history of neuromuscular disease. Family history was notable for a sister with thyroid disease. Physical examination was notable only for reduced deep tendon reflexes.
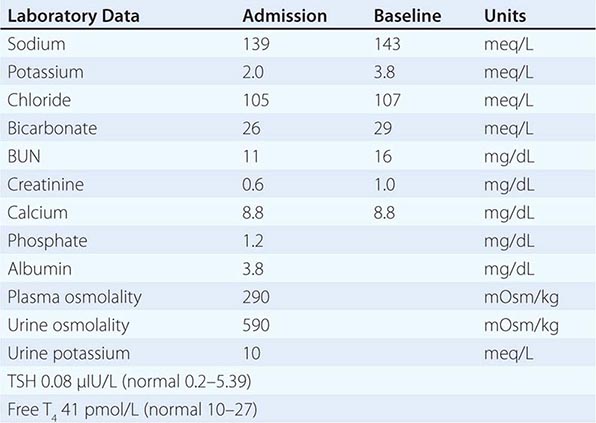
APPROACH TO DIAGNOSIS
This patient developed hypokalemia due to a redistribution of potassium between the intracellular and extracellular compartments; this pathophysiology was readily apparent following calculation of the TTKG. The TTKG is calculated as (Posmol × UPotassium)/(PPotassium × Uosmol). The expected values for the TTKG are <3 in the presence of hypokalemia and >7–8 in the presence of hyperkalemia (see also Case 2 and Case 8). Alternatively, a urinary K+-to-creatinine ratio of >13 mmol/g creatinine (>1.5 mmol/mmol creatinine) is compatible with excessive renal K+ excretion. In this case, the calculated TTKG was 2.5, consistent with appropriate renal conservation of K+ and a nonrenal cause for hypokalemia. In the absence of significant gastrointestinal loss of K+, the patient was diagnosed with a “redistributive” subtype of hypokalemia.
More than 98% of total-body potassium is intracellular; regulated buffering of extracellular K+ by this large intracellular pool plays a crucial role in the maintenance of a stable plasma K+ concentration. Clinically, changes in the exchange and distribution of intra- and extracellular K+ can cause significant hypo- or hyperkalemia. Insulin, β2-adrenergic activity, thyroid hormone, and alkalosis promote cellular uptake of K+ by multiple interrelated mechanisms, leading to hypokalemia. In particular, alterations in the activity of the endogenous sympathetic nervous system can cause hypokalemia in several settings, including alcohol withdrawal, hyperthyroidism, acute myocardial infarction, and severe head injury.
Weakness is common in severe hypokalemia; hypokalemia causes hyperpolarization of muscle, thereby impairing the capacity to depolarize and contract. In this particular patient, Graves’ disease caused hyperthyroidism and hypokalemic paralysis (thyrotoxic periodic paralysis [TPP]). TPP develops more frequently in patients of Asian or Hispanic origin. This predisposition has been linked to genetic variation in Kir2.6, a muscle-specific, thyroid hormone–induced K+ channel; however, the pathophysiologic mechanisms that link dysfunction of this ion channel to TPP have yet to be elucidated. The hypokalemia in TPP is attributed to both direct and indirect activation of the Na+/K+-ATPase by thyroid hormone, resulting in increased uptake of K+ by muscle and other tissues. Thyroid hormone induces expression of multiple subunits of the Na+/K+-ATPase in skeletal muscle, increasing the capacity for uptake of K+; hyperthyroid increases in β-adrenergic activity are also thought to play an important role in TPP.
Clinically, patients with TPP present with weakness of the extremities and limb girdle, with paralytic episodes that occur most frequently between 1 and 6 AM. Precipitants of weakness include high carbohydrate loads and strenuous exercise. Signs and symptoms of hyperthyroidism are not always present, often leading to delays in diagnosis. Hypokalemia is often profound and usually accompanied by redistributive hypophosphatemia, as in this case. A TTKG of <2–3 separates patients with TPP from those with hypokalemia due to renal potassium wasting, who will have TTKG values that are >4. This distinction is of considerable importance for therapy; patients with large potassium deficits require aggressive repletion with K+-Cl–, which has a significant risk of rebound hyperkalemia in TPP and related disorders.
APPROACH TO MANAGEMENT
Ultimately, definitive therapy for TPP requires treatment of the associated hyperthyroidism. In the short term, however, potassium replacement is necessary to hasten muscle recovery and prevent cardiac arrhythmias. The average recovery time of an acute attack is reduced by ~50% in patients treated with intravenous K+-Cl– at a rate of 10 meq/h; however, this incurs a significant risk of rebound hyperkalemia, with up to 70% developing a plasma K+ concentration of >5.0 meq/L. This potential for rebound hyperkalemia is a general problem in the management of all causes of redistributive hypokalemia, resulting in the need to distinguish these patients accurately and rapidly from those with a large K+ deficit due to renal or extrarenal loss of K+. An attractive alternative to K+-Cl– replacement in TPP is treatment with high-dose propranolol (3 mg/kg), which rapidly reverses the associated hypokalemia, hypophosphatemia, and paralysis. Notably, rebound hyperkalemia is not associated with this treatment.
CASE 8
A 66-year-old man was admitted to hospital with a plasma K+ concentration of 1.7 meq/L and profound weakness. The patient had noted progressive weakness over several days, to the point that he was unable to rise from bed. Past medical history was notable for small-cell lung cancer with metastases to brain, liver, and adrenals. The patient had been treated with one cycle of cisplatin/etoposide 1 year before this admission, which was complicated by acute kidney injury (peak creatinine of 5, with residual chronic kidney disease), and three subsequent cycles of cyclophosphamide/doxorubicin/vincristine, in addition to 15 treatments with whole-brain radiation.
On physical examination, the patient was jaundiced. Blood pressure was 130/70 mmHg, increasing to 160/98 mmHg after 1 L of saline, with a JVP at 8 cm. There was generalized muscle weakness.
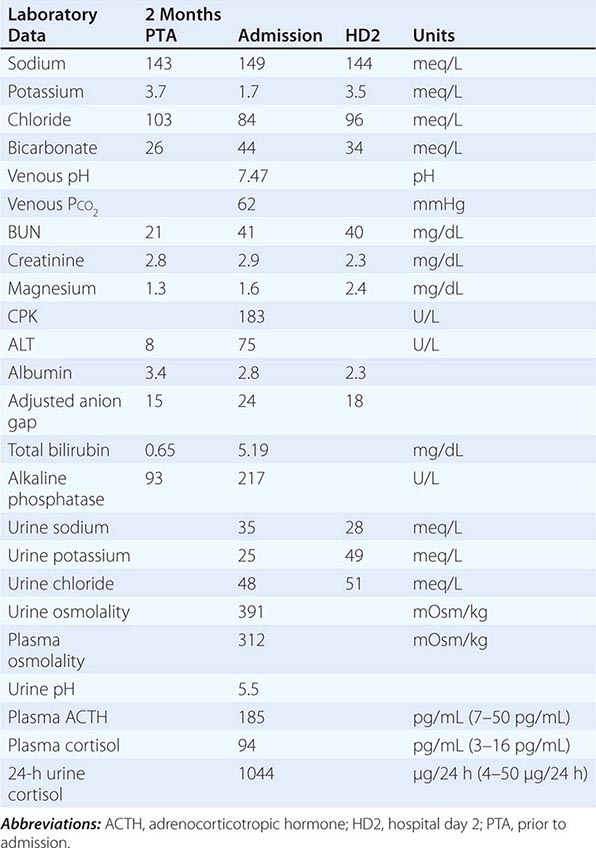
The patient’s hospital course was complicated by acute respiratory failure attributed to pulmonary embolism; he died 2 weeks after admission.
APPROACH TO DIAGNOSIS
Why was this patient hypokalemic? Why was he weak? Why did he have an alkalosis?
This patient suffered from metastatic small-cell lung cancer, which was persistent despite several rounds of chemotherapy and radiotherapy. He presented with profound hypokalemia, alkalosis, hypertension, severe weakness, jaundice, and worsening liver function tests.
With respect to the hypokalemia, there was no evident cause of nonrenal potassium loss, e.g., diarrhea. The urinary TTKG was 11.7, at a plasma K+ concentration of 1.7 meq/L; this TTKG value is consistent with inappropriate renal K+ secretion, despite severe hypokalemia. The TTKG is calculated as (Posmol × UPotassium)/(PPotassium × Uosmol). The expected values for the TTKG are <3 in the presence of hypokalemia and >7–8 in the presence of hyperkalemia (see also Case 2 and Case 6).
The patient had several explanations for excessive renal loss of potassium. First, he had had a history of cisplatin-associated acute kidney injury, with residual chronic kidney disease. Cisplatin can cause persistent renal tubular defects, with prominent hypokalemia and hypomagnesemia; however, this patient had not previously required potassium or magnesium repletion, suggesting that cisplatin-associated renal tubular defects did not play a major role in this presentation with severe hypokalemia. Second, he was hypomagnesemic on presentation, suggesting total-body magnesium depletion. Magnesium depletion has inhibitory effects on muscle Na+/K+-ATPase activity, reducing influx into muscle cells and causing a secondary increase in K+ excretion. Magnesium depletion also increases K+ secretion by the distal nephron; this is attributed to a reduction in the magnesium-dependent, intracellular block of K+ efflux through the secretory K+ channel of principal cells (ROMK, Fig. 64e-1). Clinically, hypomagnesemic patients are refractory to K+ replacement in the absence of Mg2+ repletion. Again, however, this patient had not previously developed significant hypokalemia, despite periodic hypomagnesemia, such that other factors must have caused the severe hypokalemia.
The associated hypertension in this case suggested an increase in mineralocorticoid activity, causing increased activity of ENaC channels in principal cells, NaCl retention, hypertension, and hypokalemia. The increase in ENaC-mediated Na+ transport in principal cells would have led to an increase in the lumen-negative potential difference in the connecting tubule and cortical collecting duct, driving an increase in K+ secretion through apical K+ channels (Fig. 64e-1). This explanation is compatible with the very high TTKG, i.e., an increase in K+ excretion that is inappropriate for the plasma K+ concentration.
What caused an increase in mineralocorticoid activity in this patient? The patient had bilateral adrenal metastases, indicating that primary hyperaldosteronism was unlikely. The clinical presentation (hypokalemia, hypertension, and alkalosis) and the history of small-cell lung cancer suggested Cushing’s syndrome, with a massive increase in circulating glucocorticoids, in response to ectopic adrenocorticotropic hormone (ACTH) secretion by his small-cell lung cancer tumor. Confirmation of this diagnosis was provided by a very high plasma cortisol level, high ACTH level, and increased urinary cortisol (see the laboratory data above).
Why would an increase in circulating cortisol cause an apparent increase in mineralocorticoid activity? Cortisol and aldosterone have equal affinity for the mineralocorticoid receptor (MLR); thus, cortisol has mineralocorticoid-like activity; however, cells in the aldosterone-sensitive distal nephron (the distal convoluted tubule [DCT]), connecting tubule (CNT), and collecting duct are protected from circulating cortisol by the enzyme 11β-hydroxysteroid dehydrogenase-2 (11βHSD-2), which converts cortisol to cortisone (Fig. 64e-2); cortisone has minimal affinity for the MLR. Activation of the MLR causes activation of the basolateral Na+/K+-ATPase, activation of the thiazide-sensitive Na+-Cl– cotransporter in the DCT, and activation of apical ENaC channels in principal cells of the CNT and collecting duct (Fig. 64e-2). Recessive loss-of-function mutations in the 11βHSD-2 gene lead to cortisol-dependent activation of the MLR and the syndrome of apparent mineralocorticoid excess (SAME), comprising hypertension, hypokalemia, hypercalciuria, and metabolic alkalosis, with suppressed plasma renin activity (PRA) and suppressed aldosterone. A similar syndrome is caused by biochemical inhibition of 11βHSD-2 by glycyrrhetinic/glycyrrhizinic acid (found in licorice, for example) and/or carbenoxolone.
FIGURE 64e-2 11β-Hydroxysteroid dehydrogenase-2 (11βHSD-2) and syndromes of apparent mineralocorticoid excess. The enzyme 11βHSD-2 protects cells in the aldosterone-sensitive distal nephron (the distal convoluted tubule [DCT ], connecting tubule [CNT], and collecting duct) from the illicit activation of mineralocorticoid receptors (MLR) by cortisol. Binding of aldosterone to the MLR leads to activation of the thiazide-sensitive Na+-Cl– cotransporter in DCT cells and the amiloride-sensitive epithelial sodium channel (ENaC) in principal cells (CNT and collecting duct). Aldosterone also activates basolateral Na+/K+-ATPase and, to a lesser extent, the apical secretory K+ channel ROMK (renal outer medullary K+ channel). Cortisol has equivalent affinity for the MLR to that of aldosterone; metabolism of cortisol to cortisone, which has no affinity for the MLR, prevents these cells from activation by circulating cortisol. Genetic deficiency of 11βHSD-2 or inhibition of its activity causes the syndromes of apparent mineralocorticoid excess (see Case 8).
In Cushing’s syndrome caused by increases in pituitary ACTH, the incidence of hypokalemia is only 10%, whereas it is ~70% in patients with ectopic secretion of ACTH, despite a similar incidence of hypertension. The activity of renal 11βHSD-2 is reduced in patients with ectopic ACTH compared with Cushing’s syndrome, resulting in SAME; the prevailing theory is that the much greater cortisol production in ectopic ACTH syndromes overwhelms the renal 11βHSD-2 enzyme, resulting in activation of renal MLRs by unmetabolized cortisol (Fig. 64e-2).
Why was the patient so weak? The patient was profoundly weak due to the combined effect of hypokalemia and increased cortisol. Hypokalemia causes hyperpolarization of muscle, thereby impairing the capacity to depolarize and contract. Weakness and even ascending paralysis can frequently complicate severe hypokalemia. Hypokalemia also causes a myopathy and predisposes to rhabdomyolysis; notably, however, the patient had a normal creatine phosphokinase (CPK) level. Cushing’s syndrome is often accompanied by a proximal myopathy, due to the protein-wasting effects of cortisol excess.
The patient presented with a mixed acid-base disorder, with a significant metabolic alkalosis and a bicarbonate concentration of 44 meq/L. A venous blood gas was drawn soon after his presentation; venous and arterial blood gases demonstrate a high level of agreement in hemodynamically stable patients, allowing for the interpretation of acid-base disorders with venous blood gas results. In response to his metabolic alkalosis, the PCO2 should have increased by 0.75 mmHg for each 1-meq/L increase in bicarbonate; the expected PCO2 should have been ~55 mmHg. Given the PCO2 of 62 mmHg, he had an additional respiratory acidosis, likely caused by respiratory muscle weakness from his acute hypokalemia and subacute hypercortisolism.
The patient’s albumin-adjusted AG was 21 + ([4 – 2.8] × 2.5) = 24; this suggests a third acid-base disorder, AG acidosis. Notably, the measured AG can increase in alkalosis, due to both increases in plasma protein concentrations (in hypovolemic alkalosis) and to the alkalemia-associated increase in net negative charge of plasma proteins, both causing an increase in unmeasured anions; however, this patient was neither volume-depleted nor particularly alkalemic, suggesting that these effects played a minimal role in his increased AG. Alkalosis also stimulates an increase in lactic acid production, due to activation of phosphofructokinase and accelerated glycolysis; unfortunately, however, a lactic acid level was not measured in this patient. It should be noted in this regard that alkalosis typically increases lactic acid levels by a mere 1.5–3 meq/L and that the patient was not significantly alkalemic. Regardless of the underlying pathophysiology, the increased AG was likely related to the metabolic alkalosis, given that the AG had decreased to 18 by hospital day 2, coincident with a reduction in plasma bicarbonate.
Why did the patient have a metabolic alkalosis? The activation of MLRs in the distal nephron increases distal nephron acidification and net acid secretion. In consequence, mineralocorticoid excess causes a saline-resistant metabolic alkalosis, which is exacerbated significantly by the development of hypokalemia. Hypokalemia plays a key role in the generation of most forms of metabolic alkalosis, stimulating proximal tubular ammonium production, proximal tubular bicarbonate reabsorption, and distal tubular H+/K+-ATPase activity.
APPROACH TO MANAGEMENT
The first priority in the management of this patient was to increase his plasma K+ and magnesium concentrations rapidly; hypomagnesemic patients are refractory to K+ replacement alone, resulting in the need to correct hypomagnesemia immediately. This was accomplished via the administration of both oral and intravenous K+-Cl–, giving a total of 240 meq over the first 18 h; 5 g of intravenous magnesium sulfate was also administered. Multiple 100-mL “minibags” of saline containing 20 meq each were infused, with cardiac monitoring and frequent measurement of plasma electrolytes. Of note, intravenous K+-Cl– should always be given in saline solutions because dextrose-containing solutions can increase insulin levels and exacerbate hypokalemia.
This case illustrates the difficulty in predicting the whole-body deficit of K+ in hypokalemic patients. In the absence of abnormal K+ redistribution, the total deficit correlates with plasma K+ concentration, which drops by approximately 0.27 mM for every 100-mmol reduction in total-body stores; this would suggest a deficit of ~650 meq of K+ in this patient, at the admission plasma K+ concentration of 1.7 meq/L. Notably, however, alkalemia induces a modest intracellular shift of circulating K+ such that this patient’s initial plasma K+ concentration was not an ideal indicator of the total potassium deficit. Regardless of the underlying pathophysiology in this case, close monitoring of plasma K+ concentration is always essential during the correction of severe hypokalemia in order to gauge the adequacy of repletion and to avoid overcorrection.
Subsequent management of this patient’s Cushing’s syndrome and ectopic ACTH secretion was complicated by the respiratory issues. The prognosis in patients with ectopic ACTH secretion depends on the tumor histology and the presence or absence of distant metastases. This patient had an exceptionally poor prognosis, with widely metastatic small-cell lung cancer that had failed treatment; other patients with ectopic ACTH secretion caused by more benign, isolated tumors, most commonly bronchial carcinoid tumors, have a much better prognosis. In the absence of successful surgical resection of the causative tumor, management of this syndrome can include surgical adrenalectomy or medical therapy to block adrenal steroid production.
CASE 9
A stuporous 22-year-old man was admitted with a history of behaving strangely. His friends indicated he experienced recent emotional problems stemming from a failed relationship and had threatened suicide. There was a history of alcohol abuse, but his friends were unaware of recent alcohol consumption. The patient was obtunded on admission, with no evident focal neurologic deficits. The remainder of the physical examination was unremarkable.
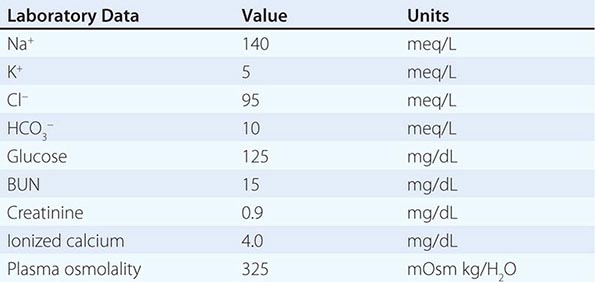
Urinalysis revealed crystalluria, with a mixture of envelope-shaped and needle-shaped crystals.
APPROACH TO DIAGNOSIS
This patient presented with CNS manifestations and a history of suspicious behavior, suggesting ingestion of a toxin. The AG was strikingly elevated at 35 meq/L. The ΔAG of 25 significantly exceeded the ΔHCO3– of 15. The fact that the Δ values were significantly disparate indicates that the most likely acid-base diagnosis in this patient is a mixed high-AG metabolic acidosis and a metabolic alkalosis. The metabolic alkalosis in this case may have been the result of vomiting. Nevertheless, the most useful finding is that the osmolar gap is elevated. The osmolar gap of 33 (difference in measured and calculated osmolality or 325 – 292) in the face of a high-AG metabolic acidosis is diagnostic of an osmotically active metabolite in plasma; a difference of >10 mOsm/kg indicates a significant concentration of an unmeasured osmolyte. Examples of toxic osmolytes include ethylene glycol, diethylene glycol, methanol, and propylene glycol.
Several caveats apply to the interpretation of the osmolar gap and AG in the differential diagnosis of toxic alcohol ingestions. First, unmeasured, neutral osmolytes can also accumulate in lactic acidosis and alcoholic ketoacidosis; i.e., an elevated osmolar gap is not specific to AG acidoses associated with toxic alcohol ingestions. Second, patients can present having extensively metabolized the ingested toxin, with an insignificant osmolar gap but a large AG; i.e., the absence of an elevated osmolar gap does not rule out toxic alcohol ingestion. Third, the converse can also be seen in patients who present earlier after ingestion of the toxin, i.e., a large osmolar gap with minimal elevation of the AG. Finally, clinicians should be aware of the effect of co-ingested ethanol, which can itself elevate the osmolar gap and can reduce metabolism of the toxic alcohols via competitive inhibition of alcohol dehydrogenase (see below), thus attenuating the expected increase in the AG.
Ethylene glycol is commonly available as antifreeze or solvents and may be ingested accidently or as a suicide attempt. The metabolism of ethylene glycol by alcohol dehydrogenase generates acids such as glycoaldehyde, glycolic acid, and oxalic acid. The initial effects of intoxication are on the CNS and, in the earliest stages, mimic inebriation, but may quickly progress to full-blown coma. Delay in treatment is one of the most common causes of mortality with toxic alcohol poisoning. The kidney shows evidence of acute tubular injury with widespread deposition of calcium oxalate crystals within tubular epithelial cells. Cerebral edema is common, as is crystal deposition in the brain; the latter is irreversible.
The co-occurrent crystalluria is typical of ethylene glycol intoxication; both needle-shaped monohydrate and envelope-shaped dihydrate calcium oxalate crystals can be seen in the urine as the process evolves. Circulating oxalate can also complex with plasma calcium, reducing the ionized calcium as in this case.
Although ethylene glycol intoxication should be verified by measuring ethylene glycol levels, therapy must be initiated immediately in this life-threatening situation. Although therapy can be initiated with confidence in cases with known or witnessed ingestions, such histories are rarely available. Therapy should thus be initiated in patients with severe metabolic acidosis and elevated anion and osmolar gaps. Other diagnostic features, such as hypocalcemia or acute renal failure with crystalluria, can provide important confirmation for urgent, empiric therapy.
APPROACH TO MANAGEMENT
Because all four osmotically active toxic alcohols—ethylene glycol, diethylene glycol, methanol, and propylene glycol—are metabolized by alcohol dehydrogenase to generate toxic products, competitive inhibition of this key enzyme is common to the treatment of all four intoxications. The most potent inhibitor of alcohol dehydrogenase, and the drug of choice in this circumstance, is fomepizole (4-methyl pyrazole). Fomepizole should be administered intravenously as a loading dose (15 mg/kg) followed by doses of 10 mg/kg every 12 h for four doses, and then 15 mg/kg every 12 h thereafter until ethylene glycol levels have been reduced to <20 mg/dL and the patient is asymptomatic with a normal pH. Additional important components of the treatment of toxic alcohol ingestion include fluid resuscitation, thiamine, pyridoxine, folate, sodium bicarbonate, and hemodialysis. Hemodialysis is used to remove both the parent compound and toxic metabolites, but it also removes administered fomepizole, necessitating adjustment of dosage frequency. Gastric aspiration, induced emesis, or the use of activated charcoal is only effective if initiated within 30–60 min after ingestion of the toxin. When fomepizole is not available, ethanol, which has more than 10-fold affinity for alcohol dehydrogenase compared to other alcohols, may be substituted and is quite effective. Ethanol must be administered IV to achieve a blood level of 22 meq/L (100 mg/dL). A disadvantage of ethanol is the obtundation that follows its administration, which is additive to the CNS effects of ethylene glycol. Furthermore, if hemodialysis is used, the infusion rate of ethanol must be increased because it is rapidly dialyzed. In general, hemodialysis is indicated for all patients with ethylene glycol intoxication when the arterial pH is <7.3 or the osmolar gap exceeds 20 mOsm/kg H2O.
65 |
Hypercalcemia and Hypocalcemia |
The calcium ion plays a critical role in normal cellular function and signaling, regulating diverse physiologic processes such as neuromuscular signaling, cardiac contractility, hormone secretion, and blood coagulation. Thus, extracellular calcium concentrations are maintained within an exquisitely narrow range through a series of feedback mechanisms that involve parathyroid hormone (PTH) and the active vitamin D metabolite 1,25-dihydroxyvitmin D [1,25(OH)2D]. These feedback mechanisms are orchestrated by integrating signals between the parathyroid glands, kidney, intestine, and bone (Fig. 65-1; Chap. 423). Disorders of serum calcium concentration are relatively common and often serve as a harbinger of underlying disease. This chapter provides a brief summary of the approach to patients with altered serum calcium levels. See Chap. 424 for a detailed discussion of this topic.
FIGURE 65-1 Feedback mechanisms maintaining extracellular calcium concentrations within a narrow, physiologic range (8.9–10.1 mg/dL [2.2–2.5 mM]). A decrease in extracellular (ECF) calcium (Ca2+) triggers an increase in parathyroid hormone (PTH) secretion (1) via the calcium sensor receptor on parathyroid cells. PTH, in turn, results in increased tubular reabsorption of calcium by the kidney (2) and resorption of calcium from bone (2) and also stimulates renal 1,25(OH)2D production (3). 1,25(OH)2D, in turn, acts principally on the intestine to increase calcium absorption (4). Collectively, these homeostatic mechanisms serve to restore serum calcium levels to normal.
HYPERCALCEMIA
ETIOLOGY
The causes of hypercalcemia can be understood and classified based on derangements in the normal feedback mechanisms that regulate serum calcium (Table 65-1). Excess PTH production, which is not appropriately suppressed by increased serum calcium concentrations, occurs in primary neoplastic disorders of the parathyroid glands (parathyroid adenomas; hyperplasia; or, rarely, carcinoma) that are associated with increased parathyroid cell mass and impaired feedback inhibition by calcium. Inappropriate PTH secretion for the ambient level of serum calcium also occurs with heterozygous inactivating calcium sensor receptor (CaSR) or G protein mutations, which impair extracellular calcium sensing by the parathyroid glands and the kidneys, resulting in familial hypocalciuric hypercalcemia (FHH). Although PTH secretion by tumors is extremely rare, many solid tumors produce PTH-related peptide (PTHrP), which shares homology with PTH in the first 13 amino acids and binds the PTH receptor, thus mimicking effects of PTH on bone and the kidney. In PTHrP-mediated hypercalcemia of malignancy, PTH levels are suppressed by the high serum calcium levels. Hypercalcemia associated with granulomatous disease (e.g., sarcoidosis) or lymphomas is caused by enhanced conversion of 25(OH) D to the potent 1,25(OH)2D. In these disorders, 1,25(OH)2D enhances intestinal calcium absorption, resulting in hypercalcemia and suppressed PTH. Disorders that directly increase calcium mobilization from bone, such as hyperthyroidism or osteolytic metastases, also lead to hypercalcemia with suppressed PTH secretion as does exogenous calcium overload, as in milk-alkali syndrome, or total parenteral nutrition with excessive calcium supplementation.
|
CAUSES OF HYPERCALCEMIA |
Abbreviations: CaSR, calcium sensor receptor; FHH, familial hypocalciuric hypercalcemia; PTH, parathyroid hormone; PTHrP, PTH-related peptide.
CLINICAL MANIFESTATIONS
Mild hypercalcemia (up to 11–11.5 mg/dL) is usually asymptomatic and recognized only on routine calcium measurements. Some patients may complain of vague neuropsychiatric symptoms, including trouble concentrating, personality changes, or depression. Other presenting symptoms may include peptic ulcer disease or nephrolithiasis, and fracture risk may be increased. More severe hypercalcemia (>12–13 mg/dL), particularly if it develops acutely, may result in lethargy, stupor, or coma, as well as gastrointestinal symptoms (nausea, anorexia, constipation, or pancreatitis). Hypercalcemia decreases renal concentrating ability, which may cause polyuria and polydipsia. With long-standing hyperparathyroidism, patients may present with bone pain or pathologic fractures. Finally, hypercalcemia can result in significant electrocardiographic changes, including bradycardia, AV block, and short QT interval; changes in serum calcium can be monitored by following the QT interval.
DIAGNOSTIC APPROACH
The first step in the diagnostic evaluation of hyper- or hypocalcemia is to ensure that the alteration in serum calcium levels is not due to abnormal albumin concentrations. About 50% of total calcium is ionized, and the rest is bound principally to albumin. Although direct measurements of ionized calcium are possible, they are easily influenced by collection methods and other artifacts; thus, it is generally preferable to measure total calcium and albumin to “correct” the serum calcium. When serum albumin concentrations are reduced, a corrected calcium concentration is calculated by adding 0.2 mM (0.8 mg/dL) to the total calcium level for every decrement in serum albumin of 1.0 g/dL below the reference value of 4.1 g/dL for albumin, and, conversely, for elevations in serum albumin.
A detailed history may provide important clues regarding the etiology of the hypercalcemia (Table 65-1). Chronic hypercalcemia is most commonly caused by primary hyperparathyroidism, as opposed to the second most common etiology of hypercalcemia, an underlying malignancy. The history should include medication use, previous neck surgery, and systemic symptoms suggestive of sarcoidosis or lymphoma.
Once true hypercalcemia is established, the second most important laboratory test in the diagnostic evaluation is a PTH level using a two-site assay for the intact hormone. Increases in PTH are often accompanied by hypophosphatemia. In addition, serum creatinine should be measured to assess renal function; hypercalcemia may impair renal function, and renal clearance of PTH may be altered depending on the fragments detected by the assay. If the PTH level is increased (or “inappropriately normal”) in the setting of elevated calcium and low phosphorus, the diagnosis is almost always primary hyperparathyroidism. Because individuals with FHH may also present with mildly elevated PTH levels and hypercalcemia, this diagnosis should be considered and excluded because parathyroid surgery is ineffective in this condition. A calcium/creatinine clearance ratio (calculated as urine calcium/serum calcium divided by urine creatinine/serum creatinine) of <0.01 is suggestive of FHH, particularly when there is a family history of mild, asymptomatic hypercalcemia. In addition, sequence analysis of the CaSR gene is now commonly performed for the definitive diagnosis of FHH, although in some families, FHH may be caused by mutations in G proteins that mediate signaling by the CaSR. Ectopic PTH secretion is extremely rare.
A suppressed PTH level in the face of hypercalcemia is consistent with non-parathyroid-mediated hypercalcemia, most often due to underlying malignancy. Although a tumor that causes hypercalcemia is generally overt, a PTHrP level may be needed to establish the diagnosis of hypercalcemia of malignancy. Serum 1,25(OH)2D levels are increased in granulomatous disorders, and clinical evaluation in combination with laboratory testing will generally provide a diagnosis for the various disorders listed in Table 65-1.
HYPOCALCEMIA
ETIOLOGY
The causes of hypocalcemia can be differentiated according to whether serum PTH levels are low (hypoparathyroidism) or high (secondary hyperparathyroidism). Although there are many potential causes of hypocalcemia, impaired PTH production and impaired vitamin D production are the most common etiologies (Table 65-2) (Chap. 424). Because PTH is the main defense against hypocalcemia, disorders associated with deficient PTH production or secretion may be associated with profound, life-threatening hypocalcemia. In adults, hypoparathyroidism most commonly results from inadvertent damage to all four glands during thyroid or parathyroid gland surgery. Hypoparathyroidism is a cardinal feature of autoimmune endocrinopathies (Chap. 408); rarely, it may be associated with infiltrative diseases such as sarcoidosis. Impaired PTH secretion may be secondary to magnesium deficiency or to activating mutations in the CaSR or in the G proteins that mediate CaSR signaling, which suppress PTH, leading to effects that are opposite to those that occur in FHH.
|
CAUSES OF HYPOCALCEMIA |
Abbreviations: CaSR, calcium sensor receptor; PTH, parathyroid hormone.
Vitamin D deficiency, impaired 1,25(OH)2D production (primarily secondary to renal insufficiency), or vitamin D resistance also cause hypocalcemia. However, the degree of hypocalcemia in these disorders is generally not as severe as that seen with hypoparathyroidism because the parathyroids are capable of mounting a compensatory increase in PTH secretion. Hypocalcemia may also occur in conditions associated with severe tissue injury such as burns, rhabdomyolysis, tumor lysis, or pancreatitis. The cause of hypocalcemia in these settings may include a combination of low albumin, hyperphosphatemia, tissue deposition of calcium, and impaired PTH secretion.
CLINICAL MANIFESTATIONS
Patients with hypocalcemia may be asymptomatic if the decreases in serum calcium are relatively mild and chronic, or they may present with life-threatening complications. Moderate to severe hypocalcemia is associated with paresthesias, usually of the fingers, toes, and circumoral regions, and is caused by increased neuromuscular irritability. On physical examination, a Chvostek’s sign (twitching of the circumoral muscles in response to gentle tapping of the facial nerve just anterior to the ear) may be elicited, although it is also present in ~10% of normal individuals. Carpal spasm may be induced by inflation of a blood pressure cuff to 20 mmHg above the patient’s systolic blood pressure for 3 min (Trousseau’s sign). Severe hypocalcemia can induce seizures, carpopedal spasm, bronchospasm, laryngospasm, and prolongation of the QT interval.
DIAGNOSTIC APPROACH
In addition to measuring serum calcium, it is useful to determine albumin, phosphorus, and magnesium levels. As for the evaluation of hypercalcemia, determining the PTH level is central to the evaluation of hypocalcemia. A suppressed (or “inappropriately low”) PTH level in the setting of hypocalcemia establishes absent or reduced PTH secretion (hypoparathyroidism) as the cause of the hypocalcemia. Further history will often elicit the underlying cause (i.e., parathyroid agenesis vs. destruction). By contrast, an elevated PTH level (secondary hyperparathyroidism) should direct attention to the vitamin D axis as the cause of the hypocalcemia. Nutritional vitamin D deficiency is best assessed by obtaining serum 25-hydroxyvitamin D levels, which reflect vitamin D stores. In the setting of renal insufficiency or suspected vitamin D resistance, serum 1,25(OH)2D levels are informative.
GLOBAL CONSIDERATIONS
![]() In countries with more limited access to health care or screening laboratory testing of serum calcium levels, primary hyperparathyroidism often presents in its severe form with skeletal complications (osteitis fibrosa cystica) in contrast to the asymptomatic form that is common in developed countries. In addition, vitamin D deficiency is paradoxically common in some countries despite extensive sunlight (e.g., India) due to avoidance of sun exposure and poor dietary vitamin D intake.
In countries with more limited access to health care or screening laboratory testing of serum calcium levels, primary hyperparathyroidism often presents in its severe form with skeletal complications (osteitis fibrosa cystica) in contrast to the asymptomatic form that is common in developed countries. In addition, vitamin D deficiency is paradoxically common in some countries despite extensive sunlight (e.g., India) due to avoidance of sun exposure and poor dietary vitamin D intake.
66 |
Acidosis and Alkalosis |
NORMAL ACID-BASE HOMEOSTASIS
Systemic arterial pH is maintained between 7.35 and 7.45 by extracellular and intracellular chemical buffering together with respiratory and renal regulatory mechanisms. The control of arterial CO2 tension (PaCO2) by the central nervous system (CNS) and respiratory system and the control of plasma bicarbonate by the kidneys stabilize the arterial pH by excretion or retention of acid or alkali. The metabolic and respiratory components that regulate systemic pH are described by the Henderson-Hasselbalch equation:
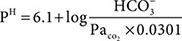
Under most circumstances, CO2 production and excretion are matched, and the usual steady-state PaCO2 is maintained at 40 mmHg. Underexcretion of CO2 produces hypercapnia, and overexcretion causes hypocapnia. Nevertheless, production and excretion are again matched at a new steady-state PaCO2. Therefore, the PaCO2 is regulated primarily by neural respiratory factors and is not subject to regulation by the rate of CO2 production. Hypercapnia is usually the result of hypoventilation rather than of increased CO2 production. Increases or decreases in PaCO2 represent derangements of neural respiratory control or are due to compensatory changes in response to a primary alteration in the plasma [HCO3–].
DIAGNOSIS OF GENERAL TYPES OF DISTURBANCES
The most common clinical disturbances are simple acid-base disorders; i.e., metabolic acidosis or alkalosis or respiratory acidosis or alkalosis.
SIMPLE ACID-BASE DISORDERS
Primary respiratory disturbances (primary changes in PaCO2) invoke compensatory metabolic responses (secondary changes in [HCO3–]), and primary metabolic disturbances elicit predictable compensatory respiratory responses (secondary changes in PaCO2). Physiologic compensation can be predicted from the relationships displayed in Table 66-1. In general, with one exception, compensatory responses return the pH toward, but not to, the normal value. Chronic respiratory alkalosis when prolonged is an exception to this rule and often returns the pH to a normal value. Metabolic acidosis due to an increase in endogenous acids (e.g., ketoacidosis) lowers extracellular fluid [HCO3–] and decreases extracellular pH. This stimulates the medullary chemoreceptors to increase ventilation and to return the ratio of [HCO3–] to PaCO2, and thus pH, toward, but not to, normal. The degree of respiratory compensation expected in a simple form of metabolic acidosis can be predicted from the relationship: PaCO2 = (1.5 × [HCO3–]) + 8 ± 2. Thus, a patient with metabolic acidosis and [HCO3–] of 12 mmol/L would be expected to have a PaCO2 between 24 and 28 mmHg. Values for PaCO2 <24 or >28 mmHg define a mixed disturbance (metabolic acidosis and respiratory alkalosis or metabolic alkalosis and respiratory acidosis, respectively). Compensatory responses for primary metabolic disorders move the PaCO2 in the same direction as the change in [HCO3–], whereas, conversely, compensation for primary respiratory disorders moves the [HCO3–] in the same direction as the primary change in PaCO2 (Table 66-1). Therefore, changes in PaCO2 and [HCO3–] in opposite directions (i.e., PaCO2 or [HCO3–] is increased, whereas the other value is decreased) indicate a mixed disturbance. Another way to judge the appropriateness of the response in [HCO3–] or PaCO2 is to use an acid-base nomogram (Fig. 66-1). While the shaded areas of the nomogram show the 95% confidence limits for normal compensation in simple disturbances, finding acid-base values within the shaded area does not necessarily rule out a mixed disturbance. Imposition of one disorder over another may result in values lying within the area of a third. Thus, the nomogram, while convenient, is not a substitute for the equations in Table 66-1.
|
PREDICTION OF COMPENSATORY RESPONSES ON SIMPLE ACID-BASE DISTURBANCES AND PATTERN OF CHANGES |
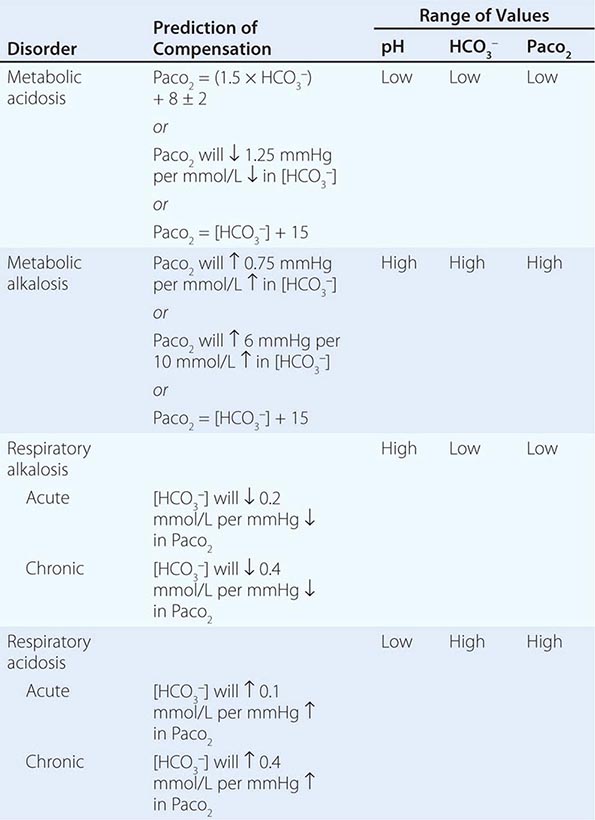
FIGURE 66-1 Acid-base nomogram. Shown are the 90% confidence limits (range of values) of the normal respiratory and metabolic compensations for primary acid-base disturbances. (From TD DuBose Jr: Acid-base disorders, in Brenner and Rector’s The Kidney, 8th ed, BM Brenner [ed]. Philadelphia, Saunders, 2008, pp 505–546, with permission.)
MIXED ACID-BASE DISORDERS
Mixed acid-base disorders—defined as independently coexisting disorders, not merely compensatory responses—are often seen in patients in critical care units and can lead to dangerous extremes of pH (Table 66-2). A patient with diabetic ketoacidosis (metabolic acidosis) may develop an independent respiratory problem (e.g., pneumonia) leading to respiratory acidosis or alkalosis. Patients with underlying pulmonary disease (e.g., chronic obstructive pulmonary disease) may not respond to metabolic acidosis with an appropriate ventilatory response because of insufficient respiratory reserve. Such imposition of respiratory acidosis on metabolic acidosis can lead to severe acidemia. When metabolic acidosis and metabolic alkalosis coexist in the same patient, the pH may be normal or near normal. When the pH is normal, an elevated anion gap (AG; see below) reliably denotes the presence of an AG metabolic acidosis at a normal serum albumin of 4.5 g/dL. Assuming a normal AG of 10 mmol/L, a discrepancy in the ΔAG (prevailing minus normal AG) and the ΔHCO3– (normal value of 25 mmol/L minus abnormal HCO3– in the patient) indicates the presence of a mixed high-gap acidosis—metabolic alkalosis (see example below). A diabetic patient with ketoacidosis may have renal dysfunction resulting in simultaneous metabolic acidosis. Patients who have ingested an overdose of drug combinations such as sedatives and salicylates may have mixed disturbances as a result of the acid-base response to the individual drugs (metabolic acidosis mixed with respiratory acidosis or respiratory alkalosis, respectively). Triple acid-base disturbances are more complex. For example, patients with metabolic acidosis due to alcoholic ketoacidosis may develop metabolic alkalosis due to vomiting and superimposed respiratory alkalosis due to the hyperventilation of hepatic dysfunction or alcohol withdrawal.
|
EXAMPLES OF MIXED ACID-BASE DISORDERS |
Abbreviations: AG, anion gap; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
METABOLIC ACIDOSIS
Metabolic acidosis can occur because of an increase in endogenous acid production (such as lactate and ketoacids), loss of bicarbonate (as in diarrhea), or accumulation of endogenous acids (as in renal failure). Metabolic acidosis has profound effects on the respiratory, cardiac, and nervous systems. The fall in blood pH is accompanied by a characteristic increase in ventilation, especially the tidal volume (Kussmaul respiration). Intrinsic cardiac contractility may be depressed, but inotropic function can be normal because of catecholamine release. Both peripheral arterial vasodilation and central venoconstriction can be present; the decrease in central and pulmonary vascular compliance predisposes to pulmonary edema with even minimal volume overload. CNS function is depressed, with headache, lethargy, stupor, and, in some cases, even coma. Glucose intolerance may also occur.
There are two major categories of clinical metabolic acidosis: high-AG and non-AG, or hyperchloremic, acidosis (Table 66-3 and Table 66-4).