179 Hydrocarbons
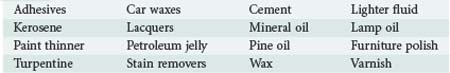
Hydrocarbons are a diverse array of chemicals composed exclusively of hydrogen and carbon atoms. Some hydrocarbon derivatives such as various halogenated hydrocarbons also contain other elements. They are ubiquitous in daily life and include plant and animal fats, alcohols, solvents, natural gas, petroleum derivates, and a host of industrial chemicals (Table 179-1). Many exist in complex mixtures. This chapter focuses on the toxicity of petroleum distillates, which represents several hundred compounds arising from crude oil.1
 Chemistry
Chemistry
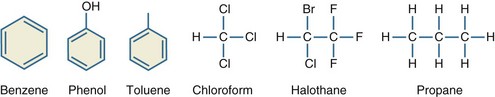
Halogenated hydrocarbons are hydrocarbons with fluorine, chlorine, bromine, or iodine substitutions. Medicinal uses for halogenated hydrocarbons include anesthetics such as halothane, propellants for inhalers, and chloral hydrate for sedation. Refrigerants such as Freon are mixtures of halogenated hydrocarbons (Figure 179-1).2
 Epidemiology
Epidemiology
The Toxic Exposures Surveillance System database maintained by the American Academy of Poison Control Centers reported 46,357 hydrocarbon exposures and 11 deaths in 2008. Eighty-seven percent of all exposures were unintentional or accidental, and 31% occurred among patients younger than 6 years of age.3 The number of hydrocarbon-related calls made by the public or healthcare providers to poison centers over the past decade are decreasing.4 This number certainly underrepresents the actual number of annual exposures.
Determining the incidence of chronic exposures is even more difficult. A 1993 World Health Organization report estimated that 238,000 U.S. workers annually were exposed to benzene.5 Petrochemical workers, rubber workers, shoe manufacturers, and printers all have workplace exposures to benzene,6 but second-hand cigarette smoke, products from gasoline combustion, and industrial emissions expose virtually everyone to benzene, at least occasionally.7,8 Because of its known tendency to promote development of hematologic malignancies, benzene has been extensively studied in terms of its toxic effects. Many other hydrocarbons are encountered in daily life, and the effects of low-level exposures to these compounds are unclear. More than 1 million workers are exposed annually to kerosene and its byproducts.9 Toluene is present in the air in most urban and suburban environments at concentrations up to 6.6 parts per billion (ppb), and it can be found in higher concentrations in soil and water.10
Intentional abuse of inhaled hydrocarbons is a particularly dangerous form of hydrocarbon exposure. In 2007, 13.3% of high school students reported inhalant abuse.11 Among delinquent youth, 38.5% reported inhalant abuse, and 28.3% of inhalant abusers met DSM-IV dependence criteria.12,13 Determining the epidemiology of inhalant abuse is difficult because of the poor reliability of self-reported data and the wide availability of inhalants.14 The use of inhalants as a “gateway drug” is concerning because of the potential for more serious drug abuse later in life.15 Among students, the highest rates of abuse occur in women, Hispanics, and people in rural communities.16
Depending on the type of chemical abused, inhalants are generally categorized as gases, nitrates, solvents, or aerosols.17 Gases are compressed hydrocarbons such as refrigerants, propane, butane, and inhalational anesthetics. Nitrates, or “poppers,” are used as smooth-muscle relaxants to heighten sexual experiences. Solvents are a diverse group of liquids with relatively high vapor pressures and include glues, fuels, paint thinners, and the liquid in felt-tip markers. Aerosols are hydrocarbon-based propellants found in spray bottles. These bottles contain both a gas propellant and a solvent, either of which may be abused. Inverting the can and activating the nozzle selectively releases the gas propellant. Once the gas is released, puncturing the can yields access to the liquid solvent.
Differing methods of inhalant abuse include sniffing, snorting, huffing, and bagging. Sniffing is the passive inhalation of gaseous fumes from a container; snorting refers to insufflation of liquid hydrocarbons into the nasal passageways; huffing is the inhalation of fumes from a rag soaked in solvent; and bagging is the inhalation of fumes from a solvent placed into a paper or plastic bag.18,19
 General Management
General Management
Management of hydrocarbon toxicity depends upon the route of exposure (Table 179-2). Hydrocarbon ingestion without aspiration typically results in mild symptoms. Hydrocarbons are gastric irritants, and 35% to 51% of patients will spontaneously vomit.20–23 Hemorrhagic gastritis following ingestion has been reported.24 If aspiration does not occur, the outcome from ingestion of a hydrocarbon is usually good, especially if respiratory symptoms are absent for 6 to 8 hours and the chest radiograph is normal.3,20–23,25,26
| Agent | Toxicity |
|---|---|
| Pentachlorophenol | Oxidative phosphorylation uncoupler |
| Formaldehyde | Irritant, respiratory sensitizer, allergen |
| Diisocyanates | Respiratory sensitizer, allergen |
| Perchloroethylene (PERC) | Central nervous system depression, cardiac sensitization |
| Bromomethane | Severe neurotoxicity |
| Hydrazine-containing fuel | Seizures, pyridoxine depletion |
Given that hydrocarbon toxicity primarily results from pulmonary aspiration, gastric lavage and induced emesis with ipecac should be avoided.26–28 Lavage, spontaneous emesis, and ipecac all increase the risk for hydrocarbon aspiration with subsequent pneumonitis.20,22,26,29 However, it is appropriate to perform gastric lavage or induce emesis when the ingested hydrocarbon is known to cause systemic toxicities, the volume of hydrocarbon ingested is very large, or the hydrocarbon has been ingested along with one or more other dangerous substances.28 Activated charcoal fails to adsorb most hydrocarbons, and its use is not routinely recommended.30,31 Material Safety Data Sheets (MSDS), the Micromedex database, or poison control centers can help identify hydrocarbons that warrant decontamination efforts.
 Nonspecific Symptoms of Aspiration
Nonspecific Symptoms of Aspiration
Fever and leukocytosis are common after hydrocarbon aspiration. Fever occurs in up to 73% of ingestions and 93% of intentional aspirations.20,23,27,32 Approximately 30% of patients with fever are otherwise asymptomatic.22,23 In one study, fever resolved after 24 hours in 41% of patients; 5% of patients had persistent fever lasting longer than 5 days.27 Another study found that fever resolved after an average of 1.25 days.33 Heating or burning hydrocarbons results in the production of many airborne molecules. Inhalation of these molecules can result in a prolonged fever referred to as polymer fume fever.34,35 One study of patients with hydrocarbon ingestion found leukocytosis in 75% of those with clinical pneumonia, versus only 32% in those without pneumonia. Both groups had a similar percentage of patients with a left shift on the differential white blood cell count.23
 Pulmonary Toxicity
Pulmonary Toxicity
Aspiration of hydrocarbons results in a lipoid pneumonia. Chronic ingestion of hydrocarbons among patients with gastroesophageal reflux disease can result in slowly developing symptoms.36,37 Intravenous injection of hydrocarbons can result in a lipoid pneumonia or vascular hydrocarbon emboli.38–41 A case of lipoid pneumonia due to dermal absorption of hydrocarbons in a patient with severe psoriasis was reported.42 This patient suffered from severe psoriasis and applied large amounts of petroleum jelly to her skin for 10 days prior to evidence of lipoid pneumonia. Some halogenated hydrocarbons such as trichloroethylene are mucosal irritants which can induce caustic pneumonitis.43–45 Respiratory tract sensitization and reactive airway disease can occur following repeated exposure to certain hydrocarbons.46–51 Rarely, the irritant effects of some hydrocarbons can result in upper airway injury and obstruction.52
Gastrointestinal absorption plays a minor role in toxicity. Experimental canine and primate models in which esophageal ligation was performed prior to instillation of kerosene by gastrostomy failed to demonstrate pulmonary injury in any of the animals.25,53,54 However, small doses of hydrocarbons administered intratracheally resulted in severe pulmonary toxicity.55–58
Aspiration occurs with the inhalation of a hydrocarbon that exists as a liquid under ambient conditions of temperature and pressure. The risk for pulmonary toxicity is determined in part by the physical properties of liquid hydrocarbons, including surface tension, viscosity, and volatility.59,60 Surface tension refers to the cohesion of molecules generated by van der Waals forces. Materials with low surface tension tend to spread over an area, and therefore these substances are more likely to be aspirated. Viscosity measures the resistance of a fluid to flow. Liquids with low viscosity are more likely to be aspirated.61 Volatility refers to the tendency of a liquid to vaporize into a gaseous state. Volatile hydrocarbons are more lipid soluble and more easily disrupt surfactant layers and/or cell membranes, thereby predisposing to toxicity.61,62 However, hydrocarbons that exist as gases in ambient conditions cannot be aspirated and do not cause lipoid pneumonia. An example is propane, a gas that is purchased as a compressed liquid but which volatilizes completely and rapidly upon return to normal atmospheric pressure. The clinical effects of these gases result from hypoxia and central nervous system (CNS) depression.63–65 European regulation of the allowable viscosity, volatility, and surface tension of lamp oils has not led to an appreciable decline in the incidence of patients developing lipoid pneumonia.66
Multiple mechanisms of pulmonary injury occur in hydrocarbon aspiration. Microscopic findings include thick hyaline membranes in air spaces, capillary distension, vascular thrombosis, intraalveolar hemorrhage, hyperemia, neutrophilic or lymphocytic alveolitis, and bronchial necrosis.37,67,68 The most characteristic finding is the presence of lipid-laden macrophages.67 Foreign body granulomas or “parafinomas” following aspiration have been reported.70,71 Bronchoalveolar lavage (BAL) reveals thick or greasy fluid. Oil red O staining of the fluid can confirm the presence of exogenous lipids, and polymorphonuclear exudates or hemorrhagic secretions can be present.38,72,73 Animal models reveal an early exudative phase characterized by the presence of red blood cells, macrophages, and edema fluid in alveolar airspaces along with diminished lung compliance. This early phase is followed by a secondary phase of proliferative bronchiolitis.68,74 Disruption of the pulmonary surfactant layer from hydrocarbons exacerbates ventilation/perfusion mismatching and decreases pulmonary compliance.75,76
The diagnosis of hydrocarbon aspiration is usually suggested by the history. Coughing, gagging, or choking following ingestion of hydrocarbons portends the development of pulmonary injury, although nearly a third of patients with early symptoms do not develop significant toxicity.22,23,77 Hypoxemia, respiratory distress, and physical examination evidence of pneumonia develop rapidly, although delayed onset of these symptoms has been reported.32,78 Lung function studies reveal a restrictive or obstructive pattern.37 In cases of respiratory distress where it is unknown if hydrocarbon aspiration occurred, bronchoalveolar lavage or lung biopsy can be diagnostic. Uncommon complications of aspiration include the development of pneumatoceles, cavitary lesions, abscesses, lung necrosis, bronchopleural fistula, pneumothorax or empyema.23,71,79–82
Radiographic findings are variable. Ninety percent of patients with pulmonary symptoms have abnormal radiographs on arrival, and nearly all develop abnormalities by 6 hours.22 Interestingly, chest radiograph abnormalities in the absence of respiratory symptoms are common. Fifty percent of asymptomatic patients have abnormal chest radiographs, and of these patients with abnormal roentgenographic findings, only 5% go on to develop significant toxicity.22,27 Chest radiographs can reveal areas of consolidation, atelectasis, fibrosis, ground-glass opacities, or pleural effusions.23,37,83 Bibasilar interstitial or right lobar findings are the most common and can develop within an hour of aspiration.20,23,27 Computerized tomography (CT) reveals airspace consolidation with areas of low attenuation and air bronchograms. Ground-glass opacities, airspace nodules, and/or crazed paving patterns can be seen.84,85 Areas of fat attenuation within pulmonary opacities can be diagnostic, although inflammatory infiltrates can mask this finding.37,83 Magnetic resonance imaging (MRI) reveals T1 hyperintensities consistent with, though not specific for, lipid content.86,87 Chemical shift MR with opposed-phase imaging is sensitive for detecting lipids and can provide a specific test for lipoid pneumonia if available.88 Positron emission tomography (PET) scanning of a patient suspected to have a malignancy but later found to have exogenous lipoid pneumonia revealed a high standard uptake value.89
There are limited data on outcomes following hydrocarbon aspiration. A follow-up of 17 children 8 to 14 years after exposure found that 82% had one or more pulmonary function abnormalities.90 A separate study found normal pulmonary function in 3 children exposed 8 to 10 years earlier.91 A retrospective review of 44 adult patients with chronic lipoid pneumonia found that 21% developed complications including pulmonary fibrosis, recurrent infections in the region of injury, and Aspergillus-related diseases.37
Management of Pulmonary Toxicity
Management of hydrocarbon aspiration focuses on respiratory support. β-Adrenergic agonists are indicated for treatment of bronchospasm.67 Ventilation with high levels of positive end-expiratory pressure and recruitment maneuvers can improve gas exchange.38 High-frequency percussive ventilation resulted in significant clinical improvement in a patient who deteriorated after multiple modes of ventilation had failed, and mobilized a large amount of thick oily secretions.92 Clinical improvement has been reported with high-frequency oscillation or high-frequency jet ventilation93–95 and extracorporeal membrane oxygenation.96
Therapy with corticosteroids remains controversial because human data are limited. A double-blind placebo-controlled trial of 71 children with hydrocarbon poisoning did not reveal any difference between treatment groups.33 There are many case reports with variable outcomes following both oral and inhaled corticosteroid use.37,68,72,97–99 Various animal models have shown no difference in outcome100–102 or worsened outcome due to increased infectious complications.103
Aspiration and the subsequent presence of pneumonia, fever, radiographic findings, and leukocytosis make antibiotic use common, but no controlled human data demonstrate the value of antibiotics. Various animal models have shown no difference in rates of infection when prophylactic antibiotics were given.100,101,103 Given the limitations in data, the authors feel that routine administration of antibiotics is not supported by the literature. We recommend antibiotics only for patients with persistent fever lasting longer than 24 hours, patients with peripheral white blood cell count higher than 20,000 cells/µL, or patients with deteriorating clinical status after 24 hours.
Many additional therapies have been used for lipoid pneumonia. Surfactant therapy for acute respiratory distress syndrome (ARDS) is controversial, but there are reports of successful use of this strategy in cases of hydrocarbon aspiration.75,104,105 An ovine hydrocarbon aspiration model found 100% survival with surfactant therapy versus 25% survival with saline, although all animals were sacrificed at 6 hours.106 A patient with prolonged respiratory compromise underwent lung lavage on hospital day 49. Polysorbate 80 in Ringer’s lactate was used until the effluent was clear of lipid, followed by surfactant instillation. This resulted in clinical and lung aeration improvements.107 Nitric oxide along with high-frequency oscillatory ventilation was used successfully in a pediatric patient.94 A rabbit model using partial liquid ventilation and inhaled nitric oxide showed improvements in gas exchange.108 Animal models of hyperbaric oxygen demonstrated transient improvement in oxygenation followed by rapid decline.100
 Nervous System Toxicity
Nervous System Toxicity
CNS effects vary depending on the route and intent of exposure. Among those with hydrocarbon aspiration secondary to ingestion, one-third have signs of CNS toxicity ranging from drowsiness to stupor and seizures. In this setting, the presence of CNS symptoms correlates strongly with the development of fever, hypoxemia, and pneumonitis.23 Intentional hydrocarbon inhalation produces euphoric effects that mimic ethanol inebriation. Symptoms include mydriasis, nystagmus, hallucinations, increased libido, and delirium. Severe or prolonged exposures can result in tremors, seizures, and hypoxic encephalopathy.109–112 These effects usually resolve within a few hours, although prolonged symptoms can occur in some cases.113
The neurophysiologic effects of inhalants are not completely understood. Inhalation leads to CNS depression via enhanced γ-aminobutyric acid (GABA)-mediated neurotransmission, antagonism of N-methyl-D-aspartic acid receptors, inhibition of normal cell-cell signaling, and enhanced serotonergic transmission.115–119 The release of dopamine reinforces abuse patterns.120 Chronic abusers develop tolerance to these effects and may increase the amount inhaled to compensate.111,121 Because of physical dependence, chronic users can develop inhalant withdrawal symptoms such as craving, irritability, and insomnia.122 Baclofen and lamotrigine have been advocated as treatments for inhalant withdrawal syndromes.123,124
Chronic exposure to solvents, whether intentional or unintentional, can cause a broad spectrum of CNS disorders. Initial symptoms are nonspecific and include memory difficulties, fatigue, loss of concentration, and personality changes that can be reversible.114,125–127 Continued exposure leads to an irreversible leukoencephalopathy that can present as cerebellar ataxia, parkinsonism, encephalopathy, convulsions, and/or deficits in higher functioning.112,128,129 MRI reveals changes in the basal ganglia and thalamus along with cortical and cerebellar atrophy.112,128,130,131 Single photon emission computerized tomography (SPECT) findings have demonstrated prominent abnormalities with areas of hypoperfusion and hyperperfusion.132 Many hydrocarbons are associated with the development of peripheral neuropathy, most notably n-hexane and methyl-n-butyl ketone.126,133–135
 Cardiac Toxicity
Cardiac Toxicity
Sudden sniffing death refers to cardiac arrest following the inhalation of volatile hydrocarbons, especially halogenated derivatives.63,64,136,137 Ingestion or inhalation of halogenated hydrocarbons can cause dysrhythmias that persist for days.138 Sixty-four percent of inhalant-related deaths result from arrhythmias, and most of the remainder result from hypoxia and/or hypercapnia.65,139 Toluene has been shown to prolong the QT interval and inhibit cardiac sodium currents.140,141 Electrophysiologic studies on animals identified concentration-dependent suppression of spontaneous pacemaker activity, resulting in asystole, though some animals developed ventricular tachydysrhythmias. Cardiotoxicity worsens in the setting of acidosis or hypoxemia,142–144 and toxicity persists for hours after exposure.144 Autopsy findings are usually nonspecific,65 although myocardial fibrosis induced by hydrocarbon abuse can increase the risk of dysrhythmias.145 Coronary artery spasm and infarction contribute to toxicity.146,147 The myocardium may be sensitized to catecholamines following inhalant abuse, and thus sudden excitation or exercise can trigger ventricular dysrhythmias.148,149 Administration of epinephrine worsened inhalant-induced cardiotoxicity in a canine model.150 Therefore the management of cases of hydrocarbon-induced toxicity should eschew the use of epinephrine or other adrenergic agonists.137 β-Adrenergic blockers can blunt myocardial sensitization and have been used successfully in the treatment of ventricular dysrhythmias secondary to hydrocarbon toxicity.138 Amiodarone and lidocaine also have been used successfully to terminate ventricular arrhythmias.137,151
 Hepatotoxicity
Hepatotoxicity
Hepatitis and liver failure can occur following hydrocarbon exposure.152,153 Halogenated hydrocarbons are particularly dangerous in this regard, whereas most other hydrocarbons induce only a mild hepatitis.154–156 Carbon tetrachloride is a prototypical example; it induces centrilobular liver necrosis via cytochrome 2E1 metabolism in a manner similar to the way acetaminophen induces hepatocellular damage.157–159 Chloroform, 1,1,1-trichloroethane, and other halogenated hydrocarbons can cause significant hepatic injury.160–162 Treatment with N-acetylcysteine has provided hepatoprotection in animal models and in case reports of human exposure.160,161,163 Although data are limited regarding the use of N-acetylcysteine therapy in patients exposed to hepatotoxic hydrocarbons, it should be used because of its low cost and wide safety profile.
 Renal Effects
Renal Effects
Both halogenated and nonhalogenated hydrocarbons can cause acute renal failure.164–166 Acute tubular injury is primarily responsible,164,167 although interstitial nephritis has been reported.168 Chronic exposure can lead to a slow decline in renal function via progressive tubular injury.169,170 Albuminuria can be a useful marker to gauge renal injury in chronic exposures.171
Toluene inhalation is notorious for inducing a renal tubular acidosis-like syndrome and ureteral calculi; in addition, toluene can cause direct kidney injury and acute renal failure.172–175 Toluene is metabolized first to benzoic acid and then to hippuric acid, which can be measured in the blood or urine to confirm recent exposure.176 Acutely, toluene abusers can present with a widened anion gap due to the formation of the unmeasured anions, benzoate and hippurate.175 However, chronic use can result in a renal tubular acidosis-like syndrome, and patients can present with life-threatening hypokalemia and resultant muscle weakness or paresis.177 Hippurate-induced acidification of the glomerular filtrate disrupts the normal pH gradient and prevents the distal tubule from excreting hydrogen ions in exchange for potassium ions. Thus, potassium excretion increases along with retention of endogenous hydrogen ions. This combination results in development of hyperchloremic metabolic acidosis with profound hypokalemia and hypophosphatemia.174,178,179 A serum potassium concentration as low as 0.8 meq/L, which required infusion of 260 mEq potassium over 6 hours, has been reported.180 Total body stores are depleted, and supplementation with hundreds of mEq of potassium may be required.178 Treatment includes hydration and repletion of electrolytes; hemodialysis may be required for reversing severe hypokalemia.181 Prognosis is good, and most patients recover completely.177
An unusual toxin is nitromethane, which is commonly found in model engine fuel along with methanol in a 50 : 50 mixture. Nitromethane interferes with laboratory assays for creatinine, and the presence of nitromethane in samples can yield falsely elevated results.182–184 Management of concurrent methanol poisoning or renal disease is challenging in the setting of this laboratory interference.
 Hematologic Effects
Hematologic Effects
Hydrocarbons can cause many acute hematologic abnormalities. Hemolysis occasionally follows hydrocarbon ingestion.185,186 Most cases are mild and do not require treatment, although red cell transfusion and exchange transfusion rarely are required.187,188 Naphthalene found in some mothballs can induce profound and prolonged hemolysis.189 Methemoglobinemia has been reported following hydrocarbon ingestion, usually with agents containing nitro side groups.190,191 Methylene chloride and methylene iodide are slowly metabolized to carbon monoxide and cause prolonged carbon monoxide poisoning.192,193 Aplastic anemia can result from exposure to high concentrations of benzene, typically following chronic occupational exposures.194
 Dermatologic Effects
Dermatologic Effects
Hydrocarbon skin exposure typically results in a mild irritant dermatitis that can be treated by cleansing the area with soap and water to remove residual hydrocarbons, followed by lotion application.195 However, prolonged exposure over a few hours can lead to chemical burns complicated by blistering and partial or full-thickness skin necrosis.196 Allergic contact dermatitis can occur in patients with chronic exposures.195,197 Ingestion of chlorobenzenes such as dioxins causes specific lesions known as chloracne.198 Compressed hydrocarbons can cause cold burns due to the endothermic reaction that occurs during rapid vaporization.199 Hot tar or asphalt can cause prolonged burning. Application of a petroleum-based solvent or antibiotic ointment facilitates tar removal.200,201 High-pressure hydrocarbon injection injuries, especially to the hand, can result in severe disability and warrant immediate surgical consultation.202
 Carcinogenicity
Carcinogenicity
In 1775, Percivall Pott noted that chimney sweeps commonly develop testicular cancer, and this association was later found to result from exposure to polyaromatic hydrocarbons.203 Since then, many hydrocarbon-induced cancers have been discovered, and this problem is a matter of significant public concern owing to the ubiquitous exposure to hydrocarbon products. Various organizations categorize the carcinogenicity of chemicals, including hydrocarbon products. Among these organizations are the National Toxicology Program (NTP) under the U.S. Department of Health and Human Services and the International Agency for Research on Cancer (IARC) under the World Health Organization. IARC divides chemicals into 5 groups; group 1 consists of 107 known human carcinogens (Table 179-3),204 group 2A consists of 58 probable human carcinogens, group 2B consists of 249 possible human carcinogens, group 3 consists of 512 unclassified carcinogens, and group 4 consists of 1 chemical that is probably not carcinogenic.205 Thus many thousands of chemicals remain unstudied.
| Agent | Cancer |
|---|---|
| Benzene | Acute myelogenous leukemia |
| Vinyl chloride | Hepatic angiosarcoma |
| Formaldehyde | Nasal cancer |
| Mineral oils | Squamous cell carcinomas |
| Coal tar pitch | Skin cancer |
| Ortho-toluidine | Bladder cancer |
From the International Agency for Research on Cancer, World Health Organization. Available at: http://monographs.iarc.fr/ENG/Classification/index.php.
Key Points
Anas N. Criteria for hospitalizing children who have ingested products containing hydrocarbons. JAMA. 1981;246:840-843.
Press E, Co-operative Kerosene Poisoning Study. Evaluation of gastric lavage and other factors in the treatment of accidental ingestion of petroleum distillate products. Pediatrics. 1962;29:648-674.
Lifshitz M, Sofer S, Gorodischer R. Hydrocarbon poisoning in children: a 5-year retrospective study. Wilderness Environ Med. 2003;14:78-82.
Marks M. Adrenocorticosteroid treatment of hydrocarbon pneumonia in children: a cooperative study. J Pediatr. 1972;81:366-369.
Carlisle EJ, Donnelly SM, Vasuvattakul S, Kamel S, Tobe S, Halperin ML. Glue-sniffing and distal renal tubular acidosis: sticking to the facts. J Am Soc Nephrol. 1991;1:1019-1027.
1 Agency for Toxic Substances and Disease Registry. Toxicological profile for total petroleum hydrocarbons. Washington DC: U.S. Public Health Service; 1999.
2 National Institute of Standards and Technology, TRC Group. NIST/TRC Web Thermo Tables (WTT). Available from. http://wtt-lite.nist.gov/cgi-bin/openindex.cgi.
3 Bronstein AC, et al. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila). 2009;47(10):911-1084.
4 American Association of Poison Control Centers. Annual data reports. Available from. http://www.aapcc.org/dnn/NPDSPoisonData/AnnualReports/tabid/125/Default.aspx.
5 International Programme on Chemical Safety. Benzene environmental health criteria 150. Geneva: World Health Organization; 1993.
6 Weisel CP. Benzene exposure: an overview of monitoring methods and their findings. Chem Biol Interact. 2010;184(1-2):58-66.
7 Rappaport SM, et al. Human benzene metabolism following occupational and environmental exposures. Chem Biol Interact. 2010;184(1-2):189-195.
8 Agency for Toxic Substances and Disease Registry. ToxFAQ for benzene. Washington DC: U.S. Department of Health and Human Services; 2007.
9 Agency for Toxic Substances and Disease Registry. Toxicological profile for fuel oils. Washington DC: U.S. Department of Health and Human Services; 2007.
10 Agency for Toxic Substances and Disease Registry. Toxicological profile for toluene. Washington DC: U.S. Department of Health and Human Services; 2000.
11 Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance System. Atlanta: CDC; 2007.
12 Howard MO. Inhalant use among incarcerated adolescents in the United States: Prevalence, characteristics, and correlates of use. Drug Alcohol Depend. 2008;93:197-209.
13 Wu LT. Inhalant Abuse and Dependence Among Adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
14 Martino SC, et al. Recanting of life-time inhalant use: how big a problem and what to make of it. Addiction. 2009;104(8):1373-1381.
15 Wu LT. Is inhalant use a risk factor for heroin and injection drug use among adolescents in the United States. Addict Behav. 2007;32:265-281.
16 Edwards R. Disparities in young adolescent inhalant use by rurality, gender, and ethnicity. Subst Use Misuse. 2007;42:643-670.
17 Ridenour TA, Bray BC, Cottler LB. Reliability of use, abuse, and dependence of four types of inhalants in adolescents and young adults. Drug Alcohol Depend. 2007;91(1):40-49.
18 Kurtzman TL, Otsuka KN, Wahl RA. Inhalant abuse by adolescents. J Adolesc Health. 2001;28(3):170-180.
19 Lacy BW, Ditzler TF. Inhalant abuse in the military: an unrecognized threat. Mil Med. 2007;172(4):388-392.
20 Press E, Co-operative Kerosene Poisoning Study. Evaluation of gastric lavage and other factors in the treatment of accidental ingestion of petroleum distillate products. Pediatrics. 1962;29:648-674.
21 Byard RW, Simpson E, Gilbert JD. Temporal trends over the past two decades in asphyxial deaths in South Australia involving plastic bags or wrapping. J Clin Forensic Med. 2006;13(1):9-14.
22 Anas N. Criteria for hospitalizing children who have ingested products containing hydrocarbons. JAMA. 1981;246(8):840-843.
23 Lifshitz M, Sofer S, Gorodischer R. Hydrocarbon poisoning in children: a 5-year retrospective study. Wilderness Environ Med. 2003;14(2):78-82.
24 Caravati EM, Bjerk PJ. Acute toluene ingestion toxicity. Ann Emerg Med. 1997;30(6):838-839.
25 Dice WH, et al. Pulmonary toxicity following gastrointestinal ingestion of kerosene. Ann Emerg Med. 1982;11(3):138-142.
26 Beamon RF, et al. Hydrocarbon ingestion in children: a six-year retrospective study. JACEP. 1976;5(10):771-775.
27 Cachia EA, Fenech FF. Kerosene Poisoning in Children. Arch Dis Child. 1964;39:502-504.
28 Mofenson HC, Greensher J. The new correct answer to an old question on kerosene ingestion. Pediatrics. 1977;59(5):788.
29 Ng RC. Emergency treatment of petroleum distillate and turpentine ingestion. Can Med Assoc J. 1974;111:537-538.
30 Laass W. Therapy of acute oral poisoning by organic solvents: treatment by activated charcoal in combination with laxatives. Arch Toxicol Suppl. 1980;4:406-409.
31 Jones J, et al. Repetitive doses of activated charcoal in the treatment of poisoning. Am J Emerg Med. 1987;5(4):305-311.
32 Gentina T, et al. Fire-eater’s lung: seventeen cases and a review of the literature. Medicine (Baltimore). 2001;80(5):291-297.
33 Marks M. Adrenocorticosteroid treatment of hydrocarbon pneumonia in children—a cooperative study. J Pediatr. 1972;81(2):366-369.
34 Shusterman D, Neal E. Prolonged fever associated with inhalation of multiple pyrolysis products. Ann Emerg Med. 1986;15(7):831-833.
35 Delgado JH, Waksman JC. Polymer fume fever-like syndrome due to hairspray inhalation. Vet Hum Toxicol. 2004;46(5):266-267.
36 Strachan P. Pneumonia in a Patient With Gastroesophageal Reflux and Chronic Constipation: Lipoid Pneumonia. Clin Pulm Med. 2006;13(6):321-323.
37 Gondouin A, et al. Exogenous lipid pneumonia: a retrospective multicentre study of 44 cases in France. Eur Respir J. 1996;9(7):1463-1469.
38 Behrends M, Beiderlinden M, Peters J. Acute lung injury after peppermint oil injection. Anesth Analg. 2005;101(4):1160-1162.
39 Harris AM, et al. Pneumonitis following grease gun injury. Injury. 2004;35(12):1303-1305.
40 Santos JW. Pneumonitis after Intravenous Self-Administration of Solvent. Respir Care. 2001;46(1):53-55.
41 Seifert SA, Dart RC, Kaplan EH. Accidental, intravenous infusion of a peanut oil-based medication. J Toxicol Clin Toxicol. 1998;36(7):733-736.
42 Cohen MA, Galbut B, Kerdel FA. Exogenous lipoid pneumonia caused by facial application of petrolatum. J Am Acad Dermatol. 2003;49(6):1128-1130.
43 Wallace G. Horse rug lung: toxic pneumonitis due to fluorocarbon inhalation. Occup Environ Med. 2005;62:414-416.
44 Morimatsu Y. Acute Pulmonary Injury due to Exposure to a High Concnetration of Trichloroethylene Vapor. J Occup Health. 2006;48:271-272.
45 Schloneger M, Stull A, Singer JI. Inhalant abuse: a case of hemoptysis associated with halogenated hydrocarbons abuse. Pediatr Emerg Care. 2009;25(11):754-757.
46 Nguyen B, et al. Time course of onset of sensitization to common and occupational inhalants in apprentices. J Allergy Clin Immunol. 2003;111(4):807-812.
47 Saygun M, et al. Five annual observations of respiratory findings in gun factory workers exposed to solvents. J Occup Environ Med. 2007;49(8):909-912.
48 Fujimaki H, et al. Effect of long-term exposure to low-level toluene on airway inflammatory response in mice. Toxicol Lett. 2007;168(2):132-139.
49 Schweigert M. Investigation of Pulmonary Function Among Employees Exposed to Low Levels of Monomeric Isocyanates and Solvents at an Automobile Finishings Plant. J Occup Environ Med. 2002;44(11):1083-1090.
50 Sodeyama N, et al. [A case of chronic thinner intoxication developing hyperkinesie volitionnelle three years after stopping thinner abuse]. Rinsho Shinkeigaku. 1993;33(2):213-215.
51 Yoshizawa Y, et al. Hypersensitivity pneumonitis induced by toluene diisocyanate: sequelae of continuous exposure. Ann Intern Med. 1989;110(1):31-34.
52 Grufferman S, Walker FW. Supraglottitis following gasoline ingestion. Ann Emerg Med. 1982;11(7):368-370.
53 Wolfe BM, Brodeur AE, Shields JB. The role of gastrointestinal absorption of kerosene in producing pneumonitis in dogs. J Pediatr. 1970;76(6):867-873.
54 Mann MD, Pirie DJ, Wolfsdorf J. Kerosene absorption in primates. J Pediatr. 1977;91(3):495-498.
55 Wolfsdorf J, Paed D. Kerosene intoxication: an experimental approach to the etiology of the CNS manifestations in primates. J Pediatr. 1976;88(6):1037-1040.
56 Scharf SM, Prinsloo I. Pulmonary mechanics in dogs given different doses of kerosene intratracheally. Am Rev Respir Dis. 1982;126(4):695-700.
57 Bratton L, Haddow JE. Ingestion of charcoal lighter fluid. J Pediatr. 1975;87(4):633-636.
58 Scharf SM, Heimer D, Goldstein J. Pathologic and physiologic effects of aspiration of hydrocarbons in the rat. Am Rev Respir Dis. 1981;124(5):625-629.
59 Marcinkowski AL. Postdeposition Dispersion of Aerosol Medications Using Surfactant Carriers. J Aerosol Med Pulm Drug Deliv. 2008;21(4):361-369.
60 Amoruso MA, et al. Review of the toxicology of mineral spirits. Int J Toxicol. 2008;27(1):97-165.
61 Pham K, Sverchek J, McPheeters RA. Chemical pneumonitis from hydrocarbon aspiration. West J Emerg Med. 2008;9(3):165.
62 Nave C. Hyperphysics. Available from. http://hyperphysics.phy-astr.gsu.edu/Hbase/hframe.html.
63 Bowen SE, Daniel J, Balster RL. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug Alcohol Depend. 1999;53(3):239-245.
64 Maxwell JC. Deaths related to the inhalation of volatile substances in Texas: 1988-1998. Am J Drug Alcohol Abuse. 2001;27(4):689-697.
65 Wick R, et al. Inhalant deaths in South Australia: a 20-year retrospective autopsy study. Am J Forensic Med Pathol. 2007;28(4):319-322.
66 Van Gorcum TF, et al. Lamp oil poisoning: did the European guideline reduce the number and severity of intoxications? Clin Toxicol (Phila). 2009;47(1):29-34.
67 Griffin JW, et al. Hydrocarbon pneumonitis following furniture polish ingestion; a report of fifteen cases. J Pediatr. 1954;45(1):13-26.
68 Segev D, et al. Kerosene-induced severe acute respiratory failure in near drowning: reports on four cases and review of the literature. Crit Care Med. 1999;27(8):1437-1440.
69 Volk BW, et al. Diagnosis of lipoid pneumonia. Am J Surg. 1955;89(1):158-165.
70 Borrie J, Gwynne JF. Paraffinoma of lung: lipoid pneumonia. Report of two cases. Thorax. 1973;28(2):214-221.
71 Kadakal F, Uysal MA, Gülhan NB, Turan NG, Bayramoğlu S, Yilmaz V. Fire-eater’s pneumonia characterized by pneumatocele formation and spontaneous resolution. Diagn Interv Radiol. 2010;16:201-203. Epub 2009 Oct 5
72 Kim ES, et al. Squalene-induced exogenous lipoid pneumonia in an infant. Pediatr Int. 2009;51(5):751-753.
73 Hoffman LR. Lipoid Pneumonia Due to Mexican Folk Remedies. Arch Pediatr Adolesc Med. 2005;159:1043-1048.
74 Goodwin SR, et al. Kerosene aspiration: immediate and early pulmonary and cardiovascular effects. Vet Hum Toxicol. 1988;30(6):521-524.
75 Horoz OO, Yildizdas D, Yilmaz HL. Surfactant therapy in acute respiratory distress syndrome due to hydrocarbon aspiration. Singapore Med J. 2009;50(4):130-132.
76 Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59(2):201-222.
77 Machado B, Cross K, Snodgrass WR. Accidental hydrocarbon ingestion cases telephoned to a regional poison center. Ann Emerg Med. 1988;17(8):804-807.
78 Jayashree M. Predictors of Outcome in Children with Hydrocarbon Poisoning Receiving Intensive Care. Indian Pediatr. 2006;43:715-719.
79 Khan AJ, Akhtar RP, Faruqui ZS. Turpentine oil inhalation leading to lung necrosis and empyema in a toddler. Pediatr Emerg Care. 2006;22(5):355-357.
80 Thalhammer GH, Eber E, Zach MS. Pneumonitis and pneumatoceles following accidental hydrocarbon aspiration in children. Wien Klin Wochenschr. 2005;117(4):150-153.
81 Rodricks A, et al. Turpentine-induced chemical pneumonitis with broncho-pleural fistula. J Assoc Physicians India. 2003;51:729-730.
82 Bray A, Pirronti T, Marano P. Pneumatoceles following hydrocarbon aspiration. Eur Radiol. 1998;8(2):262-263.
83 Baron SE, Haramati LB, Rivera VT. Radiological and clinical findings in acute and chronic exogenous lipoid pneumonia. J Thorac Imaging. 2003;18(4):217-224.
84 Marchiori E, et al. Lipoid pneumonia in 53 patients after aspiration of mineral oil: comparison of high-resolution computed tomography findings in adults and children. J Comput Assist Tomogr. 2010;34(1):9-12.
85 Yi MS, et al. CT findings in hydrocarbon pneumonitis after diesel fuel siphonage. AJR Am J Roentgenol. 2009;193(4):1118-1121.
86 Laurent FM, Tunon de Lara M. [Exposure to asbestos. Role of thoracic imagery in screening and follow-up]. Rev Mal Respir. 1999;16(6 Pt 2):1193-1202.
87 Brechot JM, et al. Computed tomography and magnetic resonance findings in lipoid pneumonia. Thorax. 1991;46(10):738-739.
88 Cox JE, Choplin RH, Chiles C. Case report. Chemical-shift MRI of exogenous lipoid pneumonia. J Comput Assist Tomogr. 1996;20(3):465-467.
89 Talwar A, et al. False-positive PET scan in a patient with lipoid pneumonia simulating lung cancer. Clin Nucl Med. 2004;29(7):426-428.
90 Gurwitz D, et al. Pulmonary function abnormalities in asymptomatic children after hydrocarbon pneumonitis. Pediatrics. 1978;62(5):789-794.
91 Taussig LM, et al. Pulmonary function 8 to 10 years after hydrocarbon pneumonitis. Normal findings in three children carefully studied. Clin Pediatr (Phila). 1977;16(1):57-59.
92 Mabe TG, et al. High-frequency percussive ventilation in a pediatric patient with hydrocarbon aspiration. Pediatr Crit Care Med. 2007;8(4):383-385.
93 Yu MC, et al. Multiple organ failure following lamp oil aspiration. Clin Toxicol (Phila). 2007;45(3):304-306.
94 Patwari P. Use of inhaled nitric oxide for hydrocarbon aspiration. Chest. 2005;128(4):445.
95 Bysani GK. Treatment of Hydrocarbon Pneumonitis : High Frequency Jet Ventilation as an Alternative to Extracorporeal Membrane Oxygenation. Chest. 1994;106(1):300-303.
96 Chyka PA. Benefits of extracorporeal membrane oxygenation for hydrocarbon pneumonitis. J Toxicol Clin Toxicol. 1996;34(4):357-363.
97 Hussain IR, et al. Severe lipoid pneumonia following attempted suicide by mineral oil immersion. Thorax. 1996;51(6):652-653. discussion 656-7
98 Kamijo Y, et al. Pulse steroid therapy in adult respiratory distress syndrome following petroleum naphtha ingestion. J Toxicol Clin Toxicol. 2000;38(1):59-62.
99 Gurkan F. Use of Nebulized Budesonide in Two Critical Patients with Hydrocarbon Intoxication. Am J Ther. 2005;12:366-367.
100 Schwartz SI, et al. Effects of Drugs and Hyperbaric Oxygen Environment on Experimental Kerosene Pneumonitis. Dis Chest. 1965;47:353-359.
101 Steele RW, Conklin RH, Mark HM. Corticosteroids and antibiotics for the treatment of fulminant hydrocarbon aspiration. JAMA. 1972;219(11):1434-1437.
102 Wolfsdorf J, Kundig H. Dexamethasone in the management of kerosene pneumonia. Pediatrics. 1974;53(1):86-90.
103 Brown JIII. Experimental kerosene pneumonia: Evaluation of some therapeutic regimens. J Pediatr. 1974;84(3):396-401.
104 Moya F. Synthetic surfactants: where are we? Evidence from randomized, controlled clinical trials. J Perinatol. 2009;29:523-528.
105 Davidson WJ, et al. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit Care. 2006;10(2):R41.
106 Widner LR, et al. Artificial surfactant for therapy in hydrocarbon-induced lung injury in sheep. Crit Care Med. 1996;24(9):1524-1529.
107 Russo R, et al. Case of exogenous lipoid pneumonia: steroid therapy and lung lavage with an emulsifier. Anesthesiology. 2006;104(1):197-198.
108 Uchida T, et al. The combination of partial liquid ventilation and inhaled nitric oxide in the severe oleic acid lung injury model. Chest. 1998;113(6):1658-1666.
109 Crocetti M. Inhalants. Pediatr Rev. 2008;29(1):33-34. discussion 34
110 Press E, Done AK. Solvent sniffing. Physiologic effects and community control measures for intoxication from the intentional inhalation of organic solvents. I. Pediatrics. 1967;39(3):451-461.
111 Press E, Done AK. Solvent sniffing. Physiologic effects and community control measures for intoxication from the intentional inhalation of organic solvents. II. Pediatrics. 1967;39(4):611-622.
112 Cairney S, et al. The neurobehavioural consequences of petrol (gasoline) sniffing. Neurosci Biobehav Rev. 2002;26(1):81-89.
113 Finch CK, Lobo BL. Acute inhalant-induced neurotoxicity with delayed recovery. Ann Pharmacother. 2005;39(1):169-172.
114 van Valen E, et al. The course of chronic solvent induced encephalopathy: a systematic review. Neurotoxicology. 2009;30(6):1172-1186.
115 MacIver MB. Abused inhalants enhance GABA-mediated synaptic inhibition. Neuropsychopharmacology. 2009;34(10):2296-2304.
116 lubman D. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008;154:316-326.
117 Del Re AM, Woodward JJ. Inhibition of gap junction currents by the abused solvent toluene. Drug Alcohol Depend. 2005;78(2):221-224.
118 Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131(7):1303-1308.
119 Lopreato GF, et al. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;70(1):11-15.
120 Riegel AC, et al. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32(7):1558-1569.
121 Bowen SE, Balster RL. Tolerance and sensitization to inhaled 1,1,1-trichloroethane in mice: results from open-field behavior and a functional observational battery. Psychopharmacology (Berl). 2006;185(4):405-415.
122 Perron BE, et al. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935-937.
123 Shen YC. Treatment of inhalant dependence with lamotrigine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):769-771.
124 Muralidharan K, et al. Baclofen in the management of inhalant withdrawal: a case series. Prim Care Companion J Clin Psychiatry. 2008;10(1):48-51.
125 Kaukiainen A, et al. Symptoms of chronic solvent encephalopathy: Euroquest questionnaire study. Neurotoxicology. 2009;30(6):1187-1194.
126 Dick FD. Solvent neurotoxicity. Occup Environ Med. 2006;63(3):221-226. 179
127 Feldman RG, Ratner MH, Ptak T. Chronic toxic encephalopathy in a painter exposed to mixed solvents. Environ Health Perspect. 1999;107(5):417-422.
128 Papageorgiou SG, et al. Severe dopaminergic pathways damage in a case of chronic toluene abuse. Clin Neurol Neurosurg. 2009;111(10):864-867.
129 Pezzoli G, et al. Clinical and pathological features in hydrocarbon-induced parkinsonism. Ann Neurol. 1996;40(6):922-925.
130 Alkan A, et al. Occupational prolonged organic solvent exposure in shoemakers: brain MR spectroscopy findings. Magn Reson Imaging. 2004;22(5):707-713.
131 Ridgway P, Nixon TE, Leach JP. Occupational exposure to organic solvents and long-term nervous system damage detectable by brain imaging, neurophysiology or histopathology. Food Chem Toxicol. 2003;41(2):153-187.
132 Kucuk NO, et al. Brain SPECT findings in long-term inhalant abuse. Nucl Med Commun. 2000;21(8):769-773.
133 Sendur O. Toxic neuropathy due to n-hexane: report of three cases. Inhal Toxicol. 2009;21:210-214.
134 n-Hexane-related peripheral neuropathy among automotive technicians–California, 1999-2000. MMWR Morb Mortal Wkly Rep. 2001;50:1011-1013.
135 Spencer PS. Aromatic as well as aliphatic hydrocarbon solvent axonopathy. Int J Hyg Environ Health. 2002;205:131-136.
136 Jones GR, Singer PP. An unusual trichloroethanol fatality attributed to sniffing trichloroethylene. J Anal Toxicol. 2008;32(2):183-186.
137 Adgey AA, Johnston PW, McMechan S. Sudden cardiac death and substance abuse. Resuscitation. 1995;29(3):219-221.
138 Mortiz F, et al. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
139 Byard RW, Chivell WC, Gilbert JD. Unusual facial markings and lethal mechanisms in a series of gasoline inhalation deaths. Am J Forensic Med Pathol. 2003;24(3):298-302.
140 Alper AT, et al. Glue (toluene) abuse: increased QT dispersion and relation with unexplained syncope. Inhal Toxicol. 2008;20(1):37-41.
141 Cruz SL, et al. Inhibition of cardiac sodium currents by toluene exposure. Br J Pharmacol. 2003;140(4):653-660.
142 Flowers NC, Hand RC, Horan LG. Concentrations of fluoroalkanes associated with cardiac conduction system toxicity. Arch Environ Health. 1975;30(7):353-360.
143 Flowers NC, Horan LG. Acid-base relationships and the cardiac response to aerosol inhalation. Chest. 1973;63(1):74-78.
144 Taylor GJT, Harris WS. Cardiac toxicity of aerosol propellants. JAMA. 1970;214(1):81-85.
145 Pfeiffer H, et al. Sudden death after isobutane sniffing: a report of two forensic cases. Int J Legal Med. 2006;120(3):168-173.
146 El-Menyar AA, El-Tawil M, Al Suwaidi J. A teenager with angiographically normal epicardial coronary arteries and acute myocardial infarction after butane inhalation. Eur J Emerg Med. 2005;12(3):137-141.
147 Carder JR, Fuerst RS. Myocardial infarction after toluene inhalation. Pediatr Emerg Care. 1997;13(2):117-119.
148 Steffee CH, Davis GJ, Nicol KK. A whiff of death: fatal volatile solvent inhalation abuse. South Med J. 1996;89(9):879-884.
149 Shepherd RT. Mechanism of sudden death associated with volatile substance abuse. Hum Toxicol. 1989;8(4):287-291.
150 Zink J, Sasyniuk BI, Dresel PE. Halothane-epinephrine-induced cardiac arrhythmias and the role of heart rate. Anesthesiology. 1975;43(5):548-555.
151 Edwards KE, Wenstone R. Successful resuscitation from recurrent ventricular fibrillation secondary to butane inhalation. Br J Anaesth. 2000;84(6):803-805.
152 Franco G, Fonte R, Candura F. Hepatotoxicity of organic solvents. Br J Ind Med. 1986;43(2):139.
153 Hamada M, et al. Occupational liver injury due to N,N-dimethylformamide in the synthetics industry. Intern Med. 2009;48(18):1647-1650.
154 Janssen S, et al. Impairment of organ function after oral ingestion of refined petrol. Intensive Care Med. 1988;14(3):238-240.
155 Singh N, et al. Outcome of sixty-four cases of ethylene dibromide ingestion treated in tertiary care hospital. J Assoc Physicians India. 2007;55:842-845.
156 Atkinson L, et al. Toxic reaction to inhaled paint fumes. Postgrad Med J. 1989;65(766):559-561. discussion 561-2
157 Takahashi S, et al. Increased cytotoxicity of carbon tetrachloride in a human hepatoma cell line overexpressing cytochrome P450 2E1. J Int Med Res. 2002;30(4):400-405.
158 Manno M, et al. Potentiation of occupational carbon tetrachloride toxicity by ethanol abuse. Hum Exp Toxicol. 1996;15(4):294-300.
159 Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(3):185-209.
160 Lin CH, et al. Saved by a material safety data sheet. Occup Med (Lond). 2005;55(8):635-637.
161 Choi SH, et al. Diagnostic radiopacity and hepatotoxicity following chloroform ingestion: a case report. Emerg Med J. 2006;23(5):394-395.
162 Takaki A, et al. A 27-year-old man who died of acute liver failure probably due to trichloroethylene abuse. J Gastroenterol. 2008;43(3):239-242.
163 Maksimchik YZ, et al. Protective effects of N-acetyl-L-cysteine against acute carbon tetrachloride hepatotoxicity in rats. Cell Biochem Funct. 2008;26(1):11-18.
164 Choi YH, et al. ARF requiring hemodialysis after accidental perchloroethylene ingestion. Am J Kidney Dis. 2003;41(3):E11.
165 Carrieri M, et al. Acute, nonfatal intoxication with trichloroethylene. Arch Toxicol. 2007;81(7):529-532.
166 Erickson TB, et al. Acute renal toxicity after ingestion of Lava light liquid. Ann Emerg Med. 1996;27(6):781-784.
167 Ravnskov U. Hydrocarbons and renal failure: primary damage in glomerulonephritis is tubular, not glomerular. Nephron. 1992;61(2):243.
168 Taverner D, Harrison DJ, Bell GM. Acute renal failure due to interstitial nephritis induced by “glue-sniffing” with subsequent recovery. Scott Med J. 1988;33(2):246-247.
169 Gonzalez-Yebra AL, et al. Occupational exposure to toluene and its possible causative role in renal damage development in shoe workers. Int Arch Occup Environ Health. 2006;79(3):259-264.
170 Hotz P, et al. Serum laminin, hydrocarbon exposure, and glomerular damage. Br J Ind Med. 1993;50(12):1104-1110.
171 Voss JU, et al. Nephrotoxicity of organic solvents: biomarkers for early detection. Int Arch Occup Environ Health. 2005;78(6):475-485.
172 Gupta RK, van der Meulen J, Johny KV. Oliguric acute renal failure due to glue-sniffing. Case report. Scand J Urol Nephrol. 1991;25(3):247-250.
173 Kroeger RM, et al. Recurrent urinary calculi associated with toluene sniffing. J Urol. 1980;123(1):89-91.
174 Tang HL, et al. Renal tubular acidosis and severe hypophosphataemia due to toluene inhalation. Hong Kong Med J. 2005;11(1):50-53.
175 Kamijima M, et al. Metabolic acidosis and renal tubular injury due to pure toluene inhalation. Arch Environ Health. 1994;49(5):410-413.
176 Thiesen FV. Laboratory diagnosis of toluene-based inhalants abuse. Clin Toxicol. 2007;45:557-562.
177 Kao KC, et al. Hypokalemic muscular paralysis causing acute respiratory failure due to rhabdomyolysis with renal tubular acidosis in a chronic glue sniffer. J Toxicol Clin Toxicol. 2000;38(6):679-681.
178 Baskerville JR, Tichenor GA, Rosen PB. Toluene induced hypokalaemia: case report and literature review. Emerg Med J. 2001;18(6):514-516.
179 Carlisle EJ, et al. Glue-sniffing and distal renal tubular acidosis: sticking to the facts. J Am Soc Nephrol. 1991;1(8):1019-1027.
180 Kirk LM, Anderson RJ, Martin K. Sudden death from toluene abuse. Ann Emerg Med. 1984;13(1):68-69.
181 Gerkin RDJr, LoVecchio F. Rapid reversal of life-threatening toluene-induced hypokalemia with hemodialysis. J Emerg Med. 1998;16(5):723-725.
182 Leonard CP, Akhtar J. Co-ingestion of methanol and nitromethane: using falsely elevated creatinine as indicator for methanol antidote use. Pediatr Crit Care Med. 2007;8(4):392-393.
183 Rastogi A, et al. Spurious elevation in serum creatinine caused by ingestion of nitromethane: implication for the diagnosis and treatment of methanol intoxication. Am J Kidney Dis. 2008;52(1):181-187.
184 Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be “fueled” by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):315-320.
185 Adler R, Robinson RG, Bindin J. Intravascular hemolysis: an unusual complication of hydrocarbon ingestion. J Pediatr. 1976;89(4):679-680.
186 Stockman JA3rd. More on hydrocarbon-induced hemolysis. J Pediatr. 1977;90(5):848.
187 Tauscher JW, Polich JJ. Treatment of pine oil poisoning by exchange transfusion. J Pediatr. 1959;55:511-515.
188 Algren JT, Rodgers GCJr. Intravascular hemolysis associated with hydrocarbon poisoning. Pediatr Emerg Care. 1992;8(1):34-35.
189 Lim HC, Poulose V, Tan HH. Acute naphthalene poisoning following the non-accidental ingestion of mothballs. Singapore Med J. 2009;50(8):e298-e301.
190 Verma S, Gomber S. Thinner intoxication manifesting as methemoglobinemia. Indian J Pediatr. 2009;76(3):315-316.
191 Schimelman MA, Soler JM, Muller HA. Methemoglobinemia: nitrobenzene ingestion. JACEP. 1978;7(11):406-408.
192 Weimerskirch PJ, et al. Methylene iodide poisoning. Ann Emerg Med. 1990;19(10):1171-1176.
193 Sturmann K, Mofenson H, Caraccio T. Methylene chloride inhalation: an unusual form of drug abuse. Ann Emerg Med. 1985;14(9):903-905.
194 Gross SA, et al. A hospital-based case control study of aplastic anemia in Shanghai, China. Chem Biol Interact. 2010;184(1-2):165-173.
195 Goon AT, Goh CL. Epidemiology of occupational skin disease in Singapore 1989-1998. Contact Dermatitis. 2000;43(3):133-136.
196 Shibata K, Yoshita Y, Matsumoto H. Extensive chemical burns from toluene. Am J Emerg Med. 1994;12(3):353-355.
197 Reichert-Penetrat S, et al. Allergic contact dermatitis from surgical paints. Contact Dermatitis. 2001;45(2):116-117.
198 Rosas Vazquez E, et al. Chloracne in the 1990s. Int J Dermatol. 1996;35(9):643-645.
199 Bonamonte D, et al. Cold burn from contact with a propane and butane gas blend inside a spray canister used as a hooter. Contact Dermatitis. 2008;59(1):61-62.
200 Stratta RJ, et al. Management of tar and asphalt injuries. Am J Surg. 1983;146(6):766-769.
201 Levy DB, et al. Unibase and triple antibiotic ointment for hardened tar removal. Ann Emerg Med. 1986;15(6):765-766.
202 Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20(7):503-511.
203 Kipling MD, Waldron HA. Percivall Pott and cancer scroti. Br J Ind Med. 1975;32(3):244-246.
204 International Agency for Research on Cancer, World Health Organization. Available from http://monographs.iarc.fr/ENG/Classification/crthgr01.php Accessed 2010
205 International Agency for Research on Cancer, World Health Organization. Available from http://monographs.iarc.fr/ENG/Classification/index.php Accessed 2010