Chapter 8 Hybrid Cardiac Imaging
INTRODUCTION (See Chapter 22)
The constant technological developments of noninvasive imaging over the past decades have contributed to the enhancement of our pathophysiologic understanding of many cardiac conditions. Particularly in coronary artery disease (CAD), management is based upon assessment of both the presence of coronary stenoses and their hemodynamic consequences.1,2 Hence, noninvasive imaging helps to guide therapeutic decisions by providing complementary information on coronary morphology and on myocardial perfusion and metabolism using different imaging tools,3,4 including nuclear techniques such as single-photon emission computed tomography (SPECT) or positron emission tomography (PET), computed tomography (CT) techniques such as electron beam CT (EBCT) and multislice CT (MSCT), or cardiac magnetic resonance (CMR).
Thus, the interest in hybrid imaging has rapidly spread onto cardiac applications and has changed the landscape of noninvasive cardiac imaging by bringing the different clinical specialties (i.e., cardiology, radiology, nuclear medicine) closer together.5 Additionally, it has driven the development and production of dedicated hybrid scanners in an effort to simplify image coregistration and improve patient throughput for specialized cardiac imaging centers. For the evaluation of a patient with heart disease, hybrid imaging introduces a multifaceted approach to cardiovascular assessment. This approach facilitates the detection and quantification of coronary atherosclerosis through the use of coronary artery calcium scores (CACS) and coronary angiography, the quantification of vascular reactivity and endothelial integrity, the identification of flow-limiting coronary stenoses, and the assessment of myocardial viability and metabolism by means of PET. Thus, by revealing the burden of anatomic CAD and its physiologic relevance, hybrid imaging can provide noninvasively unique information that may help to improve diagnosis and risk stratification and guide management decisions in CAD patients.
TECHNICAL CONSIDERATIONS OF HYBRID IMAGING
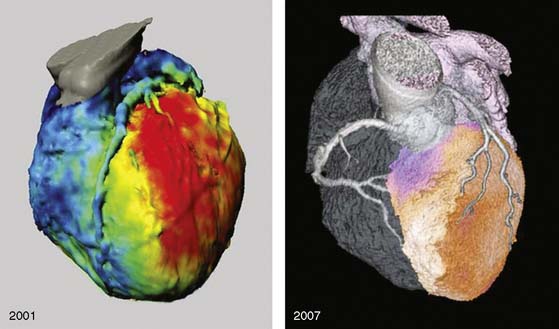
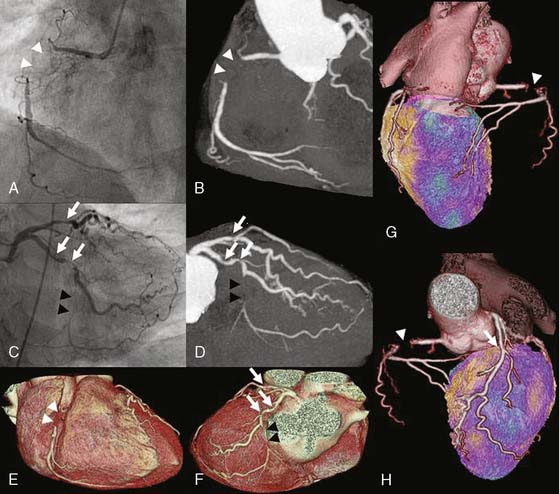
Several pioneering attempts of software-based image fusion from conventional coronary angiography (CA) and SPECT paved the way for hybrid imaging but were not implemented into clinical practice because their invasiveness precluded use for noninvasive preinterventional decision making.6–9 Fusion of CT and nuclear techniques for three-dimensional hybrid imaging was initially cumbersome, and the quality of the images suffered from several limitations (Fig. 8-1). The temporal and spatial resolution required for consistently good delineation of the coronary tree was not met by early 4- or 16-slice CT devices. Furthermore, the lack of dedicated cardiac fusion software rendered image processing tedious and time-consuming. Many new developments in hardware (e.g., 64-detector CT devices) and software (dedicated cardiac fusion software, high-powered postprocessing workstations) have helped to improve image quality and promote clinical availability of hybrid imaging (Fig. 8-2).
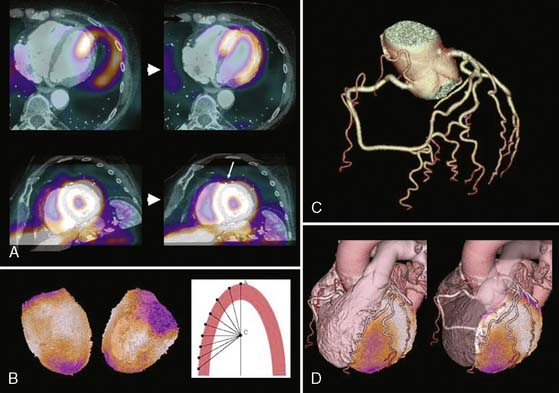
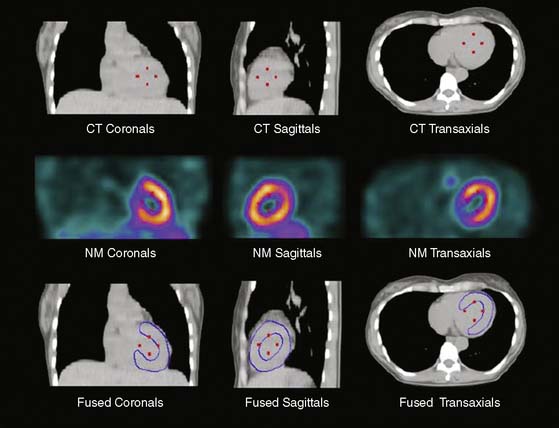
For cardiac applications, a three-dimensional display of the fused images (generated by volume-rendering technique)10 is preferable compared to oncologic or neurologic applications, because it allows the best evaluation of myocardial territories and their respective tributaries. Thus, an important prerequisite of hybrid imaging is accurate image coregistration. Misalignment may result in erroneous allocation of perfusion defects and coronary artery territories. From a computational perspective, image coregistration can be achieved by a software-based or hardware-based approach.11 Hardware-based image coregistration permits the acquisition of coregistered anatomic and functional images using hybrid scanners (such as PET/CT or SPECT/CT devices) with the capability to perform nuclear and CT image acquisition almost simultaneously, with the patient’s position fixed. Inherently, image fusion is performed fully or semiautomatically by superposition of image data sets. With software-based coregistration, image data sets can be obtained on standalone scanners and fused manually through the use of landmark-based coregistration techniques (Fig. 8-3). Intuitively, the hardware-based approach appears preferable, since manual coregistration may be hampered by issues of accuracy and user interaction. This is why, to date, hybrid PET/CT devices are widely used for whole-body PET/CT imaging, predominantly in oncology.
However, in contrast to whole-body PET/CT, the routine use of fully automated hardware-based image coregistration for cardiac hybrid applications is limited by organ-specific characteristics. Despite fixation of the patient’s position and orientation, minor beat-to-beat variations in the heart’s position may interfere with accurate image coregistration. Furthermore, CT image acquisition and analysis requires electrocardiographic gating, and images are generally reconstructed in mid-diastolic phases for obtaining optimal image quality.12 By contrast, for sufficient quality of the SPECT images, the nongated data set is used, resulting in a slight mismatch of ventricular size between CT and SPECT images. Finally, the position of the heart is highly susceptible to respiratory motion. The CT scan is performed during a single inspiratory breath hold, but SPECT images are acquired during normal breathing, without accounting for respiratory motion unless respiratory gating is used. In fact, whole-body PET/CT studies have shown significant misalignment of the heart between superimposed PET and CT acquired during inspiration.13,14
These factors contribute to the notion that despite the integration of high-end CT devices (with the capability to perform state-of-the-art coronary CT angiography) with nuclear scanners to form dedicated cardiac hybrid scanners, manual image coregistration may remain indispensable.15 Published reports with x-ray-based attenuation correction have taught us that automated coregistration of CT and SPECT images is often unreliable, and manual correction for misalignment is needed in the vast majority of cases.16,17 Dedicated cardiac fusion software packages are now commercially available, allowing software-based hybrid imaging with an excellent interobserver reproducibility and short processing durations (see Fig. 8-3). In a validation study using software-based SPECT/CT fusion from standalone scanners, reproducibility and feasibility of this method were evaluated by assessing cardiac surface landmarks in 15 patients with a single coronary stenosis and a single perfusion defect.18 The authors reported an excellent interobserver variability (r = 0.99; P < 0.0001) for landmark detection and reasonably short processing times of less than 15 minutes per patient. The full integration of these fusion software packages into the regular postprocessing applications for CT angiography will further minimize time expenditure and improve workflow for hybrid imaging (by avoiding repeat actions such as coronary artery tracking from CT angiography images).
HYBRID IMAGING: COMPREHENSIVE “ONE-STOP SHOP”
Atherosclerotic disease accounts for the majority of fatalities reported in industrialized countries. The diagnostic gold standard for establishing the presence of CAD is still x-ray coronary angiography, with all its drawbacks. A major limitation of this technique is its invasive nature, with considerable procedure-related morbidity (1.5%) and mortality (0.15%). In addition, the physiologic significance of any lesion is frequently difficult to assess from angiographic information alone. Furthermore, up to 75% of all invasive angiograms in the United States remain purely diagnostic, and between 20% and 40% of all diagnostic invasive coronary angiograms reveal no clinically significant disease,19 so noninvasive procedures have the potential to play an important role in the future by eliminating a substantial fraction of these invasive angiograms (e.g., in cases of atypical chest pain, equivocal stress test results, and low to intermediate clinical probability of coronary disease).
Multislice CT angiography has rapidly evolved from an experimental technique to the most promising imaging modality for the noninvasive visualization of coronary arteries. Using the latest generation of CT scanners (dual-source CT, 256 detectors and more), excellent image quality can be achieved in the vast majority of patients, with values for sensitivity, specificity, and positive and negative predictive values averaging 92%, 96%, 79%, and 99%, respectively.20 With its high negative predictive value, noninvasive angiography may play an important role when the clinical goal is to rule out CAD in patient populations with low clinical probability for CAD. Nonetheless, a minor drawback of CT angiography remains the moderate positive predictive value, insofar as stenoses tend to be overestimated owing to partial volume effects from coronary artery–wall calcifications and other image artifacts.
The value of hybrid imaging originates from the spatial correlation of structural and functional information on the fused images, which facilitates a comprehensive interpretation of coronary lesions and their pathophysiologic relevance. Although this can be achieved by mental integration, coronary artery anatomy may vary considerably, therefore standard myocardial distribution territories correspond in only 50% to 60% with the real anatomic coronary tree.7 First clinical results with noninvasive hybrid imaging were presented by Namdar and coworkers using fusion of myocardial perfusion PET with 13N-ammonia and 4-slice CT angiography in 25 patients with CAD. Sensitivity, specificity, and positive and negative predictive value of hybrid PET/CT for the detection of flow-limiting stenoses in the main coronary vessels were 90%, 98%, 82%, and 99%, respectively, compared to the clinical gold standard of PET and conventional coronary angiography. These encouraging results were confirmed by a similar study by Rispler and coworkers.21 The authors compared the diagnostic accuracy of hybrid SPECT/CT imaging for the detection of flow-limiting coronary artery stenoses with CT angiography alone in 56 patients with angina pectoris. Hybrid SPECT/CT resulted in a significant improvement in specificity (from 63% to 95%) and positive predictive value (from 31% to 77%) compared to CT alone, without any change in sensitivity and negative predictive value. A similar study by Santana and colleagues showed an improved diagnostic accuracy of hybrid SPECT/CT imaging compared to SPECT alone (P < 0.001) and to the side-by-side analysis of SPECT and CT (P = 0.007) for diagnosis of obstructive CAD on conventional coronary angiography.22 These data show that hybrid SPECT/CT imaging may play an important role in the noninvasive diagnosis of CAD as a decision-making tool for assessing the need for revascularization in coronary artery stenoses.
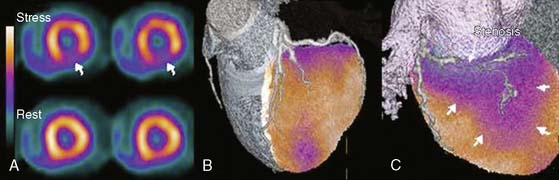
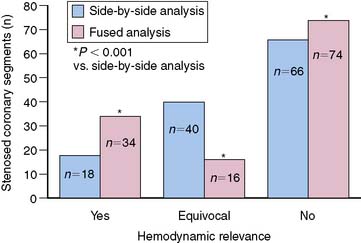
A recent publication focused on the role of hybrid SPECT/CT imaging for assessing the hemodynamic relevance of coronary artery stenoses and its potential added clinical value over side-by-side analysis in 38 high-risk patients with abnormal perfusion on SPECT (Fig. 8-4).3 The main clinical benefit of hybrid SPECT/CT imaging was a significant reduction in the number of coronary lesions with equivocal hemodynamic significance (from n = 40 to n = 16; P < 0.001) (Fig. 8-5). Added diagnostic information of SPECT/CT fusion was more commonly found in patients with stenoses of small vessels such as diagonal or posterolateral branches. Hence, the hybrid approach allows reliable allocation of perfusion defects to its subtending coronary artery, compared to the side-by-side analysis—a finding that might be particularly useful to guide revascularization strategies in symptomatic patients with multivessel disease and/or intermediate-degree stenoses.
The role of elective PCI in patients with stable CAD is a matter of ongoing debate.23,24 Guidelines recommend proof of ischemia prior to elective revascularization of coronary stenoses,23,25,26 and several reports have demonstrated that PCI fails to improve prognosis in patients with stable CAD compared to conservative treatment.24,27 These limitations have prompted the need for a comprehensive noninvasive CAD assessment prior to coronary revascularization. Hybrid cardiac imaging has the potential to fill this gap and become the long-awaited “One-Stop Shop,” helping clinicians to more fully incorporate clinical evidence into their decision-making process in patients with CAD. Elective interventional therapy can be planned carefully, helping to avoid overuse of angioplasty and stent placement. This is extremely relevant because overuse of expensive intravascular stents is a key cost driver in invasive cardiology practice.28 When lesion anatomy appears unsuitable for angioplasty, bypass grafting may be considered directly, without the need for further preoperative diagnostic coronary angiography, provided the noninvasive CT angiography images are of diagnostic quality.
VALUE OF HYBRID IMAGING FOR ATTENUATION CORRECTION
Strictly speaking, the term hybrid imaging refers to the combined or fused imaging of two data sets where both modalities equally contribute to image information. In a wider sense, however, the use of low-dose CT information29 for x-ray-based attenuation correction of myocardial perfusion images may also be considered “hybrid imaging.” In this setting, the CT images do not provide added anatomic or functional information but are used to improve image quality of the other modality (i.e., PET or SPECT).
Whereas the accuracy of cardiac PET imaging has long benefited from correction methods for tissue attenuation, in SPECT imaging, commercial methods have only recently been made available. Various techniques with different line sources, such as 241-americium (241Am), 153-gadolinium (153Gd), and 99m-technetium (99mTc) have been proposed. Initial reports indicate that some of these methods have achieved significant improvements, but that others have created more artifacts than they have remedied and have varied greatly in their clinical success.30
Certainly the use of CT for attenuation correction could well be an important step forward in solving some of the major attenuation-correction problems of the SPECT technique, allowing consistent image reading in male and female patients. Because of the recent introduction of this technique, only limited data are available. Although initial experiences seem promising,31,32 there are some drawbacks that need to be overcome. For example, misalignment between SPECT and the attenuation map can lead to artifacts in the apical, septal, and anterior walls that will appear as defects. It also can cause overcorrection in the basal inferior and lateral segments. There is evidence that mismatches along the other directions may have a similar effect. The coregistration of SPECT and the attenuation map need to be verified for every patient, even when using integrated dual-modality imaging devices.17
In PET, the major advantage of using CT for attenuation correction is the short time duration (less than 5 seconds) compared with conventional correction with germanium sources (10 to 20 minutes). This allows the separation of the acquisitions of perfusion and viability PET scans, since the time loss for an additional transmission scan is minimal. Koepfli and coworkers reported a good feasibility and repeatability of this method for quantitative PET myocardial perfusion measurements. The results show that myocardial blood flow quantification is largely independent of CT tube current and document that electrocardiogram (ECG) gating of the CT beam seems not to be necessary.29 Attenuation correction with CT provided results highly comparable to those obtained using germanium attenuation correction.
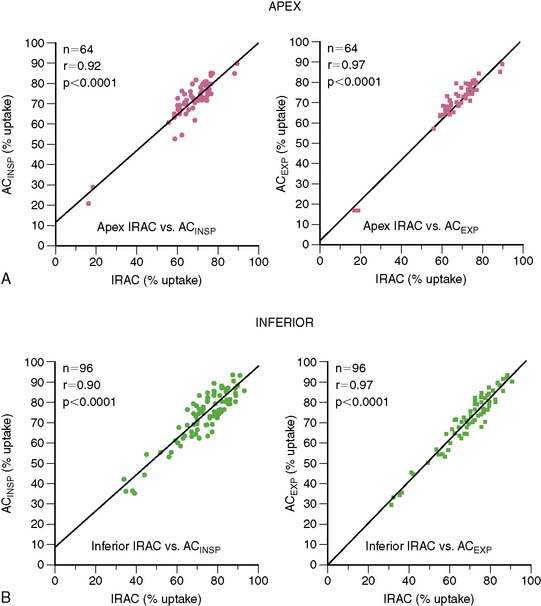
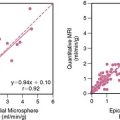
Although in SPECT, photon energies are lower (70 keV with 201-thallium [201Tl], 140 keV with 99mTc versus 511 keV with positron-emitting radionuclides) and thus more susceptible to photon attenuation, the use of x-ray-based attenuation correction is less well established compared to PET, because in general, attenuation correction for SPECT is more complex. Therefore, the latest developments in SPECT technology have aimed in a different direction: at reducing acquisition times and/or lowering radiation exposures, rather than addressing the issue of attenuation correction. Thus no attenuation correction is available in most dedicated cardiac SPECT scanners.33 The lack of an inherent attenuation correction facility can be overcome by using low-dose native coronary artery CACs scans performed on a standalone high-end CT device for attenuation correction of the SPECT images. In contrast to present low-dose CT facilities of hybrid SPECT/CT scanners, CACS scans are ECG-triggered and acquired during a single breath hold. Furthermore, this approach requires the use of interface software for manual coregistration of SPECT and CT information to avoid misalignment between both images (Fig. 8-6). The feasibility and reproducibility of this method have been demonstrated in a recent publication by Schepis and coworkers using CACS scans during full inspiratory and expiratory breath hold.16 Parametric attenuation maps from CACS scans provided accurate and reliable attenuation correction of SPECT images, resulting in a very good correlation compared to the established attenuation correction with the low-dose low-resolution CT facility included in the hybrid SPECT/CT scanner. Interestingly, the expiratory CACS scan proved slightly superior to the inspiratory scan, particularly for regional tracer uptake values in apical and inferior segments, notably those segments most affected by attenuation artifacts (Fig. 8-7). This finding suggests that in hybrid scans, CACS scan may be performed during normal expiration to allow its additional use for attenuation correction of SPECT images.
PROGNOSTIC AND DIAGNOSTIC VALUE OF CORONARY ARTERY CALCIUM SCORES (See Chapter 20)
CACS provides an estimate of coronary atherosclerotic plaque burden and correlates strongly with the overall amount of coronary plaque (calcified and noncalcified) as determined at postmortem examination.34 Assessment of the presence of subclinical coronary atherosclerosis with CACS provides an opportunity to identify asymptomatic patients who are at risk of developing clinical coronary artery disease (CAD) over the long term. Several studies in asymptomatic subjects have consistently shown that CACS provides accurate risk estimates for cardiac death and ischemic events beyond the risk calculations derived from clinical parameters.35 These observations have led to the implementation of CACS in current recommendations for coronary risk assessment.35,36 Particularly in patients at intermediate clinical risk, CACS may prove helpful in further stratifying those patients into low-, intermediate-, and high-risk categories.37
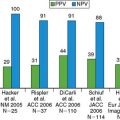
In addition to its prognostic value, CACS may also offer diagnostic information. Coronary artery calcifications are almost always extant in the presence of angiographically significant CAD, so a CACS of 0 virtually excludes any significant angiographic CAD. In the largest study to date comparing CACS with conventional coronary angiography, sensitivity and negative predictive values of CACS for detecting angiographically significant CAD were 99% and 97%, respectively, indicating an excellent ability of CACS to rule out obstructive CAD.38 However, specificity and positive predictive value (23% and 62%) were low and increased only moderately when shifting the CACS cutoff from 0 to higher values. These limitations are the reason why CACS is not recommended as a single first-line imaging tool for the evaluation of symptomatic patients with suspected CAD.
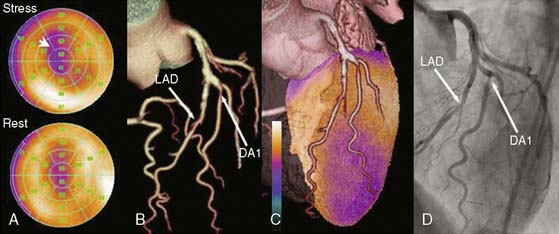
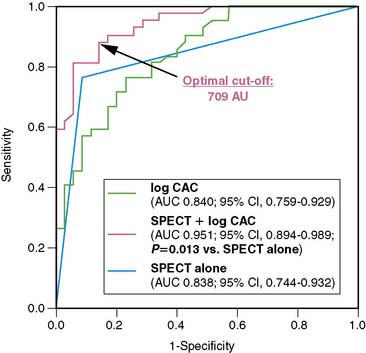
An alternative approach, however, is using CACS in combination with myocardial perfusion imaging for the diagnosis of CAD, inasmuch as CACS is being made increasingly available with the introduction of hybrid scanners. In a recent study by Schepis and colleagues, the combination of SPECT and CACS resulted in a significant improvement in sensitivity and specificity for the diagnosis of angiographically significant CAD compared to SPECT alone (Fig. 8-8). These findings suggest a potential role of CACS as an adjunct to SPECT for the noninvasive evaluation of CAD. It seems reasonable to elaborate algorithms where the combination of CACS with myocardial perfusion studies may help refine the interpretation of the latter. On the one hand, the presence of high atherosclerotic burden in a symptomatic patient with a normal perfusion scan may suggest significant multivessel disease with balanced ischemia. On the other hand, an equivocal perfusion result in the presence of very low CACS may raise the suspicion of an attenuation or respiratory artifact, justifying further CAD rule-out with CT angiography. However, despite encouraging results, the real role of the calcium score in the cascade of noninvasive investigations of CAD remains to be determined.
HYBRID SCANNERS VERSUS HYBRID IMAGING
Despite the widespread use of coronary CT angiography and myocardial perfusion imaging (MPI) with SPECT or PET, both techniques vary considerably in their image acquisition times. Whereas coronary CT angiography with the newest generation 64-slice or dual-source CT devices is performed in less than 12 seconds,12 emission scans for stress and rest gated SPECT with 99mTc-based radiotracers at standard doses take at least 45 minutes.39 This discrepancy between emission and transmission scan times determines that high-end CT facilities constituting the CT component of hybrid cardiac scanners will be blocked by long emission scan times and therefore operate at low capacity. Many advances in nuclear medicine, such as newly developed dedicated cardiac detector systems33 and novel image reconstruction algorithms,40 may contribute to reducing emission scan times considerably. However, to date, in hybrid scanners with high-end CT facilities, the rather long emission scan times preclude operating the high-end CT device at full capacity. Additionally, despite the promise of hybrid cardiac imaging, first clinical experiences with hybrid SPECT/CT imaging have shown that in an usual population referred for noninvasive workup of CAD, only a minority benefit from hybrid imaging, compared to side-by-side interpretation of MPI and CT.3 Thus at present, it appears that seen from the standpoint of patient throughput, a dedicated cardiac hybrid scanner is less profitable than two standalone devices for normal-volume nuclear diagnostic centers. Nonetheless, it will depend on the individual setting of each institution to determine the type of approach—that is, software-based fusion or hybrid scanner—that is best tailored for its particular purpose, and highly specialized cardiac centers may prefer hybrid scanners for integrative cardiac imaging.
PERSPECTIVES OF HYBRID IMAGING
Hybrid imaging is a new and highly dynamic field of continuing research driven by constant advances in technology, innovations in noninvasive imaging, and increasing clinical interest in this promising tool. Efforts to improve techniques and implement hybrid imaging in daily clinical routine are ongoing. The advent of ultrafast dedicated cardiac SPECT scanners with short acquisition times33 and their incorporation with multislice CT devices into hybrid scanners will reduce dead-time issues and allow for higher patient throughput and improvement of scanner efficiency. Furthermore, the increasing use of prospective ECG-gating protocols for coronary CT angiography will help to reduce radiation burden to approximately 2.0 to 2.5 mSv,41 a dose that allows hybrid scanning at a reasonable radiation exposure (Fig. 8-9). First trials using hybrid imaging with PET/CT reported a reduction of 60% to 73% in radiation exposure using a prospective ECG-triggered protocol compared to retrospectively triggered spiral acquisition, without any loss in image quality.42,43 In a recent SPECT/CT trial, a comparable reduction of 78% was reported for hybrid stress-only SPECT/CT in a low-pretest probability population. The resulting total effective dose was 5.4 mSv for hybrid imaging with prospective ECG-triggering for CT angiography.44
Myocardial perfusion imaging is by far the most important application of nuclear studies in cardiology. However, software-based hybrid imaging allows free combination of morphologic images with any nuclear study. This widens the potential use of hybrid imaging by including studies for cardiac innervation, metabolism, gene expression and stem cell imaging, and plaque imaging, and by combining different modalities such as CT, nuclear techniques, or cardiac magnetic resonance (Fig. 8-10).45,46 Nevertheless, atherosclerotic disease is responsible for high morbidity and mortality in industrialized countries. Despite major advances in treatment of CAD patients, a large number of victims of the disease who are apparently healthy die suddenly without prior symptoms. The recognition of the role of the vulnerable plaque has opened new avenues of opportunity in the field of cardiovascular medicine.47 The hybrid technology has the unique potential to enable detection and quantification of the burden of calcified and noncalcified plaques, quantification of vascular reactivity and endothelial health, identification of flow-limiting coronary stenoses, and (potentially) identification of high-risk plaques using fusion of morphology and biology with molecularly targeted PET imaging.48 By this means, in the future, hybrid imaging may allow easy and comprehensive noninvasive assessment of coronary plaque burden, its pathophysiologic relevance, and biological plaque activity, providing accurate individual risk estimates upon which further management decisions can be based.
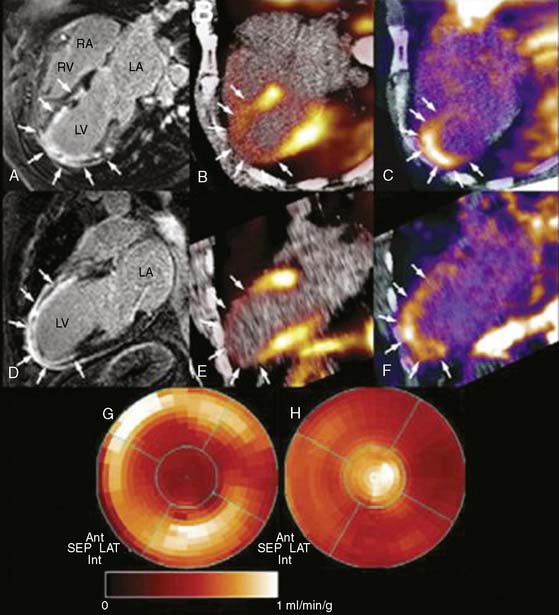
Figure 8-10 Multimodality imaging with cardiac magnetic resonance (CMR) and PET. A and D show horizontal and vertical long-axis slices of CMR. Late gadolinium hyperenhancement in the apex demonstrates a nearly transmural myocardial scar. B and E reveal diminished perfusion assessed with 13N-ammonia PET in the apical, apicoanterior, and inferoseptal regions. In images C and F, PET with 18F-RGD (a novel PET agent targeting αvβ3 integrin, a key mediator of angiogenesis) demonstrates focal tracer uptake in the infarcted area. This signal co-localizes to the regions of delayed hyperenhancement on CMR and indicates the presence of myocardial repair processes after ischemic injury. The complementary representation of perfusion with 13N-ammonia (G) and angiogenesis in the infarcted apical myocardium (H) is visualized with polar maps.
(From Mankowski RM, Ebersberger U, Nekolla S, Schwaiger M: In vivo molecular imaging of angiogenesis, targeting alphav beta3 integrin expression in a patient after acute myocardial infarction. Eur Heart J 29:2201, 2008. Reprinted with permission of Oxford University Press.)
1. Topol E.J., Nissen S.E. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92(8):2333-2342.
2. Silber S., Albertsson P., Aviles F.F., Camici P.G., Colombo A., Hamm C., et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26(8):804-847.
3. Gaemperli O., Schepis T., Valenta I., Husmann L., Scheffel H., Duerst V., et al. Cardiac image fusion from stand-alone SPECT and CT: clinical experience. J Nucl Med. 2007;48(5):696-703.
4. Namdar M., Hany T.F., Koepfli P., Siegrist P.T., Burger C., Wyss C.A., et al. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med. 2005;46(6):930-935.
5. Gourtsoyiannis N., McCall I., Reiser M., Silberman B., Bischof Delaloye A., Carrio I., et al. White paper of the European Society of Radiology (ESR) and the European Association of Nuclear Medicine (EANM) on multimodality imaging. Eur Radiol. 2007;17(8):1926-1930.
6. Faber T.L., Santana C.A., Garcia E.V., Candell-Riera J., Folks R.D., Peifer J.W., et al. Three-dimensional fusion of coronary arteries with myocardial perfusion distributions: clinical validation. J Nucl Med. 2004;45(5):745-753.
7. Schindler T.H., Magosaki N., Jeserich M., Oser U., Krause T., Fischer R., et al. Fusion imaging: combined visualization of 3D reconstructed coronary artery tree and 3D myocardial scintigraphic image in coronary artery disease. Int J Card Imaging. 1999;15(5):357-368. discussion 369–70
8. Peifer J.W., Ezquerra N.F., Cooke C.D., Mullick R., Klein L., Hyche M.E., et al. Visualization of multimodality cardiac imagery. IEEE Trans Biomed Eng. 1990;37(8):744-756.
9. Nishimura Y., Fukuchi K., Katafuchi T., Sagou M., Oka H., Ishida Y., et al. Superimposed display of coronary artery on gated myocardial perfusion scintigraphy. J Nucl Med. 2004;45(9):1444-1449.
10. Fishman E.K., Ney D.R., Heath D.G., Corl F.M., Horton K.M., Johnson P.T. Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why. Radiographics. 2006;26(3):905-922.
11. Bax J.J., Beanlands R.S., Klocke F.J., Knuuti J., Lammertsma A.A., Schaefers M.A., et al. Diagnostic and clinical perspectives of fusion imaging in cardiology: is the total greater than the sum of its parts? Heart. 2007;93(1):16-22.
12. Leschka S., Scheffel H., Desbiolles L., Plass A., Gaemperli O., Valenta I., et al. Image quality and reconstruction intervals of dual-source CT coronary angiography: recommendations for ECG-pulsing windowing. Invest Radiol. 2007;42(8):543-549.
13. Gilman M.D., Fischman A.J., Krishnasetty V., Halpern E.F., Aquino S.L. Hybrid PET/CT of the thorax: when is computer registration necessary? J Comput Assist Tomogr. 2007;31(3):395-401.
14. Gould K.L., Pan T., Loghin C., Johnson N.P., Guha A., Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48(7):1112-1121.
15. Gaemperli O., Kaufmann P.A. Hybrid cardiac imaging: more than the sum of its parts? J Nucl Cardiol. 2008;15(1):123-126.
16. Schepis T., Gaemperli O., Koepfli P., Ruegg C., Burger C., Leschka S., et al. Use of coronary calcium score scans from stand-alone multislice computed tomography for attenuation correction of myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34(1):11-19.
17. Goetze S., Wahl R.L. Prevalence of misregistration between SPECT and CT for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol. 2007;14(2):200-206.
18. Gaemperli O., Schepis T., Kalff V., Namdar M., Valenta I., Stefani L., et al. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging. 2007;34(7):1097-1106.
19. Achenbach S., Daniel W.G. Noninvasive coronary angiography—an acceptable alternative? N Engl J Med. 2001;345(26):1909-1910.
20. Schuijf J.D., Jukema J.W., van der Wall E.E., Bax J.J. The current status of multislice computed tomography in the diagnosis and prognosis of coronary artery disease. J Nucl Cardiol. 2007;14(4):604-612.
21. Rispler S., Keidar Z., Ghersin E., Roguin A., Soil A., Dragu R., et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49(10):1059-1067.
22. Santana C.A., Garcia E.V., Faber T.L., Sirineni G.K., Esteves F.P., Sanyal R., et al. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. J Nucl Cardiol. 2009;16:201-211.
23. Fox K., Garcia M.A., Ardissino D., Buszman P., Camici P.G., Crea F., et al. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(11):1341-1381.
24. Boden W.E., O’Rourke R.A., Teo K.K., Hartigan P.M., Maron D.J., Kostuk W.J., et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503-1516.
25. Smith S.C.Jr, Feldman T.E., Hirshfeld J.W.Jr, Jacobs A.K., Kern M.J., King S.B.3rd, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47(1):e1-121.
26. Gibbons R.J., Abrams J., Chatterjee K., Daley J., Deedwania P.C., Douglas J.S., et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 2003;41(1):159-168.
27. Bucher H.C., Hengstler P., Schindler C., Guyatt G.H. Percutaneous transluminal coronary angioplasty versus medical treatment for non-acute coronary heart disease: meta-analysis of randomised controlled trials. BMJ. 2000;321(7253):73-77.
28. Kaiser C., Brunner-La Rocca H.P., Buser P.T., Bonetti P.O., Osswald S., Linka A., et al. Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitats Trial (BASKET). Lancet. 2005;366(9489):921-929.
29. Koepfli P., Hany T.F., Wyss C.A., Namdar M., Burger C., Konstantinidis A.V., et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med. 2004;45(4):537-542.
30. Corbett J.R., Ficaro E.P. Attenuation corrected cardiac perfusion SPECT. Curr Opin Cardiol. 2000;15(5):330-336.
31. Utsunomiya D., Tomiguchi S., Shiraishi S., Yamada K., Honda T., Kawanaka K., et al. Initial experience with x-ray CT based attenuation correction in myocardial perfusion SPECT imaging using a combined SPECT/CT system. Ann Nucl Med. 2005;19(6):485-489.
32. Fricke E., Fricke H., Weise R., Kammeier A., Hagedorn R., Lotz N., et al. Attenuation correction of myocardial SPECT perfusion images with low-dose CT: evaluation of the method by comparison with perfusion PET. J Nucl Med. 2005;46(5):736-744.
33. Patton J.A., Slomka P.J., Germano G., Berman D.S. Recent technologic advances in nuclear cardiology. J Nucl Cardiol. 2007;14(4):501-513.
34. Rumberger J.A., Simons D.B., Fitzpatrick L.A., Sheedy P.F., Schwartz R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157-2162.
35. Berman D.S., Hachamovitch R., Shaw L.J., Friedman J.D., Hayes S.W., Thomson L.E., et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med. 2006;47(7):1107-1118.
36. Grundy S.M., Cleeman J.I., Merz C.N., Brewer H.B.Jr, Clark L.T., Hunninghake D.B., et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
37. Greenland P., Bonow R.O., Brundage B.H., Budoff M.J., Eisenberg M.J., Grundy S.M., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49(3):378-402.
38. Haberl R., Becker A., Leber A., Knez A., Becker C., Lang C., et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37(2):451-457.
39. Hansen C.L., Goldstein R.A., Berman D.S., Churchwell K.B., Cooke C.D., Corbett J.R., et al. Myocardial perfusion and function single photon emission computed tomography. J Nucl Cardiol. 2006;13(6):e97-e120.
40. Borges-Neto S., Pagnanelli R.A., Shaw L.K., Honeycutt E., Shwartz S.C., Adams G.L., et al. Clinical results of a novel wide beam reconstruction method for shortening scan time of Tc-99m cardiac SPECT perfusion studies. J Nucl Cardiol. 2007;14(4):555-565.
41. Husmann L., Valenta I., Gaemperli O., Adda O., Treyer V., Wyss C.A., et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29(2):191-197.
42. Kajander S., Ukkonen H., Sipila H., Teras M., Knuuti J. Low radiation dose imaging of myocardial perfusion and coronary angiography with a hybrid PET/CT scanner. Clin Physiol Funct Imaging. 2009;29(1):81-88.
43. Javadi M., Mahesh M., McBride G., Voicu C., Epley W., Merrill J., et al. Lowering radiation dose for integrated assessment of coronary morphology and physiology: first experience with step-and-shoot CT angiography in a rubidium 82 PET-CT protocol. J Nucl Cardiol. 2008;15(6):783-790.
44. Husmann L., Herzog B.A., Gaemperli O., Tatsugami F., Burkhard N., Valenta I., et al. Diagnostic accuracy of computed tomography coronary angiography and evaluation of stress-only single-photon emission computed tomography/computed tomography hybrid imaging: comparison of prospective electrocardiogram-triggering vs. retrospective gating. Eur Heart J. 2008.
45. Nekolla S.G., Martinez-Moeller A., Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging. 2008.
46. Makowski M.R., Ebersberger U., Nekolla S., Schwaiger M. In vivo molecular imaging of angiogenesis, targeting alphavbeta3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J. 2008;29(18):2201.
47. Naghavi M., Libby P., Falk E., Casscells S.W., Litovsky S., Rumberger J., et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664-1672.
48. Di Carli M.F., Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115(11):1464-1480.