Hyaline Molds, Mucorales (Zygomycetes), Dermatophytes, and Opportunistic and Systemic Mycoses
1. Describe where mucorales (zygomycetes) are found, how they are transmitted to humans, and the diseases they cause.
2. Describe the characteristic colony morphology of the mucorales (zygomycetes).
3. Outline the tests needed to diagnose a Trichophyton species.
4. List the key features that distinguish Trichophyton rubrum and Trichophyton mentagrophytes.
5. Compare and contrast the ways Microsporum audouinii and Microsporum canis are spread and the populations at risk.
6. Define ectothrix and endothrix.
7. Explain why diagnosing an opportunistic fungal infection in an immunocompromised patient is difficult.
8. Compare and contrast Aspergillus and Penicillium spp., both macroscopically and microscopically.
9. Discuss the dimorphic molds in relation to their endemic areas, disease states, and associated diagnostic methods for identification.
The Mucorales
General Characteristics
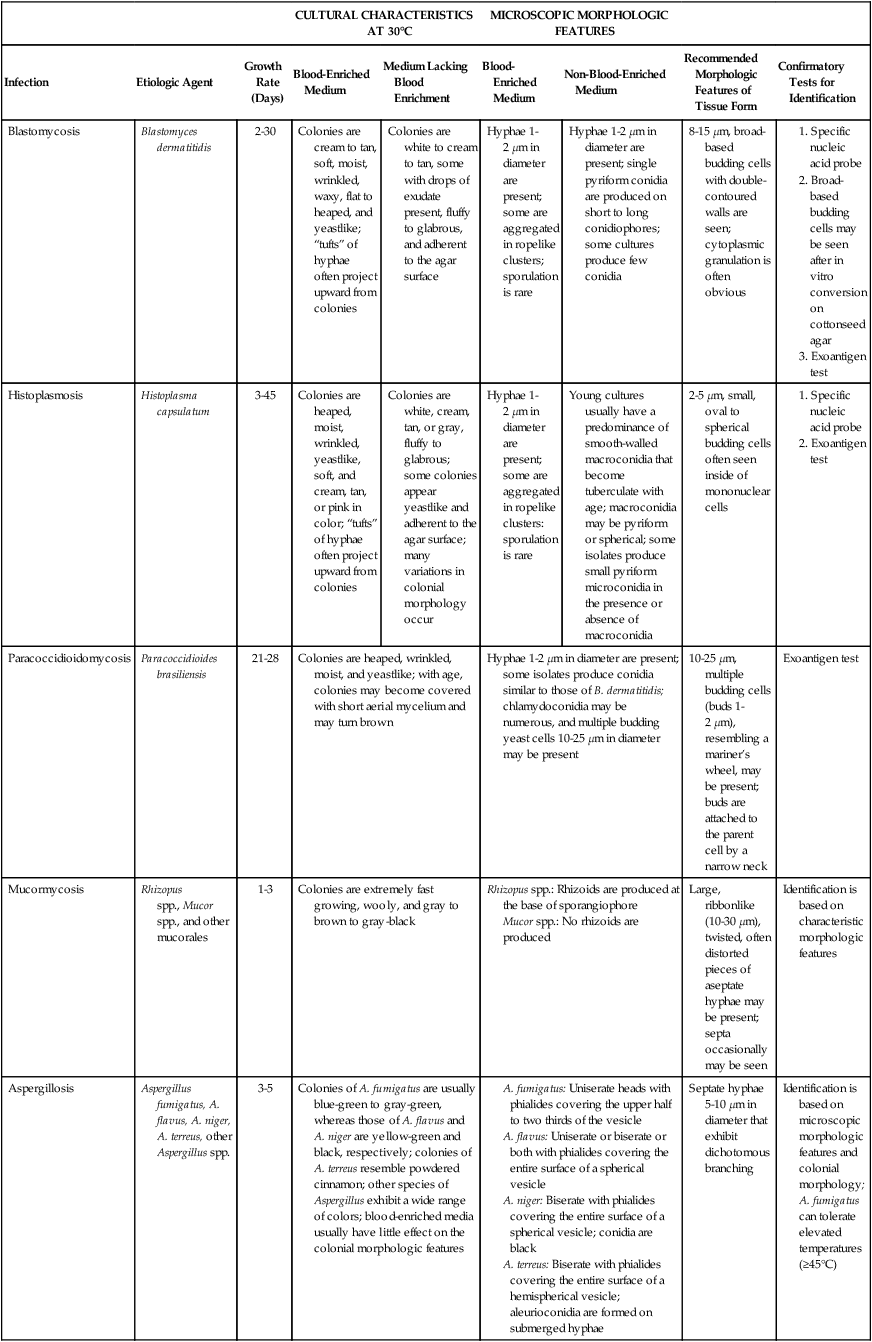
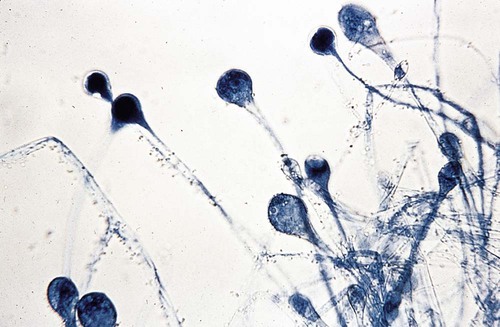
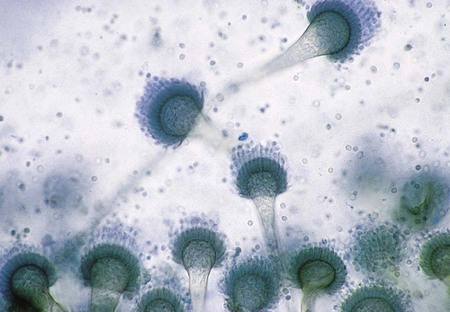
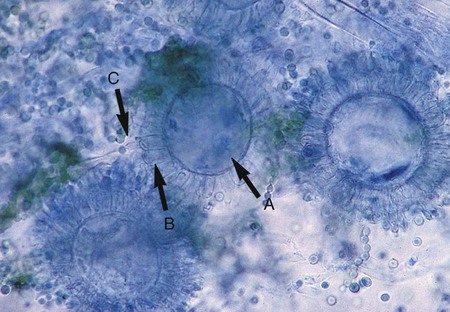
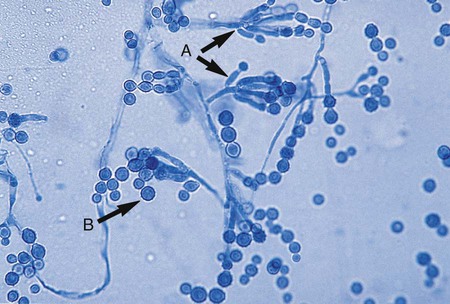
The mucorales (zygomycetes) characteristically produce large, ribbonlike hyphae that are irregular in diameter and contain occasional septa. Because the septa may not be apparent in some preparations, this group sometimes has been characterized as aseptate. The specific identification of these organisms is confirmed by observing the characteristic saclike fruiting structures (sporangia), which produce internally spherical, yellow or brown spores (sporangiospores) (Figure 60-1). Each sporangium is formed at the tip of a supporting structure (sporangiophore). During maturation, the sporangium becomes fractured and sporangiospores are released into the environment. Sporangiophores are usually connected to one another by occasionally septate hyphae called stolons, which attach at contact points where rootlike structures (rhizoids) may appear and anchor the organism to the agar surface. Identification of the mucorales (Mucor, Rhizopus, Lichtheimia, and Absidia spp.) is partly based on the presence or absence of rhizoids and the position of the rhizoids in relation to the sporangiophores.
Laboratory Diagnosis
Specimen Collection and Transport
See General Considerations for the Laboratory Diagnosis of Fungal Infections in Chapter 59.
Direct Detection Methods
Stains.
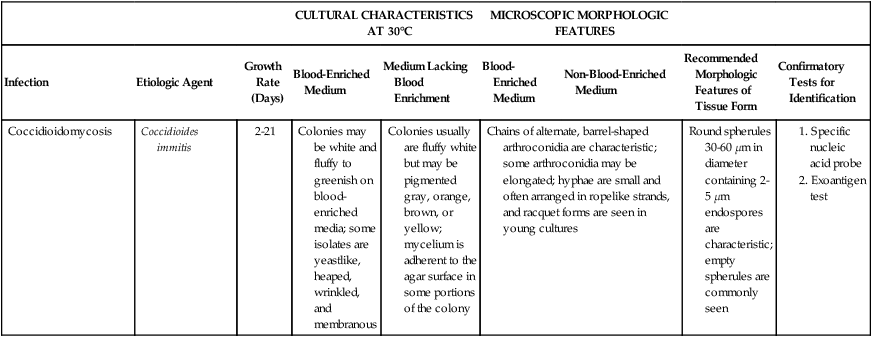
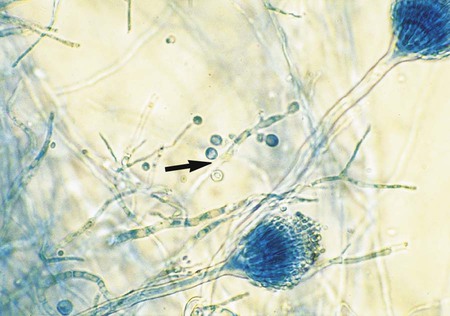
A mucormycosis may be diagnosed rapidly by examining tissue specimens or exudate from infected lesions in a calcofluor white or potassium hydroxide preparation. Branching, broad-diameter, predominantly nonseptate hyphae are observed (Figure 60-2). It is important that the laboratory notify the clinician of these findings, because mucorales grow rapidly, and vascular invasion occurs at a rapid rate.
Cultivation.
The colonial morphologic features of the mucorales allow immediate suspicion that an organism belongs to this group. Colonies characteristically produce a fluffy, white to gray or brown hyphal growth that resembles cotton candy and that diffusely covers the surface of the agar within 24 to 96 hours (Figure 60-3). The hyphae can grow very fast and may lift the lid of the agar plate (also known as a “lid lifter”). The hyphae appear to be coarse. The entire culture dish or tube rapidly fills with loose, grayish hyphae dotted with brown or black sporangia. The different genera and species of mucorales cannot be differentiated by colonial morphologic features, because most are identical.

Approach to Identification
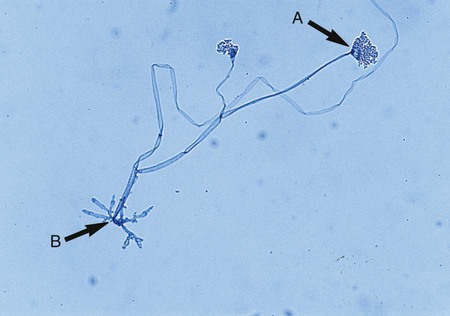
Rhizopus spp. have unbranched sporangiophores with rhizoids that appear opposite the point where the stolon arises, at the base of the sporangiophore (see Figure 60-1). In contrast, Mucor spp. are characterized by sporangiophores that are singularly produced or branched and have a round sporangium at the tip filled with sporangiospores. They do not have rhizoids or stolons, which distinguishes this genus from the other genera of the mucorales (Figure 60-4). Lichtheimia spp. and Absidia spp. are characterized by the presence of rhizoids that originate between sporangiophores (Figure 60-5). The sporangia of Lichtheimia spp. are pyriform and have a funnel-shaped area (apophysis) at the junction of the sporangium and the sporangiophore. Usually a septum is formed in the sporangiophore just below the sporangium. Other genera of Glomeromycota that are encountered much less frequently in the clinical laboratory are Rhizomucor, Saksenaea, Cunninghamella, Apophysomyces, Conidiobolus, and Basidiobolus spp.
The Dermatophytes
General Characteristics
Laboratory Diagnosis
Specimen Collection and Transport
See General Considerations for the Laboratory Diagnosis of Fungal Infections in Chapter 59.
Direct Detection Methods
Stains.
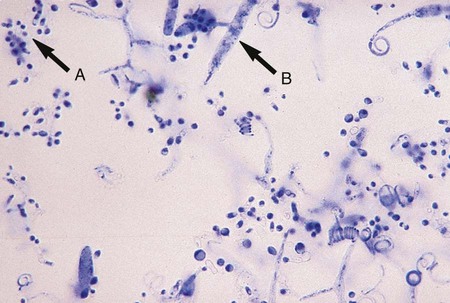
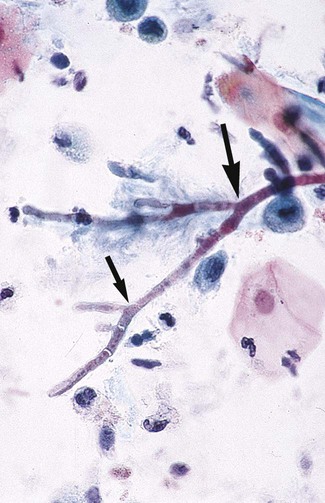
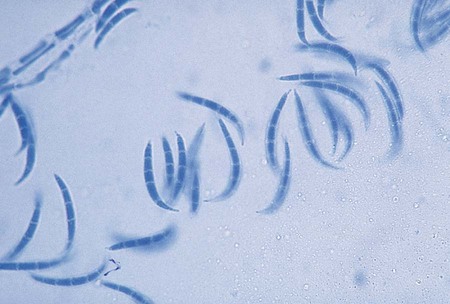
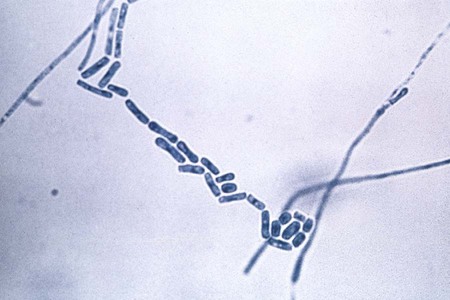
Calcofluor white or potassium hydroxide preparations reveal the presence of hyaline septate hyphae and/or arthroconidia (see Figures 59-4 and 60-6). Direct microscopic examination of infected hairs may reveal the hair shaft to be filled with masses of large arthroconidia (4 to 7 µm) in chains, characteristic of an endothrix type of invasion. In other instances, the hair shows external masses of spores that ensheath the hair shaft; this is characteristic of the ectothrix type of hair invasion. Hairs infected with Trichophyton schoenleinii reveal hyphae and air spaces within the shaft.
Cultivation.
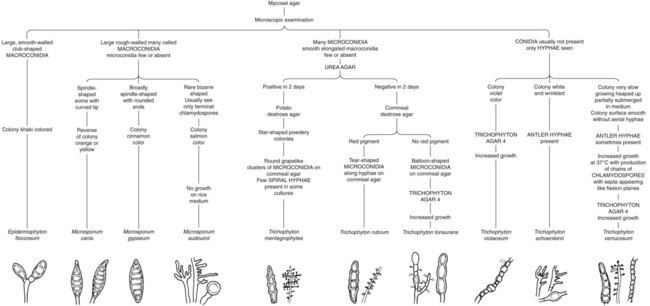
Because the dermatophytes generally present a similar microscopic appearance in infected hair, skin, or nails, final identification typically is made by culture. A summary of the colonial and microscopic morphologic features of these fungi is presented in Table 60-1. Figure 60-7 presents an identification schema useful to the clinical laboratory for identification of commonly encountered dermatophytes. The schema begins with the microscopic features of the dermatophytes that may be visible in the initial examination of the culture. In many instances, the primary recovery medium fails to function as well as a sporulation medium. Often the initial growth must be subcultured onto cornmeal agar or potato dextrose agar to induce sporulation.
TABLE 60-1
Characteristics of Dermatophytes Commonly Recovered in the Clinical Laboratory
| Dermatophyte | Colonial Morphology | Growth Rate | Microscopic Identification |
| Microsporum audouinii* | Downy white to salmon-pink colony; reverse tan to salmon-pink | 2 weeks | Sterile hyphae; terminal chlamydoconidia, favic chandeliers, and pectinate bodies; macroconidia rarely seen (bizarre shaped if seen); microconidia rare or absent |
| Microsporum canis | Colony usually membranous with feathery periphery; center of colony white to buff over orange-yellow; lemon-yellow or yellow-orange apron and reverse | 1 week | Thick-walled, spindle-shaped, multiseptate, rough-walled macroconidia, some with a curved tip; microconidia rarely seen |
| Microsporum gypseum | Cinnamon-colored, powdery colony; reverse light tan | 1 week | Thick-walled, rough, elliptical, multiseptate macroconidia; microconidia few or absent |
| Epidermophyton floccosum | Center of colony tends to be folded and is khaki green; periphery is yellow; reverse yellowish brown with observable folds | 1 week | Macroconidia: large, smooth walled, multiseptate, clavate, and borne singly or in clusters of two or three; microconidia not formed by this species |
| Trichophyton mentagrophytes | Different colonial types; white, granular, and fluffy varieties; occasional light yellow periphery in younger cultures; reverse buff to reddish brown | 7-10 days | Many round to globose microconidia, most commonly borne in grapelike clusters or laterally along the hyphae; spiral hyphae in 30% of isolates; macroconidia are thin walled, smooth, club shaped, and multiseptate; numerous or rare, depending upon strain |
| Trichophyton rubrum | Colonial types vary from white downy to pink granular; rugal folds are common; reverse yellow when colony is young, but wine/ red color commonly develops with age | 2 weeks | Microconidia usually teardrop-shaped, most commonly borne along sides of the hyphae; macroconidia usually absent but when present are smooth, thin walled, and pencil shaped |
| Trichophyton tonsurans | White, tan to yellow or rust, suedelike to powdery; wrinkled with heaped or sunken center; reverse yellow to tan to rust red | 7-14 days | Microconidia are teardrop or club shaped with flat bottoms; vary in size but usually larger than other dermatophytes; macroconidia rare(balloon forms found when present) |
| Trichophyton schoenleinii* | Irregularly heaped, smooth, white to cream colony with radiating grooves; reverse white | 2-3 weeks | Hyphae usually sterile; many antler-type hyphae seen (favic chandeliers) |
| Trichophyton violaceum* | Port wine to deep violet colony, may be heaped or flat with waxy-glabrous surface; pigment may be lost on subculture | 2-3 weeks | Branched, tortuous. sterile hyphae; chlamydoconidia commonly aligned in chains |
| Trichophyton verrucosum | Glabrous to velvety white colonies; rare strains produce yellow-brown color; rugal folds with tendency to skin into agar surface | 2-3 weeks | Microconidia rare, large and teardrop-shaped when seen; macroconidia extremely rare but form characteristic rat-tail types when seen; many chlamydoconidia seen in chains, particularly when colony is incubated at 37°C |
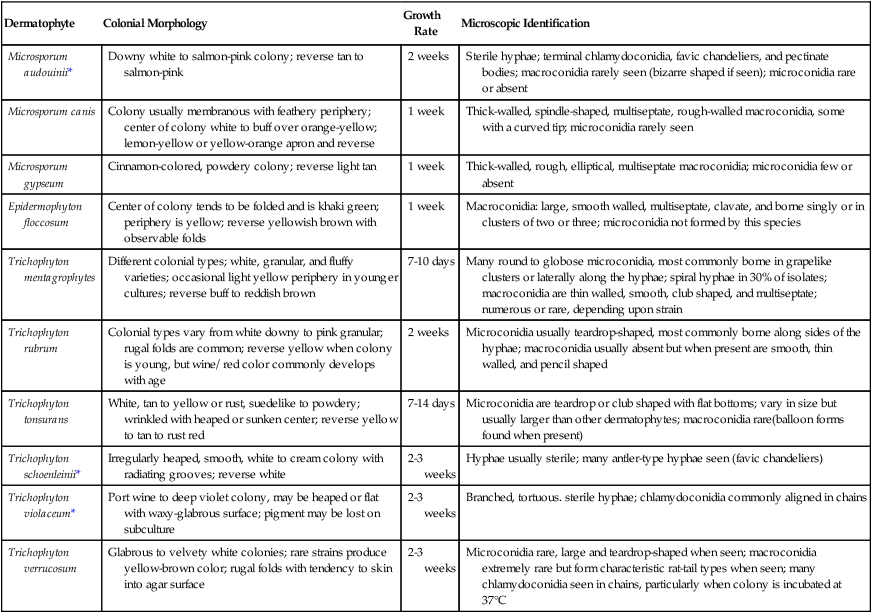
*These organisms are not commonly seen in the United States.
Approach to Identification
Trichophyton spp.
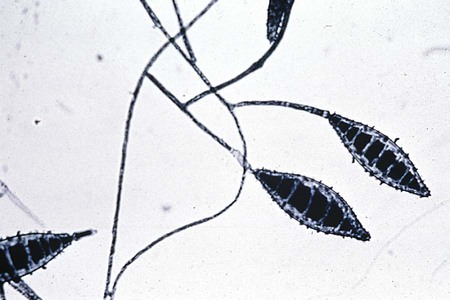
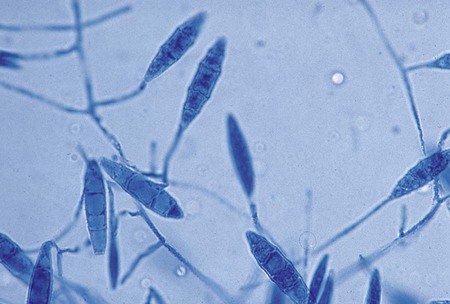
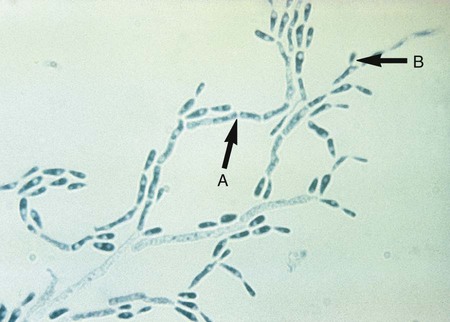
Microscopically, Trichophyton organisms are characterized by smooth, club-shaped, thin-walled macroconidia with three to eight septa ranging from 4 × 8 µm to 8 × 15 µm. The macroconidia are borne singly at the terminal ends of hyphae or on short conidiophores; the microconidia (which may be described as “birds on a fence”) predominate and are usually spherical, pyriform (teardrop shaped), or clavate (club shaped), and 2 to 4 µm (Figure 60-8). Only the common Trichophyton species are described here.
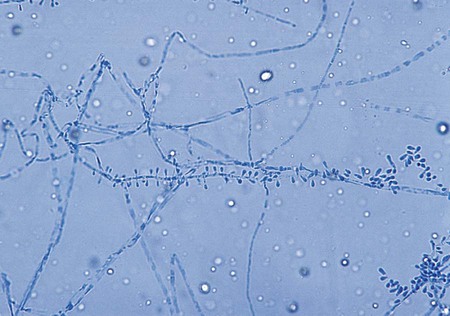
T. rubrum and T. mentagrophytes are the most common species recovered in the clinical laboratory. T. rubrum is a slow-growing organism that produces a flat or heaped-up colony, generally white to reddish, with a cottony or velvety surface. The characteristic cherry-red color is best observed on the reverse side of the colony; however, this is produced only after 3 to 4 weeks of incubation. Occasional strains may lack the deep red pigmentation on primary isolation. Two types of colonies may be produced: fluffy and granular. Microconidia are uncommon in most of the fluffy strains and more common in the granular strains; they occur as small, teardrop-shaped conidia often borne laterally along the sides of the hyphae (see Figure 60-8). Macroconidia are seen less commonly, although they are sometimes found in the granular strains, where they appear as thin-walled, smooth-walled, multicelled, cigar-shaped conidia with three to eight septa. T. rubrum has no specific nutritional requirements. It does not perforate hair in vitro or produce urease.
T. mentagrophytes produces two distinct colonial forms: the downy variety recovered from patients with tinea pedis and the granular variety recovered from lesions acquired by contact with animals. The rapidly growing colonies may appear as white to cream-colored or yellow, cottony or downy, and coarsely granular to powdery. They may produce a few spherical microconidia. The granular colonies may show evidence of red pigmentation. The reverse side of the colony is usually rose-brown, occasionally orange to deep red, and may be confused with T. rubrum. Granular colonies sporulate freely, with numerous small, spherical microconidia in grapelike clusters and thin-walled, smooth-walled, cigar-shaped macroconidia measuring 6 × 20 µm to 8 × 50 µm, with two to five septa (Figure 60-9). Macroconidia characteristically exhibit a definite narrow attachment to their base. Spiral hyphae may be found in one third of the isolates recovered.
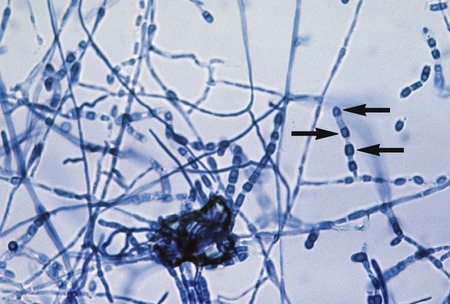
T. mentagrophytes produces urease within 2 to 3 days after inoculation onto Christensen’s urea agar. Unlike T. rubrum, T. mentagrophytes perforates hair (Figure 60-10), a feature that may be used to distinguish between the two species when differentiation is difficult.
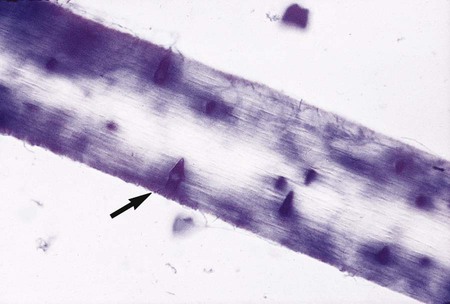
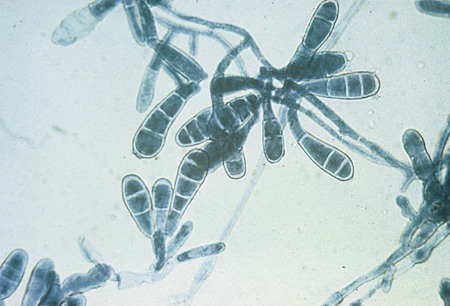
Cultures of T. tonsurans develop slowly and are typically buff to brown, wrinkled and suedelike in appearance. The colony surface shows radial folds and often develops a craterlike depression in the center with deep fissures. The reverse side of the colony is yellowish to reddish brown. Microscopically, numerous microconidia with flat bases are produced on the sides of hyphae. With age, the microconidia tend to become pleomorphic, are swollen to elongated, and are referred to as balloon forms (Figure 60-11). Chlamydoconidia are abundant in old cultures; swollen and fragmented hyphal cells resembling arthroconidia may be seen. T. tonsurans grows poorly on media lacking enrichments (casein agar); however, growth is greatly enhanced by the presence of thiamine or inositol in casein agar.
Microscopically, chlamydoconidia in chains and antler hyphae may be the only structures observed microscopically in cultures of T. verrucosum (see Figures 59-10 and 59-16). Chlamydoconidia may be abundant at 35° to 37°C. Microconidia may be produced by some cultures if the medium is enriched with yeast extract or a vitamin (Figure 60-12). Conidia, when present, are borne laterally from the hyphae and are large and clavate. Macroconidia are rarely formed, vary considerably in size and shape, and are referred to as “rat tail” or “string bean” in appearance.
T. schoenleinii is a slowly growing organism (30 days or longer) that produces a white to light gray colony with a waxy surface. Colonies have an irregular border consisting mostly of submerged hyphae, which tend to crack the agar. The surface of the colony is usually nonpigmented or tan, furrowed, and irregularly folded. The reverse side of the colony is usually tan or nonpigmented. Microscopically, conidia commonly are not formed. The hyphae tend to become knobby and club shaped at the terminal ends, with the production of many short lateral and terminal branches (Figure 60-13). Chlamydoconidia are generally numerous. All strains of T. schoenleinii may be grown in a vitamin-free medium and grow equally well at room temperature or at 35° to 37°C.
Microsporum spp.
Species of the genus Microsporum are immediately recognized by the presence of large (8-15 × 35-150 µm), spindle-shaped, echinulate, rough-walled macroconidia with thick walls (up to 4 µm) containing four or more septa (Figure 60-14). The exception is Microsporum nanum, which characteristically produces macroconidia having two cells. Microconidia, when present, are small (3 to 7 µm) and club shaped and are borne on the hyphae, either laterally or on short conidiophores. Cultures of Microsporum spp. develop either rapidly or slowly (5 to 14 days) and produce aerial hyphae that may be velvety, powdery, glabrous, or cottony, varying in color from whitish, buff, to a cinnamon brown, with varying shades on the reverse side of the colony.
Colonies of M. canis grow rapidly, are granular or fluffy with a feathery border, white to buff, and characteristically have a lemon-yellow or yellow-orange fringe at the periphery. On aging, the colony becomes dense and cottony and a deeper brownish-yellow or orange and frequently shows an area of heavy growth in the center. The reverse side of the colony is bright yellow, becoming orange or reddish-brown with age. In rare cases, strains are recovered that show no reverse side pigment. Microscopically, M. canis shows an abundance of large (15-20 × 60-125 µm), spindle-shaped, multisegmented (four to eight) macroconidia with curved ends (see Figure 60-14). These are thick walled with spiny (echinulate) projections on their surfaces. Microconidia are usually few in number, but large numbers occasionally may be seen.
M. gypseum grows rapidly as a flat, irregularly fringed colony with a coarse, powdery surface that appears to be buff or cinnamon color. The underside of the colony is conspicuously orange to brownish. Microscopically, macroconidia are seen in large numbers and are characteristically large, ellipsoidal, have rounded ends, and are multisegmented (three to nine) with echinulated surfaces (Figure 60-15). Although they are spindle shaped, these macroconidia are not as pointed at the distal ends as those of M. canis. The appearance of the colonial and microscopic morphologic features is sufficient to make the distinction between M. gypseum and M. canis.
Epidermophyton sp.
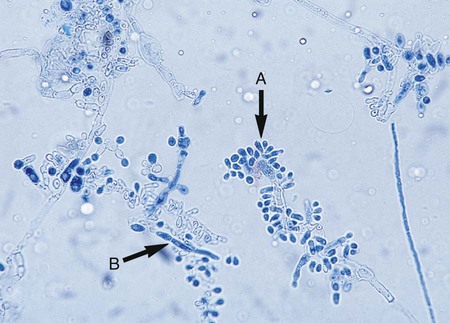
E. floccosum, the only member of the genus Epidermophyton, is a common cause of tinea cruris and tinea pedis. Because this organism is susceptible to cold, specimens submitted for dermatophyte culture should not be refrigerated before culture, and cultures should not be stored at 4°C. In direct examination of skin scrapings using the calcofluor white or potassium hydroxide preparation, the fungus is seen as fine branching hyphae. E. floccosum grows slowly; the growth appears olive green to khaki, with the periphery surrounded by a dull orange-brown. After several weeks, colonies develop a cottony white aerial mycelium that completely overgrows the colony; the mycelium is sterile and remains so even after subculture. Microscopically, numerous smooth, thin-walled, club-shaped, multiseptate (2 to 4 µm) macroconidia are seen (Figure 60-16). They are rounded at the tip and are borne singly on a conidiophore or in groups of two or three. Microconidia are absent, spiral hyphae are rare, and chlamydoconidia are usually numerous. The absence of microconidia is useful for differentiating this organism from Trichophyton spp.; the morphology of the macroconidia (smooth, thin walled) is useful for differentiating it from Microsporum spp.
The Opportunistic Mycoses
General Characteristics
Epidemiology and Pathogenesis
Aspergillus spp.
Several Aspergillus spp. are among the most frequently encountered fungi in the clinical laboratory (Table 60-2); any is potentially pathogenic in the immunocompromised host, but some species are more frequently associated with disease than others. The aspergilli are widespread in the environment, where they colonize grain, leaves, soil, and living plants. Conidia of the aspergilli are easily dispersed into the environment, and humans become infected by inhaling them. Assessing the significance of Aspergillus organisms in a clinical specimen may be difficult. They are found frequently in cultures of respiratory secretions, skin scrapings, and other specimens.
TABLE 60-2
Species of Aspergillus Recovered from Clinical Specimens During a 10-Year Period at the Mayo Clinic
| Organisms | CLINICAL SPECIMEN SOURCE | ||||
| Respiratory Secretions | Gastrointestinal | Genitourinary | Skin, Subcutaneous Tissue | Blood, Bone, CNS, Other | |
| A. clavatus | *97/93* | 1/1 | – | 1/1 | – |
| A. flavus | 1298/740 | 10/10 | 11/11 | 177/131 | 2/2 |
| A. fumigatus | 3247/2656 | 11/9 | 14/14 | 175/137 | 8/8 |
| A. glaucus | 503/307 | 1/1 | – | 8/8 | 1/1 |
| A. nidulans | 52/48 | – | – | 5/3 | – |
| A. niger | 1484/1376 | 18/18 | 17/17 | 151/124 | 11/11 |
| A. terreus | 164/146 | – | – | 23/21 | 3/3 |
| A. versicolor | 1237/1202 | 6/6 | 24/22 | 226/224 | 16/16 |
| Other Aspergillus species | 3463/3418 | 18/14 | 32/32 | 319/314 | 16/16 |
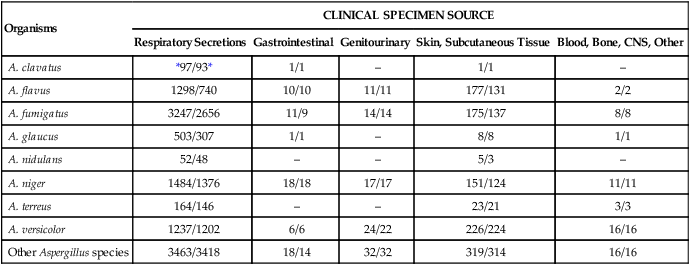
CNS, Central nervous system.
*Numerator, Number of cultures; denominator, number of patients.
Laboratory Diagnosis
Specimen Collection and Transport
See General Considerations for the Laboratory Diagnosis of Fungal Infections in Chapter 59.
Direct Detection Methods
Stains.
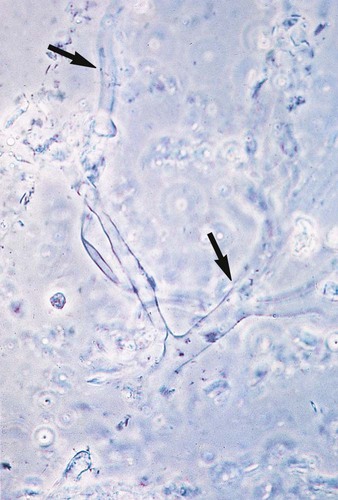
Specimens submitted for direct microscopic examination containing organisms in this group demonstrate septate hyphae that usually show evidence of dichotomous branching, often of 45 degrees (Figure 60-17). In addition, some hyphae may have rounded, thick-walled cells. Although often considered to represent an Aspergillus species, these cannot be reliably distinguished from hyphae of Fusarium spp., Pseudallescheria boydii, or other hyaline molds.
MALDI-TOF (Matrix-Assisted Laser Desorption Ionization).
MALDI-TOF is a biophysical method that significantly reduces the time required to specifically identify fungal isolates and is being implemented in some large reference laboratories. An overview of the technique is discussed in more detail in Chapter 7.
Approach to Identification
Aspergillus spp.
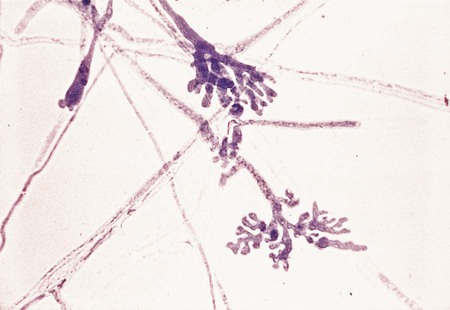
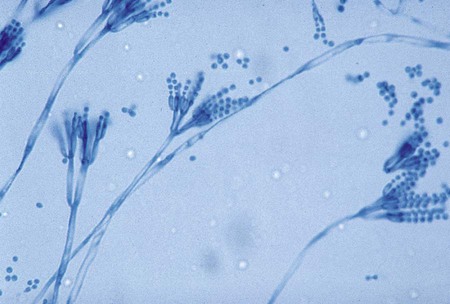
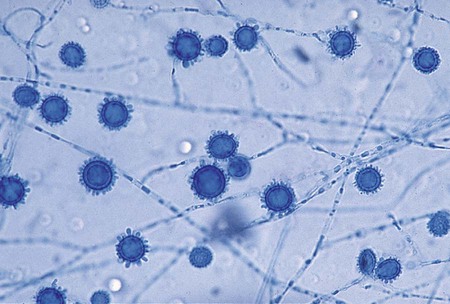
A. fumigatus is a rapidly growing mold (2 to 6 days) that produces a fluffy to granular, white to blue-green colony. Mature sporulating colonies most often have a blue-green, powdery appearance. Microscopically, A. fumigatus is characterized by the presence of septate hyphae and short or long conidiophores with a characteristic “foot cell” at their base. The foot cell is T or L shaped at the base of the conidiophore, but it is not a separate cell. The tip of the conidiophore expands into a large, dome-shaped vesicle with bottle-shaped phialides covering the upper half or two thirds of its surface. Long chains of small (2 to 3 µm in diameter), spherical, rough-walled, green conidia form a columnar mass on the vesicle (Figure 60-18). Cultures of A. fumigatus are thermotolerant and able to withstand temperatures up to 45°C.
A. flavus is a somewhat more rapidly growing species (1 to 5 days) that produces a yellow-green colony. Microscopically, vesicles are globose, and phialides are produced directly from the vesicle surface (uniserate) or from a primary row of cells called metulae (biserate). The phialides give rise to short chains of yellow-orange elliptical or spherical conidia that become roughened on the surface with age (Figure 60-19). The conidiophore of A. flavus is also coarsely roughened near the vesicle.
A. niger produces darkly pigmented, roughened spores macroscopically, but microscopically its hyphae are hyaline and septate, as are those of other aspergilli (i.e., it is not melanized). A. niger produces mature colonies within 2 to 6 days. Growth begins initially as a yellow colony that soon develops a black, dotted surface as conidia are produced. With age, the colony becomes jet black and powdery but the reverse remains buff or cream colored; this occurs on any culture medium. Microscopically A. niger shows septate hyphae, long conidiophores supporting spherical vesicles giving rise to large metulae, and smaller phialides (biserate), from which long chains of brown to black, rough-walled conidia are produced (Figure 60-20). The entire surface of the vesicle is involved in sporulation.
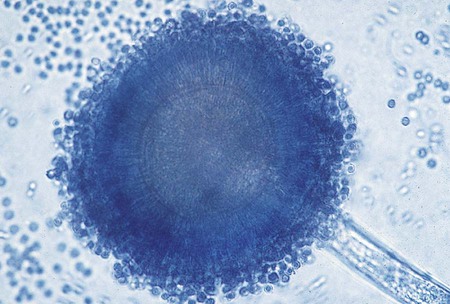
A. terreus is less commonly seen in the clinical laboratory; it produces tan colonies that resemble cinnamon. Vesicles are hemispherical, as seen microscopically, and phialides cover the entire surface and are produced from a primary row of metulae (biserate). Phialides produce globose to elliptical conidia arranged in chains. This species produces larger cells, aleurioconidia, which are found on submerged hyphae (Figure 60-21).
Fusarium spp.
Colonies of Fusarium spp. grow rapidly, within 2 to 5 days, and are fluffy to cottony and may be pink, purple, yellow, green, or other colors, depending on the species. Microscopically the hyphae are small and septate and give rise to phialides producing either single-celled microconidia, usually borne in gelatinous heads similar to those seen in Acremonium spp. (see Figure 59-17) or large, multicelled macroconidia that are sickle or boat shaped and contain numerous septations (Figure 60-22). Some cultures of Fusarium spp. commonly produce numerous chlamydoconidia. The most common medium used to induce sporulation is cornmeal agar. The keys to identification of Fusarium spp. are based on growth on potato dextrose agar.
Geotrichum candidum.
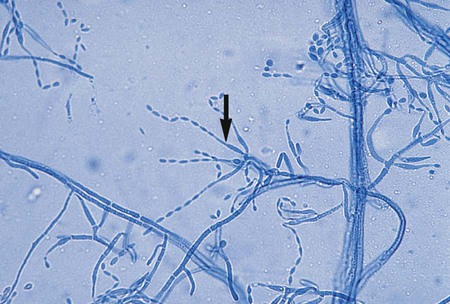
G. candidum often initially appears as a white to cream-colored, yeastlike colony; some isolates may appear as white, powdery molds. Hyphae are septate and produce numerous rectangular to cylindrical to barrel-shaped arthroconidia (Figure 60-23). Arthroconidia do not alternate but are contiguous, in contrast to Coccidioides immitis (Figure 60-24). Blastoconidia are not produced.
Acremonium spp.
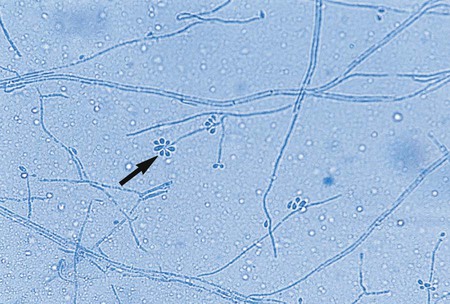
Colonies of Acremonium spp. are rapid growing and also may appear yeastlike when initial growth is observed. Mature colonies become white to gray to rose or reddish-orange. Microscopically, small septate hyphae that produce single, unbranched, tubelike phialides are observed. Phialides give rise to clusters of elliptical, single-celled conidia contained in a gelatinous cluster at the tip of the phialide (see Figure 59-17).
Penicillium spp.
Colonies of Penicillium spp. are most commonly shades of green or blue-green, but pink, white, or other colors may be seen. The surface of the colonies may be velvety to powdery because of the presence of conidia. Microscopically hyphae are hyaline and septate and produce brushlike conidiophores (i.e., penicilli). Conidiophores produce metulae from which flask-shaped phialides producing chains of conidia arise (Figure 60-25). P. marneffei, a particularly virulent species in this genus, is discussed in the section on hyaline, septate, dimorphic molds.
Paecilomyces spp.
Colonies of Paecilomyces spp. are often velvety, tan to olive brown, and somewhat powdery. Colonies of P. lilacinus exhibit shades of lavender to pink. Microscopically Paecilomyces spp. resemble Penicillium spp. in that a penicillus is formed. However, the phialides of Paecilomyces spp. are long, delicate, and tapering (Figure 60-26), in contrast to the more blunted phialides of Penicillium spp.. The penicillus produces numerous chains of small, oval conidia that are easily dislodged. Single phialides producing chains of conidia may also be present.
Scopulariopsis spp.
Scopulariopsis spp. have been associated with onychomycosis, pulmonary infection, fungus ball and, more recently, invasive fungal disease in the immunocompromised host. Colonies of Scopulariopsis spp. initially appear white but later become light brown and powdery. Colonies often resemble those of M. gypseum. Microscopically a Scopulariopsis organism resembles a large Penicillium organism at first glance, because a rudimentary penicillus is produced. Annellophores produce the flask-shaped annellides, which support the lemon-shaped conidia in chains. Conidia are large, have a flat base, and are rough walled (Figure 60-27). The hyaline and septate species is S. brevicaulis. S. brumptii is a dematiaceous species and is occasionally recovered in the clinical laboratory; it has been reported to have caused a brain abscess in a liver transplant recipient.
Systemic Mycoses
General Characteristics
Laboratory Diagnosis
Specimen Collection and Transport
See General Considerations for the Laboratory Diagnosis of Fungal Infections in Chapter 59.
Direct Detection Methods
Stains.
Blastomyces dermatitidis.
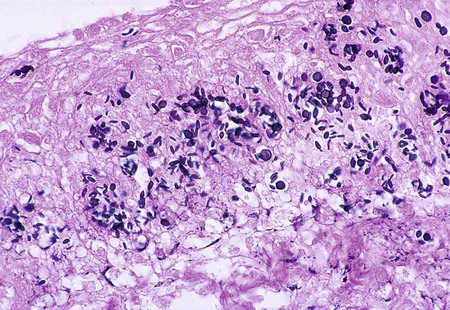
The diagnosis of blastomycosis may easily be made when a clinical specimen is observed by direct microscopy. B. dermatitidis appears as large, spherical, thick-walled yeast cells 8 to 15 µm in diameter, usually with a single bud that is connected to the parent cell by a broad base (Figures 60-28 to 60-30). A smaller form (2-8 µm) is seen in rare cases.
Coccidioides immitis.
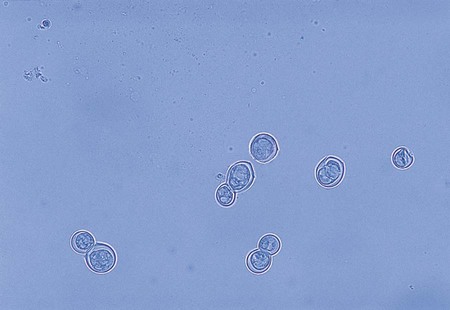
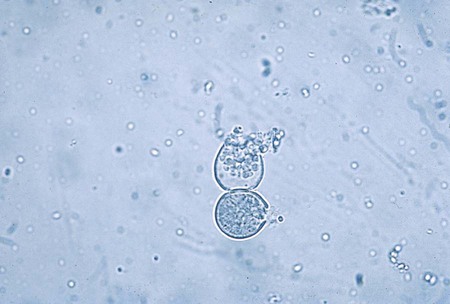
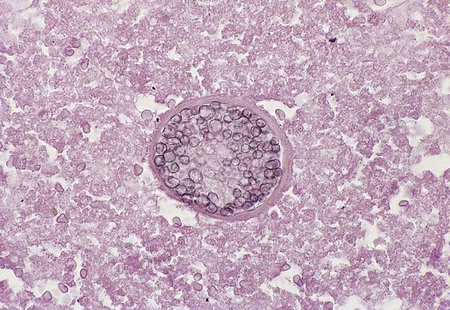
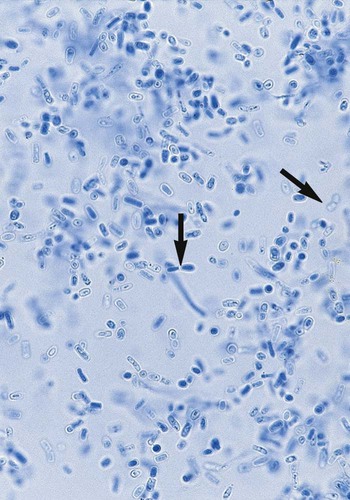
In direct microscopic examinations of sputum or other body fluids, C. immitis appears as a nonbudding, thick-walled spherule, 20 to 200 µm in diameter, that contains either granular material or numerous small (2 to 5 µm in diameter), nonbudding endospores (Figures 60-31 to 60-33). The endospores are freed by rupture of the spherule wall; therefore, empty and collapsed “ghost” spherules may also be present. Small, immature spherules measuring 5 to 20 µm may be confused with H. capsulatum or B. dermatitidis. Two endospores or immature spherules lying adjacent to one another may give the appearance that budding yeast is present. When identification of C. immitis is questionable, a wet preparation of the clinical specimen may be made using sterile saline, and the edges of the coverglass may be sealed with petrolatum and incubated overnight. When spherules are present, the endospores produce multiple hyphal strands.
Histoplasma capsulatum.
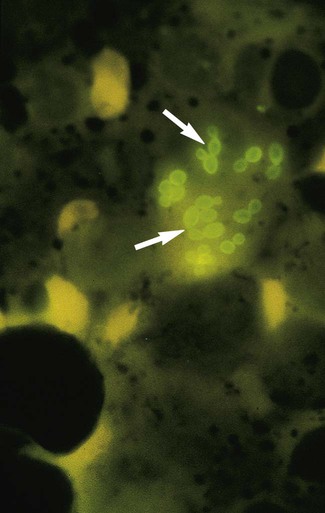
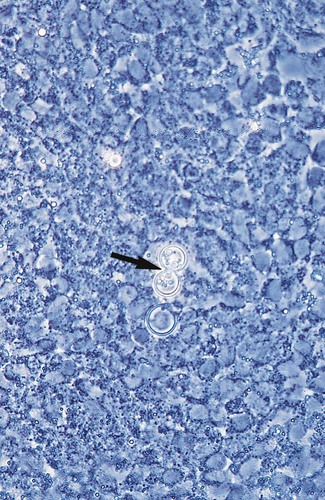
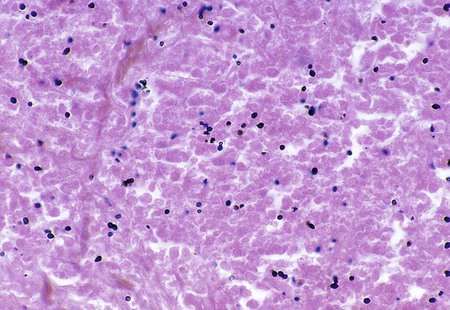
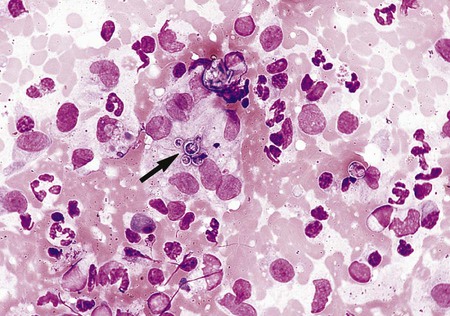
Direct microscopic examination of respiratory tract specimens and other similar specimens often fails to reveal the presence of H. capsulatum. However, an astute laboratorian may detect the organism when examining Wright- or Giemsa-stained specimens of bone marrow and, in rare cases, peripheral blood. H. capsulatum is found intracellularly in mononuclear cells as small, round to oval yeast cells 2 to 5 µm in diameter (Figure 60-34; also see Figure 60-6).
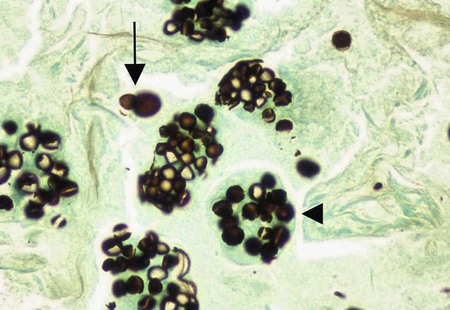
Paracoccidioides brasiliensis.
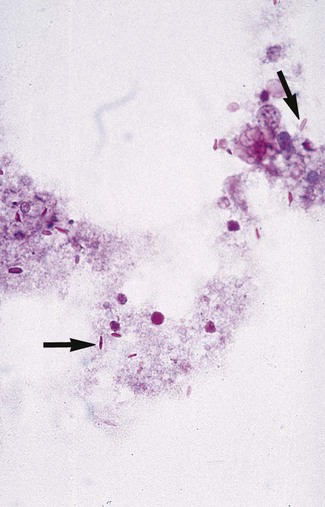
Specimens submitted for direct microscopic examination are important for the diagnosis of paracoccidioidomycosis. Large, round or oval, multiple budding yeast cells (8 to 40 µm in diameter) are usually recognized in sputum, mucosal biopsy specimens, and other exudates. Characteristic multiply budding yeast forms resemble a “mariner’s wheel” (Figure 60-35). The yeast cells surrounding the periphery of the parent cell range from 8 to 15 µm in diameter. Some cells may be as small as 2 to 5 µm but still exhibit multiple buds.
Penicillium marneffei.
Direct examination of infected tissues and exudates reveals that P. marneffei produces small, yeastlike cells (2 to 6 µm) that have internal cross-walls; no budding cells are produced (Figure 60-36). Like H. capsulatum, P. marneffei may be detected in peripheral blood smears with disseminated disease.
Sporothrix schenckii.
Exudate aspirated from unopened subcutaneous nodules or from open draining lesions often is submitted for culture and direct microscopic examination. Direct examination of this material usually has little diagnostic value, because demonstrating the rare characteristic yeast forms is difficult. S. schenckii usually appears as small (2 to 5 µm in diameter), round to oval, to cigar-shaped yeast cells (Figure 60-37). If stained using the periodic acid-Schiff (PAS) method in histologic section, an amorphous pink material may be seen surrounding the yeast cells (Figure 60-38).
Nucleic Acid Amplification.
Nucleic acid amplification assays are not routinely performed but are available in some reference laboratories and in research settings. Real-time or homogeneous, rapid-cycle polymerase chain reaction (PCR) assays have been described for H. capsulatum and C. immitis. These assays have proved suitable for isolate identification. Perhaps the most significant advance in clinical mycology in the past few decades was the development of specific nucleic acid probes for identifying some of the dimorphic fungi (see Procedure 60-2 on the Evolve site). DNA probes (Gen-Probe, San Diego, California) are commercially available that are complementary to species-specific ribosomal RNA. Fungal cells are heat killed and disrupted by a lysing agent and sonication, and the nucleic acid is exposed to a species-specific DNA probe, which has been labeled with a chemiluminescent tag (acridinium ester). The labeled DNA probe combines with a ribosomal RNA of the organism to form a stable DNA : RNA hybrid. All unbound DNA probes are “quenched,” and light generated from the DNA : RNA hybrids is measured in a luminometer. The total testing time is less than 1 hour.
Cultivation.
The definitive traditional identification method for dimorphic fungus includes observing both the mold and tissue or parasitic forms of the organism. In general 25° to 30°C is the optimal temperature for recovery and identification of the dimorphic fungi from clinical specimens. Temperature (35° to 37°C), certain nutritional factors, and stimulation of growth in tissue independent of temperature are among the factors necessary to initiate the transformation of the mold form to the tissue form. Previously, B. dermatitidis and H. capsulatum were identified definitively by the in vitro conversion of a mold form to the corresponding yeast form through in vitro conversion on a blood-enriched medium incubated at 35° to 37°C; definitive identification of C. immitis involved conversion to the spherule form by animal inoculation. Except for C. immitis, the conversion of dimorphic molds to the yeast form can be accomplished with some difficulty (see Procedure 60-3 on the Evolve site). Some laboratories use the exoantigen test (see Procedure 60-4 on the Evolve site) to identify the dimorphic pathogens. However, this test requires extended incubation before cultures may be identified.
Approach to Identification
Blastomyces dermatitidis.
Microscopically, hyphae of the mold form of B. dermatitidis are septate and delicate and measure approximately 2 µm in diameter. Commonly, ropelike strands of hyphae are seen; however, these are found with most of the dimorphic fungi. The characteristic microscopic morphologic features are single, circular to pyriform conidia produced on short conidiophores that resemble lollipops (Figure 60-39); less commonly, the conidiophores may be elongated. The production of conidia in some isolates is minimal or absent, particularly on a medium containing blood enrichment.
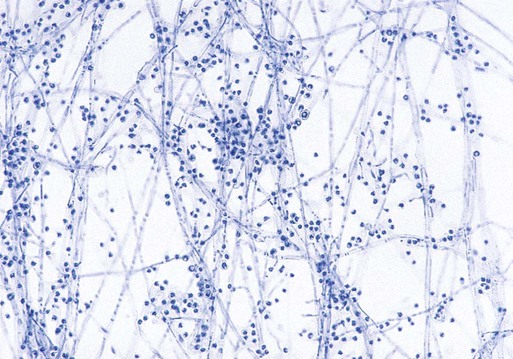
When incubated at 37°C, colonies of the yeast form develop within 7 days and appear waxy and wrinkled and cream to tan. Microscopically, large, thick-walled yeast cells (8 to 15 µm in diameter) with buds attached by a broad base are seen (see Figure 60-28). Some strains may produce yeast cells as small as 2 to 5 µm, called microforms. These small forms may resemble C. neoformans var. neoformans or H. capsulatum. Although these microforms may be present, a thorough search should reveal more typical yeast forms. During conversion, swollen hyphal forms and immature cells with rudimentary buds are also likely to be present. Because the conversion of B. dermatitidis is easily accomplished, this is feasible in the clinical laboratory; however, this is the most appropriate instance in which mold to yeast conversion should be attempted. B. dermatitidis may also be identified by the presence of a specific band (i.e., A band) in the exoantigen test or by nucleic acid probe testing. In some instances, H. capsulatum, P. boydii, or T. rubrum might be confused microscopically with B. dermatitidis. The site of infection and the relatively slow growth rate of B. dermatitidis and careful examination of the microscopic morphologic features usually differentiates it from these fungi.
Coccidioides immitis.
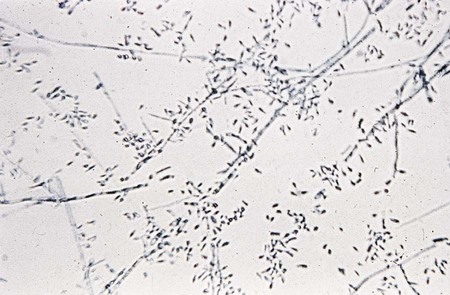
Microscopically some C. immitis cultures show small, septate hyphae that often exhibit right-angle branches and racquet forms. With age, the hyphae form arthroconidia that are characteristically rectangular to barrel shaped. The arthroconidia are larger than the hyphae from which they were produced and stain darkly with lactophenol cotton or aniline blue. The arthroconidia are separated by clear or lighter staining, nonviable cells (disjunctor cells). These types of conidia are referred to as alternate arthroconidia (see Figure 60-24). Arthroconidia have been reported to range from 1.5 to 7.5 µm in width and 1.5 to 30 µm in length, whereas most are 3 to 4.5 µm in width and 3 µm in length. Variation has been reported in the shape of arthroconidia, ranging from rounded to square or rectangular to curved; however, most are barrel shaped. Even if alternate arthroconidia are observed microscopically, definitive identification should be made using nucleic acid probe testing. If a culture is suspected of being C. immitis, it should be sealed with tape to prevent chances of laboratory-acquired infection. Because C. immitis is the most infectious of all the fungi, extreme caution should be used in handling cultures of this organism. Safety precautions include the following:
1. If culture dishes are used, they should be handled only in a Level 3 BSC. Cultures should be sealed with tape if the specimen is suspected to contain C. immitis.
2. The use of cotton plug test tubes is discouraged, and screw-capped tubes should be used if culture tubes are preferred. All handling of cultures of C. immitis in screw-capped tubes should be performed inside a BSC.
3. All microscopic preparations for examination should be performed in a Level 3 BSC.
4. Cultures should be autoclaved as soon as final identification of C. immitis is made.
Other, usually nonvirulent fungi that resemble C. immitis microscopically may be found in the environment. Some molds, such as Malbranchea sp., also produce alternate arthroconidia, although these tend to be more rectangular; however, such species must be considered when making the identification. G. candidum and Trichosporon spp. produce hyphae that disassociate into contiguous arthroconidia; these should not be confused with C. immitis (Figure 60-40; see Figure 60-23). The colonial morphologic features of older cultures of these fungi may resemble C. immitis, but as noted, the arthroconidia are not alternate. It is also important to remember that if confusion in identification does arise, or when occasional strains of C. immitis that fail to sporulate are encountered, identification by exoantigen or nucleic acid probe testing may be performed.
Histoplasma capsulatum.
Microscopically the hyphae of H. capsulatum are small (approximately 2 µm in diameter) and are often intertwined to form ropelike strands. Commonly, large (8 to 14 µm in diameter) spherical or pyriform, smooth-walled macroconidia are seen in young cultures. With age, the macroconidia become roughened or tuberculate and provide enough evidence to make a tentative identification (Figure 60-41). The macroconidia are produced either on short or long conidiospores. Some isolates produce round to pyriform, smooth microconidia (2 to 4 µm in diameter), in addition to the characteristic tuberculate macroconidia. Some isolates of H. capsulatum fail to sporulate despite numerous attempts to induce sporulation.
Paracoccidioides brasiliensis.
Microscopically the mold form is similar to that seen with B. dermatitidis. Small hyphae (approximately 2 µm in diameter) are seen, along with numerous chlamydoconidia. Small (3 to 4 µm), delicate, globose or pyriform conidia may be seen arising from the sides of the hyphae or on very short conidiophores (Figure 60-42). Most often cultures reveal only fine septate hyphae and numerous chlamydoconidia.
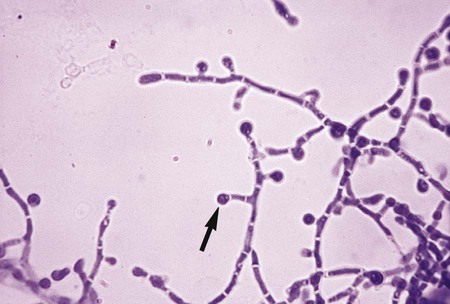
After temperature-based conversion on a blood-enriched medium, the colonial morphology of the yeast form is characterized by smooth, soft-wrinkled, yeastlike colonies that are cream to tan. Microscopically the colonies are composed of yeast cells 10 to 40 µm in diameter surrounded by narrow-necked yeast cells around the periphery, as previously described (see Figure 60-35). If in vitro conversion to the yeast form is unsuccessful, the exoantigen test (see Procedure 60-4 on the Evolve site) should be used to make the definitive identification of P. brasiliensis. Nucleic acid probe testing is not available for this organism. However, P. brasiliensis is known to cross react with B. dermatitidis. This cross reaction, in conjunction with microscopic and colonial morphology, epidemiologic data, and clinical features, may be used for definitive identification of this fungus.
Penicillium marneffei.
At 25°C, P. marneffei grows rapidly and produces blue-green to yellowish colonies. A soluble red to maroon pigment, which diffuses into the agar, is highly suggestive of P. marneffei. At 37°C, conversion of mycelium to the infective, yeastlike form occurs in approximately 2 weeks. Oval, yeastlike cells (2 to 6 µm in diameter) with septa are seen; abortive, extensively branched, and highly septate hyphae may also be present (see Figure 60-36).
Sporothrix schenckii.
Microscopically, hyphae are delicate (approximately 2 µm in diameter), septate, and branching. Single-celled conidia 2 to 5 µm in diameter are borne in clusters from the tips of single conidiophores (flowerette arrangement). Each conidium is attached to the conidiophore by an individual, delicate, threadlike structure (denticle) that may require examination under oil immersion to be visible. As the culture ages, single-celled, thick-walled, black-pigmented conidia may also be produced along the sides of the hyphae, simulating the arrangement of microconidia produced by T. rubrum (sleeve arrangement) (Figure 60-43).
Because of similar morphologic features, saprophytic species of the genus Sporotrichum may be confused with S. schenckii, and they must be differentiated. During incubation of a culture at 37°C, a colony of S. schenckii transforms to a soft, cream-colored to white, yeastlike appearance. Microscopically singly or multiply budding, spherical, oval, or elongate, cigar-shaped yeast cells are observed without difficulty (Figure 60-44). Conversion from the mold form to the yeast form is easily accomplished and usually occurs within 1 to 5 days after transfer of the culture to a medium containing blood enrichment; most isolates of S. schenckii are converted to the yeast form within 12 to 48 hours at 37°C. Sporotrichum spp. do not produce a yeast form.
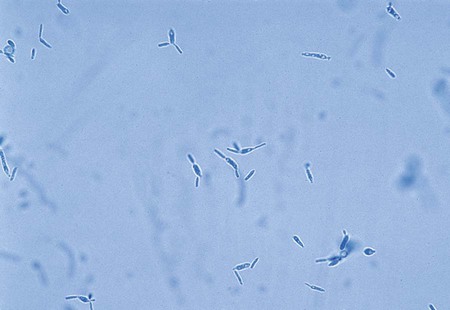
Table 60-3 presents a summary of the colonial and microscopic morphologic features of the dimorphic fungi in addition to other organisms previously discussed.
TABLE 60-3
| CULTURAL CHARACTERISTICS AT 30°C | MICROSCOPIC MORPHOLOGIC FEATURES | |||||||
| Infection | Etiologic Agent | Growth Rate (Days) | Blood-Enriched Medium | Medium Lacking Blood Enrichment | Blood-Enriched Medium | Non-Blood-Enriched Medium | Recommended Morphologic Features of Tissue Form | Confirmatory Tests for Identification |
| Blastomycosis | Blastomyces dermatitidis | 2-30 | Colonies are cream to tan, soft, moist, wrinkled, waxy, flat to heaped, and yeastlike; “tufts” of hyphae often project upward from colonies | Colonies are white to cream to tan, some with drops of exudate present, fluffy to glabrous, and adherent to the agar surface | Hyphae 1-2 µm in diameter are present; some are aggregated in ropelike clusters; sporulation is rare | Hyphae 1-2 µm in diameter are present; single pyriform conidia are produced on short to long conidiophores; some cultures produce few conidia | 8-15 µm, broad-based budding cells with double-contoured walls are seen; cytoplasmic granulation is often obvious | |
spp., Mucor
spp., and other mucorales
Mucor spp.: No rhizoids are produced
A. fumigatus: Uniserate heads with phialides covering the upper half to two thirds of the vesicle
A. flavus: Uniserate or biserate or both with phialides covering the entire surface of a spherical vesicle
A. niger: Biserate with phialides covering the entire surface of a spherical vesicle; conidia are black
A. terreus: Biserate with phialides covering the entire surface of a hemispherical vesicle; aleurioconidia are formed on submerged hyphae