Chapter 253 Human Metapneumovirus
Epidemiology
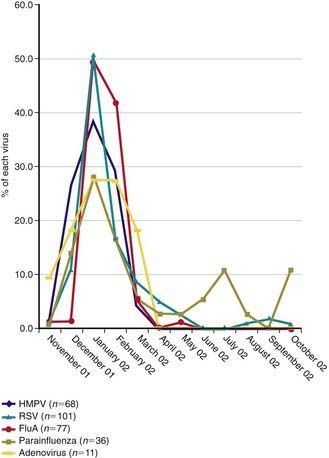
HMPV outbreaks occur in annual epidemics during late winter and early spring in temperate climates, often overlapping with the second half of the annual RSV epidemic (Fig. 253-1). Sporadic infection does occur year round. The usual period of viral shedding is likely to be several weeks after primary infection in infants. The incubation period is 3-5 days. Humans are the only source of virus. Transmission is thought to occur by close or direct contact with contaminated secretions involving large-particle aerosols, droplets, or contaminated surfaces. Nosocomial infections have been reported, and contact isolation with excellent handwashing for health care providers is indicated in medical settings. This virus affects the elderly, immunocompromised patients, and patients with reactive airways disease more severely than otherwise healthy individuals.
Clinical Manifestations
HMPV is associated with the common cold (complicated by otitis media in about 30% of cases) and with lower respiratory tract illnesses such as bronchiolitis, pneumonia, croup, and exacerbation of reactive airways disease. The profile of signs and symptoms caused by HMPV is very similar to that caused by RSV (Table 253-1). Approximately 5-10% of outpatient lower respiratory tract illnesses in otherwise healthy young children is associated with HMPV infection, which is 2nd in incidence only to RSV. Children with RSV or HMPV infection require supplemental oxygen and medical intensive care at similar frequencies.
Table 253-1 CLINICAL MANIFESTATIONS OF HUMAN METAPNEUMOVIRUS IN CHILDREN
COMMON (>50%)
LESS COMMON
RARE
Arnold JC, Singh KK, Milder E, et al. Human metapneumovirus associated with central nervous system infection in children. Pediatr Infect Dis J. 2009;28:1057-1060.
Bao X, Liu T, Shan Y, et al. Human meta-pneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008;4:e1000077.
Crowe JEJr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23(Suppl):S215-S221.
Foulongne V, Buyon G, Rodiere M, et al. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:34-39.
Klein MI, Coviello S, Bauer G, et al. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J Infect Dis. 2006;193:1544-1551.
Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382-386.
Williams JV, Crowe JEJr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192:1149-1153.
Williams JV, Tollefson SJ, Heymann PW, et al. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115:1311-1312.
Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387-395.
Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower reparatory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320-324.