Human immunodeficiency virus infection: living with a chronic illness
KERRI SOWERS, PT, DPT, MARY LOU GALANTINO, PT, PhD, MSCE and DAVID M. KIETRYS, PT, PhD, OCS
After reading this chapter the student or therapist will be able to:
1. Appreciate the role of the immune system in chronic HIV disease.
2. Discuss the neuropathological features of HIV infection and understand potential neurocognitive and neuropsychological alterations that may occur.
3. Understand the various systems (integumentary, musculoskeletal, cardiopulmonary, and neurological) that affect function in HIV-infected adult and pediatric patients.
4. Appreciate the role of psychoneuroimmunology in HIV rehabilitation management.
5. Establish safe exercise parameters in the HIV-positive population.
Identification of the clinical problem
Initially recognized in 1982, acquired immunodeficiency syndrome (AIDS) has been one of the leading causes of death among young adults in the United States since that time. Even with significant advancements in the medical management of the disease, there continues to be a devastating impact in the developing world.1,2 The course of human immunodeficiency virus (HIV) disease in industrialized nations, including the United States, has changed dramatically as a result of advancements in medications used to treat the disease, as well as increased public awareness and the expansion of programs in poverty-stricken areas. Although the disease was once considered a death sentence, the long-term prognosis for those diagnosed with HIV/AIDS has drastically changed in most industrialized countries. In the United States, HIV is no longer found in the top 10 causes of death for the entire adult population. Yet, HIV is the seventh leading cause of death in young adults (age 20 to 24 years); for both older teens (ages 15 to 19 years) and young teens (ages 10 to 14 years), HIV is ranked fourteenth; for children (ages 5 to 9 years), HIV drops to nineteenth on the list.3 Although still alarming, this is actually an improvement compared with the 1990s. Most epidemiologists and clinicians attribute improved life expectancy to the impact of new, highly active antiretroviral therapies (HAART). Implementation of these medications has resulted in a decline in AIDS deaths nationally.4,5 However, the incidence of HIV disease, which demonstrated some decrease in the 1990s, demonstrated an increase from 1999 to 2006. HAART regimens have fostered longevity for many, resulting in the evolution of HIV infection into a chronic disease. Individuals previously disabled by the disease now have the potential to return to work and functional activities and often can expect to live a normal life expectancy. Despite these gains, HIV disease, related comorbidities, and the side effects of medications used to treat the disease have a great impact on rehabilitative medicine because of the multisystem involvement, which often progresses slowly throughout the life span. The advancements in medications that have led to increased life expectancies and improved functional capabilities have also led to a greater demand for rehabilitative services.
HAART has slowed and prevented the progression from HIV infection to AIDS and from AIDS to death.6 In communities with access to antiretroviral medications, the incidence of perinatally acquired AIDS has declined significantly as a result of administration of HAART during pregnancy.7 Unfortunately, perinatal transmission of the virus in developing nations continues to be a crisis.
In 2008 the Centers for Disease Control and Prevention (CDC) revised its definition of AIDS and its classification system of HIV disease. To reflect current scientific knowledge, the new system elucidates the importance of CD4+ T-lymphocyte cell counts as indicators for pharmacological disease management. Based on laboratory criteria and clinical presentation, the disease is classified into four stages. Stage 1 has no AIDS-defining condition and either a CD4+ T-lymphocyte count greater than or equal to 500 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes greater than or equal to 29%. Stage 2 also has no AIDS-defining condition and either a CD4+ T-lymphocyte count of 200 to 499 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes of 14% to 28%. Stage 3 is classified by the CDC as AIDS; it is defined as a CD4+ T-lymphocyte count less than 200 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes less than 14% or documentation of an AIDS-defining condition (Box 31-1). A fourth stage was also identified as HIV Infection, Stage Unknown (for cases in which no information is obtained regarding the CD4+ T-lymphocyte counts or ratios or regarding any AIDS-defining conditions); the primary use of this stage is for surveillance purposes.8 The entire spectrum of illness from initial diagnosis to AIDS can be covered by the term HIV disease. In addition, the terms acute HIV infection, asymptomatic HIV disease, symptomatic HIV disease, and advanced HIV disease (AIDS) are used throughout this chapter. In general, asymptomatic HIV disease corresponds with Stage 1, symptomatic HIV disease with Stage 2, and advanced HIV disease (AIDS) with Stage 3. Table 31-1 presents the various modifiers of quality of life throughout the various stages of HIV disease.
TABLE 31-1 
QUALITY-OF-LIFE ISSUES FOR HIV DISEASE STAGES
| STAGE | CD4+ COUNT | PHYSICAL INDICATORS | MODERATORS OF QUALITY OF LIFE | GENERAL QUALITY-OF-LIFE ISSUES |
| Stage 1: Asymptomatic Disease, HIV infection | >500 cells/mcL | May have persistent generalized lymphadenopathy |
Appraisals: Anticipatory grieving, catastrophizing, and other cognitive distortions; changed expectations of future; identity and self-esteem issues Coping: Dealing with present and future uncertainties; at risk for denial, disengagement, substance abuse, risky sex, suicide; issues of eliciting social support |
Emotional functioning: Anxiety, anger, often increasing at diagnosis and diminishing and recycling as individual confronts realities of living with HIV disease
Role functioning: Often able to work; possible decrements in job mobility and career opportunities; job loss
Social functioning: Fear, isolation, issues of trust in relationships; stigmatization; changes in social support networks because of deaths; relationship and sexual changes; isolation, withdrawal
Physical functioning: Normal but may be altered because of depression or anxiety; may have hypervigilance regarding all physical symptoms
Spiritual functioning: Opportunity to direct attention inward, thus yielding to contemplation of life’s meaning, reassessment of spiritual and existential issues
Appraisals: Anticipatory grieving, catastrophizing, and other cognitive distortions; changed expectations of future; identity and self-esteem issues related to threats to occupational and functional abilities
Coping: Dealing with present and future uncertainties; at risk for denial, disengagement, substance abuse, and risky sex
Emotional functioning: Anxiety, anger, often increasing on emergence of symptoms and then fluctuating with challenges and threats to present and future functioning
Role functioning: Often able to work; may take on new roles as part of HIV support–related network
Social functioning: Changes in social support networks resulting from deaths, isolation, withdrawal, relationship and sexual changes, and stigmatization
Physical functioning: May have reduced energy levels; moderate symptomatology; possible cognitive deficits; pain; wasting
Spiritual functioning: Anticipatory grieving, sense of relatedness to something greater than the self, unavoidable confrontation with one’s own mortality
Appraisals: Facing chronic illness and death; grieving about current and anticipated losses; catastrophizing and other cognitive distortions; reassessment of spiritual and existential issues
Coping: Coping strategies may be overwhelmed in dealing with current difficulties such as financial losses, medical costs, treatment and side effects, housing; may lose some traditional coping strategies such as recreational outlets
Emotional functioning: Anxiety, anger may cycle according to fluctuations in disease status and appraisals; relief from uncertainty
Role functioning: Diminished capacity for work; role changes—often need care instead of being a caretaker
Social functioning: May have diminished social networks because of lack of mobility, illness, and deaths among friends
Physical functioning: Self-care difficulties; fatigue; wasting; much time spent in medical care; debilitation from infection and treatments; possible cognitive deficits
Spiritual functioning: Essential worth is to provide a framework from which to pose and seek responses to metaphysical questions generated by presence of life-threatening disease; integration and transcending of biological and psychosocial nature, which gives access to nonphysical realms as prophecy, love, artistic inspiration, completion, and healing actions

Epidemiology
It is currently estimated that over 30 million people are infected with HIV globally. In the United States, the CDC estimated that 1.1 million adults and adolescents were HIV positive at the end of 2006. Because of complex social and economic factors, African Americans are disproportionally affected, with approximately half of the cases in the United States involving this minority group. Alarmingly, it is estimated that approximately 25% of individuals in the United States infected with HIV are unaware of the infection.9
In the most recent publication of the World Health Report from the World Health Organization (WHO), HIV/AIDS is the sixth leading cause of death worldwide, with an estimated 2.04 million deaths per year.10 Worldwide, of the 33 million people (all ages) living with HIV, 30.8 million are adults, 15.5 million are women, and 2.0 million are children (under the age of 15 years). New HIV infections in 2007 totaled 2.7 million, with 2.3 million in adults and 370,000 in children under 15 years old. Global AIDS deaths totaled nearly 2.0 million; adult deaths were 1.8 million, whereas children under age 15 years totaled 270,000.11 It was estimated that in 2006 the United States had approximately 14,561 deaths from AIDS-related illnesses.9
Tuberculosis (TB) is a former leading microbial killer; it is caused by infectious bacteria that spread through the air in microscopic droplets. WHO estimates that there were 9.27 million new TB cases in 2007; of those, 1.37 million cases (14.8%) were in HIV-positive individuals. Approximately 456,000 deaths caused by TB occurred in HIV-infected individuals (23% of the estimated 2 million HIV deaths were caused by TB).12
Normal immunity
The immune system is complex and dynamic, comprising a multitude of components and subsystems, all of which interact continuously. The normal immune system has two main components, or lines of defense, against illness (Figure 31-1). The first is the innate, or inborn, component, which includes the skin, the cilia and mucosal linings of the respiratory and digestive systems, the gastric fluids and enzymes of the stomach, and the phagocyte cells. This innate component of the immune system keeps pathogens out of the body by creating barriers against them, by ejecting them, or by enveloping them and eliminating them. The second, the acquired component of the immune system develops defenses against specific pathogens, starts in utero, and continues throughout life. It is acquired (or antibody) immunity that is most pertinent to understanding HIV infection and its progression.

 Main components of immunity.
Main components of immunity.Acquired immunity
Acquired immunity is divided into humoral and cell-mediated responses. Humoral immunity depends on the production of antibodies. This response is effective for disposing of free-floating or cell-surface pathogens. The cell-mediated response is required to destroy infected cells, those with intracellular pathogens. Cell-mediated immunity is essential for destroying pathogens responsible for the opportunistic infections and neoplasms that are associated with AIDS.13,14
For the study of HIV pathology, it is important to consider three types of immune system cells: macrophages, T lymphocytes (T cells), and B lymphocytes (B cells). Macrophages originate in the bone marrow and then migrate to the organs in the lymphatic system. Macrophages recognize and then phagocytize antigens—substances deemed foreign to the body. All but a fragment of the antigen is digested by the macrophage. This remaining fragment protrudes from the cellular surface, where it is then recognized by T and B cells, allowing those cells to develop an appropriate immune response.15
Both of the lymphocytes (T and B cells) originate in the bone marrow. Their differentiation into T and B cells depends on where they develop immunocompetence. Immunocompetence is the ability of the immune system to mobilize in response to an antigen; it can be weakened secondary to age-related changes, radiation therapy, chemotherapy, or viral infections. T cells migrate to the thymus to develop this ability. B cells develop it before leaving the bone marrow. T cells travel to lymph nodes, the spleen, and connective tissues, where they wait to phagocytize the antigens in the manner previously described. B cells function in the same way against free-floating blood-borne pathogens.15
In the process of identifying and destroying antigens, the acquired immune system retains a memory of the antigen. This allows the immune system to respond more rapidly and effectively to the pathogen if it is reintroduced into the body. Herein lies the pertinence of vaccination and the phenomenon of being immune to an illness.15
Pathogenesis of HIV disease
HIV belongs to a class of viruses known as retroviruses, which carry their genetic material in the form of ribonucleic acid (RNA) rather than deoxyribonucleic acid (DNA). HIV primarily infects the mononuclear cells, especially CD4 and macrophages, but B cells are also infected.16 HIV binds to the receptor sites on the surface of the CD4 lymphocytes, eventually fusing with and then entering the cells. Reverse transcriptase released from the HIV allows a DNA copy of the virus to be made within the host cell, which then becomes integrated into the host cell genome. Other enzymes, such as integrase and protease, turn the lymphocyte into a “virus factory,” and replicated virions bud out of the cell to infect others.
When the CD4 cell count drops below 200 cells/mcL, the individual is diagnosed with an opportunistic infection or other AIDS-defining illness, or the individual demonstrates wasting syndrome or HIV-related dementia, he or she is reclassified as being in Stage 3—advanced HIV disease or AIDS. It is possible for patients in this stage to demonstrate remarkable recovery in terms of both laboratory values and function with HAART. Individuals who do not have access to HAART, or individuals in whom HAART has failed, will eventually die as a result of the effects of opportunistic infections that inevitably occur. Quality-of-life issues throughout the stages of HIV disease are described in Table 31-1.
Medical management
Cell counts and prophylaxis
For the healthy HIV-negative adult, the average CD4 cell count is approximately 1000 cells/mcL. However, counts fluctuate over time and may range from 500 to 1600 cells/mcL.17 A CD4 cell count of 200 cells/mcL marks a critical point in the course of HIV infection, often indicating that the stage of advanced HIV infection or AIDS has been reached. Serious opportunistic infections are likely to occur once this level of immune depletion has been attained.18–20
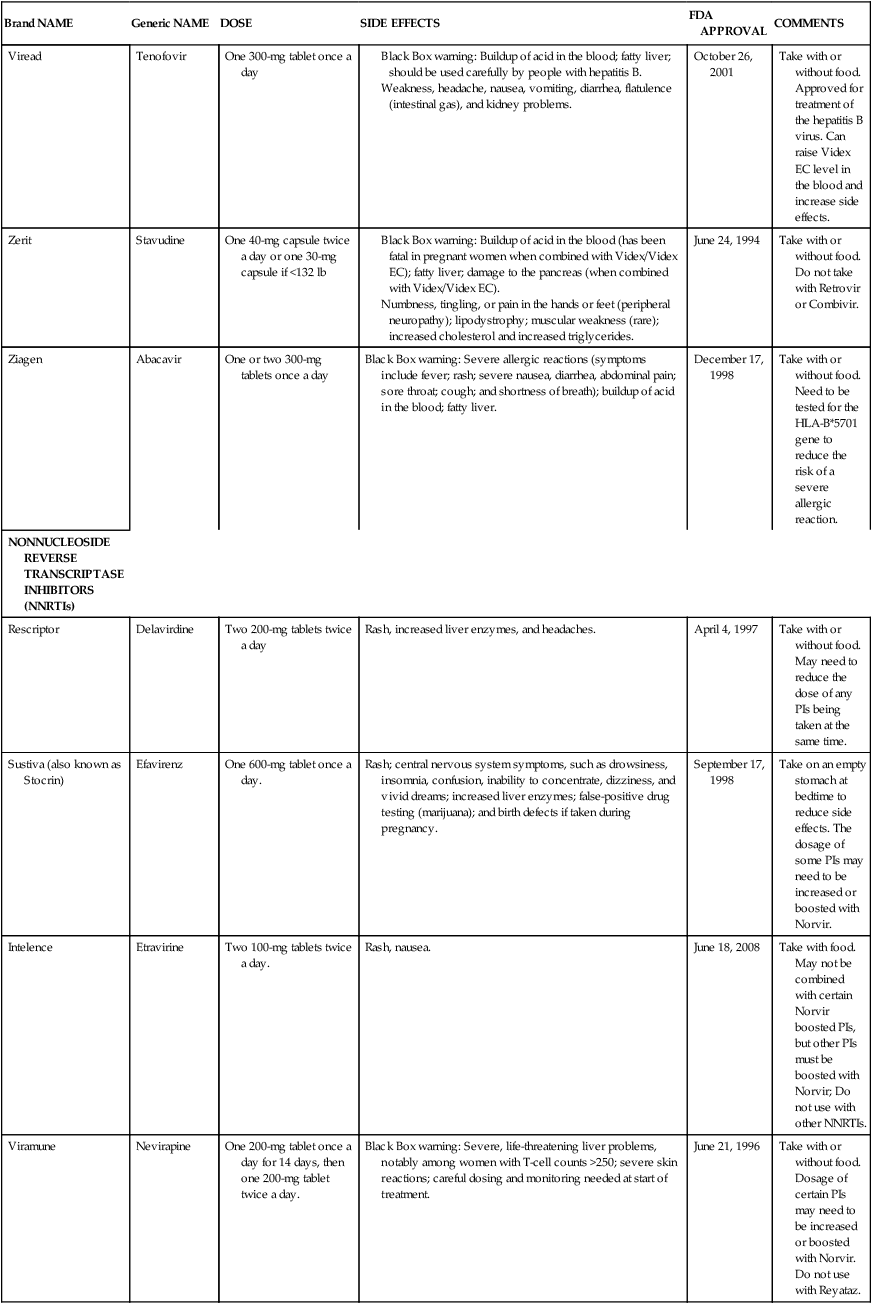
Exercise, stress, seasons of the year, serum cortisol level, and the presence of acute or chronic illness and infection have all been reported to affect CD4 cell counts. Thus the initial CD4 lymphocyte numbers should be confirmed by repeat testing. Caution should be exercised to avoid overinterpreting small changes in CD4 lymphocyte test results. The overall trend of CD4 counts is more important than any single value. Testing is typically done at a frequency of four times annually. In addition to CD4 cell counts, CD4/CD8 ratios are used to evaluate the status of the immune system. CD4 counts above 500 cells/mcL indicate no need for antiretroviral therapy because individuals are generally asymptomatic. It is currently recommended that HAART be initiated when CD4 levels are below 350 cells/mcL, with individual parameters influencing the decision.21 CD4 cell counts below 200 cells/mcL are an indication for prophylactic Pneumocystis jirovechi (previously referred to in the literature as Pneumocystis carinii; this text will refer to the current terminology) pneumonia (PCP) and toxoplasmosis measures. Persons with counts below 100 cells/mcL may also receive prophylactic agents against cytomegalovirus (CMV) infection, infection with Mycobacterium avium complex (MAC), and fungal infections such as cryptococcosis and candidiasis.13 In addition, it is recommended that HIV-positive pregnant women, those with HIV-associated nephropathy, and those co-infected with the hepatitis B virus be started on a HAART regimen immediately.22 Table 31-2 is a summary of common pharmacological agents prescribed to combat opportunistic infections and, most pertinent to rehabilitation, their potential side effects.
TABLE 31-2 
| Brand NAME | Generic NAME | DOSE | SIDE EFFECTS | FDA APPROVAL | COMMENTS |
| NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS (NRTIs) | |||||
| Combivir | Zidovudine and lamivudine |
Black Box warning: Buildup of acid in the blood (has been fatal in pregnant women when combined with Videx/Videx EC); fatty liver; damage to the pancreas (when combined with Videx/Videx EC).
Numbness, tingling, or pain in the hands or feet (peripheral neuropathy); lipodystrophy; muscular weakness (rare); increased cholesterol and increased triglycerides.



Viral load measurement
Testing for the amount of HIV in plasma by measuring viral RNA has become a standard component of the management of HIV-infected patients.23 There are important prognostic implications for the amount of viral load in persons with HIV disease.24 In patients with higher viral loads, disease progression is more rapid, both immunologically, in terms of the rate of CD4 cell count decline, and clinically, in terms of development of AIDS-defining illness. In addition, the plasma levels in HIV-positive pregnant women directly correlate with the risk of perinatal transmission.25 Viral load is an important useful marker for judging the effectiveness of various antiretroviral drug interventions.26,27
There are several assays available for testing HIV for resistance to antiretroviral agents. Genotype or phenotype testing is used to determine whether the virus has mutated. The results of genotype or phenotype testing provide important information about resistance to specific antiretroviral drugs. If a mutant form is resistant to a particular antiretroviral drug, the HAART regimen may be altered so that the potential for viral suppression is maximized. Changes in the drug combinations used for HAART to respond to viral resistance are referred to as salvage therapy. Like genotypic testing, phenotypic testing may not detect small subpopulations of resistant HIV.28
Researchers continue to work on developing effective HAART components and vaccines. The primary goal of antiretroviral therapy is to achieve prolonged suppression of HIV replication.23,29 At this time, there are six classes of HIV medications. Two classes of drugs, receptor site inhibitors and fusion inhibitors (FIs), work to prevent HIV from successfully entering the cell. CCR5 inhibitors (CIs) include maraviroc (Selzentry), a CCR5 co-receptor antagonist (receptor site inhibitor). Receptor site inhibitors are the most recently approved class of drugs. FIs such as enfuvirtide (Fuzeon) or T-20 act outside the T cells by blocking the entry of HIV into the cell. T-20 is often used as part of salvage therapy; it is a twice-daily injectable drug with a cost of more than $25,000 per year.
The four other classes of drugs work within the cell by interfering with one of three enzymes that are involved with the replication process: reverse transcriptase, integrase, and protease. These classes include nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors (INIs), and protease inhibitors (PIs). In 1987, zidovudine (AZT), an NRTI, was first approved by the U.S. Food and Drug Administration. Since that time, several more NRTI drugs have been approved.30 Other drugs, such as nevirapine and efavirenz, also inhibit the reverse transcriptase enzyme, but they are not nucleoside analogs. These NNRTIs bind to the enzymatic binding pocket of the reverse transcriptase gene and block binding by the nucleosides.31 Like reverse transcriptase, integrase is an enzyme that is active in the early stages of the replication process, and INIs can be used to interrupt its function by preventing the integration of the virus in the host cell’s DNA.32 INIs, such as elvitegravir or raltegravir (Isentress), are one of the most recently approved classes of drugs. Another drug target for anti-HIV agents is the protease enzyme. The PI drugs are structurally different from other drugs and include agents such as ritonavir, indinavir, nelfinavir, and saquinavir.33
HAART may be NNRTI or PI based (i.e., NNRTI and PI drugs are used in combination with an NRTI such as AZT). There has been a gradual evolution of pharmacology that has allowed for multiple drugs to be combined into one pill. Thus the number of pills required per day as well as the administration schedule have become increasingly more manageable over recent years. However, drugs from different classes (NRTI, NNRTI, and PI) are typically included in HAART. The current recommendation from the Department of Health and Human Services for a treatment-naïve patient is either one NNRTI and two NRTIs or a PI (boosted with ritonavir) and two NRTIs.22 Because of the rapidly evolving nature of HAART, the reader is advised to consult with the CDC for the most current clinical practice guidelines.
Current medication regimens can significantly reduce the HIV level not only in the peripheral blood but also in the lymphoid tissue and the central nervous system (CNS).34 The goal of HAART is to reduce HIV viral load to undetectable levels in serum. The greatest challenge with HAART is resistance to one drug in a class of agents, which may induce partial or complete resistance with other agents, depending on the specific mutations involved.28,35 In a field that is rapidly changing, specific recommendations for antiretroviral therapy are best made by an infectious disease specialist with experience in the management of patients with HIV disease. The major therapeutic decisions include (1) when to initiate therapy, (2) what drugs to prescribe, (3) when to change therapy, and (4) which drugs to change to. When PIs were introduced as a complement to already existing NRTI and NNRTI drugs, the mortality rate of HIV-infected patients and the incidence of opportunistic infections decreased, most likely as a result of the increased use of combination HAART.36 The role of drugs with immunomodulating activity in combination with HAART is also undergoing extensive research.37,38 Drug regimens for HIV disease are dynamic, and clinical practice guidelines are consistently updated; many changes in the approach to drug interventions can be expected as HIV infection continues to be a chronic disease.39
Vaccines
HIV-positive individuals respond less well than do uninfected persons to most vaccines. The degree of immunodeficiency present at the time of vaccination has an impact on the response to hepatitis A or B, pneumococcal, and influenza A and B vaccines.40 Patients with a CD4 count of more than 200 cells/mcL have a more successful response to the vaccine. Patients should be informed that the extent and duration of the protective efficacy of these vaccines are still uncertain.
Vaccination for HIV has the potential to prevent or control disease progression. The development of an effective preventative vaccine for HIV is an area of continuing research. The first human immunizations with the potential AIDS vaccine took place in 1986 in healthy seropositive volunteers in France and Zaire. Low levels of both humoral and cell-mediated immune responses resulted. One conclusion of this study is that booster vaccinations could be effective.41 Several vaccine candidates have been developed and tested in human phase 1 or 2 trials. To date, at least 13 vaccine candidates have been created with use of different forms of recombinant proteins that target the HIV envelope. Research has found that the vaccine candidates introduced antibodies that rarely neutralized HIV progression, as evidenced by assessment of patient blood counts (i.e., CD4 counts). Furthermore, these recombinant proteins rarely produced a cellular response that would target and destroy cells already infected with HIV.42 Currently there is no evidence of a vaccine that produces extended, high-titer neutralization across a variety of HIV strains.42 The most recent clinic trial, the Thai Phase III HIV vaccine trial (also known as RV 144), was completed in September 2009. The study incorporated two vaccines, a prime vaccine (ALVAC-HIV) and a booster vaccine (AIDSVAX B/E), which were based on strains found in Thailand, where the clinical trial was conducted. The clinical trial involved over 16,000 volunteers who received either the vaccine combination or a placebo. The clinical trial found that the vaccine regimen was safe and modestly effective, demonstrating that the vaccine combination lowered the HIV infection rate by 31.2% compared with the placebo. The study also found that the vaccine had no effect on the viral load of those volunteers who became infected during the clinical trial.43
Genetic mutation of the virus further complicates attempts to disable it. Genetically similar but distinguishable strains of HIV can exist in one individual. Furthermore, drug-resistant strains of HIV have been identified.44 Yet another difficulty with vaccination development is a lack of animal models. Chimpanzees replicate simian immunodeficiency virus, a similar but not identical disease. In addition, an average of 12 years and $231 million is required for a new drug to gain Food and Drug Administration approval. Many major pharmaceutical companies seem wary of the immense research expenses and potential liability risks linked to vaccine development. The result is that smaller biotechnology companies with fewer resources are assailing the complicated problems of HIV infection.13 Recent advancements and plans for future clinical trials are largely supported by military or government programs. Researchers are optimistic that the vaccine will induce both humoral and cellular immune responses and have no toxic effects. It will protect against initial infection and retard disease onset in infected individuals.
Nutrition
Involuntary loss of more than 10% of baseline body weight in a 12-month period or a 5% loss in baseline body weight in a 6-month period with chronic diarrhea or unexplained weakness and fever constitute HIV wasting syndrome.45 Retrospective demographic research in the United States found that 17.8% of individuals with AIDS had wasting syndrome.46,47 The ensuing malnutrition contributes to further immunosuppression.48 Nutritional consultation is critical for those patients experiencing wasting syndrome and as a preventative measure for those who are HIV positive. Studies have been done investigating the effects of nutritional counseling and other measures such as medications, hormone supplementation, and exercise on lean body mass in patients with HIV wasting syndrome. It has been shown that nutritional counseling, medications to inhibit tumor necrosis factor, androgen supplementation, growth hormone administration, and resistance strength training have all been effective in improving lean body mass. Increased caloric intake alone increases lean body mass, but primarily through fat stores. Resistance strength training may prove to be the most beneficial in increasing lean body mass with minimal side effects and minimal cost.49
Weight loss or reduction in lean body mass is also a problem for patients using HAART. Comprehensive nutritional intervention is advocated during the early stages of HIV infection to maintain nutritional status. HAART compromises nutrition in HIV patients because of complicated drug and nutrient interactions, adverse side effects including diarrhea and nausea, and in some cases excessive pill loads that must be consumed. Furthermore, HAART has been linked to a condition identified as HIV-associated lipodystrophy. This syndrome is marked by various combinations of insulin resistance, hyperlipidemia, visceral adiposity, loss of peripheral fat stores, and dorsocervical fat accrual.50 Lipodystrophy is a syndrome that makes the nutritional management of HIV more difficult and may necessitate exercise, pharmacological intervention, and diet modifications.51
Systemic manifestations
Integumentary system and neoplasms
Cutaneous disorders develop in 64% to 90% of all individuals infected with HIV. Most HIV-induced skin findings develop only when the CD4 count falls below 500 cells/mcL. As the CD4 cell count decreases further, multiple cutaneous disorders may develop.52 There are three AIDS-defining malignancies: Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), and cervical cancer. KS was the first neoplastic condition to be related to HIV infection and it remains the most common. However, over the past decade the incidence of KS has diminished as a result of the use of more powerful antiretroviral therapy and maintenance of immune status.52 KS can involve almost every part of the body, but the most common site of initial KS presentations is the skin or mucous membranes.53 The disorder manifests as cutaneous purple nodular lesions or as rife visceral lesions. AIDS-KS has been intimately associated with the lymphatic system, specifically, deficient lymphatic transport, nodal dysfunction, and tumors, which contribute to lymphedema.54
In KS there is a broad therapeutic spectrum from cryotherapy to systemic chemotherapy.55 In NHL, early therapeutic intervention is necessary because of the fast progression of the tumor.56 The cervical cancer in HIV-positive women seems to be more aggressive than in HIV-negative women and requires early therapeutic intervention.57 The cancer incidence in patients with HIV is reported to be higher among non-black patients.58
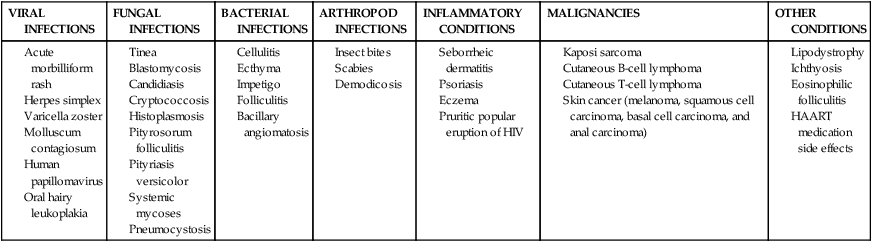
Several other tumors occur in people with HIV infection: anorectal cancer, lung cancer, malignant testicular tumor, Hodgkin lymphoma, basal cell carcinoma, and malignant melanoma.56,59 It is beyond the scope of this chapter to detail all aspects of cancer and dermatological concerns; however, the therapist needs to be aware of the importance of differential diagnosis because the skin is the first line of defense of the immune system and further workup may be warranted. See Table 31-3 for integumentary conditions associated with HIV.
TABLE 31-3 
HIV AND INTEGUMENTARY CONDITIONS*
| VIRAL INFECTIONS | FUNGAL INFECTIONS | BACTERIAL INFECTIONS | ARTHROPOD INFECTIONS | INFLAMMATORY CONDITIONS | MALIGNANCIES | OTHER CONDITIONS |
HAART, Highly active antiretroviral therapies.
*Some skin conditions listed in this table are seen in the general population but may be more severe or more difficult to treat in HIV-infected patients.
Musculoskeletal system
Musculoskeletal manifestations of HIV infection are not as common as manifestations seen in other body systems, including the CNS, pulmonary system, and GI tract. Musculoskeletal disorders tend to occur in advanced HIV disease. Knowledge of the different abnormalities that may occur in the musculoskeletal system is crucial to patient management and affects morbidity and mortality. Primary abnormalities are seen as osseous and soft tissue infections, polymyositis, myopathy, and arthritis. Spinal infections such as pyogenic discitis, osteomyelitis, spinal TB, and epidural abscesses are more likely to occur in HIV-positive individuals than in those who are HIV negative. Discitis and osteomyelitis are more common in patients with CD4 counts >200 cells/mcL, whereas spinal TB and epidural abscesses are more common in patients with CD4 counts <200 cells/mcL.60 Secondary musculoskeletal complications are often a result of the various compensatory patterns of gait as a result of HIV-related peripheral neuropathy syndrome or the change in biomechanics of the foot and ankle from KS and NHL.61 These lead to potential spinal changes and back pain.
HIV-positive patients with acute myopathy typically have proximal muscle weakness and elevated creatine phosphokinase levels.62 Patients may have initial symptoms of difficulty with basic activities of daily living (ADLs), such as rising from a chair or climbing stairs. If myopathy is in an acute inflammatory stage, resisted exercise is contraindicated.
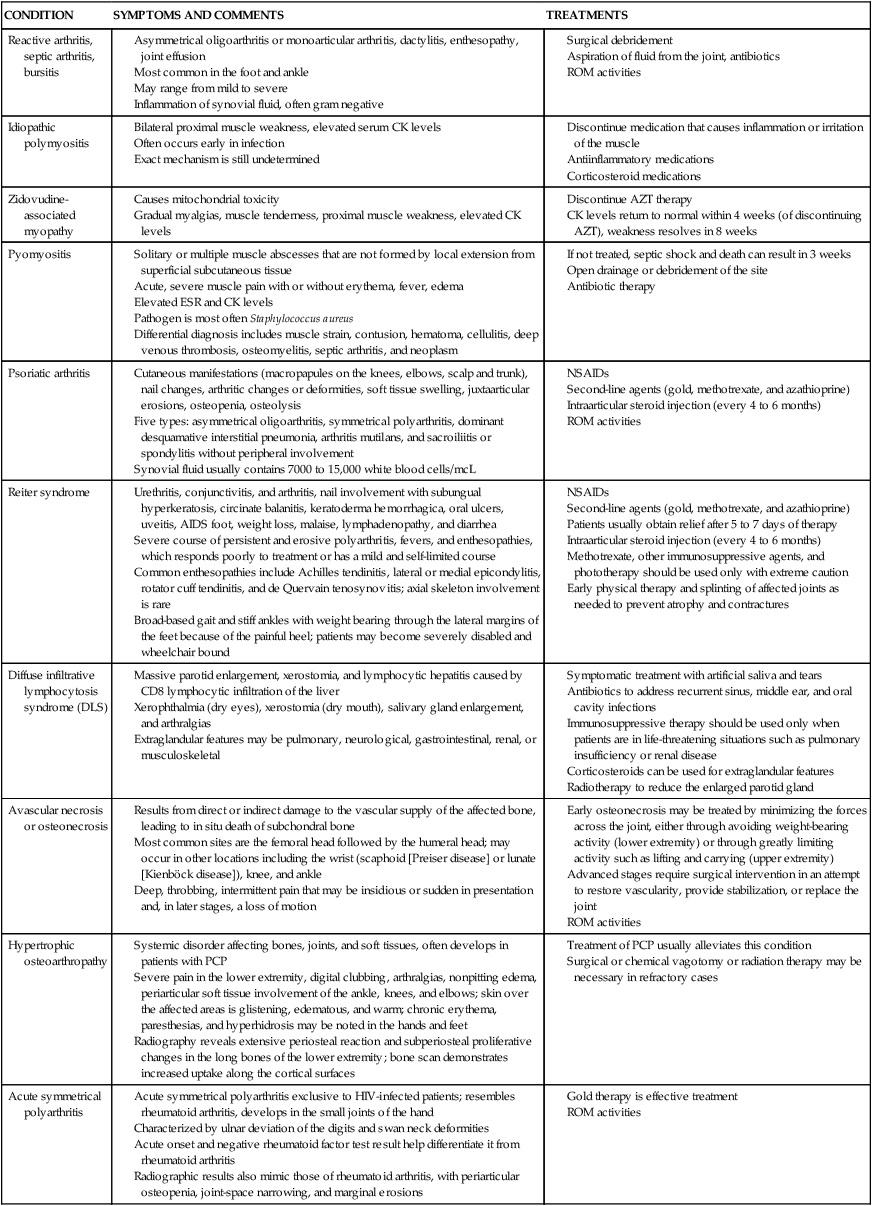
Arthritis in HIV-positive individuals has a wide spectrum of presentations ranging from mild arthralgias to severe joint disability.63 Arthritides seen in patients with AIDS have been classified into five groups on the basis of clinical presentation: (1) painful articular syndrome, (2) acute symmetrical polyarthritis, (3) spondyloarthropathic arthritis (Reiter syndrome, psoriatic arthritis), (4) HIV-associated arthritis, and (5) septic arthritis.64 Standardized diagnostic tests and treatments are the same for HIV-positive individuals with musculoskeletal impairments. A key difference to consider is the effect of HAART medications. Side effects of HAART may evoke symptoms that may complicate the differential diagnosis. The HAART drugs taken by HIV-positive patients may limit pharmaceutical treatment options for musculoskeletal conditions, namely immunosuppressant medications. See Table 31-4 for musculoskeletal conditions associated with HIV.
TABLE 31-4 
HIV AND MUSCULOSKELETAL CONDITIONS
| CONDITION | SYMPTOMS AND COMMENTS | TREATMENTS |
| Reactive arthritis, septic arthritis, bursitis |
Solitary or multiple muscle abscesses that are not formed by local extension from superficial subcutaneous tissue
Acute, severe muscle pain with or without erythema, fever, edema
Pathogen is most often Staphylococcus aureus
Differential diagnosis includes muscle strain, contusion, hematoma, cellulitis, deep venous thrombosis, osteomyelitis, septic arthritis, and neoplasm
Cutaneous manifestations (macropapules on the knees, elbows, scalp and trunk), nail changes, arthritic changes or deformities, soft tissue swelling, juxtaarticular erosions, osteopenia, osteolysis
Five types: asymmetrical oligoarthritis, symmetrical polyarthritis, dominant desquamative interstitial pneumonia, arthritis mutilans, and sacroiliitis or spondylitis without peripheral involvement
Synovial fluid usually contains 7000 to 15,000 white blood cells/mcL
Urethritis, conjunctivitis, and arthritis, nail involvement with subungual hyperkeratosis, circinate balanitis, keratoderma hemorrhagica, oral ulcers, uveitis, AIDS foot, weight loss, malaise, lymphadenopathy, and diarrhea
Severe course of persistent and erosive polyarthritis, fevers, and enthesopathies, which responds poorly to treatment or has a mild and self-limited course
Common enthesopathies include Achilles tendinitis, lateral or medial epicondylitis, rotator cuff tendinitis, and de Quervain tenosynovitis; axial skeleton involvement is rare
Broad-based gait and stiff ankles with weight bearing through the lateral margins of the feet because of the painful heel; patients may become severely disabled and wheelchair bound
Second-line agents (gold, methotrexate, and azathioprine)
Patients usually obtain relief after 5 to 7 days of therapy
Intraarticular steroid injection (every 4 to 6 months)
Methotrexate, other immunosuppressive agents, and phototherapy should be used only with extreme caution
Early physical therapy and splinting of affected joints as needed to prevent atrophy and contractures
Massive parotid enlargement, xerostomia, and lymphocytic hepatitis caused by CD8 lymphocytic infiltration of the liver
Xerophthalmia (dry eyes), xerostomia (dry mouth), salivary gland enlargement, and arthralgias
Extraglandular features may be pulmonary, neurological, gastrointestinal, renal, or musculoskeletal
Symptomatic treatment with artificial saliva and tears
Antibiotics to address recurrent sinus, middle ear, and oral cavity infections
Immunosuppressive therapy should be used only when patients are in life-threatening situations such as pulmonary insufficiency or renal disease
Results from direct or indirect damage to the vascular supply of the affected bone, leading to in situ death of subchondral bone
Most common sites are the femoral head followed by the humeral head; may occur in other locations including the wrist (scaphoid [Preiser disease] or lunate [Kienböck disease]), knee, and ankle
Deep, throbbing, intermittent pain that may be insidious or sudden in presentation and, in later stages, a loss of motion
Early osteonecrosis may be treated by minimizing the forces across the joint, either through avoiding weight-bearing activity (lower extremity) or through greatly limiting activity such as lifting and carrying (upper extremity)
Advanced stages require surgical intervention in an attempt to restore vascularity, provide stabilization, or replace the joint
Systemic disorder affecting bones, joints, and soft tissues, often develops in patients with PCP
Severe pain in the lower extremity, digital clubbing, arthralgias, nonpitting edema, periarticular soft tissue involvement of the ankle, knees, and elbows; skin over the affected areas is glistening, edematous, and warm; chronic erythema, paresthesias, and hyperhidrosis may be noted in the hands and feet
Radiography reveals extensive periosteal reaction and subperiosteal proliferative changes in the long bones of the lower extremity; bone scan demonstrates increased uptake along the cortical surfaces
Acute symmetrical polyarthritis exclusive to HIV-infected patients; resembles rheumatoid arthritis, develops in the small joints of the hand
Characterized by ulnar deviation of the digits and swan neck deformities
Acute onset and negative rheumatoid factor test result help differentiate it from rheumatoid arthritis
Radiographic results also mimic those of rheumatoid arthritis, with periarticular osteopenia, joint-space narrowing, and marginal erosions
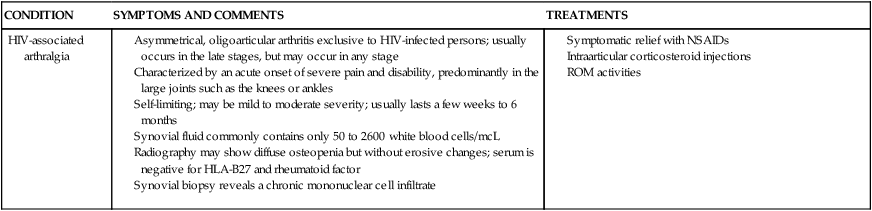
Asymmetrical, oligoarticular arthritis exclusive to HIV-infected persons; usually occurs in the late stages, but may occur in any stage
Characterized by an acute onset of severe pain and disability, predominantly in the large joints such as the knees or ankles
Self-limiting; may be mild to moderate severity; usually lasts a few weeks to 6 months
Synovial fluid commonly contains only 50 to 2600 white blood cells/mcL
Radiography may show diffuse osteopenia but without erosive changes; serum is negative for HLA-B27 and rheumatoid factor
Synovial biopsy reveals a chronic mononuclear cell infiltrate
Cardiopulmonary system
Pulmonary diseases continue to be important causes of illness and death in patients with HIV infection, but changes in therapy and demographics of HIV-infected populations are changing their manifestations. The risk for development of specific disorders is related to the degree of immunosuppression, HIV risk group, area of residence, and use of prophylactic therapies.65 Sinusitis and bronchitis occur frequently in the HIV-positive population, more so than in the general public. The increasing population of HIV-positive drug users is reflected in the increasing incidence of TB and bacterial pneumonia.
Anti-Pneumocystis prophylaxis has reduced the incidence of and mortality from PCP. The PCP-causing organism is usually acquired in childhood, and 65% to 85% of healthy adults possess PCP antibodies. Reactivation of latent infection is responsible for the recurrent fever, dyspnea, and hypoxia that characterize PCP.66,67 Adjunctive corticosteroid therapy has improved the outlook for respiratory failure.65 Multiple studies have shown that the use of corticosteroids in HIV-positive patients in acute respiratory failure has not increased the risk for the development of opportunistic infections.68
Mycobacterial infections in HIV-infected individuals usually manifest as either MAC infection or TB.5 Steadily increasing incidence of infection by Mycobacterium tuberculosis is likely the result of two factors: better medical management of HIV as a whole and the development of multidrug-resistant strains of mycobacteria. MAC infection tends to appear late in the course of HIV infection. Initial infection involves the GI and pulmonary tracts and eventually disseminates throughout the body. This disorder probably is caused not by latent reactivation of the organism but rather by primary infection by ingestion or inhalation.69 Signs and symptoms of MAC infection include pneumonia, fever, weight loss, malaise, sweats, anorexia, abdominal pain, and diarrhea.
As in many other infections, initial signs and symptoms of TB include fever, weight loss, malaise, cough, lymph node tenderness, and night sweats. Pulmonary involvement accounts for 75% to 100% of cases of TB infection in HIV-positive patients, but extrapulmonary infection, especially in lymph nodes and bone marrow, occurs in up to 60% of these individuals as well.66,69–71 Less common areas of infection include the CNS, cardiac, and mucosal tissues. TB is communicable, preventable, and treatable. Tuberculin skin testing with follow-up chest radiographs when appropriate should be available and routinely offered to individuals at HIV testing sites. Individuals at highest risk for concomitant HIV and TB infections include the homeless, intravenous drug users, and prison inmates.66,71 The risk of infection in health care personnel and in the general public is a concern. Isolation rooms that provide negative-pressure, nonrecirculated ventilation, specific air filters, and higher air exchange rates offer the best protection to health care providers exposed to TB-infected individuals. Properly fitted face masks that filter droplet nuclei should be worn. Monitoring of personnel who work with these populations will identify the need for necessary preventive therapy.66 The majority of health care facilities require personnel to have yearly screenings and have established guidelines to prevent the spread of TB in their patient population and within their workforce.
CMV can affect the GI and respiratory tracts but primarily targets optic structures and the CNS; 40% to 100% of healthy adults possess CMV antibodies.72 However, an individual who is immunosuppressed becomes more vulnerable to symptoms of infection with CMV. Predominant consequences of HIV-CMV co-infection are unilateral or bilateral deficits in visual acuity, visual field cuts, and blindness.
Although most other organ system involvement has been extensively described in studies and reviews, cardiac complications related to HIV infection have remained less characterized. Most studies have described cardiac problems as postmortem findings, although some clinical series have been reported. It is now clear that cardiac involvement in people living with HIV infection is quite common. Pericardial effusion and myocarditis are among the most commonly reported cardiac abnormalities. Cardiomyopathy, endocarditis, and coronary vasculopathy have also been reported. It is now apparent that HIV infection itself, the medical management of HIV disease, and secondary opportunistic infections can all affect the myocardium, pericardium, endocardium, and blood vessels.72,73 Cardiovascular risk in HIV-positive patients depends on several factors: direct and indirect vascular effects of chronic exposure to the virus, metabolic effects from prolonged HAART, the normal aging process (important to consider given the increased life expectancy of HIV-positive patients), and other cardiovascular risk factors (such as diet and genetics).74
Body fat changes and lipid abnormalities have been reported in individuals with HIV disease.75 Known as lipodystrophy or fat redistribution syndrome, these body fat and metabolic changes have been connected to PI use.76 It is estimated that 50% of HIV-positive individuals taking HAART develop these metabolic conditions.77 These body fat changes may have strong implications for patients undergoing rehabilitation interventions. Signs and symptoms of the syndrome vary, and not all need to be present in any particular patient. However, in both men and women, three main components of the syndrome have emerged. These include changes in body shape, hyperlipidemia, and insulin resistance. Clinically, distinct body shape changes are apparent. The most prevalent include increased abdominal growth, dorsocervical fat pad, benign symmetrical lipomatosis, lipodystrophy, and breast hypertrophy in women.78,79 The increased abdominal growth is characterized by a redistribution and accumulation of fat in the central visceral areas of the body.79,80 Corresponding symptoms include GI discomfort, bloating, distention, and fullness.80
In addition to visible signs and symptoms, adverse changes in lipid, glucose, and insulin levels have been reported.81 A number of studies have revealed hyperlipidemia to be present in HIV-positive patients, many of whom, but not all, were undergoing PI therapy.82
To date, the exact cause of lipodystrophy has not been determined, but two main theories have been hypothesized. Each is still in the process of being studied.75,83 As individuals live longer with HIV disease, they are at greater risk for development of cardiac disease. Therapists need to be apprised of various changes in laboratory results and signs and symptoms of cardiac disease when designing an exercise program and facilitating the return to functional activities. Screening guidelines (from the Infectious Diseases Society of America HIV Medicine Association [IDSA HIVMA]) include the following:
 Monitor fasting lipid levels before beginning HAART and during the first 4 to 6 weeks of treatment
Monitor fasting lipid levels before beginning HAART and during the first 4 to 6 weeks of treatment
 Monitor fasting glucose levels before and during HAART
Monitor fasting glucose levels before and during HAART
 Monitor body weight and body shape changes on a routine basis
Monitor body weight and body shape changes on a routine basis
See Table 31-5 for effects of HIV treatment on cardiovascular factors and Table 31-6 for cardiovascular risk factors associated with HIV.
TABLE 31-5 
EFFECTS OF HIV TREATMENT ON CARDIOVASCULAR FACTORS
| CARDIOVASCULAR FACTOR | INCIDENCE WHEN TREATED AND UNTREATED | EFFECTS |
| Lipid metabolism (HDL-C) |
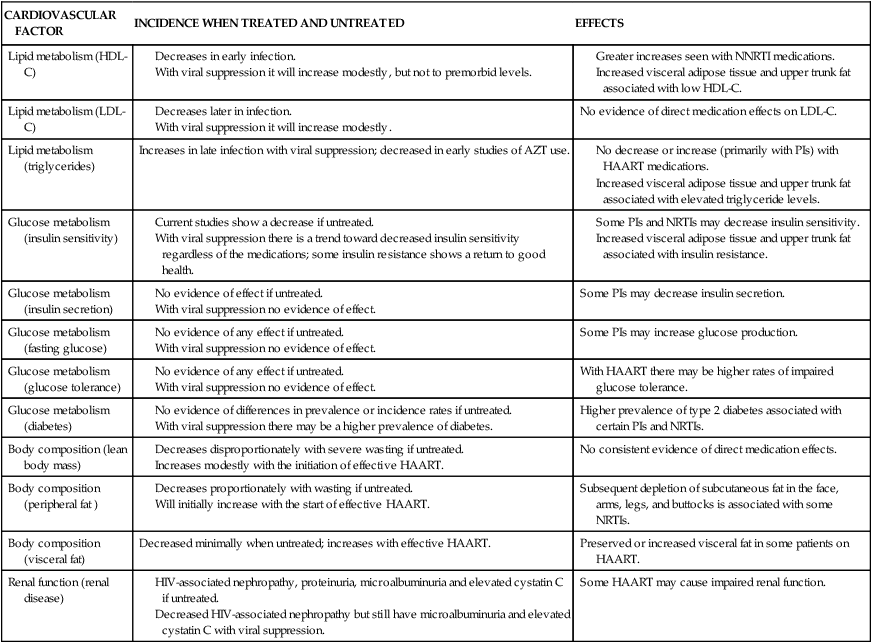
TABLE 31-6 
CARDIOVASCULAR RISK FACTORS AND HIV
| RISK FACTOR | INTERVENTION | RESULT |
| Cigarette smoking | Interpersonal counseling | Increased quitting rates. |
| HTN | ||
| Dyslipidemia | ||
| Disordered glucose metabolism | ||
| Use of HAART |
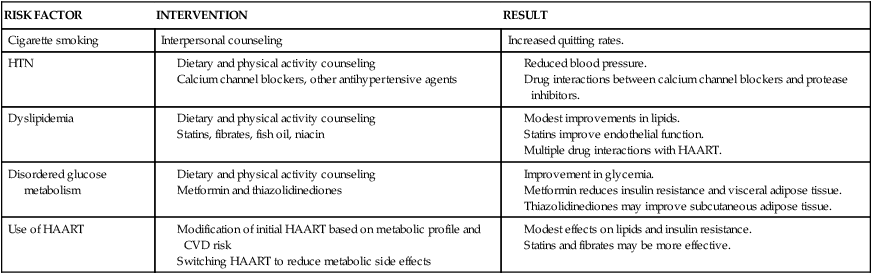
CVD, Cardiovascular disease; HAART, highly active antiretroviral therapies; HTN, hypertension.
Neurological system
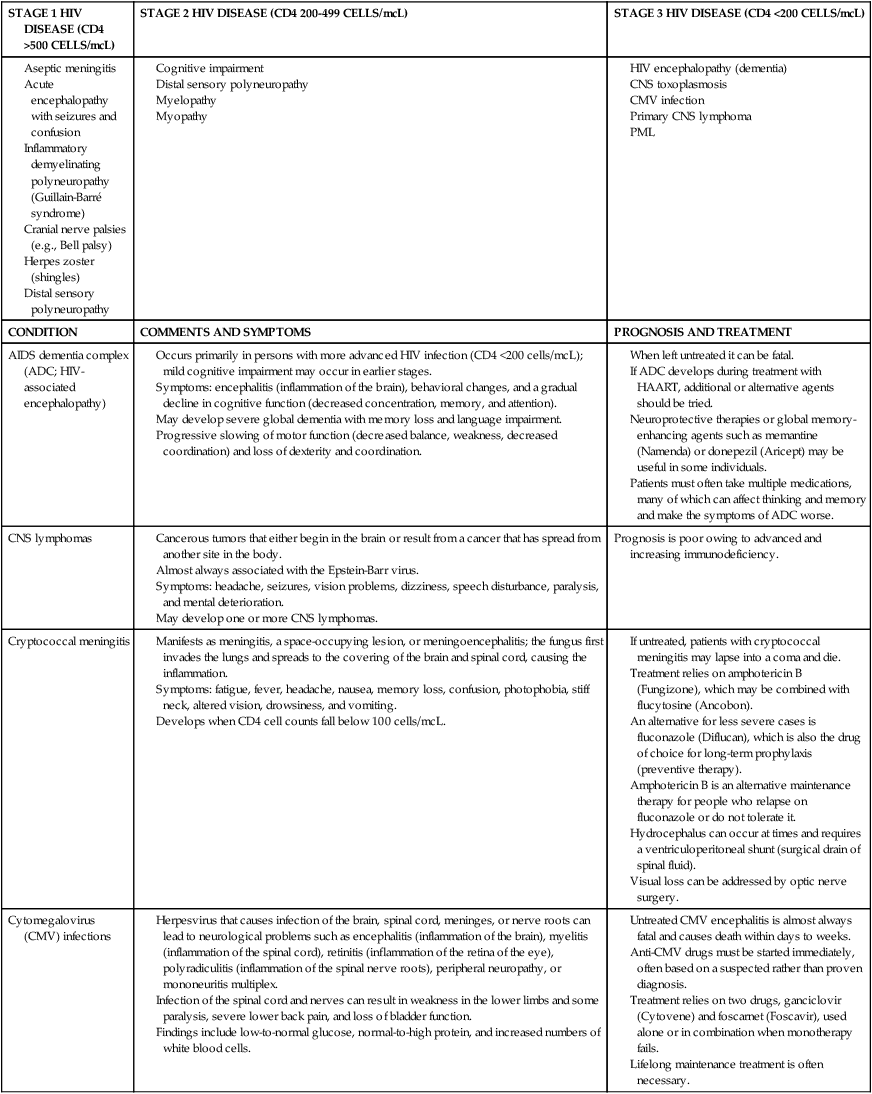
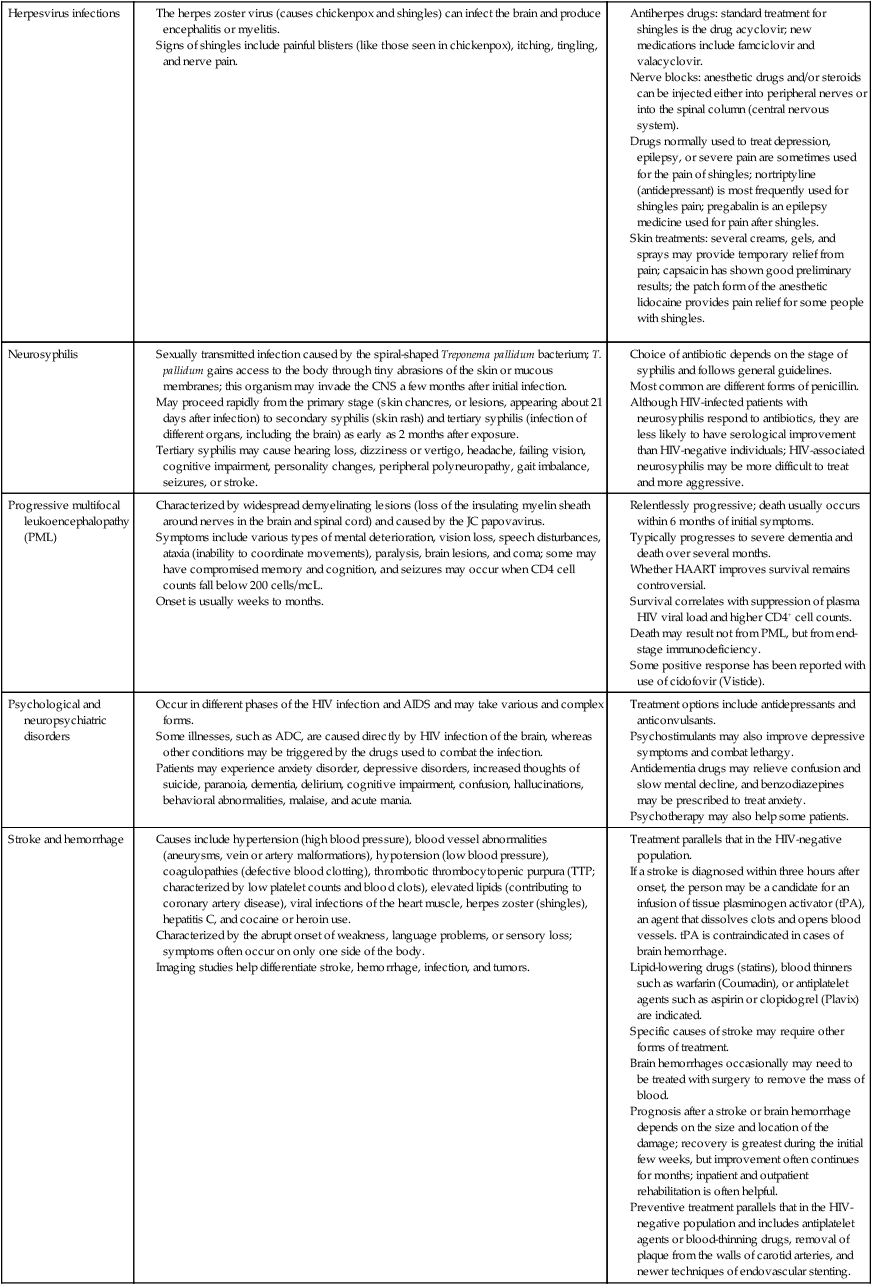
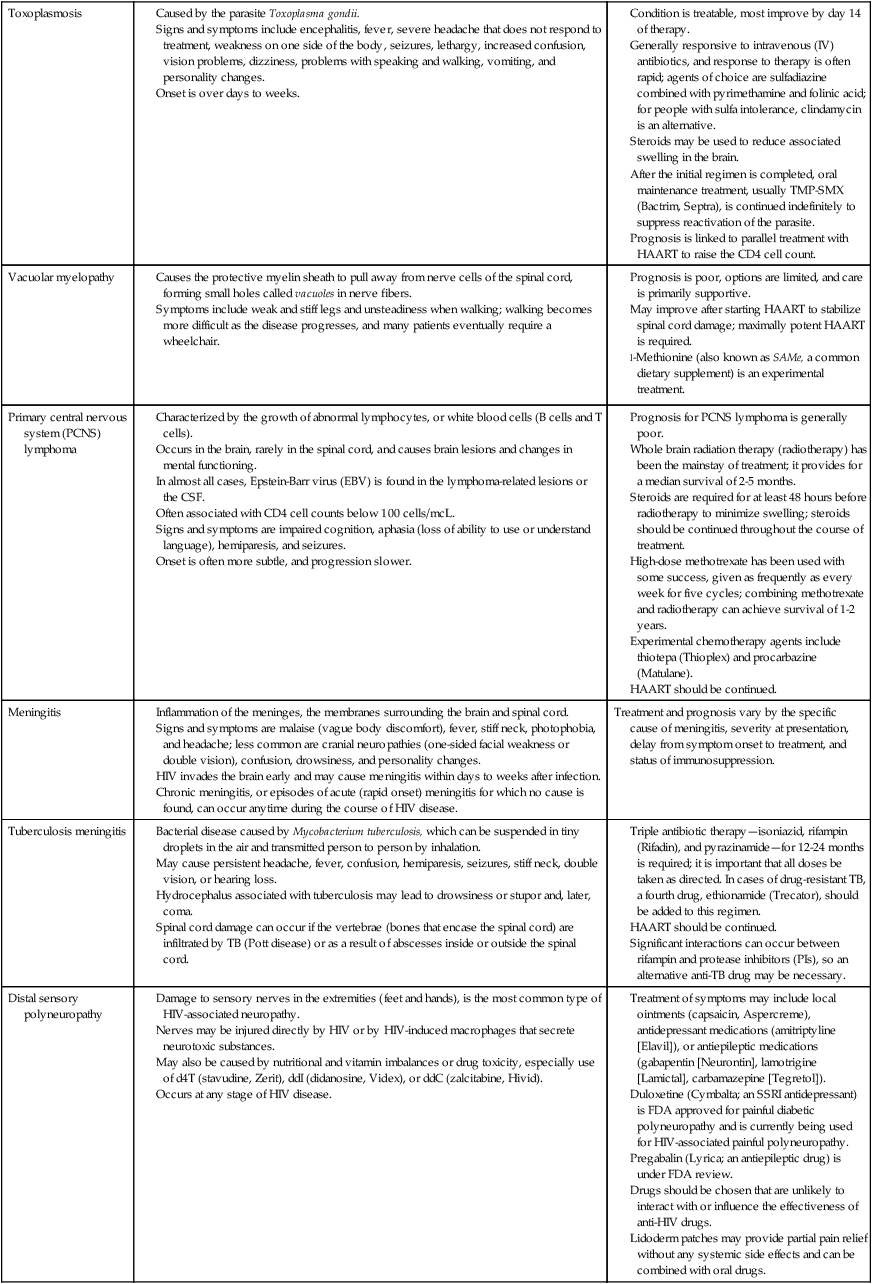
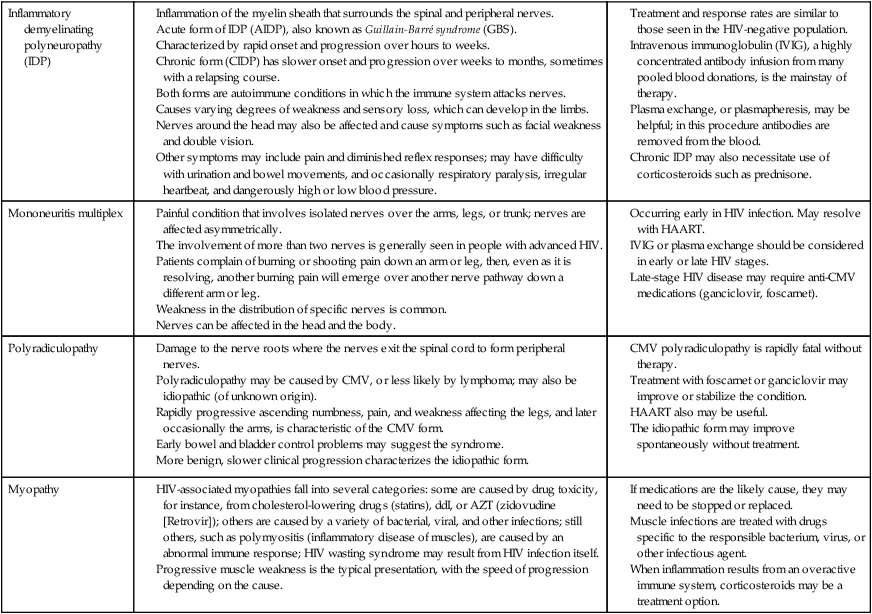
The neurological manifestations of HIV disease are numerous and involve the autonomic nervous system (ANS), CNS, and peripheral nervous system (PNS).84 Over the course of the disease, up to 70% of patients have some form of neurological symptom.85 Significant progress in understanding and treating the neurologically involved HIV patient has been made over the past decade.86 However, HIV continues to affect every division of the human nervous system (Box 31-2). HIV-positive infants show early, catastrophic encephalopathy, loss of brain growth, motor deficits, and cognitive dysfunction.87 Unfortunately, neurobehavioral dysfunction in early pediatric AIDS remains unchanged after therapy. Dementia develops in some adult patients in spite of the multidrug therapies, and other patients have subtle neurobehavioral changes that diminish the quality of their prolonged lives. Thus HIV infection of the CNS remains an important clinical concern. A variety of host and viral factors are associated with an increased risk of developing HIV-associated neurocognitive disorders (HANDs). Studies are demonstrating similarities between factors that predispose HIV-positive patients to HANDs and the risk factors of Alzheimer dementia, suggesting the potential for a common pathological mechanism.88 Evidence has shown that HIV-infected monocytes are carried across the blood-brain barrier and infect the macrophages and microglia in the CNS.89,90 HIV enters the CNS early, yet HANDs often do not occur until advanced stages when the patient is categorized as having AIDS. Hypotheses for the development of HANDs in the advanced stages include the loss of immune control with disease progression, heightened immune activation, increased transfer of infected monocytes into the CNS, and variations or mutations in the virus. Because current HAART medications have poor CNS penetration, HANDs continues to pose significant challenges for advanced HIV/AIDS patients.91 See Table 31-7 for neurological conditions associated with HIV.
TABLE 31-7 
HIV AND NEUROLOGICAL CONDITIONS
| STAGE 1 HIV DISEASE (CD4 >500 CELLS/mcL) | STAGE 2 HIV DISEASE (CD4 200-499 CELLS/mcL) | STAGE 3 HIV DISEASE (CD4 <200 CELLS/mcL) |
Occurs primarily in persons with more advanced HIV infection (CD4 <200 cells/mcL); mild cognitive impairment may occur in earlier stages.
Symptoms: encephalitis (inflammation of the brain), behavioral changes, and a gradual decline in cognitive function (decreased concentration, memory, and attention).
May develop severe global dementia with memory loss and language impairment.
Progressive slowing of motor function (decreased balance, weakness, decreased coordination) and loss of dexterity and coordination.
When left untreated it can be fatal.
If ADC develops during treatment with HAART, additional or alternative agents should be tried.
Neuroprotective therapies or global memory-enhancing agents such as memantine (Namenda) or donepezil (Aricept) may be useful in some individuals.
Patients must often take multiple medications, many of which can affect thinking and memory and make the symptoms of ADC worse.
Manifests as meningitis, a space-occupying lesion, or meningoencephalitis; the fungus first invades the lungs and spreads to the covering of the brain and spinal cord, causing the inflammation.
Symptoms: fatigue, fever, headache, nausea, memory loss, confusion, photophobia, stiff neck, altered vision, drowsiness, and vomiting.
If untreated, patients with cryptococcal meningitis may lapse into a coma and die.
Treatment relies on amphotericin B (Fungizone), which may be combined with flucytosine (Ancobon).
An alternative for less severe cases is fluconazole (Diflucan), which is also the drug of choice for long-term prophylaxis (preventive therapy).
Amphotericin B is an alternative maintenance therapy for people who relapse on fluconazole or do not tolerate it.
Hydrocephalus can occur at times and requires a ventriculoperitoneal shunt (surgical drain of spinal fluid).
Herpesvirus that causes infection of the brain, spinal cord, meninges, or nerve roots can lead to neurological problems such as encephalitis (inflammation of the brain), myelitis (inflammation of the spinal cord), retinitis (inflammation of the retina of the eye), polyradiculitis (inflammation of the spinal nerve roots), peripheral neuropathy, or mononeuritis multiplex.
Infection of the spinal cord and nerves can result in weakness in the lower limbs and some paralysis, severe lower back pain, and loss of bladder function.
Findings include low-to-normal glucose, normal-to-high protein, and increased numbers of white blood cells.
Untreated CMV encephalitis is almost always fatal and causes death within days to weeks.
Anti-CMV drugs must be started immediately, often based on a suspected rather than proven diagnosis.
Treatment relies on two drugs, ganciclovir (Cytovene) and foscarnet (Foscavir), used alone or in combination when monotherapy fails.
Antiherpes drugs: standard treatment for shingles is the drug acyclovir; new medications include famciclovir and valacyclovir.
Nerve blocks: anesthetic drugs and/or steroids can be injected either into peripheral nerves or into the spinal column (central nervous system).
Drugs normally used to treat depression, epilepsy, or severe pain are sometimes used for the pain of shingles; nortriptyline (antidepressant) is most frequently used for shingles pain; pregabalin is an epilepsy medicine used for pain after shingles.
Skin treatments: several creams, gels, and sprays may provide temporary relief from pain; capsaicin has shown good preliminary results; the patch form of the anesthetic lidocaine provides pain relief for some people with shingles.
Sexually transmitted infection caused by the spiral-shaped Treponema pallidum bacterium; T. pallidum gains access to the body through tiny abrasions of the skin or mucous membranes; this organism may invade the CNS a few months after initial infection.
May proceed rapidly from the primary stage (skin chancres, or lesions, appearing about 21 days after infection) to secondary syphilis (skin rash) and tertiary syphilis (infection of different organs, including the brain) as early as 2 months after exposure.
Tertiary syphilis may cause hearing loss, dizziness or vertigo, headache, failing vision, cognitive impairment, personality changes, peripheral polyneuropathy, gait imbalance, seizures, or stroke.
Choice of antibiotic depends on the stage of syphilis and follows general guidelines.
Most common are different forms of penicillin.
Although HIV-infected patients with neurosyphilis respond to antibiotics, they are less likely to have serological improvement than HIV-negative individuals; HIV-associated neurosyphilis may be more difficult to treat and more aggressive.
Characterized by widespread demyelinating lesions (loss of the insulating myelin sheath around nerves in the brain and spinal cord) and caused by the JC papovavirus.
Symptoms include various types of mental deterioration, vision loss, speech disturbances, ataxia (inability to coordinate movements), paralysis, brain lesions, and coma; some may have compromised memory and cognition, and seizures may occur when CD4 cell counts fall below 200 cells/mcL.
Relentlessly progressive; death usually occurs within 6 months of initial symptoms.
Typically progresses to severe dementia and death over several months.
Whether HAART improves survival remains controversial.
Survival correlates with suppression of plasma HIV viral load and higher CD4+ cell counts.
Death may result not from PML, but from end-stage immunodeficiency.
Some positive response has been reported with use of cidofovir (Vistide).
Occur in different phases of the HIV infection and AIDS and may take various and complex forms.
Some illnesses, such as ADC, are caused directly by HIV infection of the brain, whereas other conditions may be triggered by the drugs used to combat the infection.
Patients may experience anxiety disorder, depressive disorders, increased thoughts of suicide, paranoia, dementia, delirium, cognitive impairment, confusion, hallucinations, behavioral abnormalities, malaise, and acute mania.
Causes include hypertension (high blood pressure), blood vessel abnormalities (aneurysms, vein or artery malformations), hypotension (low blood pressure), coagulopathies (defective blood clotting), thrombotic thrombocytopenic purpura (TTP; characterized by low platelet counts and blood clots), elevated lipids (contributing to coronary artery disease), viral infections of the heart muscle, herpes zoster (shingles), hepatitis C, and cocaine or heroin use.
Characterized by the abrupt onset of weakness, language problems, or sensory loss; symptoms often occur on only one side of the body.
Imaging studies help differentiate stroke, hemorrhage, infection, and tumors.
Treatment parallels that in the HIV-negative population.
If a stroke is diagnosed within three hours after onset, the person may be a candidate for an infusion of tissue plasminogen activator (tPA), an agent that dissolves clots and opens blood vessels. tPA is contraindicated in cases of brain hemorrhage.
Lipid-lowering drugs (statins), blood thinners such as warfarin (Coumadin), or antiplatelet agents such as aspirin or clopidogrel (Plavix) are indicated.
Specific causes of stroke may require other forms of treatment.
Brain hemorrhages occasionally may need to be treated with surgery to remove the mass of blood.
Prognosis after a stroke or brain hemorrhage depends on the size and location of the damage; recovery is greatest during the initial few weeks, but improvement often continues for months; inpatient and outpatient rehabilitation is often helpful.
Preventive treatment parallels that in the HIV-negative population and includes antiplatelet agents or blood-thinning drugs, removal of plaque from the walls of carotid arteries, and newer techniques of endovascular stenting.
Caused by the parasite Toxoplasma gondii.
Signs and symptoms include encephalitis, fever, severe headache that does not respond to treatment, weakness on one side of the body, seizures, lethargy, increased confusion, vision problems, dizziness, problems with speaking and walking, vomiting, and personality changes.
Condition is treatable, most improve by day 14 of therapy.
Generally responsive to intravenous (IV) antibiotics, and response to therapy is often rapid; agents of choice are sulfadiazine combined with pyrimethamine and folinic acid; for people with sulfa intolerance, clindamycin is an alternative.
Steroids may be used to reduce associated swelling in the brain.
After the initial regimen is completed, oral maintenance treatment, usually TMP-SMX (Bactrim, Septra), is continued indefinitely to suppress reactivation of the parasite.
Prognosis is linked to parallel treatment with HAART to raise the CD4 cell count.
Causes the protective myelin sheath to pull away from nerve cells of the spinal cord, forming small holes called vacuoles in nerve fibers.
Symptoms include weak and stiff legs and unsteadiness when walking; walking becomes more difficult as the disease progresses, and many patients eventually require a wheelchair.
Characterized by the growth of abnormal lymphocytes, or white blood cells (B cells and T cells).
Occurs in the brain, rarely in the spinal cord, and causes brain lesions and changes in mental functioning.
In almost all cases, Epstein-Barr virus (EBV) is found in the lymphoma-related lesions or the CSF.
Often associated with CD4 cell counts below 100 cells/mcL.
Signs and symptoms are impaired cognition, aphasia (loss of ability to use or understand language), hemiparesis, and seizures.
Prognosis for PCNS lymphoma is generally poor.
Whole brain radiation therapy (radiotherapy) has been the mainstay of treatment; it provides for a median survival of 2-5 months.
Steroids are required for at least 48 hours before radiotherapy to minimize swelling; steroids should be continued throughout the course of treatment.
High-dose methotrexate has been used with some success, given as frequently as every week for five cycles; combining methotrexate and radiotherapy can achieve survival of 1-2 years.
Experimental chemotherapy agents include thiotepa (Thioplex) and procarbazine (Matulane).
Inflammation of the meninges, the membranes surrounding the brain and spinal cord.
Signs and symptoms are malaise (vague body discomfort), fever, stiff neck, photophobia, and headache; less common are cranial neuropathies (one-sided facial weakness or double vision), confusion, drowsiness, and personality changes.
HIV invades the brain early and may cause meningitis within days to weeks after infection.
Chronic meningitis, or episodes of acute (rapid onset) meningitis for which no cause is found, can occur anytime during the course of HIV disease.
Bacterial disease caused by Mycobacterium tuberculosis, which can be suspended in tiny droplets in the air and transmitted person to person by inhalation.
May cause persistent headache, fever, confusion, hemiparesis, seizures, stiff neck, double vision, or hearing loss.
Hydrocephalus associated with tuberculosis may lead to drowsiness or stupor and, later, coma.
Spinal cord damage can occur if the vertebrae (bones that encase the spinal cord) are infiltrated by TB (Pott disease) or as a result of abscesses inside or outside the spinal cord.
Triple antibiotic therapy—isoniazid, rifampin (Rifadin), and pyrazinamide—for 12-24 months is required; it is important that all doses be taken as directed. In cases of drug-resistant TB, a fourth drug, ethionamide (Trecator), should be added to this regimen.
Significant interactions can occur between rifampin and protease inhibitors (PIs), so an alternative anti-TB drug may be necessary.
Damage to sensory nerves in the extremities (feet and hands), is the most common type of HIV-associated neuropathy.
Nerves may be injured directly by HIV or by HIV-induced macrophages that secrete neurotoxic substances.
May also be caused by nutritional and vitamin imbalances or drug toxicity, especially use of d4T (stavudine, Zerit), ddI (didanosine, Videx), or ddC (zalcitabine, Hivid).
Treatment of symptoms may include local ointments (capsaicin, Aspercreme), antidepressant medications (amitriptyline [Elavil]), or antiepileptic medications (gabapentin [Neurontin], lamotrigine [Lamictal], carbamazepine [Tegretol]).
Duloxetine (Cymbalta; an SSRI antidepressant) is FDA approved for painful diabetic polyneuropathy and is currently being used for HIV-associated painful polyneuropathy.
Pregabalin (Lyrica; an antiepileptic drug) is under FDA review.
Drugs should be chosen that are unlikely to interact with or influence the effectiveness of anti-HIV drugs.
Lidoderm patches may provide partial pain relief without any systemic side effects and can be combined with oral drugs.
Inflammation of the myelin sheath that surrounds the spinal and peripheral nerves.
Acute form of IDP (AIDP), also known as Guillain-Barré syndrome (GBS).
Characterized by rapid onset and progression over hours to weeks.
Chronic form (CIDP) has slower onset and progression over weeks to months, sometimes with a relapsing course.
Both forms are autoimmune conditions in which the immune system attacks nerves.
Causes varying degrees of weakness and sensory loss, which can develop in the limbs.
Nerves around the head may also be affected and cause symptoms such as facial weakness and double vision.
Other symptoms may include pain and diminished reflex responses; may have difficulty with urination and bowel movements, and occasionally respiratory paralysis, irregular heartbeat, and dangerously high or low blood pressure.
Treatment and response rates are similar to those seen in the HIV-negative population.
Intravenous immunoglobulin (IVIG), a highly concentrated antibody infusion from many pooled blood donations, is the mainstay of therapy.
Plasma exchange, or plasmapheresis, may be helpful; in this procedure antibodies are removed from the blood.
Chronic IDP may also necessitate use of corticosteroids such as prednisone.
Painful condition that involves isolated nerves over the arms, legs, or trunk; nerves are affected asymmetrically.
The involvement of more than two nerves is generally seen in people with advanced HIV.
Patients complain of burning or shooting pain down an arm or leg, then, even as it is resolving, another burning pain will emerge over another nerve pathway down a different arm or leg.
Damage to the nerve roots where the nerves exit the spinal cord to form peripheral nerves.
Polyradiculopathy may be caused by CMV, or less likely by lymphoma; may also be idiopathic (of unknown origin).
Rapidly progressive ascending numbness, pain, and weakness affecting the legs, and later occasionally the arms, is characteristic of the CMV form.
Early bowel and bladder control problems may suggest the syndrome.
More benign, slower clinical progression characterizes the idiopathic form.
HIV-associated myopathies fall into several categories: some are caused by drug toxicity, for instance, from cholesterol-lowering drugs (statins), ddI, or AZT (zidovudine [Retrovir]); others are caused by a variety of bacterial, viral, and other infections; still others, such as polymyositis (inflammatory disease of muscles), are caused by an abnormal immune response; HIV wasting syndrome may result from HIV infection itself.
Progressive muscle weakness is the typical presentation, with the speed of progression depending on the cause.
Autonomic nervous system.
Dysfunction of the ANS has been associated with HIV infection. This has implications for overall function and the design of a rehabilitation program for people living with HIV disease. In one study, individuals with the greatest ANS involvement had dementia, myelopathy, and sensory peripheral neuropathy. Variations in heart rate, including resting tachycardia, were common. Abnormal blood pressure readings were identified in response to isometric exercise and positional changes (sit to stand and tilting).92
Central nervous system.
HIV enters the CNS during the early stages of the disease and is hypothesized to traverse the blood-brain barrier during the initial acute primary infection stage. Although the initial CNS invasion by HIV is asymptomatic in most individuals, affective and cognitive deficits may develop.93 It is not possible in this context to discuss the neuropathological features of each of the many secondary infections and neoplasms of HIV illness. It is important to realize, however, that the clinical manifestations of these pathological processes overlap with one another and with the signs and symptoms of primary HIV infection of the CNS; lesions of the CNS can be the site of more than one opportunistic disease process simultaneously. In Table 31-8, a wide variety of organisms or conditions responsible for the neurological manifestations associated with HIV infection are listed. These include primary and secondary viral, protozoan, fungal, and Mycobacterium infections, as well as neoplasms and iatrogenic conditions. Infectious processes may cause large lesions in the brain, such as meningitis, encephalitis, or both. Such infections cause neurocognitive impairments that develop as dementia, amnesia, or delirium.93 Thirty percent to 40% of healthy adults have contracted toxoplasmosis, caused by Toxoplasma gondii.34,94 Unchecked by the immune system, toxoplasmosis results in CNS dysfunction—namely, altered cognition, headache, focal neurological deficits, encephalitis, and seizures. Cerebellar disorders associated with HIV infection are typically the result of discrete cerebellar lesions resulting from opportunistic infections such as toxoplasmosis and progressive multifocal leukoencephalopathy or primary CNS lymphoma.95 CNS lymphoma results in cognitive dysfunction and presentation of fever, focal neurological impairments, headache, seizures, and motor deficits.93
TABLE 31-8 
COMMON OPPORTUNISTIC DISEASES IN HIV INFECTION
Isoniazid: paresthesia and peripheral neuropathy, elevated liver function test values, anorexia, nausea, vomiting, fatigue, malaise, weakness
Rifabutin: hepatotoxicity, neutropenia, nausea, vomiting, diarrhea, rash, itching
Clofazimine: reddish-brown discoloration of skin, conjunctiva, sweat, hair, urine, and feces; abdominal pain, diarrhea
Ethambutol: reversible blurring of vision, anaphylaxis, skin irritation, nausea, vomiting, fever
Cycloserine: convulsions, drowsiness, headache, tremor, other CNS disturbances
Ethionamide: nausea, vomiting, peripheral and optic neuritis, mental depression, postural hypotension, rash
Rifampin: urine discoloration, heartburn, nausea, vomiting, abdominal cramps, headache, drowsiness, fatigue
Streptomycin: nausea, vomiting, vertigo, numbness of the face, rash, fever, itching, elevated white blood cell count
Clotrimazole: abdominal pain, diarrhea, nausea, vomiting
Nystatin: diarrhea, nausea, vomiting, stomach pain
Ketoconazole: hepatitis, gynecomastia, nausea, vomiting, decreased libido, diarrhea, dizziness, drowsiness, photophobia, rash, itching, sleepiness
Amphotericin B: fever, shaking chills, hypotension, anorexia, nausea, vomiting, headache and tachypnea
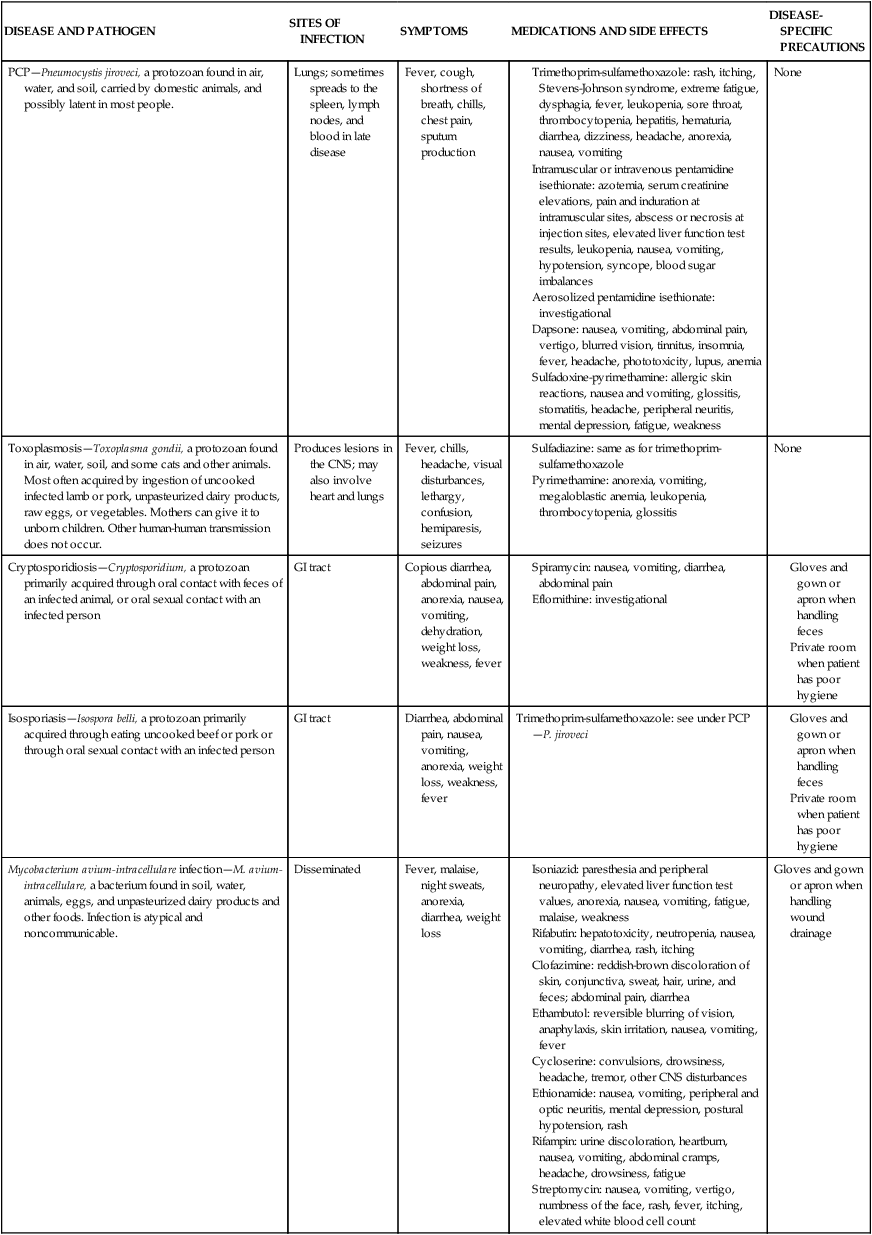
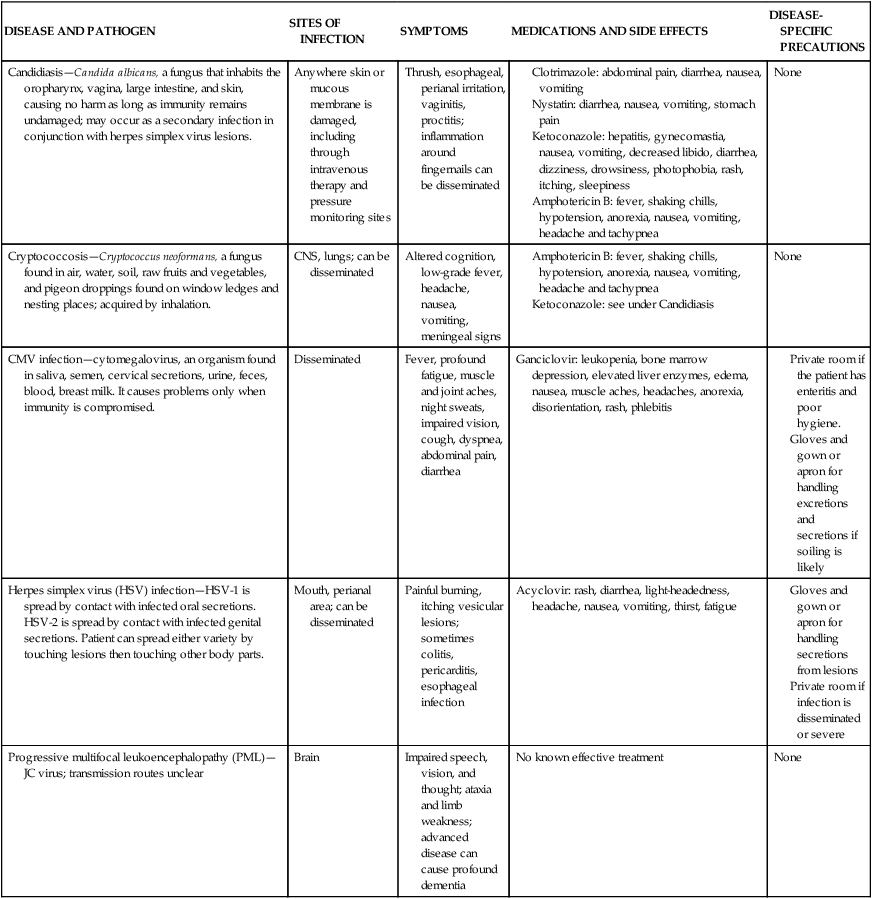
CMV, Cytomegalovirus; CNS, central nervous system; GI, gastrointestinal.
Evidence supports that the neurotoxic effect of HIV is more likely to affect the basal ganglia, the frontal neocortex, the white matter tracts connecting the regions (such as the fronto-striato-thalamocortical loops), the temporal cortices (including the hippocampus), and the parietal cortices.96 A relationship between stroke and AIDS has been reported.97,98 The most common cause of cerebral infarction in both clinical and autopsy series was nonbacterial thrombotic endocarditis. Intracerebral hemorrhages were usually associated with thrombocytopenia, primary CNS lymphoma, and metastatic KS.
HIV-related conditions in the spinal cord include not only HIV myelitis, opportunistic infections, and lymphomas but also vacuolar myelopathy, which affects predominantly the dorsolateral white matter tracts. The cause of vacuolar myelopathy is not understood, and it has not been unequivocally linked with HIV infection.99 Vacuolar myelopathy may affect up to 30% of untreated adults with advanced HIV/AIDS, and the incidence may be even higher in children infected with HIV.100 Unless it is treated with effective antiretroviral therapy, vacuolar myelopathy of the spinal cord associated with moderate clinical disability develops in many patients with AIDS.101
Treatment for CNS impairments includes an eclectic blend of rehabilitation strategies. Neuromuscular disturbances may first appear as movement disorders. Subtleties of altered movement can be detected early and during subsequent treatment phases. A neurological examination can be performed to determine a diagnosis and prognosis. This may include the level of the lesion, neuromuscular deficits, need for assistive devices, ability to perform ADLs, and functional abilities. Various quality-of-life assessments used with the HIV population can be found in Table 31-9.
TABLE 31-9 
QUALITY-OF-LIFE ASSESSMENTS IN HIV DISEASE
| INSTRUMENT | AUTHOR | DIMENSIONS | LENGTH | ADMINISTRATION |
| AIDS Health Assessment Questionnaire (AIDS-HAQ) | Lubeck and Fries (1991-1992) | Physical function, mental health, cognitive function, social health, energy and fatigue | 30 items | Self-administered (5 minutes) |
| AIDS Specific Functional Assessment (ASFA) | Rapkin et al (1991-1993) | Evaluates usefulness of functional assessment | Varies | Self-administered, care provider |
| Idiographic Functional Status Assessment (IFSA) | Rapkin et al (1991-1992) | Patient-generated activities associated with pursuit of following goal types: | 75 items | Self-administered |
| Medical Outcomes Study HIV Instrument (MOS-HIV or MOS-30) | Wu et al (1991) | Health, pain, physical functioning, role functioning, social functioning, mental health, fatigue, energy, health distress, cognitive functioning, health transition, general quality of life | 30 items | Self-administered (5 minutes) |
| HIV Patient-Assessed Report of Status and Experience (HIV-PARSE) | Berry et al (1991) | Physical health, mental health, general health | 38 items | Self-administered (5 minutes) |
| Functional Multidimensional Evaluation of People with HIV (VFM/HIV) | Marazzi et al (1992) | Self-sufficiency with ADL, economic resources, social resources, physical health, mental health | 12 items | Self-administered |
| Neuropsychiatric AIDS Rating Scale (NARS) | Boccellari et al (1992) | Assesses patient’s orientation, memory, motor ability, behavioral changes, problem-solving ability, and ADL | Varies | Health care provider |
| HIV Overview of Problems Evaluation System (HOPES) | Schag et al (1992) | Global, physical, psychosocial, medical interaction, significant others, sexual components | 139 items | Self-administered (15 minutes) |
| HIV-Related Quality-of-Life Questions (HIV-QOL) | Cleary et al (1993) | Mental health, energy and fatigue, fever, limitations of basic ADL and intermediate ADL, disability days, all symptoms, sleep symptoms, neurological symptoms, memory symptoms, pain | 30 items | Self-administered (5 minutes) |
| HIV Quality Audit Marker (HIV-QAM) | Holzemer et al (1993) | Captures nurse data collector’s judgment of status of patient based on observations, interviews, and recorded interviews | Varies based on duration of interview | Nurse |
| HIV Visual Analog Scale | Nokes et al (1994) | Rates HIV-related symptom severity and general well-being | Varies | |
| HIV Assessment Tool (HAT) | Nokes et al (1994) | Physical symptoms related to HIV disease, social and role functioning, psychological well-being, and personal attitudes related to well-being | 34 items | Self-administered |
| Multidimensional Quality of Life Questionnaire for Persons with HIV (MQOL-HIV) | Avis and Smith (1994) | Mental health, physical health, physical functioning, social functioning, social support, cognitive functioning, financial status, partner intimacy, sexual functioning, medical care | 40 items | Self-administered (10 minutes) |
| Medical Outcomes Study HIV Health Survey (MOS-HIV) | Wu, Rivicki, Jacobson, and Maltz (1997) | General health perceptions, role functioning, mental health, quality of life, pain, social functioning, health distress, physical functioning, energy and fatigue, cognitive function, and health transition | 35 items | Self-administered or via interview (5-10 minutes) |
| HIV/AIDS-Targeted Quality of Life (HAT-QoL) Instrument | Holmes and Shea (1998) | Overall function, sexual function, disclosure worries, health worries, financial worries, HIV mastery, life satisfaction, medication concerns, provider trust | 42 items | Self-administered (15 minutes) |
| World Health Organization-Quality of Life HIV Instrument (WHOQOL-HIV) | Mental Health: Evidence and Research Department of Mental Health and Substance Dependence, World Health Organization, Geneva | Physical, psychological, level of independence, social, environmental, spiritual, general quality of life and health, symptoms of HIV, social inclusion, death and dying, forgiveness and fear of death | 115 items | Self-administered |
| Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being (FACIT-Sp-12) | Brady et al; Peterman (2002) | Faith, meaning, and peace | 12 items | Self-administered |
| The Self-Efficacy for Managing Chronic Disease 6-Item Scale | Lorig and Sobel (2001) | Covers several domains that are common across many chronic diseases—symptom control, role function, emotional functioning, and communicating with physicians—with less subject burden than other surveys | 6 items | Self-administered |
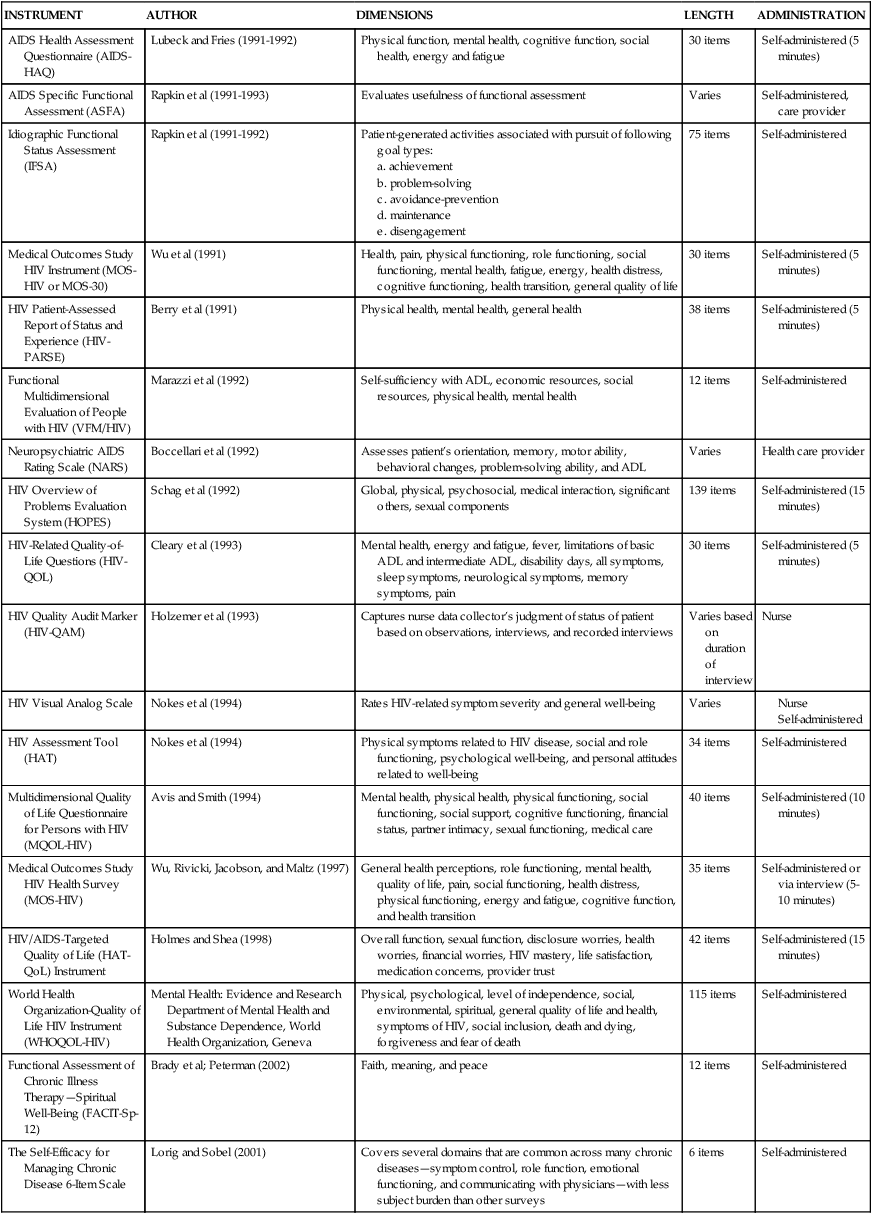
Peripheral nervous system.
Possible neurological complications associated with HIV disease that may affect the PNS include meningitis, ataxia, myelopathy, and encephalitis. PNS diseases have been reported in up to 50% of HIV-infected individuals, resulting in distal polyneuropathy, Guillain-Barré syndrome, and mononeuropathy.102
Distal symmetrical polyneuropathy (DSP) is the most common form of neuropathy in HIV infection. The most frequent complaints in DSP are numbness, burning, and paresthesias in the feet. These symptoms are typically symmetrical and often so severe that patients have contact hypersensitivity and gait disturbances. Involvement of the upper extremities and distal weakness may occur later in the course of DSP. Neurological examination shows sensory loss to pain and temperature in a stocking-glove distribution, increased vibratory thresholds, and diminished ankle reflexes compared with knee reflexes.85,103 Patients with AIDS frequently have concurrent CNS disorders and neuropathy, characterized by hyperactive knee reflexes and depressed ankle reflexes.
The incidence of DSP increases with advancing immunosuppression, in parallel with decreased CD4 counts.104 Thirty-five percent of patients with AIDS may have electrophysiological or clinical abnormalities.105 Furthermore, pathological evidence of DSP is present in almost all patients who die of AIDS.106 Various theories regarding the mechanism of DSP have been proposed. It was formerly thought that direct HIV invasion of the nervous system caused DSP94; however, most investigators now believe that this is not the sole cause.104 A “dying-back” neuropathy affecting all fiber types, with prominent macrophage infiltration of the peripheral nerve, has been described.106 Additional theories include HAART drug toxicity, neurotoxic effects of cytokines, toxicity of HIV proteins, and mitochondrial damage.107 Cytokines, tumor necrosis factor, and interleukin-1 have been identified in the peripheral nerves of patients with AIDS.108
Balance and postural mechanisms.
Balance disturbances may be seen with HIV involvement of either the CNS or the PNS. Polyneuropathy caused by AZT (AZT polyneuropathy) and CMV, which is a common pathogen in AIDS (inflammatory polyneuropathy), may manifest in the form of a generalized asymmetrical demyelination and chronic denervation of muscles.109 Demyelination and denervation of nerves that supply postural muscles may weaken such muscles and result in balance problems (e.g., distal pain, paresthesia, numbness, or core weakness). It is also possible that, apart from muscle demyelination and denervation, the pathological process, which also includes macrophage infiltration of neural structures, could spread to affect the vestibular neural complex of the inner ear, which is important in the maintenance of both static and dynamic balance. Our clinical experience shows that sensory changes are common in the lower limbs of neuropathic HIV/AIDS patients. The balance problems of these patients are likely to be connected to a lack of adequate proprioception from the legs during stance, and it is well known that diminished sensory information makes gait control more difficult. Refer to Chapter 22 for a discussion of balance dysfunction.
Peripheral neuropathy weakens the neuromuscular system and causes a limitation in functional activities. These effects on the neuromuscular system manifest in disturbances of postural control. An appropriate posture should be regarded as the starting position for a functional activity. However, compromise of the postural pattern is so characteristic of HIV peripheral neuropathy that it is diagnostic for HIV-1 infection.110 The neurological abnormality resulting from peripheral neuropathy in HIV/AIDS produces postural disturbances111 that may take various forms that worsen with the severity of the neuropathy112 and compromise functional activity at various levels. This means that as the condition of HIV/AIDS patients deteriorates, balance deficits may increase.
According to Husstedt and colleagues,113 peripheral neuropathy in HIV disease progresses much more rapidly than that associated with diabetes or hereditary polyneuropathies. Again, because of demyelination as the HIV infection progresses, distal symmetrical peripheral neuropathy increases, resulting in a depression of certain motor functions such as gait and manual dexterity, and a worsening of the condition is caused by demyelination.113 There is therefore a need to treat HIV neuropathy as soon as it is diagnosed, to avoid complications that lead to impairments in functional mobility.
One group61 has identified peripheral neuropathy and its complications as causes of functional limitations in individuals with HIV disease. A patient who, for instance, has balance impairment resulting from peripheral neuropathy may not function effectively with ADLs. Functional impairments may impede a patient’s ability to return to gainful employment. For individuals with HIV disease, peripheral neuropathy and its resulting pain may be a limiting factor in the ability to return to work. Any intervention that can aid in the reduction of functional limitations should be incorporated into the rehabilitation plan of care.
Pain
A factor closely related to HIV neuropathy is pain. Pain is one of the most prominent and distressing symptoms in patients with HIV disease, and it has a significant effect on quality of life and psychological state. Pain may affect patients at any stage of the disease process; however, it is more frequent during the advanced stages. The occurrence of pain during HIV infection varies from 30% to 80%. Pain is the result of a complex process that involves psychological and neurophysiological mechanisms, and therefore it should be assessed with use of sensitive tools that examine its multidimensional nature. One model that evaluates the evaluative, affective, and sensory aspects of pain is the McGill Pain Questionnaire. This assessment tool is useful in evaluating HIV disease–related pain because different causes and nonsensorial factors related to the disease often make clinical assessment of pain difficult.114
Most HIV patients require various pain treatment interventions. Distal symmetrical peripheral neuropathy has been shown to be the most common peripheral neuropathy complaint in patients with HIV-1 infection.115–117 Peripheral neuropathy is one of the most common types of pain in HIV-infected men,118 and peripheral neuropathies occur in as many as 40% to 60% of patients with HIV disease. Peripheral neuropathy is the most prevalent neurological complication associated with HIV. CNS or PNS involvement has been found in 30% to 63% of patients across the arena of HIV and is often related to antiretroviral therapy.119 When neuropathy results in distal painful paresthesia, imbalance in stance and gait may result from compensatory measures aimed at relieving pain in dynamic standing activities. Postural compensations may further exacerbate musculoskeletal, cervical, thoracic, or lumbosacral back pain.
Pain management is a critical part of the overall care of individuals with HIV disease. Pain is the second most common reason for hospitalization of patients with AIDS.120 A study of 72 AIDS patients found that 97% had pain related to the disease process.121 Newshan and Wainapel,121 who surveyed 100 patients who had pain associated with AIDS, showed that the two reported pain types were abdominal and neuropathic pain. In a longitudinal study of HIV-positive men, painful peripheral neuropathy was one of the most common types of pain.118
DSP results in painful paresthesias that are challenging to treat with pharmacological interventions. Oral gabapentin and cutaneous lidocaine patches are often prescribed to manage pain associated with peripheral neuropathy. Clinical experience shows that conventional transcutaneous electrical nerve stimulation may exacerbate or relieve peripheral pain in patients with HIV infection. Another consideration for treatment is low-voltage electroacupuncture.122 Manual therapy to improve ankle and foot range of motion along with other compensatory areas is recommended for pain management and return to function. (See Chapters 32 and 39 for additional information.)
Psychopathology
Medical and neuropsychiatric sequelae of HIV infection present a spectrum of diagnostic and treatment challenges to health care practitioners. Both HIV infection and the various opportunistic infections that manifest in patients as the result of an immunocompromised state can affect the CNS. Epidemiological studies indicate that greater than 60% of HIV-positive individuals will experience at least one major psychiatric disorder during the course of their infection. Depression is the most common disorder, closely followed by anxiety and substance abuse disorders.123 Therefore therapists need to be familiar with the diagnosis and management of HIV infection–related medical and psychiatric disorders. These disorders have a great impact on the outcomes of rehabilitation.
Careful consideration of psychological function is warranted during clinical encounters with HIV-infected persons. AIDS-related psychopathologies mimic many previously described consequences of primary HIV infection, opportunistic infections, and drug side effects. These psychiatric complications can be affective or organic. Indicators include disturbances in sleep and appetite patterns, diminished memory and energy, psychomotor retardation, withdrawal, apathy, and emotional liability. Anxiety disorders (particularly post-traumatic stress disorder), adjustment reactions, reactive and endogenous depressions, and obsessive disorders frequently result.124–126 Refer to Chapter 6 for additional recommendations regarding psychological adaptation and adjustments to nervous system dysfunction.
With use of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, one study found Axis I disorders (excluding substance abuse) in 61.9% of the subjects.127 Indeed, the virus’s affinity with subcortical structures of the CNS that regulate affect and mood supports research indicating a prevalence of manic episodes that is 10 times higher than in the general population.128 Manic episodes have been identified at all stages of the disease process and may also occur in response to AZT therapy.16,46,129 When associated with HIV infection, mania appears to be secondary to structural CNS changes.130,131 Described manic episodes generally respond well to psychiatric medications and may not recur.46,132–134
Analyses of new-onset psychosis among HIV-positive individuals yielded the following information. Psychotic episodes are preceded by a period (days to months) of affective and behavioral changes.135 Admitting diagnoses to psychiatric units included “undifferentiated schizophrenia, schizophreniform disorder, ‘reactive psychosis,’ atypical psychosis, depression with psychotic features and mania.”135 Some psychiatric diagnoses were revised during the course of hospitalization to “AIDS encephalitis, cryptococcal meningitis, or ‘organic psychosis.’”136 Eighty-seven percent of the subjects in one study displayed delusions that were usually persecutory, grandiose, or somatic. Affective disturbances were present in 81% of the subjects. Hallucinations and thought process disorders were each prominent in 61% of patients. Several subjects received the diagnosis of AIDS during their psychiatric hospitalization.136
Remarkable progress has been made in recent years in the therapeutics of HIV-associated dementia. Viral replication in and outside the CNS has been reduced by HAART. This has resulted in partial repair of cellular immune function with improvement in, and prevention of, neurological deficits associated with HIV disease.137 Extensive use of PIs is associated with dramatic declines in overall mortality and morbidity, including HIV-associated dementia.37,138
Neuropathological abnormalities seen in the brain tissue of patients with HIV-associated dementia are usually diffuse and predominantly localized to the white and deep gray matter regions. Myelin pallor and inflammatory infiltrates composed of macrophages and multinucleated giant cells are the hallmarks of this disease process, although a spectrum of lesions has been identified from encephalitis to leukoencephalopathy.139,140 The characteristic clinical feature of HIV-associated dementia is disabling cognitive impairment, often accompanied by behavioral changes, motor dysfunction, or both.140 Degrees of impairment have been recorded, and a five-part staging system was subsequently developed.141–143 Motoric manifestations of AIDS dementia complex include gait disturbances, intention tremor, and abnormal release of reflexes.
Pediatric HIV infection
Pediatric HIV infection differs from that most commonly seen in adults. Symptoms develop much earlier in pediatric patients compared with adults. Children infected with HIV may be classified as “rapid progressors” or “slow progressors.” Rapid progressors are children infected with HIV who manifest symptoms within the first 12 to 24 months of life. These children progress quickly to AIDS-defining conditions and have a rapid decline in CD4 count. Children who are slow progressors have a more gradual progression of symptoms and are likely to show evidence of immune system compromise by 7 to 8 years of age. A small percentage of children remain healthy and have only nominal or no symptoms of the disease and a normal to slightly decreased CD4 count through 9 to 10 years of age.144
The prediction of 6 million pregnant women and 5 to 10 million children infected with HIV-1 by the year 2000145 may have been an underestimate. An accurate understanding of the timing of HIV transmission from mother to fetus is important for the design of intervention strategies. The AIDS Clinical Trial Group Study (ACTG) 076 trial, which included treatment from the fourteenth week of gestation in women with CD4 counts of more than 200/mm3, prompts other considerations.146 Onset of HIV-1 infection in children has a wide spectrum of clinical manifestations.147 Thus prevention of transmission from mother to fetus via HAART is a critical component of managing this worldwide epidemic.
Pediatric HIV is neurotrophic in nature in that the virus most often initially affects the CNS rather than the PNS. As the virus spreads, pediatric HIV patients can have CNS disorders that include encephalopathy, pyramidal tract signs, receptive and expressive language difficulties, cognitive deficits, psychomotor impairments, and upper respiratory infections.144 Neuroimaging shows HIV has an influence on neurological function in the basal ganglia, frontal cortex, and other connecting structures in the CNS. Studies also support that there is an environmental component which contributes to developmental and behavioral issues in HIV-positive children.148
In the first year of life, severe immunodeficiency develops in 15% to 20% of pediatric patients with serious recurrent infections or neurological dysfunction, whereas in school-age children the disease progresses more slowly and the risk for development of HIV-related encephalopathy becomes less.149 Some infants have features of severe immunodeficiency, whereas others have nonspecific findings, such as hepatosplenomegaly, failure to thrive, unexplained fever, parotitis, and recurrent gastroenteritis. Adenopathy is common, and salivary gland enlargement occurs more frequently than in adults. Otitis media and measles, despite immunization, are also more frequent complications in children.56,81 Cardiac involvement in children with HIV infection is a well-known entity and occurs clinically more often in patients with advanced disease.150
Studies have shown that children with HIV demonstrate behaviors often associated with attention-deficit/hyperactivity disorder (ADHD) including impulsivity, hyperactivity, difficulty attending, and decreased ability to focus on stimuli. In one study, the most common behavioral issues were psychosomatic disorders (28%), learning disorders (25%), hyperactivity (20%), impulsive-hyperactive disorder (19%), conduct problems (16%), and anxiety (8%); standardized intelligence scores were lower compared with established population norms. Hyperactivity was more common in children with a Wechsler IQ lower than 90, anxiety issues were more common in children older than 9 years, and conduct disorders were more often seen in children with CD4 counts less than 660 cells/mcL.148
Children are susceptible to disorders seen in adults—herpesvirus infection, pneumonia, toxoplasmosis, meningitis, and encephalitis. HIV encephalopathy is noted to have the most serious side effects because of its progressive deteriorating pattern and associated CNS abnormalities,17 although static encephalopathy can be characterized by severely delayed cognitive functioning and neuromotor skills without deterioration.151 Manifestations in children include cerebral atrophy, ataxia, rigidity, hyperreflexia, and the inability to achieve or sustain developmental milestones. Although the HIV neurodevelopmental involvement causes a prognostic worsening, most studies of pediatric cases of neuro-AIDS demonstrate that an early diagnosis followed by adequate antiretroviral therapeutic regimens can lead to significant, even if temporary, improvement.149
Rehabilitation of the pediatric patient requires a multidisciplinary approach to meet the medical, emotional, and psychosocial needs of these children and their families. Children are encouraged to give form to their psychological experiences through play, writing or telling stories, and creating works of art.152