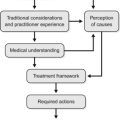
10 How to use the monographs
General considerations
In this book, the monographs on individual herbs are designed to be as user-friendly as possible and hence are divided into two sections:
• A summary monograph which provides at a glance a definition, background material and clinically relevant information.
• A technical data section which extensively reviews the botany, pharmacology, clinical trial data, safety data and regulatory status in selected countries.
Common and botanical names
For example, the Echinacea species are currently being revised. A potential change includes E. angustifolia→E. pallida var. angustifolia.1 Changes which have been proposed, but will not be enacted due to possible detriment to the pharmaceutical, herbal and agricultural industries, include:2
With the use of gene-sequencing techniques, changes may increase in the future as a greater understanding of taxonomy at a genetic level develops. Of an estimated 5 million species (of living things), only about 1.5 million are documented at present and they are constantly being renamed and moved in the 20 or so categories of the Linnaean classification system. A new approach (phylogenetic nomenclature), which names groups of organisms that descend from a common ancestor is gaining popularity.3
• Mabberley DJ. The Plant Book, 2nd Edn. Cambridge University Press, Cambridge, 1997. In the preparation of this book the authors followed the system of Cronquist (1981) as modified by Kubitzki (1990) (pp. ix-xiii)
• PLANTS database, United States Department of Agriculture, USDA
• GRIN Taxonomy database, Agricultural Research Service, USDA
• Global Plant Checklist, International Organisation for Plant Information
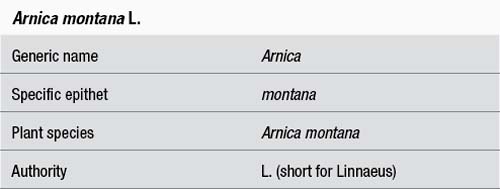
The botanical name is usually a Latin binomial consisting of a generic name, which comes first and then the specific epithet. Both components of the name are italicised. The generic name, which is capitalised, defines the genus to which the plant belongs. The authority that follows the specific epithet further defines the species. It indicates the taxonomist credited with naming the species (and hence the author of the name) and is often abbreviated (e.g. ‘Linnaeus’ becomes ‘L’). The authority has been included in the initial identifying information in these monographs and if necessary in the Adulteration section, but is not retained throughout the remainder of the monograph.
Summary actions
The actions are important because they encompass the traditional pharmacology of herbal medicine and link the therapeutic requirements of the patient to the choice of herbs (see the Chapter 7). Only those actions which are considered to be well supported are listed.
Can be used for
This section effectively covers the clinical uses that can be recommended in practice.
Dosage
The authoritative texts used mainly comprise the following:
• British Herbal Medicine Association’s Scientific Committee. British Herbal Pharmacopoeia. BHMA, Bournemouth, 1983
• British Herbal Medicine Association’s Scientific Committee. British Herbal Pharmacopoeia, 4th Edn. BHMA, Bournemouth, 1996
• Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China, English Edn. Chemical Industry Press, Beijing, 1997
• Bensky D, Gamble A. Chinese Herbal Medicine Materia Medica. Eastland Press, Seattle, 1986
• Scientific Committee of ESCOP (European Scientific Cooperative on Phytotherapy). ESCOP Monographs. European Scientific Cooperative on Phytotherapy, ESCOP Secretariat, UK, March 1996–October 1999
• Blumenthal M, et al (eds). The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. American Botanical Council, Austin, 1998.
• Kapoor LD. CRC Handbook of Ayurvedic Medicinal Plants. CRC Press, Boca Raton, 1990
• Regional Research Laboratory and Indian Drug Manufacturers’ Association. Indian Herbal Pharmacopoeia. Indian Drug Manufacturers’ Association, Mumbai and Regional Research Laboratory, Jammu-Tawi, 1998
In addition, dosages used in clinical trials were also taken into account.
Summary assessment of safety
This section summarises the safety data detailed in the Technical data section.
Technical data
Adulteration
CITES (the Convention on International Trade in Endangered Species of Wild Fauna and Flora) currently provides the only international instrument for the listing of species considered to be sufficiently endangered to the extent that commercial trade must be either monitored and controlled or prohibited.4
Pharmacodynamics
When extrapolating from animal studies a common misconception is that the dose used in the animal (mg/kg) directly relates to the human dose on a body weight basis. In other words, the mg/kg dose in the animal is to be multiplied by the average human weight of 70 kg to give the corresponding human dose in mg. Such considerations are important in toxicology and new drug development (where an effective human dose needs to be worked out from prior animal studies). But animals have much faster metabolism, so a correction factor needs to be applied. One publication has defined the scale-up factors for common animal models. This is around 6 for the mouse and 11 for the rat.5 In other words if a rat study used 100 mg/kg of extract, the corresponding human dose (for 70 kg) is 1.135 g, not 7.0 g. This can only apply if oral doses were used in the animal model. Other assumptions are that the model is relevant to the human disease and the animal metabolises the agent in the same way as humans.
Pharmacokinetics
The known pharmacokinetics of key constituents is reviewed, giving emphasis to human studies.
Clinical trials
Emphasis is given to randomised, controlled, double blind clinical trials. However, open (not blinded) and uncontrolled (no control group) trials are also briefly summarised. Where a number of clinical trials have been subjected to meta-analysis, or systematic review, this publication is primarily reviewed rather than those individual trials included in the meta-analysis or review, although important individual studies may also be highlighted. Also see Appendix E.
Relevance of scientific studies to clinical choices
• In vivo, herb given by injection: no relevance unless known active components have established bioavailability, even then of limited value
• In vivo, herb given orally: some relevance, depending on dose and validity of the animal model
• Clinical study: highly relevant, but depending on the design of the trial and being able to reproduce the treatment used.
Toxicology and other safety data
Toxicology
| ig* | intragastric |
| im | intramuscular |
| ip | intraperitoneal |
| iv | intravenous |
| LD50 | lethal dose for 50% of the tested population |
| LOAEL | lowest observed adverse effects level |
| MLD | minimum lethal dose |
| The least amount of a chemical that can produce death | |
| MOAEL | minimum observed adverse effect level |
| MTD | maximum tolerated dose |
| NOAEL | no observed adverse effect level |
| The highest dosage administered that does not produce toxic effects | |
| Sc | subcutaneous |
| TLV | threshold limit value |
| TTL | threshold toxic limit |
* In some cases ‘oral’ has been used for ‘gavage’ and ‘ig’ administration.
LD50 test
The LD50 test was introduced in 1927 for the biological standardisation of drugs.6 With the mean lethal dose (LD50) test, groups of experimental animals are treated with graduated doses of a test substance with the aim of obtaining a 50% or even higher mortality at the highest doses. The scientific significance of the classical LD50 test has been questioned on the basis of the relatively broad variability of the test results (more than 2-fold and up to 11-fold differences) and for animal welfare reasons.7 Three recently developed alternative animal tests that significantly improve animal welfare – the fixed dose procedure, the acute toxic class method, and the up and down procedure – can now be used within a strategy of acute toxicity testing for all types of test substances and for regulatory and in-house purposes. In vitro cytotoxicity tests could be used as adjuncts to these alternative animal tests to improve dose level selection and reduce (at least modestly) the number of animals used. However, the total replacement of animal tests requires a considerable amount of further development8 and such modern data are not yet currently available for most herbs.
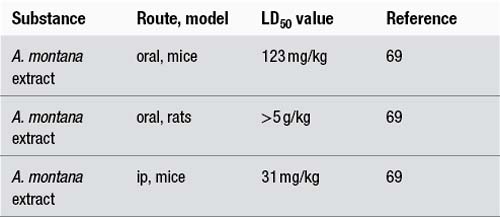
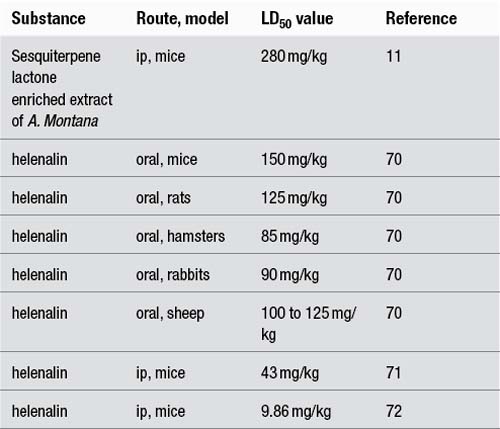
The LD50 values can be grouped into toxicity levels,9 as outlined in the following table with examples.
| Lethal dose | Toxicity level | Example(s) |
|---|---|---|
| <1 mg/kg | Dangerously toxic | Dioxin – 0.045 mg/kg (oral, rat (female)) |
| 1–50 mg/kg | Extremely toxic | Indomethacin – 12.6 mg/kg (oral, rat) |
| Dieldrin – 46 mg/kg (oral, rat) | ||
| 50–500 mg/kg | Very toxic | Aristolochic acid – 55.9 mg/kg (oral, mouse (male)) |
| Curare – 270 mg/kg (oral, rabbit) | ||
| Paracetamol – 338 mg/kg (oral, mouse) | ||
| Caffeine – 355 mg/kg (oral, rat (male)) | ||
| 500–5000 mg/kg | Moderately toxic | Atropine – 622 mg/kg (oral, rat) |
| Aspirin – 1500 mg/kg (oral, rat) | ||
| Baking soda – 4220 mg/kg (oral, rat) | ||
| 5000–15 000 mg/kg | Slightly toxic | Sodium cyanide – 6444 mg/kg (oral, rat) |
| Monosodium succinate (food additive) –>8 g/kg (oral, rat) | ||
| >15000 mg/kg | Practically non-toxic | Propylene glycol (cosmetics) – 20 000 mg/kg (oral, rat) |
Ames salmonella/microsome mutagenicity assay (Salmonella test, Ames test)
This is a short-term bacterial reverse mutation assay specifically designed to detect a wide range of chemical substances producing genetic damage leading to gene mutations.10 The test was developed by Ames and colleagues in the mid-1970s and became the most used test because of its initial promise of high qualitative (yes/no) predictivity for cancer in rodents and, by extension, in humans. The relationship between mutagenic potency prediction and quantitative carcinogenicity, however, is now known to be weak,11 despite the fact that early studies with this assay indicated that greater than 90% of the known carcinogens tested were mutagenic and that 90% of the non-carcinogens tested were non-mutagenic. The power of this assay was derived from the use of a liver microsome fraction (S9 mix) containing the mixed function oxidase (cytochrome P450) enzymes required to activate the test substance into precarcinogens (as might occur in the body after phase I metabolism by the liver). As the basis of the selection of chemicals for mutagenicity testing shifted to relative environmental importance, the sensitivity of the Salmonella assay for detecting carcinogens decreased. A negative result does not imply that the chemical will be non-carcinogenic. There are a large number of false-negatives produced (i.e. non-genotoxic carcinogens).12 Some plant components such as flavonoids give a false positive on this test.
Use in pregnancy and lactation
(The pregnancy categories are adapted from the Australian publication Medicines in Pregnancy, 4th Edn, 1999.)
| Category A | No proven increase in the frequency of malformation or other harmful effects on the fetus despite consumption by a large number of women. |
| Category B1 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. No evidence of increased fetal damage in animal studies. |
| Category B2 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Animal studies are lacking. |
| Category B3 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Evidence of increased fetal damage in animal studies exists, although the relevance to humans is unknown. |
| Category C | Has caused or is associated with a substantial risk of causing harmful effects on the fetus or neonate without causing malformations. |
| Category D | Has caused or is associated with a substantial risk of causing fetal malformation or irreversible damage. |
| Category X | High risk of damage to the fetus. |
Regulatory status in selected countries
The regulatory status of the herb in Australia, China, Germany, the UK and the USA is presented.
Australia
Part 4 of Schedule 4 of the Therapeutic Goods Regulations specifies herbs which cannot be contained in any products listed on the Australian Register of Therapeutic Goods (ARTG), or which can only be present in minute doses or if other specified conditions are met. In other words, herbs in Part 4 of Schedule 4 are considered to be more toxic and cannot be included in over-the-counter herbal products without further safety evaluations (see below).
References
1. Binns SE, Baum BR, Arnason JT. Syst Bot. 2002;27(3):610–632.
2. Binns SE, Baum BR, Arnason JT. Taxon. 2001;50(4):1199–1200.
3. Milius S. Sci News. 1999;156(17):268–270.
4. Lewington A. Traffic Int. 1993:31–32.
5. Reagan-Shaw S, Nihal M, Ahmad N. FASEB J. 2008;22(3):659–661.
6. DePass LR. Toxicol Lett. 1989;49(2-3):159–170.
7. Schlede E, Mischke U, Roll R, et al. Arch Toxicol. 1992;66:455–470.
8. Botham PA. ILAR J. 2002;43(suppl):S27–S30.
10. Mortelmans K, Zeiger E. Mutat Res. 2000;455(2):29–60.
11. Fetterman BA, Kim BS, Margolin BH, et al. Environ Mol Mutagen. 1997;29(3):312–322.
Andrographis
Synonyms
Chiretta, King of bitters (Engl), kalmegh (Bengali, Hindi), kirata (Sanskrit), chuan xin lian (Chin), senshinren (Jap).
Traditional view
In Ayurvedic medicine, the herb is used for its bitter tonic, stomachic, antipyretic and laxative properties. It is said to increase appetite, strengthen digestion and diminish flatulence, hyperacidity and biliousness.1 The herb is also utilised for treatment of many other conditions, including diabetes, debility and hepatitis.2 The roots and leaves have a reputation for being depurative and anthelmintic.3 In traditional Chinese medicine, Andrographis is bitter and ‘cold’, and is used to clear Heat from the Blood (especially in the lungs, throat and urinary tract) and to detoxify Fire Poison (manifesting as skin sores and carbuncles). In addition to gastrointestinal complaints, it is prescribed for throat infections, cough with thick sputum and snake bites.4,5 Since Andrographis is regarded as a ‘cold’ herb, it is ideally suited to treating acute infections, which are ‘hot’ conditions.
Dosage
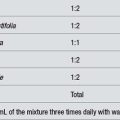
Being very bitter, some people may find Andrographis difficult to take in liquid preparations. Whichever way it is taken, the daily preventative dose for an adult is about 2 to 3 g or its equivalent (for example, 4 to 6 mL/day of a 1:2 fluid extract). During infection, the effective dose is nearer to 6 g/day (up to 12 mL/day of the 1:2). Standardisation for andrographolides is preferable.
Technical data
Botany
Andrographis paniculata is an annual shrub belonging to the Acanthaceae family. It grows to a height of 1 metre with branches that are sharply quadrangular, often narrowly winged towards the apical region. The leaves are lanceolate (5 to 8 cm long), and the flowers are small, solitary in panicles, with a corolla ranging from white to rose pink in colour and hairy externally. The fruit is approximately 2 cm long, linear-oblong in shape and acute at both ends. The seeds are numerous, yellowish-brown and glabrous.6 It grows wild as an undershrub in tropical moist deciduous forests6 and is also cultivated as a rainy season crop.2
Key constituents
• Diterpenoid lactones, collectively referred to as andrographolides, and consisting of aglycones (such as andrographolide) and glucosides (such as neoandrographolide and andrographiside)7,8
• Diterpene dimers,7 flavonoids9
Many of the diterpenoid lactones (such as andrographolide) are bitter; however, neoandrographolide is not.8
Adulteration
Andrographis echioides is an adulterant of A. paniculata.11 Purchased extracts of Andrographis paniculata are sometimes devoid of any andrographolide content, despite claims to the contrary on product specifications.12
Pharmacodynamics
Anti-infective and immunomodulating activity
Although Andrographis is widely used in infections and infestations, the most likely opinion is that its value here is mainly as an immune-enhancing treatment. Early reports in China attributed an antibacterial activity to the plant that was not supported in a later review.5 No direct antibacterial activity could be demonstrated for an aqueous extract of Andrographis against Salmonella, Shigella, Escherichia coli, group A streptococci and Staphylococcus aureus in vitro. Animal studies using orally administered Andrographis (0.12 to 0.24 g/kg) for 6 months failed to demonstrate bactericidal activity.13 Serum taken from 10 healthy volunteers after a single oral dose of Andrographis (ranging from 1 g to 6 g) showed no bactericidal activity against a number of organisms.13
However, an alcoholic extract of Andrographis did show significant activity against an E. coli enterotoxin-induced secretory response (that causes diarrhoea) in vivo,14 and in another study effected in vitro inhibition of adherence of Streptococcus mutans.15 Andrographolide potentiated the sensitivity of two strains of Pseudomonas aeruginosa to several antibiotic drugs in vitro.16 Significant growth inhibition (compared to five other herbs) was recently demonstrated for an Andrographis aqueous extract in vitro against Streptococcus agalactiae.17 Feeding Nile tilapia fish (Oreochromis niloticus) with Andrographis reduced mortality following infection with this species of Streptococcus (although such an outcome could also reflect enhanced immunity).
Liquid extract of Andrographis root demonstrated strong in vitro anthelmintic activity against human Ascaris lumbricoides.18 Subcutaneous administration of a decoction of Andrographis leaves to infected dogs reduced nematode larvae in the blood by 85%.19
Early uses as a substitute for quinine in malaria have been supported by recent research. An in vitro study revealed that xanthones from Andrographis root bearing a hydroxyl group at position 2 demonstrated the most potent antimalarial activity, while xanthones with a hydroxyl group at position 1, 4 or 8 possessed very low activity. Further, in vivo antimalarial testing of the most active xanthone on Swiss Albino mice with Plasmodium berghei infection demonstrated a substantial reduction (62%) in parasitaemia after treatment with 30 mg/kg.10 However, more relevant to current herbal use are studies on Andrographis leaf or whole plant. An earlier study showed that a chloroform extract of the whole plant demonstrated complete parasite growth inhibition within a 24 hour incubation period in vitro at concentrations as low as 0.05 mg/mL. There was significant reduction in mortality rates in mice administered Andrographis whole plant extract (5 mg/kg/day, ip for 4 days) just after malarial infection.20 Methanolic extracts of Andrographis leaves21 and aerial parts22 were active in vitro against both chloroquine resistant and sensitive strains of Plasmodium falciparum.
Dehydroandrographolide succinic acid monoester (DASM), a drug derived from andrographolide, has been found to inhibit HIV in vitro. This effect was observed on several HIV strains and DASM was non-toxic to other cells in the concentration range. However, the diterpenoid lactones of Andrographis (dehydroandrographolide and andrographolide) were devoid of anti-HIV activity.23 Moreover, in vitro studies with aqueous extracts of Andrographis showed little or no inhibition of HIV-1. Modes of inhibition studied comprised inhibition of HIV-1 protease,24 inhibition of the interaction between HIV-1 gp 120 and immobilised CD4 receptor, inhibition of HIV-1 reverse transcriptase and inhibition of glycohydrolase enzymes.25
Andrographolides showed in vitro activity against herpes simplex virus 1 in vitro26 and both Andrographis ethanolic extract (25 μg/mL) and andrographolide (5 μg/mL) inhibited the expression of Epstein-Barr virus lytic proteins in vitro, thereby inhibiting viral maturation.27 Andrographolide showed significant activity against influenza A viruses in vitro, including the H5N1 strain.28 Administration of andrographolide (100 to 200 mg/kg/day, oral) to mice infected with avian influenza A strains H9N2 and H5N1 and the human strain H1N1 significantly reduced death rate, prolonged life, inhibited lung consolidation and reduced viral titres in the lung.28 However, such in vivo activity might also be the consequence of immune effects.
Early research suggested an immunostimulant action for Andrographis. Enhanced phagocytosis was demonstrated in vitro for a decoction of the herb and in vivo after injection of the soluble derivatives.5 Isolated andrographolide (4 mg/kg/day, ip for 2 days) and an ethanolic Andrographis extract (25 mg/kg/day, oral for 7 days) significantly stimulated both antigen-specific and innate immune responses in mice. The whole extract produced stronger immunostimulation.29 Prolonged survival in animals after snakebite was observed after pretreatment with extracts of Andrographis.30
Four later studies (three from the same research group) have demonstrated a downregulation of immune response by andrographolide using both in vitro and in vivo models. In vitro, andrographolide reduced T cell activation in splenocytes,31 reduced IL-2 production in stimulated Jurkat T cells by interfering with nuclear factor of activated T cells (NFAT) and mitogen-activated protein kinases (MAPK),32 reduced IL-2 and interferon-gamma in stimulated murine T cells,33 and downregulated macrophage immune responses and cytokine expression.34 In vivo, andrographolide (4 mg/kg/day, ip) reduced T cell function and significantly reduced the severity of experimental autoimmune encephalomyelitis in mice, including antimyelin T cell and antibody responses.31 Andrographolide (1 mg/kg/day for 7 days, ip) also reduced antibody production and the number of IL-4-producing splenocytes in mice after antigen challenge.34
However, several other later publications have observed an enhanced immune response from andrographolide or Andrographis extract, most notably the in vivo studies summarised in the Antitumour activity section that follows. In addition, andrographolide and a combination of Andrographis and Siberian ginseng extracts (Kan Jang) demonstrated lymphocyte proliferation and stimulation of some cytokines in vitro in a whole blood cell culture.35 Andrographis extract (25 or 50 mg/kg/day, oral for 14 or 28 days) and andrographolide (1 or 4 mg/kg/day, oral for 14 or 28 days) enhanced specific antibody and cell-mediated immune responses in mice inoculated with an inactivated Salmonella vaccine.36 A mixture of andrographolides (1.0, 1.5 and 2.5 mg/kg, oral) potentiated delayed-type hypersensitivity (DTH) in mice inoculated with sheep red blood cells, but also countered the increase in DTH after cyclophosphamide treatment.37 This suggests an immunomodulatory activity. Further experimentation at the same doses revealed that the andrographolide mixture significantly stimulated phagocytic activity, white blood cell counts and spleen and thymus weights in mice.
Antitumour activity
A methanol extract of Andrographis showed potent cell differentiation-inducing activity on mouse leukaemia cells in vitro. Some of the isolated diterpenes also demonstrated this activity.7,38 Andrographolide was shown to inhibit the in vitro proliferation of more than 30 tumour cell lines representing various types of cancers, specifically breast, CNS, colon, lung, melanoma, ovarian, prostate, renal and leukaemia. The compound was found to exert direct anticancer activity on cancer cells by cell-cycle arrest via induction of p27 and decreased expression of cyclin-dependent kinase 4 (CDK4). It also enhanced tumour necrosis factor-alpha (TNF-alpha) in lymphocytes, and possibly via this mechanism increased their cytotoxic activity against cancer cells in vitro.39 Andrographolide (100 and 200 mg/kg/day for 10 days, oral) significantly inhibited the growth of B16 melanoma and HT-29 colon tumours in mice.39
Since these publications there has been a considerable number of in vitro investigations of the antineoplastic activity of andrographolide against a wide variety of cancer cell lines. An extensive review of material up to 2008 by Varma and co-workers identified the key mechanisms involved.40 These included induction of cell-cycle arrest (possibly due to increased levels of p21) and apoptosis (via a variety of mechanisms involving caspase-3, caspase-8, BcL-2 and TNF-alpha-related apoptosis inducing ligand). Other mechanisms have been proposed from recent research, including a downregulation of epidermal growth factor receptors,41 a decrease of cell-cycle related proteins,42 changes in the intracellular redox system43 and a novel cell differentiating activity.44 Other andrographolides have also been shown to induce cell-cycle arrest in vitro.45
Neoandrographolide sensitised the cytotoxic action of etopiside against a leukaemia cell line46 and andrographolide was found to sensitise cancer cells (such as colorectal, cervical and hepatic) to doxorubicin.47
Other researchers have investigated inhibition of angiogenesis as an anticancer prospect for Andrographis extract and andrographolide. Intraperitoneal administration of both in angiogenesis-induced mice led to substantial reductions in elevated proinflammatory cytokines such as IL-1beta, IL-6, TNF-alpha and granulocyte-macrophage colony-stimulating factor (GM-CSF) and the most potent angiogenic factor, vascular endothelial growth factor (VEGF). Antiangiogenic factors such as tissue inhibitor of metalloproteinase 1 (TIMP-1) and IL-2 levels were elevated after treatment.48 The inhibitory effect on VEGF production was supported by a later in vitro study.49
Andrographolide inhibited the adhesion of gastric cancer cells to endothelial cells in vitro by blocking E-selectin expression in the latter.50 Taiwanese scientists have found inhibition of migration and invasion of cancer cell lines in vitro via the downregulation of matrix metalloproteinase-7 (MMP-7)51,52 and MMP-2.53
There have also been additional in vivo anticancer studies on andrographolide and Andrographis extract (both administered intraperitoneally). A 70% ethanolic extract of Andrographis (10 mg/animal for 10 days, ip) substantially reduced tumour growth, helped maintain total white cell count, improved IL-2 and GM-CSF levels and reduced TNF-alpha in mice inoculated with Dalton’s lymphoma ascites cells.54 These effects were maintained for 11 to 20 days after the final herb dose and were also observed for animals concurrently treated with a combination of cyclophosphamide, radiation and whole body hyperthermia. The same authors also observed that both Andrographis extract (10 mg/animal for 10 days, ip) and andrographolide (0.5 mg/animal for 10 days, ip) substantially prolonged the survival times of mice inoculated with EL4 thymoma cells.55 IL-2 and interferon-gamma levels were increased and the authors concluded, based on this and a series of complex experiments, that the two treatments increased cytotoxic T lymphocyte activity. In general, the Andrographis extract was more active than andrographolide. The same research group using similar models and treatments has also demonstrated enhanced natural killer cell activity and antibody-dependent cytotoxicity in normal and tumour-bearing (Ehrlich ascites carcinoma) mice.56
Hepatoprotective and choleretic activity
Andrographolide showed protective activity against chemically induced toxicity in rat hepatocytes in vitro. The observed hepatoprotective effect was greater than silymarin.57 Intraperitoneal administration of andrographolide, andrographiside and neoandrographolide (100 mg/kg) to mice protected against hepatotoxic damage caused by carbon tetrachloride and tert-butylhydroperoxide. Andrographiside and neoandrographolide had the greatest effect on reducing lipid peroxidation and were comparable to silymarin.58 Similar studies suggest that andrographolide is the major active antihepatotoxic principle in Andrographis.59 Intraperitoneal administration of three diterpene constituents of Andrographis showed protective effects on hepatotoxicity induced in mice by various chemicals. The protective effect of andrographiside and neoandrographolide was as strong as silymarin, and could be due to the glucoside groups acting as strong antioxidants.60 Andrographolide exhibited hepatoprotective activity after oral or intraperitoneal administration to rats with chemically induced acute hepatitis. Treatment with the herb led to complete normalisation of five biochemical parameters and improved histopathological changes in the liver.61
Intraperitoneal pretreatment of mice with different doses of andrographolide or arabinogalactan proteins from Andrographis for 7 days was followed by intraperitoneal injection of ethanol (7.5 g/kg of body weight). At 500 mg/kg and 125 mg/kg, respectively, the protective activity of these two preparations against hepatic and renal alcohol toxicity was comparable to silymarin.62
Oral administration of Andrographis extract and andrographolide to rats demonstrated a protective action against carbon tetrachloride-induced hepatotoxicity. The leaf extract showed stronger activity than andrographolide.63 Pre- and post-treatment with oral doses of Andrographis (0.5 g/kg/day) normalised alcohol-induced increases in serum transaminase activity in rats. The researchers concluded that Andrographis has a protective as well as a curative effect on alcohol-induced toxic liver damage.64
Andrographolide (5, 7 and 10 mg/kg, oral) improved levels of antioxidant parameters such as glutathione, superoxide dismutase and catalase in mice treated with the liver carcinogen hexachlorocyclohexane (BHC).65 It also reduced parameters of liver damage and the development of liver tumours in BHC-treated mice at the same doses.66
Significant hepatoprotective activity was demonstrated for an alcohol extract of Andrographis and two of its diterpenes – andrographolide and neoandrographolide – against the hepatotoxicity caused by Plasmodium berghei infection in animals. The protective effect of Andrographis was thought to be partially due to reactivation of superoxide dismutase, which in turn counteracted peroxidative damage caused by the infection. Andrographis may also induce drug metabolising systems that detoxify hepatotoxins.67 Administration of Andrographis (0.5 g/kg/day) or andrographolide (5.0 mg/kg/day) to rats for 7 to 30 consecutive days induced the liver microsomal drug-metabolising enzymes aniline hydroxylase, N-demethylase and O-demethylase.68
Andrographolide produced a dose-dependent choleretic effect (increased bile flow, bile salt and bile acids) in rats and guinea pigs after oral administration69 and by intraperitoneal injection in rats.70 The effect was stronger than silymarin.69 Aqueous extract of Andrographis orally administered to rats at 3.75 mL/kg increased bile flow and liver weight. A maximal increase in flow and weight was reached after 2 days.71
Cardiovascular activity
An early study investigating an aqueous extract by intravenous administration suggested that Andrographis may limit the expansion of the ischaemic focus, may exert a marked protective effect on the reversible ischaemic myocardium and could demonstrate a weak fibrinolytic action.72 Andrographis alleviated myocardial ischaemia-reperfusion injury in vivo.73 It upregulated cellular reduced glutathione and protected cardiomyocytes against hypoxia/reoxygenation injury in vitro.74 The mechanism was probably via a decrease in the harmful effect of oxygen free radicals.75 A study using rabbits found Andrographis alleviated atherosclerotic arterial stenosis induced by both de-endothelialisation and a high cholesterol diet. In addition, it lowered the restenosis rate after experimental angioplasty.76 Andrographolide (5 mg/kg, presumably ip) suppressed the hyperplasia of arterial neointima (about a 60% reduction) in a murine model of arterial restenosis. This was via the downregulation of NF-kappaB target genes that are critical in thrombosis and inflammation.77
An aqueous extract of Andrographis given by intraperitoneal infusion to rats exhibited a dose-dependent reduction in systolic blood pressure in spontaneously hypertensive rats and normotensive controls.78 A crude water extract of Andrographis, and two semi-purified n-butanol and aqueous fractions, significantly reduced mean arterial blood pressure in anaesthetised rats without decreasing heart rate after ip administration. The hypotensive substance in the crude water extract appeared to be concentrated in the butanol fraction.79
Following the observation that some patients exhibited a hypotensive response while taking Andrographis, the in vitro and in vivo actions of the herb and three of its diterpenoids were investigated.80 The diterpenoid 14-deoxy-11,12-didehydroandrographolide (DIAP) was most active at reducing the chronotropic response of isolated rat atria and exerting spasmolytic activity in rat aortic rings. An Andrographis aqueous extract with the highest levels of DIAP (delivering 19 mg/kg of this compound, oral doses for 7 days) was the most potent (compared to two other extracts with lower levels of DIAP) at reducing systolic blood pressure in rats. Mechanistic studies suggested that vascular smooth muscle was the major site of the hypotensive effect.
Andrographolide inhibited PAF-induced human platelet aggregation81 and deoxyandrographolide antagonised PAF-mediated processes in neutrophils,82 as did andrographolide,83 all in vitro.
Anti-inflammatory, antipyretic, antiallergic and analgesic activity
Several early in vivo studies found antipyretic and anti-inflammatory effects for andrographolides (after oral administration or injection). The anti-inflammatory activity of the andrographolides may be due to the promotion of ACTH (adrenocorticotrophic hormone) and consequent enhancement of adrenocortical function.5 Andrographolide administered orally (30, 100 and 300 mg/kg) significantly reduced inflammation in a number of animal models including adjuvant-induced arthritis.84 The addition of andrographolide to an endothelial cell culture together with TNF-alpha caused a concentration-dependent reduction of the enhancement of endothelial monocyte adhesion, which is part of the inflammatory process.85 Another in vitro study found that andrographolide inhibited NF-kappaB binding to DNA, thereby reducing the expression of pro-inflammatory proteins such as COX-2 in neutrophils.83 Andrographolide prevented oxygen radical production by human neutrophils in vitro.86
As well as demonstrating antioxidant activity in vitro and in vivo, a 70% methanolic extract of Andrographis (10 mg/animal for 5 days, ip) completely inhibited carrageenan-induced paw oedema in mice.87 Oral administration of neoandrographolide (150 mg/kg) also demonstrated anti-inflammatory activity in mice as well as in vitro, using several experimental models.88
In a murine model of asthma, andrographolides (30 mg/kg, ip) inhibited the elevation of bronchoalveolar fluid (BAF) levels of TNF-alpha and GM-CSF, and almost abolished the accumulation in BAF of lymphocytes and eosinophils.89 In a similar model, andrographolide (0.1, 0.5 and 1 mg/kg, ip) dose-dependently inhibited increases in total cell count, eosinophil count and IL-4, IL-5 and IL-13 levels in BAF.90 It also attenuated IgE responses, eosinophilia and airway mucus production and hyper-responsiveness. Examination of lung tissue specimens and further in vitro investigations suggested that andrographolide might act by inhibiting the NF-kappaB pathway. An anti-inflammatory mechanism mediated by reduced NF-kappaB expression was also observed for andrographolide (2 mg/kg/day for 7 days, ip) in another study in mice, using a similar experimental model of asthma.91
Oral doses of andrographolide at 300 mg/kg demonstrated analgesic activity; at 100 and 300 mg/kg significant antipyretic effects were also observed after 3 h. In addition, this dose exhibited significant protective activity against aspirin-induced ulceration in rats.92 An aqueous extract of Andrographis (40 and 100 mg/kg, oral) and andrographolide (25, 50 and 100 mg/kg, oral), but not a 95% ethanolic extract (100 and 200 mg/kg, oral), demonstrated significant analgesic activity in mice.93 The aqueous extract and andrographolide were also active at oral doses of 100 mg/kg in reducing carrageenan-induced rat paw oedema. A similar activity profile (analgesic and antioedema) was demonstrated for subcutaneous injection of andrographolide (10, 25 and 50 mg/kg).94 Analgesia was probably mediated via non-opioid pathways, since naloxone failed to antagonise the activity of andrographolide. A methanolic extract of Andrographis (100 to 300 mg/kg, ip) slightly lowered body temperature, increased pentobarbitone sleeping time, demonstrated analgesic activity, reduced exploratory behaviour and curiosity, and exhibited some muscle-relaxing activity in mice.95 The same doses in rats also reduced exploratory behaviour in the Y-maze test.
Hypoglycaemic activity
An aqueous extract of Andrographis (10 mg/kg) was found to prevent glucose-induced hyperglycaemia in rabbits, but failed to prevent glucose absorption from the gut.96 In a screening of several traditional remedies, only Andrographis (as aqueous extract, and especially freeze-dried extract, administered at 50 mg/kg and 6.25 mg/kg body weight) significantly lowered blood glucose levels in streptozotocin-induced hyperglycaemic rats.97 In a further study Andrographis decoction was orally administered to alloxan-induced diabetic rats, with a significant reduction in blood glucose levels observed compared with controls.98
In a study comparing normal and streptozotocin-induced diabetic rats, an ethanolic extract of Andrographis not only demonstrated hypoglycaemic effects, but also reduced oxidative stress in the diabetic rats. Normal and diabetic rats were randomly divided into groups and treated orally with distilled water, metformin (500 mg/kg) or Andrographis (400 mg/kg) twice daily for 14 days. Both Andrographis and metformin significantly increased body weight and reduced fasting serum glucose in the diabetic rats, but had no such effects in the normal rats. Both treatments also significantly increased the activity of the antioxidant enzymes superoxide dismutase (SOD) and catalase in the diabetic rats, but again not in the normal rats.99 In another study, Andrographis extract (400 mg/kg, oral) decreased blood glucose and increase activities of the antioxidant enzymes SOD and catalase in streptozotocin-induced diabetic rats.100
Inhibition of the digestive enzymes alpha-glucosidase and alpha-amylase can significantly decrease the postprandial increase in blood glucose. In vitro testing demonstrated that a 20% ethanolic extract of Andrographis possessed an appreciable alpha-glucosidase inhibitory effect, but demonstrated only weak inhibition of alpha-amylase.101 Supporting this observation, a single oral dose of Andrographis extract (250, 500 or 1000 mg) dose-dependently and significantly reduced blood glucose in streptozotocin-induced diabetic rats challenged with starch and sucrose, but not after a glucose challenge.101 In contrast, andrographolide (0.5 to 1.5 mg/kg, oral) dose-dependently decreased plasma glucose in streptozotocin-induced diabetic rats, and at 1.5 mg/kg (oral) significantly attenuated plasma glucose after glucose challenge in normal rats.102
As a possible contradiction of the above results for sucrose, oral administration of Andrographis extract and andrographolide produced a dose- and time-dependent activation of brush-border membrane-bound hydrolases (lactase, maltase, sucrase) in rats, suggesting it accelerated the intestinal digestion and absorption of disaccharides.103
Other activity
An aqueous extract of Andrographis (200 mg/kg, oral) largely countered the nephrotoxic impact of gentamicin in rats, in terms of tending to normalise serum creatinine and urea, blood urea nitrogen and urine volume.104 Whether this effect was due to antioxidant activity or represented a specific renoprotective activity is not clear.
Three studies cited above attest to significant in vivo and in vitro antioxidant activity for the herb,87,93,100 as do some of the hepatoprotection studies. Oral administration of an aqueous extract of Andrographis (10, 20 and 30 mg per mouse) caused a significant elevation of catalase, SOD and glutathione-S-transferase activities in lymphoma-bearing mice, as well as exhibiting some antitumour activity.105
Other studies have demonstrated protection against toxins, in some cases linked to antioxidant activity. Andrographolide and an aqueous extract of Andrographis (250 mg/kg/day for 7 days, ip) protected against nicotine-induced neurotoxicity in rats by reducing oxidative stress.106 A similar protective activity against nicotine-induced oxidative stress was demonstrated in vitro for lymphocytes.107
A 70% ethanolic extract of Andrographis (10 mg/animal/day for 10 days, ip) was also shown to protect against cyclophosphamide toxicity in mice in two separate but similar studies conducted by the same research group.108,109 The elevation of TNF-alpha induced by the drug was lowered by Andrographis treatment. Andrographolide was also active.109
Topical application of Andrographis extract (10%) improved wound healing in rats.110 Wounds dressed with Andrographis showed markedly less scar width, higher fibroblast proliferation, more collagen, less angiogenesis and an absence of inflammatory cells.
Pharmacokinetics
An early study found that oral doses of radiolabelled andrographolide given to mice were rapidly absorbed and distributed to organs, especially gallbladder, kidney, ovary and lung. Andrographolide levels appeared to be low in spleen, heart and brain. Approximately 90% was excreted in the urine and faeces after 24 h, and 94% after 48 h. At 48 h, radiolabelled andrographolide only accounted for approximately 11% of urine and liver fractions, the remainder consisted of metabolites.111
Using isolated rat small intestine it was observed that P-glycoprotein (P-gp) was involved in the intestinal transport and absorption of andrographolide.112 One recent study using in vitro models and rats calculated that andrographolide had low absolute bioavailability (2.67%) because of its rapid biotransformation and efflux by P-gp.113 Metabolites of andrographolide found in rats included sulphates114 and an unusual sulphonic acid derivative (in urine).115
Studies in human volunteers have identified sulphate116 and glucuronide117 conjugates in urine after ingestion of andrographolide. Administration of a single 200 mg dose of andrographolide to each of 20 healthy volunteers revealed mean values of Tmax and Cmax of 1.6 h (range 1.5 to 2.0 h) and 58.6 ng/mL (range 29.3 to 81.2 ng/mL), respectively.118 The elimination half-life of andrographolide was 10.5±2.1 h. These results are consistent with a relatively low bioavailability for andrographolide.
Andrographolides might exhibit better bioavailability from Andrographis extracts. After oral administration of 1 g/kg of an extract (containing 4.52% andrographolide and 2.95% 14-deoxy-11,12-didehydroandrographolide (DIAP)) to rats, the respective Cmax and Tmax values observed were 1.42±0.09 μg/mL and 3.0±0.12 h for andrographolide and 1.31±0.05 μg/mL and 3.0±0.15 h for DIAP.119 Similarly in rabbits, 2 mL/kg of a liquid extract of Andrographis (containing 35.2 mg andrographolide and 20.7 mg DIAP) resulted in respective Cmax and Tmax values of 2.28 μg/mL and 1.0 h for andrographolide and 1.33 μg/mL and 0.75 h for DIAP.120
The amount of andrographolide was determined in the blood plasma of rats and 15 human volunteers following the oral administration of a 60% ethanolic Andrographis extract to rats and its combination with Siberian ginseng to humans (Kan Jang).121 In rats it was found that andrographolide is rapidly and almost totally (91%) absorbed after oral administration of 20 mg/kg of the Andrographis extract. Less than 10% was found in the urine, presumably because of extensive metabolism. The pharmacokinetics of andrographolide was found to be highly variable in humans after the single oral administration of 20 mg via Kan Jang, although it was reasonably rapidly absorbed (Tmax 1.37 h). The calculated steady-state plasma concentration after the normal multiple doses of the herbal combination was around 0.66 μg/mL, with a Cmax after each dose of about 1.34 μg/mL.
Clinical trials
Respiratory infections
A recent systematic review of two systematic reviews and eight clinical trials concluded that there was evidence that Andrographis was useful in the treatment of upper respiratory tract infections, but expressed concerns about publication bias and particularly the fact that most of the trials had been conducted in association with product manufacturers.122 The authors called for more independent clinical trials.
The two systematic reviews included in the above were as follows. In one, seven double blind, controlled trials (n=896) that met inclusion criteria for evaluation of efficacy were considered. All trials scored at least three (out of a maximum of five) for methodological quality on the Jadad scale. Collectively, the data suggested that Andrographis was superior to placebo in alleviating the subjective symptoms of uncomplicated upper respiratory tract infection. There was also preliminary evidence of a preventative effect. Adverse events reported following the herb administration were generally mild and infrequent.123 In the second review, 433 patients from three trials were included in the meta-analysis. Andrographis either alone or in combination with Siberian ginseng was more effective than placebo in the treatment of uncomplicated acute upper respiratory tract infection.124
In a general review of the literature for evidence of the efficacy and safety of complementary and alternative medicine for the prevention and treatment of upper respiratory tract infection in children, the authors concluded that Andrographis decreased nasal secretions (p<0.01), but not other symptoms.125
The subjects of these reviews as well as other studies follow.
Uncontrolled early Chinese clinical studies in patients with bacterial and viral respiratory infections suggested beneficial effects after oral administration of Andrographis or andrographolides, implying an immune enhancing action.5 Investigations from the Sichuan Traditional Medicine Research Institution found Andrographis exerted a beneficial effect in the treatment of infectious diseases associated with cold symptoms. The major finding was the lowered body temperature within 48 h after treatment with Andrographis. Of 84 cases of common cold, 70 achieved normal body temperature within 48 h.126
A randomised double blind study of 152 patients with pharyngotonsillitis found Andrographis (6 g/day) for 1 week to be as effective as paracetamol (acetaminophen) in relieving fever and sore throat. For both groups the difference between baseline symptoms and final evaluation was significant (p<0.0001). Lower doses of Andrographis were not as effective.127
Tablets containing a total of 1200 mg Andrographis extract (standardised to 4% andrographolides) or placebo were given to 61 patients suffering symptoms of common cold in a double blind, placebo-controlled clinical trial. After 4 days of treatment, measured symptoms were significantly reduced in the Andrographis-treated group compared to placebo: strength of disease (p=0.0001), tiredness (p=0.0001), sweating/shivering (p=0.001), sore throat (p=0.0001) and muscular ache (p=0.0001). In terms of clinical signs (rhinitis, sinus pains and headaches, lymphatic swellings), there was no significant difference between the treated and placebo groups at day 4. However, when the groups were compared over time (specifically day 0 versus day 4), there was a significant decrease in the intensity of these signs only for the Andrographis group (p<0.05). The overall reduction in the symptom score over time was also significant (p<0.01). The authors concluded that, based on their findings, Andrographis can significantly reduce the symptoms and duration of the common cold.128
In a randomised, double blind, placebo-controlled clinical trial, 107 healthy children received either Andrographis extract tablets (200 mg/day of extract, standardised to 11.2 mg andrographolide) or placebo for 3 months during the winter season. This dose corresponds to about 1 g of original herb. Analysis after the first month indicated no significant change for Andrographis treatment. However, by the third month there was a significant decrease in the incidence of colds compared with placebo (30% versus 62%; p<0.01). The relative risk of catching a cold was 2.1 times lower for the Andrographis group.129 The same research team conducted a further randomised, placebo-controlled, double blind study of an Andrographis extract at 1200 mg/day over 5 days in 158 adult patients. Visual analogue scale evaluations of the intensity of headache, tiredness, earache, sleeplessness, sore throat, nasal secretion, phlegm and frequency and intensity of cough were performed by the patients at days 0, 2 and 4 of the treatment. Using a logistic regression model of assessment, there was a significant decrease in the intensity of the symptoms of tiredness, sleeplessness, sore throat and nasal secretion in the Andrographis group at day 2, as compared with the placebo group. By day 4, a significant decrease in the intensity of all symptoms was observed for the Andrographis group. No adverse effects were observed or reported.130
In another randomised, double blind, placebo-controlled pilot study, 50 outpatients with symptoms of common cold were treated with tablets containing Andrographis extract (1020 mg/day, about 6 g of herb). The patients were advised to make their first clinic visit not later than 3 days after the occurrence of cold symptoms. After 5 days of therapy, subjective evaluation demonstrated a significantly reduced number of sick leave days (p<0.03), improved symptoms (p<0.025) and hastened recovery (p<0.05). Side effects were very few and mild.131
Recently, a randomised, double blind, placebo-controlled clinical trial observed that treatment with a standardised extract of Andrographis reduced the symptoms of uncomplicated upper respiratory tract infection.132 A total of 223 patients received either 200 mg/day of an Andrographis extract (about 2.5 g of herb, containing 60 mg of andrographolides) or a matching placebo for 5 days after experiencing the typical symptoms of a common cold. Nine self-evaluated symptoms were used to assess the efficacy of the herbal treatment: cough, expectoration, nasal discharge, headache, fever, sore throat, earache, malaise/fatigue and sleep disturbance. Both groups showed improvement in these scores from days 1 to 3. However, from days 3 to 5 most of the symptoms in the placebo group were unchanged, whereas symptom improvement continued for the Andrographis group. The difference in the overall symptom score between the two groups was significant at day 5 (p<0.05). For individual symptoms on day 5, all were significantly improved for the Andrographis group versus placebo (p<0.05), except for earache. The overall efficacy of Andrographis was a significant 2.1 times higher than placebo (p<0.05), and the herbal treatment was well tolerated. One weakness of the trial design was that patients were not treated for longer than 5 days. Hence the impact of Andrographis on shortening the duration of the common cold could not be assessed.
In two randomised, parallel-group clinical studies, a standardised extract of Andrographis (as the combination Kan Jang) was compared with amantadine in the treatment of diagnosed viral influenza infection.133 Each tablet comprised 85 mg of Andrographis extract (containing 5 mg andrographolides) and 10 mg of Siberian ginseng extract (from 120 mg root). The typical acute dose was four tablets three times daily. In the first pilot study, 71 Kan Jang-treated patients were compared with 469 patients on amantadine; in the second phase 66 patients were enrolled. Duration of sick leave and frequency of post-influenza complications were used as outcome measures and indicated that the herbal combination contributed to a quicker recovery. It reduced the risk of post-influenza complications and was also well tolerated.
A three-arm study compared the efficacy of standard treatment, Kan Jang and a preparation containing Echinacea purpurea extract in patients with uncomplicated common colds.134 Of the 130 children aged between 4 and 11 years studied over a period of 10 days, 39 patients received only standard treatment, 53 were treated with the Andrographis combination plus standard treatment and 41 were treated with Echinacea plus standard treatment. It was found that adjuvant treatment with the Andrographis combination was significantly more effective than the Echinacea preparation when started at an early stage of uncomplicated common colds. The effect was particularly pronounced in terms of the amount of nasal secretion and congestion. It also accelerated the recovery time compared to other treatments. The need for standard medication was significantly less in the Andrographis combination group compared with the others and it was well tolerated, with no adverse reactions reported.
The same Andrographis combination was also tested in a phase III randomised, double blind, placebo-controlled parallel group clinical trial in the treatment of uncomplicated upperrespiratory tract infections. After an initial pilot trial involving 46 patients over 3 to 8 days, 179 patients completed the 3-day study according to protocol. Both the total symptom score (from patients’ evaluation) and the total diagnosis score (from physicians’ evaluation) showed highly significant improvements (p<0.0006 and p=0.003, respectively), as compared with placebo. Throat signs and symptoms demonstrated the most significant improvement.135
A double blind, placebo-controlled clinical study evaluated the impact of Kan Jang treatment for 5 days in the management of acute upper respiratory symptoms (including sinusitis) in 185 patients. At the end of the treatment, significant differences compared with placebo were in evidence for total symptoms in the group as a whole (p<0.001) and in the acute and recurrent sinusitis subgroups. In terms of individual symptoms in the whole group, significant differences against placebo (p<0.001) were observed for sore throat, headache, malaise and catarrh.136
Enteric infections
Many early Chinese studies used oral administration of Andrographis or andrographolides in acute bacillary dysentery and enteritis and observed a marked benefit.5 Patients with acute diarrhoea were treated with powdered leaves and stems of Andrographis. The Andrographis was more effective in reducing the number of Shigella, but was less effective for cholera compared with tetracycline. Oral administration of 1 g every 12 h for 2 days was more effective than giving a dose of 500 mg every 6 h for 2 days.137
Inflammatory disorders
Familial Mediterranean fever (FMF) is a recessively inherited inflammatory disorder characterised by recurrent attacks of fever and serositis (inflammation of the serous tissues such as pleura, pericardium and peritoneum). A combination of Andrographis extract 600 mg/day (containing 48 mg andrographolide), Siberian ginseng extract 120 mg/day (standardised to >9.6 mg eleutheroside E), Schisandra chinensis extract 600 mg/day (standardised to >9.6 mg schisandrins) and licorice extract 120 mg/day (standardised to >7.2 mg glycyrrhizin) was assessed over 30 days in a pilot study involving 24 children with FMF.138 Using a double blind, placebo-controlled design, it was found that duration, frequency and severity of attacks were all significantly less compared with placebo following the herbal treatment (p=0.0003). A separate publication investigated the impact of the herbal combination on plasma nitric oxide (NO) levels during the trial.139 Additional control groups were used, consisting of healthy volunteers and FMF patients treated with colchicine. Basal levels of NO in FMF patients during attack-free periods over the 30-day trial were found to be no different to the healthy controls. Surprisingly, NO levels fell during attacks. The herbal formulation with Andrographis was found to normalise blood levels of NO and decrease IL-6 in FMF patients during attacks.
A 14-week randomised, double blind, placebo-controlled clinical trial in 60 patients examined the impact of a 75% ethanolic extract of Andrographis (300 mg/day corresponding to 3 g of herb and containing 90 mg of andrographolides) in active rheumatoid arthritis.140 All trial patients were given methotrexate and were allowed to take prednisone or chloroquine in stable doses if already prescribed. Compared with baseline there were significant improvements observed in the Andrographis group by week 14 for tender joints (p=0.001), number of swollen joints (p=0.02), severity of swollen joints (p=0.01), severity of tender joints (p=0.002), levels of rheumatoid factor (p=0.01) and quality of life measures (p<0.001). However, compared with the placebo group these changes were not statistically significant. Perhaps a larger trial with a higher dose might have yielded results that were more definite.
A randomised, double blind trial was conducted at five centres in Shanghai to compare a standardised extract of Andrographis with the non-steroidal anti-inflammatory drug mesalazine (4.5 g/day, in slow release form) in patients with mildly to moderately active ulcerative colitis (confirmed by colonoscopy).141 One hundred and eight patients completed the trial. The aqueous-ethanolic extract provided about 108 mg/day of andrographolide. Treatment with Andrographis extract demonstrated similar efficacy to mesalazine. Scores for clinical symptoms (fever, stool frequency, stool consistency, stool blood, abdominal pain, mucous stool, tenesmus (straining) and abdominal tension) were assessed throughout treatment. Symptom scores decreased over time in both groups. Clinical efficacy was also assessed by the percentage of patients attaining remission, partial remission or improvement in symptoms. Mucosal healing was evaluated by colonoscopy and, in the 34 patients with biopsies available, histopathology was evaluated. Such outcomes were significantly better (p<0.001) for both groups compared with baseline, and there was no significant difference between the two treatment groups. Thirteen per cent of patients in the Andrographis group and 27% of patients in the mesalazine group had at least one adverse event. Most adverse events appeared to be related to the underlying disease.
Other conditions
In a phase I clinical trial, 13 HIV positive patients and 5 healthy volunteers took 5 mg/kg andrographolide for 3 weeks, escalating to 10 mg/kg for 3 weeks, which was then intended to rise to 20 mg/kg for a final 3 weeks. However, the trial was interrupted at 6 weeks due to adverse events, including an anaphylactic reaction in one patient. All adverse events had resolved by the end of observation. A significant rise in the mean CD4+ lymphocyte level in HIV patients occurred after administration of 10 mg/kg andrographolide (from 405 to 501 cells/mm3, p=0.002). There were no statistically significant changes in mean plasma HIV-1 RNA levels throughout the trial.142
An open study in Thailand compared parameters of urinary tract infection in patients undergoing shock wave dissolution of kidney stones (lithotripsy). The study found that 1 g of Andrographis was as effective as the antibiotics co-trimoxazole and norfloxacin in reducing pyuria and haematuria.143
A phase I clinical study of Kan Jang in healthy men revealed a slightly positive benefit on sperm count, sperm activity and other indices of fertility when it was taken at three times the normal dose.144
Sixty-three patients with cardiac and cerebrovascular diseases were observed at 3 h and/or 1 week after taking Andrographis extract. Results showed that platelet aggregation induced by ADP was significantly inhibited (p<0.001). The aggregation rate was lower at 1 week. In other volunteers taking Andrographis, serotonin release from platelets was decreased (p<0.01), but plasma serotonin levels remained unchanged. A rise in platelet cAMP levels might be the mechanism behind the antiplatelet activity of Andrographis.145
A majority of 20 patients with infective hepatitis showed marked improvement in symptoms after approximately 24 days of treatment with an Andrographis decoction (equivalent to 40 g of herb per day). Significant decreases in various liver function tests were also observed. Overall, 80% of cases were ‘cured’ and 20% were relieved.146
Toxicology and other safety data
Toxicology
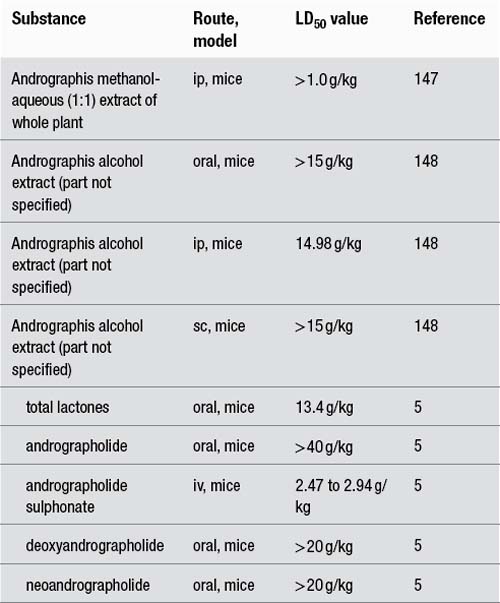
The following LD50 data have been recorded for Andrographis extract and its constituents:
In acute toxicity studies, no toxic effects were observed in mice after oral administration of a suspension of Andrographis leaf powder (2 g/kg), a suspension of leaf alcohol extract (2.4 g/kg) or andrographolide (3 g/kg).149 Similarly, subcutaneous administration of Andrographis leaf decoction (0.33 g/kg) to rabbits did not exhibit toxic effects.19 Female rats treated for 14 days with an oral dose of 5 g/kg of a methanolic Andrographis extract also demonstrated no adverse effects.150
No toxic effects were observed in subacute oral toxicity tests when either a leaf powder suspension (200 and 400 mg/kg) or straight leaf powder (50 to 150 mg/kg) was administered on alternate days for 4 weeks to mice or for 14 weeks to rats, respectively.149 Also andrographolide (1 g/kg/day) administered to rats and rabbits for 7 days did not cause toxic effects.5 Rats administered Andrographis powder (part and route not specified) at dosages of 0.12, 1.2 and 2.4 g/kg/day for 6 months exhibited no abnormalities in growth rate, food consumption, clinical signs, serum biochemical parameters or histology.148
Oral administration of andrographolide (2 g/day) for 4 days caused a transient elevation of the liver enzyme ALT in healthy volunteers. Levels normalised upon discontinuation of the andrographolide. Hepatic and renal functions were not impaired after doses of 0.9 g/day for 5 days.5
Effects of Andrographis on male fertility show conflicting results, but there is probably no cause for concern based on the more recent studies. Reduced fertility and prolongation of gestation were observed in mice when the male was fed Andrographis stem powder (0.75% of diet) prior to mating. As mating rates were not confirmed, these effects may have been due to a reduction in libido. Treated females mated with untreated males showed no appreciable change in fertility or gestational period.151 In contrast, antifertility effects were not observed in mice fed the powdered leaf or root (1% of diet; approximately 2 g/kg/day) for 2 weeks prior to mating and for 3 weeks during mating.151,152
Oral administration of Andrographis leaf powder (50 and 100 mg/kg/day) for 24 to 60 days to male rats resulted in the cessation of spermatogenesis and biochemical and degenerative changes in the testes and male accessory organs.153,154 Decreased sperm counts, spermatozoa abnormalities, histopathological changes in the testes and lack of fertility were observed after oral administration of high doses of andrographolide (25 and 50 mg/kg) for 48 days.155 However, no significant differences were observed in reproductive organ weights, testicular histology or serum testosterone levels after oral administration of an Andrographis dried herb (5:1) ethanol extract (containing 5.6% andrographolide) at dosages of 20, 200 and 1000 mg/kg/day for 60 days. The authors concluded that the above variation in results might be due to differences in the preparation of the plant material used.156
Recently Andrographis extract (20, 200 and 1000 mg/kg/day orally for 65 days)157 and andrographolide (50 mg/kg/day for 2 to 8 weeks)158 demonstrated no significant effects on sperm morphology, motility and counts and were without significant adverse effects. There is also the phase I clinical study in men cited earlier.144
After nitrosation with nitrite under acidic conditions, an ethanol extract of Andrographis became mutagenic to strains TA 98 and TA 100 (Salmonella/microsome test) tested either in the presence or absence of S-9 mix.159 A methanolic extract of Andrographis was devoid of significant genotoxic effects in three different in vitro models.150
Interactions
Antiplatelet activity was demonstrated ex vivo in the blood from patients with cardiac and cerebrovascular diseases taking Andrographis extract.145 This could possibly lead to an adverse interaction with antiplatelet and anticoagulant drugs. However, oral doses of Andrographis with Siberian ginseng exhibited no interaction with warfarin in rats.160
There have been several studies examining the in vitro or in vivo impact of either Andrographis extract or andrographolide on drug metabolising enzymes. However, the clinical relevance of these findings remains uncertain. In vitro, andrographolide induced the expression of cytochrome P450 superfamily 1 members CYP1A1 and CYP1A2, but not CYP1B1, in a concentration-dependent manner in murine hepatocytes.161,162 In isolated human and rat liver microsomes Andrographis extract and andrographolide inhibited CYP3A4 and 2C9 activity.163 Andrographis extracts and andrographolide significantly induced glutathione-S-transferase activity in rat primary hepatocytes.164,165
Of more relevance are the in vivo studies. An aqueous or an ethanolic extract of Andrographis (equivalent to 5 mg/kg/day andrographolide orally for 14 to 30 days) induced CYP1A1 and CYP2B in mice.166 Similar doses of Andrographis extract and andrographolide given orally to rats decreased CYP2C11 activity.167 Andrographolide (5 mg/kg/day, sc) enhanced CYP1A1 expression only in polycyclic aromatic hydrocarbon responsive mice, and only in males (probably via an interaction with testosterone).168
Theophylline is a typical substrate of CYP1A2 in rats. Oral Andrographis extract (1 or 2 g/day) or andrographolide (154 mg/kg/day) pretreatment for 3 days in rats increased the clearance of subsequently administered theophylline, confirming results from in vitro studies.169 The doses used were relatively high and do not reflect typical human doses.
Use in pregnancy and lactation
However, Andrographis is best avoided during early pregnancy until more information is available regarding its antifertility activity. A product containing standardised extract of Andrographis leaf has been used to treat the common cold in Scandinavia for over 20 years and no cases of pregnancy termination have been reported.170 Results from experiments regarding possible antifertility effects in female animals are conflicting (see below).151,152,170–173
Oral administration of an Andrographis extract (200, 600 and 2000 mg/kg) for the first 19 days of pregnancy did not impact progesterone levels in pregnant rats.170 Female mice fed high doses of Andrographis powder (2 g/kg/day) for 6 weeks failed to conceive when mated with males of proven fertility in a controlled experiment.171 Intraperitoneal injection of Andrographis whole plant decoction prevented implantation in mice and caused abortion at different stages of gestation in mice and rabbits. The decoction also terminated early pregnancy when administered by oral, intravenous, subcutaneous, intramuscular and intrauterine routes in mice.172 However, oral administration of Andrographis extract to rats at doses less than 2 g/kg during the first 9 days of pregnancy failed to interrupt pregnancy, induce fetal resorption or alter the number of live offspring.173 Andrographis stem powder (0.75% of diet) had no appreciable effect on fertility when fed to female mice for up to 4 weeks prior to mating.151 Antifertility effects were not observed in mice fed the powdered leaf or root (1% of diet; approximately 2 g/kg/day) for 2 weeks prior to mating and for 3 weeks during mating.151,152
No teratogenic or toxic effects were observed when a suspension of Andrographis leaf powder (200 and 400 mg/kg) was orally administered on alternate days for 4 weeks to mice in a controlled experiment.149
In vitro tests, which are of uncertain relevance to normal human use, have shown the following effects. Andrographis chloroform extract and andrographolide sodium succinate suppressed hormonal secretion and had a cytotoxic effect on cultured human placental chorionic trophoblastic tissue (aged between 6 to 8 weeks of pregnancy) in vitro.174 Andrographis extract demonstrated uterine relaxant activity in vitro.175
There are no data available on the use of Andrographis during lactation.
Side effects
In general, Andrographis has been well tolerated in clinical trials. One of 90 patients receiving Andrographis extract reported unpleasant sensations in the chest and intensified headache,131 and 2 of 50 patients reported urticaria,135 in randomised, double blind, placebo-controlled trials investigating respiratory infections. Andrographis extract was administered for 3 to 5 days at a dose of 1020 mg/day (containing 63 mg andrographolide and deoxyandrographolide).
A high incidence of adverse effects, including headache, fatigue, pruritus/rash, metallic/decreased taste and diarrhoea, was reported in a trial of pure andrographolide in HIV patients. One patient experienced an anaphylactic reaction.142 The oral dose of andrographolide, 15 mg/kg/day for 3 weeks followed by 30 mg/kg/day for a further 3 weeks, was very high compared with normal therapeutic dosages of Andrographis extract. Cases of anaphylactic shock after injection of Andrographis extract have been reported in China.5
Regulatory status in selected countries
Andrographis is not on the UK General Sale List and is not covered by a Commission E monograph.
References
2. Kapoor LD. CRC Handbook of Ayurvedic Medicinal Plants. Boca Raton: CRC Press, 1990. p. 39
4. Bensky D, Gamble A. Chinese Herbal Medicine Materia Medica. Seattle: Eastland Press, 1986. p. 136
7. Matsuda T, Kuroyanagi M, Sugiyama S, et al. Chem Pharm Bull. 1994;42(6):1216–1225.
8. Tang W, Eisenbrand G. Chin Drugs of Plant Origin. Berlin: Springer Verlag, 1992. pp. 97–103
9. Zhu PY, Liu GQ. Chin Trad Herb Drugs. 1984;15:373–376.
10. Dua VK, Ojha VP, Roy R, et al. J Ethnopharmacol. 2004;95(2–3):247–251.
13. Leelarasamee A, Trakulsomboon S, Sittisomwong N. J Med Assoc Thai. 1990;73(6):299–304.
14. Gupta S, Chaudhry MA, Yadava JNS. Int J Crude Drug Res. 1990;28(4):273–283.
15. Limsong J, Benjavongkulchai E, Kuvatanasuchati J. J Ethnopharmacol. 2004;92(2–3):281–289.
16. Wu CM, Cao JL, Zheng MH, et al. J Int Med Res. 2008;36(1):178–186.
17. Rattanachaikunsopon P, Phumkhachorn P. J Biosci Bioeng. 2009;107(5):579–582.
18. Raj RK. Ind J Physiol Pharmacol. 1975;19(1):47–49.
19. Dutta A, Sukul NC. J Helminthol. 1982;56(2):81–84.
20. Najib Nik ARN, Furuta T, Kojima S, et al. J Ethnopharmacol. 1999;64(3):249–254.
21. Siti Najila MJ, Noor Rain A, Mohamad Kamel AG, et al. J Ethnopharmacol. 2002;82(2–3):239–242.
22. Mishra K, Dash AP, Swain BK, et al. Malar J. 2009;8:26.
23. Chang RS, Ding L, Chen GQ, et al. Proc Soc Exp Biol Med. 1991;197(1):59–66.
24. Xu H, Wan M, Loh B, et al. Phytother Res. 1996;10:207–210.
25. Collins RA, Ng TB, Fong WP, et al. Life Sci. 1997;60(23):345–351.
26. Wiart C, Kumar K, Yusof MY, et al. Phytother Res. 2005;19(12):1069–1070.
27. Lin TP, Chen SY, Duh PD, et al. Biol Pharm Bull. 2008;31(11):2018–2023.
28. Chen JX, Xue HJ, Ye WC, et al. Biol Pharm Bull. 2009;32(8):1385–1391.
29. Puri A, Saxena R, Saxena RP, et al. J Nat Prod. 1993;56(7):995–999.
30. Martz W. Toxicon. 1992;30(10):1131–1142.
31. Iruretagoyena MI, Tobar JA, González PA, et al. J Pharmacol Exp Ther. 2005;312(1):366–372.
32. Carretta MD, Alarcón P, Jara E, et al. Eur J Pharmacol. 2009;602(2–3):413–421.
33. Burgos RA, Seguel K, Perez M, et al. Planta Med. 2005;71(5):429–434.
34. Wang W, Wang J, Dong SF, et al. Acta Pharmacol Sin. 2010;31(2):191–201.
35. Panossian A, Davtyan T, Gukassyan N, et al. Phytomedicine. 2002;9(7):598–605.
36. Xu Y, Chen A, Fry S, et al. Int Immunopharmacol. 2007;7(4):515–523.
37. Naik SR, Hole A. Planta Med. 2009;75(8):785–791.
38. Kumar RA, Sridevi K, Kumar NV, et al. J Ethnopharmacol. 2004;92(2–3):291–295.
39. Rajagopal S, Kumar RA, Deevi DS, et al. J Exp Ther Oncol. 2003;3(3):147–158.
40. Varma A, Padh H, Shrivastava N. eCAM, 2009;9.
41. Tan Y, Chiow KH, Huang D, et al. Br J Pharmacol. 2010;159(7):1497–1510.
42. Shen KK, Liu TY, Xu C, et al. Yao Xue Xue Bao. 2009;44(9):973–979.
43. Ji L, Shen K, Liu J, et al. Redox Rep. 2009;14(4):176–184.
44. Manikam SD, Stanslas J. J Pharm Pharmacol. 2009;61(1):69–78.
45. Geethangili M, Rao YK, Fang SH, et al. Phytother Res. 2008;22(10):1336–1341.
46. Pfisterer PH, Rollinger JM, Schyschka L, et al. Planta Med. 2010;76(15):1698–1700.
47. Zhou J, Ong CN, Hur GM, et al. Biochem Pharmacol. 2010;79(9):1242–1250.
48. Sheeja K, Guruvayoorappan C, Kuttan G. Int Immunopharmacol. 2007;7(2):211–221.
49. Zhao F, He EQ, Wang L, et al. J Asian Nat Prod Res. 2008;10(5–6):467–473.
50. Jiang CG, Li JB, Liu FR, et al. Anticancer Res. 2007;27(4B):2439–2447.
51. Lee YC, Lin HH, Hsu CH, et al. Eur J Pharmacol. 2010;632(1–3):23–32.
52. Shi MD, Lin HH, Chiang TA, et al. Chem Biol Interact. 2009;180(3):344–352.
53. Chao HP, Kuo CD, Chiu JH, et al. Planta Med. 2010;76(16):1827–1833.
54. Sheeja K, Kuttan G. Immunopharmacol Immunotoxicol. 2008;30(1):181–194.
55. Sheeja K, Kuttan G. Immunopharmacol Immunotoxicol. 2007;29(1):81–93.
56. Sheeja K, Kuttan G. Integr Cancer Ther. 2007;6(1):66–73.
57. Visen PK, Shukla B, Patnaik GK, et al. J Ethnopharmacol. 1993;40(2):131–136.
59. Handa SS, Sharma A. Indian J Med Res. 1990;92:276–283.
60. Kapil A, Koul IB, Banerjee SK, et al. Biochem Pharmacol. 1993;46(1):182–185.
61. Handa SS, Sharma A. Indian J Med Res. 1990;92:284–292.
62. Singha PK, Roy S, Dey S. J Ethnopharmacol. 2007;111(1):13–21.
63. Choudhury BR, Poddar MK. Methods Find Exp Clin Pharmacol. 1984;6(9):481–485.
64. Choudhury BR, Poddar MK. Methods Find Exp Clin Pharmacol. 1983;5(10):727–730.
65. Trivedi NP, Rawal UM, Patel BP. Integr Cancer Ther. 2007;6(3):271–280.
66. Trivedi NP, Rawal UM, Patel BP. Integr Cancer Ther. 2009;8(2):177–189.
67. Chander R, Srivastava V, Tandon JS. Int J Pharmacog. 1995;33(2):135–138.
68. Choudhury BR, Haque SJ, Poddar MK. Planta Med. 1987;53(2):135–140.
69. Shukla B, Visen PKS, Patnaik GK, et al. Planta Med. 1992;58(2):146–149.
70. Tripathi GS, Tripathi YB. Phytother Res. 1991;5:176–178.
71. Chaudhuri SK. Indian J Exp Biol. 1978;16:830–832.
72. Zhao HY, Fang WY. J Tongji Med Univ. 1990;10(4):212–217.
73. Guo ZL, Zhao HY, Zheng XH. J Tongji Med Univ. 1994;14(1):49–51.
74. Woo AY, Waye MM, Tsui SK, et al. J Pharmacol Exp Ther. 2008;325(1):226–235.
75. Guo ZL, Zhao HY, Zheng XH. J Tongji Med Univ. 1995;15(4):205–208.
76. Wang DW, Zhao HY. Chin Med J. 1994;107(6):464–470.
77. Wang YJ, Wang JT, Fan QX, et al. Cell Res. 2007;17(11):933–941.
78. Zhang CY, Tan BK. Clin Exp Pharmacol Physiol. 1996;23(8):675–678.
79. Zhang CY, Tan BK. J Ethnopharmacol. 1997;56(2):97–101.
80. Yoopan N, Thisoda P, Rangkadilok N, et al. Planta Med. 2007;73(6):503–511.
81. Amroyan E, Gabrielian E, Panossian A, et al. Phytomedicine. 1999;6(1):27–31.
82. Burgos RA, Hidalgo MA, Monsalve J, et al. Planta Med. 2005;71(7):604–608.
83. Hidalgo MA, Romero A, Figueroa J, et al. Br J Pharmacol. 2005;144(5):680–686.
84. Madav S, Tandan SK, Lal J. Fitoterapia. 1996;67(5):452–458.
85. Habtemariam S. Phytother Res. 1998;12:37–40.
86. Shen YC, Chen CF, Chiou WF. Br J Pharmacol. 2002;135(2):399–406.
87. Sheeja K, Shihab PK, Kuttan G. Immunopharmacol Immunotoxicol. 2006;28(1):129–140.
88. Liu J, Wang ZT, Ji LL. Am J Chin Med. 2007;35(2):317–328.
89. Abu-Ghefreh AA, Canatan H, Ezeamuzie CI. Int Immunopharmacol. 2009;9(3):313–318.
90. Bao Z, Guan S, Cheng C, et al. Am J Respir Crit Care Med. 2009;179(8):657–665.
91. Li J, Luo L, Wang X, et al. Cell Mol Immunol. 2009;6(5):381–385.
92. Madav S, Tripathi HC. Tandan. Ind J Pharm Sci. 1995;57(3):121–125.
93. Lin FL, Wu SJ, Lee SC, et al. Phytother Res. 2009;23(7):958–964.
94. Sulaiman MR, Zakaria ZA, Abdul RA, et al. Biol Res Nurs. 2010;11(3):293–301.
95. Mandal SC, Dhara AK, Maiti BC. Phytother Res. 2001;15(3):253–256.
96. Borhanuddin M, Shamsuzzoha M, Hussain AH. Bangladesh Med Res Counc Bull. 1994;20(1):24–26.
97. Husen R, Pihie AH, Nallappan M. J Ethnopharmacol. 2004;95(2–3):205–208.
98. Reyes BA, Bautista ND, Tanquilut NC, et al. J Ethnopharmacol. 2006;105(1–2):196–200.
99. Zhang XF, Tan BK. Clin Exp Pharmacol Physiol. 2000;27(5–6):358–363.
100. Dandu AM, Inamdar NM. Pak J Pharm Sci. 2009;22(1):49–52.
101. Subramanian R, Asmawi MZ, Sadikun A. Acta Biochim Pol. 2008;55(2):391–398.
102. Yu BC, Hung CR, Chen WC, et al. Planta Med. 2003;69(12):1075–1079.
103. Choudhury BR, Poddar MK. Methods Find Exp Clin Pharmacol. 1985;7(12):617–621.
104. Singh P, Srivastava MM, Khemani LD. Ups J Med Sci. 2009;114(3):136–139.
105. Verma N, Vinayak M. Mol Biol Rep. 2008;35(4):535–540.
106. Das S, Gautam N, Dey SK, et al. Appl Physiol Nutr Metab. 2009;34(2):124–135.
107. Das S, Neogy S, Gautam N, et al. Toxicol In Vitro. 2009;23(1):90–98.
108. Sheeja K, Kuttan G. Asian Pac J Cancer Prev. 2006;7(4):609–614.
109. Sheeja K, Kuttan G. Integr Cancer Ther. 2006;5(3):244–251.
110. Al-Bayaty FH, Abdulla MA, Hassan MI, et al. Nat Prod Res. 2011:1–7. [Epub ahead of print]
111. Zheng ZY, Wan YD, He GX. Chin Trad Herb Drugs. 1982;13:417–420.
112. Daodee S, Wangboonskul J, Jarukamjorn K, et al. Pak J Biol Sci. 2007;10(12):2078–2085.
113. Ye L, Wang T, Tang L, et al. J Pharm Sci, 2011. [Epub ahead of print]
114. He X, Li J, Gao H, et al. Chem Pharm Bull (Tokyo). 2003;51(5):586–589.
115. He X, Li J, Gao H, et al. Drug Metab Dispos. 2003;31(8):983–985.
116. Cui L, Qiu F, Wang N, Yao X. Chem Pharm Bull (Tokyo). 2004;52(6):772–775.
117. Cui L, Qiu F, Yao X. Drug Metab Dispos. 2005;33(4):555–562.
119. Akowuah GA, Zhari I, Mariam A, et al. Food Chem Toxicol. 2009;47(9):2321–2326.
120. Chen L, Yu A, Zhuang X, et al. Talanta. 2007;74(1):146–152.
121. Panossian A, Hovhannisyan A, Mamikonyan G, et al. Phytomedicine. 2000;7(5):351–364.
122. Kligler B, Ulbricht C, Basch E, et al. Explore (NY). 2006;2(1):25–29.
123. Coon JT, Ernst E. Planta Med. 2004;70(4):293–298.
124. Poolsup N, Suthisisang C, Prathanturarug S, et al. J Clin Pharm Ther. 2004;29(1):37–45.
125. Carr RR, Nahata MC. Am J Health Syst Pharm. 2006;63(1):33–39.
127. Thamlikitkul V, Dechatiwongse T, Theerapong S, et al. J Med Assoc Thai. 1991;74(10):437–442.
128. Hancke J, Burgos R, Caceres D. Phytother Res. 1995;9:559–562.
129. Caceres DD, Hancke JL, Burgos RZ, et al. Phytomedicine. 1997;4(2):101–104.
130. Caceres DD, Hancke JL, Burgos RA, et al. Phytomedicine. 1999;6(4):217–223.
131. Melchior J, Palm S, Wikman G. Phytomedicine. 1996/7;3(4):315–318.
132. Saxena RC, Singh R, Kumar P, et al. Phytomedicine. 2010;17(3–4):178–185.
133. Kulichenko LL, Kireyeva LV, Malyshkina EN, et al. J Herb Pharmacother. 2003;3(1):77–93.
134. Spasov AA, Ostrovskij OV, Chernikov MV, et al. Phytother Res. 2004;18(1):47–53.
135. Melchior J, Spasov AA, Ostrovskij OV, et al. Phytomedicine. 2000;7(5):341–350.
136. Gabrielian ES, Shukarian AK, Goukasova GI, et al. Phytomedicine. 2002;9(7):589–597.
138. Amaryan G, Astvatsatryan V, Gabrielyan E, et al. Phytomedicine. 2003;10(4):271–285.
139. Panossian A, Hambartsumyan M, Panosyan L, et al. Nitric Oxide. 2003;9(2):103–110.
140. Burgos RA, Hancke JL, Bertoglio JC, et al. Clin Rheumatol. 2009;28(8):931–946.
141. Tang T, Targan SR, Li ZS, et al. Aliment Pharmacol Ther. 2011;33(2):194–202.
142. Calabrese C, Berman SH, Babish JG, et al. Phytother Res. 2000;14(5):333–338.
143. Muangman V, Viseshsindh V, Ratana-Olarn K, et al. J Med Assoc Thai. 1995;78(6):310–313.
144. Mkrtchyan A, Panosyan V, Panossian A, et al. Phytomedicine. 2005;12(6–7):403–409.
145. Zhang YZ, Tang JZ, Zhang YJ. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14(1):28–30, 24, 35.
146. Chaturvedi GN, Tomar GS, Tiwari SK, et al. Ancient Sci Life. 1982;2:208–215.
147. Nakannishi K, Sasaki SI, Kiang AK, et al. Chem Pharm Bull. 1965;13:822.
148. Sithisomwongse N, Pengchata J, Cheewapatana S, et al. Thai J Pharm Sci. 1989;14(2):109–117.
151. Shamsuzzoha M, Rahman MS, Ahmed MM. Bangladesh Med Res Conc Bull. 1979;5(1):14–18.
152. Shamsuzzoha M, Shamsur RM, Mohiuddin AM, et al. Lancet. 1978;2(8095):900.
153. Akbarsha MA, Manivannan B, Hamid KS, et al. Indian J Exp Biol. 1990;28(5):421–426.
154. Akbarsha MA, Manivannan B. Indian J Comp Animal Physiol. 1993;11(2):103–108.
155. Akbarsha MA, Murugaian P. Phytother Res. 2000;14(6):432–435.
156. Burgos RA, Caballero EE, Sanchez NS, et al. J Ethnopharmacol. 1997;58(3):219–224.
157. Allan JJ, Pore MP, Deepak M, et al. Int J Toxicol. 2009;28(4):308–317.
158. Sattayasai J, Srisuwan S, Arkaravichien T, et al. Food Chem Toxicol. 2010;48(7):1934–1938.
159. Ieamworapong C, Kangsadalumpai K, Rojanapo W. Environ Mol Mutagen. 1989;14(suppl 15):93.
160. Hovhannisyan AS, Abrahamyan H, Gabrielyan ES, et al. Phytomedicine. 2006;13(5):318–323.
162. Chatuphonprasert W, Jarukamjorn K, Kondo S, et al. Chem Biol Interact. 2009;182(2–3):233–238.
163. Pekthong D, Martin H, Abadie C, et al. J Ethnopharmacol. 2008;115(3):432–440.
164. Chang KT, Lii CK, Tsai CW, et al. Food Chem Toxicol. 2008;46(3):1079–1088.
165. Yang AJ, Li CC, Lu CY, et al. J Agric Food Chem. 2010;58(3):1993–2000.
166. Jarukamjorn K, Don-in K, Makejaruskul C, et al. J Ethnopharmacol. 2006;105(3):464–467.
167. Pekthong D, Blanchard N, Abadie C, et al. Chem Biol Interact. 2009;179(2–3):247–255.
168. Jarukamjorn K, Kondo S, Chatuphonprasert W, et al. Eur J Pharm Sci. 2010;39(5):394–401.
169. Chien CF, Wu YT, Lee WC, et al. Chem Biol Interact. 2010;184(3):458–465.
170. Panossian A, Kochikian A, Gabrielian E, et al. Phytomedicine. 1999;6(3):157–162.
171. Zoha MS, Hussain AH, Choudhury SA. Bangladesh Med Res Counc Bull. 1989;15(1):34–37.
172. But PPH. Abstr Chin Med. 1988;2(2):247–269.
174. Zhang X, Zhuang L, Li S, et al. Acta Zool Sin. 1985;31(1):52–58.
175. Burgos RA, Aguila MJ, Santiesteban ET, et al. Phytother Res. 2001;15(3):235–239.
Arnica flowers
Synonyms
Leopard’s or wolf’s bane, Mountain tobacco (Engl), Arnicae flos (Lat), Arnikablüten, Berwohlverleih (Ger), Fleurs d’arnica (Fr), Polmonaria di montagna (Ital), Guldblomme (Dan).
Traditional view
Traditionally Arnica has been a popular remedy used externally for sprains, bruises, painful swellings, injuries and wounds. However, topical application was avoided in cases of tender or broken skin. It was often applied diluted and the surface deliberately not covered with bandages. Arnica was also used internally as a stimulant and diuretic, but to a much lesser extent due to its allergenic and irritant effects.1–3
Can be used for
May also be used for
Preparations
‘Arnica oil’ is obtained by macerating 1 part Arnica flowers in 5 parts vegetable oil.
Arnica tincture (1:5, 45% ethanol) is also used topically as a lotion or incorporated into creams or ointments. Recently a supercritical carbon dioxide extract with a high sesquiterpene lactone content has been introduced to the market.6
Technical data
Botany
Arnica, a member of the Compositae or Asteraceae (daisy) family, is a perennial herb with a horizontally growing rhizome. It grows to 30 to 60 cm, the lower leaves are up to 17 cm long, elliptical in shape, 5-veined and form a rosette at the base of the stem. The flower heads number 1 to 3, and are up to 8 cm wide. They consist of peripheral, ligulate flowers, which are yellow-orange and central flowers which are tubular.7,8
Adulteration
Since Arnica montana is a protected species in many countries, it is liable to adulteration with various yellow flowering Compositae plants.2,9–11 Arnica flower does not contain rutin and can therefore be easily distinguished from the most commonly occurring adulterant Heterotheca inuloides (Mexican Arnica).9,10 In addition to this species, the European Pharmacopoeia regards Calendula officinalis as an adulterant.
Shortage of A. montana has led to a more widespread use of related species including A. chamissonis, A. alpina, A. cordifolia, A. sororia, A. fulgens, A. longifolia and A. sachalinensis. The latter three are particularly used for tinctures.12,13 However, only A. montana is listed as official in the British Herbal Pharmacopoeia, British Pharmacopoeia and European Pharmacopoeia.4,14,15A. chamissonis subsp. foliosa is accepted by the German Commission E.16
Key constituents
• Sesquiterpene lactones (SLs) of the pseudoguaianolide type (0.2% to 1.5%), including helenalin and 11alpha, 13-dihydrohelenalin and their ester derivatives17,18
• Triterpenes, including arnidiol19
• Flavonoids (0.4% to 0.6%)17 including quercitin 3-O-glucuronic acid20
• Lignans including pinoresinol21
• Non-toxic pyrrolizidine alkaloids (tussilagine and isotussilagine)22
• Essential oil (0.23% to 0.35%) containing sesquiterpenes, thymol derivatives and other monoterpenes19
• Caffeoylquinic acids (phenolic acids) including 3,5- and 1,5-dicaffeoylquinic acids.23
One study found that the mean total SL levels were higher in the disk florets of Arnica (0.87%) than in the ray florets (0.71%), lower in the flower receptacles (0.35%) and lowest in the stems (0.03%).24 The total SL content increased progressively as the flowers matured, from 0.51% in buds to 0.94% in withered flowers. An investigation of the SL content of Arnica tincture stored for 3 years at 4, 25 and 30°C found a decrease in content of 13%, 32% and 37% respectively.25
Pharmacodynamics
Anti-inflammatory and immune regulatory activity
Like parthenolide from feverfew, helenalin and certain other SLs in Arnica exert potent anti-inflammatory activity in vitro by inhibiting the activation of the transcription factor nuclear factor (NF)-kappaB. This was first demonstrated in 1997 and found to be quite selective, as the activity of four other transcription factors was not affected.26 Follow-up research indicated that helenalin acts by selectively alkylating the p65 subunit of NF-kappaB, rather than by inhibiting the degradation of IkappaB.27 (NF-kappaB, composed of a p50 and a p65 subunit, is retained in an inactive cytoplasmic complex by binding to a third (inhibitory) subunit IkappaB. Degradation of IkappaB results in its activation.)
Inhibition of NF-kappaB has also been demonstrated in vitro for various extracts of Arnica species, including Arnica tincture.28–30 Consistent with inhibition of NF-kappaB activation, an Arnica extract inhibited nitric oxide production and cyclo-oxygenase (COX)-2 activity in activated murine macrophages.31
Other related anti-inflammatory activity has been found for the SLs in Arnica. The transcription factor nuclear factor of activated T cells (NFAT) is also inhibited in vitro by Arnica extract.30 Suppression of matrix metalloproteinase (MMP)-1 and MMP-13 mRNA levels in bovine and human articular chondrocytes was also observed.32 This latter effect may be due to inhibition of activation of the transcription factors activator protein (AP)-1 and NF-kappaB, and implies possible activity in osteoarthritis.
Arnica SLs have also demonstrated additional anti-inflammatory mechanisms in vitro. Two derivatives of 11alpha, 13-dihydrohelenalin inhibited the stimulated release of the pro-inflammatory enzyme neutrophil elastase from human neutrophils.33 Helenalin provoked irreversible inhibition of leukotriene C4 (LTC4) synthase in human platelets and inhibited both 5-lipoxygenase and LTC4 synthase in human granulocytes.34 Polymorphonuclear neutrophil chemotaxis was inhibited at low concentrations, whereas prostaglandin synthetase activity was inhibited at a higher concentration by a series of sesquiterpene lactones, including helenalin and dihydrohelenalin.35
Helenalin also appears to modulate immune activity in vitro. It induced apoptosis in activated CD4+ T cells by triggering the mitochondrial pathway of apoptosis, or otherwise inhibiting their proliferation.36 As previously demonstrated, activation of NFAT (specifically NFATc2) was also found.
Additional results from in vitro studies suggest that the mechanism of the anti-inflammatory activity of Arnica and its constituents also possibly occurs at several other sites:35
• Uncoupling of oxidative phosphorylation in polymorphonuclear neutrophils
• Elevation of cAMP in neutrophils and liver cells
• Inhibition of lysosomal enzymatic activity in neutrophils and liver cells.
Several sesquiterpene lactones, including those from Arnica, have demonstrated anti-inflammatory activity by intraperitoneal injection (2.5 mg/kg/day) in animal models such as carrageenan-induced paw oedema and chronic adjuvant arthritis. The alpha-methylene-gamma-lactone structure was required for inhibitory activity in both models and the 6-hydroxy group of helenalin was required for potency in the former model. Inhibition of writhing reflex (an indication of analgesic activity) was also demonstrated.37
Topical Arnica was shown to be an effective anti-inflammatory in a rat model of acute muscle damage.38 The Arnica gel (containing 200 mg/g of an unspecified Arnica tincture) was either applied by massage or ultrasound, with the latter failing to exert an anti-inflammatory effect above the control group.
Antimicrobial activity
Components of the essential oil of Arnica have demonstrated potent activity against Gram-positive and Gram-negative bacteria and against Candida spp. in vitro.39 Helenalin and helenalin acetate were also active in vitro against Gram-positive bacteria and Proteus vulgaris.40 The polyacetylenes have shown broad-range antimicrobial activity against pathogenic fungi (Trichophyton spp., Microsporum gypseum, Epidermophyton spp.) and bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli) in vitro.41 However, compared with propolis, an Arnica extract showed little antibacterial activity against oral pathogens.42 Some modest activity for Arnica was demonstrated against periodontal bacteria.43 SLs including helenalin were also quite active against Leishmania mexicana with IC50 values of 2 to 4 μM.44
Helenalin and related sesquiterpene lactones have anti-trypanosomal activity (with an IC50 as low as 0.05 μM for helenalin)45 and the pseudoguaianolide SLs show high activity against asexual blood forms of Plasmodium falciparum.46
Staph. aureus is a major udder pathogen causing bovine mastitis. Some pro-inflammatory cytokines, including tumour necrosis factor (TNF)-alpha, enhance its growth. Helenalin markedly reduced the growth of Staph. aureus in the presence of TNF-alpha in bovine mammary epithelial cells.47 Helenalin also reduced Staph. aureus infection and associated inflammation in a murine model of mastitis following ip administration (20 mg/kg).
Antitumour activity
Like parthenolide from feverfew, helenalin has exhibited antitumour activity in vitro, although it has been less extensively studied. In a screening of 21 flavonoids and five SLs from Arnica species, helenalin demonstrated the strongest cytotoxic activity of the SLs when tested on lung carcinoma cells. The flavonoids showed moderate to low cytotoxicity compared with the reference compound.48
Helenalin strongly induced apoptosis in leukaemia Jurkat T cells,49 even if they lacked the CD95 death receptor or over-expressed antiapoptotic proteins.50 It probably acts by inducing a mitochondria-dependent pathway. The cytotoxicity of helenalin against KB tumour cells was decreased by cysteine, but the cytotoxicity of 11alpha,13-dihydrohelenalin was not affected.51
The effect of flavones and flavonols from Arnica spp. on the cytotoxicity of helenalin was investigated in the human lung carcinoma cell line. At non-toxic concentrations, all flavonoids (except kaempferol) significantly reduced helenalin-induced cytotoxicity.52
Arnica SLs inhibited Walker 26 carcinosarcoma and Ehrlich ascites tumour growth in vivo (2.5 mg/kg day for rats and 25 mg/kg/day for mice, both ip).53
Other activity
The stabilisation of the lysosomal membrane in liver cells by SLs was dependent on the alpha-methylene-gamma-lactone structure.35
Helenalin and 11alpha,13-dihydrohelenalin in vitro have inhibited human platelet function via thiol-dependent pathways. The reduction in platelet sulphydryl groups is probably associated with reduced phospholipase A2 activity.54
Helenalin, dihydrohelenalin and epoxyhelenalin (20 mg/kg/day in mice) produced a lowering of serum cholesterol (30%) and serum triglycerides (25%). Thiol-bearing enzymes of lipid synthesis were inhibited by these compounds in vitro, suggesting they alkylate thiol nucleophiles by a Michael-type addition.55
Pharmacokinetics
In vitro studies using pig skin stratum corneum investigated the penetration kinetics of different preparations of Arnica. It was observed that SLs penetrate the skin in amounts sufficient to confer an anti-inflammatory effect.56 SLs in tinctures penetrated better than isolated SLs. A later study by the same group using a similar model suggested that the degree of total penetration depended more on the type of the formulation (ointment being superior to gel) and its SL content, rather than the SL composition or extraction method used.57
Oleic acid and dimethylsulphoxide acted as skin permeation enhancers of SLs from a supercritical carbon dioxide extract of Arnica, as assessed using human stratum corneum and epidermis as an in vitro membrane.58 Using a similar model, 11,13-dihydrohelenalin derivatives were found to exhibit the most permeation from an Arnica tincture.59 A study using an in vitro pig skin membrane observed that a micro-emulsion was a good vehicle for the permeation of helenalin.60
Clinical trials
A 6-week, open, multicentre trial involving 26 men and 53 women with mild to moderate osteoarthritis of the knee concluded that an Arnica gel (containing 50 g/100 g of a 1:20 fresh plant tincture) applied mornings and evenings was an effective treatment, as assessed using the WOMAC scale.61 The overall local adverse event rate of 7.6% included only one allergic reaction.
In a randomised, double blind study over 3 weeks, 204 patients with active osteoarthritis of interphalangeal joints of hands were treated topically with gel preparations of either ibuprofen (5%) or Arnica (50 g/100 g of 1:20 fresh plant tincture). There were no differences between the two groups in terms of pain and hand function improvements, or for any secondary endpoints evaluated. Adverse events reports were comparable and rare.62 The study was planned and performed according to strict international guidelines for studies of multiple sites of osteoarthritis of the fingers: OARSI (Osteoarthritis Research Society International), EMEA (European Agency for the Evaluation of Medicinal Products) guidelines for controlled studies and their statistical evaluation as well as according to good clinical practice rules. This was the very first herbal study looking at this condition to be performed according to these strict guidelines.
A double blind, randomised, controlled trial was conducted in healthy volunteers with laser-induced bruises who were given either a 20% Arnica ointment, a 5% vitamin K preparation, a 1% vitamin K/0.3% retinol mix or white petrolatum (as a placebo) twice a day under occlusion for 2 weeks. Independent assessments of bruise resolution observed that the Arnica ointment was comparable to vitamin K and significantly more effective than the petrolatum (p=0.003) or vitamin K/retinol mix (p=0.01).63
In a double blind study, 570 patients with acute ankle joint distortion were randomised to four treatment groups: Arnica tincture (diluted 10 g tincture/100 mL solution), hydroxyethyl salicylate (HES, 3%), their combination, and a placebo.64 The medication was applied four to five times daily (0.5 mL each time) as a spray. Efficacy was assessed on days 3 to 4 by evaluating pain on motion using a visual analogue scale (VAS). The base preparation for all four treatments contained essential oils and camphor, ensuring that the different study preparations were not discernible by their organoleptic properties. Pain improvement for the combination was highly significant compared with all other groups, and approximately corresponded to the cumulative effect of the single constituents. According to the VAS assessment, the Arnica tincture contributed about one-third to the treatment effect of the combination. Interestingly, no adverse local reaction occurred in the group using the Arnica spray (unlike any other treatment group). The same research group conducted an earlier preliminary clinical study that demonstrated an additive analgesic effect of Arnica and HES. This was a single blind trial involving healthy volunteers following the creation of pain by transcutaneous electrostimulation.65
A placebo-controlled, double blind, randomised clinical study involving 89 patients with pronounced symptoms of chronic venous insufficiency tested the efficacy of an Arnica gel (containing 20% Arnica tincture). After 3 weeks, the symptom of feeling heaviness in the legs (which is strongly associated with peripheral oedema), together with objective measurements of oedema and venous tone, were assessed. The ‘heavy leg’ feeling improved significantly more in the Arnica group compared with placebo. In addition, venous tone and oedema were improved. This efficacy of Arnica for the treatment of symptoms associated with varicose veins is believed to be due to a protective effect against oedema.66
Twelve male volunteers externally applied preparations for muscle ache. Arnica gel was more effective than placebo gel.67
A randomised, double blinded, placebo-controlled study showed no difference between an Arnica gel and its vehicle on post laser treatment burns, with 19 patients acting as their own controls.68
Toxicology and other safety issues
Toxicology
The following LD50 data have been recorded for Arnica extract and its constituents:
An aqueous-ethanolic extract of Arnica did not demonstrate irritating, sensitising or phototoxic activity after topical application to the skin of rabbits or guinea pigs. Minimal irritant activity was observed when the 50% extract was instilled in the eyes of rabbits.69 In contrast, a short chain ether extract of Arnica demonstrated strong dermal sensitising activity in guinea pigs.73
Arnica absolute is an alcohol extract of Arnica concrete, which is obtained from the fresh flowers by an organic solvent extraction process. Arnica absolute (5% to 100%) induced slight patchy to moderate erythema in guinea pigs upon topical application. However, 75% absolute was neither irritating nor phototoxic to the skin of mice. Also, 4% absolute was neither irritating nor sensitising to human volunteers in a maximisation study. The absolute did not induce dermal sensitisation in guinea pigs.69
Arnica tincture was weakly mutagenic in the Ames test in vitro. The mutagenic activity was thought to be due to the flavonoid content and was found to vary, depending on plant origin and method of preparation.74 Helenalin was inactive in this test.75
In male mice, a single injection of helenalin (25 mg/kg) increased serum alanine aminotransferase, lactate dehydrogenase, urea nitrogen and sorbitol dehydrogenase within 6 h. Intraperitoneal injection of helenalin (25 mg/kg/day) increased differential polymorphonuclear leukocyte counts and decreased lymphocyte counts and liver, thymus and spleen weights. Histological evaluation revealed substantial effects on lymphocytes in the thymus, spleen and mesenteric lymph nodes. Multiple helenalin exposure (25 mg/kg/day) also inhibited hepatic microsomal enzyme activities and decreased cytochrome P450 and cytochrome B5 contents.71 Helenalin and helenalin acetate demonstrated dermal sensitising activity in guinea pigs at concentrations of 0.1% to 1.0%.76
The activity of helenalin has been attributed to its ability to alkylate sulphydryl groups.19 In vitro and in vivo studies suggest that the cytotoxicity of helenalin is strongly dependent on hepatic glutathione levels, which can be rapidly depleted by even low concentrations of helenalin.77,78 Helenalin demonstrated chromosome-damaging activity in vitro in Chinese hamster ovarian cells.11 The relevance of this result to the in vivo activity of Arnica has not been established.
A recent review on the safety of Arnica montana and its extract concluded that there are insufficient data to support their safe use in cosmetic formulations.69
Special warnings and precautions
Not for prolonged external application. Individuals with known sensitivity to other members of the Asteraceae family (such as ragweed, daisies and chrysanthemums), or to plants from other families with SLs, chemically related to Arnica (such as the Lauraceae), should use Arnica cautiously.79
Use in pregnancy and lactation
Miscarriage has been reported after overdose of ingested Arnica tincture or infusion11,80 (see Overdosage). Constituents of Arnica have been shown to increase uterine tone and contraction, but Arnica tincture has not demonstrated these actions. Arnifolin (1 to 5 mg/kg) increased the tone and strengthened periodical contractions of the rabbit uterus in situ and 6-O-acetyl-11,13-dihydrohelenalin contracted isolated rat uterus.11,81 Arnica tincture did not increase tone or contraction of isolated pregnant rabbit uteri.11 Similar negative results were demonstrated in the cat after intravenous administration of 0.3 mL of fresh Arnica extract.82
Internal use of Arnica is contraindicated in breastfeeding. Do not apply near the nipple.
Side effects
Cases of allergic or irritant contact dermatitis caused by topical application of Arnica were first reported in 1844.83 A review of the literature up to 1980 found more than 100 cases of contact dermatitis due to sensitisation with Arnica. Most cases were induced by self-treatment with Arnica tincture.13 Reactions have also been reported after the use of other Arnica preparations, including ointments, creams, soaps, lotions and shampoos.79 Arnica ointments and plasters are considered to pose a much lower risk of reaction than other types of applications.12,84 Several case reports of allergic contact dermatitis from the topical use of Arnica, proven by patch testing, have been published since the 1980 review.85–87
Arnica-sensitive individuals are known to cross-react with other Compositae and Lauraceae species.79 SL and epoxythymol-diester constituents of the plant have been proven to be both sensitising agents and to act as allergens.13,88 It is likely that other constituents of Arnica also contribute to the acquired hypersensitivity.89 SL-sensitive individuals tend to develop cross-reactions to chemically related SLs in other species.90 The presence of an alpha-methylene-gamma-lactone group has been shown to be important for cross-reactivity between SLs.12
A 1992 review reported that Arnica contact allergy was recognised in 11% to 75% of patients at dermatology clinics.79 However, several studies published since this review have reported much lower percentages, only up to 1.14%.84,89,91,92 Further to this, a survey of 38 physicians provided data for 18 830 patients who received 42 378 Asteraceae-containing products.93 Adverse reactions to the topical use of Arnica were rare and not serious.
A relatively recent study investigated eight patients with a recent history of Arnica allergy.94 Although all eight had previously exhibited a positive reaction to an Arnica test preparation, this could only be reproduced in five. Moreover, the majority of the patients were not sensitive to Arnica SLs. This demonstrates that the issues governing contact sensitivity to Arnica are complex. One experimental study suggested that the anti-inflammatory and immune modulating properties of Arnica SLs (see above under Pharmacodynamics) reduce their potential for contact sensitisation.95 As examples of this, contact hypersensitivity to Arnica could not be induced in a standard murine model and Arnica tinctures suppressed contact hypersensitivity to the strong sensitiser trinitrochlorobenzene.95
The Commission E advises that prolonged treatment of damaged skin with Arnica can cause oedematous dermatitis with the formation of pustules. Extended use may cause eczema. In treatment involving higher concentrations, primary toxic skin reactions with the formation of vesicles or even necrosis may occur.16 Extensive oral mucosal ulceration was reported in a 48-year-old woman who misused a mouthwash containing 70% ethanol, Arnica tincture and oil of peppermint.96 The preparation should be diluted five times with water, but was instead used neat.
A case of leukaemia-related Sweet’s syndrome, reportedly triggered by topical application of a cream containing 1.5% Arnica, has been published. Pathergy (skin hyper-reactivity) to Arnica was suspected.97
Overdosage
The symptoms of overdose after oral ingestion of Arnica include nausea, vomiting, diarrhoea, dizziness, trembling, increased heart rate, cardiac rhythm disturbances, difficulty with breathing and collapse.98 Arnica poisoning has been observed to cause death due to circulatory paralysis with secondary respiratory arrest.98
A 19-year-old male mistakenly consumed an unknown amount of tea made from the leaves and flowers of Arnica. Two hours later he experienced myalgia, headache and shaking chills. He developed hyperthermia, tachycardia, hypotension and raised serum levels of creatinine, aspartate aminotransferase and alanine aminotransferase. After treatment with fluid and dopamine, he was discharged 6 days later when his symptoms had improved.99 A man experienced stomach cramping and died within 36 h of consuming 70 g of Arnica tincture.11
Miscarriage has been reported after overdose with ingested Arnica tincture or infusion.11,80 A woman in the second month of pregnancy miscarried after a few days when she ingested an infusion of 20 g Arnica flower. Ingestion of three tablespoons of a self-prepared tincture of Arnica flowers led to miscarriage within 24 h.11 Multisystem failure has been reported following the ingestion of Arnica with abortive intent.100
References
1. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;Vol 1. p. 55
5. Sanderson L. How to Make Your Own Herbal Cosmetics. New Canaan: Keats Publishing Inc, 1977. p. 51
6. Bilia AR, Mc Bergonzi, Mazzi G, et al. J Pharm Biomed Anal. 2006;41(2):449–454.
7. Chiej R. The Macdonald Encyclopedia of Medicinal Plants. London: Macdonald, 1984. Entry No. 40
12. Hausen BM, Herrmann HD, Willuhn G. Contact Dermatitis. 1978;4(1):3–10.
13. Hausen BM. Hautarzt. 1980;31(1):10–17.
14. British Pharmacopoeia 2002. CD–ROM. Crown Copyright, 2002.
15. European Pharmacopoeia, 4th ed. Strasbourg: Council of Europe; 2002;(suppl 4.3):672–674.
18. Staneva J, Denkova P, Todorova M, et al. J Pharm Biomed Anal. 2011;54(1):94–99.
20. Ganzera M, Egger C, Zidorn C, et al. Anal Chim Acta. 2008;614(2):196–200.
21. Schmidt TJ, Stausberg S, Raison JV, et al. Nat Prod Res. 2006;20(5):443–453.
23. Lin LZ, Harnly JM. J Agric Food Chem. 2008;56(21):10105–10114.
24. Douglas JA, Smallfield BM, Burgess EJ, et al. Planta Med. 2004;70(2):166–170.
25. Schmidt TJ, Matthiesen U, Willuhn G. Planta Med. 2000;66(7):678–681.
26. Lyss G, Schmidt TJ, Merfort I, et al. Biol Chem. 1997;378(9):951–961.
27. Schaffner W. Biol Chem. 1997;378(9):935.
28. Lyss G, Schmidt TJ, Pahl HL, et al. Pharm Pharmacol Lett. 1999;9(1):5–8.
29. Ekenäs C, Zebrowska A, Schuler B, et al. Planta Med. 2008;74(15):1789–1794.
30. Klaas CA, Wagner G, Laufer S, et al. Planta Med. 2002;68(5):385–391.
31. Verma N, Tripathi SK, Sahu D, et al. Mol Cell Biochem. 2010;336(1–2):127–135.
32. Jäger C, Hrenn A, Zwingmann J, et al. Planta Med. 2009;75(12):1319–1325.
33. Siedle B, Gustavsson L, Johansson S, et al. Biochem Pharmacol. 2003;65(5):897–903.
34. Tornhamre S, Schmidt TJ, Näsman–Glaser B, et al. Biochem Pharmacol. 2001;62(7):903–911.
35. Hall IH, Starnes CO, Lee KH, et al. J Pharm Sci. 1980;69(5):537–543.
36. Berges C, Fuchs D, Opelz G, et al. Mol Immunol. 2009;46(15):2892–2901.
37. Hall IH, Lee KH, Starnes CO, et al. J Pharm Sci. 1979;68(5):537–542.
38. Alfredo PP, Anaruma CA, Pião AC, et al. Ultrasonics. 2009;49(4–5):466–471.
39. Kellner W, Kober W. Arzneimittelforschung. 1955;5:224.
40. Willuhn G, Rottger PM, Quack W. Pharm Ztg. 1982;127:2183–2185.
41. Reisch J, et al. Arzneimittelforschung. 1967;17:816.
42. Koo H, Gomes BP, Rosalen PL, et al. Arch Oral Biol. 2000;45(2):141–148.
43. Iauk L, Lo Bue AM, Milazzo I, et al. Phytother Res. 2003;17(6):599–604.
44. Barrera PA, Jimenez–Ortiz V, Tonn C, et al. J Parasitol. 2008;94(5):1143–1149.
45. Schmidt TJ, Brun R, Willuhn G, et al. Planta Med. 2002;68(8):750–751.
46. Grancois G, Passreiter CM. Phytotherapy Res. 2004;18(2):184–186.
47. Boulanger D, Brouillette E, Jaspar F, et al. Vet Microbiol. 2007;119(2–4):330–338.
48. Woerdenbag HJ, Merfort I, Passreiter CM, et al. Planta Med. 1994;60(5):434–437.
49. Gertsch J, Sticher O, Schmidt T, et al. Biochem Pharmacol. 2003;66(11):2141–2153.
50. Dirsch VM, Stuppner H, Vollmar AM. Cancer Res. 2001;61(15):5817–5823.
51. Heilmann J, Wasescha MR, Schmidt TJ. Bioorg Med Chem. 2001;9(8):2189–2194.
52. Woerdenbag HJ, Merfort I, Schmidt TJ, et al. Phytomedicine. 1995;2(2):127–132.
53. Hall IH, Lee KH, Starnes CO, et al. J Pharm Sci. 1978;67(9):1235–1239.
54. Schroder H, Losche W, Strobach H, et al. Thromb Res. 1990;57(6):839–845.
55. Hall IH, Lee KH, Starnes CO, et al. J Pharm Sci. 1980;69(6):694–697.
56. Wagner S, Suter A, Merfort I. Planta Med. 2004;70(10):897–903.
57. Wagner S, Merfort I. J Pharm Biomed Anal. 2007;43(1):2–38. 3
58. Bergonzi MC, Bilia AR, Casiraghi A, et al. Pharmazie. 2005;60(1):36–38.
59. Tekko IA, Bonner MC, bowen RD, et al. J Pharm Pharmacol. 2006;58(9):1167–1176.
60. Bergamante V, Ceschel GC, Marazzita S, et al. Drug Deliv. 2007;14(7):427–432.
61. Knuesel O, Weber M, Suter A. Adv Ther. 2002;19(5):209–218.
62. Widrig R, Suter A, Saller R, et al. Rheumatol Int. 2007;27(6):585–591.
63. Leu S, Havey J, White LE, et al. Br J Dermatol. 2010;163(3):557–563.
64. Kučera M, Kolar P, Barna M, et al. Pain Res Treat, 2011;7. Article ID 365625
65. Kučera M, Horácek O, Kálal J, et al. Arzneimittelforschung. 2003;53(12):850–856.
67. Moog-Schulze JB. Tijdschr Integr Geneeskunde. 1993;9:105–112.
68. Alonso D, Lazarus MC, Baumann L. Dermatol Surg. 2002;28(8):686–688.
70. Witzel DA, Ivie GW, Dollahite JW. Am J Vet Res. 1976;37(7):859–861.
71. Chapman DE, Roberts GB, Reynolds DJ, et al. Fundam Appl Toxicol. 1988;10(2):302–312.
72. Kim HL. Res Commun Chem Pathol Pharmacol. 1980;28(1):189–192.
73. Hausen BM. Contact Dermatitis. 1978;4(5):308.
74. Goggelmann W, Schimmer O. Prog Clin Biol Res. 1986;206:63–72.
75. MacGregor JT. Food Cosmet Toxicol. 1977;15(3):225–228.
76. Herrmann HD, Willuhn G, Hausen BM. Planta Med. 1978;34(3):299–304.
77. Merrill J, Kim HL, Safe S. Adv Exp Med Biol. 1986;197:891–896.
78. Merrill JC, Kim HL, Safe S, et al. J Toxicol Environ Health. 1988;23(2):159–169.
80. Merdinger O. MMW. 1938;85:1469–1470.
81. Rybalko KS, Trutneva EA, Kibal’chich PN. Aptetschnoje Delo. 1965;14:32–33.
82. Kreitmair H. Mercks Jahresber. 1936;50:106–107.
83. Ochsenheimer J. Osterr Med Wschr. 1844:226–227.
84. Bruynzeel DP, van Ketel WG, Young E, et al. Contact Dermatitis. 1992;27(4):278–279.
85. Hormann HP, Korting HC. Occup Environ Dermatoses. 1994;42(6):246–249.
86. Hormann HP, Korting HC. Phytomedicine. 1995;4:315–317.
87. Pirker C, Moslinger T, Koller DY, et al. Contact Dermatitis. 1992;26(4):217–219.
88. Passreiter CM, Florack M, Willuhn G, et al. Derm Ber Umwelt. 1988;36(3):79–82.
89. Hausen BM. Am J Contact Dermatitis. 1996;7(2):94–99.
91. Paulsen E, Andersen KE, Hausen BM. Contact Dermatitis. 1993;29(1):6–10.
92. Reider N, Komericki P, Hausen BM, et al. Contact Dermatitis. 2001;45(5):269–272.
93. Jeschke E, Ostermann T, Lüke C, et al. Drug Saf. 2009;32(8):691–706.
94. Jocher A, Nist G, Weiss JM, et al. Contact Dermatitis. 2009;61(5):304–306.
95. Lass C, Vocanson M, Wagner S, et al. Exp Dermatol. 2008;17(10):849–857.
96. Moghadam BK, Gier R, Thurlow T. Cutis. 1999;64(2):131–134.
97. Delmonte S, Brusati C, Parodi A, et al. Dermatology. 1998;197(2):195–196.
98. Hänsel R, Haas H. Therapie mit Phytopharmaka. Berlin: Springer-Verlag, 1983. p. 272
99. Topliff A, Grande G. J Toxicol Clin Toxicol. 2000;38(5):518.
100. Ciganda C, Laborde A. J Toxicol Clin Toxicol. 2000;39(3):318–319.
Astragalus
Synonyms
Membranous milk-vetch root (Engl), Astragali Radix (Lat), huang qi (Chin), ogi (Jap), hwanggi (Kor), astragel (Dan).
Traditional view
In traditional Chinese medicine Astragalus is classified as a herb that tonifies the Qi (energy) and Blood (nutrition), hence it is used for postpartum fever and recovery from severe loss of blood. It tonifies the Spleen (being used for fatigue linked to decreased appetite), raises the Yang Qi of the Spleen and Stomach (used for organ prolapse and uterine bleeding) and promotes urination, tissue healing and the discharge of pus. Its properties are sweet and slightly warm.1 Traditionally, Astragalus is taken as the powdered dried root or decoction. This is reflected in the body of pharmacological research on the properties of the Astragalus polysaccharides, which would occur in these dosage forms.
Other applications
Skin care, cosmetics and hair tonics for its healing, nourishing and vasodilating properties.3
Dosage
Duration of use
May be taken long term for most applications but is contraindicated during acute infection.
Summary assessment of safety
No adverse effects are expected if used as recommended. Astragalus might aggravate acute infection.
Technical data
Botany
Astragalus is a member of the Leguminosae (pea) family, the Papilionoideae subfamily, and grouped in the same tribe as the licorice genus.4Astragalus mongholicus is a perennial herb growing 60 to 150 cm high. The leaves are pinnate, with 25 to 37 leaflets, and elliptic. The racemes are axillary, the calyx is 5 mm long and tubular. The root is flexible, long and covered with a tough, wrinkled, yellowish-brown epidermis. The woody interior is of a yellowish-white colour.5
Adulteration
Astragalus propinquus, A. lepsensis, A. aksuensis, A. hoantchy, A. hoantchy subsp. dshimensis, A. lehmannianus, A. sieversianus and A. austrosibiricus have all been identified as adulterants of Astragalus membranaceus, whilst Hedysarum polybotrys is a substitute. Astragaloside IV is normally used as a marker for quality control. In the Japanese Pharmacopoeia 1996, substitutes including A. chrysopterus, A. floridus and A. tongolensis are officially permitted, but these are not accepted in China.6 More recently Glycyrrhiza pallidiflora has been suggested as a common adulterant.7
Key constituents
• Triterpenoid saponins (including astragalosides I to VIII), isoflavonoids (including formononetin), polysaccharides8
• Phytosterols, flavonoids, essential oil, amino acids (gamma-aminobutyric acid, canavanine).9
The important biologically active constituents in Astragalus are the polysaccharides and saponins.8 Depending on the method of preparation, their levels will vary in extracts. Polysaccharides are mainly present in aqueous extracts such as a decoction, and can be isolated from these. Hot water extracts will also contain saponins. Ethanolic extracts will contain only low levels of polysaccharides, with the solubility of Astragalus saponins more or less increasing with the ethanol content used (for an extract made at room temperature).
Several pharmacological studies have investigated ‘Astragalus polysaccharides’. However, the purity and composition of such isolates are likely to vary considerably. One recent Chinese study described isolation by sequential decoction with subsequent removal of proteins and colour.10 Another described the preparation of a decoction of the defatted root, followed by protein removal and preliminary isolation using ethanol precipitation. This crude polysaccharide extract was then further purified by ionexchange, followed by gel filtration.11 As a result, two polysaccharides (APS-I and APS-II) were isolated (consisting of arabinose and glucose, or rhamnose, arabinose and glucose, respectively).
The chemical composition of Astragalus was found to vary with the region of cultivation.12 Moreover, the content of astragalosides in the root bark is up to 74 times that in the xylem, hence thin roots contain higher levels than thick roots.12
Pharmacodynamics
Since polysaccharides are very large molecules with relatively low oral bioavailability, the relevance of any in vitro outcomes is questionable, especially to the oral use of an Astragalus decoction. This also applies for in vivo models where the polysaccharides were administered by injection. Hence, this research is mainly noted in passing and emphasis has been placed on the in vivo studies where the polysaccharides were administered orally. Also it should be kept in mind that the oral polysaccharide research is only relevant to the use of Astragalus as a decoction.
Saponins do have reasonable oral bioavailability, but they are generally changed by gut flora before systemic absorption (see Chapter 2). Hence, the relevance of the saponin in vitro research is also uncertain, but probably higher than for the polysaccharides. Again emphasis has been placed on in vivo studies where the saponins were given orally.
Immune function
Astragalus markedly enhanced the cytotoxicity of natural killer cells,13 potentiated interleukin-2-generated LAK (lymphokine-activated killer) cell cytotoxicity manifested by tumour cell lysis14 and reversed tumour-associated macrophage suppression in urological tumours,15 all in vitro. Using an in vitro local graft-versus-host reaction as a test assay for T-cell function, Astragalus extract restored the reaction in cells taken from 9 of 10 cancer patients.16 Saponins from Astragalus stimulated the natural killer (NK) cell activity of human peripheral blood lymphocytes and restored steroid-inhibited NK cell activity, both in vitro.17 Astragalus saponins reduced nicotinic acetylcholine receptor antibodies in blood cell cultures from myasthenia gravis patients.18
More recently, Astragalus was found in vitro to correct the immunological dysfunction in peripheral dendritic cells taken from children with Henoch–Schönlein purpura19 and modified responses from lipopolysaccharide-stimulated macrophages, reducing cytokines in a dose-dependent manner.20 Astragalosides significantly increased phagocytic activity against Mycobacterium tuberculosis in vitro.21 Peripheral blood mononuclear cells (PBMCs) from 27 children with recurrent tonsillitis were stimulated and cultured with Astragalus in vitro for 48 hours.22 Astragalus improved interferon-gamma output. Similar results were seen for Astragalus in PBMCs from 15 asthma patients, suggesting a reversal of Th2 predominant status.23
Oral doses of Astragalus in mice (200 mg/kg) enhanced several aspects of immunity, including increased thymus weight, potentiation of phagocytic function, superoxide anion production by peritoneal macrophages and proliferation of splenocytes.24 Protective effects on immune suppression in mice were observed after co-administration of Astragalus with a carcinogen. Macrophage numbers and white cell function were raised to the same as or greater than normal levels.25 Co-administration of Astragalus with an antitumour agent resulted in protection against the immunosuppression induced by the antitumour agent.26 Oral administration of Astragalus (5 g/kg/day for 7 days) increased phagocytic activity27 and significantly increased the lymphocyte transformation rate in a suppressed cellular immunity model.28 Astragalus promoted interleukin-2 production in splenic lymphocytes of blood-deficient mice.29 A protective effect of Astragalus extract after oral administration against Japanese encephalitis virus infection in mice was demonstrated. The authors proposed that the protective effect of Astragalus is based on a non-specific mechanism during the early stage of infection, before shifting to antibody production, and that macrophages play an important role by inducing the production of active oxygen.30
Astragaloside IV (50 mg/kg/day, iv) mitigated the development of the characteristic features of ovalbumin-induced chronic experimental asthma in mice.31 Astragalus saponins (400 mg/kg, oral) exerted a protective effect against microbial sepsis in mice.32 An ethanolic extract of Astragalus modified Th1/Th2 cytokine secretion patterns in vitro and in vivo (1.25 g/kg/day, oral for 7 days), favouring Th2 responses.33 In contrast, oral doses of Astragalus attenuated the expression of IgA nephropathy in rats, possibly by diminishing Th2 cytokine responses.34 Astragalus improved the phagocytic activity of peritoneal macrophages in mice.35 The effect of ip injection was higher than for oral doses, but the difference was not significant. The immune response of carp (including phagocytic activity) was enhanced after including Astragalus at 0.5% of diet.36
Astragalus injection (at the equivalent of 6 g/kg/day of root by oral gavage) protected against cyclophosphamide-induced thymus injury in mice.37 In other experiments, injection of Astragalus protected the immune organs of rats with obstructive jaundice,38 and improved haematopoiesis in myelosuppressed mice.39,40 Astragalus extract by injection demonstrated potential as a vaccine adjuvant41 (this is a common property of saponins, including those in Astragalus).42 The injection of an Astragalus decoction (10 g/kg) prevented airway hyper-reactivity in the ovalbumin mouse model of chronic asthma by inhibiting Th2 cytokine release.43
High oral doses of Astragalus decoction given to healthy subjects (15.6 g/day for 20 days) significantly increased serum IgM, IgE and cAMP.44 Two months of oral treatment in people susceptible to the common cold greatly increased levels of IgA and IgG in nasal secretions, and administration for 2 weeks or 2 months enhanced the induction of interferon by peripheral white blood cells.45 Healthy human volunteers treated with Astragalus fresh root tincture (15 mL/day, equivalent to 1.23 g of root for 7 days) demonstrated significant increases in white blood cell CD69 expression (a marker for lymphocyte activation) after 1 and 7 days compared with placebo in a small, double blind trial.46 Patients receiving chronic haemodialysis treated with intravenous Astragalus injection (30 mL two to three times a week for 2 months) exhibited significantly greater levels of interleukin-2 than patients in the control group in a small trial involving 31 people, suggesting a favourable effect on immune function.47 The expression of CD25 on T cells was also significantly increased in a similar clinical study using the same doses after 24 hours (p<0.02), but not after 7 days.48
There is a substantial number of in vitro studies on the immune effects of Astragalus polysaccharides of varying composition. Astragalus polysaccharides in vitro potentiated the immune-mediated antitumour activity of interleukin-249 and the activity of monocytes.50 Astragalus polysaccharides improved the in vitro responses of lymphocytes taken from normal volunteers and cancer patients51 and enhanced the NK cell activity in vitro of blood samples from normal volunteers and systemic lupus erythematosus (SLE) patients.52 The polysaccharide fraction F3 potentiated the lymphokine-activated killer cell-inducing activity of interleukin-2 in blood taken from cancer and AIDS patients.53 Other studies have shown that Astragalus polysaccharides in vitro activated and exerted mitogenic activity on B cells,54,55 promoted neutrophil adhesion to vascular endothelial cells,56 activated macrophages55,57 and dendritic cells,58,59 improved the innate immune response of bladder epithelial cells,60 and induced differentiation of splenic dendritic cells, followed by the shifting of Th2 to Th1 balance, with enhancement of T cell function.61
Injection of Astragalus polysaccharides downregulated the immune response in rats with glomerulonephritis62 and mice with type 1 diabetes,63 augmented antibody responses54 and restored the lymphocyte blastogenic response in older mice.54Astragalus polysaccharides (2 g/kg/day, oral) also prevented the development of type 1 diabetes in NOD mice by correcting the Th1/Th2 cytokine imbalance.64 Oral doses of Astragalus polysaccharides (2 mg per chick) improved immune responses in chickens vaccinated against Newcastle disease65 and at 220 mg/kg of feed acted synergistically with probiotic bacteria in modulating chick immune responses.66 The innate immune response (phagocytic activity) was enhanced in sea cucumbers fed Astragalus polysaccharides (0.3% of feed) and superfine root powder (3.0% of feed).67
A comparison of oral doses of the whole extracts of Astragalus membranaceus, A. membranaceus var. mongholicus and Hedysarum polybotrys (all at 560 mg/kg) and their various chemical fractions (all at 560 mg/kg) demonstrated that the polysaccharide fractions of all three herbs were the most active fractions and exerted similar levels of immune enhancement in two separate assays in mice.68 Another study found that the polysaccharides from four Chinese herbs, including Astragalus, were all active at inducing serum antibodies and promoting T cell proliferation following ip injection in chickens.69 These studies highlight a key paradox of the research into the contribution of polysaccharides to the immune activity of herbs, namely that the polysaccharide fraction from any plant is likely to have immune-enhancing activity. A key question is: why has a particular herb, such as Astragalus, developed a strong traditional reputation as an immune-enhancing herb if such activity is solely due to similarly acting components common to most other plants?
Antiviral activity
The antiviral activity of Astragalus is most likely to be due to increased immunity and possibly enhanced interferon production.2 In support of this, Astragalus demonstrated slight inhibitory activity against adenovirus type 7 in vitro. Natural and recombinant interferon enhanced the inhibitory activity of Astragalus.70 It also promoted the production of interferon by mouse lung against parainfluenza virus type I and Newcastle disease virus in vitro.71 Astragalus exhibited potent hepatitis B surface antigen-inactivating activity in vitro,72 inhibited the activity of murine retroviral reverse transcriptase and human DNA polymerases73 and had a protective effect on cultured beating heart cells infected with coxsackie B2 virus.74
Astragalus extract exhibited activity against herpes simplex virus type 1 in vitro75 and countered the growth-inhibitory effect of cytomegalovirus infection on human cord blood progenitor cells.76 Calycosin-7-O-beta-glucopyranoside, a major isoflavonoid in Astragalus, inhibited coxsackie virus B3 (CVB3) in vitro and was also active (24 mg/kg, oral) in an acute myocarditis model in mice.77
Oral or intranasal administration of Astragalus decoction protected mice from infection with parainfluenza virus type I.24,45,78 Results from a series of in vivo experiments indicated that the effect of Astragalus resembled that of both bronchitis vaccine and the interferon mediator tilorone.45 Astragalus polysaccharides had a weak inhibitory effect on hepatitis B virus in mice,79 but astragaloside IV was quite active against the virus in vitro.80
Astragalus increased the survival rate and improved some abnormal electrophysiological parameters in acute CVB3 viral myocarditis in vivo.81 In vitro and in vivo studies indicate Astragalus may act by decreasing the secondary damage caused by calcium ion influx, thereby improving abnormal myocardial electric activity, as well as inhibiting the replication of CVB3 virus RNA in the myocardium.82,83
Astragalus feeding (2.2 mg/kg/day) increased survival rate, alleviated pathological changes and reduced markers of cardiac damage in mice with CVB3 myocarditis.84 In contrast to other work, an inhibitory effect on virus RNA replication in vivo was not correlated with the induction of beta-interferon,85 but was greater than a calcium channel blocker (verapamil) and a steroidal anti-inflammatory drug (dexamethasone) in vitro.86 Routine therapy combined with oral administration of Astragalus to viral myocarditis patients significantly enhanced immune parameters when compared with patients receiving routine therapy alone.87
Adaptogenic and tonic activity
Addition of Astragalus enhanced growth, metabolism and longevity in cell cultures.2 It lowered oxygen consumption in mitochondria, enhanced tolerance to stress and prolonged the life of human embryonic kidney cells in culture.88 Two isomers of the molecule HDTIC extracted from Astragalus extended the lifespan of human fetal lung diploid fibroblasts in vitro by slowing telomere shortening, reducing DNA damage and improving DNA repair.89 They also delayed replicative senescence in the same model.90 The telomerase activator cycloastragenol (TA-65, found at low levels in Astragalus) elongated short telomeres in mouse embryonic fibroblasts and improved some healthspan indicators in adult/old mice without increasing cancer incidence.91 Such observations have been interpreted by some as suggestive that Astragalus or these components will promote human longevity, but this is clearly premature on current limited evidence.
Administration of Astragalus over 2 weeks to mice markedly increased plasma cAMP.92 Astragalus decoction improved learning performance in animal maze tests93 and improved memory in two models (50 g/kg/day for 7 days, oral).94 Administration over 15 days inhibited field search behaviour, decreased spontaneous activity and increased sleep time.93 Decoction of Astragalus improved endurance in mice and increased weight gain compared with controls.1 A mixture of ginseng and Astragalus demonstrated antifatigue activity in mice. This activity was partly due to an improvement of energy metabolism.95 Astragalus extract (400 mg/kg/day) countered the adverse effects of repeated restraint stress in rats, improving spatial learning and memory and reducing anxiety.96 Oral administration of Astragalus increased the turnover of proteins in serum and liver in animals treated daily for 10 days.97 Astragalus lowered collagen content in the aorta and lung of old rats to near levels found in young animals98 and improved the density of M-cholinergic brain receptors.99 In early research, oral administration of Astragalus increased plasma cAMP in healthy subjects.100
Cardiovascular and haemorheological activity
Astragalus saponins demonstrated a positive inotropic action on isolated heart and decreased the resting potential of cultured myocardial cells, suggesting an inotropic effect exerted through modulation of Na+,K+-ATPase.101 Astragalosides reduced intracellular calcium overload in rat cardiomyocytes and enhanced free radical removal, both suggestive of a protective effect against myocardial injury.102 Astragaloside IV improved post-ischaemic heart function and ameliorated reperfusion arrhythmias in rat hearts in vitro,103 and improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes.34 Astragalus extract prevented daunorubicin-induced apoptosis of cultured cardiomyocytes by decreasing free radical release104 and demonstrated a cardiotonic effect in isolated beating rabbit atria.105 Astragaloside IV improved homocysteine-induced endothelial dysfunction in rat aortic rings via antioxidant activity.106
Oral administration of aqueous extract of Astragalus countered the rise in blood pressure and plasma renin activity in a hypertensive model.107 Intragastric administration of Astragalus produced a hypotensive effect in another experimental model.108 Gamma-aminobutyric acid was isolated as a potential hypotensive constituent.109 Oral Astragalus improved impaired endothelial-dependent vasodilation in obese rats.110,111 Astragaloside IV has exhibited vasodilatory effects in vivo,112,113 as has the whole extract.114
Improvement of cardiac function has been demonstrated for Astragalus or its isolates in a number of in vivo models. In several such studies Astragalus or Astragalus components were given by injection. Examples of oral dose studies include the inhibitory effect of Astragalus (5, 10 and 20 g/kg/day) on left ventricular hypertrophy induced by pressure overload in rats.115 Astragalus (20 g/kg, oral) also counted abnormal cardiac function in rats with pressure overload-induced heart failure, and at oral doses of 3.3 or 10 g/kg/day improved cardiac function in doxorubicin-injured rat hearts.116 All these doses are rather high. Astragalosides have also demonstrated cardioprotective activity in vivo following administration by injection,103,117 as has Astragalus injection.118
Cardiac output increased in 20 patients with angina pectoris after 2 weeks of treatment with Astragalus.119 Astragalus strengthened left ventricular function and had an anti-OFR (oxygen free radical) effect in acute myocardial infarction patients compared with controls. The decrease in the pre-ejection period:left ventricular ejection time ratio was closely correlated with the increase in superoxide dismutase activity of red blood cells and the decrease in lipid peroxidation of plasma. This anti-OFR activity of Astragalus may be one of the mechanisms behind its cardiotonic activity.120
Astragalus extract demonstrated a protective effect on erythrocyte deformability in vitro for blood taken from normal subjects and patients with SLE.121 Astragalus significantly enriched the blood, as measured by improvement in haemorheological indices,122 and, in a ‘blood stagnation’ experimental model, decreased whole-blood specific viscosity and increased plasma specific viscosity.123
Hepatoprotective activity
Astragalus saponins were protective against chemically induced liver injury in vitro and in vivo.124 Oral doses of Astragalus polysaccharides (200 mg/kg/day) also demonstrated hepatoprotective activity in rats.125 Astragalus extracts exhibited hepatoprotective activity and increased the activity of hepatic lysozymes, tissue dehydrogenase and liver glycogen.126–128 Oral doses of an extract combination of Astragalus and Paeonia lactiflora (60, 120 and 240 mg/day) protected against immunological liver injury in mice.129 The total flavonoids of Astragalus (100 mg/kg, oral) protected against paracetamol liver damage in mice.130,131
Some studies suggest that Astragalus in combination might protect against the hepatic fibrosis associated with chronic liver damage. Oral doses of the Astragalus and Paeonia combination mentioned above (80 and 160 mg/kg/day) reduced liver damage and fibrosis in rats with carbon tetrachloride-induced liver injury and decreased the elevation of tumour growth factor (TGF)-beta1.132 Oral doses of a combination of Astragalus and Salvia miltiorrhiza (60, 120 and 240 mg/kg/day) also demonstrated antifibrotic activity in a similar model.133
Renal activity
A 2009 systematic review examined the published in vivo studies for Astragalus in early diabetic nephropathy in rats.134 Of 41 articles identified, 13 reports that fulfilled the inclusion criteria were reviewed. Meta-analysis revealed that Astragalus extract (orally or by injection depending on the study, but mainly the former) significantly reduced fasting blood glucose, glomerular filtration rate, urinary albumin excretion and thickness of the glomerular basement membrane (p values ranging from <0.00001 to 0.03).
Several other in vivo studies have demonstrated protective effects on renal function in a variety of models, including IgA nephropathy.135–140 Doses used were often relatively high and the route of administration was not always specified.
See also the Clinical trials section for diuretic activity in a human study.
Antidiabetic activity
In addition to the studies included in the meta-analysis above, the effects of Astragalus or its components have also been examined in several diabetic models. For example, oral doses of Astragalus polysaccharides (400 to 2000 mg/day) alleviated glucose toxicity and restored glucose homeostasis,141 lowered blood glucose and reduced insulin resistance142–144 and reduced cardiomyopathy145 in various diabetic models. Other components have also been shown to have antidiabetic activity, including the isoflavonoids146 and astragaloside IV, which protected against diabetic neuropathy in rats (3, 6 and 12 mg/kg twice a day, oral).147
Anticancer activity
Astragalus saponins,148–150 Astragalus injection151,152 and an Astragalus extract153 have all shown growth inhibitory activity against a variety of tumour cell lines in vitro. Mechanisms included promotion of apoptosis,148,149,153 growth inhibition150 and downregulation of Akt phosphorylation.151
Astragalus saponins (100 and 200 mg/kg/day for 20 days, oral) reduced tumour volume in a mice xenograft model of colon cancer, demonstrating activity comparable to 5-fluorouracil.150 Various fractions of Astragalus polysaccharides (prepared by decoction followed by stepwise ethanol precipitation) demonstrated weak cytotoxic activity in vitro, but promoted immune response in a mouse cancer model after ip injection.154 Astragalus extracts demonstrated chemopreventative activity in mice (10, 20 and 40 mg/kg/day, oral)155 and rats (90 and 180 mg/kg/day, oral).156
Antioxidant and anti-inflammatory activities
Astragalus flavonoids demonstrated a protective effect on mammalian cell damage caused by the hydroxyl radical, inhibited lipid peroxides and increased superoxide dismutase activity in vitro.157 Three Astragalus saponins demonstrated superoxide anion scavenging activity in vitro.158 Astragalus extract reduced free radical-mediated injury to renal tubules in rabbits (2.4 g/kg, iv).159 Astragalus injection reduced measures of oxidative stress and inflammation in a controlled trial involving 60 haemodialysis patients.160
An aqueous extract of Astragalus demonstrated a broad anti-inflammatory effect on human amnion cells in vitro.161 Astragalosides inhibited the formation of advanced glycation endproducts during the incubation of bovine serum albumin with ribose.162
Astragalus polysaccharides (250, 500 and 1000 mg/kg/day, oral) were anti-inflammatory in rats with adjuvant arthritis163 and the 8:1 dried aqueous extract of Astragalus (30 and 100 mg/kg/day, oral) reduced the expression of various inflammatory mediators in zymosan air-pouch mice.164 Astragalus decoction (100 mg/kg/day, oral) also inhibited the development of experimental atopic dermatitis in mice.165 High oral doses of an aqueous extract of Astragalus either before (2 and 4 g/kg/day) or after (4 and 8 g/kg/day) hapten provocation reduced experimental colitis in rats.166 Oral (4 and 8 g/kg/day) or intracolonic (200 and 800 mg/kg/day) doses of this Astragalus extract also demonstrated similar therapeutic activity in the same colitis model, but by modulation of different colonic cytokines, depending on the mode of administration.167
Neurological activity
Astragalus extract,168 astragaloside IV169 and Astragalus isoflavones170 exhibited neuroprotective activity in various in vitro models. Peripheral nerve regeneration was demonstrated in vitro and in vivo (in rats via local administration) for an Astragalus extract.171 An Astragalus extract (150 and 300 mg/kg, oral) also protected against pentylenetetrazole-induced seizures in mice.172
Other activity
Astragalus inhibited aldose reductase,173 promoted the replication of hepatic DNA,174 inhibited mitochondrial oxygen consumption caused by lipid peroxidation175 and stimulated the motility of human sperm,176 all in vitro. Incubating sperm from infertile men with Astragalus decoction significantly enhanced motility.177,178 Intraperitoneal injection of Astragalus extract into rats made infertile by cadmium resulted in significant increases in sperm counts and reduced sperm malformation, compared with untreated controls.179
A methanolic extract of Astragalus inhibited the growth of the human intestinal bacterium Clostridium perfringens in vitro.180 Oral administration of Astragalus normalised the imbalance in intestinal flora in an experimental model of senility.181
Oral administration of a concentrated solution of Astragalus strengthened small intestine movement and muscle tonus, especially in the jejunum.182 This activity supports its traditional use in organ prolapse.
Astragaloside IV (3, 10 and 30 mg/kg/day, oral) exerted gastroprotective effects in rats with ethanol-induced gastric mucosal damage.183
Pharmacokinetics
A pharmacokinetic investigation of astragaloside IV administered by intravenous injection to rats and dogs found highest concentrations in the lung and liver.184 The compound was relatively quickly eliminated and does not appear to cross the blood-brain barrier.
The bioavailabilities of the flavonoids in Astragalus decoction were examined using various models, namely a computational chemistry prediction method, a Caco-2 cell monolayer and an improved rat everted gut sac model.185 The computational model suggested that 26 compounds in Astragalus, including 12 flavonoids, were potentially bioavailable. The two in vitro models found that 21 compounds were absorbed, including flavonoid and isoflavonoid aglycones and some of their glycosides and metabolites. Following a multiple-dose study in a healthy male volunteer who ingested 60 g of the crude herb via decoction twice a day for 5 days, several of the same compounds were also detected in his urine, but mainly the metabolites (as might be expected for urine).
Clinical trials
Immune function
In an open, randomised clinical trial, 115 patients with leucopenia received a high dose of a concentrated Astragalus preparation (equivalent to 30 g/day of Astragalus) or a low dose (equivalent to 10 g/day) over a period of 8 weeks. There was a significant rise of average white blood cell (WBC) counts in both groups after treatment (p<0.001). The average WBC count for the high-dose group was significantly higher than for the low-dose group (p<0.05). On the basis of these findings, the author suggested that Astragalus is an effective treatment for leucopenia and increasing the dosage could enhance its effectiveness.186 In an open study, 1000 volunteers received Astragalus either orally, as a nasal spray or in a compound formula. A prophylactic effect for the common cold was observed, as evidenced by decreased incidence and shortened duration of infection.45
A combination of Astragalus (320 mg/day of an 18:1 extract containing 40% polysaccharides) and calcium-aluminium silicate as a mineral carrier and possible synergist was investigated in a 6-week double blind, placebo-controlled clinical trial involving 48 patients with seasonal allergic rhinitis.187 Both the attending physicians and the patients judged the active treatment as more efficacious than placebo (p=0.003 and p=0.026, respectively). However, no statistically significant differences were detected between the groups for serum IgE, IgG and nasal eosinophils, although there was a trend to lower IgG for Astragalus plus minerals (p=0.18). In a post hoc analysis of 21 patients with weed pollen allergy, Astragalus plus minerals significantly improved total symptom score (p=0.022) and quality of life (p<0.001) compared with placebo. All adverse events were mild and not considered to be connected to the active treatment.
Astragalus in combination with other herbs has also been investigated for clinical effects on immune function. In a double blind, placebo-controlled trial, 85 children aged 7 to 15 years with asthma were randomly assigned to receive either an oral herbal formula (0.619 g/day, comprising equal weights of dried aqueous extracts from Astragalus, Cordyceps, Stemona, Fritillaria and Scutellaria baicalensis), or placebo for 6 months.188 There was no significant difference recorded for any of the trial endpoints between the active treatment and placebo groups. The impact of Compound Astragalus Recipe was investigated in 60 patients with myasthenia gravis in a controlled trial.189 While clinical outcomes were similar in the herbal and control groups, the herbal treatment significantly lowered the CD4+/CD8+ ratio (p<0.05).
Astragalus injection has demonstrated a range of effects on immune function in open label clinical trials. For example, it improved cellular immunity in patients with serious abdominal trauma,190 promoted recovery of haematopoietic function in patients with chronic aplastic anaemia,191 improved immune function in patients with congestive heart failure192 and enhanced the efficacy of conventional treatment for SLE.193
Antiviral activity
Administration of Astragalus to a large number of patients with chronic viral hepatitis resulted in a success rate of 70% in an open trial. In most cases, elevated serum ALT levels returned to normal after 1 to 2 months.194
In a double blind clinical trial, 235 patients with typical chronic cervicitis (associated with viral infection) received one of the following treatments applied locally by gauze: recombinant interferon-alpha1 (at 5 or 10 mg), combined interferon (5 mg) and Astragalus (0.5 mL of a 1:1 extract), or Astragalus alone (0.5 mL of a 1:1 extract). These treatments were applied twice per week for 3 weeks. The Astragalus plus interferon group showed a similar improvement to the higher dose interferon group, with approximately 60% of patients demonstrating complete resolution or marked improvement. Only 8% of patients treated with Astragalus alone exhibited marked improvement and no patients were completely cured. These results suggest that Astragalus acted synergistically with interferon therapy.195 An earlier double blind trial showed a similar result for 164 patients with cervical erosion associated with herpes simplex virus infection.196 In 106 patients with herpes simplex keratitis, those randomly assigned to Astragalus treatment (dose and route not specified in the English abstract) exhibited significant improvement in the rebalancing of Th1/Th2 cytokines, an effect not observed in the comparative control group receiving ribavirin.197
An infused formulation of six herbs with Astragalus as the main component was significantly better than a control treatment (including silymarin) in terms of clinical improvement and greater negative seroconversions in an open label trial involving 208 patients with chronic viral hepatitis B.198
A formulation containing extracts of Astragalus combined with Glycyrrhiza glabra, Artemisia capillaris, Morus alba and Carthamus tinctorius was investigated in HIV positive patients in Thailand. In an open label study, 28 HIV-1 infected adults (CD4 count >200 cells/mm3 and HIV-1 RNA >20 000 copies/mL) received 5 g/day of the combination plus sulfamethozaxole and trimethoprim for 12 weeks.199 Up to 36% of patients demonstrated a reduction in plasma HIV-1 RNA of more than 0.5 log during the trial, but CD4 counts were unchanged. In a subsequent randomised, double blind, 24-week clinical trial, the efficacy of the herbal combination (7.5 g/day) given with zidovudine and zalcitabine was compared against the two drugs plus a herbal placebo in 60 HIV-1 positive patients.200 The decline in HIV RNA was significantly greater in the group receiving the herbal combination (p<0.001) and CD4 cell counts were higher compared with baseline (p<0.05), versus no change in the control group. Serious adverse events were not observed.
A 2004 Cochrane review of herbal medicines for viral myocarditis found 40 randomised trials involving 3448 patients.201 Twenty-five different herbal treatments were tested in the included trials. Astragalus was given as a single treatment in 10 trials (oral doses in three, by injection in seven) and as part of a herbal combination in another 13 trials (all doses oral). Analysis of the 10 Astragalus-only trials (plus one where the formulation was mainly Astragalus) found Astragalus significantly improved premature beat, cardiac output, ejection fraction and some levels of myocardial enzymes (indicating cardiac damage). Overall the authors stressed the low methodological quality, but suggested some herbal medicines such as Astragalus deserve further examination in this context in rigorous trials.
Cardiovascular conditions (other than viral myocarditis)
In a comparative trial, 92 patients suffering from ischaemic heart disease were treated with Astragalus, Salvia miltiorrhiza or the anti-anginal drug nifedipine. Results were superior for the Astragalus-treated group, as demonstrated by marked relief from angina pectoris and improvement in several objective clinical parameters. Treatment of ischaemic heart disease by Astragalus was significantly more effective compared with the control group (p<0.05).202 In an open label trial in 20 patients with angina pectoris, Astragalus (60 g/day, presumably by decoction) significantly improved cardiac output by 16.9% after 2 weeks (p<0.01), with no improvement of left ventricular diastolic function.203 Unlike digitalis, the herb did not inhibit ATP activity.
Forty-five patients with chronic heart failure (defined in TCM (traditional Chinese medicine) terms by Xin-qi or Xin-yang deficiency) were randomised to receive conventional medicine or conventional medicine plus 4.5 g/day (oral) of an Astragalus granule containing only Astragalus.204 While improvements from baseline in TNF-alpha, left ventricular ejection fraction (LVEF) and walking distance were observed in both groups, they were all significantly higher in the group receiving Astragalus (p<0.05).
Ninety chronic heart failure patients (TCM diagnosis as above) were randomly assigned to receive perindopril (4 mg/day) and one of three different doses of Astragalus granule (15, 9 or 4.5 g/day).205 Clinical improvements, including for LVEF, walking distance and quality of life, were greatest in the highest dose group (p<0.01).
Ninety-four patients with vascular disease secondary to type 2 diabetes mellitus were randomly assigned to either Astragalus with saponins from Panax notoginseng, or simvastatin, in an open label trial. Blood cholesterol and triglyceride levels and a measure of vascular inflammation (MMP-9) improved similarly in the two groups.206
Astragalus injection has demonstrated a range of effects in cardiac patients in clinical trials. For example, it improved cardiac function and haemodynamics in children with tetralogy of Fallot,207 improved heart function parameters in patients with congestive heart failure208,209 and acute myocardial infarction,210 and decreased inflammatory cytokine production in patients undergoing heart valve replacement.211 Injected doses typically contained high levels of the crude herb (up to 80 g).
Kidney disease
A randomised, double blind, placebo-controlled crossover study in 12 healthy men assessed the impact of a single oral dose of Astragalus (0.3 g/kg of a dried 4:1 aqueous extract) on diuresis.212 Compared with placebo, Astragalus markedly increased urinary sodium excretion, fractional sodium excretion and urinary excretion of chloride during the first 4 hours. The authors concluded that Astragalus induces marked natriuresis in healthy men and attributed this to enhanced renal responses to endogenous ANP (atrial natriuretic peptide).
A US group of doctors described two separate cases (published 3 years apart) of remission of idiopathic membranous nephropathy (IMN, probably autoimmune in origin) after therapy with Astragalus. The first case described a 77-year-old woman with nephrotic syndrome secondary to IMN who was largely unresponsive to conventional treatments.213 After beginning Astragalus (15 g/day as part of the formulation Shen-Yan Siwei Pian) there was a marked decrease in proteinuria. Nephrotic syndrome recurred after a temporary cessation of the formulation, with complete remission after its reintroduction. Remission persisted even after stopping the herbal treatment. The second case was a 63-year-old man with nephrotic syndrome due to IMN.214 In addition to conventional treatments (which had not resolved his proteinuria) he took Astragalus (15 g/day herb equivalent of a 4:1 extract – presumably aqueous) for around 12 months, after which he experienced complete remission of nephrotic syndrome.
In an open clinical trial, a combination of Arctium lappa fruit and tincture of Astragalus orally for 3 months with losartan was compared with losartan alone in 54 patients with diabetic nephropathy. The herbal combination reduced hyperlipidaemia, proteinuria and postprandial hyperglycaemia significantly compared with losartan alone.215 Patients with diabetic nephropathy were randomly assigned to take simvastatin or a combination of Astragalus and Panax saponins. Both treatments reduced hyperlipidaemia and various markers of renal damage.206 In an open label trial, 21 patients with type 2 diabetes and microalbuminuria received 150 mL four times a day of an unspecified Astragalus and Ligusticum decoction for 6 months.216 The herbal combination improved both urinary albumin excretion and endothelial dysfunction.
Astragalus injection improved renal tubular function in patients with IgA nephropathy217 and primary nephrotic syndrome.218
Cancer therapy
A meta-analysis of 34 randomised clinical trials involving patients with non-small-cell lung cancer treated with platinum-based chemotherapy and Astragalus-based Chinese herbs suggested a benefit from the combination.219 Most trials involved formulas featuring Astragalus, but two were of Astragalus alone. The herbs were administered by injection in around one-third of the trials. Twelve trials measuring such outcomes reported significantly lower mortality rates after 12 months when Astragalus was combined with chemotherapy (risk ratio 0.67). Nine studies reported significantly lower mortality rates after 24 months when Astragalus was combined with chemotherapy (risk ratio 0.73). Most of the studies included were of low methodological quality. A Cochrane review indentified four relevant trials where a decoction of Astragalus and a formulation featuring Astragalus was combined with chemotherapy regimens in patients with colorectal cancer.220 Chemotherapy-induced nausea, vomiting and leucopenia were all decreased by concomitant administration of Astragalus decoction, and immune function was improved. The trials were of low quality, suggesting larger, more rigorous trials are needed to confirm these results.
A systematic review and meta-analysis of Chinese herbs in the treatment of hepatocellular carcinoma found that formulations containing Astragalus had a larger treatment effect than the pooled broad estimate (odds ratio 1.35, p=0.048).221 Products containing Astragalus significantly improved 12-month survival rates (odds ratio 1.28, p<0.0001). While there were questions over trial design, the authors considered this to be compelling evidence that needs to be evaluated in high-quality clinical trials. The herbal treatments were often administered by injection.
In an open label trial, 20 children with acute leukaemia in remission received Astragalus (90 g/day, mode of administration not specified in the English abstract) plus chemotherapy for 1 month, while another 24 in the control group received chemotherapy alone.222 The herbal treatment increased dendritic cell induction of peripheral mononuclear cells and enhanced antigen-presenting ability.
Other conditions
In a double blind, placebo-controlled clinical trial involving 507 elderly people, oral administration of Astragalus in combination with Polygonum multiflorum and Salvia miltiorrhiza demonstrated significant antiageing effects. Improvements were noted in vigour, strength, vision, cellular immunity and serum lipofuscin levels. The total effective rate was 76.6% compared with 34.5% for placebo (p<0.001).223
Eighty-four patients with liver cirrhosis and portal hypertension were randomly assigned to receive either conventional treatment or a combination of Astragalus and Salvia miltiorrhiza (dan shen) for 3 months in an open label trial.224 There were significant improvements in haemodynamic measures and indices of liver fibrosis in the group treated with the herbal combination compared with the control group.
A 4-week randomised, double blind, placebo-controlled clinical trial was conducted with 36 adults with chronic fatigue (but not necessarily chronic fatigue syndrome).225 The trial participants were divided into a control group (receiving a ‘placebo’ Chinese herbal formulation known as Hyangsapyunweesan (in Korean), 3 g/day) or 3 or 6 g/day of a 3.3:1 concentrated aqueous extract from equal parts of Astragalus and dan shen. This combination significantly decreased subjective fatigue severity scores compared with the control group (p<0.05).
Toxicology and other safety data
Toxicology
No adverse effects were observed within 48 hours after oral administration of Astragalus at doses of 75 and 100 g/kg. The ip LD50 of Astragalus has been reported to be 40 g/kg in mice. However, ip injection of 50 g/kg elicited no significant toxic reactions in mice in another study.2 A 3-month subchronic toxicity evaluation of Astragalus given by injection found no distinct toxicity at doses up to 39.9 g/kg in rats and 19.95 g/kg in dogs.226
The aqueous extract of Astragalus (1.25 mg/mL) modestly increased the incidence (16%) of aberrant cells in the Ames test in vitro.227 In contrast, aqueous-methanolic extract of Astragalus showed no mutagenic effects228 and an aqueous extract demonstrated antimutagenic activity in vitro.229 A Chinese herbal formula (Man-Shen-Ling) that contains Astragalus did not exhibit toxic, mutagenic, teratogenic or carcinogenic effects in acute and chronic toxicity tests in animal models.230