Chapter 1 How does an embryo form?
Human development begins when a spermatozoon fertilizes an oocyte. By definition, an embryo comprises the tissues formed once mitosis of an ovum (a fertilized oocyte) begins; thus even at the two-cell stage it is an embryo. These few cells multiply in number over an 8-week period into a fetus, by which time it will consist of many millions of cells. During the first two weeks (the pre-embryonic period) the embryo moves through the uterine tube to the uterus where it will implant. The differentiation that establishes the organ systems takes place between 3 and 8 weeks in the first 8 weeks following fertilization (the embryonic period). The period of time from the end of week 8 to full term (38 weeks) is a phase of growth and enlargement (the fetal period). The crucial phase during which there is potential for malformation is in the first 8 weeks, and this period is when the embryo is most vulnerable to environmental agents such as viruses and other teratogens. Table 1.1 summarizes the major events of the prenatal stages of development. The stages of the formation of an embryo are often described in relation to weeks of development.
Table 1.1 Stages of development before birth
| Time period | Stage | Main events |
|---|---|---|
| Conception to week 2 | Pre-embryonic period | |
| 2nd to 8th week | Embryonic period | |
| 9th week to birth | Fetal period |
The 1st week—Fertilization and formation of the blastocyst
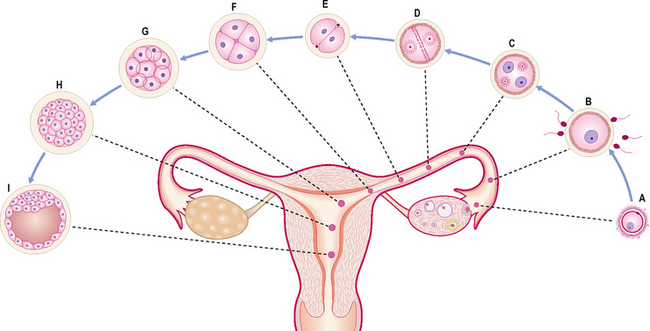
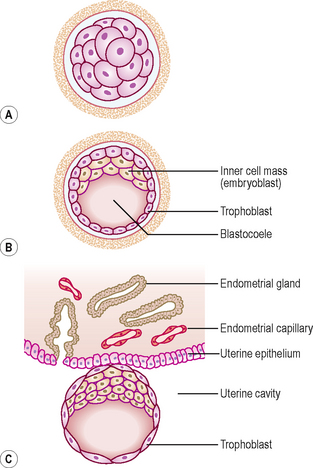
A fertilized ovum has a diploid number of chromosomes and once the second meiotic division has been completed, the stage of cleavage can begin. This consists of a series of rapid mitotic cell divisions in which the ovum divides over a period of about 3 days, resulting in the so-called 16-cell-stage embryo (Figs 1.1 and 1.2A). Each cell is known as a blastomere. After each cleavage division, whilst the number of cells increases, the size of each cell diminishes. The solid sphere of cells that forms is known as a morula, because it was thought to resemble a mulberry! Each of these new daughter cells is, at this stage, pluripotential. In other words, each daughter cell has the potential to differentiate into cells of any lineage.
The morula soon shows signs of further differentiation. Cavities appear within the centre of the sphere of cells, forming a blastocyst, the cavity itself being the blastocoele (Fig. 1.2B, C). Once this stage has been reached the outer layer of the blastocyst soon thins to single-cell thickness to become the trophoblast, enclosing the enlarging fluid-filled blastocyst cavity. The central group of cells move to one pole of the blastocyst (the embryonic pole) to form the inner cell mass from which the whole embryo itself will form. The trophoblast contributes to the fetal component of the placenta (Fig. 1.3).
The process of morula and blastocyst formation occurs whilst the sphere of dividing cells is in transit along the uterine tube (Fig. 1.1). Fertilization takes place in the ampulla of the uterine tube, approximately 12–24 hours after ovulation. The first mitotic division of cleavage will be completed by the time that the two-cell stage embryo reaches the middle of the tube, at about 30 hours post-fertilization. By 3 days the morula of 12–16 cells will have reached the junction of the uterine tube and the uterus. By 4–5 days the fully formed blastocyst reaches the uterine lumen in preparation for implantation, which occurs a day later.
The 2nd week—Implantation and formation of bilaminar embryonic disc
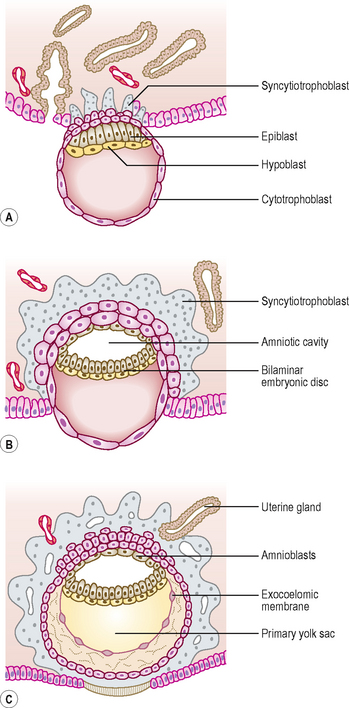
At this stage the embryo is partly implanted in the endometrium. The implantation process initiates the decidual reaction or decidualization in the uterine stroma, the cells of which contribute the maternal component of the placenta. The trophoblast begins to differentiate: its inner part becomes a single layer of cells, hence its name the cytotrophoblast (Fig. 1.3A). The outer layer is more extensive and is the invasive layer. It is a syncytium, and at this stage, although it has invaded the endometrium, it has not invaded endometrial blood vessels. It is known as the syncytiotrophoblast. By this stage, the inner cell mass of the blastocyst has differentiated into two layers: the epiblast and the hypoblast (Fig. 1.3A). These two layers are in contact and form a bilaminar embryonic disc. Within the epiblast a cavity develops, the amniotic cavity, which fills with amniotic fluid. Some epiblast cells become specialized as amnioblasts, and they secrete the amniotic fluid. The exocoelomic membrane is derived from the hypoblast and lines the cavity that appears beneath the endoderm, the primary yolk sac (Fig. 1.3C). The fluid contained in this sac is the source of nutrition for the embryo before the placenta is fully formed and functional.
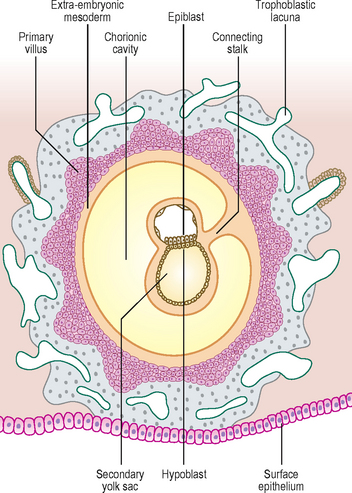
By 12 days there has been significant change particularly in the trophoblast. Small clefts appear in the syncytiotrophoblast called lacunae which communicate with the maternal endometrial sinusoids, thereby deriving nutritional support for the developing embryo (Fig. 1.4). Concurrent with this is the further development of the cytotrophoblast which is thickest at the embryonic pole of the conceptus. Clefts appear between the exocoelomic membrane and the cytotrophoblast (Fig. 1.4). These merge to form the extra-embryonic coelom and this cavity comes to almost completely surround the embryo. This is the chorionic cavity (Fig. 1.5).
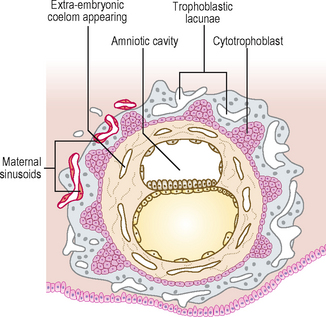
Fig. 1.4 Implanted blastocyst. At 12 days cavities develop which coalesce to form the extra-embryonic coelom.
By day 13 the lacunae have enlarged further. The cytotrophoblast has begun to form primary chorionic villi, which are finger-like protrusions into the lacunae. The embryo proper consists of two layers, the epiblast and the hypoblast, still closely applied to each other. The two cavities continue to enlarge, with the amniotic cavity above the epiblast and the yolk sac below the hypoblast, now known as the secondary yolk sac because of the presence of the chorionic cavity (Fig. 1.5). The embryo is connected to the cytotrophoblast by a connecting stalk of extra-embryonic mesoderm (primitive connective tissue) (Fig. 1.5). This stalk is the forerunner of the umbilical cord. By this stage the uterine epithelium has reformed, thus completely engulfing the conceptus. The largest development of trophoblastic lacunae is on the deepest surface of the conceptus. By the end of the second week the syncytiotrophoblast produces the hormone human chorionic gonadotrophin (HCG), which maintains the corpus luteum in the ovary, which in turn sustains the thickness of the endometrium. The hormone is secreted in the urine and thus its presence is an early indicator of pregnancy. This is the basis upon which pregnancy test kits work.
The 3rd week—Further development of the embryo and formation of trilaminar embryonic disc
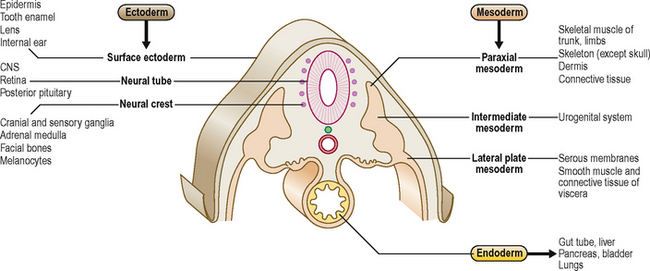
The embryo develops further by forming three germ layers, the process being known as gastrulation. Two layers have already formed: the epiblast and the hypoblast (Fig. 1.5). These two closely apposed layers take up the form of two elliptical plates, and together are termed the bilaminar embryonic disc. From this point the epiblast becomes known as the ectoderm, and the hypoblast as the endoderm. The ectoderm gives rise to the third layer that comes to lie between the two original germ layers, the intra-embryonic mesoderm. As a useful generalization, the ectoderm (the outer skin) forms the covering of the body (the epidermis) as well as the nervous system; the endoderm (the inner skin) forms the lining of the gastrointestinal and respiratory systems; and the mesoderm (the middle skin) forms the skeletal, connective and muscle tissues of the body. The main tissue derivatives of the germ layers are shown in Figure 1.6.
Development of the ectoderm
Primitive streak formation
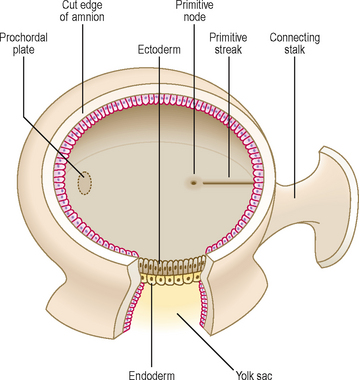
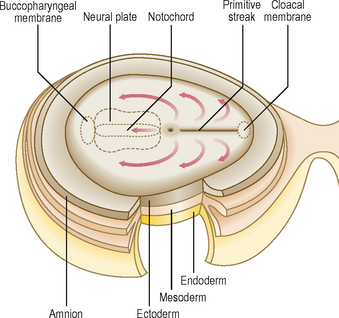
By the end of the second week of development there is a groove-like midline depression in the caudal end of the bilaminar embryonic disc. This marks the appearance of the primitive streak (Fig. 1.7). By the beginning of week 3 the streak deepens. At the cephalic end of the streak the primitive node develops (Fig. 1.7). Cells of the ectoderm layer migrate towards the streak and then detach from it, spreading out laterally beneath it. This migration forms a new germ layer, the intra-embryonic mesoderm. The new germ layer spreads out in all directions to lie between the ectoderm and the endoderm, except in two locations, where the original two germs layers remain in contact: the prochordal plate, at the cephalic end of the disc, and the cloacal plate at the caudal end of the disc (Fig. 1.8). The prochordal plate is soon replaced by the buccopharyngeal membrane, which forms a temporary seal for the future oral cavity. In week 4 this membrane breaks down to establish communication between the gut tube and the amniotic cavity. The cloacal plate is replaced by the cloacal membrane.
Notochord formation
Cells derived from the primitive node migrate cranially towards the buccopharyngeal membrane (Fig. 1.8). This results in the appearance of the notochordal plate, which in turn folds in to form the solid cylinder of the notochord. The notochord thus comes to underlie the future neural tube (the future brain and spinal cord—see below and Chapter 10), and forms both a longitudinal axis for the embryo, and the centres (nuclei pulposi) of the intervertebral discs of the vertebral column.
Neurulation
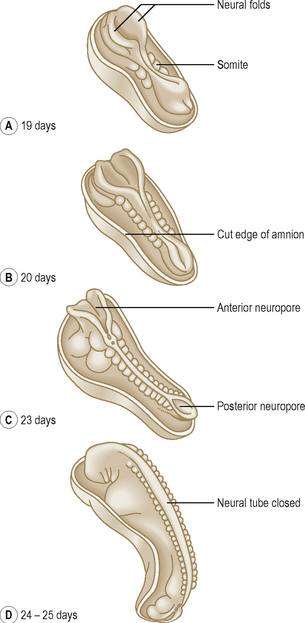
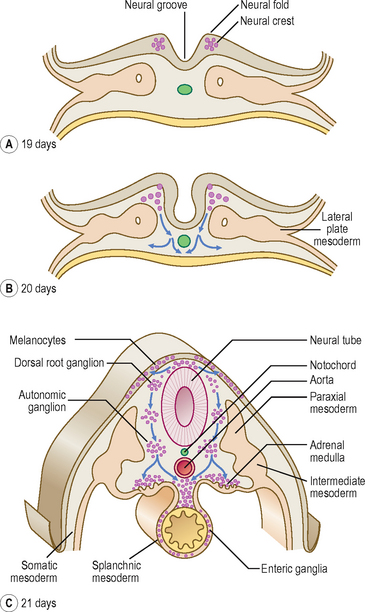
The process of the formation of the brain and spinal cord is known as neurulation. The ectoderm germ layer gives rise to neuroectoderm, which gives rise to most of the major components of the nervous system. At about 19 days, at the cranial end of the primitive streak, the underlying mesoderm and notochord induce the ectoderm to form the neural plate (Fig. 1.8), which rounds up to form the neural folds (Fig. 1.9A, B). The neural plate enlarges initially at the cranial end. At 20 days, the neural plate in the mid-region of the embryo remains narrowed, but it expands at the caudal end. The plate deepens to form the neural groove from which the neural tube forms. The cranial and caudal ends of the tube are open and are known as the anterior and posterior neuropores; these eventually close (Fig. 1.9C, D). At the edges of the neural tube, where the neuroectoderm is continuous with the surface ectoderm, the neural crests are formed. The neural crest cells detach themselves from the rest of the neural groove, before the tube forms, forming discrete aggregations of neural crest cells (Fig. 1.10). They contribute to the formation of dorsal root, cranial, enteric and autonomic ganglia, connective tissues of the face and bones of the skull, the adrenal medulla, glial cells, Schwann cells, melanocytes, parts of the meninges and parts of teeth. The further development of the nervous system will be dealt with in Chapter 10.
Further development of the mesoderm
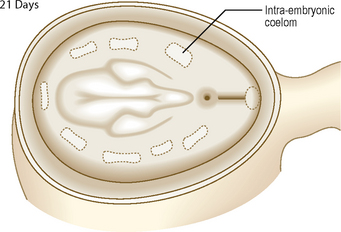
As the numbers of cells increase each side of the notochord, by day 17 the layer is thickest closest to the midline, and is known as the paraxial mesoderm. The parts further out are known as the intermediate and lateral plate mesoderm (Fig. 1.10C). At day 19, clefts begin to appear in the lateral plate. The mesoderm of the lateral plate is continuous with the extra-embryonic mesoderm covering the amniotic sac and the yolk sac (Fig. 1.10A, B). The mesoderm covering the amniotic sac is termed the parietal or somatic layer and that covering the yolk sac the visceral or splanchnic layer (Fig. 1.10B). The diverging limbs of the extra-embryonic mesoderm open into the extra-embryonic coelom (space or cavity). The clefts that appear within the lateral plate merge to form the intra-embryonic coelom, a cavity which is the forerunner of the serous cavities (Fig. 1.11) (i.e. the pericardial, pleural and peritoneal cavities). The intra-embryonic and extra-embryonic coeloms are therefore continuous.
Arising between the paraxial and lateral plate mesoderm is the intermediate mesoderm from which the urogenital system develops (Fig. 1.10C) (see Chapters 8 and 9).
Segmentation of the mesoderm
Paraxial mesoderm
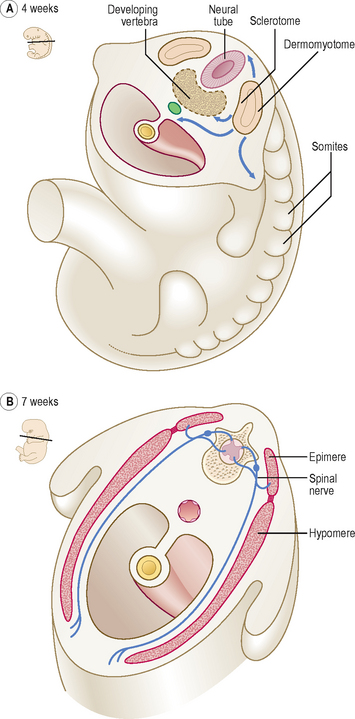
The paraxial mesoderm undergoes further differentiation in paired blocks of tissue in a craniocaudal direction on each side of the notochord called somites. The first pair of somites form at about 20 days; thereafter at a rate of about three per day until 42–44 pairs are formed. Not all therefore exist at the same time, and all will differentiate further. The age of an embryo is often related to the number of somite pairs present. From the beginning of the fourth week the somites undergo further differentiation to form dermomyotomes (form connective tissue and skeletal muscle) and sclerotomes (form bone and cartilage) (Fig. 1.12A). Cells from the sclerotomes surround the notochord and spinal cord, and give rise to the vertebral column. Details of the formation of vertebrae, and the contribution made by the sclerotomes, may be found in Chapter 4.
Somite development
In the fourth week, the medially placed mesenchymal cells of the somites migrate towards the notochord to form sclerotomes (mesenchyme is the loosely arranged embryonic connective tissue in the embryo). The ventrolateral cells of the somites become the myotomes, and those remaining become the dermatomes (Fig. 1.12A). The myotomes split into dorsal epimeres and ventral hypomeres. The dorsal epimeres give rise to epaxial muscles, the erector spinae muscles. The ventral hypomeres give rise to hypaxial muscles, which include muscles of the body wall (Fig. 1.12B). The ventrolateral parts of the somites in the regions of the future limb buds that remain after the sclerotomal portion has formed migrate to differentiate into limb musculature (see Chapter 4). The dermatomes form the dermis of the skin, and thus these cells come to lie beneath the surface ectoderm, which forms the uppermost layer of the skin, the epidermis. It is important to appreciate that as the myotomes and dermatomes migrate to their adult position they bring with them their innervation, from the segment of origin of the developing spinal cord (see Chapter 10).
The lateral plate mesoderm
The two layers of the lateral plate mesoderm enclose the intra-embryonic coelom. The mesodermal cells of the lateral plate arrange themselves as thin layers, which become the serous membranes of the body: the pleura, pericardium and peritoneum. These two continuous layers differentiate therefore to become a lining for the future body wall, a covering for the future endodermal gut tube (see Chapter 7) and the smooth muscle and connective tissue of the gut wall. The development of the body cavities is dealt with in Chapter 3. The serous layer in contact with the future body wall is the parietal layer because parietes means ‘walls’ in Latin; the serous layer in contact with the endodermal gut tube, for instance, is the visceral layer because viscera means ‘organs’. Other names for these two layers are somatopleure (meaning ‘membrane associated with the body side’) and splanchnopleure (meaning ‘membrane associated with the organ side’) (Fig. 1.10B).
The endoderm
The epithelial lining of the gastrointestinal tract is derived from the endoderm germ layer. The formation of the endodermal gut tube depends on the transverse and longitudinal folding of the embryo. In addition to the lining of the gastrointestinal and respiratory systems, the endoderm also gives rise to the parenchymal cells of the liver and pancreas (see Chapter 7), and of the thyroid and parathyroids, as well as the lining of the urinary bladder.
The 4th week—Folding of the embryo
Folding takes place in two directions: longitudinal (also referred to as cephalocaudal) and lateral (or transverse). Longitudinal folding occurs mainly as a consequence of the rapid enlargement of the cranial end of the neural tube to form the brain (see Chapter 10). Lateral folding is a consequence of the enlargement of the somites. The process of lateral folding is illustrated in Figure 1.13.
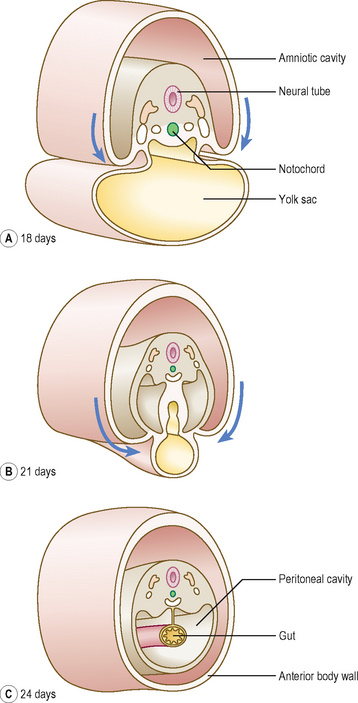
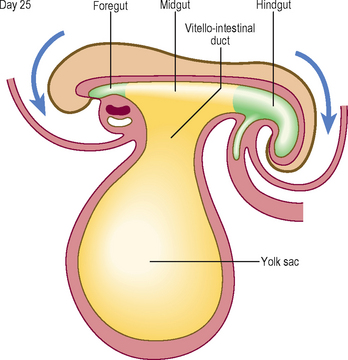
Longitudinal folding, which occurs between days 21 and 24, results in bending of the embryo so that the head and tail are brought closer together (Fig. 1.14). The endoderm forms a tube-like structure with an initially wide communication with the yolk sac: this communication narrows as the longitudinal folding increases. The amniotic cavity pushes in at the cranial and caudal ends of the embryonic disc, thus increasing the degree of longitudinal folding at the head and tail folds. The amniotic cavity also pinches the connection of the yolk sac and gut to form the narrowed communication of the vitello-intestinal (or vitelline) duct (Fig. 1.14). Later this duct is lost.
 The series of rapid mitotic divisions of the ovum result in the formation of a morula, and then a blastocyst. The blastocyst is a fluid-filled hollow sphere, which consists of an inner cell mass at one pole, surrounded by a thin wall of trophoblast cells.
The series of rapid mitotic divisions of the ovum result in the formation of a morula, and then a blastocyst. The blastocyst is a fluid-filled hollow sphere, which consists of an inner cell mass at one pole, surrounded by a thin wall of trophoblast cells. The trophoblast cells attach the blastocyst to the uterine wall, and form the fetal portion of the placenta.
The trophoblast cells attach the blastocyst to the uterine wall, and form the fetal portion of the placenta. The trophoblast differentiates into syncytiotrophoblast and cytotrophoblast. Lacunae appear in the syncytiotrophoblast, and merge to communicate with maternal blood sinusoids. By this the embryo derives nutrition from the maternal circulation. The cytotrophoblast forms villi, which are finger-like protrusions into the lacunae.
The trophoblast differentiates into syncytiotrophoblast and cytotrophoblast. Lacunae appear in the syncytiotrophoblast, and merge to communicate with maternal blood sinusoids. By this the embryo derives nutrition from the maternal circulation. The cytotrophoblast forms villi, which are finger-like protrusions into the lacunae. The amniotic and chorionic cavities come to surround the embryo and their walls constitute the fetal membranes.
The amniotic and chorionic cavities come to surround the embryo and their walls constitute the fetal membranes. The endometrium responds to implantation by mounting the decidual reaction. The decidua basalis forms the maternal component of the placenta.
The endometrium responds to implantation by mounting the decidual reaction. The decidua basalis forms the maternal component of the placenta. The inner cell mass differentiates into two of the three primary germ layers: the epiblast (later the ectoderm) and the hypoblast (later the endoderm). This process is known as gastrulation, and results in a bilaminar embryonic disc.
The inner cell mass differentiates into two of the three primary germ layers: the epiblast (later the ectoderm) and the hypoblast (later the endoderm). This process is known as gastrulation, and results in a bilaminar embryonic disc. The ectoderm further differentiates and forms the primitive streak, which gives rise to the third germ layer, the mesoderm. Thus, the trilaminar embryonic disc forms. The mesoderm differentiates further into paraxial, intermediate and lateral plate mesoderm. The lateral plate mesoderm encloses the intra-embryonic coelom.
The ectoderm further differentiates and forms the primitive streak, which gives rise to the third germ layer, the mesoderm. Thus, the trilaminar embryonic disc forms. The mesoderm differentiates further into paraxial, intermediate and lateral plate mesoderm. The lateral plate mesoderm encloses the intra-embryonic coelom. The paraxial mesoderm forms the segmented somites, which give rise to muscles, skeletal structures and dermis. The intermediate mesoderm contributes to the urogenital system, and the lateral plate mesoderm forms the parietal and visceral layers of the serous membranes of the body.
The paraxial mesoderm forms the segmented somites, which give rise to muscles, skeletal structures and dermis. The intermediate mesoderm contributes to the urogenital system, and the lateral plate mesoderm forms the parietal and visceral layers of the serous membranes of the body. Neurulation is the process by which the ectoderm gives rise to the neural tube and neural crests of the developing brain and spinal cord.
Neurulation is the process by which the ectoderm gives rise to the neural tube and neural crests of the developing brain and spinal cord. The notochord forms a midline axis of the body: it largely disappears before birth, but persists as the centres of the intervertebral discs of the vertebral column (the nucleus polposus).
The notochord forms a midline axis of the body: it largely disappears before birth, but persists as the centres of the intervertebral discs of the vertebral column (the nucleus polposus). At the cranial end of the primitive streak, at the primitive node, the notochord forms, and this grows cranially and constitutes a midline axis of the body: it largely disappears before birth, but persists as the centres of the intervertebral discs of the vertebral column.
At the cranial end of the primitive streak, at the primitive node, the notochord forms, and this grows cranially and constitutes a midline axis of the body: it largely disappears before birth, but persists as the centres of the intervertebral discs of the vertebral column.
 Clinical box
Clinical box