Chapter 2 How do the placenta and fetal membranes form?
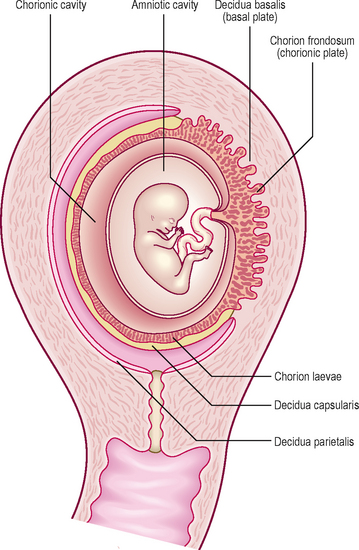
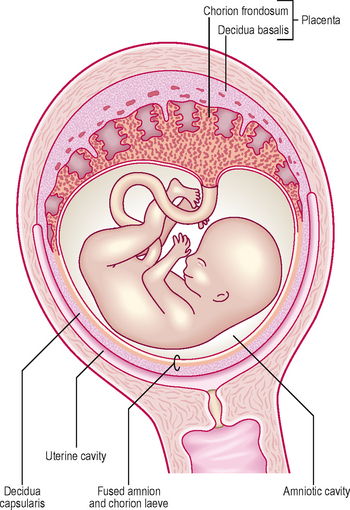
In response to the circulating progesterone and to the blastocyst, the stromal cells of the endometrium become large and accumulate glycogen. At 12 days the syncytiotrophoblast of the blastocyst begins to erode the endometrium and the maternal vessels at the implantation site become congested and dilated. These cellular changes, together with an increase in endometrial vascularization, are known as the decidual reaction. Within a few days the decidual reaction spreads throughout the endometrium, which now is known as the decidua. As the implanted embryo bulges into the uterine lumen the decidua becomes identifiable as three discrete areas at the implantation site (Fig. 2.1). The decidua underlying the embryo is called the decidua basalis, which forms the maternal face of the placenta. The decidua capsularis lines the superficial part of the embryo bulging into the uterine lumen and the remainder of the decidua is called the decidua parietalis.
What are the fetal membranes?
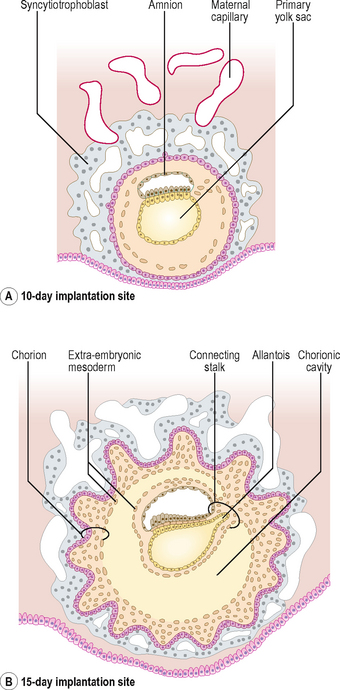
The term fetal membrane is applied to those structures derived from the blastocyst which do not contribute to the embryo. The amnion, the chorion, the yolk sac and the allantois make up the fetal membranes (Fig. 2.2). The amnion lines the amniotic sac and protects the embryo from physical injury. The amniotic sac enlarges rapidly due to an increase in the volume of amniotic fluid. The chorion is a double-layered membrane formed by the trophoblast and the extra-embryonic mesoderm, which eventually will give rise to the fetal part of the placenta. From 12 days until the end of embryonic period the developing embryo is suspended in the chorionic cavity. The expansion of the amniotic sac obliterates the chorionic cavity and the only connection between the embryo and the chorion is via a thick plate of mesoderm called the connecting stalk.
Amniotic cavity disorders
Amniotic fluid serves as a cushion for the embryo and fetus, and prevents attachments between the skin of the embryo and surrounding tissues. In a condition called oligohydramnios, the volume of amniotic fluid is too low, resulting in compression of the fetus by the uterine wall. If the amniotic fluid is in excess, then a polyhydramnios results (see Chapter 5). Tears or ruptures in the amnion result in amniotic bands forming which can surround limbs and may result in various fetal anomalies.
Development of the uteroplacental circulation
The umbilical arteries carry the deoxygenated blood from the embryo to the chorion and umbilical veins return the oxygenated blood to the embryo. The uterine vessels supply the maternal blood to the trophoblastic lacunae. When the cytotrophoblast cells grow into the lacunae as the primary chorionic villi, the primitive uteroplacental circulation is established. The initial formation of primary chorionic villi is explained in Chapter 1.
Further development of chorionic villi
From day 15, the primary chorionic villi begin to branch, and the extra-embryonic (chorionic) mesoderm invades the core of the primary villi, thus converting them into secondary chorionic villi (Fig. 2.3). The secondary villi line the entire surface of the chorion, and soon blood vessels develop within the mesenchyme of these villi that connect to the umbilical vessels in the embryo. Villi containing blood vessels are called tertiary villi (Fig. 2.4).
As the embryo and its coverings grow, the chorionic villi on the abembryonic pole become compressed against the decidua capsularis and become avascular (Figs 2.1 and 2.5). This area of chorion is known as the smooth chorion or chorion laeve. As the chorion laeve regresses, the villi on the embryonic pole increase in size and number. Because of its leafy appearance, this portion of the chorion is known as the chorion frondosum (Figs 2.1 and 2.5).
Formation of the placenta and placental circulation
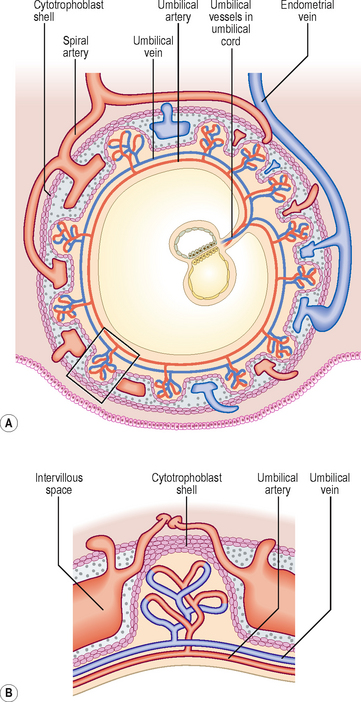
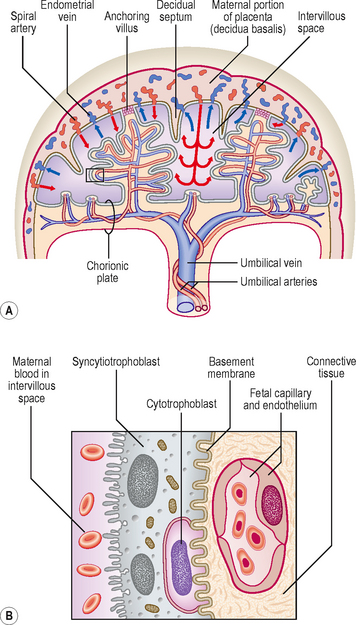
The placenta has both maternal and fetal components. The maternal portion is called the basal plate and is derived from the decidua basalis, and the fetal portion is called the chorionic plate, formed by the chorion frondosum (Fig. 2.1). The tertiary villi of the chorion frondosum grow towards the basal plate, and attach to the decidual tissue via the cytotrophoblast shell (Fig. 2.4B). Because these villi secure the attachment of the fetal placenta to the basal plate, they are known as anchoring villi (Fig. 2.6A). The blood-filled spaces between the anchoring villi, which were trophoblastic lacunae, now become the intervillous spaces (Fig. 2.6A).
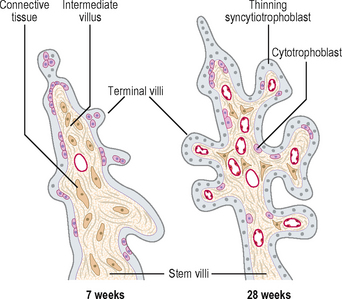
The anchoring villi give rise to numerous side branches called intermediate villi, which in turn produce small rounded terminal or floating villi (Fig. 2.7). The terminal villi develop as sprouts of syncytiotrophoblast. During the third trimester, the terminal villi take over the functions of nutrient and gaseous exchange from the intermediate villi. The continuous branching of the villi into the intervillous spaces therefore results in an extensive placental villous tree. As the placenta grows during the last trimester of pregnancy, the placental villi show maturation changes. The capillaries become larger and come to lie close to a much thinner syncytiotrophoblast. Most of the cells of the cytotrophoblast layer disappear, thus facilitating diffusional exchange mechanisms.
As the villous trees grow into the intervillous spaces, the decidual tissue sends wedge-like placental septa to divide the placenta into 15–20 irregular convex areas called cotyledons. Since the placental septa do not reach the chorionic plate, the maternal circulation between the cotyledons is maintained (Fig. 2.6A).
The exchange of nutrients, respiratory gases and waste products between the maternal and fetal blood takes place across the placental membrane (see below) within the intervillous spaces. Maternal blood enters these spaces from the spiral arteries, branches of the uterine artery, bringing nutrients and oxygen for the embryo and fetus, and the endometrial veins drain the waste products to the maternal circulation (Figs 2.4A and 2.6A). The oxygenated fetal blood from the placenta passes into the umbilical vein, and the deoxygenated blood from the fetus is returned to the placenta by way of the umbilical arteries.
Placental membrane and placental functions
The partition between the maternal and fetal circulations is the placental membrane, often called the placental barrier (Fig. 2.6B). This membrane is composed of fetal tissues and is initially four layers thick: (1) the fetal capillary endothelium, (2) the connective tissue of the villus, (3) the cytotrophoblast and (4) the syncytiotrophoblast. From the fourth month the placental membrane becomes progressively thinner as the fetal capillaries enlarge, the amount of connective tissue in the core of the villus is reduced, and the cytotrophoblast largely disappears.
Structure of the full-term placenta
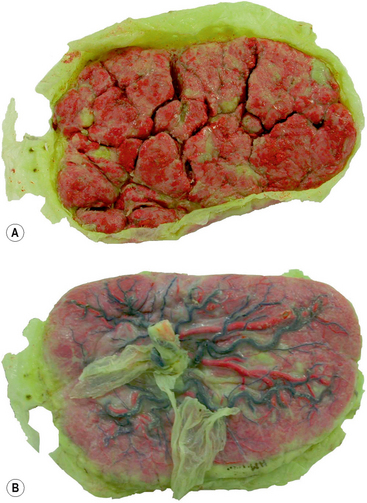
At term the placenta appears as a disc, and has fetal and maternal surfaces (Fig. 2.8). The fetal surface is smooth, and the umbilical cord is inserted into the centre of this surface. The amnion invests the umbilical cord, and covers the fetal surface of the placenta. The umbilical vessels radiate from the umbilical cord, and run between the transparent amnion and smooth chorion before supplying the chorionic villi. At birth the amnion and chorion are torn from the margins of the placenta. The maternal surface is covered by a thin layer of decidua basalis torn from the uterine wall during birth. Beneath this layer, the maternal surface shows 15–20 roughened cotyledons (Fig. 2.8A).
Twins and their fetal membranes
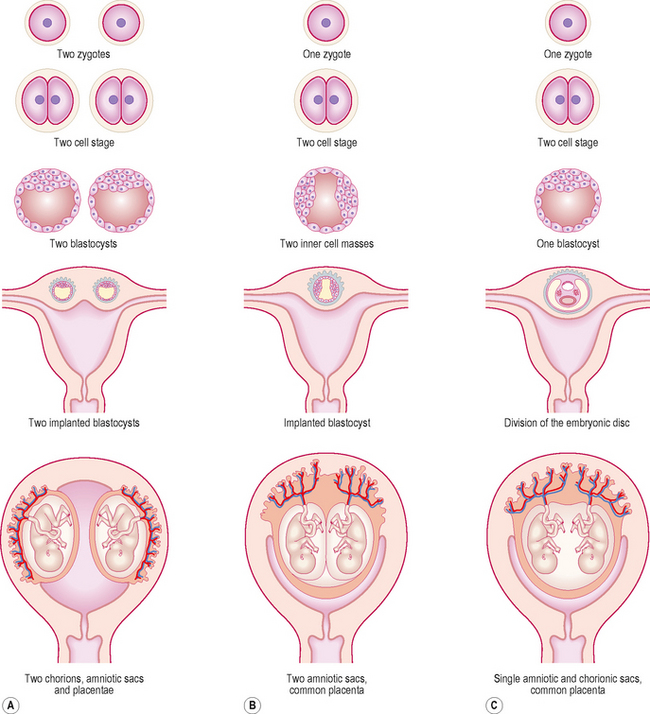
Dizygotic twins implant separately and develop separate placentae and fetal membranes (Fig. 2.9A). Sometimes, however, because of their proximity a secondary fusion between the two amniotic and chorionic sacs may occur.
The sharing of placenta and fetal membranes in monozygotic twins depends on the stage at which a single zygote divides. If the splitting occurs at the two-cell stage of the embryo, the resulting twins will implant separately with their own fetal membranes and placentae (Fig. 2.9B). In such cases, although the arrangement of fetal membranes is the same as that of the non-identical dizygotic twins, their identity as monozygotic twins can only be revealed by conducting blood and other diagnostic tests. In most cases of identical twinning, the inner cell mass splits within a single blastocyst. Subsequently, two embryos will share a single chorionic sac and placenta but will be enclosed by separate amniotic sacs (Fig. 2.9B). If the splitting occurs after the formation of the bilaminar embryonic disc, the twins will occupy a single amnion and chorionic sac, and share the same placenta (Fig. 2.9C). Such twins usually fail to survive, as the umbilical cords may get entangled within the amniotic sac thus blocking the fetal circulation.

 Clinical box
Clinical box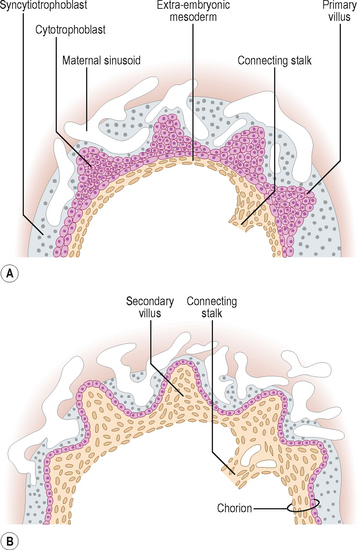
 Clinical box
Clinical box Clinical box
Clinical box Clinical box
Clinical box