CHAPTER 41 History of Pediatric Anesthesia
The pediatric anesthesiologist does not generally diagnose or cure patients but rather guides and supports each young patient through the operative experience with the least possible mental and physical stress. The history of pediatric anesthesia is best told by tracing the steps others have taken; steps toward increased precision in regulation and control of neurologic, respiratory, cardiovascular, and other body systems to serve both the surgeon and child (Smith, 1991).
Phase I: pediatric anesthesia before 1940
Primitive Period
Before the introduction of ether in 1846, circumcisions, amputations, tumor excisions, and correction of gross deformities were performed on infants and children without any relief of pain. Struggling was controlled by use of force, but pain was accepted as an unavoidable part of life. Crude attempts were occasionally made with alcoholic “spirits,” nerve compression, or even brief strangling, coupled with headlong surgical speed; however, these attempts resulted in predictably poor outcomes for both operation and patient. Harelip repair had been attempted without pain relief measures in many parts of the world for hundreds of years. In Japan, general anesthesia with the herb mixture tsu san sen was used successfully for breast cancer operations in 1804 by Seishu Hanaoka. In 1837, Gancho Homma reported a series of general anesthesia procedures with the use of the same herb mixture for children over 5 years of age for harelip repair, but it was withheld from use in younger patients because of its toxicity (Iwai and Satoyoshi, 1992). The conviction that small infants did not need anesthesia was not effectively suppressed until recently (Anand and Hickey, 1987). For many years, the “whiskey nipple” had been used widely as a sedative supplement to local anesthesia in infants undergoing abdominal procedures, and wine has been given for ritual circumcisions for millennia.
Early Control of Pain: Ether and Chloroform
The introduction of ether was the first giant step in the history of anesthesia. Although Crawford Long used ether in his rural practice in Georgia beginning in 1842, and his third ether-anesthesia procedure was for a toe amputation in a 7-year-old boy (Long, 1849), it was not until the famous public demonstration of ether anesthesia at the Massachusetts General Hospital in Boston in 1846 that ether was widely accepted for use during surgery (Morton, 1847). The discovery that sensation, or pain, and along with it consciousness and motion, could be abolished temporarily by ether was widely acclaimed; however, little was known about ether’s actions, how to use it, or what its dangers might be. Ether was accepted only gradually over several years, with many surgeons retaining the belief that ordinary men (the wealthy excluded) should be able to tolerate surgery without anesthesia! However, for children and ladies, who were considered to be “more sensitive,” anesthesia was considered appropriate, although Morton himself was reluctant to administer it to young subjects because of the high incidence of nausea and vomiting in this population (Bigelow, 1846; Warren, 1847; Pernick, 1975).
It was soon found that pouring ether onto a handkerchief or small cloth was a practical method of administration with small children. One simply pressed the cloth to the patient’s face until the child was quiet and limp. The cloth was then withdrawn, and the surgeon was granted 3 or 4 minutes to operate as the child regained consciousness. The use of continuous administration of ether caught on slowly with gradual familiarization with the new agent. The early impression that ether was easy to administer, effective, and safe led to the belief that it was a trivial service that any inexperienced person, often an orderly or a parent, could perform. The unfortunate result was that throughout the rest of the century, the administration of anesthesia continued to be held in poor repute as a medical activity, rarely attracting physicians with special interest or ability in the field. Nurses eventually began to assume increasing responsibilities for providing anesthesia care. As late as 1940, a physician, in the lead article published in the first edition of the new journal Anesthesiology, noted, “During my internship I was trained by a nurse. I was given a cone, a can of ether, and a few empirical tricks” (Haggard, 1940).
In England, chloroform was accepted more readily because of its smoother and more rapid action. Soon, however, the incidence of deaths became so alarming that the British established a dictum that only physicians should be allowed to administer anesthesia (Eckenhoff, 1966). The fortunate result was that throughout the British Empire, anesthesia flourished as a medical specialty and its workers gained equal status with other physicians and became early leaders in the field.
Another great advantage for the British in the early development of anesthesia was the presence of the astounding John Snow (1813 to 1858), who made epidemiologic advances of national importance, ran an active medical practice, and kept notes on hundreds of anesthesia experiences and research experiments, mostly in the last 10 years of his life (1846 to 1856) (Griffith, 1934). Snow first described signs by which a practitioner could monitor and control the depth of anesthesia in patients of all ages (Snow, 1847). His five stages of anesthesia (excitement, loss of consciousness, relaxation, eye movement, and depth of respiration) served as a guideline throughout the remainder of the century and formed the basis of Guedel’s important guide, Inhalation Anesthesia, published in 1937. Snow explored both ether and chloroform, preferring the latter, which he found well suited to infants and children. However, he warned of chloroform’s danger with excessive depth (Snow, 1858). His record of successfully anesthetizing 147 infants for harelip repair is hardly conceivable in view of the mortality that this operation continued to bear well into the next century.
In the United States, the special needs of children were given slight consideration for many years. The child was treated as “a little adult”; surgeons operated with large instruments, and all equipment was adult sized. Ether remained the principal agent. Although criticism of chloroform became more vehement, its use was advocated in the United States as recently as 1957 (Kopetsky, 1903; Schwartz, 1957). Progress was made by trial and error, with little communication among those using anesthesia. Most literature in the United States concerning anesthesia for children was written by surgeons before 1900.
Interest grew slowly around the turn of the century, and nurses and surgeons began to develop skills sufficient to carry children through longer and more difficult procedures. Thousands of tonsillectomies were being practiced by 1900, and appendectomy was an accepted, although often dangerous, procedure. Orthopedic surgery was the most active type of pediatric surgery during this time, and most procedures were easily managed by simple ether techniques. One of the first signs of concern for the child’s anxiety when undergoing anesthesia was voiced by James Gwathmey in 1907. He recommended that one should “add a few drops of the mother’s cologne to the ether mask and induce the child in the mother’s arms.” Another step toward easing induction came in 1928 with the introduction of tribromoethanol, the German Avertin, which was used widely as a rectal agent. It provided almost certain sleep in 7 to 8 minutes and was of special value before ether induction, because unlike the barbiturates used later, it had a bronchodilating effect that facilitated rather than retarded induction. However, the drug required preparation immediately before use. That, in addition to the repeated occurrence of fecal incontinence, led to its abandonment.
Between 1925 and 1940, activity in both pediatric surgery and anesthesia began to accelerate. William Ladd, whose interest stemmed from his experience in caring for children injured in a massive explosion in Halifax, Nova Scotia in 1917, led the development of pediatric surgery in North America (Goldbloom, 1917; Steward, 1983; Smith, 1959). His work at Children’s Hospital Boston was devoted to the correction of neonatal defects, including harelip. Ladd performed harelip repair seated, with the infant held facing him in the lap of a nurse. The anesthetist stood behind the nurse, directing ether from a vaporizing bottle into the infant’s mouth via a metal mouth hook.*
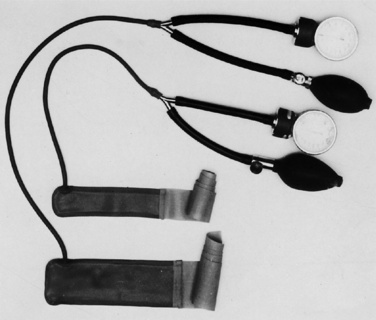
The introduction of cyclopropane in 1930 proved particularly helpful for pediatric anesthetists in the management of infants, although it required assembly of a closed-system apparatus. Lamont and Harmel developed a miniaturization of the to-and-fro canisters Waters described in Wisconsin, and they used this technique for Blalock’s “blue baby” (tetralogy of Fallot) operations at Johns Hopkins Hospital. In Boston, Betty Lank,† an enterprising nurse anesthetist, further redesigned the miniature to-and-fro apparatus with less dead space and shrank adult celluloid masks to infant size, enabling her to provide anesthesia, relaxation, and controlled respiration for Ladd’s infants as well as for Robert Gross’ widely heralded division of a patent ductus arteriosus in 1938—without endotracheal intubation (Fig. 41-1).

FIGURE 41-1 Ms. Betty Lank served as chief nurse anesthetist at Children’s Hospital Boston from 1935 to 1969.
By 1940, considerable progress had been made in the ability of minimally trained anesthetists to provide satisfactory operating conditions for the surgeons of that time (Smith, 1959). Ladd strenuously corrected the previous concept by establishing the dictum “The child is not a little man.” Supportive warming, preoperative correction of electrolyte balance, and intraoperative charting became standardized. Clinical signs of anesthetic depth, described by Guedel in 1937, served well. This might be termed the height of the art of pediatric anesthesia in the United States, where simple expedients still prevailed.
In England, there had been more progress in airway control. After World War I, Magill and Rowbotham popularized tracheal intubation for adult procedures, and in 1937, Philip Ayre of Newcastle-Upon-Tyne reported his classic method of endotracheal intubation with a T-tube device for harelip repair in neonates (Ayre, 1937). Although Robson of Toronto had described intubation of children using digital guidance rather than a laryngoscope, it had received little attention (Robson, 1936).
Phase II: emergence of pediatric anesthesia, 1940 to 1960
Factors in the Rapid Development of Interest
Before and during this period, activity accelerated in related fields, and much information became available defining normal and abnormal infants in such texts as Clement Smith’s The Physiology of the Newborn Infant (1945), Taussig’s Congenital Malformations of the Heart (1947), and Nelson’s Textbook of Pediatrics (1950). The practice of adult anesthesia had become established, providing fresh information on new agents and techniques easily adaptable to children. As yet, the only established pediatric anesthesiologist in North America was Charles Robson in Toronto; in England, it was Robert Cope at London’s Hospital for Sick Children. Among those interested in pediatric anesthesia, M. Digby Leigh made himself well known (Fig. 41-2). Trained by Waters in Wisconsin, Leigh was appointed head of anesthesia at Montreal Children’s Hospital, where he taught and (with his invaluable associate Kathleen Belton) authored Paediatric Anaesthesia (1948) the first North American text on this subject. Leigh and Belton described the use of spinal anesthesia for intrathoracic procedures, an original pediatric circle absorption apparatus, and a nonrebreathing valve. Leigh moved to Vancouver, British Columbia in 1947 and then to Los Angeles in 1954, where he started the first annual pediatric anesthesia teaching conference in America. He was a brilliant technician and a stern teacher, and he delighted his audiences with his stinging repartee. His foresighted attempts to monitor exhaled carbon dioxide in 1952, however, were rebuffed by incredulous scoffers (Conn, 1992).
In the meantime, in Liverpool, G. Jackson Rees (Fig. 41-3) had been named anesthetist at the Alder Hey Children’s Hospital by his mentor and teacher, Cecil Grey. Together they conceived the idea that practically all surgery could be performed under the simple and nonexplosive combination of nitrous oxide and curare. Rees, adapting the Ayre T-tube system by adding an expiratory limb and breathing bag (the well-known Jackson-Rees system), proceeded to carry out this concept with astounding success. With minor alterations this technique was to survive through years of short-lived, complicated types of apparati. Rees’ conviction that respiration should be controlled in infants with reduced tidal volumes and rates of 60 to 80 breaths per minute also met with criticism, but it proved to be rational when increased tidal volumes were found to cause barotrauma (volutrauma) and surfactant inactivation.
Foundations of Clinical Control and Support
Neonatal Surgery and Anesthesia
Among the surgical challenges in the beginning of the 1940s, three congenital defects were dominant: tracheoesophageal fistula (TEF), omphalocele, and congenital diaphragmatic hernia (CDH). Both Leven and Ladd performed secondary multiprocedure repair of TEF in 1939 (Smith, 1959). Primary repair, first accomplished by Haight in 1941, then became the most important challenge in pediatric surgery, each case demanding all-day and all-night efforts of all participants. In Boston, the operation was carried out with the patient under the influence of cyclopropane via mask with a to-and-fro apparatus and the endotracheal intubation being reserved for emergency use during operation. Control of the exposed pleura during esophageal anastomosis required complete immobility, and the operation, in the words of Ladd, was “like stitching the wing of a butterfly” (Smith, 1959). Supportive management played a large part in the survival of these infants, both during and after the operation. Warmth was maintained by heating and humidifying the operating room, wrapping limbs in sheet-wadding, and using a semiclosed to-and-fro absorption technique. Blood pressure was measured by a locally introduced cuff with a latex bladder encircling the arm (Fig. 41-4). Fluids and blood were administered via a open-top burette with rubber tubing and “cut down” metal cannula in the saphenous vein. Postoperative survival depended largely on the remarkably able services of one or two very special nurses.
Early Attempts to Control Fear
It soon became evident that for the small child, the fear of needles and the horrors of anesthetic induction were deeply upsetting and of long duration. Concern about this most unfortunate anesthetic by-product was voiced by psychologists, pediatricians, anesthesiologists, and others, as well as numerous mothers reporting prolonged night terrors, bed-wetting, and dependence (Levy, 1945; Jackson, 1951; Eckenhoff, 1953).
Some attention had been paid to premedication shortly before this time. French Armand-Delille (1932) recommended morphine, and Waters (1938) promoted the combination of morphine and scopolamine, but the response to the outburst of concern came in a flood of reports on a variety of ineffective agents. The basic error in most studies was, and still is, the use of age or weight for estimation of drug dosage, when neither reflects the child’s state of mind. General use of intramuscular barbiturates plus morphine mixed with either atropine or scopolamine resulted in severe horror of needles, an uncomfortably dry mouth, and an unpredictable degree of sedation, seldom better than 65% successful. Attempts to improve this record continued to play a large part in the activities and literature of pediatric anesthesiologists with only slight improvement with regard to the effectiveness of sedative drugs. However, the concentration of attention of numerous investigators on this problem did result in the development of close personal interest in each child studied, possibly responsible for much of the benefit credited to the drug being promoted.
Methods of induction showed somewhat more success than those of sedation. Thiopental replaced rectal tribromoethanol, providing greater ease of administration via either the intravenous or the rectal route, whereas induction with nitrous oxide, cyclopropane, or divinyl ether eliminated much use of the dreaded ether (Weinstein, 1939). With the repeated failure of sedative agents, greater skills were developed by caring anesthetists to gain the confidence of children in preoperative visits and then to divert their attention at induction by telling them stories or by simply lulling them to sleep. Hypnosis was used for induction by Betcher (1958), Marmer (1959), and a few others for the total operation in short procedures. It was particularly valuable for the repair of facial lacerations in small children who had recently eaten. Unfortunately, this potentially useful and harmless method gained only limited acceptance.
Endotracheal Intubation
The greatest advance in pediatric anesthesia between 1940 and 1960 was in control of the airway by tracheal intubation. Early use in England and Canada met with little resistance. In the United States, however, opposition by surgeons raised the first major obstacle to progress in the new specialty. (It must be admitted that reasonable concern had been aroused in those who had witnessed the traumatic attempts of inexperienced individuals to perform unnecessary intubations.) It was the efforts of Rees in England; Leigh, then in Canada; the British-American Gillespie (1939); Americans Deming (1952), Pender (1954), and others; and their supportive younger surgeons that brought forth grudging acceptance of tracheal intubation of infants and children in the United States in the 1940s and 1950s.
The ongoing development of this technique led to an increased understanding of laryngeal anatomy; replacement of the “classic” hyperextension of the head by use of the “sniffing” position for intubation; a succession of different types of tracheal tubes, including the tapered tube of Cole (1945) that enjoyed more than a decade of popularity; a variety of tube materials progressing from coarse rubber to nonreactive plastic; and laryngoscopes of several types and sizes (Eckenhoff, 1951).
As with ether and other major advances, the advent of tracheal intubation brought a host of disadvantages and a few real dangers that in turn led to a glut of literature concerning complications, including subglottic stenosis, laryngeal irritation from large tubes, and tracheitis caused by contamination (Baron and Kohlmoos, 1951; Flagg, 1951; Smith, 1953a; Colgan and Keats, 1957). This proved to be just the beginning.
“Total Control” of Respiration: The Muscle Relaxants
After the first clinical use of d-tubocurarine by Griffith and Mitchell in Canada in 1942, Canadians and British accepted it readily and began extensive use in both children and adults, to be followed by much investigation in later years (Anderson, 1951; Stead, 1955; Leigh et al., 1957; Rees, 1958). Again, in the United States there was much opposition, this time by anesthesiologists as well as surgeons, to whom the concept of total “takeover” of an essential body function, termed controlled respiration, appeared to be a dangerous and unacceptable “physiologic trespass” (Gross, 1953; Beecher and Todd, 1954). Beecher threatened one of the authors of this chapter that “heads would roll” if he and others persisted in support of its use. In the meantime, Cullen (1943) had found it quite safe for adults and children, using it as a sole agent for infant surgery. This practice was abandoned after Scott Smith of Utah was tested under total curarization and suffered acutely on painful stimulation (Smith, 1947; Smith et al., 1947). As with tracheal intubation, the total acceptance of neuromuscular blocking agents in the United States required many years. By 1960, however, the terms controlled respiration and assisted respiration had gained widespread use.
Pediatric Breathing Systems: Assisted and Controlled Respiration
With the stimulating effect of ether on respiration in light surgical planes, assisted respiration was seldom needed. Open chest surgery, cyclopropane, and particularly muscle relaxants definitely changed this picture and led to a succession of considerably diverse devices (Dorsch and Dorsch, 1975). The to-and-fro absorption method, using soda-lime canisters of graduated sizes, was particularly adaptable to infants but caused heat retention and the aspiration of lime dust. For larger children, the canisters were bulky and heat retention was even more troublesome.
Special interest was taken in infant circle absorption systems. The Leigh, Ohio, and Bloomquist models were not only difficult to handle but also introduced the problems of valve resistance and dead space (Leigh and Belton, 1948).
To eliminate problems of carbon dioxide accumulation, several nonrebreathing valves were designed by Leigh and Belton (1948), Stephen and Slater (1948), and others. Although compact in design, they were not easy to manage and were definitely ill-suited for use with explosive agents.
The saga of apparatus variously called rebreathing (British), nonrebreathing (United States), and partial rebreathing (general) is complicated and involved numerous studies and modifications of the basic Ayre T system over 30 years (Fig. 41-5) (Ayre, 1937). After the Rees elongation of the expiratory limb with an attached breathing bag, the addition of exhaust valves placed either proximal (Mapleson A) or distal (Mapleson D) to the face brought intensive examination, as did the estimation of proper flow rates of incoming gases. Evaluation by Mapleson (1954) and Inkster (1956) did much to clarify these issues at the time, but more problems lay ahead.
Cardiovascular and Thermal Control
Between 1940 and 1960, revolutionary advances were made in several areas involving the combined efforts of anesthesiologists and surgeons. The intentional reduction of arterial blood pressure, extensively explored by Enderby (1950) and others in England, was cautiously extended to pediatric use by Anderson (1955) with trimethaphan camphorsulfonate (Arfonad). This served as a reasonably safe agent. It was the initial step in induced hypotension to reduce surgical blood loss in major pediatric surgery and to prevent excessive blood pressure elevation during correction of coarctation of the aorta. However, the agent was unpredictable and soon was replaced by more controllable agents and techniques.
Controlled Reduction of Body Temperature and Cardiopulmonary Arrest
After Gross’ ligation of a patent ductus arteriosus in 1938, correction of coarctation of the aorta, repair of vascular rings, and shunt procedures for tetralogy of Fallot were successfully performed under closed or semiclosed inhalation anesthesia, usually with cyclopropane (Harmel and Lamont, 1948; Harris, 1950; Smith, 1952). McQuiston, endeavoring to reduce the oxygen requirements of Dr. Potts’ cyanotic infants, cooled them 3° to 4° C on a simple ice-water mattress, thereby introducing the practice of hypothermic control of body metabolism into pediatric anesthesia (McQuiston, 1949). Efforts to reduce oxygen demand by further lowering temperatures to 30° C with immersion in ice water provided surgeons time for simple intracardiac aortic or pulmonary valvotomy (Lewis and Taufic, 1953; Virtue, 1955).
The drive to bypass both the heart and lungs initiated by Gibbon in 1937 became exciting in the early 1950s, with competing surgeons Lillihei, Kirklin, and Kay and their respective anesthesiologists Matthews et al. (1957), Patrick et al. (1957), and Mendelsohn et al. (1957) all contributing toward the first practical use of the pump oxygenator in 1955, 2 years before publication of the articles cited.
Mild and moderate hypothermia techniques were also used in this period for neurosurgery, orthopedic surgery, and harelip repair (Kilduff et al., 1956).
Control During Maintenance of Anesthesia
As more extensive procedures were developed and surgeons began to prefer accuracy to speed, 4-hour operations became more common and the methods of maintenance and support grew more demanding. Experience, skill, and constant observation were still primary factors that were assisted by a few simple devices (Fig. 41-6). During this time the precordial stethoscope became essential for use with every infant or child throughout the field of anesthesia (Smith, 1953b). A precordial or esophageal stethoscope served first to keep the anesthetist in direct contact with the child at all times, providing unaltered information as to the clarity and strength of breath sounds and the rate, rhythm, and strength of heart sounds (Smith, 1991). Strength of heart sounds was an important guide to the degree of blood loss at that time. Arterial blood pressure could be obtainable with standard apparatus for larger children and with a specially constructed latex cuff with an inflatable bladder for infants (the Smith cuff). During this phase, the electrocardiograph was occasionally brought into operating rooms, encased in antiexplosive shielding (shaped like a torpedo) and serving relatively little purpose. Body temperature was measured intermittently at oral, nasal, or rectal sites, the standard glass thermometer giving way to the safer but less accurate thermostat devices. The anesthesia chart was considered a necessary item, gaining in importance as procedures grew increasingly complex and legal suits more common.
Progress in Local Anesthesia
Ladd had used local infiltration for abdominal procedures in premature infants in the late 1930s, and Leigh wrote of spinal anesthesia for open chest work in the 1940s, but improved inhalation methods outmoded both. Except for brachial plexus block, little attention was paid to these methods in the United States (Small, 1951; Eather, 1958; Smith, 1959). In many other countries, however, where inhalation anesthesia was less advanced, there was considerable dependence on regional and spinal anesthesia for both infants and children.
Halothane Opens a New Era
Although anesthesia with relaxants and nitrous oxide permitted the use of electrical instruments in the operating room, explosive gases were still popular until the introduction of halothane. After its first use in England by Johnstone in 1956 (in 10% concentration), this nonflammable, nonirritating, and potent agent was promptly introduced in Canada by Junkin et al. (1957) and in the United States by Stephen and colleagues (1958). Subsequently, flammable anesthetic agents were totally abolished in the United States, thus opening the way to revolutionary changes, first in the control of blood loss by cautery and then in the development of electronic devices for monitoring and physiologic control.
Supportive Care and Oxygen Therapy
Related fields brought important aid to pediatric anesthesiologists during this period. The time-honored rule developed by Holliday and Segar (1957) for pediatric fluid administration based on metabolic requirements serves to this day. Also of great importance, particularly in reducing the morbidity and mortality of small infants undergoing surgery, was the control of infection by the use of antibiotic agents.
In related areas, Virginia Apgar introduced her scoring system for neonatal assessment, Peter Safar launched his crusade for mouth-to-mouth ventilation and further work in cardiopulmonary resuscitation, and the first multidisciplinary adult intensive care unit in North America was developed (Apgar, 1953; Safar, 1958).
The first pediatric intensive care unit was established in Goteburg, Sweden, in 1955.* Similar units were established in Stockholm (Feychtung), Liverpool (Rees), and Melbourne (MacDonald and Stocks) between 1960 and 1964. In North America, the first pediatric intensive care unit was established by Downes at the Children’s Hospital of Philadelphia in 1967, followed by Children’s Hospital of Pittsburgh (Kampschulte), Yale-New Haven Hospital (Gilman), Massachusetts General Hospital (Todres and Shannon), and the Hospital for Sick Children in Toronto (Conn) within the next 4 years (Downes, 1992).
Teaching and Research
On the heels of Leigh and Belton’s Pediatric Anesthesia (1948), Stephen’s Elements of Pediatric Anesthesia (1954) and Smith’s Anesthesia for Infants and Children (1959) were published, with details of advances to date. Articles on new agents and techniques steadily increased in the anesthesia-related literature. Whereas some reported personal early experiences based on limited numbers, many were of lasting value, including those involving tracheal abnormalities by Eckenhoff (1951) and Colgan and Keats (1957), tracheoesophageal repair by Zindler and Deming (1953), and the important warning of Leigh et al. (1957) concerning bradycardia after the intravenous administration of succinylcholine.
Research, on the other hand, was still in its infancy, there being little space, time, or funds for sophisticated investigation. As previously mentioned, many clinical studies offered practical current value concerning new agents and techniques. Whereas those concerning preoperative sedation outnumbered others, many reports covered airway resistance, valves, and dead space (Macon and Bruner, 1950; Mapleson, 1954; Orkin et al., 1954; Hunt, 1955; Inkster, 1956). The introduction of muscle relaxants initiated other studies, including those of Hodges (1955), Stead (1955), Telford and Keats (1957), and Bush and Stead (1962). The addition of halothane led to a more critical analysis of this agent than had been done for previous anesthetics.
This period established such fundamental techniques and basic concepts that Rees subsequently (1991) stated, “Paediatric anaesthesia in Great Britain and Ireland shows that by 1950 it had reached a point at which current practice is recognizable…. Future pediatric anaesthetists are therefore unlikely to experience the great excitement their predecessors enjoyed between 1930 and 1950, but will derive satisfaction from changes less dramatic as the curve of improvement approaches perfection.”
Phase III: era of nonflammable anesthetics, 1960 to 1980
Unfortunately, progress was not marked in the control of fear. Despite continued efforts to address this problem by many skilled workers, sedatives were still unpredictable and intramuscular needles were still in general use (Poe and Karp, 1946; Rackow and Salanitre, 1962; Root, 1962). The introduction of ketamine (Ketalar) by Domino et al. (1965) created mixed feelings based on its early postoperative psychological reactions, but the agent found a place in pediatric use for uncontrollable patients and to accomplish minor but painful procedures.
A major change in the methods for controlling fear was in giving permission to parents to be at the child’s bedside at all times, including “sleep-in” privileges, and later, to preinduction and induction areas. Whereas many parents were assuaged by these moves, statistics failed to show great help for the children (Schulman et al., 1967).
Increasing Clinical Precision
Modification of the partial rebreathing systems by Bain and Spoerel (1972), by which the exhalation tube passes inside the inhalation arm, provided a means of scavenging expired gases, thereby greatly enhancing the use of this popular pediatric method. This marked a major evolution of airway systems for pediatric anesthesia.
Steps toward greater precision in monitoring were taken in the measurement of infant blood pressure by Doppler sonography and by oscillotonometry (Dinamap) (Marcy and Cook, 1988). In the determination of arterial oxygen saturation, the transcutaneous electrode (ear oximetry) was used with limited success (Saunders et al., 1976). It was during this period that “control by the numbers” gained predominance over the unreliable art of anesthesia. Led by Downes of Philadelphia and others, arterial blood gas determinations, blood sugar, hemoglobin, electrolytes, and other measurements were serially evaluated intraoperatively in adjacent laboratories (Smith, 1990). Insertion of arterial and central venous catheters became commonplace, and urinary catheterization became an important guide to fluid and electrolyte replacement.
Progress in controlling fluid balance included the recognition of the importance of electrolytes in all intravenous solutions (Bennett et al., 1970; Herbert et al., 1971). New concepts concerning blood replacement included Davenport’s practical recommendation to give blood when loss reached 10% of blood volume, followed later by Furman’s more precise suggestion to maintain the hematocrit level above 28% to 30% in children and 40% in the newborn (Davenport and Barr, 1963; Furman et al., 1975). At this time, the concept of replacement of preoperative fluid deficit was widely adopted.
Airway problems presented ongoing challenges, and their management continued to register improved methods of control. Great emphasis was placed on the prevention of food aspiration and the damaging effects of hypoxia. The Sellick maneuver (1961) and many warnings from Salem (1970) about “the full stomach” were forever fixed in the mind of each new resident.
The treatment of acute epiglottitis by nasotracheal intubation instead of the former mandatory use of tracheostomy was a major change and clear advance, speeding recovery and significantly reducing serious complications (Oh and Motoyama, 1977).
Management of “the difficult airway” began to assume a larger role in both adult and pediatric anesthesia as more complicated procedures were undertaken on more deformed patients. By this time, some 120 different types of laryngoscope blades had been invented, each with some minor modifications to suit the designer, usually with no major advantage. It was the introduction of the fiberoptic laryngoscope that enabled anesthesiologists to intubate infants and children for whom this had been virtually impossible (Taylor and Towley, 1972; Stiles, 1974).
The startling appearance of what became known as malignant hyperthermia caused great concern, but widespread warnings about “triggering agents” and the discovery of a specific counteragent, dantrolene, usually brought it under reasonable control, at least when it was recognized early (Denborough et al., 1960; Britt, 1979; Gronert, 1980).
Surgical Progress
Interesting progress occurred in the evolution of infant surgery. TEF repair became a standardized procedure, and mortality was generally limited to infants with serious cardiac defects. Problems related to omphalocele repair were largely overcome by the introduction of Schuster’s staged mesh sac closure (1967). CDH, however, brought about increasing difficulties as smaller and more premature infants were encountered. Acidosis, shunting, and pulmonary hypertension posed problems yet to be solved (Raphaely and Downes, 1973; Dibbins, 1976). Attempts to use extracorporeal membrane oxygenation (ECMO) predicted later value for this procedure (Bartlett et al., 1979).
After the breakthrough in cardiac surgery accomplished by the establishment of extracorporeal circulation in larger children, the next step was to find a means of operating within the hearts of neonates without obstruction by intracardiac catheters. In 1965, the use of hyperbaric oxygenation served as a temporary answer, providing surgeons 3 to 5 minutes of inflow occlusion for the performance of aortic valvotomy and other brief procedures (Bernhard et al., 1966). The use of oxygen at 3 to 4 atmospheres of pressure substantially increased plasma oxygen-carrying capacity, while presenting increased potency of nitrous oxide and increased flammability (Smith et al., 1964).
The final hurdle in the approach to infant cardiac surgery was passed by the successful combination of deep hypothermia and extracorporeal circulation, providing time for the most complicated reconstruction of neonatal cardiac defects (Horiuchi et al., 1963; Hikasa et al., 1967). Rendering an infant virtually dead by complete cessation of respiration and circulation at a body temperature of 15° C appeared to be the ultimate in physiologic control. Agents and techniques have undergone several changes in subsequent years but remain fundamentally similar.
Among many well-known tenets established, two of particular interest to anesthesiologists were the danger of succinylcholine in patients with elevated serum potassium levels and the evident tolerance to anesthesia in patients with hemoglobin levels as low as 6 g/dL (Powell and Miller, 1975).
The craniofacial repair devised by Tessier and others (1967) for correction of the disfiguring deformities of Apert syndrome and Crouzon disease was a bold undertaking. It was equally challenging for the anesthesiologists involved with difficult airway management, prolonged maintenance, marked fluid and blood loss, and a particularly precarious period of recovery while the child’s head was so completely swathed in tight bandages that only the endotracheal tube was visible.
Anesthetic management to separate conjoined twins at birth was of such unique interest that any report of a single case was welcomed for publication. A separate team of surgeons and anesthesiologists was assigned to each twin, and multiple problems of shared organ systems, hemorrhage, and airway obstruction were noted (Furman et al., 1971; Winston et al., 1987).
Postoperative Patient Management: Ventilation, Resuscitation, and Intensive Care
One of the first needs to be met was the ability to provide prolonged tracheal intubation, fostered by Brandstater (1962), MacDonald and Stocks (1965), and Hatch (1968). The continuation of earlier efforts to devise ventilation machines for infants and children with cardiopulmonary pathology brought a succession of models, some as adaptations of those made for adults and others designed specifically for younger patients.
Recovery rooms or postanesthesia care units (PACUs) for routine postoperative care had been established in many hospitals over previous decades, but areas staffed by highly trained personnel and fully equipped for high-risk patient care became mandatory. The earliest units of this type, termed critical care or intensive care units, appeared in Goteburg, Sweden, in 1955; others were established in France in 1962, England in 1964, and Australia in 1963. In America they appeared in Philadelphia in 1967, Pittsburgh and Boston in 1969, and Toronto in 1971, where Conn established a prototype of the modern multidisciplinary unit for infants and children, including near-drowning survivors (Downes and Raphaely, 1975; Conn et al., 1980; Downes, 1992).
Important new approaches to resuscitation replaced such outmoded maneuvers as anal dilation, drugs promoted as respiratory stimulants, and the prone pressure method. During the trial-and-error development of pediatric cardiac surgery and the poliomyelitis epidemics, apparent death became an indication for immediate slashing into the chest and manual cardiac compression. When it became evident that the patients occasionally survived both the original insult and the therapeutic assault, the term “cardiac arrest” was coined (Singer, 1977). Because the exact cause of these mishaps commonly was uncertain, each was considered “an act of God,” and successful resuscitation was considered a feather in the cap of any anesthesiologist who had been associated with one. Intelligent procedures of ventilation and closed-chest cardiac compression, combined with electric and pharmacologic stimulation, brought far greater reason, order, and success.
Organized Teaching
Teaching clinics were established for residents and others throughout the United States, Canada, and England. Edward Eger stood out as teacher par excellence over 3 decades. Annual symposia on pediatric anesthesia initiated by Leigh in 1962 were followed by those organized by Conn in Toronto, Downes in Philadelphia, Salem in Chicago, Ryan in Boston, and others. Literature became increasingly available in periodicals, including both new editions of previously published texts and added texts such as those by Davenport (1967) of Canada and an excellent Australian text by Brown and Fisk (1979). Early exponents of the specialty became popular at local and national meetings, and an international exchange of speakers grew rapidly, stimulating interest and exchanging information on the rapidly developing scene.
International Progress
At this time, it became evident that considerable progress was being made in many parts of the world. In France, Delegue pioneered the modern stage, her text Memento a l’Usage de l’Anesthesiologiste-Reanimateur Pediatrique passing through several editions. Rapid advances were taking place in France in many aspects of pediatric anesthesia. A marked difference in their approach appeared with the use of combinations of intravenous phenothiazines, antihistamines, and barbiturates in place of inhalation agents and had remarkable success (G. Durand-Gurry, personal communication, 1993). Their work in regional anesthesia and pharmacology was also outstanding (Saint Maurice et al., 1986; Murat et al., 1988). Early leaders in Europe included Suuterinen of Finland; Swensson, Feyting, and Ekstrom-Jodal of Sweden; and Rondio and Wezyk of Poland. South African and Australian workers, adopting British methods, also kept abreast or ahead of other areas. Douglas Wilson has been called the real pioneer of pediatric anesthesia in Western Australia, and Margaret McLellan, John Stocks, Ian McDonald, M.A. Denborough, and others contributed on local and international levels. Japanese interest in pediatric anesthesia began later, but proceeded vigorously beginning in 1958; the publication of Pediatric Anesthesia in 1958 by Onchi and Fujita served as a valuable guide (Iwai and Satoyoshi, 1992). Throughout Latin America, Brazilian physicians and others followed North American methods to some extent, but in these countries, especially in Mexico, local and regional anesthetic techniques were depended on and consequently more highly developed than inhalation anesthesia (Fortuna, 1967; Melman et al., 1975). The great number of students who studied in North America and Europe from India, the Philippine Islands, and other Asiatic areas resulted in the growth of this organized activity and clinical excellence in their respective nations.
Research Stimulated by Clinical Advances
The introduction of various breathing devices, new muscle relaxants, and halogenated agents provided material for extensive investigation. The demonstration of the more rapid uptake of anesthetic gases by infants than by adults became a classic study, as did Motoyama’s measurements of pulmonary mechanics, with some of his contributions illuminating the pulmonary physiology of infants and children (Salanitre and Rackow, 1969; Motoyama, 1977; Motoyama and Cook, 1980). Bush and Stead (1962), Cook (1974), Goudsouzian et al. (1975), and other researchers did much to establish the comparable actions of successive neuromuscular blocking agents in the search for safer, shorter-acting, and reversible types (hence, greater control). The advent of new halogenated agents aroused the pharmacologic investigation of metabolism, toxicity, cardiovascular depression, and seizures, and the increased potency demanded vaporizers of greater accuracy (Brennan, 1957; Fabian et al., 1958; Lomaz, 1965). Other notable achievements included the establishment of minimum effective doses (ED50) of halothane by Nicodemus and others (1969) and the minimum alveolar concentration (MAC) as a standard measure of potency, followed by the application of MAC to demonstrate the higher halothane requirement for infants than for adults (Eger et al., 1965; Gregory et al., 1969).
Phase IV: progress and sophistication, 1980 to present
Recognition of Risk
During this phase, there has been a widespread appreciation that infants and small children are at increased risk for complications from anesthesia and surgery. Previous reports had shown that these were often related to cardiovascular factors (including hypovolemia and anemia), respiratory difficulties (e.g., airway obstruction, hypoxia, and inadequate ventilation), or electrolyte imbalance (e.g., hyperkalemia, hyponatremia, and hypoglycemia) (Salem et al., 1975). Additional reports from the United States described some of the problems in greater detail, as did others from France, Scandinavia, Canada, and England (Olsson and Hallen, 1988; Tiret et al., 1988; Cohen et al., 1990; Lunn, 1992; Morray et al., 1993; Keenan et al., 1994; Holzman, 1994). In particular, postoperative apnea, especially in very premature infants, was noted by several investigators, and overnight hospitalization in these situations was widely adopted (Steward, 1982; Gregory and Steward, 1983; Liu et al., 1983). In addition, other groups of patients were noted to be at increased risk of postoperative respiratory complications, such as children with obstructive sleep apnea who appear more sensitive to the respiratory depressant effects of narcotics (Brown et al., 2004, 2006; Sanders et al., 2006). Furthermore, there were indications that risk in small children could be decreased if individuals specially trained and experienced in pediatric anesthesia provided the anesthesia care (Keenan et al., 1991; Morray, 1994; Berry, 1995; Downes, 1995). Although this concept remains controversial, surgeons, pediatricians, and parents increasingly have begun to appreciate the important role of pediatric anesthesiologists.
Growth in Pediatric Facilities
In part because the post-World War II “baby boomers” were now having their own children, pediatric facilities exhibited enormous growth during this period. Freestanding children’s hospitals, which had existed for more than a century, expanded, and new pediatric hospitals were established. Some of these evolved as pediatric “hospitals within a hospital,” whereby large general hospitals developed specific buildings, wings, or floors to provide specialized care for children. Currently, several large children’s hospitals in the United States are performing more than 20,000 surgical procedures per year. Some children’s hospitals perform more operations and have more operating rooms and staff than major hospitals caring for adult patients. Reports have been published indicating that the outcome (at least for some conditions in small children) is better when surgery is performed by pediatric specialists in large pediatric centers (Bratton et al., 2001; Kososka et al., 2001). The American Academy of Pediatrics (2002, 2003) has disseminated several policy statements emphasizing the need to have proper personnel and facilities available whenever children require surgery or anesthesia.
Changing Patterns of Care
There has also been a great impetus to perform surgical procedures in younger and younger patients. Whereas this was not possible or was fraught with danger in earlier eras, better equipment and surgeons well trained in pediatric subspecialties have made this not only feasible but also desirable for children. Outcome studies in several areas have shown that corrective procedures performed in infancy, rather than palliative procedures done initially and followed by full repair later in life, lead to improved long-term results. For example, in 1975 the American Academy of Pediatrics advocated surgical repair of elective urologic defects in children after the age of 4 years but by 1996 was recommending these repairs occur in infancy. Likewise, repair of congenital cardiac anomalies shifted from early palliative procedures (largely shunts) to corrective repairs in the neonatal period (Jenkins et al., 1995). This tendency to perform more complex surgical procedures in younger patients undoubtedly contributed to the growth of major pediatric centers.
When anesthesiologists are unable or unwilling to assist in these areas, physicians from other specialties (including pediatricians, intensivists, and hospitalists) or nurses have stepped in (Malviya et al., 1997; Lowrie et al., 1998; Lightdale et al., 2008). Whether this affords a comparable degree of safety to administration of anesthesia by anesthesiologists in these situations remains to be seen. In any case, the large number of children requiring sedation or immobilization for nonoperative procedures has led to the development of sedation guidelines by several organizations representing anesthesiologists, pediatricians, emergency physicians, dentists, and others, with some variability among them (American Academy of Pediatrics, 1992; American Society of Anesthesiologists, 1996; American College of Emergency Physicians, 1997; American Academy of Pediatric Dentistry, 1997). A group of pediatric anesthesiologists at Dartmouth Hitchcock Medical Center convened in 2000 to develop a consensus among physicians from varied disciplines in this regard, and they continue to disseminate a bulletin via email to individuals interested in this ongoing issue (Dartmouth Sedation Project, 2000).
New Developments in Anesthesia
Although the scope of new developments in anesthesia is the subject of much of the remainder of this book, there are several major changes worth noting. The age-old, commonly used, restriction of preoperative intake ultimately underwent scrutiny, which resulted in changes in concept and reduction of fasting time (Coté, 1990; Ferrari et al., 1999). New, potent, inhalational anesthetic agents have been developed, virtually replacing halothane as the “standard” for the previous generation. To induce anesthesia via mask, sevoflurane is used almost exclusively, because it acts faster and results in less bradycardia and hypotension (Sarner et al., 1995; Holzman et al., 1996). This has been especially important in infants. Isoflurane is often used for maintenance of anesthesia, in part because it is currently cost effective. Desflurane may be used when particularly rapid awaking is desired, although the need for special, temperature-regulated vaporizers has limited its popularity. None of these newer agents, however, has the smooth induction properties of sevoflurane. Concerns have been raised about breakdown products that may develop when sevoflurane interacts with some carbon dioxide absorbents, especially when desiccated, leading to overheating of the absorbent system, carbon monoxide production, or both (Holak et al., 2003). The clinical significance of this remains unclear.
A new, major feature in airway management has been the promotion of the laryngeal mask airway to eliminate tracheal intubation for many simple procedures, as well as provide airway access in emergency situations when intubation is difficult (Brain, 1983; Mason and Bingham, 1990; Pennant and White, 1993). Endoscopes have also been developed that permit fiberoptic intubation even in small children.
Several “descendents” of fentanyl have been developed, with remifentanil capable of providing potent and transient analgesia when administered via constant intravenous infusion (Davis et al., 2001; Galinkin et al., 2001; Ross et al., 2001). Fentanyl transcutaneous “patches” have also been developed, largely to provide analgesia for chronic pain, and fentanyl is sometimes administered transnasally or transorally (Friesen et al., 1995; Viscusi et al., 2004). Potent opioids have been particularly useful for cardiac procedures when inhaled anesthetic agents are not well tolerated (Hickey and Hansen, 1984). Perhaps more significant, the prolonged debate over the need for anesthesia at all during surgery on small infants was finally terminated when Anand and Hickey (1987) produced evidence of physiologic stress in infants under light anesthesia. This brought general agreement that all infants should receive anesthesia during surgery, although the best method for doing this, especially when the patient is a fetus, remains to be fully elucidated.
However, concern has been expressed in recent years about potential adverse effects of many anesthetic agents on brain development. Studies in young animals receiving ketamine, midazolam, nitrous oxide, isoflurane, and other commonly used anesthetic agents have indicated increased apoptosis and cell death, at least in some experimental models, raising fears that similar findings could possibly occur when these drugs are administered to young children (Jevtovic-Todorovic and Olney, 2008). In view of the apparent increase in the incidence of autism and related disorders, much investigation is ongoing in this regard (Sun et al., 2008; Davidson et al., 2008). However, current consensus is that the benefits of a well-conducted anesthesia of the relatively brief duration necessary to perform most surgical procedures far outweigh the theoretical risk of anesthetic neurotoxicity (Soriano et al., 2005; Mellon et al., 2007; Loepke et al., 2008).
Propofol has become the most common intravenous induction agent for adults, but has not completely replaced thiopental in children because propofol causes some discomfort when injected into small peripheral veins. Midazolam has replaced diazepam as the intravenous benzodiazepine of choice for sedation in children; it is also the most popular oral sedative in the preoperative setting (Kain et al., 2000). Midazolam can be administered via several other routes (including intranasal) and is sometimes used rectally because production of methohexital, which is often used for this purpose, has ceased for economic reasons. The use of topical agents including lidocaine-prilocaine (EMLA; Freeman et al., 1993) or lidocaine-tetracaine (Synera; Sethna et al., 2005) for skin desensitization eases the discomfort of venipuncture for intravenous induction but requires adequate time to become effective. Intramuscular injections are now rarely necessary in pediatric anesthesia practice.
Several new, nondepolarizing muscle relaxants have replaced curare. Although pancuronium is commonly used for lengthy procedures, several shorter-acting agents (especially cisatracurium and vecuronium) are often administered for brief procedures and have fewer side effects. Rocuronium is often used to facilitate emergency endotracheal intubation, and succinylcholine is no longer administered without good cause because masseter spasm, malignant hyperthermia, or both occasionally develop after its administration, especially in the presence of potent inhaled anesthetic agents (Schwartz et al., 1984). In addition, the Food and Drug Administration (1997) issued a “black box” warning because of serious complications (including cardiac arrest resulting from acute hyperkalemia) associated with its use, particularly in young boys with unrecognized muscular dystrophy.
Although there was some use of local and regional anesthesia for children before 1940, the development of improved inhalation methods soon largely displaced other forms of anesthesia. Regional anesthesia became more commonplace in children, beginning in Europe with “single-shot” techniques (e.g., spinal, caudal, or peripheral nerve block) reducing the requirements for general anesthesia and providing postoperative analgesia (Abajian et al., 1984; Yaster and Maxwell, 1989; Dalens, 1989; Bosenberg and Ivani, 1998; Williams et al., 2006). Small, portable ultrasound machines have been introduced, which add great precision to the ability to deliver local anesthetic solutions to the desired location (Willschke et al., 2005, 2006; Marhofer et al., 2005; Marhofer and Chan, 2007; Tran et al., 2008). In addition, continuous infusions of local anesthetics with or without fentanyl are often delivered intraoperatively and postoperatively via catheters placed during surgery, especially via the epidural route. This reflects the much greater emphasis on the needs of the pediatric patient in the postoperative period. Pain control after surgery has been a major focus of pediatric anesthesiologists with patient-, parent-, or nurse-controlled analgesia available via computer-controlled infusion pumps for delivery of medications by the intravenous, epidural, or perineural route (Ganesh et al., 2007). Pain treatment services have become more important aspects of the mission of most departments of pediatric anesthesia to provide care for children with medical and surgical pain (Zeltzer et al., 1989; Berde and Sethna, 2002; Schecter et al., 2002).
In addition, outpatient pain treatment clinics evaluate and treat many chronic pain conditions in childhood; although largely directed by anesthesiologists, these are multidisciplinary (using nerve blocks, oral medications, acupuncture, hypnosis, and behavioral modification) and involve the participation of neurologists, neurosurgeons, physiatrists, physical therapists, psychologists, and many other ancillary medical and nursing personnel. Greater attention has been directed toward assessing and allaying preoperative fears and anxiety of patients and their families. Preoperative clinics are used to evaluate many patients in advance of their procedures, and significant attention has been devoted to methods of easing induction of anesthesia, especially the use of oral premedicants and parental presence during mask induction (Kain et al., 1998a, 1998b, 2000, 2003). In all instances, kindness remains the essential feature in preoperative management.
Progress in Monitoring
Technical advances have also greatly enhanced patient monitoring. Smaller equipment is now readily available so that young patients can be monitored as carefully as critically ill adults. Percutaneous catheters can be inserted directly into virtually any peripheral vein or artery; the Seldinger technique (if necessary with ultrasound guidance) can be used to insert central catheters (Ganesh and Jobes, 2008). Echocardiography can be performed transthoracically or transesophageally in small children as well as in adults. Its use has greatly facilitated the ability of cardiac surgeons to assess the repair of congenital heart lesions intraoperatively (Ungerleider et al., 1990). “Standard” monitoring is now quite extensive and sophisticated as promulgated by the American Society of Anesthesiologists (1986) and includes continuous pulse oximetry and capnography. It appears that the average anesthesiologist is often able to manage a procedure with little or no direct observation of the patient, although a precordial or an esophageal stethoscope is still considered valuable by many pediatric anesthesiologists.
Advances in the intensive care unit also have been significant during this time and extend into the operating rooms. Patients with severe lung injury are now managed with several new forms of respiratory support, including advanced mechanical ventilators, inhaled nitric oxide, and extracorporeal membrane oxygenation or ECMO (Neonatal Inhaled Nitric Oxide Study Group, 1997; Bartlett et al., 2000; Aharon et al., 2001; Campbell et al., 2003). This latter technique can be used for weeks at a time and as an emergency method of resuscitation from cardiac arrest when a reversible condition is suspected (Laussen, 2002).
Advances in Surgery
Numerous developments have occurred in surgical techniques that have greatly influenced anesthesia practice and are discussed elsewhere in this text. However, it is worthwhile noting that laparoscopic techniques, robotics, and intraoperative imaging have progressed so extensively and rapidly into pediatric practice that anesthesiologists have had to accommodate the special problems and challenges presented by these situations. Perhaps nowhere is this more apparent than the field of fetal surgery where surgeons, obstetricians, anesthesiologists, and neonatologists must collaborate to care for two patients simultaneously, as techniques for surgical repair of prenatal anomalies are developed and assessed (Harrison et al., 1982, 1993; Rosen, 1992; Cauldwell, 2002). Controversy remains, however, about the best way to provide analgesia during fetal procedures (Benatar and Benatar, 2001; Myers et al., 2002; Schwartz and Galinken, 2003; Lee et al., 2005).
Organ transplantation has extended beyond kidneys, and transplantation of the liver has become a major challenge for pediatric surgeons and anesthesiologists (Starzl et al., 1963). To support a young patient with hepatic failure through a repeat liver transplant potentially requiring replacement of multiple blood volumes while monitoring and maintaining cardiac function, temperature, and many components of blood chemistry during an 8- to 12-hour operation is one of the most demanding anesthesia-related tasks known. The record of 1000 liver transplantations performed at the Children’s Hospital of Pittsburgh between 1980 and 1990 with a nearly 70% survival rate stands as a remarkable accomplishment of both surgeons and anesthesiologists (Robertson and Borland, 1990). Transplantation of the heart, lungs, or both appears similarly impressive, although it is not associated with the same degree of blood loss (Jamieson et al., 1984). Intestinal transplants more recently have emerged as a potential method for treating children with short bowel syndrome (Mazariegos et al., 2008). In any case, a shortage of available organs, especially those of a small size, greatly limits pediatric transplantation, and attempts to grow organs in cell culture has evolved into the new and fascinating field of tissue engineering (Langer and Vacanti, 1993; Lanza et al., 2000; Dunn, 2008).
Research Efforts
Activity in research is reaching a new level of excellence and productivity, whereas demands for clinical service and limitations of funding make this an ongoing challenge, and old problems persist. As noted by Berry (1993), failure in the endless search for better preoperative sedation lies in controversies regarding basic ground rules for investigation, especially in children. In many fields, understanding of the problems has been gradually extended, with resultant improvement in the practical management of patients. Physiologic studies of cerebral circulation in the neonate by Rogers and colleagues (1980), gas exchange in cardiac patients, pharmacologic biotransformation of sedatives, the infant and the myoneural junction, and hypoxia in children who have undergone anesthesia are but a few of the important initial studies (Goudsouzian and Standaert, 1986; Motoyama and Glazener, 1986; Saint-Maurice et al., 1986; Lindahl, 1989; Fletcher, 1993). The report by Lerman and others (1986) concerning postanesthetic vomiting after strabismus surgery is another illustration of one of the problems of the conscious child that remains particularly difficult to control. Advances in molecular biology, mapping the human genome, and genomic pharmacology offer great hope that it will be possible to “customize” care for individual patients in a continuing attempt to drive the curve of improvement mentioned by Rees (1991) “closer to perfection” (Holtzman and Marteau, 2000).
Development of the Subspecialty
Pediatric anesthesia organizations have advanced greatly over the recent generation. Although a small group of anesthesiologists from the United States and Canada have had a section within the American Academy of Pediatrics since 1966 (now called the Section on Anesthesiology and Pain Medicine), it was not until 1973 that pediatric activities formally became a part of the American Society of Anesthesiologists. At this same time, a separate organization devoted to pediatric anesthesiology was established in the United Kingdom, but it was not until 1986 that the Society for Pediatric Anesthesia (SPA) was created in the United States. This is now the largest organization in the world devoted to pediatric anesthesiology with more than 4000 members largely from the United States. The SPA has held annual meetings since 1987 in association with the annual meeting of the American Society of Anesthesiologists. For several years, it has also organized an additional winter educational meeting. The goals of the SPA are listed in Box 41-1; the SPA’s presidents are listed in Table 41-1.
Box 41-1 Goals of the Society for Pediatric Anesthesia
TABLE 41-1 Presidents of the Society for Pediatric Anesthesia
| Year | President | Location |
| 1986 to 1988 | Myron Yaster, MD | Baltimore, Md. |
| 1988 to 1990 | Robert Crone, MD | Seattle, Wash. |
| 1990 to 1992 | Aubrey Maze, MD | Phoenix, Ariz. |
| 1992 to 1994 | Charles Lockhart, MD | Denver, Colo. |
| 1994 to 1996 | William Greeley, MD | Durham, N.C. |
| 1996 to 1998 | Mark Rockoff, MD | Boston, Mass. |
| 1998 to 2000 | Steven Hall, MD | Chicago, Ill. |
| 2000 to 2002 | Peter Davis, MD | Pittsburgh, Pa. |
| 2002 to 2004 | Anne Lynn, MD | Seattle, Wash. |
| 2004 to 2006 | Francis McGowan, MD | Boston, Mass. |
| 2006 to 2008 | Jayant Deshpande, MD | Nashville, Tenn. |
| 2008 to 2010 | Joseph Tobin, MD | Winston-Salem, N.C. |
The SPA collaborated with other pediatric anesthesiology groups in developing a proposal to have fellowship training formally accredited by the Accreditation Council for Graduate Medical Education (Rockoff and Hall, 1997). This was successful in 1997, and there are currently 45 programs in the United States that offer 1 year of training in pediatric anesthesiology to individuals who have completed a basic residency in anesthesiology (Accreditation Council for Graduate Medical Education, 2008). Furthermore, subspecialization within pediatric anesthesiology continues to advance informally with individuals developing additional experience in areas such as pediatric pain medicine, pediatric intensive care, pediatric cardiac anesthesiology, and others.
Finally, many additional textbooks directed to pediatric anesthesia have been published, and several have been updated into multiple editions; these include texts by Gregory (2002), Coté et al. (2009), Motoyama and Davis (2006), Berry (1990), Brown and Fisk (1992), Sumner and Hatch (1999), Steward and Lerman (2001), Bisonnette and Dalens (2002), and others. The further development of pediatric anesthesiology into subspecialties became evident in texts on topics such as uncommon diseases, neonatal anesthesia, pediatric pain regional anesthesia, cardiac anesthesia, and intensive care (Dalens et al., 1990; Saint-Maurice and Steinberg, 1990; Bush and Harkins, 1991; Katz and Steward, 1993; Hatch et al., 1995; Rogers, 1996; Tobias and Deshpande, 1996; Todres and Fugate, 1996; Schechter et al., 2002; Lake and Booker, 2004). The journal Paediatric Anaesthesia, originally edited by Bush in Liverpool and Saint-Maurice in Paris, became the first independent monthly publication devoted to this field. With its international editorial board members, it has led to enhanced worldwide relationships among pediatric anesthesiologists. Anesthesia & Analgesia, one of the largest anesthesia journals in the world, has also developed a separate section devoted to pediatric anesthesia. Furthermore, pediatric anesthesiologists are members of the editorial boards of Anesthesiology and several other well-respected international anesthesia journals.
Abajian J.C., Mellish R.W., Browne A.F., et al. Spinal anesthesia for the high-risk infant. Anesth Analg. 1984;63:359.
Accreditation Council for Graduate Medical Education. Available at www.ACGME.org Accessed December 29, 2008
Aharon A.S., Drinkwater D.C., Churchwell K.B., et al. Extracorporeal membrane oxygenation after repair of congenital cardiac lesions. Ann Thorac Surg. 2001;72:2095.
American Academy of Pediatric Dentistry. Guidelines for the elective use of pharmacologic conscious sedation and deep sedation in pediatric dental patients. Pediatr Dent. 1997;19:48.
American Academy of Pediatrics, Clinical Report. Facilities and equipment for the care of pediatric patients in a community hospital. Pediatrics. 2003;111:1120.
American Academy of Pediatrics, Committee on Drugs, Section on Anesthesiology. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:1110.
American Academy of Pediatrics, Section on Urology. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychologic effects of surgery and anesthesia. Pediatrics. 1996;97:590.
American Academy of Pediatrics, Surgical Advisory Panel. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110:187.
American College of Emergency Physicians. The use of pediatric sedation and analgesia. Ann Emerg Med. 1997;29:834.
American Society of Anesthesiologists House of Delegates. Standards for basic intraoperative monitoring. October 21, 1986. Park Ridge, III
American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 1996;84:459.
Anand K.J.S., Hickey P.R. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321.
Anderson S.M. Controlled hypotension with Arfonad in pediatric surgery. Br Med. 1955;72:103.
Anderson S.M. Use of depressant and relaxant drugs in infants and children. Lancet. 1951;2:965.
Apgar V. Proposal for new method of evaluation of newborn infants. Anesth Analg. 1953;32:260.
Armand-Delille P.F. Morphine injection before induction of general anesthesia in children. Bull Acad Natl Med. 1932;107:890.
Ayre P. Endotracheal anesthesia for babies with special reference to harelip and cleft lip operations. Anesth Analg. 1937;16:330.
Bain J.A., Spoerel W.E. A streamlined anaesthetic system. Can Anaesth Soc J. 1972;19:426.
Baron S.H., Kohlmoos H.W. Laryngeal sequelae of endotracheal anesthesia. Ann Otol Rhinol Laryngol. 1951;60:67.
Bartlett R.H., Gazzaniga A.B., Huxtable R.H. Extracorporeal membrane oxygenation (ECMO) in newborn respiratory failure: technical consideration. Trans Am Soc Artif Organs. 1979;25:473.
Bartlett R.H., Roloff D.W., Custer J.R., et al. Extracorporeal life support: the University of Michigan experience. JAMA. 2000;283:904.
Beecher H.K., Todd D.P. A study of the deaths associated with anesthesia and surgery. Ann Surg. 1954;140:2.
Benatar D., Benatar M. A pain in the fetus: toward ending confusion about fetal pain. Bioethics. 2001;15:57.
Bennett E.J., Dougherty M.J., Jenkins M.T. Fluid requirements for neonatal anesthesia and operation. Anesthesiology. 1970;32:343.
Berde C.B., Sethna N.F. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094.
Bernhard W.F., Navarro R.V., Yagi H., et al. Cardiovascular surgery in infants performed under hyperbaric conditions. Vasc Dis. 1966;3:33.
Berry F.A. Anesthetic management of difficult and routine pediatric patients, ed 2. New York: Churchill Livingstone, 1990.
Berry F.A. Editorial: when is a control group not a control? Paediatr Anaesth. 1993;3:63.
Berry F.A. The winds of change. Paediatr Anaesth. 1995;5:279.
Betcher A.M. Hypno-induction techniques in pediatric anesthesia. Anesthesiology. 1958;19:279.
Bigelow H.J. Insensibility during surgical operations produced by inhalation. Boston Med Surg J. 1846;35:16.
Bissonnette B., Dalens B.J. Pediatric anesthesia: principles and practice. New York: McGraw-Hill, Medical Pub. Division, 2002.
Bosenberg A.T., Ivani G. Regional anesthesia-children are different. Paediatr Anaesth. 1998;8:447.
Brain A.I.J. The laryngeal mask: a new concept in airway management. Br J Anaesth. 1983;55:801.
Brandstater B.M.. Prolonged intubation: an alternative to tracheostomy in infants. Proceedings of the European Congress of Anaesthesia. 1962.
Bratton S.L., Haberkern C.M., Waldhausen J.H.T., et al. Intussusception: hospital size and risk of surgery. Pediatrics. 2001;107:299.
Brennan H.J. Vaporizer for fluothane. Br J Anaesth. 1957;29:332.
Britt B.A. Etiology and patho-physiology of malignant hypothermia. Fed Proc. 1979;38:44.
Brown K.A., Laferrière A., Moss I.R. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806.
Brown K.A., Laferrière A., Lakheeram I., et al. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105:665.
Brown T.C.K., Fisk G.C. Anaesthesia for children, ed 2. Oxford: Blackwell, 1992.
Brown T.C.K., Fisk G.C. Anaesthesia for children. Oxford: Blackwell Publishing, 1979.
Bush G.H., Stead A.L. The use of d-tubocurarine in neonatal anaesthesia. Br J Anaesth. 1962;34:721.
Bush J.P., Harkins S.W. Children in pain: clinical and research issues from a developmental perspective. New York: Springer-Verlag, 1991.
Buxton D.W. Anaesthetics: their use and administration. London: HK Lewis Co Ltd, 1888.
Campbell B.T., Braun T., Schumacher R., et al. Impact of ECMO on neonatal mortality in Michigan.1980–1999. J Pediatr Surg. 2003;38:290.
Cauldwell C.B. Anesthesia for fetal surgery. Anesthesiol Clin North America. 2002;20:211.
Cohen M.M., Cameron C.B., Duncan P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70:160.
Cole F. A new endotracheal tube for infants. Anesthesiology. 1945;6:87.
Colgan F.C., Keats A.S. Subglottic stenosis, a cause of difficult intubation. Anesthesiology. 1957;18:265.
Conn A.W. Origins of paediatric anaesthesia in Canada. Paediatr Anaesth. 1992;2:179.
Conn A.W., Montes J.E., Barker G.A., et al. Cerebral salvage in near-drowning following neurological classification by triage. Can Anaesth Soc J. 1980;27:201.
Cook D.R. Neonatal anesthetic pharmacology: a review. Anesth Analg. 1974;53:544.
Coté C.J. NPO after midnight for children: a reappraisal. Anesthesiology. 1990;72:589.
Coté C.J., Lerman J., Todres I.D. A practice of anesthesia for infants and children, ed 4. Philadelphia: Elsevier, 2009.
Cullen S.C. The use of curare for the improvement of abdominal muscle relaxation during inhalation anesthesia. Surgery. 1943;14:261.
Dalens B. Regional anesthesia in children. Anesth Analg. 1989;68:654.
Dalens B.J., Monnet J.P., Harmand Y. Pediatric regional anesthesia. CRC Press, 1990.
Dartmouth. Pediatric Sedation Project Site. http://an.hitchcock.org/PediSedation, 2000. Available at
Davenport H.T. Paediatric anaesthesia. Philadelphia: Lea & Febiger, 1967.
Davenport H.T., Barr M.N. Blood loss during pediatric operations. Can Med Assoc. 1963;789:1309.
Davidson A.J., McCann M.E., Morton N.S., et al. Anesthesia and outcome after neonatal surgery: the role for randomized trials. Anesthesiology. 2008;109:941.
Davis P.J., Galinkin J., McGowan F.X., et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. Anesth Analg. 2001;93(1380):1387.
Deming M.V. Agents and techniques for induction of infants and young children. Anesth Analg. 1952;31:113.
Denborough M.A., Forster F.A., Lovell R.R.H., et al. Anaesthetic deaths in a family. Lancet. 1960;2:45.
Dibbins A.W. Neonatal diaphragmatic hernia: a physiologic challenge. Am J Surg. 1976;131:408.
Domino E.F., Chodoff P., Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic in man. Clin Pharm Ther. 1965;6:279.
Dorsch J.A., Dorsch S.E. Understanding anesthetic equipment. Baltimore: Williams & Wilkins, 1975.
Downes J.J. The historical evolution, current status, and prospective development of pediatric critical care. Crit Care Clin. 1992;8:1.
Downes J.J. What is a paediatric anaesthesiologist? The American perspective. Paediatr Anaesth. 1995;5:277.
Downes J.J., Raphaely R.C. Pediatric intensive care. Anesthesiology. 1975;43:238.
Dunn J.C.Y. Tissue engineering and regenerative science in pediatrics. Pediatr Res. 2008;63:459.
Eather K.E. Axillary brachial plexus block. Anesthesiology. 1958;19:683.
Eckenhoff J.E. Anesthesia from Colonial times. Philadelphia: JB Lippincott, 1966.
Eckenhoff J.E. Relationship of anesthesia to postoperative personality changes in children. Am J Dis Child. 1953;86:587.
Eckenhoff J.E. Some anatomic considerations of the infant larynx influencing endotracheal intubation. Anesthesiology. 1951;12:401.
Eger E.I., Saidman L.J., Branstater B. Minimum alveolar concentration: a standard of anesthetic potency. Anesthesiology. 1965;26:756.
Enderby G.E.H. Controlled circulation with hypotensive drugs and posture to reduce bleeding in surgery. Lancet. 1950;1:1145.
Fabian L.W., Newton G.W., Stephen C.R. Simple and accurate fluothane vaporizer. Anesthesiology. 1958;19:284.
Ferrari L.R., Rooney F.M., Rockoff M.A. Preoperative fasting practices in pediatrics. Anesthesiology. 1999;90:978.
Flagg P.J. Endotracheal inhalation anesthesia: special reference to postoperative reaction and suggestions for their elimination. Laryngoscope. 1951;61:1.
Fletcher R. Gas exchange during anaesthesia and controlled ventilation in children with congenital heart disease. Paediatr Anaesth. 1993;3:5.
Food and Drug Administration. cardiac arrest in children and adolescent patients receiving succinylcholine: succinylcholine-induced hyperkalemia. 1997.http://www.fda.gov/medwatch/safety/1997/succin.htm. Available at
Fortuna A. Caudal analgesia: a simple and safe technique in paediatric surgery. Br J Anaesth. 1967;39:165.
Freeman J.A., Doyle E., Im N.G., et al. Topical anaesthesia of the skin. Paediatr Anaesth. 1993;3:129.
Friesen R.H., et al. Oral transmucosal fentanyl citrate for preanaesthetic medication for pediatric cardiac surgery patients. Paediatr Anaesth. 1995;5:29.
Furman E.B., Roman D.G., Hairabet J., et al. Management for surgical separation of newborn conjoined twins. Anesthesiology. 1971;34:95.
Furman E.B., Roman D.G., Hemmer E., et al. Specific therapy in water, electrolyte and blood volume replacement during pediatric surgery. Anesthesiology. 1975;42:187.
Galinkin J.L., Davis P.J., McGowan F.X., et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. Anesth Analg. 2001;93:1387.
Ganesh A., Jobes D.R. Ultrasound guided catheterization of the internal jugular vein. Anesthesiology. 2008;108:1155.
Ganesh A., Rose J.B., Wells L., et al. Continuous peripheral nerve blockade for inpatient and outpatient post-operative analgesia in children. Anesth Analg. 2007;105:1234.
Gibbon J.H. Artificial maintenance of circulation during experimental occlusion of pulmonary artery. Arch Surg. 1937;34:1105.
Gillespie N.A. Endotracheal anaesthesia in infants. Br J Anaesth. 1939;17:2.
Goldbloom R.B. Halifax and the precipitate birth of pediatric surgery. Pediatrics. 1917;11:164.
Goudsouzian N.G., Standaert F.G. The infant and the myoneural junction. Anesth Analg. 1986;65:1208.
Goudsouzian N.G., Donlon J.N., Savarese J.J. Re-evaluation of dosage and duration of action of d-tubocurarine in the pediatric age group. Anesthesiology. 1975;43:416.
Gregory G.A. Pediatric anesthesia, ed 4. Philadelphia: Churchill Livingstone, 2002.
Gregory G.A., Steward D.J. Life-threatening perioperative apnea in the ex-premie. Anesthesiology. 1983;59:495.
Gregory G.A., Eger E.I., Munson E.S. The relationship between age and halothane requirement. Anesthesiology. 1969;31:344.
Griffith H.R. John Snow, pioneer specialist in anesthesia. Anesth Analg. 1934;13:45.
Gronert G.A. Malignant hyperthermia. Anesthesiology. 1980;53:395.
Gross R.E. The surgery of infancy and childhood. Philadelphia: WB Saunders, 1953.
Guedel A. Inhalation anesthesia. New York: The Macmillan Co, 1937.
Gwathmey J.T. Anesthesiology in infants and children. Pediatrics. 1907;19:734.
Haggard H.W. The place of the anesthetist in American medicine. Anesthesiology. 1940;1:1.
Harmel H.H., Lamont A. Anesthesia in the surgical treatment of congenital pulmonary stenosis. Anesthesiology. 1948;7:477.
Harris A.J. Management of anesthesia for congenital heart operation in children. Anesthesiology. 1950;11:328.
Harrison M.R., Golbus M.S., Filly R.A., et al. Open fetal surgery was performed at UCSF. N Engl J Med. 1982;308:591.
Harrison M.R., Adzick N.S., Flake A.W., et al. Correction of congenital diaphragmatic hernia in utero: VI. Hard lessons learned. J Pediatr Surg. 1993;28:1411.
Hatch D.J. Prolonged nasotracheal intubation in infants and children. Lancet. 1968;1:1272.
Hatch D., Sumner E., Hellmann J. Surgical neonate: anaesthesia and intensive care, ed 3. New York: Oxford University Press, 1995.
Herbert W.I., Scott E.B., Lewis G.B. Fluid management of pediatric patients. Anesth Analg. 1971;50:376.
Hewer C.L. Anaesthesia in children. London: HK Lewis Co. Ltd, 1923.
Hickey P.R., Hansen D.D. Fentanyl- and sufentanil-oxygen-pancuronium anesthesia for cardiac surgery in infants. Anesth Analg. 1984;63:117.
Hikasa Y., Shirocani H., Satomura K., et al. Open-heart surgery in infants with an aid of hypothermic anesthesia. Arch Jpn Chir. 1967;36:495.
Hodges R.J.H. Suxamethonium tolerance and pseudocholinesterase levels in children. In: Proceedings of World Conference of Anaesthetists. the Netherlands: Sheveningen; 1955:247-251. September 5-10
Holak E.J., Mei D.A., Dunning M.B., et al. Carbon monoxide production from sevoflurane breakdown: modeling of exposures under clinical conditions. Anesth Analg. 2003;96:757.
Holliday M.A., Segar W.E. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823.
Holtzman N.A., Marteau T.M. Will genetics revolutionize medicine? N Engl J Med. 2000;343:141.
Holzman R.S. Morbidity and mortality in pediatric anesthesia. Pediatr Clin North Am. 1994;41:239.
Holzman R.S., van der Velde M.E., Kaus S.J., et al. Sevoflurane depresses myocardial contractility less than halothane during induction of anesthesia in children. Anesthesiology. 1996;85:1260.
Horiuchi T., Koyamada K., Matano I., et al. Radical operation for ventricular septal defect in infancy. J Thorac Cardiovasc Surg. 1963;46:180.
Hunt K.H. Resistance in respiratory valves and canisters. Anesthesiology. 1955;16:190.
Inkster J.S. The T-piece technique in anaesthesia. Br J Anaesth. 1956;28:512.
Iwai S., Satoyoshi M. History of paediatric anaesthesia in Japan. Paediatr Anaesth. 1992;2:275.
Jackson K. Psychological preparation as a method of reducing the emotional trauma of anesthesia in children. Anesthesiology. 1951;12:293.
Jamieson S.W., Stinson E.B., Baldwin J.C., et al. Operative technique for heart-lung transplantation. J Thorac Cardiovasc Surg. 1984;87:930.
Jenkins K.J., Newburger J.W., Lock J.E., et al. In-hospital mortality for surgical repair of congenital heart defects: preliminary observations of variation in caseload. Pediatrics. 1995;95:323.
Jevtovic-Todorovic V., Olney J.W. Pro: Anesthesia-induced developemental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106:1659.
Johnstone M. The human cardiovascular response to fluothane anesthesia. Br J Anaesth. 1956;28:392.
Junkin C.L., Smith C., Conn A.W. Fluothane for pediatric anesthesia. Can Anaesth Soc J. 1957;4:259.
Kain Z.N., Mayes L.C., Wang S.M., et al. Parental presence during induction of anesthesia vs. sedative medication: which intervention is more effective? Anesthesiology. 1998;89:1147.
Kain Z.N., Mayes L.C., Caramico L.A., et al. Preoperative preparation programs in children: a comparative examination. Anesth Analg. 1998;87:1249.
Kain Z.N., Hofstadter M.B., Mayes L.C., et al. Midazolam: effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676.
Kain Z.N., Caldwell-Andrews A.A., Wang S.M., et al. Parental intervention choices for children undergoing repeated surgeries. Anesth Analg. 2003;96:970.
Katz J., Steward D.J. Anesthesia and uncommon pediatric diseases, ed 2. Philadelphia: WB Saunders, 1993.
Keenan R.L., Shapiro J.H., Kane F.R., et al. Bradycardia during anesthesia in infants: an epidemiologic study. Anesthesiology. 1994;80:976.
Kilduff C.J., Wyant G.M., Dale R.H. Anaesthesia for repair of cleft lip and palate in infants using moderate hypothermia. Can Anaesth Soc J. 1956;3:102.
Kopetsky S.J. The selection of anesthesia in children. Med Rec. 1903;14:534.
Kososka E.R., Minkes R.K., Silen M.L., et al. Effect of pediatric surgical practice on the treatment of children with appendicitis. Pediatrics. 2001;107:1298.
Lake C.L., Booker P.D. Pediatric cardiac anesthesia, ed 4. Philadelphia: Lippincott Williams and Wilkins, 2004.
Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920.
Lanza R.P., Langer R., Vacanti J.P. Principles of tissue engineering, ed 2. New York: Academic Press, 2000.
Laussen P.C.. Mechanical support of the circulation: The ABC&E of pediatric resuscitation, 2002. Available at http://web1.tch.harvard.edu/heartmurmurs/fall2002/art1.html Accessed March 24, 2005
Lee S.J., Ralston H.J.P., Drey E.A., et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947.
Leigh M.D., Belton M.K. Pediatric anesthesia. New York: The Macmillan Co, 1948.
Leigh M.D., McCoy D.D., Belton M.K., et al. Bradycardia following intravenous administration of succinylcholine chloride to infants and children. Anesthesiology. 1957;18:698.
Lerman J., Eustis S., Smith D.R. Effect of droperidol pretreatment on postanesthetic vomiting in children undergoing strabismus surgery. Anesthesiology. 1986;65:322.
Levy D.M. Psychic trauma of operation in children. Am J Dis Child. 1945;69:75.
Lewis F.J., Taufic M. Closure of atrial septal defect with the aid of hypothermia: Experimental accomplishments and report of one successful case. Surgery. 1953;33:52.
Lightdale J.R., Valim C., Newburg A.R., et al. Efficiency of propofol vs. midazolam and fentanyl sedation at a pediatric teaching hospital: a prospective study. Gastrointest Endosc. 2008;67:1067.
Lindahl S.G.E. Oxygen consumption and carbon dioxide elimination in infants and children during anaesthesia and surgery. Br J Anaesth. 1989;62:70.
Liu L.M.P., Coté C.J., Goudsouzian N.G., et al. Life-threatening apnea in infants recovering from anesthesia. Anesthesiology. 1983;59:506.
Loepke A.W., McGowan F.X., Soriano S.G. Con: The toxic effects of anesthetics in the develooping brain: the clinical perspective. Anesth Analg. 2008;106:1664.
Lomaz J.G. Halothane and jaundice in paediatric anesthesia. Anaesthesia. 1965;20:70.
Long C.W. An account of the first use of sulphuric ether by inhalation as an anaesthetic in surgical operations. S Med Surg J. 1849;5:45.
Lowrie L., Weiss A.H., Lacombe C. The pediatric sedation unit: a mechanism for pediatric sedation. Pediatrics. 1998;102:E30.
Lunn J.N. Implications of the national confidential enquiry into perioperative deaths for paediatric anaesthesia. Paediatr Anaesth. 1992;2:69.
MacDonald I.H., Stocks J.G. Prolonged nasotracheal intubation: a review of its development in a paediatric hospital. Br J Anaesth. 1965;37:161.
Macon E.B., Bruner H.D. The scientific aspect of endotracheal tubes. Anesthesiology. 1950;11:313.
Malviya S., Voepel-Lewis T., Tait A.R. Adverse events and risk factors associated with the sedation of children by non-anesthesiologists. Anesth Analg. 1997;85:1207.
Mapleson W.W. The elimination of rebreathing in various semi-closed anesthetic systems. Br J Anaesth. 1954;26:323.
Marcy J.H., Cook D.R. Basic neonatal anesthesia and monitoring. In: Marcy J.H., Cook D.R., editors. Neonatal anesthesia. Pasadena, Calif: Appleton Davies, 1988.
Marhofer P., Chan V.W.S. Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth Analg. 2007;104:1265.
Marhofer P., Greher M., Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth. 2005;94:7.
Marmer M.J. Hypnosis as an adjunct to anesthesia in children. Am J Dis Child. 1959;97:314.
Mason D.G., Bingham R.M. The laryngeal mask airway in children. Anaesthesia. 1990;45:760.
Matthews J.H., Buckley J.J., Van Bergen F.H. Acute effect of low-flow extracorporeal circulation on cerebral circulation. Anesthesiology. 1957;18:169.
Mazariegos G.V., Soltys K., Bond G., et al. Pediatric intestinal retransplantation: techniques, management, and outcomes. Transplantation. 2008;86:1777.
McQuiston W.O. Anesthetic problems in cardiac surgery in children. Anesthesiology. 1949;10:590.
Mellon R.D., Simone A.F., Rappaport A. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509.
Melman E., Pennelas J., Maruffo J. Regional anesthesia in children. Anaesth Analg. 1975;54:387.
Mendelsohn D.Jr, Mackrell T.N., Machlan M.A., et al. Experiences using the pump-oxygenator for open heart surgery in man. Anesthesiology. 1957;18:223.
Morray J.P. Implications for subspecialty care of anesthetized children. Anesthesiology. 1994;80:969.
Morray J.P., et al. A comparison of pediatric and adult anesthesia closed malpractice claims. Anesthesiology. 1993;78:461.
Morton W.T.G. Remarks on the proper mode of administering the sulphuric ether by inhalation. Boston: Dutton & Wentworth, Printers, 1847.
Motoyama E.K. Pulmonary mechanics during early postnatal years. Pediatr Res. 1977;11:220.
Motoyama E.K., Cook C.D. Respiratory physiology. In: Smith R.M., editor. Anesthesia for infants and children. ed 4. St. Louis: CV Mosby; 1980:38-87.
Motoyama E.K., Davis P.J., editors. Smith’s anesthesia for infants and children, ed 7, Philadelphia: Mosby, 2006.
Motoyama E.K., Glazener C.H. Hypoxemia after general anesthesia in children. Anesth Analg. 1986;65:267.
Murat I., Montay G., Delleur M.M., et al. Bupivacaine pharmacokinetics during epidural anaesthesia in children. Eur J Anaesthesiol. 1988;5:113.
Myers L.B., Cohen D., Galinkin J., et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569.
Nelson W.E. Nelson’s textbook of pediatrics. Philadelphia: WB Saunders, 1950.
Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997;99:838.
Nichols D.G. Rogers textbook of pediatric intensive care, ed 4. Philadelphia: Lippincott, Williams & Wilkins, 2008.
Nicodemus H.F., Nassiri-Rahimi C., Bachman L., et al. Median effective doses (ED50) of halothane in adults and children. Anesthesiology. 1969;31:344.
Oh T.H., Motoyama E.K. Comparison of nasotracheal intubation and tracheostomy in management of epiglottitis. Anesthesiology. 1977;46:214.
Olsson G.L., Hallen B. Cardiac arrest during anesthesia: a computerized study. Acta Anaesthesiol Scand. 1988;32:653.
Onchi O., Fujita M. Pediatric anesthesia. Tokyo: Nankodo & Co Ltd, 1958.
Orkin L.K., Siegel M., Rovenstine E.A. I. Resistance to breathing by apparatus used in anesthesia. Anesth Analg. 1954;33:217.
Patrick R.T., Theye R.A., Moffitt E.A. Studies in extracorporeal circulation. V. Anesthesia and supportive care during intracardiac surgery with the Gibbon-type pump-oxygenator. Anesthesiology. 1957;18:673.
Pender J.W. Endotracheal anesthesia in children. Anesthesiology. 1954;15:495.
Pennant J.H., White P.F. Review article: The laryngeal mask airway: its uses in anesthesiology. Anesthesiology. 1993;79:144.
Pernick M.S. A calculus of suffering. New York: Columbia University Press, 1975.
Poe M.F., Karp M. Seconal as a basal anesthetic in children: a preliminary report. Anesth Analg. 1946;25:88.
Powell D.R., Miller R.D. The effect of repeated doses of succinylcholine on serum potassium in patients with renal failure. Anesth Analg. 1975;54:746.
Rackow H., Salanitre E. A dose effect study of preoperative medication in children. Anesthesiology. 1962;23:747.
Raphaely R.C., Downes J.J.Jr. Congenital diaphragmatic hernia: prediction of survival. J Pediatr Surg. 1973;8:815.
Rees G.J. An early history of paediatric anaesthesia. Paediatr Anaesth. 1991;1:3.
Rees G.J. Paediatric anaesthesia. Br J Anaesth. 1960;32:132.
Rees G.J. The child as a subject for anaesthesia. In: Evans F.T., Gray T.C., editors. Modern trends in anaesthesia. New York: Harper & Row, Publishers, 1958.
Robertson K., Borland L.M. Anesthesia for organ transplantation. In: Motoyama E.K., Davis P.J., editors. Smith’s anesthesia for infants and children. St. Louis: CV Mosby, 1990.
Robson C.H. Anesthesia in children. Am J Surg. 1936;34:468.
Rockoff M.A., Hall S.C. Subspecialty training in pediatric anesthesiology: what does it mean? Anesth Analg. 1997;85:1185.
Rogers M.C., Nugent S., Traystman R.J. Control of cerebral circulation in the neonate and infant. Crit Care Med. 1980;8:570.
Rogers M.C., Nichols D.G., editors. Textbook of pediatric intensive care, ed 3, Baltimore: Williams & Wilkins, 1996.
Root B. Problems in evaluating effects of premedication in children. Anesth Analg. 1962;41:180.
Rosen M.A. Anesthesia for fetal procedures and surgery. In: Snider S.M., Levinson G., editors. Anesthesia for obstetrics. ed 3. Baltimore: Williams & Wilkins; 1992:281-285.
Ross A.K., Davis P.J., Dear G., et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. 2001;93:1393.
Safar P. Ventilatory efficiency of mouth-to-mouth artificial respiration. JAMA. 1958;167:335.
Saint-Maurice C., Steinberg O.S. Regional anaesthesia in children. Fribourg, Switzerland: Mediglobe, 1990.
Saint-Maurice C., Meistelman C., Rey E., et al. The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology. 1986;65:536.
Salanitre E., Rackow H. The pulmonary exchange of nitrous oxide and halothane in infants and children. Anesthesiology. 1969;30:388.
Salem M.R. Anesthetic management of patients with a “full stomach:” a critical review. Anesth Analg. 1970;49:47.
Salem M.R., Bennett E.J., Schweiss J.F., et al. Cardiac arrest related to anesthesia: contributing factors in infants and children. JAMA. 1975;233:238-241.
Sanders J.C., King M.A., Mitchell R.B., et al. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea. Anesth Analg. 2006;103:1115.
Sarner J.B., Levine M., Davis P.J., et al. Clinical characteristics of sevoflurane in children: a comparison with halothane. Anesthesiology. 1995;82:38.
Saunders N.A., Powles A.C.P., Rebuck A.S. Ear oximetry accuracy practicability in the assessment of arterial oxygenation. Am Rev Respir Dis. 1976;113:745.
Schechter N.L., Berde C.B., Yaster M. Pain in infants, children, and adolescents, ed 2. Baltimore: Williams & Wilkins, 2002.
Schulman J.L., Foley J.M., Vemon D.T.A., et al. A study of the effect of the mother’s presence during anesthesia induction. Pediatrics. 1967;39:111.
Schuster S.R. A new method for the staged repair of large omphaloceles. Surg Gynecol Obstet. 1967;125:837.
Schwartz H. Chloroform anesthesia for ophthalmic examination. Am J Ophthalmol. 1957;43:27.
Schwartz L., Rockoff M.A., Koka B.V. Masseter spasm with anesthesia: incidence and implications. Anesthesiology. 1984;61:772.
Schwarz U., Galinkin A.L. Anesthesia for fetal surgery. Semin Pediatr Surg. 2003;12:196.
Sellick B.A. Cricoid pressure to control the regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:204.
Sethna N.F., Verghese S.T., Hannallah R.S., et al. A randomized controlled trial to evaluate S-Caine patch for reducing pain associated with vascular access in children. Anesthesiology. 2005;102:403.
Singer J.J. Cardiac arrest in children. J Am Coll Emerg Physicians. 1977;6:198.
Small G.A. Brachial plexus block anesthesia in children. JAMA. 1951;147:1648.
Smith C.A. The physiology of the newborn infant. Springfield, IL: Charles C Thomas, 1945.
Smith R.M. The prevention of tracheitis in children following endotracheal anesthesia. Anesth Analg. 1953;32:102.
Smith R.M. Anesthesia for pediatric surgery. In: Gross D.E., editor. Surgery of infants and children. Philadelphia: WB Saunders, 1953.
Smith R.M. Circulatory factors affecting anesthesia in surgery for congenital heart disease. Anesthesiology. 1952;13:38.
Smith R.M. Anesthesia for infants and children. St. Louis: CV Mosby, 1959.
Smith R.M. History of pediatric anesthesia. In Motoyama E.K., Davis P.J., editors: Smith’s anesthesia for infants and children, ed 5, St. Louis: Mosby, 1990.
Smith R.M. Progress in paediatric anaesthesia in the United States. Paediatr Anaesth. 1991;1:63.
Smith R.M., Crocker D.C., Adams J.G. Anesthetic management of patient under hyperbaric oxygenation. Anesth Analg. 1964;43:766.
Smith S.M. The use of curare in infants and children. Anesthesiology. 1947;8:176.
Smith S.M., Brown H., Toman J., et al. The lack of cerebral effects of d-tubocurarine. Anesthesiology. 1947;8:1.
Snow J. On chloroform and other anesthetics. London: John Churchill, 1858.
Snow J. On the inhalation of the vapour of ether. London: John Churchill, 1847.
Soriano S.G., Anand K.J., Rovnaghi C.R., et al. Of mice and men: should we extrapolate rodent experimental data to the care of human neonates? Anesthesiology. 2005;102:866.
Starzl T.E., Marchioro T.L., von Kaulla K.N., et al. Homo-transplantation of the liver in human. Surg Gynecol Obstet. 1963;117:659.
Stead A.L. The response of the newborn infant to muscle relaxants. Br J Anaesth. 1955;27:124.
Stephen C.R. Elements of paediatric anaesthesia. Springfield, IL: Charles C Thomas, 1954.
Stephen C.R., Slater H.M. A non-rebreathing, non-resisting valve. Anesthesiology. 1948;9:550.
Stephen C.R., Lawrence J.H., Fabian L.W., et al. Clinical experience with fluothane: 1,400 cases. Anesthesiology. 1958;19:197.
Steward D.J. History of pediatric anesthesia. In: Gregory G.A., editor. Pediatric anesthesia. New York: Churchill Davidson, 1983.
Steward D.J. Preterm infants are more prone to complications following minor surgery than are term infants. Anesthesiology. 1982;56:304.
Steward D.J., Lerman J. Manual of pediatric anesthesia, ed 5. New York: Churchill Livingstone, 2001.
Stiles C.M. A flexible fiberoptic bronchoscope for endotracheal intubation of infants. Anesth Analg. 1974;53:1017.
Sumner E., Hatch D. Paediatric anaesthesia, ed 2. New York: Oxford University Press, 1999.
Sun L.S., Li G., DiMaggio C., et al. Anesthesia and neurodevelopment in children: time for an answer. Anesthesiology. 2008;109:757.
Taussig H.B. Congenital malformations of the heart. New York: The Commonwealth Fund, 1947.
Taylor P.A., Towley R.M. The broncho-fiberscope as an aid to endotracheal intubation. Br J Anaesth. 1972;46:611.
Telford J., Keats A.S. Succinylcholine in cardiovascular surgery in infants and children. Anesthesiology. 1957;18:841.
Tessier P., Guiot G., Delbet J.P., et al. Osteotomies cranio-naso-orbito-faciales hypertelorisme. Ann Chir Plast Esthet. 1967;12:103.
Tiret L., Nivoche Y., Hatton F., et al. Complications related to anaesthesia in infants and children. Br J Anaesth. 1988;61:263.
Tobias J.D., Deshpande J.K. The pediatric pain handbook. St. Louis: Mosby, 1996.
Todres I.D., Fugate J.H. Critical care of infants and children. Boston: Little, Brown and Co, 1996.
Tran D.Q.H., Mun?oz L., Russo G., et al. Ultrasonography and stimulating perineural catheters for nerve blocks: a review of the evidence. Can J Anaesth. 2008;55:447.
Ungerleider R.M., Greeley W.J., Sheikh K.H., et al. Routine use of intraoperative epicardial echocardiography and Doppler color flow imaging to guide and evaluate repair of congenital heart lesions: a prospective study. J Thorac Cardiovasc Surg. 1990;100:297.
Virtue R.W. Hypothermic anesthesia. Springfield, IL: Charles C Thomas, 1955.
Viscusi E.R., Reynolds L., Chung F., et al. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA. 2004;291:1333.
Warren J.M. Inhalation of ether. Boston Med Surg J. 1847;36:160.
Waters R.M. Pain relief for children. Am J Surg. 1938;39:470.
Weinstein M.L. Rectal pentothal sodium: a new pre- and basal anesthetic drug in the practice of surgery. Anesth Analg. 1939;18:221.
Williams R.K., Adams D.C., Aladjem E.V., et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont infant spinal registry. Anesth Analg. 2006;102:67.
Willschke H., Marhofer P., Bosenberg A., et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth. 2005;95:226.
Willschke H., Marhofer P., Bosenberg A., et al. Epidural catheter placement in children: comparing a novel approach using ultrasound guidance and a standard loss-of-resistance technique. Br J Anaesth. 2006;97:200.
Winston K.R., Rockoff M.A., Mullikan J.B., et al. Surgical division of craniopagi. Neurosurgery. 1987;21:782.
Yaster M., Maxwell L.G. Pediatric regional anesthesia. Anesthesiology. 1989;70:324.
Zeltzer L.K., Jay S.M., Fisher D.M. The management of pain associated with pediatric procedures. Pediatr Clin North Am. 1989;36:941.
Zindler M., Deming M.V. The anesthetic management of infants for repair of congenital atresia of the esophagus with tracheo-esophageal fistula. Anesth Analg. 1953;32:180.
* An accurate description of the first anesthetic agents given by one of the writers (RMS) for Dr. Ladd at Children’s Hospital Boston in 1946.
† Ms. Lank, who served as chief nurse anesthetist at Children’s Hospital Boston from 1935 to 1969, was one of several remarkable nurses who provided much of the anesthesia care for children at major pediatric centers in the United States until physician anesthesiologists trained and experienced during World War II returned home and began developing interest and expertise in pediatric care.
* The excellent first-hand report of Downes (1992) on the development of pediatric critical care is recommended.