12 High-Risk Percutaneous Coronary Interventions
Identifying the High-Risk PCI Patient
Angiographic Factors
The American College of Cardiology/American Heart Association (ACC/AHA) created a scoring system that classified lesions according to their complexity, likelihood of successful dilation, and the likelihood of an adverse event. Lesions were classified as either type A, B, or C, with C the highest risk lesions, based on lesion characteristics (Tables 12-1 and 12-2).
| Type A Lesions |
ACC, American College of Cardiology; AHA, American Heart Association.
Table 12-2 Lesion Characteristics and the Increased Risk of Ischemic Complications (Based on Multivariate Analysis)
| Lesion Characteristic | Odds Ratio |
|---|---|
| Nonchronic total occlusion | 4.74 (2.69–8.38) |
| Degenerated saphenous vein graft | 4.18 (2.39–7.31) |
| Length ≥20 mm | 2.77 (1.51–5.09) |
| Irregularity | 1.88 (1.32–2.66) |
| Large filling defect | 1.41 (1.17–1.70) |
| Length 10–20 mm | 1.88 (1.26–2.82) |
| Moderate calcification with angulation >45° | 4.44 (1.24–15.96) |
| Eccentric | 2.12 (1.04–4.57) |
| Severe calcification | 2.19 (1.04–4.57) |
| Saphenous vein graft age ≥10 years | 1.81 (1.00–3.31) |
Adapted from Ellis SG, Guetta V, Miller D, et al. Relation between lesion characteristics and risk with percutaneous intervention in the stent and glycoprotein IIb/IIIa era. Circulation 1999;100:1971–1976.
In the mid-1990s, an era in which coronary stents and platelet glycoprotein IIb/IIIa inhibitors were frequently utilized, Ellis and coworkers analyzed a large database of patients undergoing PCI. Ten angiographic factors were identified that correlated with greater risk of complication. The two factors associated with the greatest increased risk were degenerated saphenous vein grafts (relative risk 4.18) and nonchronic total occlusion (relative risk 4.74). Other factors included long lesions, lesions with large filling defects, calcified angulated lesions, eccentric lesions, and old saphenous vein grafts (Table 12-2). The finding of marked increased risk in degenerated vein grafts supports the practice of using distal protection devices during PCI of such lesions.
Angiographic Risk Assessment Using the SYNTAX Score
The SYNTAX score, an angiographic grading tool to determine the complexity of coronary artery disease (CAD), was derived from pre-existing risk assessment classifications from numerous studies and expert consensus. The SYNTAX score is the sum of the points assigned to each individual lesion identified in the coronary tree with >50% diameter narrowing in vessels >1.5 mm diameter. The coronary tree is divided into 16 segments according to the AHA classification (see Fig. 12-1). Each segment is given a score of 1 or 2 based on the presence of disease, and this score is then weighted based on a chart, with values ranging from 3.5 for the proximal left anterior descending artery (LAD) to 5.0 for left main, and 0.5 for smaller branches. The branches <1.5 mm in diameter, despite having severe lesions, are not included in the SYNTAX score. The percent diameter stenosis is not a consideration in the SYNTAX score, only the presence of a stenosis from 50% to 99% diameter, <50% diameter narrowing, or total occlusion. A multiplication factor of 2 is used for non-occlusive lesions and 5 is used for occlusive lesions, reflecting the difficulty of PCI. Further characterization of the lesions adds points. (Fig. 12-1 shows the diagram of vessel segments used in the SYNTAX score.)
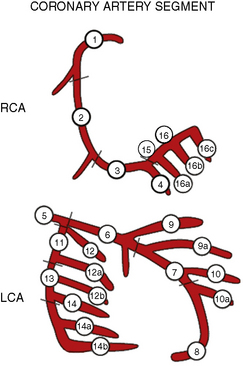
Figure 12-1 Definition of the coronary tree segments from the SYNTAX study
1. RCA (right coronary artery) proximal: from the ostium to one half the distance to the acute margin of the heart.
2. RCA mid: from the end of first segment to acute margin of heart.
3. RCA distal: from the acute margin of the heart to the origin of the posterior descending artery.
4. Posterior descending artery: running in the posterior interventricular groove.
5. Left main: from the ostium of the LCA (left coronary artery) through bifurcation into left anterior descending and left circumflex branches.
6. LAD (left anterior descending) proximal: proximal to and including first major septal branch.
7. LAD mid: LAD immediately distal to origin of first septal branch and extending to the point where LAD forms an angle (RAO [right anterior oblique] view). If this angle is not identifiable, this segment ends at one half the distance from the first septal to the apex of the heart.
8. LAD apical: terminal portion of LAD, beginning at the end of previous segment and extending to or beyond the apex.
9. First diagonal: the first diagonal originating from segment 6 or 7.
9a. First diagonal a: additional first diagonal originating from segment 6 or 7, before segment 8.
10. Second diagonal: originating from segment 8 or the transition between segment 7 and 8.
10a. Second diagonal a: additional second diagonal originating from segment 8.
11. Proximal circumflex artery: main stem of circumflex from its origin of left main and including origin of first obtuse marginal branch.
12. Intermediate/anterolateral artery: branch from trifurcating left main other than proximal LAD or LCX (left circumflex). It belongs to the circumflex territory.
12a. Obtuse marginal a: first side branch of circumflex running in general to the area of obtuse margin of the heart.
12b. Obtuse marginal b: second additional branch of circumflex running in the same direction as 12.
13. Distal circumflex artery: the stem of the circumflex distal to the origin of the most distal obtuse marginal branch, and running along the posterior left atrioventricular groove. Caliber may be small or artery absent.
14. Left posterolateral: running to the posterolateral surface of the left ventricle. May be absent or a division of obtuse marginal branch.
14a. Left posterolateral a: distal from 14 and running in the same direction.
14b. Left posterolateral b: distal from 14 and 14a and running in the same direction.
15. Posterior descending: most distal part of dominant left circumflex when present. It gives origin to septal branches. When this artery is present, segment 4 is usually absent.
16. Posterolateral branch from RCA: posterolateral branch originating from the distal coronary artery distal to the crux.
16a. Posterolateral branch from RCA: first posterolateral branch from segment 16.
16b. Posterolateral branch from RCA: second posterolateral branch from segment 16.
16c. Posterolateral branch from RCA: third posterolateral branch from segment 16.
(From Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–227.)
The SYNTAX score algorithm then sums each of these features for a total SYNTAX score. Table 12-3 summarizes the SYNTAX grade categories. A computer algorithm (available online at www.syntaxscore.com) is then queried, and a summed value is produced. Figure 12-2 shows two patients each with three-vessel CAD but very different PCI risk based on SYNTAX scores.
Reprinted from Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of CAD. EuroIntervention 2005;1:219–227.
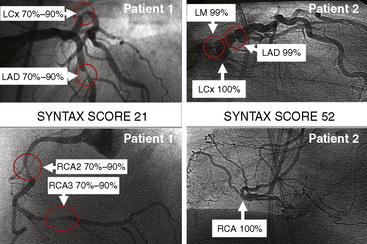
Figure 12-2 Angiographic examples of SYNTAX scores in patients undergoing PCI. Selected angiograms.
(Reprinted from Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of CAD. EuroIntervention 2005;1:219–227 with permission.)
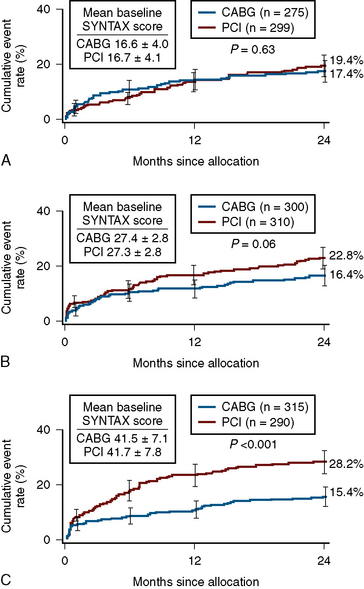
In patients with SYNTAX score <33, equal outcomes after revascularization were obtained with regard to major adverse cardiac events for both PCI and CABG. For higher SYNTAX scores, CABG had fewer adverse events than PCI. The conclusion of the SYNTAX study showed that the overall safety outcomes (death, cerebrovascular accident, MI) were similar in CABG and PCI patients at 12 months (7.7 vs. 7.6%). There was a higher rate of revascularization in the PCI group (13.7 vs. 5.9%), balanced by a higher rate of cerebrovascular accident in the CABG group (2.2 vs. 0.6%). Of note, the overall PCI major adverse cardiac and cerebrovascular event rate (MACCE) was higher (17.8 vs.12.1%) primarily due to an excess need for repeat revascularization. If one accepts a repeat revascularization as part of the natural history of PCI and not an adverse event, then the difference between revascularization strategies becomes even less (Fig. 12-3).
Patient-Related Factors
Several clinical factors can be utilized to identify high-risk PCI, such as the presence of multivessel disease, angioplasty to more than one lesion, suboptimal activated clotting time (ACT), residual stenosis above 30%, depressed ejection fraction, old age (>65 years), unstable angina and recent MI (Table 12-4). A retrospective study from the Mayo Clinic, examining the risk of PCI with the use of glycoprotein IIb/IIIa inhibitors and coronary stents, found that clinical factors such as left main or multivessel disease, an ejection fraction below 35%, or a recent MI were more important than angiographic factors for predicting complications. Other studies have identified the presence of diabetes mellitus and renal disease as indicators of high-risk patients (Table 12-5).
Table 12-4 Patient and Clinical Factors Associated With Higher Risk PCI
PCI, percutaneous coronary intervention.
Table 12-5 Elective PCI Patient and Lesion High-Risk Characteristics
CHF, congestive heart failure; SVG, saphenous vein graft.
Modified from King SB, III, Walford G, for the New York State Cardiac Advisory Committee. Percutaneous coronary interventions in New York State, 2005–2007. Albany: New York State Department of Health, April 2010;1–52; and Dehmer GJ, Blankenship J, Wharton TP, Jr., et al. The current status and future direction of percutaneous coronary intervention without on-site surgical backup: an expert consensus document from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2007;69:471–478.
PCI for AMI
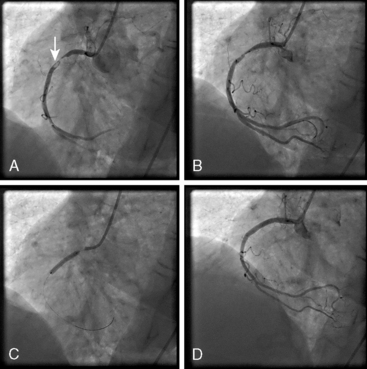
PCI for AMI can be performed before (primary PCI) or after thrombolysis (facilitated or rescue PCI). Primary PCI is the approach of choice for acute ST-segment elevation MI, but is not available in all facilities. Primary PCI for AMI is indicated in patients who present within 12 hours from the onset of symptoms and in whom the infarct vessel can be recanalized within 90 minutes of presentation. Primary PCI is also indicated for patients in whom thrombolytics are contraindicated and for patients in cardiogenic shock. Figure 12-4 shows angiograms of PCI for AMI.
PCI in AMI patients immediately after successful thrombolysis (called facilitated PCI) has shown no benefit with regard to salvage of jeopardized myocardium or prevention of reinfarction or death. In some studies, this approach was associated with increased adverse events, including bleeding, recurrent ischemia, emergency coronary artery surgery, and death. Routine PCI immediately after thrombolysis may increase the chance of vascular complications at the access site and hemorrhage into the infarct related vessel wall (Table 12-6).
Technical Considerations for PCI in AMI
Cardiogenic Shock
The SHOCK trial provided randomized data of the role of PCI in patients with cardiogenic shock. In this multicenter trial, urgent revascularization was compared to initial medical stabilization in 302 patients with cardiogenic shock from AMI. In patients who underwent urgent revascularization, 64% had angioplasty and 36% were revascularized via CABG. At 30 days, the mortality was 46.7% and 56.0% for the revascularization and the medical therapy groups, respectively (P = NS). At 6 months, however, the mortality was significantly lower in the revascularized group (50.3% vs. 63.1%), and this difference remained significant at 1-year follow-up. Of note, in the prespecified group of patients older than 75 years, there was no benefit from revascularization. Based on the SHOCK trial and prior reports, PCI for elderly cardiogenic shock patients may not be beneficial (Table 12-7).
PCI, percutaneous coronary intervention; VT/VF, ventricular tachycardia/ventricular fibrillation.
Pharmacologic Support
Antiplatelet Agents (Intravenous)
The platelet glycoprotein IIb/IIIa inhibitors block platelet aggregation and adhesion. Intravenous administration of these agents usually leads to 80% to 95% inhibition of platelet aggregation. These agents reduce ischemic complications in patients undergoing PCI. The greatest absolute of risk reduction is in those with high-risk angiographic and/or clinical features. Therefore, these agents should be strongly considered during high-risk PCI. Chapter 5 provides detailed information on platelet glycoprotein IIb/IIIa inhibitor characteristics and dosing regimens.
Vasopressors and Inotropic Agents
For high-risk PCI, vasopressors and inotropic agents should be readily available. In very high–risk PCI procedures, at least one vasopressor agent should be premixed and available for immediate infusion (Table 12-8). Commonly used vasopressors include dopamine and norepinephrine.
Table 12-8 Medications and Dosing Regimens for Arrhythmias and Hypotension During High-Risk PCI
| Bradycardia |
• Amiodarone—can administer in one of two regimens, as dictated by clinical setting
• Lidocaine 1.0–1.5 mg/kg IV bolus—can give additional boluses of 0.5–0.75 mg/kg IV as indicated, up to total dose of 3.0 mg/kg
• Epinephrine 1 mg IV bolus—can repeat every 3–5 min
• Amiodarone 300 mg IV rapid infusion after dilution in 20–30 mL fluid—can give additional 150-mg rapid infusions in similar manner as indicated
• Lidocaine 1.0–1.5 mg/kg IV bolus—can give additional boluses of 0.5–0.75 mg/kg IV as indicated, up to total dose of 3.0 mg/kg
Transvenous Pacing
• Heart block greater than first degree
• PCI involving the (dominant) artery supplying the arteriovenous node (particularly if the right coronary artery is occluded during a left circumflex artery PCI and vice versa).
A balloon-tipped pacing catheter reduces the potential for right ventricular perforation.
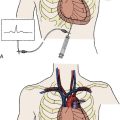
Intra-aortic Balloon Pump (IABP)
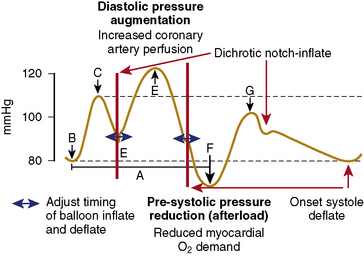
IABP counterpulsation increases myocardial oxygen supply and decreases myocardial oxygen demand. IABP balloon inflation at the onset of diastole (at the dicrotic notch on the central arterial pressure tracing) results in augmentation of diastolic pressure, which increases coronary artery (and systemic) perfusion. Deflation of the balloon just before systole (end diastole on the arterial pressure tracing) results in decreased ventricular afterload, which decreases myocardial oxygen consumption and increases cardiac output. These effects are illustrated in Figure 12-5. An example of the arterial waveform during correctly timed intra-aortic balloon counterpulsation is shown in Figure 12-6.
The indications and contraindications for IABP counterpulsation during high-risk PCI are shown in Table 12-9. Before a diagnostic cardiac catheterization or interventional procedure, the patient should be treated medically to optimize hemodynamics and reduce myocardial ischemia. Hypotension (not responding to volume loading or intravenous vasopressors) and medically refractory angina are important indications for IABP placement. Contraindications to IABP placement must be factored into decisions on whether to proceed with a high-risk PCI.
Table 12-9 Indications and Contraindications for IABP Counterpulsation During High-Risk PCI
| Indications for Prophylactic Balloon Pump Placement |
IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Notes on the IABP insertion technique:
As discussed previously, iliac-femoral angiography (or distal aortic angiography with runoff) should be performed at the time of diagnostic catheterization, or at least, preceding high-risk PCI. The IABP balloon is inserted into either groin using standard Seldinger technique, as described in detail by Kern (2011). The Cardiac Catheterization Handbook, 5th edition. Some manufacturers are making small caliber or “sheathless” IABP balloon catheters, which are especially useful in the elderly and those with peripheral vascular disease. Fluoroscopic observation of the balloon inflated above the renal arteries confirms optimal placement.
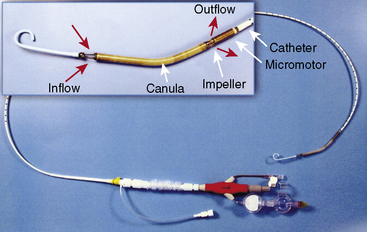
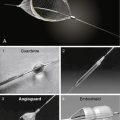
Impella LV Support Device
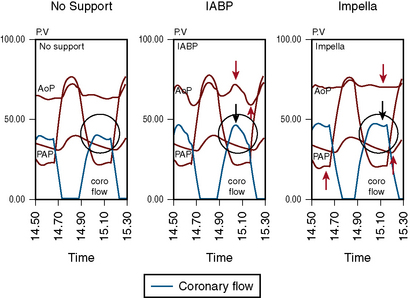
The Impella 2.5 can be inserted into the left ventricle over a 0.018-inch stiff guidewire through the femoral artery, across the aortic valve and into the left ventricle. The tip of the catheter has a “pigtail” that facilitates safe positioning in the left ventricle. The impeller motor draws blood into the cannula and expels it into the aorta, thereby generating pressure, and flows up to 2.5 L/min (Fig. 12-7). This compares with the IABP, which provides 0.2-0.4 L/min of support. A 13F femoral sheath is required for insertion of the Impella 2.5. Peripheral vascular disease and aortic valve disease are contraindications to the Impella.
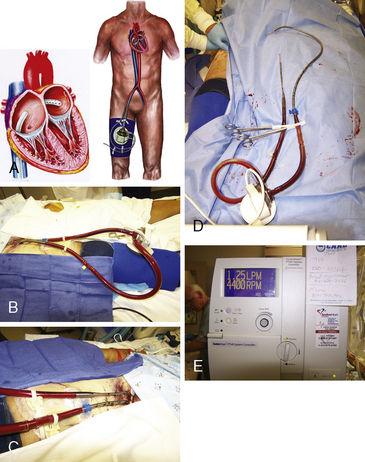
The TandemHeart Pump System
The TandemHeart percutaneous ventricular assist device is designed for short-term mechanical LV support. The TandemHeart involves the placement of a 21F catheter inserted into the left atria from the femoral vein via a transseptal puncture. Blood is withdrawn from the left atrium by an external centrifugal pump and infused into the femoral artery via a 14–19F catheter. The TandemHeart can provide up to 4.5 L/min of cardiac support. As with IABP and Impella, iliac-femoral angiography must be performed prior to canula insertion. Figure 12-8 shows the TandemHeart system.
Management of Complications in High-Risk PCI Patients
Hypotension
Hypotension may result from myocardial ischemia, coronary perforation with cardiac tamponade, arrhythmia, contrast- or medication-induced anaphylaxis, or acute occult bleeding (e.g., retroperitoneal hematoma). This differential diagnosis of hypotension must be evaluated quickly and therapy directed to the cause of the hypotension. After the first step of aggressive fluid resuscitation with normal saline, support blood pressure with inotropic agents such as dopamine (initial starting infusion rate of 5 to 10 mcg/kg/min depending on degree of hypotension) or bolus dose norepinephrine (2.5–5 mcg IV boluses as needed). An emergent echocardiogram will help assess for cardiac tamponade or valvular dysfunction. The treatment of anaphylaxis is discussed below (Table 12-10).
Table 12-10 Treatment of Severe Anaphylactoid Reactions: Recommendations From the Society of Cardiac Angiography and Interventions
| Initial Pharmacological Therapy |
Bradyarrhythmias and Heart Block
Bradyarrhythmias may occur as a result of ischemia of the sinus node (most commonly supplied via the right coronary artery). Symptomatic sinus bradycardia can initially be treated with atropine (0.5–1.0 mg IV), with repeat doses every 3 to 5 min as indicated, up to a usual total maximal dose of 2.0 mg (see Table 12-8).
Ventricular Tachyarrhythmias
Ventricular tachycardia (VT) and ventricular fibrillation (VF) may result from severe myocardial ischemia. Patients who develop VT and are hemodynamically stable can first be treated with intravenous antiarrhythmic therapy, specifically amiodarone and lidocaine (see Table 12-8). Stable patients not responding to antiarrhythmic therapy can be treated with synchronized cardioversion (beginning at 100 J and increasing stepwise up to 360 J as necessary).
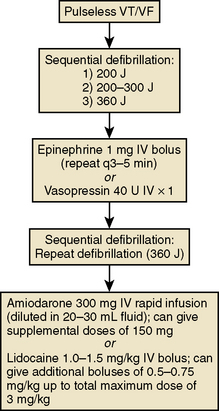
Patients who develop unstable or pulseless VT or VF have been treated with a precordial thump. However, immediate defibrillation is indicated (at 200 J biphasic energy). Chest compressions are indicated to support coronary perfusion pressure and should be continued with minimal interruptions. Patients who do not convert after defibrillation should be treated with epinephrine (1 mg IV, repeated every 3–5 min) or vasopression (40 units IV × 1) and repeat defibrillation afterward. Patients who still do not respond can then be treated with amiodarone (300 mg IV bolus after dilution in 20 mL fluid) or lidocaine (1.0–1.5 mg/kg IV bolus). The advanced cardiac life support (ACLS) algorithm for the treatment of pulseless VT/VF is presented in Figure 12-9.
Pulselessness
Underlying causes of PEA include:
1. Hypovolemia, especially resulting from bleeding.
2. Pericardial tamponade, especially in patients with AMI recent cardiac biopsy, recent endocardial pacer insertion, or uremia; if tamponade is suspected, emergency pericardiocentesis is warranted.
3. Enhanced vagal tone in patients with ischemic heart disease. Consider this whenever the heart rate is inappropriate for the degree of hypotension. Atropine is indicated.
4. Massive pulmonary embolism.
5. Tension pneumothorax, especially in patients on ventilators or in patients with central venous access above the diaphragm. Emergency needle decompression is indicated.
Anaphylactoid Reactions
Anaphylactoid reactions to contrast agents may include hypotension and shock. Although such reactions are exceedingly rare during PCI procedures, the operator should understand the treatment of this life-threatening condition. Recommendations by the Society for Cardiac Angiography and Intervention for treatment include intravenous epinephrine with large volumes of normal saline, diphenhydramine, hydrocortisone, and, if unresponsive to therapy, an H2 blocker and dopamine. Specific dosing regimens are given in Table 12-10.
Slow Flow and No Reflow
• Verapamil (125–250 mcg boluses)
• Nitroglycerin (100–200 mcg boluses)
Preparation and administration of these medications is given in Table 12-11.
Table 12-11 Preparation and Administration Guidelines for Intracoronary Vasodilators Used for “Slow Flow” and “No Reflow”
| Verapamil |
Botman C.J., Schonberger J., Koolen S., et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83:2093–2097.
Garg S., Sarno G., Garcia-Garcia H.M., et al. A new tool for the risk stratification of patients with complex coronary artery disease: the clinical SYNTAX score. Circ Cardiovasc Interv. 2010;3:317–326.
Hoffman S.N., TenBrook J.A., Wolf M.P., et al. A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one- to eight-year outcomes. J Am Coll Cardiol. 2003;41:1293–1304.
Kelly R.V., Cohen M.G., Stouffer G.A. Mechanical thrombectomy options in complex percutaneous coronary interventions. Catheter Cardiovasc Interv. 2006;68:917–928.
Kern M.J. The cardiac catheterization handbook, 5th ed. Philadelphia: Saunders; 2011.
Naidu H. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123:533–543.
Neumann F.J., Blasini R., Schmitt C., et al. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction, De. Circulation, 98;24. 1998:2695–701 15.
Patel M.R., Dehmer G.J., Hirshfeld J.W., et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization. A Report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Circulation. 2009;119:1330–1352.
Remmelink M., Sjauw K.D., Henriques J.P., et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70(4):532–537.
Serruys P.W., Unger F., Sousa J.E., et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–1124.
Sianos G., Morel M.A., Kappetein A.P., et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227.
Smith C.R. Surgery, not percutaneous revascularization, is the preferred strategy for patients with significant left main coronary stenosis. Circulation. 2009;119:1013–1020.
Stone G.W., Witzenbichler B., Guagliumi G., et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230.
Svilaas T., Vlaar P.J., van der Horst I.C., et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567.
Teirstein P.S. Percutaneous revascularization is the preferred strategy for patients with significant left main coronary stenosis. Circulation. 2009;119:1021–1033.
Tonino P.A., Fearon W.F., De Bruyne B., et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study: fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821.
Valgimigli M., Serruys P.W., Tsuchida K., et al. Cyphering the complexity of coronary artery disease using the SYNTAX score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99(8):1072–1081.