CHAPTER 7 Herbs and Dietary Supplements
I. Complementary and Alternative Medicine (CAM) and Integrative Medicine
A. CAM is variably defined, but the National Center for Complementary and Alternative Medicine (NCCAM) defines CAM as a group of diverse medical and health care systems, practices, and products that are not normally considered to be conventional or allopathic medicine.
B. The Consortium of Academic Health Centers for Integrative Medicine defines integrative medicine as the practice of medicine that reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, providers, and disciplines to achieve optimal health and healing.
II. Formulation and Regulation of Dietary Supplements
A. A dietary supplement may include vitamins, minerals, herbs or other botanicals, amino acids, and substances such as enzymes, organ tissues, and metabolites taken in addition to a normal dietary intake. Supplements are available in many dosage forms including extracts, concentrates, tablets, capsules, gel caps, liquids, teas, and powders. Herbs used for medicinal purposes are sold in many different forms, including capsules, tablets, tinctures, teas, powders, whole herbs, and creams.
2. Decoction: A decoction is the product of boiling the roots or tough plant material in water to extract the “active principle.”
3. Extracts: Pills, powders, liquids, or other forms of herbs that contain a concentrated and usually standard amount of therapeutic ingredients. The most commonly used extracts are fluid extracts (tinctures), solid extracts, powdered extracts, and essential oils.
4. Essential oil: Highly volatile, aromatic, concentrated oil extracted from plants made by steam distilling volatile compounds from plant material. Also known as volatile oils, ethereal oils, or essences, they are usually complex mixtures of a wide variety of organic compounds, mostly volatile terpene derivatives that evaporate when exposed to air.
5. Fluid extracts (tinctures): A concentrated solution of the soluble constituents of plant drugs, containing alcohol as both a solvent and a preservative. Fluid extracts are typically hydroalcoholic solutions, but they can also have a glycerin, nonalcoholic base. Alcohol content varies with each product.
6. Infusion (tea): An herbal product or tea made by steeping (soaking) herbs in hot water (water of any temperature) to extract the herbal qualities. Delicate parts of the plant, like flowers or leaves, are most often used.
7. Poultices: Topical application of a thick paste of hot, moist herb or a soft mush, prepared by wetting powders or other absorbent substances with oils or water for the purpose of alleviating pain, reducing inflammation, and promoting healing.
8. Powdered extracts: Prepared from native extracts by diluting to the specified strengths with diluents (e.g., starch, lactose) with or without anticaking agents (e.g., magnesium carbonate) followed by drying, usually under vacuum, to yield dry solids. These are ground into powders to yield powdered extracts or are granulated to produce granular extracts.
B. Regulation of health claims is largely monitored by the Dietary Supplement Health and Education Act (DSHEA), although the Food and Drug Administration (FDA) and other health alliances play minor roles.
1. The Dietary Supplement Health and Education Act (DSHEA) was passed by the US Congress in October 1994. Topics discussed in DSHEA include product labeling and content, structure and function, and health claims. Health claims describe a relationship between food, food component, or dietary supplement ingredient, or health-related condition, and are not about treating, mitigating, or curing diseases (Table 7-1).
a) Product labeling: Labels must contain a descriptive name of the product stating that it is a “supplement”; the name and place of business of the manufacturer, packer, or distributor; a complete list of ingredients; and the net contents of the product. Additionally, each dietary supplement must have nutrition labeling in the form of a “supplement facts” panel and identify each dietary ingredient contained in the product in order of abundance in the product (Figure 7-1).
b) Nutrient content claims: Nutrient content claims characterize the level of a nutrient in a food. These types of claims describe the level of a nutrient or dietary substance in the product using terms like free, high, and low, or they compare the level of a nutrient in a food to that of another food using terms such as more, reduced, and light. For example, a product containing 500 mg of calcium may claim “high in calcium.”
c) If a dietary supplement label includes such a claim, this following statement must appear on labels: “THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FDA. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.” The content of the label must be provided to the FDA 30 days before labeling.
d) Structure and function claims explain the role of a nutrient or dietary ingredient intended to affect normal structure or function in humans, for example, “calcium builds strong bones” or “fiber maintains bowel regularity.” Structure and function claims may also describe a benefit related to a nutrient deficiency disease (e.g., vitamin C and scurvy), as long as the statement also tells how widespread such a disease is in the United States. The manufacturer is responsible for ensuring the accuracy and truthfulness of these claims; they are not preapproved by the FDA, but they must be truthful and not misleading.
2. The FDA has a limited role in regulating the quality of individual products.
a) The 1990 Nutrition Labeling and Education Act (NLEA) provides for the FDA to issue regulations authorizing health claims for foods and dietary supplements after the FDA’s careful review of the scientific evidence submitted in health claim petitions.
b) The 1997 Food and Drug Administration Modernization Act (FDAMA) provides for health claims based on an authoritative statement of a scientific body of the United States government or the National Academy of Sciences; such claims may be used after submission of a health claim notification to FDA.
c) The 2003 FDA Consumer Health Information for Better Nutrition Initiative provides for qualified health claims for which the quality and strength of the scientific evidence falls below that required for FDA to issue an authorizing regulation. Such health claims must be qualified to ensure accuracy of presentation to consumers.
3. In the United States, the FDA does not function to guarantee the strength, purity, or safety of dietary supplements. Companies are expected to prove the efficacy and safety of their products.
a) The Office of Dietary Supplements (ODS) at the National Institutes of Health (NIH) was designed to strengthen knowledge and understanding of dietary supplements by evaluating scientific information, stimulating and supporting research, disseminating research results, and educating the public to foster an enhanced quality of life and health for the United States population.
b) The United States Department of Agriculture (USDA) provides leadership on food, agriculture, natural resources, and related issues based on sound public policy, the best available science, and efficient management. The USDA developed a Dietary Supplement Ingredient Database, which monitors the levels of ingredients in dietary supplement products.
4. Third party testing: Independent laboratories that test products to ensure that the label matches the product include Consumer Lab, Consumer Reports, Natural Products Association, NSF International, and the United States Pharmacopeia (USP). Quantitative and qualitative testing often finds that brand-to-brand and batch-to-batch variation exists in products.
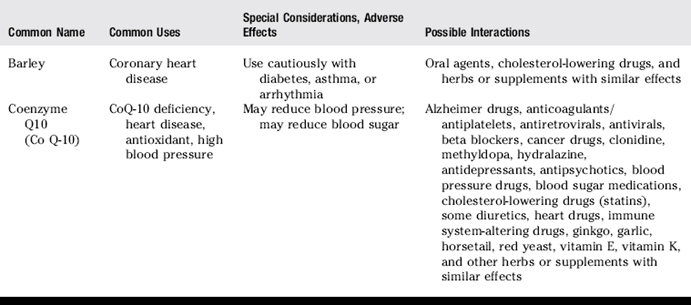
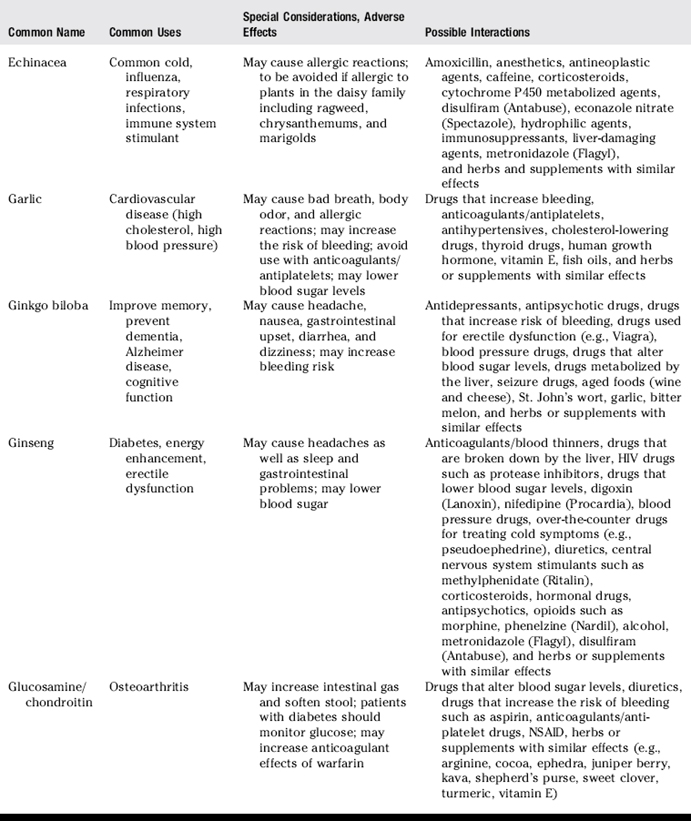
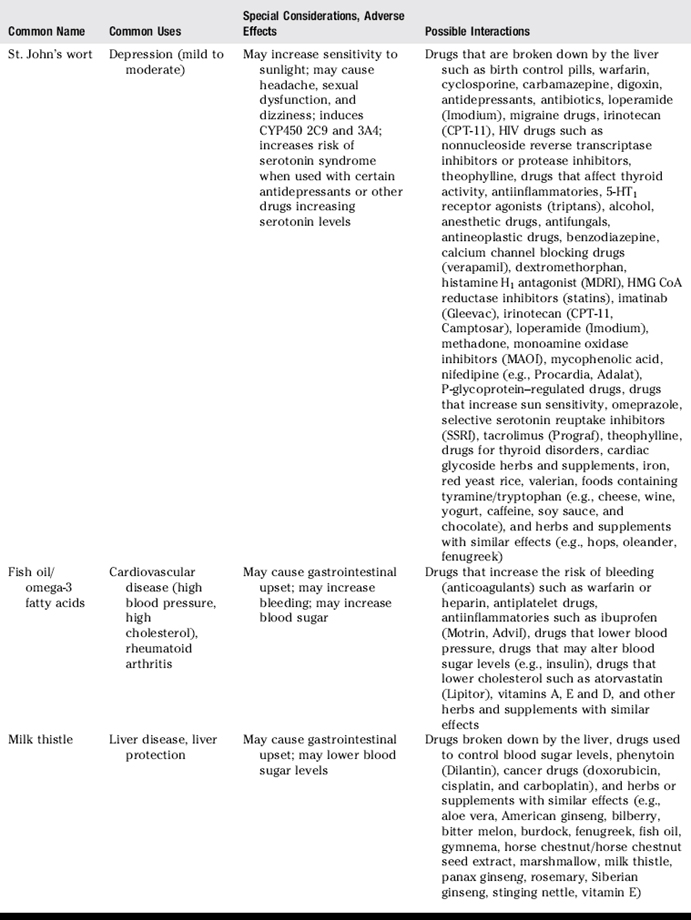
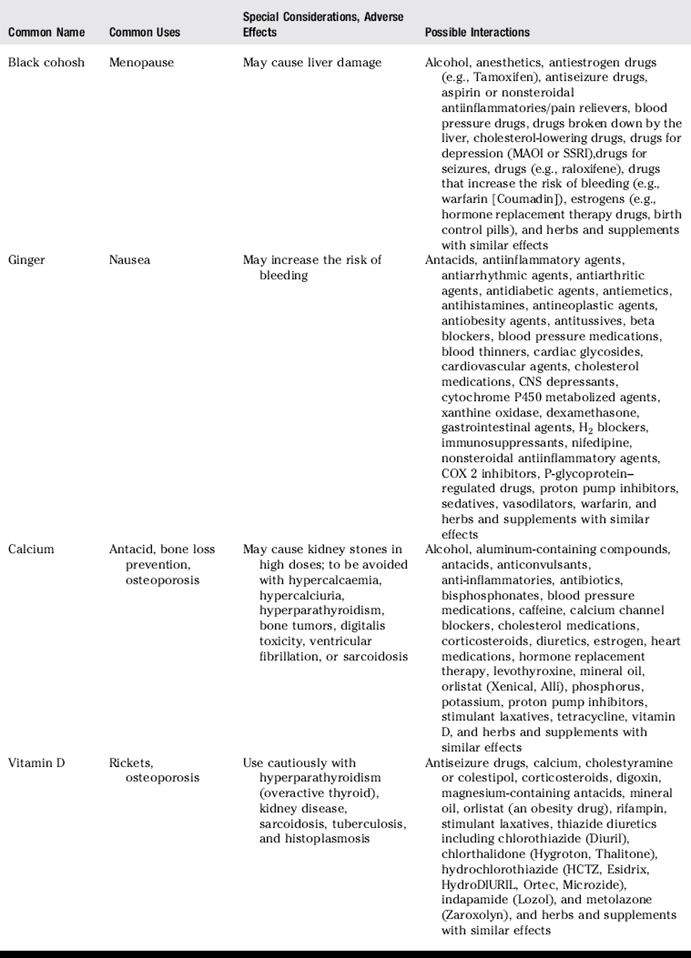
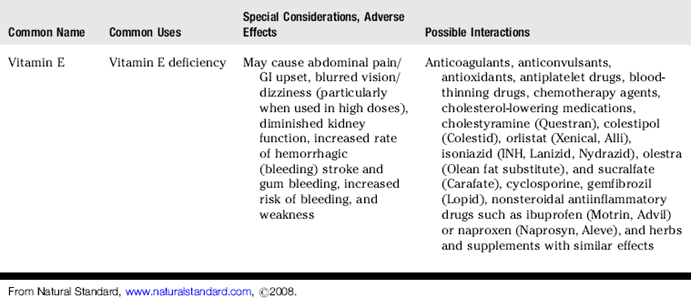
III. Common uses, adverse effects, and potential interactions of popular herbs and supplements, as well as manual therapies
A. Clinicians are encouraged to recommend doses of herbs and supplements based on those most commonly used in human trials and clinical experience. However, with natural products it is often not clear what the optimal doses are to balance effectiveness and safety due to a lack of scientific data and the lack of standardization.
B. Why is this knowledge important for patient counseling?
Table 7-1 Health Claims Approved by the FDA
| Supplement | Health Claim |
|---|---|
| Calcium | Reduced risk of Osteoporosis |
| Dietary sugar alcohol (polyols) | Does not promote tooth decay |
| Dietary fats | Increased risk of Cancer |
| Dietary saturated fat and cholesterol | Increased risk of coronary heart disease |
| Fiber-containing grain products, fruits, and vegetables | Reduced risk of coronary heart disease |
| Folate | Prevention of Neural tube defects (in pregnancy) |
| Fruits and vegetables | Reduced risk of Cancer |
| Plant sterol/stanol esters | Reduced risk of coronary heart disease |
| Potassium | Reduced risk of high blood pressure and stroke |
| Sodium | Increases Hypertension |
| Soy protein | Reduced risk of coronary heart disease |
| Vitamin B3 | Treatment of Pellagra |
| Vitamin C | Treatment of Scurvy |
| Whole grain foods | Reduced risk of heart disease and certain cancers |
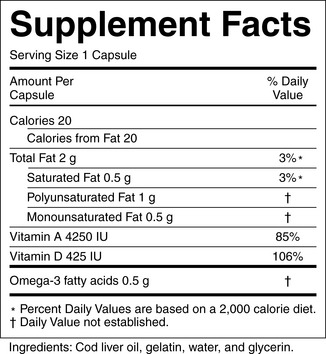
Figure 7-1 Dietary supplement label. Requirements of the label are found at: http://ods.od.nih.gov/factsheets/dietarysupplements.asp#h6
A ficitious example of a supplement label is available at: http://vm.cfsan.fda.gov/~acrobat/fdsuppla.pdf
(Courtesy of U.S. Food and Drug Administration.)
Table 7-3 Manual Therapies and Adverse Effects
| Manual Therapy | Special Considerations, Adverse Effects |
|---|---|
| Acupuncture | Needles must be sterile to avoid disease transmission. Avoid with valvular heart disease, infections, bleeding disorders or with drugs that increase the risk of bleeding (anticoagulants), medical conditions of unknown origin, neurological disorders. Avoid on areas that have received radiation therapy and during pregnancy. Use cautiously with pulmonary disease (e.g., asthma or emphysema). Use cautiously in elderly or medically compromised patients and in those with diabetes or with history of seizures. Avoid electroacupuncture with arrhythmia (irregular heartbeat) or in patients with pacemakers. |
| Acupressure | Forceful acupressure may cause bruising. |
| Chiropractic (manual adjustments) | Use extra caution during cervical adjustments. Use cautiously with acute arthritis, conditions that cause decreased bone mineralization, brittle bone disease, bone softening conditions, bleeding disorders, or migraines. Use cautiously with the risk of tumors or cancers. Avoid with symptoms of vertebrobasilar vascular insufficiency, aneurysms, unstable spondylolisthesis or arthritis. Avoid with agents that increase the risk of bleeding. Avoid in areas of paraspinal tissue after surgery. |
| Yoga | Avoid some inverted poses with disc disease of the spine, fragile, or atherosclerotic neck arteries, risk for blood clots, extremely high or low blood pressure, glaucoma, detachment of the retina, ear problems, severe osteoporosis, or cervical spondylitis. Certain yoga breathing techniques should be avoided by people with heart or lung disease. |
REVIEW QUESTIONS
(Answers and Rationales on page 335.)
5. A patient with poison ivy asks the pharmacist if she can use a skin lotion that contains comfrey. What should the pharmacist tell the patient?
8. Which of the following has been shown to interact with medications by inhibition of cytochrome P450 3A4 in the intestinal wall?
11. There is good evidence to suggest that which of the following is effective in treating Benign Prostatic Hyperplasia (BPH)?
13. Which of the following statements about yohimbine is true?
18. What is the mechanism by which glucosamine and chondroitin are suggested to treat osteoarthritis?
20. Patients with which of the following food allergies should use caution when choosing glucosamine supplements?
26. True or False: The DSHEA is an organization that regulates good manufacturing practice for dietary supplements.
29. Which of the following statements is FALSE?
37. All of the following statements are true about the Dietary Supplement Health and Education Act of 1994 (DSHEA) EXCEPT: