9 Herbal approaches to system dysfunctions
Chapter contents
Digestive system and bowel
Scope
Apart from their use to provide non-specific support for recuperation and repair, specific phytotherapeutic strategies include the following.
• functional disorders such as dyspepsia, gastro-oesophageal reflux, irritable bowel syndrome
• inflammatory conditions of the upper tract, such as aphthous ulcers, oesophagitis, gastritis
• digestive deficiency and anorexia
• food intolerances and allergies
• inflammatory diseases of the gut such as Crohn’s disease and ulcerative colitis
Orientation
An intelligent self-correcting disassembly line
The gut is a long passage designed to break down and process food, absorb nutrients and reject waste. Like an assembly line in reverse, it will only work efficiently if the delivery of material to the next stage is coordinated closely with the optimum rate of process at that stage. This subtle coordination is achieved by a robust and remarkably reliable network of control systems, orchestrated by a range of neurochemical and endocrine responses reacting to the material in the gut and managed by the complex circuitry and programmes of the enteric nervous system. Smooth muscle cells and the recently reviewed nerve-like interstitial cells of Cajal1 spontaneously generate electrophysiological slow waves that are then modulated by a network of nerves, ganglia and neurohormones within the abdomen and the intestinal wall that is complex enough to merit the description ‘intelligent’. Yet this is a decentralised system, not controlled entirely by the autonomic or higher nervous systems (the gut has approximately 108 nerve cells but only thousands of nerve fibres connecting to the central nervous system, CNS), and most ‘decisions’ are made at a very local level rather than relying on central controls.2
Law of the gut: coordinated absorption, secretion and motility
Normal peristalsis is a simple example of the self-correcting and automatous nature of the digestive system. Gut activity is coordinated by a vast complex of nerve fibres, intrinsic fibres within the digestive tract linking to networks of extrinsic fibres, these in turn linked to ganglia within the abdominal cavity. Ascending intrinsic pathways are excitatory on the gut musculature and descending pathways inhibitory, but both are activated by distension. Peristalsis follows the activation by a bolus of food of ascending excitatory pathways proximal to each point along the intestine and the simultaneous inactivation of the descending inhibitory pathways distal to the contraction. The propagation of the circular muscle contraction stops when there is no longer a sufficient distension stimulus ahead.3 The result of this arrangement is known as the ‘law of the gut’. Any bolus of food material in the gut, simply by being there, is normally propelled in one direction, towards the lower bowel;4 the absence of such a bolus leads to quiescence.
As well as provoking muscular activity, or motility, the presence of food in the gut also stimulates secretomotor reflexes via submucous neurons and causes a proportion of water and electrolytes that are absorbed with nutrients such as glucose to be returned to the lumen or instead for more to be lost in diarrhoea.5 Observations in healthy volunteers point to innate rhythms of absorption, secretion and motility in the small intestine and biliary tract during fasting, these marked by variations in plasma levels of gastrointestinal hormones. Current evidence indicates that this periodicity is generated within the intrinsic innervation,6 that is probably by local environmental factors, and that disturbances in these rhythms are potent factors in the aetiology of gastrointestinal and hepatic disease.7 There is a significant correlation between motility and secretory modes, so that they are often coupled as the ‘secretomotor’ mode. As elaborated below, cholinergic neurons seem to mediate the shift in their direction from the absorptive mode.8
Carminative herbal remedies, such as the warming spices, reduce motility9 (and thus, possibly, secretory activity) and, at least in the case of ginger, increase absorptive activity (see the ginger monograph). Increases in secretory activity and motility may be seen after the prescription of bitters, cholagogues and stimulating laxatives. However, these responses are not always predictable (in the case of bitters and cholagogues they may even be the opposite reaction in some cases) and it is also observed that reactive bowel looseness may follow a much wider variety of treatments. It appears that increases in gut activity are programmed as a response to any potentially perturbing influence, presumably as a simple defence mechanism.
Gut activity and its immune system
If there is gut infection or food intolerance, luminal antigens or bacterial products may be detected by the immune system. This may trigger a cascade of events associated with the release of inflammatory mediators. These mediators lead to increased motility and secretory patterns that are characterised by strong muscular contractions, copious secretion and diarrhoea.10,11 The close connection between the gut-associated immune responses and the enteric nervous system is confirmed as important in the symptomatology of several functional disorders.12,13 The enteric nervous system is also increasingly recognised as a regulator of epithelial barrier integrity, especially when the latter is exposed to inflammatory challenge; chronic inflammation has further disabling effects on enteric neuronal function.14 All this provides further support for an integrated functional model in managing gut and bowel disturbances.
The gut and fluid balance
Diarrhoea is an extreme pattern of fluid and electrolyte loss which, as a consequence of enteric infections and malnutrition, is still the most common immediate cause of death around the world. In developed societies it is rarely as dangerous as it is in impoverished regions, where rehydration with fluids and electrolytes is a critical life-saving measure (as seen in the sugar and salt solution widely used by aid agencies around the world for the purpose). Nevertheless, diarrhoea is relatively common, reflecting a wide variety of events in the gut, from food poisoning and enteric infection through hepatobiliary activity (bile being a prominent potential irritant of the gut wall), food intolerance, to irritable bowel behaviour.15 Often diarrhoea is of short duration and is not explained. It is even seen as a benign transient cleansing event in some healing strategies (‘better out than in’).
Some herbal traditions use remedies that loosen or bulk the bowel contents to reduce fluid accumulation and retention and even in some circumstances reduce weight. Cathartic remedies were included among those with drastic diuretic effects in the Chinese tradition, and stimulating anthraquinone laxatives clearly lead to fluid and electrolyte loss. In the French tradition, even bulk laxatives and fibre are used to reduce weight associated with fluid retention.
By contrast, there are also a range of approaches to reducing bowel looseness if this is seen as harmful. These include the temporary use of astringent tannins that reduce reflex irritation of the bowel from the higher reaches of the gut in the case of gastroenteritis, the aromatic digestives and volatile antispasmodics, and the use of demulcent and topically anti-inflammatory remedies such as Althaea (marshmallow), Filipendula (meadowsweet), Glycyrrhiza (licorice), Calendula (marigold) and, for short-term use, Symphytum (comfrey) (see Chapter 2).
Stomach activity
Healthy upper digestive function is important for maintaining health and preventing disease. Low acidity can lead to poor nutrient absorption and abnormal bowel flora. Patients with reduced gastric secretion are more susceptible to bacterial and parasitic enteric infections.16 The contribution of poor upper digestive function to the chronicity of intestinal dysbiosis is often overlooked by therapists: the herbal practitioner has bitters and pungent principles to employ.
The chemical mediators of digestive activity
The coordination of digestive processes is mediated in the first instance by batteries of neurohumoral agents, chemicals that range from familiar endocrine hormones, standard neurotransmitters and chemical mediators, found also in the CNS and other body tissues, to gut-specific chemical agents. Each may be elicited by several receptor types in the gut wall and thus may be sensitive to different secreted, metabolised, dietary or pharmacological agents. These interactions, although highly complex and multilayered, are all integrated into a whole control mechanism for digestive activity.17 Many constituents of herbal remedies are likely to interact with chemical mediators and this may provide some mechanisms for their effect on gut function. When these interactions are combined with similar examples in the nervous, endocrine, immunological and reproductive systems it appears that herbal medicine might genuinely provide a unique ‘psychoneuroimmunological’ strategy for the widest body disharmonies, one moreover that is centred on the gut.18
One common phytotherapeutic constituent has already established a number of contrasting effects on several neurohumoral mechanisms. Capsaicin, from Capsicum spp., at quite low concentrations blocks the release of calcitonin gene-related peptide, a peptide found in enteric ganglia and known to be elicited directly by local mucosal irritation leading to increased peristaltic and secretomotor activity. Capsaicin thus results in a concentration-dependent decrease in peristaltic activity following mucosal stimulation.19 Another recent discovery is the reflex release by capsaicin-stimulated nerve fibres of somatostatin, a systemic anti-inflammatory and analgesic with modulating effects on various digestive and pancreatic secretions.20 Capsaicin also blocks an alternative stimulant to intestinal contraction, vasoactive intestinal peptide (VIP): VIP mediates the effects of noradrenaline and local stressors (such as acute hypoxia in vitro) which both depress cholinergic transmission and stimulate non-cholinergic contractions.21,22 On the other hand, capsaicin is also known to induce intestinal contractions as a stimulant of sensory substance P-containing neurons,23 which are also activated by various luminal stimuli such as the presence of food metabolites and simple physical distension to stimulate intestinal contractions. The consumption of hot spices has long been a feature of traditional herbal therapeutics (see also the discussion of ‘acupharmacology’ below) and the effects of Capsicum on the wider physiology have been confirmed in studies of cutaneous blood flow after ingestion of chillies; variable increases in blood flow have been observed, most consistently in the abdominal area.24
One of the most important hormonal regulators of the digestive process is cholecystokinin. This hormone is concentrated in the wall of the upper small intestine and is secreted into the blood on the ingestion of proteins and fats and has also been implicated in the action of bitters. The physiological actions of cholecystokinin include stimulation of pancreatic and gastric acid secretion and gallbladder contraction and regulation of gastric emptying, gastrointestinal motility and satiety, as well as augmenting symptoms of fear and anxiety.25 (Plants with anxiolytic reputations have been shown to bind to cholecystokinin receptors and one, gotu kola, has been shown to attenuate startle response in human subjects.26) It is produced particularly by carbohydrate foods and appears to promote a feeling of fullness.27 In humans it has been found that foods that lead to high levels of both cholecystokinin and satiety (such as bran) are associated with lower postprandial blood sugar and insulin levels, compared with refined carbohydrate.28 This may be due to the physical characteristics of the carbohydrate but may also suggest that cholecystokinin helps to suppress hyperinsulinaemia.29 Cholecystokinin has also been observed to suppress taste when feeding strongly tasting foods to animals.30 There are suggestions that an increase in cholecystokinin production with age may contribute to the relative anorexia in the elderly.31
Gastrin is a polypeptide hormone which is secreted following vagal nerve activity and by the bulk presence of food in the stomach. Some food extractives, including partially digested proteins, alcohol and caffeine have an additional effect and some of the more stimulating plant constituents such as resins, spice ingredients and some saponins are likely to compound this activity.32 Gastrin stimulates gastric acid and pepsin secretion. It extends and indeed multiplies the short-term effect of vagal stimulation on gastric secretions.
When gastric hydrochloric acid spills over into the intestine it lowers intestinal pH and stimulates the secretion of secretin. This acts to stimulate Brunner’s glands and bicarbonate-rich bile and pancreatic secretions so as to alkalinise the intestinal contents. Secretin is also a potential mediator of the antiulcer actions of mucosal protective agents; it appears that secretin inhibits gastric acid secretion via endogenous prostaglandins.33 Glycyrrhiza (licorice) stimulates the release of secretin in humans.34
Serotonin or 5-HT receptors have been shown to mediate the emetic reflex35 and antagonists of the main receptor concerned, type 5-HT3, have been developed to reduce emesis of cancer chemotherapy. (These are also found to have anxiolytic effects, confirming the overlap in enteric and higher nervous mediators.36) There is evidence, referred to in its monograph later in this book, that ginger also acts on this receptor. 5-HT is also a likely mediator in the lower bowel of both sennoside activity and the diarrhoeal response.37 It is possibly implicated in the action of the emetic plant remedies.
There are a number of herbal constituents shown to have effects on neurosynaptic mediators. These effects are also likely to impinge on gut function, as many mediators have been found common to both. Gamma-aminobutyric acid (GABA), probably affected by a number of plant constituents, is produced in the myenteric plexus in the gut wall and acts via GABA receptors to both stimulate cholinergic and relax VIP motor neurons, contributing to both components of the peristaltic reflex.38,39 Among many other neuroactive substances and receptor sites now identified as important modulators of gut function, the endocannabinoids40 and fasting-associated orexins41 are promising further templates for phytochemical activities. Certain Echinacea alkylamides are cannabinoid 2 receptor agonists (see Echinacea monograph).
Disturbed intestinal permeability
The role of the gut wall is to allow for selective absorption of nutrients while providing vital protection against intrusion into the body tissues of harmful substances from the lumen. Non-steroidal anti-inflammatory drug (NSAID) treatment has adverse effects on enterocyte mitochondria, which may predispose the mucosa to absorption of bacterial and other large molecules that provoke a local inflammatory response. A similar mechanism may operate in patients with untreated Crohn’s disease, who show abnormally high permeability. Remission of Crohn’s induced by treatment with elemental diets coincides with a reduction in permeability. Significant correlations have been seen between permeability and plasma IgA concentrates in kidney disease and between permeability and the passage of neutrophil chemotactic agents.42
It is likely that some plant constituents could reduce excessive intestinal permeability. Tannins are likely to have a limited short-term effect at least in the upper reaches of the tract and healing plants such as Matricaria recutita (chamomile), Filipendula (meadowsweet), Ulmus spp. (slippery elm),43 Glycyrrhiza (licorice), Calendula and Symphytum (comfrey) have long been applied with this effect in mind. In theory, local anti-inflammatory activity might effectively reduce some types of increased permeability. However, the most promising effect on intestinal permeability is likely to lie in changing biliary constituents, using hepatics and choleretics (see later in this chapter).
Intestinal flora
The balance of flora populations in the gut is highly complex but, in steady-state health and dietary conditions, probably reasonably stable. In humans, for example, there seems to be a moderately predictable sequence of colonisation after birth and through to adulthood, with fluctuations in the relative numbers of aerobic and anaerobic bacteria in newborns up to a total of 1010/g wet weight, reaching in adulthood between 105 and 107/g wet weight of aerobic bacteria and between 1010 and 1011/g wet weight of anaerobic bacteria.44
The benefits of a healthy bacterial population in the gut are clear. Anaerobic bacteria in particular are shown to be responsible for considerable secondary digestion and to decrease intestinal transit time.45 Normal bacteria like Escherichia coli, Enterococcus faecalis and Bacteroides distasonis have been shown to help protect the gut from pathogenic infiltration and there are a number of other non-specific defences known.46 Mechanisms are likely to include modification of bile acids, stimulation of peristalsis, induction of immunological responses, competition for substrates and possible elaboration of various bacteriostatic substances.47 The intestinal flora also contributes to non-specific defences against immunological challenge from dietary antigens by helping to reduce their uptake across the mucosal barrier.48
The populations of bacteria and other organisms are, however, obviously dependent on their food supply, the dietary material and its metabolites, reaching the lower intestine. There is a potential for rapid responses to diet: a general reduction in bacteroidetes and increases in firmicutes, clostridia, bacilli and erysipelotrichi are apparent within 1 day of moving the diet from plant based to ‘Westernised’.49 Bowel flora in African populations with a plant-based diet are also found to be much richer in bacteroidetes than Western groups.50 Probably the most widespread impact on bacterial populations in the gut in modern times, however, is the use of antibiotics. It has long been established that this has an adverse effect on normal gut flora.51 Antibiotic use can lead to secondary hypersensitivities in the body, presumably through their effects on intestinal flora.52 For example, they can lead to increased activity of Candida, which has been shown to be a factor in driving allergic responses.53
The relationship of bile products with intestinal flora is complex and works in two directions (see also p. 209). Bile salt metabolites variably stimulate growth in bacterial populations,54 while anaerobic bacteria act on bile products to produce volatile fatty acids that control other pathogenic bacteria.55 A particularly revealing insight into the relationship is seen in the case of bowel cancer. There are three known endogenous components that affect development of colorectal cancer – colonic bacteria, the mucus layer and bile acids. The major effects of the bacteria are deconjugation and reduction of bile acids, activation of mutagen precursors, fermentation and production of volatile fatty acids, formation of endogenous mutagens and physical adsorption of hydrophobic chemicals. The mucus layer covering the surface acts as a barrier and its composition changes in premalignant and malignant colon tissue. The secretion of protective mucus is elevated by plant cell wall components in the diet. Mucus has some hydrophobic properties and its presence may alter the effect of bile components and bacterial metabolites on the gut wall.
Bowel bacteria have been linked to another cancer. In looking for reasons to explain the epidemiological link between high-fibre diets and lower risks of breast cancer, it was found that both raising fibre content in the diet and suppressing microflora with antibiotics led to reduced intestinal reabsorption of oestrogens and lower levels circulating in the blood. It was concluded that intestinal microflora raise oestrogen levels by deconjugating bound oestrogens that appear in the bile, thereby permitting the free hormones to be reabsorbed.56 The beneficial levels of a high-fibre diet are likely to be the dominant factor in women susceptible to breast cancer, especially as there is evidence that bacterial flora actually enhance some of its wider benefits.57
There are several ways popularly promoted to correct disturbed or damaging bowel flora. Current evidence indicates that varying probiotic strains mediate their effects by a variety of different effects that are dependent on the dosage employed as well as the route and frequency of delivery. Some probiotics act in the lumen of the gut by elaborating antibacterial molecules such as bacteriocins; others enhance the mucosal barrier by increasing the production of innate immune molecules; and other probiotics may mediate their beneficial effects by promoting adaptive immune responses (for example, secretory immunoglobulin A, regulatory T cells, interleukin-10). Some probiotics have the capacity to activate receptors in the enteric nervous system, which could be used to promote pain relief in the setting of visceral hyperalgesia.58 The value of a high bulk diet with reduced simple sugars intake is, however, more accepted. The phytotherapist might combine the benefits of such dietary moves with attendance to hepatic and biliary function and with bitter or aromatic digestive insurance that food matter is well rendered in the upper digestive tract. The value of direct agents such as Artemisia absinthium (wormwood), Marsdenia (condurango) and Allium sativum (garlic) on disruptive bowel flora is likely to be upheld (see also later under Intestinal dysbiosis).
Plant fructo-oligosaccharides are associated with ‘prebiotic’ properties; that is, they selectively stimulate growth and/or activities of health-giving microbial species in the gut.59 They are a mixture of oligosaccharides typically consisting of glucose linked to fructose units. They are widely distributed in plants such as onions, asparagus and particularly cereals,60 and in herbal remedies such as Cichorium (chicory) and Taraxacum (dandelion root).61 They are not hydrolysed by human digestive enzymes but are utilised by intestinal bacteria such as Bifidobacteria, Bacteroides fragilis group, Peptostreptococci and Klebsiellae. In clinical studies, improvement of faecal microflora was observed on oral administration of fructo-oligosaccharides at 8 g62 and 12.5 g63 per day; the population of bifidobacteria in faeces increased substantially compared with before the administration. Mucilages can also be used to ‘feed’ healthy bacteria.
Acupharmacology: a pharmacological basis for herbal therapeutics?
The main inputs into the decision-making processes involved in digestion are a vast array of receptors and sensory tissues along the gut wall. Each of these provides signals for some effector function elsewhere in the gut or indeed elsewhere in the body. The effects of the following archetypal plant constituents discussed in this chapter and in Chapter 2 can clearly be seen to work primarily on the digestive tract.
As a source of enteric reflexes
Through neural links forged during embryonic development, stimulation of neural receptors in the gut wall is theoretically able to effect responses in other areas. Vagal modulation can lead to wider adjustment of autonomic activity in the body and particularly in bronchial muscle activity and cardiovascular control signals originating in the thorax. Pungent principles (hot spices) increase blood flow in other areas after ingestion.24
As the body’s largest concentration of immunological activity
The body has by far its greatest exposure to immunological challenge in the digestive system.
Phytotherapeutic principles
Archetypal digestive remedies
Following Chapter 2, the approach adopted there and in the following section is to look at key chemical groups in plants, the ‘archetypal plant constituents’, rather than at individual remedies. The fragmentary nature of research support for herbal therapeutics is always a major limitation, but fortunately in the area of the digestive tract there is a more than usually reliable experience of efficacy; the digestive tract is one of the most accessible organs in the body and most traditional treatments relied on immediate clinical effects.
Dosage and other prescription practicalities
The gastrointestinal tract provides a large surface area and the processes of digestion quickly denature and dilute remedies. For most of the effects referred to, therefore, relatively large doses of plant need to be taken (and the preparation needs to contain the relevant constituent – it is no good having a convenient extract of plant without its mucky mucilage, resin or tannin if those are the constituents one needs!). Many modern prescriptions based on quantities measured in drops or milligrams would be unlikely to have much impact on this system. The main exceptions are the bitters and hot spices or other cases where the target receptors are close to the point of entry or the agent is particularly powerful.
The following summary of the main phytochemical groups with impact on the digestive system is treatment focused. In each case there is a much more substantial description in Chapter 2 and this should be also referred to before making clinical decisions, particularly for the mucilages (and bulk laxatives), bitters, anthraquinone laxatives and pungent constituents.
Bulk laxatives
Emetics
Therapeutic vomiting is much less applied than in previous centuries. Even in its usual modern application, it has been demonstrated as an inefficient way to remove ingested poisons, with appreciable amounts being forced into the small bowel.64 Activated charcoal as a non-emetic treatment for poisoning is likely to be superior,65 for example in paracetamol poisoning.66 The main reason to maintain this category is in the use of emetic plant remedies in sub-emetic doses as reflex expectorants – an important pharmacological principle explored in the Respiratory system section of this chapter and in Chapter 2.
Contraindications for emetics
Application
For poisoning emergencies use, for example, 15 mL of ipecac syrup for children and adults,67 repeating the dose in 15 to 30 minutes if necessary. If Ipecac is unavailable, soapy water or detergent with water may be used or manual stimulation of the gag reflex with finger or blunt instrument may be tried.
Mucilages
The demulcent quality of plant mucilages remains one of the most effective short-term reliefs for irritation of the upper digestive tract, notably including reflux into the oesophagus and other effects of acid dyspepsia. The fate of ingested mucilages lower down the tract is not always certain. However, some such as Ulmus (slippery elm) have persistent reputations as remedies for irritation and inflammation lower down the gut; and there may be some mucilaginous effect in some plant materials, where these merge into prebiotic polysaccharide and fibre elements. The authors in one report68 searched online databases for reports of orally ingested polysaccharides. Fifteen studies were found showing statistically significant effects in humans, although these included open label trials. They involved healthy adults and those with cancer, allergies, or aphthous stomatitis. In healthy human adults, oral arabinogalactans from Larix occidentalis (western larch) increased lymphocyte proliferation, CD8+ lymphocytes, and immunoglobulin G response to pneumococcal vaccine in placebo-controlled, randomised, clinical trials. Furanose from American ginseng (Panax quinquefolius) reduced incidence and duration of respiratory illness in a trial with healthy older adults. As suggested above, most polysaccharides are partially degraded in the digestive tract. Several are metabolised by gut bacteria and may thereby exert prebiotic effects. Fucoidans and other polysaccharides from seaweeds such as Fucus vesiculosus and agar-agar are notably resistant to breakdown and can be included in the bulk laxative category.
Saponins
Plant remedies traditionally used for saponin effects on the gut
Contraindications for saponins
The use of saponins is either contraindicated or at least inappropriate in the following:
Traditional therapeutic insights into the use of saponins
Saponins are common constituents in plants used in Chinese and Asian medicine as tonics and harmonising treatments. Modern pharmacological interest in the effects of ginseng has raised speculation that, as steroidal molecules, some saponins may modulate steroid hormone control mechanisms in the body (see also Chapter 2 and the licorice and ginseng monographs).
Tannins
The effects of this phytochemical group have been extensively covered elsewhere in this book. They can provide short-term healing and anti-inflammatory effects on the gut wall, though likely to rapidly reduce with transit through the tract unless in slowly dispersing solid form. Effects on the bowel, for example in diarrhoea, can however be significant if the symptom is a reflex consequence of irritation in the gastric or upper enteric passages (gastroenteritis). The use of tannins is not to be recommended as a long-term solution (see Chapter 2).
Plant remedies traditionally used for tannin constituents
• Hamamelis (witchhazel), Potentilla tormentilla (tormentil), Quercus (oak), Agrimonia (agrimony), Geum (avens), Krameria (rhatany), Geranium (cranesbill), Cnicus benedictus (blessed thistle), Acacia catechu (catechu), Bidens (bur-marigold), Alchemilla (ladies mantle), Polygonum (bistort), Sanguisorba (burnet).
Contraindications for tannins
The use of tannins is either contraindicated or at least inappropriate in the following:
Pungent constituents
Aromatics
Volatile spasmolytics
Contraindications for spasmolytics
The use of spasmolytics may be contraindicated or inappropriate in gastric and enteric poisoning incidents.
Resins
Anthraquinone laxatives
Bitters
This group is almost entirely focused in their impact on the digestive system, and reference to the Bitters section in Chapter 2 is particularly recommended before incorporating them into a strategic approach. Some listed below are also prominent choleretics and should be further reviewed in that section of this chapter.
Plant remedies traditionally used as bitters
• Gentiana (gentian), Centaurium (centaury), Artemisia absinthium (wormwood), Taraxacum (dandelion root), Hydrastis canadensis (golden seal), Berberis vulgaris (barberry), Aletris (unicorn root), Marsdenia (condurango), Menyanthes (bogbean), Picrasma (quassia), Swertia (chiretta), Veronicastrum (black root).
Contraindications for bitters
The use of bitters is either contraindicated or at least inappropriate in the following:
Traditional therapeutic insights into the use of bitters
Bitters were universally classified as cooling in early approaches to medicine (see Chapter 1). This insight is likely to have followed observations that administering bitters helped to contain excesses of temperature in fevers and has been associated in Chinese medicine, for example, with the view that increased digestive activity is intrinsically a cooling phenomenon. In supporting the latter, bitters were seen not only to support nourishment but to reduce the symptoms of excessive heat in some pathologies, including some headaches and migraines, skin and other inflammatory diseases and allergic or hypersensitivity conditions.
Phytotherapy for digestive conditions
Recurrent aphthous ulceration (mouth ulcers, canker sores)
Aphthous ulceration is a common recurrent condition characterised by superficial ulcers in the mouth that are round or ovoid and surrounded by inflammatory halos.69 Ulcers are often multiple and may recur. The cause is unknown, but possible factors include emotional stress, poor innate immunity, imbalanced oral flora, low serum insulin levels, food intolerances and nutrient deficiencies, some medications and dentures.69 Several of these are controversial. In some women ulcers tend to follow a cyclical pattern and occur premenstrually. The presence of serious diseases such as Crohn’s disease, ulcerative colitis, coeliac or Behçet’s disease should always be excluded. A multitude of conventional drugs have been attempted as treatments, indicating how poorly understood this condition is.69
Treatment
Case history
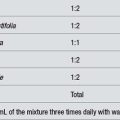
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 20 mL |
| Echinacea angustifolia root | 1:2 | 25 mL |
| Propolis | 1:10 | 25 mL |
| Valeriana officinalis | 1:2 | 20 mL |
| total | 100 mL |
Dose: 5 mL with water three times a day.
The following mixture was to be applied directly to the ulcers:
| Propolis | 1:10 | 50 mL |
| Calendula officinalis | 1:2 | 50 mL |
| total | 100 mL |
She was also advised to rinse her mouth regularly with a mixture of acidophilus yoghurt and water.
Topical application of a high alcoholic tincture or extract of Calendula, myrrh, kava or propolis is particularly beneficial. This is because such preparations are quite resinous. When they are painted on to the ulcer the alcohol dries and the resin fixes the active components so that they are not readily washed away by saliva. It is also possible that they promote a local leucocytosis (i.e. attract white blood cells). Topical application of licorice via a dissolving oral patch has been successfully used in clinical trials.70
Functional dyspepsia and gastro-oesophageal reflux disease (GORD)
The proprietary preparation Iberogast, used for over 40 years in Germany for the relief of functional dyspepsia, includes a fresh plant extract of Iberis amara (bitter candytuft) and extracts of Angelica archangelica root, Silybum marianum (milk thistle), Carum carvi (caraway), Chelidonium majus (celandine), Glycyrrhiza glabra (licorice), Matricaria recutita (German chamomile), Melissa officinalis (lemon balm) leaf, and Mentha × piperita (peppermint) with 31% ethanol. Four double blind, randomised, clinical trials have demonstrated the therapeutic effects in functional dyspepsia.71 A previous systematic review including data from the manufacturer allowed analysis of four multicentre, randomised, double blind studies against placebo or reference treatments, with a total of 595 patients; it showed significant reduction in gastrointestinal symptom scores and the conclusion that the combination seems to be an effective phytotherapeutic option without central nervous side effects.72 Another paper reported a literature search on 350 components of the mixture and found the most common pharmacological activities are motility modulating, anti-inflammatory and antioxidant effects.73 These constituents happen to overlap considerably the range of phytotherapeutic options below.
In addition to the above discussion, major factors thought to operate in GORD include:74–77
• Obesity (especially visceral adiposity) and metabolic syndrome
• Ineffective clearance of reflux from the oesophagus (including deficient saliva production)
• Impaired oesophageal mucosal defence
• Reduced lower oesophageal sphincter (LOS) pressure
• Visceral hypersensitivity, accentuated by psychological stress.
Practical measures in the treatment of GORD include:
• Elevation of the head of the bed by 10 to 15 cm. This improves oesophageal clearance at night
• Regular meal times and not eating on the run
• Avoidance of foods that reduce lower oesophageal sphincter tone. These include chocolate, carminatives (e.g. peppermint and spearmint), fatty foods, coffee, tomato concentrates and onions, but will vary from individual to individual. Spicy foods may also aggravate. Wheat can be a silent factor that seems to predispose to reflux in many patients
• Individual intolerance to certain foods may aggravate reflux, for example dairy products, and these should be avoided. These types of intolerances may not cause immediate reflux, but rather predispose to the condition. Hence an exclusion diet for at least 30 days is the only valid way to assess their role
• Avoidance of drugs which reduce LOS tone such as theophylline, calcium channel blockers and progesterone
• Giving up smoking and excessive alcohol intake (these reduce LOS tone)
• Improving mucosal resistance with Glycyrrhiza (licorice) and mucilaginous herbs such as Ulmus (slippery elm) and Althaea (marshmallow root) to assist mucoprotection. These are best taken after meals and before bed
• Bitter herbs at low doses can increase LOS tone, improve saliva output and accelerate gastric emptying. However, they also increase gastric acidity and therefore should be used cautiously. Gentiana and Artemisia (wormwood) may be too strong, hence gentler bitters such as Cynara (globe artichoke) or Achillea (yarrow) may be appropriate
• Carminative herbs and essential oils in high doses will aggravate GORD by reducing sphincter tone, but they can be indicated for functional dyspepsia. Also, in lower doses, they can improve gastrointestinal motility
• Treating associated gastroparesis with Zingiber (ginger)
• Anti-inflammatory herbs relieve symptoms and improve healing and include Filipendula (meadowsweet), Stellaria (chickweed) and Matricaria (chamomile), especially those chemotypes of Matricaria rich in bisabolol. Filipendula is also traditionally thought to reduce excess acidity and Matricaria is also spasmolytic. They are indicated in both functional dyspepsia and GORD. An in vivo study found Matricaria also lowered gastric acidity (see monograph)
• GORD and functional dyspepsia may be linked to stress, especially if associated with irritable bowel syndrome. Anxiolytic herbs such as Valeriana, and nervine tonic herbs such as Scutellaria (skullcap), are indicated if this association is evident
• Improving healing with herbs such as Calendula and Centella (gotu kola)
• Gastrointestinal spasmolytics, especially Corydalis, Matricaria and Viburnum opulus (cramp bark) may be helpful in GORD because they can exert a regulatory role on lower oesophageal function.
Example liquid formula
| Passiflora incarnata | 1:2 | 20 mL |
| Matricaria recutita (high in bisabolol) | 1:2 | 25 mL |
| Filipendula ulmaria | 1:2 | 25 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 15 mL |
| Cynara scolymus | 1:2 | 15 mL |
| total | 100 mL |
Case history
| Passiflora incarnata | 1:2 | 25 mL |
| Matricaria chamomilla | 1:2 | 25 mL |
| Filipendula ulmaria | 1:2 | 25 mL |
| Stellaria media (stabilised succus) | 25 mL | |
| total | 100 mL |
Poor upper digestive function/anorexia
Herbs that improve upper digestive function can be divided into five major groups:
• Simple bitters such as Gentiana lutea, which improve most aspects of upper digestive function
• Aromatic digestives such as Angelica archangelica, Cinnamomum (cinnamon) and Coleus forskohlii that improve gastric acid secretions. Coleus also improves exocrine pancreatic function
• Pungent herbs such as Zingiber (ginger) and Capsicum (cayenne) that are potent stimulators of gastric acid and other aspects of digestive function (as already noted)
• Choleretic herbs such as Berberis vulgaris (barberry), Silybum (St Mary’s thistle) and Taraxacum (dandelion root) that improve bile production by the liver
• Cholagogue herbs such Mentha piperita (peppermint) that improve gallbladder function.
Peptic ulcer
The herbal approach to the treatment of peptic ulcer disease should take into account all the causative and sustaining factors relevant to the individual patient. Rather than being concerned with inhibiting gastric acid, the herbal clinician stresses the support of factors that protect the mucosa and improve the capacity of the body to heal the ulcer. The prominent shift in attention towards the role of the bacterium Helicobacter pylori in causing peptic ulceration has led to many herbal approaches being mooted. These remain largely untested and some research on their role has been negative. For example, in spite of positive in vitro studies indicating an effect on H. pylori, consumption of hot spices such as chilli or of raw garlic is not known to offer any benefits in terms of human infection (for example, in Korea where these are consumed in high levels infections are notably high). In a crossover study, of 12 subjects with known H. pylori infection were tested with Jalapeno peppers, raw garlic or a bismuth-containing positive control, with clear washouts between different treatments. No benefits as assessed by urea breath test were found.78
In vitro evidence exists for an anti-adhesion effect that might reduce the infectivity of H. pylori for plant constituents such as proanthocyanidins from Vaccinium macrocarpon (cranberry), acidic polysaccharide from Camellia sinensis (green tea), carbohydrates from Panax ginseng (Korean ginseng), or the false receptors provided by fucoidans and glycosaminoglycans in medicinal algae, including Fucus vesiculosus (bladderwrack).79 All need to be confirmed in human studies (see also pp. 321–322).
A review of studies published over 30 years found that soluble fibre from fruit and vegetables was protective and refined sugars a risk factor in peptic ulcers.80 Alcohol and coffee do not seem to be related to duodenal ulcer, but gastric cancer is linked to diets high in salt and low in fresh fruit.81 Use of sugar in tea and coffee increases risk, and tea consumption reduces risk of peptic ulcers.82 Smoking is a strong risk factor.82,83 Ulcers heal more slowly in smokers and relapse is more likely. Smoking also increases the risk of infection with H. pylori84 and decreases gastric mucosal blood flow.85 Leisure time physical activity is protective,82 but skipping breakfast is a risk factor.86
The epidemiological, clinical and genetic evidence strongly suggests that host factors, especially the effects of stress (in the broadest psychosocial sense), may be decisive in determining who develops a duodenal ulcer.87
As noted above, local defences are an issue in peptic ulcer disease. Gastric mucosal blood flow is considered to be of major importance in maintaining mucosal integrity.88 Reactive oxygen species and cytokines appear to contribute to the gastric damage.89 There is an age-related decline in mucosal repair capacity, possibly related to decreased mucosal prostaglandin levels.90
Main aspects of phytotherapy include the following:
• Glycyrrhiza (licorice) and mucilaginous herbs such as Ulmus and Althaea to enhance mucoprotection. These are best taken before meals and, in the case of duodenal ulceration, should be taken at least half an hour before eating. Glycyrrhiza also improves pancreatic bicarbonate secretion
• Whilst bitter herbs such as Gentiana are contraindicated in duodenal ulcers, they may be valuable in gastric ulcers because of their trophic effect on the gastric mucous membrane
• Hydrastis canadensis (golden seal) is traditionally restorative to mucous membranes and also antibacterial, but would be best avoided in duodenal ulcers
• Immune-enhancing and antiseptic herbs such as Echinacea or high-resin remedies such as Calendula officinalis (marigold), Commiphora molmol (myrrh) and propolis may help resolve or contain Helicobacter pylori presence and improve repair mechanisms. They were traditionally used in peptic ulcer disease long before the importance of H. pylori was recognised
• Gently astringent herbs will assist ulcer healing and boost mucoprotection in the vicinity of the ulcer. Good examples are Agrimonia and Filipendula (meadowsweet). Strongly astringent herbs such as Geranium maculatum (cranesbill) will aggravate a gastric ulcer, but may be suitable for duodenal ulcer treatment
• Anti-inflammatory herbs such as Stellaria (chickweed) and bisabolol-rich Matricaria (chamomile) and vulneraries such as Calendula and Centella (gotu kola) will help break the vicious cycle of ulceration and accelerate the healing process
• Herbs benefiting the microcirculation, especially in this context Vaccinium (bilberry), Ginkgo and Vitis (grape seed extract), will also assist healing
• Pungent spices have had a common assumption of being contraindicated in peptic ulceration. However, in cultures where these are frequent components of the diet there is a contrary view that they are protective and pharmacological research tends to support this91
• Spasmolytic and carminative herbs will improve gastrointestinal motility. Carminative herbs such as Foeniculum (fennel) should be administered only in low doses. Good gastrointestinal spasmolytics include Matricaria and Viburnum opulus (cramp bark)
• Herbs that decrease the negative effects of stress can be indicated and include adaptogens, nervine tonics and anxiolytics
• Filipendula is considered by some herbalists to be a normaliser of the acidity of the stomach.92 It does appear to decrease the negative effects of acid and pepsin on the mucosa, but is probably not a potent antacid. This positive effect on the mucosa is paradoxical since it contains salicylates
• Some patients with peptic ulcer disease may experience irritation from herbal treatment and one approach with more severe or chronic cases is to start with a low dose and gradually increase over several weeks. This can especially apply with liquid preparations, in which case tablets or capsules are more appropriate.
Example liquid formulas
For gastric ulcer
| Foeniculum vulgare | 1:2 | 3 mL |
| Filipendula ulmaria | 1:2 | 25 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 20 mL |
| Matricaria recutita | 1:2 | 25 mL |
| Gentiana lutea | 1:5 | 2 mL |
| Echinacea purpurea/angustifolia root | 1:2 | 25 mL |
| total | 100 mL |
For duodenal ulcer
| Filipendula ulmaria | 1:2 | 20 mL |
| Glycyrrhiza glabra | 1:1 | 20 mL |
| Matricaria recutita | 1:2 | 20 mL |
| Vaccinium myrtillus | 1:1 | 20 mL |
| Echinacea purpurea/angustifolia root | 1:2 | 25 mL |
| total | 105 mL |
Dose: 5 mL with water three times a day half an hour before meals.
Food intolerance and allergies
Treatable factors that can contribute to a state of food allergy or intolerance include:
• Poor digestion which can lead to chemical fragments from food such as peptides or polypeptides, which are either pharmacologically active or immunogenic
• A leaky gut wall which will allow the passage of unprocessed or partially processed chemicals from food into surrounding tissue and the portal blood. Factors which can contribute to a leaky gut include alcohol, drugs, intestinal flora dysbiosis, infection and inflammation
• Poor hepatic screening of portal vein contents. Substances absorbed from the gastrointestinal tract are carried by the portal vein to the liver and can be processed by hepatocytes or phagocytosed by the Kupffer cells. In particular Kupffer cells sequester any immunologically active material and thereby dampen any potential immune reaction
• Poor immunity and detoxifying mechanisms in the body, which might lead to an excessive response to a chemical insult.
The herbal approach to the treatment of food intolerance or allergy can include the following:
• Bitter, aromatic, pungent, choleretic and cholagogue herbs to improve upper digestive function
• Hepatotrophorestoratives and stimulants of hepatic metabolism, such as Silybum and Schisandra, to improve hepatic screening and detoxification
• Depurative and lymphatic herbs, such as Arctium (burdock) and Calendula, to assist detoxification mechanisms
• Herbs with healing and protective effects on the gut wall, such as Filipendula (meadowsweet), Calendula, Matricaria (chamomile) and Althaea (marshmallow) root. These can help reduce the impact of a leaky gut
• Immune-enhancing herbs such as Echinacea, which enhances phagocytic activity. Andrographis is both bitter and immune enhancing
• Hydrastis (golden seal) as a restorative to the mucous membranes of the gut wall and a potential modifier of gut flora. Hydrastis is also bitter
• Other treatments to restore normal gut flora. This is covered in detail later in this section
• Antiallergic herbs such as Albizia and Scutellaria baicalensis (Baical skullcap) and anti-inflammatory herbs such as Matricaria and turmeric.