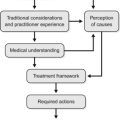
8 Herbal approaches to pathological states
Chapter Contents
Topical applications
Scope
Apart from their use to provide non-specific support for recuperation and repair, specific phytotherapeutic strategies include the following.
• seborrhoeic inflammations (such as acne vulgaris)
• cutaneous infections and infestations
• minor inflammations of mouth, throat, anus and nasal and vaginal mucosa
• certain inflammatory conditions affecting the surface of the eye.
Orientation
Background
Referral to Chapter 2 will provide much of the detail for this review. The following topical properties, however, can be highlighted.
Demulcents and healing agents
Plant material often contains apparently soothing effects on physical contact and plant remedies must have been a very early instinctive application to wounds. Plants with high mucilage content form the basis of poultices and creams. Linum (linseed, flaxseed) is one of the most impressive poultices where the skin (or subdermal tissue in even unbroken skin) is painfully inflamed. Ulmus rubra (slippery elm bark) when powdered is one of the most obviously mucilaginous plant materials available for poultices. Stellaria (chickweed) in a cream base provides effective relief for many itchy conditions. Althaea (marshmallow root) and Trigonella foenum-graecum(fenugreek) have notably soothing reputations. The expressed juice of Aloe vera also has impressive topical demulcent properties when applied directly to broken or unbroken skin.1–3
However, non-mucilaginous herbs like Alchemilla vulgaris (lady’s mantle) have also demonstrated topical healing effects, in this case on mouth ulcers.4 The anti-inflammatory remedy Boswellia has been shown topically to heal skin damaged by age and sunlight.5 The link between anti-inflammatory and healing properties is particularly well illustrated in an ex vivo study on an aqueous extract of Uncaria tomentosa (cat’s claw). The carboxy alkyl esters, in particular quinic acid esters, easily absorbed across the skin, have been shown to enhance DNA repair and skin resilience to UV damage through anti-inflammatory properties involving the inhibition of nuclear transcription factor kappaB (NF-kappaB).6
Perhaps the most thorough healing remedy is Symphytum (comfrey root) cream. When this is applied topically it combines an unparalleled local demulcent action, and tannins (see below) with an active promoter of repair, allantoin, which is known to be very rapidly absorbed into the subdermal tissue.7 Comfrey has demonstrated healing effects in controlled trials in ankle sprains,8 and even pain-relieving and anti-inflammatory effects (see below).
Astringents – tannins and OPCs
Tannins and related polyphenols are very common plant constituents with the simple property of curdling protein molecules into which they come in contact. The principle of tanning animal skins to make leather, most often using oak galls or oak bark, follows from this property. Simple washes with strong decoctions of high-tannin preparations (like broadleaved tree bark) are well-established country first-aid treatments for open wounds and third-degree burns and the technique was formally revived among ‘barefoot doctors’ in China after the Cultural Revolution in the 1960s. The aim here was to produce a sealing eschar over the exposed tissues formed from coagulated protein on the surface. In modern clinical application, suspensions of decoctions of high-tannin herbs in gum tragacanth or gum arabic can produce impressive healing effects in open wounds or skin lesions. Plants to be considered for this role include decoctions of Hamamelis (witchhazel bark), Potentilla tormentilla (tormentil root), Quercus (oak bark), Krameria (rhatany) and Geranium maculatum (American cranesbill root). The antioxidant effects of such preparations have also drawn attention.9–11 Topically applied tea extracts have been shown to help to restore skin integrity in people with radiation-induced skin damage. On the basis of in vitro cell responses to various tea extracts, these effects are suggested to involve compounds other than the polyphenols.12 The role of related polyphenols, the oligomeric proanthocyanidins (OPCs), in topical application has been suggested in a clinical study that pointed to a healing effect of combined oral and topical application of an OPC-rich pine bark extract in the treatment of ulcers of diabetic origin.13
The usefulness of application of green tea catechins for periodontal disease was investigated in a placebo-controlled trial.14 Strips containing the catechins as a slow release local delivery system were applied to oral pockets in patients once a week for 8 weeks. The pocket depth and amount of bacteria were markedly decreased in the tannin group, whereas there was no change for the placebo group.
A double blind study investigated the effect of chewing a green tea confectionery on gingival inflammation.15 A total of 47 volunteers (23 male, 24 female) were randomly assigned to chew either eight green tea or placebo candies per day for 21 days. While there was an improvement in the green tea group, the placebo group deteriorated slightly.
Anti-inflammatories
A number of plant constituents appear to possess topical anti-inflammatory effects, frequently in addition to their demulcent and astringent properties. They might be considered as alternatives to conventional steroidal and other anti-inflammatory prescriptions. For example, herbal Arnica extract compared well with topical non-steroidal anti-inflammatories in the relief of osteoarthritic pain.16 Calendula (marigold),17 at least when extracted in high-strength alcohol, and Matricaria recutita (German chamomile),18 both included in creams, have useful benefits in soothing inflamed skin lesions.19Berberis aquifolium20 and Aloe vera21 have demonstrated efficacy in the treatment of psoriatic lesions. Echinacea applied topically appears to have local anti-inflammatory effects on minor wounds.22 Hypericum (St John’s wort) extracted in oil as a red pigment is a long-standing remedy for the relief of burns and skin pain, and a hyperforin-rich extract has shown benefit over placebo in relieving atopic dermatitis.23 Other traditional remedies used topically for anti-inflammatory effects include Curcuma longa (turmeric), Juniperus (juniper oil) and Angelica archangelica (Angelica oil). Bruising is traditionally treated with external applications of Aesculus hippocastanum.24 The antiseptic properties of tea tree oil (see below) are complemented with an observed topical antihistaminic effect.25 Many of these applications are fully reviewed in the relevant monographs.
The comfrey ointment referred to above was evaluated for the treatment of knee arthritis.26 In a large placebo-controlled clinical trial, 220 patients applied 2 g of ointment three times a day for 3 weeks to their painful knee joint. The ointment either contained comfrey or was a matching placebo. In terms of self-rated pain, there was a 55% drop in the comfrey group as opposed to a drop of only 11% in the placebo group. Similar results were also seen for other measures of osteoarthritis symptoms. Overall, pain was reduced, the mobility of the knee improved and quality of life increased.
Comfrey is also good for helping to relieve muscle pain. Two strengths of comfrey cream (10% and 1%) were tested in a double blind study involving 215 people with pain in either the upper or lower back.27 The stronger cream caused significant improvements in pain on movement, pain at rest and pain on pressure.
A favourite herb for eczema and other forms of inflamed skin (dermatitis) and to promote healing is the Calendula marigold (Calendula officinalis). Radiation-induced dermatitis is a common side effect of radiation therapy. For approximately 80% of patients, irradiation induces dermatitis. Apart from the pain and inconvenience associated with the dermatitis, it can lead to interruption of the radiotherapy. There is no standard treatment for the prevention of radiation-induced dermatitis. A survey conducted in 2001 in France indicated that one-third of radiation oncologists prescribed a preventative topical agent for women undergoing irradiation for breast cancer; the most popular choice was the drug trolamine. Hence when French researchers initiated a trial of topical Calendula in patients receiving radiotherapy for breast cancer, they decided to compare its efficacy with that of trolamine.28
Antiseptics
The topical effects of herbal remedies can include some antiseptic effects in vitro, although only a few whole preparations have significant clinical prospects. The antimicrobial effects of tea tree oil have been demonstrated in controlled clinical studies in acne vulgaris,29 dandruff30 and even methicillin-resistant Staphylococcus aureus (MRSA) infections.31 The oil also has established antifungal properties.32,33 Another clinical study has demonstrated clinically relevant antifungal effects for a member of the Solanaceae (deadly nightshade) family.34
Topical antiviral properties have been demonstrated for the concentrated extract of Melissa officinalis (lemon balm) in the relief of herpes infections.35 One early study found an improved healing rate for 75% of patients, with the time between outbreaks prolonged in 50% of cases.36 Compared with conventional treatments (at the time), the average healing time of lesions was halved to about 5 days and the time between outbreaks was approximately doubled.36 In another multicentre study involving 115 patients, treatment of herpes lesions was commenced between 24 and 72 h from their outbreak.37 It was found that lesions in 87% of patients were completely healed within 6 days of treatment. The time between outbreaks was increased for 69% of patients, and this was 2.3 months with lemon balm compared with 1.3 months for conventional drug treatments such as idoxuridine and tromantadine. Minor side effects were observed in only 3% of patients. The delay in new lesions occurring was without any prophylactic application, and it is possible that a preventative application of the cream to normally affected areas would further increase the time between outbreaks. Lemon balm cream can also be used to treat herpes simplex type 2 infection, and probably other similar viral skin infections, including shingles. The cream contained 1% of a concentrated 70:1 extract of lemon balm.
Rhubarb root (Rheum officinale) and sage (Salvia officinalis) also have activity against viruses, including herpes simplex type 1. In a double blind, controlled trial involving 49 patients, the results for creams containing either 2.3% sage, or 2.3% sage and 2.3% rhubarb, were compared against the conventional antiviral cream containing acyclovir. The average time for the herpes sores to fully heal was 7.6 days with the sage cream, 6.7 days with the rhubarb-sage cream and 6.5 days for acyclovir.38 Hence, the herbal combination worked as well as the conventional drug.
Many herbs are used as a gargle, lozenge or throat spray to treat a sore throat. These include Calendula, sage, propolis, Echinacea root and golden seal. Acute viral pharyngitis is linked to the common cold and includes symptoms such as sore throat and fever. In a double blind, placebo-controlled clinical trial, the value of a sage throat spray was compared against a placebo spray in almost 300 patients with acute viral pharyngitis.39 The throat spray was used for seven applications over 3 days, and each application consisted of three sprays. A 15% strength sage spray was found to be much better than placebo for relieving throat pain. Symptomatic relief occurred within 2 h of applying the spray.
For a further review of antimicrobial effects of herbal remedies see Chapter 2.
Local anaesthetics and analgesics
As well as the well-known effects on dental pain of topical Syzygium aromaticum (clove) oil,40 and the impact on muscle pain41 and intractable itching42 of capsaicin preparations from Capsicum spp., there is now increasing evidence that comfrey (see above) also has pain-relieving properties in arthritis.43
Formulations
Liquids
Eardrops
The external ear canal can be treated with oil or alcohol/water-based preparations to help clear obstructions, to treat inflammation or infection of the canal or ear drum or to influence the middle ear by diffusion across the ear drum. Warm olive oil is a popular treatment for waxy obstructions and may be augmented by garlic or Verbascum (mullein flowers) steeped in the oil. However, bacterial contamination of such products is a concern and non-industrially produced preparations are often not to be recommended.
Solids
Plasters
Unlike the modern item, traditional plasters were impregnated dressings applied over the skin where a long-term and concentrated medication was required. The plaster mass was a waxy, rubber, resinous or other base incorporating medical agents, spread on to fabric. It was often designed to convey rubefacient, analgesic or protective effects. Cayenne (capsicum) plasters containing capsaicin are notable applications for arthritic disease.
Fever
Scope
Apart from their use to provide non-specific support for recuperation and repair, specific phytotherapeutic strategies include the following.
Particular caution is necessary in applying phytotherapy in cases of:
Orientation
Introduction
Fever is most often associated with viral and bacterial infectious illnesses of varying degrees of severity, such as ‘flu’, measles, rubella, rheumatic fever, pneumonia, malaria, scarlet fever, polio, tuberculosis and meningitis. It can also accompany a wider range of problems such as cardiovascular and autoimmune diseases, drug reactions and some cancers. Many cases, especially in children, remain mysterious.1
The fever as friend?
Perhaps because of the associations above, the fever process has come to be seen as a problem to be treated in its own right (‘We must bring the fever down’). The serious risks from hyperpyrexia (overheating) are well understood and the accompanying unpleasant symptoms are reason enough to regard fever with suspicion. However, if it is clear that fever is not part of a serious condition there are also good reasons for not suppressing the process unnecessarily. This view is gaining support in conventional medicine,2,3 particularly in reaction to the fashion for using paracetamol, and previously aspirin, to treat common childhood fevers.4
A recent example of such concerns is the New Zealand study that investigated the association between infant and childhood paracetamol use and later atopy and allergic disease.5 Children given paracetamol before the age of 15 months were 3.6 times more likely to have atopy at age 6 years than infants who had not been given the drug. Paracetamol use between the ages of 5 and 6 years showed dose-dependent associations with wheeze and asthma.
Such modern reassessment of fever treatment echoes the older traditional view that fever was not the disease itself but the body’s extraordinary efforts to resist disease. It was therefore something to be supported, or at least managed, rather than unduly suppressed. In this view, a ‘good fever’, one which went through its natural course satisfactorily, would not only lead to better resolution of the immediate crisis but would actually rearm the body’s defences and increase its resistance to future onslaughts. Indeed, it was on this issue above all others that the revivalist practices of Samuel Thomson in the 19th century were based (see p. 11). It was common practice among ‘regular’ physicians to suppress fevers with mineral drugs based on mercury, arsenic and antimony. Thomson was moved by Indian practices, especially the sweat lodge, to vehemently challenge such principles and insist instead on the view that fever was a sign of healthy defences (the ‘natural heat’ of the body resisting ‘cold’ intrusion) and should be supported in its efforts rather than suppressed. To this end, he recommended the use of heating remedies, including cayenne, and other measures to support the body through the episode, managing excesses of the febrile condition along the way. Although Thomson’s message was simple (reflecting the predominance of fevers as the main clinical priority of the times), he identified a fundamental difference between traditional practices and the new direction of orthodox medicine – supporting body defences versus attacking disease processes.
There is modern support for the traditional view.6 The body’s febrile response is accompanied by the arousal of powerful, unpleasant and debilitating defensive measures, the release of inflammatory chemicals, temperature-stimulated activity in the circulation and in various blood cells, including the scavenger white blood cells, and associated alterations in a wide range of other functions.7,8 In many ways it is like inflammation, for which an analogous traditional view applies (see p. 152): it generally proceeds in defined stages, tends to be self-limiting and is directed to mobilising defensive resources to the rapid elimination of an intrusion into the tissues. Both inflammation and fever are accompanied by what may be regarded as guarding symptoms, in the case of fever often by nausea (leading to reduced eating and unnecessary digestive, eliminative and metabolic burdens), thirst (increasing fluid consumption and compensating for fever-induced dehydration), lassitude and exhaustion (ensuring adequate rest during the process) and photophobia (encouraging withdrawal to a darkened place so as to reduce visual and other stimulation).
Practical fever management
There is a more complex story of course. For example, there are a range of cytokines, such as interleukin (IL)-1-(alpha and beta), IL-6 and tumour necrosis factor-alpha, produced by the body itself and known as endogenous pyrogens which, possibly interacting with prostaglandins, can induce relapsing and other complex fever patterns with no clear cause. More recently, it has been shown that inhibitors of cytochrome P450 exacerbate pyrexia and that inducing P450 arachidonic acid metabolism reduces fever.9–11 There are also endogenous antipyretic mediators including neuroactive substances such as glucocorticoids, vasopressin, IL-10 and melanocortins.12
1. Feeling cold, with pale cyanosed skin and shivering means that the body temperature is lower than that set in the hypothalamus and is most likely to be still rising.
2. Feeling hot, with flushed skin and sweating means that the body temperature is higher than the thermostat setting and is most likely to be coming down.
3. Having no dominant feeling of being hot or cold suggests relative equilibrium between thermostat and body temperature.
Apart from the usual techniques for bringing temperature down, such as cold wet face flannels or tepid baths, there is conventional aspirin.13 This, however, simply turns the thermostat controls down without attending to any other aspects of the fever; there is the risk of an unresolved problem with symptoms lasting for years.14
Its use in children has in any case been discontinued in recent years because of the incidence of serious side effects.15,16 Paracetamol and ibuprofen17 are still used for similar purposes; there are reports of adverse effects in the case of paracetamol particularly,18,19 but a recent systematic review indicates safety of these agents compared with nocebo effects is not a major concern.20 The wider question is the wisdom of using such agents to bring down the fever, with consequent risks to antibody production and cell repair, when no other risk is present.21
Herbal remedies, by contrast, have a number of more complex effects on the body and on the febrile response. There are a number of peripheral antipyretic mechanisms associated with plant remedies,22–24 including ginger,25 fennel,26 boldo27 and Andrographis.28 However, it is worth noting in the practical guides to fever management that follow, that the published evidence for efficacy in humans has been undermined by poor methodological quality of the studies.29
Apart from body temperature, there are other symptoms of fever that need to be watched. Many, such as nausea, vomiting, diarrhoea, headaches, coughing, pains and spasms, can usually be controlled by the appropriate herbal remedy, covered elsewhere in this book. Accepting the potential value of the febrile reaction does not mean consigning the patient to unnecessary discomfort. There are of course danger signs as well (a pulse that does not rise with temperature as expected might herald meningitis; convulsions, although common enough in children, can disguise and exacerbate polio; a dry cough of measles can resemble that of pneumonia, which can also be heralded by rapid breathing rates; malaria remains impossible to diagnose without blood tests).30 The untrained must not attempt to take full responsibility for any such treatment.
Raising fever
The patient should be advised to prepare properly. Bed rest, a minimal fresh diet and plenty of fluid are basic requirements. It is then possible to take one of the many circulatory stimulants, such as Cinnamomum zeylanicum (cinnamon,) Angelica archangelica (angelica), Zingiber (ginger), Allium (raw garlic) or even Capsicum (cayenne). A modest febrile process can then be nudged into existence, often to considerable advantage. Echinacea root, being both warming and stimulating to mucosal immune defences, may be particularly useful here.
References
3. Klein NC, Cunha BA. Treatment of fever. Infect Dis Clin North Am. 1996;10(1):211–216.
4. Adam D, Stankov G. Treatment of fever in childhood. Eur J Pediatr. 1994;153(6):394–402.
6. Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics. 2011;127(3):580–587.
8. Muzyka BC. Host factors affecting disease transmission. Dent Clin North Am. 1996;40(2):263–275.
12. Tatro JB. Endogenous antipyretics. Clin Infect Dis. 2000;31(suppl 5):190–201.
13. Bartfai T, Conti B. Fever. Sci World J. 2010;10:490–503.
14. Cuddy ML. The effects of drugs on thermoregulation. AACN Clin Issues. 2004;15(2):238–253.
16. Whelton A. Renal effects of over-the-counter analgesics. J Clin Pharmacol. 1995;35(5):454–463.
Infectious diseases
Scope
• minor to moderate acute infections of the respiratory, urinary and gastrointestinal mucosa
• minor to moderate systemic infections especially when accompanied by lymphadenopathy
• topical bacterial and fungal infections
• minor to moderate febrile infections
• minor to moderate chronic viral, bacterial and fungal infections.
Orientation
Background
Nevertheless, the herbal cupboard is not entirely bare. Most of the horrors referred to above were features of life in cities and towns, of crowded squalid conditions, far from sources of fresh food (and with no refrigerated delivery) and clean water. The historical and archaeological evidence available suggests that life was more equable in traditional rural communities, that, leaving perinatal mortality aside, average lifespans in the ancient world differed little from those reached until the middle of the 20th century1–3 and that longevity was not uncommon where land was fertile.4 In such circumstances, the case against the traditional remedies used by country people is harder to sustain.
Furthermore, if the killer diseases are taken out of the picture and more ordinary everyday infections are considered, the balance shifts in favour of historical treatment. There are a number of traditional remedies that appear to support the body in its battle against infections. From an exhaustive review of traditional cooking practices and recipes and correlation with exposure to infections, it has been concluded that the pungent spices were adopted as flavourings, consciously or unconsciously, at least in part because of their protective effect against enteric infections, with the hottest used where there is greatest exposure.5 There are other indications that chillies in particular are antimicrobial in moderate doses.6,7
As another example of a dietary antimicrobial, various publications indicate that garlic extract also has broad-spectrum antimicrobial activity against many genera of bacteria and fungi. This activity, particularly in respect of Gram-negative bacteria, seems to be stronger than that of other members of the onion family.8,9 Allicin was the early candidate as the most active constituent in garlic.10 In terms of the phytochemical world it has been verified as a potent antibacterial agent, but even then its activity is relatively modest compared with conventional antibiotics.11 Later research has demonstrated that ajoene, the prominent metabolite of allicin, also conveys particular antimicrobial activity, including against such Gram-negative bacteria as Escherichia coli and Klebsiella pneumoniae. The disulphide bond in ajoene appeared to be necessary for this activity, since reduction by cysteine, which reacts with these bonds, abolished its antimicrobial activity.12 This activity is, however, significantly exceeded by another metabolite, 10-devinylajoene, marked by substitution of the allyl group in ajoene by a methyl group and sulfinyl group.13 These findings might explain why a garlic extract was observed to have a more potent anti-staphylococcal activity than an equivalent amount of allicin.11
The incidence of stomach cancer is lower in individuals and populations with high allium vegetable intakes. To investigate this further, an aqueous extract of garlic cloves was standardised for its thiosulfinate concentration and tested for its antimicrobial activity on Helicobacter pylori. Minimum inhibitory concentration was 40 µg/mL thiosulfinate. This study may have detected a particular sensitivity of H. pylori to garlic constituents as Staphylococcus aureus tested under the same conditions was not susceptible to garlic extract up to the maximum thiosulfinate concentration tested (160 µg/mL).14 However, clinical trials of the use of garlic in eradicating H. pylori have been disappointing, although this might reflect on the form of garlic used.15,16 Systemic antifungal activity has been supported in a study where commercial garlic extract was given intravenously to two patients with cryptococcal meningitis and three patients with other types of meningitis. Plasma titres of anti-Cryptococcus neoformans activity rose two-fold over preinfusion titres.17
The position with other plant constituents is less clear. Essential oils have demonstrated antimicrobial effects in vitro (see, for example references18–20 and also Chapter 2). In one study, for example, the antimicrobial effects of some essential oils on oral bacteria were surveyed. Tea tree oil, peppermint oil and sage oil proved to be the most potent essential oils, whereas thymol and eugenol were potent essential oil components.21 Tea tree oil has demonstrated a number of antimicrobial effects, even against MRSA.22 More studies on tea tree oil are reviewed in Chapter 2. In research on antifungal effects, those of phenolics such as iso-eugenol, cinnamaldehyde, carvacrol, eugenol and thymol were linked to the presence of a free hydroxyl group linked to an alkyl substituent.23 However, all these studies generally refer to isolated oils rather than to the whole plant and it is difficult to infer from these that traditional treatments had much antimicrobial efficacy.
On the other hand, ethnobotanical reviews of traditional plants (often reported in the excellent Journal of Ethnopharmacology) have shown in vitro efficacy of whole-plant preparations (for example references24–27) and in one study a high correlation was demonstrated between traditional use and in vitro antisepsis among plants in North America.28 It is possible that modest antimicrobial effects may lie in other plant constituents: tannins (especially tannic acid and propyl gallate) have demonstrated such properties. This activity is suggested as being associated with the hydrolysis of ester linkage between gallic acid and polyols, hydrolysed after the ripening of many edible fruits. Tannins in these fruits thus may serve as a natural defence mechanism against microbial infections.29
• The powerful bitters – Artemisia absinthium (wormwood), Columbo, Marsdenia (condurango), Cinchona (quinine bark), Swertia, Berberis vulgaris (barberry bark) and Hydrastis canadensis (golden seal root) – that were likely to have been effective protectors against enteric and hepatic infections (see the monograph for the last two herbs)
• The hot pungent spices – Capsicum spp. (chillies), Zingiber (ginger) – that complement this role in enteric infections and, with Cinnamomum (cinnamon), that still today provide effective treatments in respiratory infections (see the monograph on ginger)
• Remedies like Juniperus (juniper berries), Arctostaphylos uva-ursi (bearberry), Barosma betulina (buchu) and Piper cubeba (cubeb) in the treatment of urinary infections (see the monographs on bearberry and buchu).
Phytotherapeutics
General approach to infections
• Immune-enhancing herbs like Echinacea angustifolia or E. purpurea root or Baptisia (wild indigo root) are an effective core element in the treatment of any infection acute or chronic (see below for a treatment protocol for Echinacea root). Andrographis is particularly suited to acute infections, with consistent evidence for its clinical efficacy (see the Andrographis monograph).
• Warming circulatory stimulants will act to promote defensive immune activities in many cases of acute infections. Zingiber (ginger) is particularly helpful in these circumstances; Capsicum (cayenne) and Cinnamomum (cinnamon) are respectively stronger and milder substitutes or accompaniments.
• If the infection is located in a particular organ, herbs are often prescribed to support that organ or its defensive functions: strong bitters in the case of enteric infections; Silybum (St Mary’s thistle) for liver infections; expectorants for lung infections; Juniperus for the urinary system; Serenoa (saw palmetto) for prostate infections. This is especially the case if these infections are subacute or chronic.
• Hypericum (St John’s wort) may be applied in the long-term treatment of infections with enveloped viruses; Thuja (arbor-vitae) more widely for viral infections.
• For topical treatment of accessible surfaces a different range of products can be used. Melaleuca (tea tree oil) can be applied directly to some skin infections and, in suitable carriers, for vaginitis, in the case of either bacterial or fungal origin. Melissa (lemon balm) and Glycyrrhiza (licorice) have topical antiviral activity and can be applied to herpes and similar outbreaks, as can essential oils such as tea tree (provided the virus is enveloped). Thuja and Calendula tinctures and tea tree oil are effective for fungal infections of the skin and nails. For the mouth and throat, mouthwashes and gargles can be made with Echinacea angustifolia or E. purpurea root, high-strength alcoholic tinctures of Commiphora (myrrh), Calendula, Populus gileadensis (balm of Gilead) or propolis, all best combined with fluid extract of Glycyrrhiza.
Poor immunity and recurrent infections
• Immune-enhancing herbs: Echinacea, Astragalus, Picrorrhiza, Andrographis, Phytolacca. Astragalus should not be prescribed during acute episodes and Picrorrhiza and Andrographis should not be prescribed if the patient is constitutionally cold. Astragalus is particularly indicated for chronically depleted immunity.
• Tonic and adaptogenic herbs: Panax, Eleutherococcus, Withania. It is advisable to hold back on using these during an acute infection.
• Bitter herbs: Gentiana, Artemisia absinthium especially where the patient appears anaemic or undernourished. Exercise caution if the patient is also constitutionally cold; if necessary counter the cooling effect with heating herbs.
Echinacea protocol
1. Take a 5 mL dose each day (2.5 g) as a maintenance dose (take twice this dose for maintenance if immunity is very poor).
2. If infection threatens, double or triple the daily maintenance dose until the threat passes.
3. If infection takes hold, maintain the higher dose until the infection is completely gone and then return to the normal daily dose. Alternatively, or in addition, apply the treatments noted above for acute infections.
Inflammatory and autoimmune diseases
Scope
Phytotherapy includes some unique approaches to influencing inflammatory and immunological mechanisms. Although there is an incomplete evidence base, in some cases there are enough strong themes to influence clinical practice. Phytotherapeutic strategies include the following.
• chronic inflammatory diseases of the digestive tract, including gastritis, Crohn’s disease and ulcerative colitis
• chronic inflammatory diseases of joints, and other connective tissues, including rheumatoid arthritis (RA) and ankylosing spondylitis (see also Chapter 9)
• psoriasis, scleroderma, other chronic inflammatory skin diseases (dermatitis), including complex and autoimmune conditions such as psoriasis (see also Chapter 9)
• long-term inflammatory processes underlying chronic conditions such as diabetes and atherosclerosis.
Orientation
Relevant inflammatory mechanisms
In the early stages of the inflammatory response, TNF-alpha and IL-1, among other cytokines, stimulate the endothelial cells to express the cell surface adhesion molecule P-selectin. Within a couple of hours, a second surface adhesion molecule, E-selectin, is produced. Together E- and P-selectin slow the motion of leukocytes through the bloodstream by causing them to roll along the endothelial surface, allowing other molecules to interact with the slowed leukocytes to stop them and promote their movement into the tissues (described as analogous to throwing a tennis ball at a Velcro surface). Tight adhesion to the rolling leukocyte is performed by two endothelial cell ligands, intercellular adhesion molecule-1 (ICAM-1) and vascular cellular adhesion molecule-1 (VCAM-1), which arrest the motion of the rolling leukocyte.1 Stopping the leukocyte allows it to enter the tissues by secreting proteases to breach the endothelial basement membrane, a process known as diapedesis.
There are a number of other mediators of endothelium-mediated inflammation. The major cause of the endothelial dysfunction is thought to be decreased availability of nitric oxide (NO), either due to decreased production or enhanced breakdown due to increased oxidative stress.2 It is the major factor in large arteries mediating endothelial dependent relaxation and inhibits platelet aggregation, cell adhesion and smooth muscle cell proliferation. A number of plant remedies hold promise as promoters of NO activity at the endothelium.3 The activation of nuclear transcription factor-kappa B (NF-kappaB) has also been linked with a variety of inflammatory diseases. Kappa B kinase catalyses NF-kappaB activation and is implicated in the effects of excessive free fatty acids on the induction of insulin resistance, in atherogenesis, and other inflammatory disorders.
The above are among a number of mechanisms involved in altering endothelial function in the inflammatory response. They therefore represent potential mechanisms in any inflammatory-modulating strategy, and, as will be seen below, there are many plant constituents with this potential.4 In the case of chronic deteriorations such as increased insulin resistance, diabetes and atherosclerosis, there is the prospect that many dietary plants may have long-term protective effects. The warming spices like ginger,5 cinnamon6 and turmeric7 may share cholinergic ‘endothelium-dependent’ vasodilator effects mediated by NO and counteracted for example by high glucose levels. Garlic8 and even daily beverages of tea9,10 and coffee,11 have been shown to benefit endothelial markers associated with reduced inflammation in atherosclerosis and diabetes. Among its well-attested benefits on microvascular function,12 chocolate also reduces inflammatory markers like CRP.13
Many commonly consumed herb and spice constituents may inhibit extravasation in the inflammatory response. See Table 8.1.
| Herb or spice constituemt | Properties |
|---|---|
| Ajoene | Inhibits tumour–endothelial cell adhesion, as well as the in vivo TNF-alpha response to LPS in mouse melanoma cells.14 |
| Allicin | Inhibits the spontaneous and TNF-alpha-induced secretion of IL-1beta, IL-8, IP-10 and MIG in a dose-dependent manner from intestinal epithelial cells in vitro, suppresses the expression of IL-8 and IL-1beta mRNA levels.15 |
| Allyl isothiocyanate | Significantly inhibits the cellular production of pro-inflammatory mediators such as TNF-alpha and NO.16 |
| Anethole | Inhibits NF-kappaB activation induced by TNF, TRAF2 and NIK in vitro, suppresses TNF-induced activation of the transcription factor AP-1, JNK and MAPK in vitro.17 |
| Apigenin | Inhibits TNF-alpha in LPS stimulated macrophages resulting in diminished MCP-1 and inhibition of IL-1beta in vitro.18 |
| Capsaicin | Blocks the STAT3 activation pathway in multiple myeloma cells in vitro leading to downregulation of cyclin D1, Bcl-2, Bcl-xL, survivin and VEGF.19 |
| Carnosol | Decreases LPS-induced iNOS mRNA and protein expression, reduces NF-kappaB subunits translocation and NF-kappaB DNA binding activity in activated macrophages due to inhibition of IKK, inhibits iNOS and NF-kappaB promoter activity.20 |
| Caryophyllene | Inhibits the LPS-induced NF-kappaB activation and neutrophil migration in rat paw oedema in vivo.21 |
| Cinnamaldehyde | Inhibits age-related NF-kappaB activation and targets inflammatory iNOS and COX-2, inhibits the activation of NF-kappaB via three signal transduction pathways, NIK/IKK, ERK, and p38 MAPK.22 |
| Curcumin | Downregulates the constitutive activity of NF-kappaB, decreases expression of NF-kappaB target genes COX-2 and cyclin D1, and induces apoptosis in mouse melanoma cells in vitro.23 Significantly inhibits the cellular production of proinflammatory mediators such as TNF-alpha and NO.16 |
| Diallyl sulphide | Significantly reduces the production of and serum levels of IL-1beta, IL-6, TNF-alpha and GM-CSF in mice with melanoma.24 |
| Eugenol | Blocks the release of IL-1beta, TNF-alpha and prostaglandin E2 and suppresses the mRNA expression of IL-1beta, TNF-alpha and COX-2 in LPS-stimulated human macrophages in vitro.25 |
| [6]-Gingerol | Inhibits the production of TNF-alpha, IL-1beta and IL-12 in murine peritoneal macrophages exposed to several doses of 6-gingerol in the presence of LPS stimulation.26 |
| Humulene | Inhibits the LPS-induced NF-kappaB activation and neutrophil migration in rat paw oedema, prevents the production of TNF-alpha and IL-1beta and the in vivo upregulation of kinin B(1) receptors.21 |
| Limonene / myrcene | Inhibits the LPS-induced inflammation including cell migration and production of NO along with significant inhibition of gamma-interferon and IL-4 production in mouse model of pleurisy.27 |
| Perillyl alcohol | Reduces NF-kappaB DNA-binding activity.28 |
| Phytic acid | Modulates IL-8 and IL-6 release from colonic epithelial cells stimulated with LPS and IL-1beta, suppresses IL-8 basal release, and it dose-dependently reduces IL-8 secretion by colonocytes and downregulates IL-6.29 |
| Piperine | Significantly reduces the expression of IL-1beta, IL-6, TNF-alpha, GM-CSF and IL-12p40 genes in melanoma cells.30 |
| Quercetin | Attenuates PMACI-induced activation of NF-kappaB,31 inhibits LPS-induced NO and TNF-alpha production in murine macrophages.32 |
| Ursolic acid | Inhibits IKK and p65 phosphorylation leading to the suppression of NF-kappaB activation induced by various carcinogens; this correlates with the downregulation of COX-2, MMP-9 and cyclin D1 in vitro.33 |
| Zingerone | Significantly inhibits the cellular production of proinflammatory mediators such as TNF-alpha and NO and inhibits the release of MCP-1 from 3T3-L1 adipocytes.34 |
Others may inhibit migration through inflamed endothelial cells. See Table 8.2.
Table 8.2 Inhibition of invasion
| Herb or spice constituemt | Properties |
|---|---|
| Allicin | Inhibited TNF-alpha induced ICAM-1 expression in human endothelial cells.35 |
| Allyl isothiocyanate | Downregulated mRNA level and activity of MMP-2/MMP-9 in human hepatoma SK-Hep1 cells.36 |
| Apigenin/kaempferol | Inhibited TNF-alpha induced ICAM-1 expression.37 |
| Caffeic acid | Inhibited MMP-9 activity in human hepatocellular carcinoma cell line.38 |
| Curcumin | Downregulated MMP-2 expression and activity and expression of integrin receptors, FAK and MT1-MMP in Hep2 cells.39 |
| Diallyl disulfide | Inhibited activity of MMP-2 and MMP-9 in human endothelial cells.24 |
| [6]-Gingerol | Suppressed expression and enzymatic activity of MMP-2/MMP-9 in human breast cancer cells.40 |
| Myricetin | Inhibited expression and activity of MMP-2 in colorectal cancer cells.41 |
| Quercetin | Decreased the expressions of MMP-2 and MMP-9 in PC-3 cells.42 |
However, it must be kept in mind that this research is identifying prospects only until such effects are also demonstrated in humans after oral doses. Nonetheless, there can be no harm in increasing the dietary intake of such phytochemicals in cases of chronic inflammation as a background therapy. For a fuller discussion of herbal compounds able to target defined biochemical and molecular mediators of inflammatory, autoimmune arthritis (at least from experimental models), the reader is referred to an excellent recent review.43
Infectious agents
An interesting later brush with ancient notions by modern medical research was seen in an issue of The British Medical Journal.44 It was reported that patients with RA had a significantly higher incidence or history of pulmonary disease than the wider population. It was pointed out that the lungs remain sub-clinically infected, almost indefinitely in many cases, after the incidence of pneumonia, bronchitis and pleurisy, and this persistence is obvious in conditions like emphysema and bronchiectasis. The findings were dramatic and led a linked editorial to speculate, probably reluctantly, that ‘toxins’ in the infected lung base might provoke the inflammatory changes in the rheumatoid joints.
Infectious agents are now (controversially) regarded as the major environmental factors that may cause arthritic inflammation in genetically susceptible hosts. Retroviruses, urinary and pulmonary, and especially enteric bacteria are the provocateurs most often discussed.45–48Helicobacter pylori has been associated with diseases such as autoimmune gastritis, Sjögren’s syndrome, atherosclerosis, immune thrombocytopenic purpura, inflammatory bowel diseases and autoimmune pancreatitis, in each of which it seems to play a pathogenic role in some cases. On the other hand, it has also been suggested that it may help to protect against the development of autoimmune gastritis, multiple sclerosis, systemic lupus erythematosus and inflammatory bowel diseases.49
Molecular mimicry has been found between a white blood cell protein, HLA-B27, and two molecules, nitrogenase and pullulanase D, in the anaerobic gut bacterium Klebsiella pneumoniae among ankylosing spondylitis patients,50 and between HLA-DR1/DR4 and the haemolysin molecule in another gut anaerobe, Proteus mirabilis, among RA patients.51 Molecular mimicry between HLA-B27 and K. pneumoniae molecules in the aetiology of ankylosing spondylitis patients has been extensively studied. It was first proposed by Dr Alan Ebringer in 1976. K. pneumoniae was isolated in stool samples more frequently in active phases of the disease and was linked to relapses. High antibody levels in ankylosing spondylitis patients directed against K. pneumoniae were found in several studies. Some of these antibodies were shown to cross-react with HLA-B27 as well as spinal collagens. Specific anti-Klebsiella antibodies in ankylosing spondylitis patients have now been reported from 18 different countries. ‘B27 disease’ is the new terminology proposed by Ebringer.52
In another growing association, patients with long-standing active RA have a substantially increased frequency of periodontal disease compared with that among healthy controls. High levels of oral anaerobic bacteria antibodies (such as from Porphyromonas gingivalis, Tannerella forsythensis, and Prevotella intermedia) have been found in the serum and synovial fluid of such patients. Appropriate antibiotics have been shown to be effective against the arthritis53 (leading to intriguing prospects for herbal oral antiseptics such as gum myrrh). Autoimmunity in RA has been characterised lately as an antibody response to citrullinated proteins. There is an association with periodontitis that is largely, but not exclusively, caused by Porphyromonas gingivalis infection. The citrullination of proteins by P. gingivalis and the subsequent generation of autoantigens that drive autoimmunity in RA represent a possible causative molecular mimicry link between these two diseases.54 As noted above, a further link with the aetiology of RA is with Proteus infection in the urinary tract.55 Both bacteria can be found as secondary infections elsewhere in the body and prospects for future control of rheumatoid diseases by antibiotic therapy have been raised.56
There are some strong indications from archaeology for an infectious trigger for RA. Examinations of skeletal remains from antiquity in Europe do not show signs of RA. In contrast, specimens dating back several thousand years from Native American tribes in North America show clear evidence of the disease. The prevalence of RA in this ethnic group today remains extraordinarily high, with over 5% of individuals affected in some groups. Evidence of RA in Europe first appeared in 17th century art, especially by the Dutch Masters, and the first case report was published in 1676. RA could well be linked to an infectious agent brought from the New World to the Old World.57
The site of the trigger for ankylosing spondylitis is not necessarily always the bowel. An association between ankylosing spondylitis and chronic bacterial prostatitis has long been observed. The incidence of chronic prostatitis in male ankylosing spondylitis patients was 83%, compared with 33% in patients with RA.58 This association was confirmed in a later study.59 The fact that the prostate may harbour bacteria that contribute to ankylosing spondylitis could explain the higher incidence of this disorder in males. Evidence of Chlamydial urinary tract infection is frequent in female patients with ankylosing spondylitis.60
Gut inflammation is a prime candidate for having a bacterial origin (see also below). Johne’s disease, which occurs in cattle and other ruminants, is very similar to Crohn’s disease and is caused by Mycobacterium avium subspecies paratuberculosis (MAP). In 1984, Chiodini and co-workers reported the isolation of a strain resembling MAP from the intestinal tissue of three patients with Crohn’s disease.61 This report initiated interest and controversy about a mycobacterial aetiology for Crohn’s disease that is still ongoing. MAP has also now been cultured from human milk, faeces, intestinal tissues and peripheral blood of patients with Crohn’s disease.62 Ebringer has also implicated Klebsiella in Crohn’s disease and advised a low starch diet (see later).63 In terms of Crohn’s disease, one group of researchers reflected: ‘The mycobacterial theory and the autoimmune theory are complementary; the first deals with the aetiology of the disorder, the second deals with its pathogenesis. Combined therapies directed against a mycobacterial aetiology and inflammation may be the optimal treatment …’.64 The levels of MAP found in Crohn’s disease patients is not high, so its presence does not represent a bacterial infection per se. Rather it is best viewed as a form of dysbiosis.
Viruses may also be involved in triggering an autoimmune response. For the past 25 years, a potential role of Epstein-Barr virus (EBV) in the pathogenesis of RA has been suspected. The QKRAA amino acid sequence of HLA-DRB1, a tissue marker that carries susceptibility to RA, is found in the EBV envelope. Sera from patients with RA contained higher levels of antibodies to latent and active EBV antigens.65 RA patients have more EBV-infected B cells than normal controls. Patients with RA have a 10-fold higher EBV load than healthy controls. EBV DNA is detected more frequently in immune cells, synovial fluid and saliva from RA patients than from controls.65
In contrast to Crohn’s disease, studies over 40 years have associated ulcerative colitis with cytomegalovirus (CMV).66 A clear association between onset of ulcerative colitis and primary CMV infection was confirmed by viral studies in two patients.67,68 Higher frequency and amount of antibodies to CMV were found in patients with ulcerative colitis.66 The simultaneous presence of DNA from several herpes viruses, including CMV, was much greater in ulcerative colitis patients compared to those with Crohn’s disease or normal controls.69 While it is debated that CMV infection may be a causative factor in ulcerative colitis, it is recognised by many clinicians as a complicating event that can increase its severity.70
Intestinal wall damage
There is increasing evidence of damage to the gut wall in a range of autoimmune diseases. One category of diseases, classified as spondylarthropathies, involve inflammatory damage of the joints and the skeleton, the eyes, gut, urogenital tract, skin and sometimes the heart.71,72 Ankylosing spondylitis is the prototype example of this condition; other examples include reactive arthritis, psoriatic arthritis and arthritis in patients with inflammatory bowel disease. In a series of investigations, histological signs of gut inflammation were found in a high proportion of patients with spondylarthropathies.73 Most of these patients did not present any clinical intestinal manifestations. Remission of the joint inflammation was always connected with a disappearance of the gastrointestinal inflammation. Persistence of locomotor inflammation was mostly associated with the persistence of gastrointestinal inflammation. It was proposed that some patients with a spondylarthropathy had a form of subclinical Crohn’s disease in which the locomotor inflammation was the only clinical expression.74 Further genomic work substantiates the unique relationship between gut and joint inflammation.75 These data suggest that spondylarthropathies and Crohn’s disease should be scientifically and clinically considered as distinct phenotypes of common immune-mediated inflammatory disease pathways, rather than as separate disease entities.76
This hypothesis is supported in prospective long-term studies in which ileocolonoscopied patients were reviewed over periods of up to 9 years. About 6% of spondylarthropathy patients who did not present any sign of Crohn’s disease at first investigation (but did show gut inflammation on biopsy) developed full-blown Crohn’s disease within 9 years. Electron microscopy of these lesions demonstrated an increase in the number of membranous (M) cells in inflamed mucosa. Necrosis and rupture of these M-cells, with lymphocytes entering the gut lumen, was observed. Such evidence of damage to the gut mucosa could be responsible for an increase in local antigenic stimulation and could readily lead to secondary systemic immunological disorders.77
Pathogenic forms of Escherichia coli with specific hairs or pilli adhere to the gut mucosa. In 1988, it was first demonstrated that E. coli isolated from patients with ulcerative colitis and Crohn’s disease showed a significantly greater index of adhesion when compared with normal controls.78 A new term adherent-invasive Escherichia coli (AIEC) was coined to describe this aggressive form. They can replicate intracellularly and survive within macrophages. AIEC strains were found (predominantly in the ileum) in 21.7% of Crohn’s disease chronic lesions versus 6.2% of controls.79 Crohn’s disease patients with anti-E. coli antibodies were more likely to have internal perforating disease and to require small bowel surgery.80 Because AIEC can cross and breach the intestinal barrier, move to deep tissues and continually activate macrophages, they can generate persistent inflammation.81
As mentioned above, AIEC are found in significant amounts in patients with ulcerative colitis compared with controls.78 Several workers have shown that isolates of E. coli obtained from patients with ulcerative colitis can degrade mucins and produce toxins. Results from one study suggested that connective tissue exposed by bowel ulcerations may result in selection of E. coli strains able to bind to them.82 Compared with healthy people, ulcerative colitis patients have increased levels of IgG directed against their normal flora.83 There may be an increased number of bowel bacteria in ulcerative colitis, but reduced counts of ‘protective’ bacteria such as Lactobacilli and Bifidobacteria.83 Lactobacilli numbers were certainly found to be lower in ulcerative colitis patients during the active phase.84 This was confirmed in another study that also found the bacterial diversity decreased during a relapse.85
In patients with active ulcerative colitis there is an over-production of hydrogen sulphide, which is toxic to the intestinal mucosa by competing with short-chain fatty acids.86 This appears to be due to an excess (or greater activity) of sulphate-reducing bacteria (SRB), such as Desulfovibrio desulfuricans.86 In a pilot study, a low sulphur diet for 12 months was associated with a remarkable clinical improvement in four patients with chronic ulcerative colitis.87 A recent review concluded that there is evidence to implicate SRB in the pathogenesis of ulcerative colitis.88
As noted in a Lancet review: ‘The long-standing assumption that ulcerative colitis is an autoimmune disease has been revised to incorporate evidence suggesting that commensal microflora and their products are autoantigens, and that ulcerative colitis is caused by loss of tolerance towards otherwise harmless components of the normal intestinal flora’.89
Hormonal factors
Hormonal factors in rheumatoid disease are well accepted. Risk factors include reduced childbearing and breastfeeding; these apparently contradictory influences are both associated with high prolactin levels.90
Several autoimmune diseases have been linked to higher levels of prolactin in the blood, including lupus,91 RA,92 Sjögren’s syndrome93 and juvenile arthritis.94 For systemic lupus erythematosus (SLE), serum prolactin concentrations have been correlated with both clinical activity and remission.91,95 On the other hand, preeclampsia and breast cancer have a negative association with rheumatic and other autoimmune diseases.96
Such links are confusing and even contradictory, but a defective response of the neuroendocrine system to inflammatory stimuli has been proposed as one feature of RA.97
Traditional approaches to inflammatory diseases
Anti-inflammatory remedies
The use of willow bark for rheumatism in traditional medicine is a reminder that where remedies clearly provided symptomatic relief, they were used. More recently, there has been a huge research effort to find new anti-inflammatory activities in the plant world. Many chemical subgroups from plants have shown promising experimental activity, including terpenoids and steroids, phenolics and flavonoids, fatty acids, polysaccharides and alkaloids.98 Most of this work remains on the laboratory bench as interesting markers for the effect of the whole plant; the evidence is more persuasive when demonstrated in clinical trials. For long the potential benefit of sea buckthorn (Hippophae rhamnoides) for inflammatory conditions has been assumed on the basis of its high levels of antioxidants but reinforced by clinical trials.99 Herbal remedies that have shown efficacy in clinical trials for osteoarthritis include Harpagophytum procumbens (devil’s claw),100Rosa canina (rosehip),101Uncaria tomentosa (cats claw)102 and Boswellia serrata. RA has also been relieved by Boswellia.103 The benefits of Tripterygium wilfordii in the same condition were found to be associated with an in vitro suppression of antigen-stimulated T-cell production, immunoglobulin production by B-cells104 and reduction of IL-2 production and activity.105 However, while it is a promising intervention in inflammatory disorders, this herb is potentially toxic and has been associated with serious side effects.106 The effect of the Chinese herbal formulation found effective in London trials in the treatment of atopic eczema has been shown to be associated with changes in a number of immunological functions, such as decreasing the higher levels of circulating IgE complexes and IL-2 receptors and vascular cell adhesion molecules in atopic eczema patients.107
Topical anti-inflammatory activity has been observed in clinical studies of Matricaria recutita (wild chamomile) in mucositis,108 and Melaleuca alternifolia (tea tree) oil in histamine-induced skin inflammation.109 (See earlier in this chapter regarding topical anti-inflammatory treatments.)
Diet
One of the persistent dietary notions in European tradition in the case of degenerative diseases, particularly arthritis, has been the distinction between ‘acid’ and ‘alkaline’ foods. The view is that as metabolites tend to be acidic, as acid-buffering agents are a major constituent of the body fluids and as eliminatory channels (lungs, kidney, bile and bowel) pass mainly acidic materials, inflammatory diseases may be marked by, and even result from, greater acidosis. Overt metabolic acidosis is well characterised in medicine and may follow excessive alcohol consumption, laxative abuse, excessive vitamin D and NSAIDs (most often prescribed in arthritis) and salicylate use, among other drugs.110 The case for a more subtle effect is considered further in the section on joint disease in Chapter 9. In addition to the prospects for therapy that could, for example, include diuretic herbal remedies, there are associated dietary observations that reinforce the potential for benefit in some chronic inflammatory diseases. There was, for example, a widespread traditional instinct in many inflammatory diseases to reduce animal protein, although chicken and other fowl were a common exception.
There is modern support for this approach in rheumatic diseases. A combination of fasting and vegetarian diets has been shown to reduce the ability of the urine to support the growth of Proteus mirabilis and Escherichia coli.111 A substantial placebo-controlled trial has confirmed that gamma-linolenic acid, (GLA), a component mainly of plant-based foods, has antirheumatic activity.112 The possibility of cross-reaction between dietary collagen found in animal products and the sufferer’s connective tissue has also been postulated.113,114 The effects of a low-starch diet in reducing the gut levels of anaerobic bacteria such as Klebsiella and serum IgA antibody levels in both normal subjects and those suffering ankylosing spondylitis, and in benefiting symptoms in the latter, have been supported by clinical evidence.115 Nevertheless, the quality of the research on this issue is too limited to draw firm conclusions on the value of dietary changes on RA.116
Phytotherapeutics
Plants with anti-infective properties
• Allium sativum (garlic): heating, disinfectant, especially in lungs and gut
• Berberis vulgaris (barberry): cooling and drying
• Echinacea spp. (Echinacea): warming and promoting defences, particularly of throat and upper gut wall; this applies specifically to the root
• Hypericum perforatum (St John’s wort): restorative tonic with potential antiviral support (enveloped viruses only)
• Lavandula spp. (lavender): topically warming and antiseptic
• Thuja occidentalis (arbor-vitae): warming and stimulating, with potential antiviral support (all viruses)
• Andrographis paniculata: cooling and drying, to clear heat and eliminate toxins, especially in respiratory and digestive infections.
Plants with eliminative properties
• Apium graveolens (celery): diuretic and eliminating acidic metabolites through the kidneys, particularly used in arthritic disease
• Arctium lappa (burdock): general eliminative particularly popular in skin disease
• Arctostaphylos uva-ursi (bearberry): diuretic and urinary antiseptic, most likely to be applied in arthritic disease (often to be recommended in ankylosing spondylitis, but more for the latter property)
• Betula spp. (birch): diuretic, particularly useful in arthritic disease and where inflammation leads to calcification
• Berberis aquifolium (Oregon grape): cooling and drying, cholagogue and popular in treating skin disease
• Fumaria officinalis (common fumitory): cholagogue, popular in treating skin disease
• Galium aparine (cleavers): diuretic and lymphatic, and used particularly in skin disease
• Gaultheria procumbens (wintergreen): topically warming and used over inflammations
• Inula helenium (elecampane): warming and aiding elimination (expectoration) from the lungs
• Juglans nigra (walnut bark): general alterative and laxative remedy
• Rumex crispus (yellow dock): cooling and drying with mild aperient properties for helping bile and bowel elimination
• Scrophularia nodosa (figwort): warming and generally eliminative, traditionally for aggressive skin disease
• Solidago spp. (goldenrod): traditional diuretic used for skin and sinus conditions, with some anti-inflammatory properties
• Taraxacum officinale (dandelion root): cooling and drying with cholagogue and diuretic properties, popularly combined with Apium for arthritic disease and as a remedy in skin disease
• Trifolium pratense (red clover): lymphatic and expectorant, used in skin and joint disease
• Urtica dioica (nettle leaf): warming and nutritive taken internally, popular in skin and joint disease.
Plants with digestive anti-inflammatory properties
• Aloe vera (aloe juice): reducing digestive wall inflammation
• Calendula officinalis (marigold): lymphatic and reducing inflammation in the throat and stomach
• Dioscorea villosa (wild yam): antispasmodic and anti-inflammatory on lower gut wall, possibly steroidal effect systemically
• Filipendula ulmaria (meadowsweet): demulcent and astringent effect on stomach wall overwhelming low levels of salicylates
• Hamamelis virginiana (witchhazel): astringent throughout the digestive tract
• Matricaria recutita: cooling and reducing inflammatory damage in the upper digestive tract, especially bisabolol chemotypes
• Myrica cerifera (bayberry): warming and astringent, traditionally used in fever management associated with diarrhoea and dysentery
• Symphytum officinale (comfrey leaf): potent healing effects on gut wall, to be used only in the short term
• Ulmus rubra (slippery elm): mucilaginous and healing on the upper digestive tract, best used as an early stage of, and preparatory to, wider treatment.
Plants with hormonal properties
• Aesculus hippocastanum (Horsechestnut seed): particularly useful where inflammation leads to oedema and swelling that interferes with other structures or causes compression syndromes (see monograph)
• Bupleurum falcatum (Bupleurum): (see monograph)
• Cimicifuga racemosa (black cohosh): has a reputation for arthritis, especially small joint osteoarthritis
• Pfaffia paniculata (Brazilian ginseng)
• Rehmannia glutinosa (Rehmannia): a useful adrenal tonic and anti-inflammatory in autoimmune diseases (see monograph)
Plants with general anti-inflammatory properties
• Boswellia serrata (Boswellia): a herb that appears to possess a broad range of anti-inflammatory effects (see monograph)
• Curcuma longa (turmeric): like Boswellia, a very broad acting anti-inflammatory (see monograph)
• Fraxinus excelsior (ash): moderate anti-inflammatory containing coumarins that inhibit T-cells and prostaglandin biosynthesis117
• Guaiacum spp. (lignum vitae): traditional reputation in arthritic diseases; however, is now an endangered species
• Harpagophytum procumbens (devil’s claw): mild anti-inflammatory effects (see monograph)
• Menyanthes trifoliata (bogbean): a bitter and hepatic remedy applied to rheumatic conditions where such effects are useful
• Populus spp. (poplar bark): containing salicylates with established anti-inflammatory properties117
• Salix spp. (willow bark): the original source of salicylates and with a strong traditional reputation as an antirheumatic, now supported by clinical trials (see monograph)
• Tanacetum parthenium (feverfew): potential anti-inflammatory properties (see monograph)
• Zingiber officinale (ginger): mild anti-inflammatory effects (see m onograph)
Traditional therapeutic insights into the use of treatments for inflammatory diseases
• A cold or fever, provided it could be managed within the patient’s often-diminished reserves, could lead to an effective clearing of burdens by mobilising the phagocytic defences. The occurrence of any such event would lead the practitioner to uswitch treatment rapidly and deal with the acute indications intensively until resolved (see also pp. 146–148).
• Primary eliminatory routes would be an early therapeutic focus; diuretics, aperients, depuratives and cholagogues are especially likely to be used, as indicated in the individual’s story. These might be step-like interventions, each pursued briefly for particular medium-term goals.
• The gut wall was always a priority zone. Dietary approaches would usually be combined with treatments to enhance the digestive process, like bitters or warming aromatic digestives as required, or remedies with anti-inflammatory or healing effects on the wall itself. For example, one strategy might be to start or rotate a course of treatment with a demulcent remedy like slippery elm.