17
Hepatobiliary and pancreatic trauma
Liver trauma
Blunt and penetrating trauma are the two principal mechanisms of liver injury. Road traffic accidents account for the majority of blunt injuries, whereas knife and gunshot wounds constitute the major cause of penetrating injuries. In the UK, blunt trauma predominates by a ratio of approximately 2:1 as documented in a large Scottish epidemiological study.1 Whilst this is typical for other European centres,2 it differs from the experience in South Africa, where penetrating injuries account for 66% of liver trauma,3 and in North America, where up to 86% of liver injuries are penetrating wounds.4,5
Two types of blunt liver trauma have been described – deceleration (shearing) trauma and crush injury. Deceleration injuries occur in road traffic accidents and in falls from a height where there is movement of the liver relative to its diaphragmatic attachments.6 Crush injuries are caused by direct trauma to the liver area. The two types of injury may coexist but tend to produce somewhat different types of liver injury. Deceleration or shearing injuries create lacerations in the hepatic parenchyma, typically between the right posterior section (segments 6 and 7) and the right anterior section (segments 5 and 8), which can extend to involve major vessels. In contrast, a direct blow to the abdomen may lead to a crush injury, with damage to the central portion of the liver (segments 4, 5 and 8). Compression between the right lower ribs and the spine may also cause bleeding from the caudate lobe (segment 1). Blunt trauma can rupture Glisson’s capsule and can also lead to subcapsular or intraparenchymal haematoma formation. Penetrating injuries are usually associated with gunshot or stab wounds, with the former usually resulting in more tissue damage due to the cavitation effect as the bullet traverses the liver substance.
Classification of liver injury
The severity of liver trauma ranges from a minor capsular tear, with or without parenchymal injury, to extensive disruption involving both lobes of the liver with associated hepatic vein or vena caval injury. The American Association for the Surgery of Trauma has adopted for general use the classification of liver injury described initially in 1989 by Moore and colleagues, and revised subsequently in 19947 (Table 17.1). The hepatic injury grade is calculated from assessment of the liver injury using information derived from radiological study, operative findings or autopsy report. Where there are multiple injuries to the liver, the grade is advanced by one stage. Grade I or II injuries are considered minor; they represent 80–90% of all cases and usually require minimal or no operative treatment. Grade III–V injuries are generally considered severe and may require surgical intervention, while grade VI lesions are regarded as incompatible with survival. Schweizer et al. have described a protocol-based liver trauma management system employing this classification system that permits lesser injuries to be treated non-operatively and allows more appropriate selection of patients for operative treatment.8
Table 17.1
Hepatic injury scale used by the American Association for the Surgery of Trauma
| Grade | Description | |
| I | Haematoma | Subcapsular, < 10% surface area |
| Laceration | Capsular tear, < 1 cm parenchymal depth | |
| II | Haematoma | Subcapsular, 10–50% of surface area |
| Laceration | Intraparenchymal < 10 cm in diameter, 1–3 cm parenchymal depth, < 10 cm in length | |
| III | Haematoma | Subcapsular, > 50% surface area or expanding; ruptured subcapsular or parenchymal haematoma; intraparenchymal haematoma > 10 cm or expanding |
| Laceration | > 3 cm parenchymal depth | |
| IV | Laceration | Parenchymal disruption involving 25–75% of hepatic lobe or 1–3 Couinaud segments within a single lobe |
| V | Laceration | Parenchymal disruption involving > 75% of hepatic lobe or > 3 Couinaud segments within a single lobe |
| Vascular | Juxtahepatic venous injuries – retrohepatic cava, major hepatic veins | |
| VI | Vascular | Hepatic avulsion |
Note: advance one grade for multiple injuries up to grade II.
The role of ‘aggressive’ high-volume fluid replacement in trauma victims has been questioned, with evidence suggesting that excessive fluid replacement is associated with adverse outcome.9 As this evidence came from an American series that included a large proportion of relatively young, previously fit adults suffering from penetrating trauma to the torso, with ready access to trauma centres, the results may not necessarily be applicable to practice in other countries.
Diagnosis of liver injury
In Feliciano et al.’s series of 1000 patients with liver trauma treated during a 5-year period, 45 patients underwent emergency room thoracotomy for control of haemorrhage related to their liver injury and all died.4 Similarly, in an 11-year review of 783 patients who sustained liver trauma in Scotland, 11 patients underwent an unsuccessful laparotomy or thoraco-laparotomy in the emergency room.1
An alternative investigation that has been advocated in initial trauma evaluation is focused assessment with sonography for trauma (FAST).10 This involves ultrasonographic assessment of the pericardium, right upper quadrant including Morrison’s pouch, left upper quadrant and pelvis. This evaluation is not designed to identify the degree of organ injury, but rather the presence of blood. A large meta-analysis of the use of emergency ultrasonography for blunt abdominal trauma reported sensitivity rates ranging from 28% to 97% and specificity rates close to 100%.11
Rozycki et al. demonstrated a significant correlation between haemoperitoneum in the right upper quadrant and injury to the liver, and suggested that adherence to a pre-agreed protocol increased the reliability of ultrasound assessment of abdominal trauma.12 Other centres have also reported that ultrasound is a reliable ‘first’ test for the assessment of a patient with suspected liver trauma.13 However, an important cautionary note comes from the study carried out by Richards et al.14 In a series of 1686 abdominal ultrasound scans for trauma, 71 patients had bowel or mesenteric injury and 30 patients had a negative ultrasound scan (43% false-negative rate). Limitations of FAST include operator dependence, poor assessment of the retroperitoneum, unreliable detection of pneumoperitoneum and difficulty in scanning obese patients or those with overlying wounds.
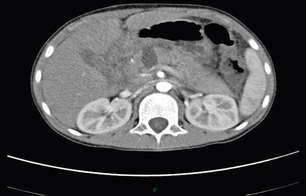
Computed tomography (CT) is the ‘gold standard’ investigation for the evaluation of a patient with suspected liver trauma (Fig. 17.1). The use of intravenous contrast may help in the detection of non-viable parenchyma. CT has high sensitivity and specificity for detecting liver injuries; these attributes increase as the time between injury and scanning increases, as haematomas and lacerations become better defined. Specific CT features of liver trauma have been reported by a number of authors. Fang et al. described intraparenchymal ‘pooling’ of intravenous contrast that correlated strongly to the presence of ongoing haemorrhage.15 Yokota and Sugimoto documented ‘periportal tracking’ to consist of a circumferential area of low attenuation around the portal triad.16 Periportal tracking is thought to represent blood or fluid within the condensation of the Glissonian sheath around the portal structures and indicates the presence of injury to structures in the portal triad. If the sign is present in the periphery of the liver it may alert the clinician to the presence of a peripheral bile duct injury that in turn may present as a bile leak. Addition of oral contrast does not appear to add to the diagnostic yield of CT in the assessment of liver injury.17
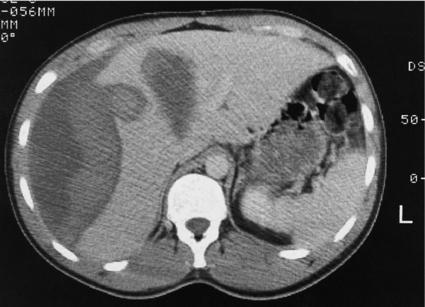
Figure 17.1 CT image of a 25-year-old male who sustained a blunt injury to the right chest wall but was admitted to hospital haemodynamically stable. The scan shows a substantial subcapsular haematoma associated with an intraparenchymal laceration. This patient was managed successfully without operation.
In order to maintain a balanced perspective, it is worthwhile considering some of the limitations of CT in the assessment of liver trauma. The CT-defined grade of injury may differ from the grade of liver injury found at operation, with the predominant tendency being to overdiagnose the grade of injury on CT as compared with subsequent operative findings. Croce et al. concluded that CT should not be used in isolation to estimate blood loss and that CT may not provide an accurate assessment of the extent of a liver laceration in some areas of the liver – specifically in the vicinity of the falciform ligament.18
Some authors recommend performing a whole-body CT as the standard diagnostic tool during the early phase for patients with polytrauma, advocating that this will alter treatment in up to 34% of patients with blunt trauma.19 A 30% reduction in mortality using this approach has also been reported.20 Other arguments in favour of an imaging survey are the reduction in time from admission to intervention and consistency in managing haemodynamically unstable patients.21 However, at present the logistics of such an approach are not universally applicable as it requires a CT scanner in, or very close to, the emergency department.
Other diagnostic/therapeutic modalities for the assessment and treatment of liver injury
Angiography plays a vital role in the conservative management of liver injuries. Extravasation of contrast seen on CT requires emergency angiography and therapeutic angiographic embolisation for ongoing blood loss or haemobilia.22 Angioembolisation is also reported following damage control surgery prior to removal of packs if re-bleeding is suspected.23,24
Other diagnostic modalities may be used in specific situations. Endoscopic retrograde cholangiopancreatography (ERCP) may help in delineation of the biliary tree in patients with liver trauma, and endoscopic transpapillary stents may be used as a therapeutic modality to treat biliary leaks.25
Management of liver injury: selection of patients for non-operative management
The feasibility of non-operative management of patients with intra-abdominal solid-organ injury was first established in paediatric surgery but was subsequently extended to adult practice. Richie and Fonkalsrud described successful conservative management of four patients with liver injury in an era before the availability of CT.26 Further indirect evidence for the feasibility of a non-operative approach came from a report published by White and Cleveland27 in the same year. They reported a consecutive series of 126 patients with liver trauma, all of whom underwent laparotomy. Interestingly, 67 patients in this series (53%) had placement of a drain to the subhepatic space as their only liver-related surgical intervention at laparotomy. Subsequent studies have recognised that 50–80% of liver injuries stop bleeding spontaneously and this has led to a non-operative approach for blunt liver trauma in selected patients.
• absence of peritoneal signs;
• availability of good-quality CT;
• ability to monitor patients in an intensive care setting;
• facility for immediate surgery (and by implication, availability of an experienced liver surgeon);
• simple liver injury with < 125 mL of free intraperitoneal blood;
Farnell et al. extended the threshold of haemoperitoneum to 250 mL and described specific liver injuries suitable for non-operative management.29 Feliciano suggested subsequently that any blunt hepatic injury, regardless of its magnitude, should be managed without operation if the patient was haemodynamically stable and had a haemoperitoneum of less than 500 mL.30 The degree of liver injury amenable to successful non-operative management has gradually extended over recent years, and most authors now believe that the ultimate decisive factor in favour of non-operative management is haemodynamic stability of the patient at presentation or after initial resuscitation, irrespective of the grade of liver injury on CT or the amount of haemoperitoneum.31,32
A 22-month prospective study from Memphis of the initial non-operative treatment of haemodynamically stable blunt hepatic trauma patients compared outcome to a matched cohort of blunt hepatic trauma patients treated operatively.33 The study reported that of 136 patients with blunt trauma, 24 (18%) underwent emergency surgery. Of the remaining 112 patients, 12 (11%) failed conservative management (for causes not related to the liver injury in seven) and the remaining 100 patients were treated successfully without operation. Of these, 30% had minor injuries (grades I and II) but 70% had major injuries (grades III–V). This study concluded that non-operative management was safe for haemodynamically stable patients and that this was independent of the CT-delineated grade of the liver injury. The blood transfusion requirement and the incidence of abdominal complications were lower in the non-operatively treated group.
Reporting a single institutional experience, Boone et al. stated that 46 (36%) of 128 consecutive patients with blunt liver trauma were successfully treated non-operatively, including 23 patients with grade III and IV injuries.31 A review of 495 patients from the published literature noted a success rate for non-operative treatment of 94%.34 This was accomplished with a mean transfusion rate of 1.9 units, a complication rate of 6% and a mean hospital stay of 13 days. There were no liver-related deaths, nor were there any missed enteric injuries.
If a non-operative strategy is selected it should be borne in mind that the risk of hollow-organ injury increases in proportion to the number of solid organs injured35 and that there is a small but significant risk of delayed haemorrhage. However, it appears that the natural course of liver injuries is more analogous to that of lung or kidney injuries, rather than splenic injuries, in that any deterioration is usually gradual, with a fall in haemoglobin level or an increase in fluid requirement, rather than acute haemodynamic decompensation. Therefore, with close supervision, patients who fail with an initial non-operative approach can be detected early and treated appropriately.
Although non-operative management of haemodynamically stable patients with liver trauma has become the standard of care over the past decade, the role of in-hospital follow-up CT to monitor the injury remains controversial. Demetriades et al. reported that follow-up CT at a mean of 10 days after surgical intervention showed a 49% incidence of liver-related complications, most of which required subsequent intervention.36 However, other authors suggest there is little evidence that follow-up CT provides additional information and rarely changes management.37 In the author’s practice, in-hospital follow-up scan is not employed routinely unless the patient develops relevant symptoms or signs, but a follow-up scan 4–6 weeks later is undertaken to ensure resolution of the injury.
The management policy for abdominal gunshot injuries in most centres continues to be a mandatory laparotomy, regardless of the clinical presentation;38 however, several studies have reported successful non-operative management of selected liver gunshot injuries.39,40 In the study by Omoshoro-Jones et al., 26.6% of patients who presented with liver gunshot injuries were managed non-operatively, with an overall success rate of 94% and a morbidity rate of 36%, of which 3% were liver related.39 This approach is associated with the risk of failure to detect concomitant intra-abdominal visceral injury and therefore should only be considered in specialist centres with experience in management of liver trauma and appropriate facilities to deal with any complications that arise.
Operative management of liver injury
Intraoperative assessment
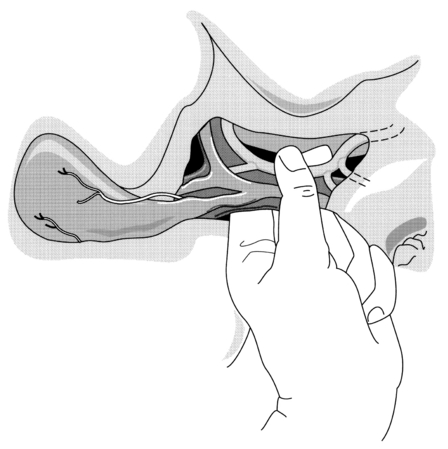
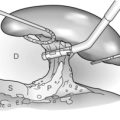
Once the abdomen has been entered, blood and clots should be removed and packs inserted into each quadrant of the abdomen. A thorough laparotomy is performed in a systematic manner to identify all intra-abdominal injuries. Any perforations in the bowel should be sutured immediately to minimise contamination. Significant liver haemorrhage can usually be controlled initially by direct pressure using packs, although additional techniques that may be employed include: temporary digital compression of the free edge of the lesser omentum (Pringle manoeuvre; Fig. 17.2); bimanual compression of the liver; or manual compression of the aorta above the coeliac trunk. At this point, further evaluation of the extent of liver injury should be delayed until the anaesthetist has replenished adequately the intravascular volume and stabilised the blood pressure. Attempts to evaluate the liver injury before adequate resuscitation may result in further blood loss, with worsening hypotension and acidosis.
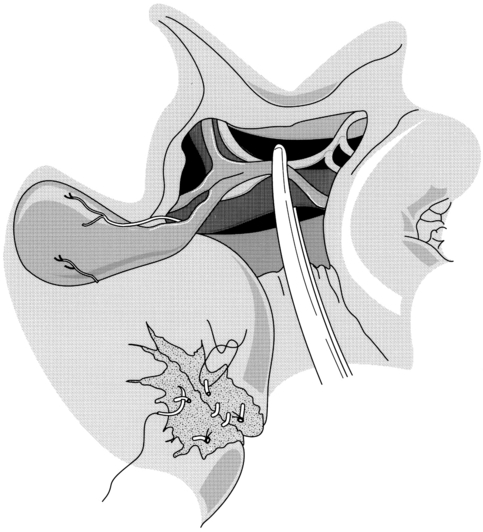
The packs can subsequently be gently removed to allow a detailed evaluation of the type and extent of the liver injury. It should be borne in mind that a subcapsular haematoma may cover an area of ischaemic tissue and that parenchymal lacerations may be associated with damage to segmental bile ducts. Many liver injuries will have stopped bleeding spontaneously by the time of surgery. However, if there is active bleeding, a Pringle manoeuvre can be used diagnostically and compression can be maintained with an atraumatic vascular clamp if haemorrhage decreases (Fig. 17.3). The clamp should be occluded only to the degree necessary to compress the blood vessels and not to injure the common bile duct. A normal liver can tolerate inflow occlusion for up to 1 hour; however, the ability of a damaged liver to tolerate ischaemia may be impaired. If haemorrhage is unaffected by portal triad occlusion, major vena cava injury or atypical vascular anatomy should be suspected. Hepatic outflow control may also be required. Access to the suprahepatic cava can be gained by an experienced liver surgeon, and slings may be placed around the hepatic veins following mobilisation of the liver from its peritoneal attachments. Total vascular occlusion of the liver requires control of the inferior vena cava below the liver in addition to the suprahepatic cava but is likely to be poorly tolerated by an injured liver.
Perihepatic packing
In situations where it is thought that definitive control of haemorrhage cannot be obtained, or patients are deemed critically unstable, coagulopathic or acidotic and therefore would not tolerate a prolonged operative procedure, perihepatic packing can be employed. This has led to the concept of damage control surgery – rapid perihepatic packing, closure of the abdominal incision with or without a Bogota bag and transferring the patient to ICU as soon as possible for continued resuscitation and rewarming. When the metabolic derangements have been corrected or improved, the patient can be taken back to theatre or transferred to a specialist centre for re-exploration and definitive treatment.41
As packing is thus a widely applicable procedure, some attention should be devoted to technical considerations. The packs should not be inserted into the liver substance itself, as this will tend to distract the edges of the parenchymal tear and encourage continued bleeding. Rather, the technique of packing involves manual closure or approximation of the parenchyma, followed by sequential placing of dry abdominal packs or a single rolled gauze around the liver and directly over the injury in an attempt to provide tamponade to a bleeding wound (Fig. 17.4). Most surgeons employ skin closure only, leaving the fascia for primary closure at the subsequent procedure for pack removal. The presence of packs, combined with massive oedema of the bowel, may lead to difficulties in wound closure. If this is encountered, a mesh can be inserted to prevent further compromise of ventilation and bowel viability, and to avoid pressure necrosis of the liver.42
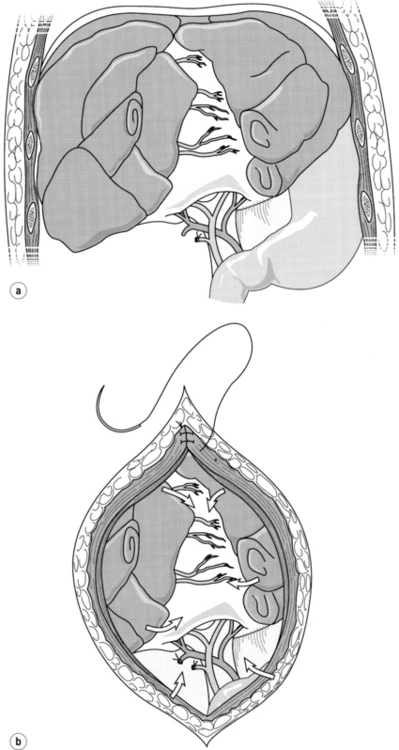
Figure 17.4 (a) Placement of gauze packs around the liver to compress the fracture. (b) Closure of the incision provides additional compression. Reproduced from Berne TV, Donovan AJ. Section 10. Injury and haemorrhage. In: Blumgart LH, Fong Y (eds) Surgery of the liver and biliary tract, 3rd edn, Vol. 2. Edinburgh: Churchill Livingstone, 1994. With permission from Elsevier.
The principal complications and limitations of perihepatic packing can be considered as ‘early’ or ‘late’. Early complications include failure to control haemorrhage. However, this is relatively uncommon as even in patients with caval or hepatic venous injuries, packing may control haemorrhage. Concerns may also be raised about the potential for compromise of caval blood flow by packing, although this may be avoided by monitoring caval pressure if this technique is available. The principal late complications of packing are infection and multiple organ dysfunction. The risk of septic complications has led to the recommendation that liver packs should be removed as soon as possible. However, Nicol et al. reported in a series of 93 patients requiring liver packing that an early re-look laparotomy at 24 hours rather than at 48 hours or later was associated with a higher incidence of re-bleeding necessitating re-packing, without any difference in the incidence of liver-related complications or intra-abdominal collections.43 Perihepatic packing is an indication for intravenous antibiotic administration.
Techniques for surgical haemostasis
Exposed bleeding vessels can be suture-ligated, clipped or repaired to achieve haemostasis. The ultrasonic dissector is useful in removing damaged and non-viable hepatic parenchyma whilst exposing blood vessels. Diathermy coagulation can also be used and in this context the argon beam coagulator, which ‘sprays’ the diathermy current on an argon beam, is invaluable as it produces surface eschar without the diathermy probe becoming adherent to the liver surface. The argon beam coagulator also has the advantage of producing less hepatic tissue necrosis than conventional diathermy, which is an advantage in a potentially contaminated operative field. Fibrin glue has been used as an adjunctive measure in some centres; however, there are concerns regarding the use of fibrin glue in humans. Fatal hypotension following application of fibrin glue into a deep hepatic laceration has been reported.44 Recently, recombinant factor VIIa has been reported as a potential adjunct in the management of liver injuries;45 however, further controlled studies are warranted to evaluate the safety and efficiency of this drug.
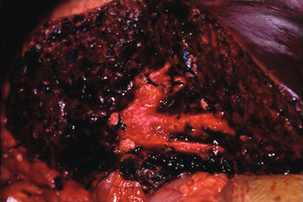
Liver sutures are absorbable sutures on a large curved blunt-tipped needle often used in conjunction with a bolster of haemostatic material. These can be used to approximate a fissured parenchymal injury and thus control haemorrhage as an alternative to exploration of the depths of the injury. The disadvantages of this technique are that vessels may continue to bleed, resulting in a cavitating haematoma, bile duct injuries may not be detected, and the suture itself may cause further bleeding, ischaemia or intrahepatic bile duct injury (Fig. 17.5).
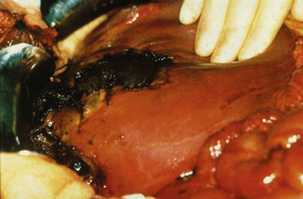
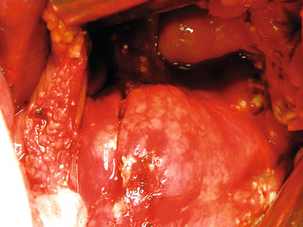
Figure 17.5 Operative photograph demonstrating a liver injury with necrosis at the site of previously inserted liver sutures that had been applied in an attempt to arrest haemorrhage.
Stone and Lamb reported that the greater omentum could be employed as a pedicled flap to fill a defect in the liver parenchyma and may help stop oozing from the low-pressure venous system of the liver parenchyma.46 The use of an absorbable polyglactin perihepatic mesh, particularly for major parenchymal disruptions, has also been reported.47 This technique is not indicated where juxtacaval or hepatic vein injury is suspected. Advocates of mesh wrapping claim that it can provide the benefits of packing without the disadvantages. In particular, a second laparotomy is not required routinely and, as mesh wrapping does not increase intra-abdominal volume or pressure, abdominal closure is much easier and respiratory or renal function is less compromised. However, there is some concern about the amount of time needed to apply the mesh wrap in a haemodynamically unstable patient who might be best treated with rapid insertion of perihepatic packs, and as yet there is insufficient general experience with this technique.
Resectional debridement
This technique involves removal of devitalised liver tissue down to normal parenchyma using the lines of the injury, rather than anatomical planes, as the boundaries of the resection.48 The optimum timing may be to combine debridement with pack removal, as necrotic tissue will be well demarcated at 48 h post-injury. Resectional debridement is by definition ‘non-anatomical’ and may expose segmental bile ducts (Fig. 17.6). Disrupted bile ducts exposed in the periphery of the liver should be sutured or ligated in order to prevent postoperative bile leaks, as this troublesome complication will not necessarily be treatable by endoscopic transampullary biliary stenting. It is better to anticipate and avoid this complication.
Anatomical liver resection
Strong et al. reported a single-centre series of 37 patients that underwent anatomical resection for liver trauma from an institutional experience of 287 patients with liver injury treated over a 13-year period.49 Twenty-seven of these patients underwent right hemihepatectomy and overall there were three postoperative deaths (8% mortality rate). However, these excellent results achieved by a technically skilled liver surgeon and his unit may not be reproduced if the technique were more widely used.
Selective ligation of the hepatic artery
Selective ligation of the hepatic artery is no longer a commonly used technique and is not mentioned frequently in contemporary reports. It may be used when intrahepatic manoeuvres have failed and when persistent re-bleeding occurs on unclamping the hepatic pedicle. In a series of 60 patients,50 Mays reported ligation of the right hepatic artery in 36 patients, the left hepatic artery in 15 patients and the main hepatic artery in the remaining nine patients. No cases of liver failure or necrosis were observed but it seems likely that modern liver surgical approaches have rendered ligation an uncommon manoeuvre in liver injury. Hepatic arterial ligation to control haemorrhage should only be performed when other manoeuvres have failed, when selective ligation has failed and when pedicle clamping has been demonstrated to arrest haemorrhage. Acute gangrenous cholecystitis is a well-recognised complication of hepatic artery ligation, and cholecystectomy should be performed if the main hepatic artery or right hepatic artery is ligated.
Management of hepatic venous and retrohepatic caval injury
Suspicion that one of these serious injuries is present should be raised if the Pringle manoeuvre fails to arrest haemorrhage. In this situation it is vital that a systematic approach be adopted. Injudicious mobilisation of the liver can cause exsanguination or embolisation of air or detached fragments of liver parenchyma. Therefore it is important to exclude anatomical vascular variants as a source of persistent bleeding. For example, there may be bleeding from the left liver due to the presence of a left hepatic artery arising from the left gastric artery or there may be bleeding from the right liver due to an aberrant right hepatic artery. The commonest anatomical variation in the origin of the right hepatic artery (occurring in approximately 15% of cases) is the persistence of the right primordial hepatic artery where the right hepatic artery arises from the sup-erior mesenteric artery and runs just to the right and slightly posterior to the structures in the porta hepatis. These anatomical variants should be considered and excluded. During this process, active bleeding can be reduced or arrested by perihepatic packing. Persistent bleeding despite exclusion of anatomical variants may then indicate the presence of hepatic venous or retrohepatic caval injury. These injuries account for about 10% of liver trauma cases, and there is no clear consensus on an optimal management strategy. Total vascular exclusion (clamping of the inferior vena cava and suprahepatic cava in addition to the Pringle manoeuvre) may be used. However, clamping the vena cava will seriously compromise venous return in a situation of major trauma and seems unwise. Veno-venous bypass (shunt from common femoral vein to left internal jugular or axillary vein) has the advantage of preserving venous return. Atriocaval shunting has also been described and, combined with a Pringle manoeuvre, allows total vascular isolation of the liver. Chen et al. reported on a series of 19 patients with blunt juxtahepatic venous injury from a group of 92 patients with blunt liver trauma over a 2-year period.51 Five patients with isolated left hepatic vein injuries were treated with the use of veno-venous bypass with no mortality. Ten of the 20 patients with isolated right hepatic vein injury were treated using an atriocaval shunt but the mortality in these 20 patients was 18 (80%), with one survivor in both the shunted and non-shunted groups. Of four patients with combined right and left hepatic vein injury, one was treated by liver transplantation but all four patients in this group died. The overall mortality rate in patients with juxtahepatic vein injury was 63%. The opportunity to optimise the outcome in patients with these serious injuries probably lies in packing followed by transfer to a specialist liver surgery unit.
Ex vivo surgery and liver transplantation
Ringe and Pichlmayr52 reported a consecutive series of eight patients with severe liver trauma treated by total hepatectomy followed by liver transplantation. These patients had all undergone prior surgery for trauma, which had been followed by severe complications – uncontrollable bleeding in four and massive necrosis in four. Where a donor liver was not immediately available a temporary portacaval shunt was used as a bridging procedure. There was a high mortality in this group, with six out of eight patients dying from multiple organ failure or sepsis. The authors conclude that total hepatectomy can be a potentially life-saving procedure in exceptional emergencies in patients with major liver injuries. Heparinised coated tubes such as the Gott shunt can be used to bridge caval defects if total hepatectomy and excision of a caval segment is required in order to obtain haemostasis.53 The shunt acts as a temporary bridge during the anhepatic phase and has been reported to remain patent over an 18-h period. Whilst experience of this sort of surgery is extremely infrequent, awareness of the therapeutic potential is useful and small series continue to report encouraging results.54
Complications of liver trauma
Complications of non-operative management
The second group of complications are those relating to coexisting injuries that have not been recognised at the time of initial presentation or become apparent after initial delay. Bile leaks may manifest as biliary peritonitis or as a localised bile collection. ERCP is useful in diagnosing the source of a bile leak in patients with liver trauma treated non-operatively and also in postoperative patients. Perforations of the intestine are also at risk of being missed as the signs of abdominal tenderness may be attributed to intra-abdominal blood from the liver injury. The risk of missing this type of injury can be minimised by regular careful clinical observation. Intestinal perforation may become apparent on serial ultrasound or CT by the presence of free intraperitoneal fluid or gas. In Sherman et al.’s series of patients with liver trauma treated non-operatively, 4 of 30 (13%) patients initially treated without operation required subsequent laparotomy.32 These were due to splenic injury in three patients and renal injury in one patient. Although the grade of injury to these organs is not specified, in all cases the injuries became apparent after a period of clinical observation. However, the authors concluded that this risk of missed solid-organ injury does not obviate the benefits of initial non-operative management.
Postoperative complications after surgery for liver trauma
The complications after liver surgery for trauma are similar to those encountered after any form of hepatic surgery. Haemorrhage in the immediate postoperative period may be due to coagulopathy related to large-volume transfusion and may require correction with fresh frozen plasma and platelet concentrates. If there is no evidence of a significant coagulopathy and bleeding continues, CT angiography may provide diagnostic information. Selective mesenteric angiography may permit therapeutic embolisation, but if this is unsuccessful, re-laparotomy will be indicated to assess and control the source of bleeding and to remove retained blood and clot. Bleeding in the later postoperative period may be due to haemobilia or bleeding from the biliary tree into the gut. It has been reported to occur in 1.2% of patients with liver trauma.55
Outcome after liver injury
The outcome after liver trauma is related not only to the severity of the injury but also to the severity of any associated injury. Most series report mortality rates of approximately 10–15%; however, the large variation in case mix between different centres makes comparison difficult. In a large series of 1000 cases of liver trauma from Houston, an overall mortality of 10.5% was reported.4 White and Cleveland documented a similar mortality rate, with eight deaths occurring in a consecutive series of 126 patients (6.3%).27 The results in the series reported by Schweizer et al. recorded an overall mortality rate of 12% (21 deaths in 175 patients), with a progressively higher mortality rate associated with an increasing grade of liver injury.8 In a series of 337 patients, Kozar et al. reported 37 hepatic-related complications in 25 patients; 63% (5 of 8) of patients with grade V injuries developed complications, 21% (19 of 92) of patients had grade IV injuries, but only 1% (1 of 130) of patients had grade III injuries.56 The mechanism of injury has an important bearing on the mortality rate, with blunt trauma carrying a higher mortality rate (10–30%) than penetrating liver trauma (0–10%). While most early deaths seem to be due to uncontrolled haemorrhage and associated injuries, most late deaths result from head injuries and sepsis with multiple organ failure.
Extrahepatic biliary tract trauma
Incidence of biliary injury
The reported incidence of injury to the extrahepatic biliary system varies between 1% and 5% of patients who sustain abdominal trauma.57 In a review of 5070 patients who sustained blunt and penetrating abdominal trauma, Penn reported a 1.9% incidence of gallbladder injury.58 Soderstrom et al. identified 31 patients (2.1%) with gallbladder injury in a group of 1449 patients who sustained blunt abdominal trauma and underwent exploratory laparotomy.59 In a further review of 949 patients undergoing laparotomy for acute trauma, there were 32 injuries to the gallbladder (3.4%) and five to the common bile duct (0.5%).60 Burgess and Fulton reported that, over a 5-year period, 24 of 184 patients with abdominal trauma had extrahepatic bile duct or gallbladder injury as well as liver injury.61 They reported that this injury was often seen with severe hepatic trauma and in association with multiple organ injury. Dawson et al. reviewed the results of treatment of all patients with porta hepatis injuries presenting to a level I trauma centre in Seattle over an 11-year period.62 A total of 21 patients (0.21% of 10 500 admissions) had injuries to the portal triad, of whom 11 (52%) died. Isolated extrahepatic bile duct injury occurred in four of these patients. Injuries to the portal vein or hepatic artery, either in isolation or in association with extrahepatic bile duct injury, were associated with the worst prognosis. Of note is the fact that in none of the 21 cases was the diagnosis of the injury made preoperatively. The male to female ratio is usually reported as approximately 5:1.63 However, Bade et al. reported a male to female ratio of 25:1, which may reflect the higher number of injuries from stab wounds seen in a South African population.64 Most series report a median age of approximately 30 years and there are many reports in children.
Classification of biliary injury
The gallbladder is the most frequently injured part of the extrahepatic biliary tract. The largest reported series of extrahepatic biliary tract injuries consists of 53 patients, of whom 45 (85%) sustained injury to the gallbladder and eight (15%) had an injury to the bile duct.64 Kitahama et al. reported the gallbladder to be involved in 32 (80%) of 40 patients, while ductal injury occurred in 12 (30%), some patients having multiple injuries.63
Injury to the gallbladder resulting from blunt trauma can be classified as contusion, avulsion or perforation. In addition to these three main types of injury, Penn added traumatic cholecystitis as a pathological entity.58 The most common type of gallbladder injury is perforation. Avulsion of the gallbladder may refer to the organ being partially or completely torn from the liver bed while still attached to the bile duct, or it may signify complete separation from all attachments with the organ lying free in the abdomen. Contusion is probably under-reported, as it will be recognised only if laparotomy is performed. The natural course of an untreated gallbladder contusion is not known, although it is likely that the majority resolve without further complication. It has been speculated that an intramural haematoma might result in necrosis of the gallbladder wall and result in a subsequent perforation. There have been a number of reports of delayed rupture of the gallbladder, and it is plausible that unrecognised contusion of the gallbladder might lead to such a delayed presentation.
Presentation and diagnosis of biliary injury
Clinical presentation of the vast majority of bile duct injuries can be divided into two broad categories. The first contains patients in whom clinical signs or associated injury lead to laparotomy with early diagnosis and surgical management (early presentation); these patients generally present with hypovolaemic shock or signs of an acute abdomen. The second category of patient has a delay (greater than 24 h) in diagnosis and definitive therapy (delayed presentation). These patients comprised over half the cases (53.2%) in a review of combined series.65 In addition, a third category of patient, representing a very small proportion of those who sustain a bile duct injury, may present with obstructive jaundice months or even years after the initial trauma (late presentation). In these patients, the bile duct injury is always isolated. Compromise of the blood supply to the duct may occur either at the time of the primary injury or at operation during the Pringle manoeuvre, and this may contribute to the development of a late biliary stricture. Bourque et al. reported that the delay between clinical presentation and surgical intervention for isolated bile duct injury averaged 18 days, with a range from several hours to 60 days.66 Michelassi and Ranson reported that biliary injury was not recognised at initial operation in 11 (12%) of 91 patients with extrahepatic biliary tract trauma,65 whereas Dawson and Jurkovich reported that 41% of bile duct injuries were missed at initial laparotomy.67
If a non-operative course of management for abdominal trauma is adopted, suspicion of an extrahepatic bile duct injury may be raised by CT evidence of a central liver injury involving the porta hepatis or the head of the pancreas, the presence of fluid collections in the subhepatic space, or evidence of periportal tracking of haematoma.16 The diagnostic procedure of choice is ERCP, and if a duct injury is identified this may be treated by endoscopic stenting.68
Operative management of biliary injury
Many patients with extrahepatic biliary tract injury present in shock due to associated haemorrhage, and the priority at laparotomy is to identify and control haemorrhage. The report of Dawson et al. demonstrates that these patients are at risk of exsanguinating on the operating table.62 Injuries to the gallbladder are best treated by cholecystectomy.69 Primary repair of a clean and simple partial or complete transection of the common duct using absorbable sutures such as 4/0 polydioxanone over a T-tube inserted through a separate choledochotomy has been described. However, this type of repair is not appropriate if there is any evidence of duct contusion, loss of ductal tissue or possible injury to the hepatic artery as this may increase the risk of late development of an ischaemic stricture. In general, it is therefore safer to recommend that most injuries should be managed by fashioning a Roux-en-Y hepatico-jejunostomy as in the management of iatrogenic bile duct injuries.
Outcome after biliary injury
Injuries of this nature are associated with a mortality rate of 10% from concomitant injuries.63 Septic complications and bile leakage account for most of the early morbidity and may require operative intervention. Late morbidity after repair of a traumatic biliary tract injury is unusual; however, jaundice or episodes of ascending cholangitis suggest a stricture of the ductal system.
Pancreatic trauma
Injuries to the pancreas are uncommon, accounting for 1–4% of severe abdominal injuries, and usually occur in young men. In a report of 51 425 patients from the Trauma Register of the German Society of Trauma Surgery, 9268 (18%) had documented abdominal injuries and 284 (3.1%) had a pancreatic injury.70
Diagnosis of pancreatic injury
In an early study, Moretz et al. found that there was no reliable correlation between serum amylase and pancreatic injury.71 In a later report, Takashima et al. retrospectively studied admission serum amylase values in a series of 73 patients with blunt pancreatic trauma treated in a single institution over a 16-year period.72 Sixty-one (84%) of these patients had a raised serum amylase level. Of interest, the serum amylase level was found to be abnormal in all patients admitted more than 3 hours after trauma.
Bearing in mind the practicality that patients with pancreatic injury will simultaneously be undergoing evaluation to exclude concomitant intra-abdominal visceral injury, contrast-enhanced CT has been the investigation of choice (Fig. 17.7). Reported CT features of pancreatic injury include free intraperitoneal fluid, localised fluid in the lesser sac, retroperitoneal fluid, pancreatic oedema or swelling and changes in the peripancreatic fat. The presence of fluid in the lesser sac between the pancreas and the splenic vein is reported by Lane et al. to be a reliable sign in blunt pancreatic injury.73 However, Sivit and Eichelberger reported that this radiological sign was rarely the only abnormal CT finding in pancreatic injury.74 It should be borne in mind that many of these CT features are also seen in acute pancreatitis (and furthermore that acute pancreatitis may occur as a result of blunt abdominal trauma). There is also evidence that CT tends to underdiagnose pancreatic injury. Akhrass et al. evaluated the clinical course of 72 patients with pancreatic injury admitted over a 10-year period.75 Seventeen of these patients underwent CT as part of their initial assessment and this was reported as normal in nine. Eight of these patients underwent laparotomy (principally for suspected associated splenic injury) and three were found to have pancreatic injury requiring distal pancreatectomy. Newer, non-invasive imaging modalities such as magnetic resonance pancreatography have been reported in the assessment of patients with suspected pancreatic trauma.76 Increased sophistication with the use of this technique may allow for accurate assessment of pancreatic ductal integrity.
Classification of pancreatic injury
Of the various proposed classification schemes, Lucas suggested in an early report that appropriate treatment be formulated according to the type of injury.77 This classification system divides pancreatic injuries into three groups:
• grade I – superficial contusion with minimal damage;
• grade II – deep laceration or transection of the left portion of the pancreas;
A more complex system of classification taking into account the frequent coexistence of duodenal and pancreatic injuries was proposed by Frey and Wardell78 (Table 17.2). The most common site of injury is the neck of the pancreas. The relative frequency of pancreatic injuries reported in collected reviews is represented in Fig. 17.9.
Table 17.2
Classification of pancreatic injury proposed by Frey and Wardell

Reproduced from Frey CF, Wardell JW. Injuries to the pancreas. In: Trede M, Carter DC (eds) Surgery of the pancreas. Edinburgh: Churchill Livingstone, 1993. With permission from Elsevier.
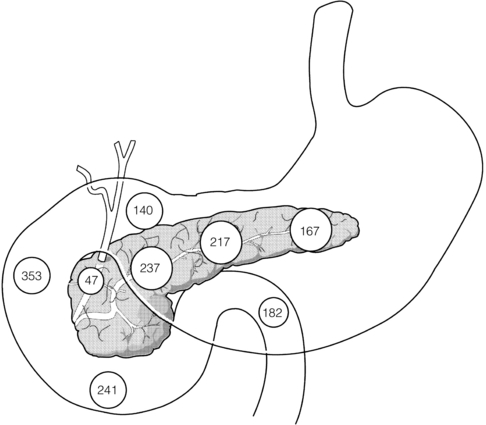
Figure 17.9 Distribution of pancreatic injuries in the world literature. Note the preponderance of injuries in the junctional area of the neck of the gland. Reproduced from Frey CF, Wardell JW. Section 9. Injuries to the pancreas. In: Trede M, Carter DC (eds) Surgery of the pancreas. Edinburgh: Churchill Livingstone, 1993. With permission from Elsevier.
Initial management of pancreatic injury
In a major retrospective clinical casenote review of pancreatic trauma from six hospitals, Bradley et al. demonstrated a significant association between pancreas-related morbidity and injury to the main pancreatic duct.79 Delayed intervention (due to delay in recognition of main pancreatic duct injury) was associated with high morbidity. CT was unreliable for the assessment of main pancreatic ductal integrity and an accurate assessment required ERCP.
Operative management of pancreatic injury
Experience of patients with pancreatic injury from Durban led to the recommendation for operative treatment of patients with penetrating or gunshot injury and signs of peritoneal irritation.80 In this large series of 152 patients with pancreatic trauma presenting during a 5-year period, 63 patients had been shot, 66 stabbed and 23 had blunt trauma. The mainstay of treatment was exploratory laparotomy followed by drainage of the pancreatic injury site. Large-bore soft silastic drains were used to minimise the risk of drain erosion into a major vessel. The mortality rates in these groups were 8% after gunshot injury, 2% after stab wounds and 10% after blunt trauma. The majority of these deaths were attributed to damage of other organs. The proportions of patients that developed pancreatic fistulas in the three groups were 14%, 9% and 13%, respectively. The authors concluded that ‘conservative’ surgical drainage (avoiding pancreatic resection) was justified after pancreatic injury. This large series lends weight to the treatment plan proposed by Lucas for grade I injuries, which consists of passive closed drainage using a wide-bore drain.
Simplified management guidelines based on the treatment protocols developed during the treatment of 124 pancreatic injuries at the University of Tennessee81 also advocate simple drainage alone for proximal pancreatic injuries. There were 37 (30%) patients with proximal injuries. The ‘pancreas-related’ morbidity was 11% – principally the sequelae of pancreatic fistulas. Of 87 distal pancreatic injuries, the integrity of the main pancreatic duct was not established in 54 (62%). Patients thought to have a high probability of duct transection were treated by distal pancreatectomy. A concern with simple drainage for injuries in the head of the pancreas is persistent pancreatic fistula, and thus a surgical alternative is to drain the head of the pancreas into a Roux-en-Y limb of jejunum.
Moncure and Goins described their experience over a 6-year period with a consecutive series of 44 patients with pancreatic injury,82 of which penetrating abdominal trauma accounted for the majority of cases. Class I pancreatic injuries occurred in 55% of patients and the majority were managed by simple drainage. Grade II injuries occurred in 18% and grade III injuries in 21%. Coexistent duodenal injuries were treated by primary closure in 21% and more complex duodenal exclusion techniques were used in 20%. The most frequent complications were intra-abdominal abscesses (31%) and pancreatic fistulas (16%).
Krige et al. reported on a series of 110 patients with pancreatic injuries after blunt trauma.83 One hundred and one patients underwent a total of 123 operations, including drainage of the pancreatic injury (n = 73), distal pancreatectomy (n = 39) and Whipple resection (n = 5). The overall complication rate was 74.5% and the mortality rate was 16.4%. Only two of the 18 deaths were attributable to the pancreatic injury. Mortality increased exponentially as the number of associated injuries increased.
Severe Lucas grade III injuries involving the head of the pancreas, duodenum and distal bile duct represent a major challenge, but fortunately are relatively rare, occurring in approximately 5% of all duodenal injuries.84 The principles of treatment are to ensure that haemorrhage from concomitant injuries is dealt with first, as this is likely to be the major source of mortality. Similarly a prolonged operative procedure should be avoided in a potentially unstable patient and the involvement of an experienced pancreatic surgeon is desirable. Duodenal injuries can be closed primarily or drained into a Roux loop. Bile duct injuries may be repaired primarily over a T-tube or drained into a Roux limb of jejunum. The large variety of operative procedures described for these complex injuries suggests that treatment has to be tailored to the individual injury complex and that no single procedure is likely to be uniformly applicable or successful. Very rarely, pancreatico-duodenectomy may be required for complex, severe pancreatic injuries with concomitant duodenal and distal bile duct injuries. Clearly, this sort of resection should not be undertaken lightly in an individual suffering from shock and its sequelae, but rather like liver transplantation for trauma it is useful to have an index of awareness of the available therapeutic options.
Complications of pancreatic injury
The most common post-traumatic complications include necrotising pancreatitis, pseudocyst formation, pancreatic abscesses and pancreatic fistula. Cerwenka et al. reported the incidence of these complications to be 15%, 9%, 6% and 4%, respectively.85 The principles regarding management are similar to those for treating these complications when they arise as a result of pancreatitis or pancreatic surgery. Inflammation of the pancreas after trauma behaves in much the same way as acute biliary or acute alcohol-induced pancreatitis, with the possible exception that there is a higher incidence of development of local complications such as pseudocyst – possibly relating to the nature of duct disruption in trauma. The Cape Town group reported that, of a series of 64 patients with pancreatic trauma, pseudocysts developed in 15 patients (23%), of whom eight had a duct injury demonstrated by endoscopic retrograde pancreatography.86 Patients with pseudocysts related to distal duct injury were treated successfully by percutaneous aspiration. Three patients with duct injuries in the neck/body region underwent distal pancreatectomy. Pseudocysts related to ductal injury in the head of the pancreas were drained internally by Roux-en-Y cyst-jejunostomy. The authors concluded that traumatic pancreatic pseudocysts associated with a peripheral duct injury may resolve spontaneously, whereas those associated with injuries to the proximal duct would more likely require surgical intervention. Alternative treatment strategies include endoscopic transpapillary or transmural drainage of the pseudocyst.
The incidence of pancreatic fistula after surgery for trauma is dependent on the type of procedure, with some evidence that the fistula rate is higher after drainage procedures than after resection. Successful insertion of pancreatic duct stents has been reported for management of major pancreatic duct disruption; however, the incidence of long-term ductal stricture is high and therefore the role of pancreatic duct stenting needs to be further defined.87
Conclusion
The contemporary management of patients with suspected liver, biliary or pancreatic injury involves detailed clinical assessment and resuscitation followed, in haemodynamically stable patients, by imaging investigations. If surgical intervention is required, the mainstay of treatment is to control haemorrhage. In European healthcare systems, the optimum care of the patient may consist of packing followed by transfer to a regional hepato-pancreatico-biliary unit. A paper by Hoyt et al. examining preventable causes of death in 72 151 admissions with abdominal trauma to North American level I trauma centres identified abdominal injury as the cause of death in 287, with liver injury being responsible for 92.88 Delays in packing were highlighted as a preventable cause of death, as was a need for better understanding of the end-points to be achieved by packing. The conclusion of this large survey was that the management of liver injury remains a major technical challenge.
References
1. Scollay, J.M., Beard, D., Smith, R., et al, Eleven years of liver trauma: the Scottish experience. World J Surg 2005; 29:744–749. 15880277
2. Talving, P., Beckman, M., Haggmark, T., et al, Epidemiology of liver injuries. Scand J Surg 2003; 92:192–194. 14582539
3. Krige, J.E., Bornman, P.C., Terblanche, J., Liver trauma in 446 patients. S Afr J Surg 1997; 35:10–15. 9164149
4. Feliciano, D.V., Mattox, K.L., Jordan, G.L., et al, Management of 1000 consecutive cases of hepatic trauma (1979–84). Ann Surg 1986; 204:438–454. 3767479
5. Fabian, T.C., Croce, M.A., Stanford, G.G., et al, Factors affecting morbidity following hepatic trauma. A prospective analysis of 482 injuries. Ann Surg 1991; 213:540–547. 2039284
6. Parks, R.W., Chrysos, E., Diamond, T., Management of liver trauma. Br J Surg 1999; 86:1121–1135. 10504364
7. Moore, E.E., Cogbill, T.H., Jurkovich, G.J., et al, Organ injury scaling: spleen and liver (1994 revision). J Trauma 1995; 38:323–324. 7897707
8. Schweizer, W., Tanner, S., Baer, H.U., et al, Management of traumatic liver injuries. Br J Surg 1993; 80:86–88. 8428303
9. Bickell, W.H., Wall, M.J., Jr., Pepe, P.E., et al, Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 1994; 331:1105–1109. 7935634
10. Scalea, T.M., Rodriguez, A., Chiu, W.C., et al, Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma 1999; 46:466–472. 10088853
11. Stengel, D., Bauwens, K., Sehouli, J., et al, Systematic review and meta-analysis of emergency ultrasonography for blunt abdominal trauma. Br J Surg 2001; 88:901–912. 11442520
12. Rozycki, G.S., Ochsner, M.G., Feliciano, D.V., et al, Early detection of hemoperitoneum by ultrasound examination of the right upper quadrant: a multi-center study. J Trauma 1998; 45:878–883. 9820696
13. McKenney, M.G., Martin, L., Lentz, K., et al, 1000 consecutive ultrasounds for blunt abdominal trauma. J Trauma 1996; 40:607–612. 8614041
14. Richards, J.R., McGahan, J.P., Simpson, J.L., et al, Bowel and mesenteric injury: evaluation with abdominal US. Radiology 1999; 211:399–403. 10228520
15. Fang, J.F., Chen, R.J., Wong, Y.C., et al, Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg 1998; 176:315–319. 9817246
16. Yokota, J., Sugimoto, T., Clinical significance of periportal tracking on computed tomographic scan in patients with blunt liver trauma. Am J Surg 1994; 168:247–250. 8080062
17. Shankar, K.R., Lloyd, D.A., Kitteringham, L., et al, Oral contrast with computed tomography in the evaluation of blunt abdominal trauma in children. Br J Surg 1999; 86:1073–1077. 10460648
18. Croce, M.A., Fabian, T.C., Kudsk, K.A., et al, AAST organ injury scale: correlation of CT graded liver injuries and operative findings. J Trauma 1991; 31:806–812. 2056544
19. Deunk, J., Dekker, H.M., Brink, M., et al, The value of indicated computed tomography scan of the chest and abdomen in addition to the conventional radiological work-up for blunt trauma patients. J Trauma 2007; 63:757–763. 18090002
20. Huber-Wagner, S., Lefering, R., Qvick, L.M., et al, The value of indicated computed tomography. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009; 373:1455–1461. 19321199
21. Chan, O., Primary computed tomography survey for major trauma. Br J Surg 2009; 96:1377–1378. 19918849
22. Forlee, M.V., Krige, J.E., Welman, C.J., et al, Haemobilia after penetrating and blunt liver injury: treatment with selective hepatic artery embolisation. Injury 2004; 35:23–28. 14728951
23. Johnston, J.W., Gracias, V.H., Reilly, P.M. Hepatic angiography in the damage control population. J Trauma. 2001; 50:176.
24. Letoublon, C., Morra, I., Chen, Y., et al, Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma 2011; 70:1032–1036. 21610421
25. Carrillo, E.H., Spain, D.A., Wohltmann, C.D., et al, Interventional techniques are useful adjuncts in the non-operative management of hepatic injuries. J Trauma 1999; 46:619–622. 10217224
26. Richie, J.P., Fonkalsrud, E.W., Subcapsular haematoma of the liver: non-operative management. Arch Surg 1972; 104:780–784. 5029409
27. White, P., Cleveland, R.J., The surgical management of liver trauma. Arch Surg 1972; 104:785–786. 5029410
28. Meyer, A.A., Crass, R.A., Lim, R.C., et al, Selective non-operative management of blunt liver injury using computed tomography. Arch Surg 1985; 120:781–784. 4015365
29. Farnell, M.B., Spencer, M.P., Thompson, E., et al, Nonoperative management of blunt hepatic trauma in adults. Surgery 1988; 104:748–756. 3175870
30. Feliciano, D.V., Continuing evolution in the approach to severe liver trauma. Ann Surg 1992; 216:521–523. 1444643
31. Boone, D.C., Federle, M., Billiar, T.R., et al, Evolution of management of major hepatic trauma: identification of patterns of injury. J Trauma 1995; 39:344–350. 7674405
32. Sherman, H.F., Savage, B.A., Jones, L.M., et al, Non-operative management of blunt hepatic injuries: safe at any grade? J Trauma 1994; 37:616–621. 7932893
33. Croce, M.A., Fabian, T.C., Menke, P.G., et al, Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg 1995; 221:744–753. 7794078
34. Pachter, H.L., Hofstetter, S.R., The current status of nonoperative management of adult blunt hepatic injuries. Am J Surg 1995; 169:442–454. 7694987
35. Nance, M.L., Peden, G.W., Shapiro, M.B., et al, Solid viscus injury predicts major hollow viscus injury in blunt abdominal trauma. J Trauma 1997; 43:618–622. 9356057
36. Demetriades, D., Karaiskakis, M., Alo, K., et al, Role of postoperative computed tomography in patients with severe liver injury. Br J Surg 2003; 90:1398–1400. 14598421
37. Cox, J.C., Fanian, T.C., Maish, G.O., et al, Routine follow-up imaging is unnecessary in the management of blunt hepatic injury. J Trauma 2005; 59:1175–1180. 16385297
38. Cogbill, T.H., Moore, E.E., Jurkovich, G.J., et al, Severe hepatic trauma: a multicenter experience with 1335 liver injuries. J Trauma 1988; 28:1433–1438. 3172301
39. Omoshoro-Jones, J.A., Nicol, A.J., Navsaria, P.H., et al, Selective non-operative management of liver gunshot injuries. Br J Surg 2005; 92:890–895. 15918164
40. Demetriades, D., Hadjizacharia, P., Constantinou, C., et al, Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg 2006; 244:620–628. 16998371
41. Calne, R.Y., McMaster, P., Pentlon, B.D., The treatment of major liver trauma by primary packing with transfer of the patient for definitive treatment. Br J Surg 1979; 66:338–339. 444853
42. Cue, J.I., Cryer, H.G., Miller, F.B., et al, Packing and planned reexploration for hepatic and retroperitoneal hemorrhage: critical refinements of a useful technique. J Trauma 1990; 30:1007–1013. 2201787
43. Nicol, A.J., Hommes, M., Primrose, R., et al, Packing for control of haemorrhage in major liver trauma. World J Surg 2007; 31:569–574. 17334868
44. Berguer, R., Staerkel, R.L., Moore, E.E., et al, Warning: fatal reaction to the use of fibrin glue in deep hepatic wounds. Case reports. J Trauma 1991; 31:408–411. 2002531
45. Vick, L.R., Islam, S., Recombinant factor VIIa as an adjunct in nonoperative management of solid organ injuries in children. J Pediatr Surg 2008; 43:195–199. 18206482
46. Stone, H.H., Lamb, J.M., Use of pedicled omentum as an autogenous pack for control of haemorrhage in major injuries of the liver. Surg Gynecol Obstet 1975; 141:92–94. 1154222
47. Brunet, C., Sielezneff, I., Thomas, P., et al, Treatment of hepatic trauma with perihepatic mesh: 35 cases. J Trauma 1994; 37:200–204. 8064916
48. Cox, E.F., Flancbaum, L., Dauterieve, A.H., et al, Blunt trauma to the liver. Analysis of management and mortality in 323 consecutive patients. Ann Surg 1988; 207:126–134. 3277545
49. Strong, R.W., Lynch, S.V., Wall, D.R., et al, Anatomic resection for severe liver trauma. Surgery 1998; 123:251–257. 9526515
50. Mays, E.T., Hepatic trauma. Curr Probl Surg 1976; 13:6–73. 780065
51. Chen, R.J., Fang, J.F., Lin, B.C., et al, Surgical management of juxtahepatic venous injuries in blunt hepatic trauma. J Trauma 1995; 38:886–890. 7602629
52. Ringe, B., Pichlmayr, R., Total hepatectomy and liver transplantation: a life-saving procedure in patients with severe hepatic trauma. Br J Surg 1995; 82:837–839. 7627526
53. Lin, P.J., Jeng, L.B., Chen, R.J., et al, Femoro-arterial bypass using Gott shunt in liver transplantation following severe hepatic trauma. Int Surg 1993; 78:295–297. 8175255
54. Ginzburg, E., Shatz, D., Lynn, M., et al, The role of liver transplantation in the subacute trauma patient. Am Surg 1998; 64:363–364. 9544151
55. Maurel, J., Aouad, K., Martel, B., et al, Post-traumatic hemobilia. How to treat? Ann Chir 1994; 48:572–575. 7847707
56. Kozar, R.A., Moore, F.A., Cothren, C.C., et al, Risk factors for hepatic morbidity following nonoperative management: multicentre study. Arch Surg 2006; 141:451–459. 16702516
57. Parks, R.W., Diamond, T., Non-surgical trauma to the extrahepatic biliary tract. Br J Surg 1995; 82:1303–1310. 7489150
58. Penn, I., Injuries of the gallbladder. Br J Surg 1962; 49:636–641. 14485100
59. Soderstrom, C.A., Maekawa, K., DuPriest, R.W., Jr., et al, Gallbladder injuries resulting from blunt abdominal trauma: an experience and review. Ann Surg 1981; 193:60–66. 7006529
60. Posner, M.C., Moore, E.E., Extrahepatic biliary tract injury: operative management plan. J Trauma 1985; 25:833–837. 4032507
61. Burgess, P., Fulton, R.L., Gall bladder and extrahepatic biliary duct injury following abdominal trauma. Injury 1992; 23:413–414. 1428171
62. Dawson, D.L., Johansen, K.H., Jurkovich, G.J., Injuries to the portal triad. Am J Surg 1991; 161:545–551. 2031534
63. Kitahama, A., Elliott, L.F., Overby, J.L., et al, The extra-hepatic biliary tract injury: perspective in diagnosis and treatment. Ann Surg 1982; 196:536–540. 6812512
64. Bade, P.G., Thomson, S.R., Hirshberg, A., et al, Surgical options in traumatic injury to the extrahepatic biliary tract. Br J Surg 1989; 76:256–258. 2720321
65. Michelassi, F., Ranson, J.H., Bile duct disruption by blunt trauma. J Trauma 1985; 25:454–457. 3999171
66. Bourque, M.D., Spigland, N., Bensoussan, A.L., et al, Isolated complete transection of the common bile duct due to blunt trauma in a child, and review of the literature. J Pediatr Surg 1989; 24:1068–1070. 2681656
67. Dawson, D.L., Jurkovich, G.J., Hepatic duct disruption from blunt abdominal trauma: case report and literature review. J Trauma 1991; 31:1698–1702. 1749047
68. Jenkins, M.A., Ponsky, J.L., Endoscopic retrograde cholangiopancreatography and endobiliary stenting in the treatment of biliary injury resulting from liver trauma. Surg Laparosc Endosc 1995; 5:118–120. 7773456
69. Ball, C.G., Dixon, E., Kirkpatrick, A.W., et al, A decade of experience with injuries to the gallbladder. J Trauma Manag Outcomes 2010; 4:3. 20398307
70. Heuer, M., Hussmann, B., Lefering, R., et al, Pancreatic injury in 284 patients with severe abdominal trauma: Outcome, course and treatment algorithm. Arch Surg 2011; 396:1067–1076. 21847623
71. Moretz, J.A., III., Campbell, D.P., Parker, D.E., et al, Significance of serum amylase in evaluating pancreatic trauma. Am J Surg 1975; 130:739–741. 1200292
72. Takashima, T., Sugimoto, K., Hirata, M., et al, Serum amylase level on admission in the diagnosis of blunt injury to the pancreas: its significance and limitations. Ann Surg 1997; 226:70–76. 9242340
73. Lane, M.J., Mindelzun, R.E., Sandhu, J.S., CT diagnosis of blunt pancreatic trauma: importance of detecting fluid between the pancreas and the splenic vein. AJR Am J Roentgenol 1994; 163:833–835. 7503824
74. Sivit, C.J., Eichelberger, M.R., CT diagnosis of pancreatic injury in children: significance of fluid separating the splenic vein and the pancreas. AJR Am J Roentgenol 1995; 165:921–924. 7676993
75. Akhrass, R., Kim, K., Brandt, C., Computed tomography: an unreliable indicator of pancreatic trauma. Am Surg 1996; 62:647–651. 8712562
76. Nirula, R., Velmahos, G.C., Demetriades, D., Magnetic resonance cholangiopancreatography in pancreatic trauma: a new diagnostic modality? J Trauma 1999; 47:585–587. 10498321
77. Lucas, C.E., Diagnosis and treatment of pancreatic and duodenal injury. Surg Clin North Am 1977; 57:49–65. 854854
78. Frey, C.F., Wardell, J.W. Injuries to the pancreas. In: Trede M., Carter D.C., eds. Surgery of the pancreas. Edinburgh: Churchill Livingstone; 1993:565–589.
79. Bradley, E., Young, P.R., Jr., Chang, M.C., et al, Diagnosis and initial management of pancreatic trauma: guidelines from a multi-institutional review. Ann Surg 1998; 227:861–869. 9637549
80. Madiba, T.E., Mokoena, T.R., Favourable prognosis after surgical drainage of gunshot, stab or blunt trauma of the pancreas. Br J Surg 1995; 82:1236–1239. 7552005
81. Patton, J.H., Jr., Lyden, S.P., Croce, M.A., et al, Pancreatic trauma: a simplified management guideline. J Trauma 1997; 43:234–239. 9291366
82. Moncure, M., Goins, W.A., Challenges in the management of pancreatic and duodenal injuries. JAMA 1993; 85:767–772. 8254694
83. Krige, J.E., Kotze, U.K., Hameed, M., et al, Pancreatic injuries after blunt abdominal trauma: an analysis of 110 patients treated at a level 1 trauma centre. S Afr J Surg 2011; 49:58–67. 21614975
84. Feliciano, D.V., Martin, T.D., Cruse, P.A., et al, Management of combined pancreatoduodenal injuries. Ann Surg 1987; 205:673–680. 3592810
85. Cerwenka, H., Bacher, H., El-Shabrawi, A., et al, Management of pancreatic trauma and its consequences – guidelines or individual therapy? Hepatogastroenterology 2007; 54:581–584. 17523326
86. Lewis, G., Krige, J.E., Bornman, P.C., et al, Traumatic pancreatic pseudocysts. Br J Surg 1993; 80:89–93. 8428304
87. Lin, B.C., Liu, N.J., Fang, J.F., et al, Long-term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc 2006; 20:1551–1555. 16897285
88. Hoyt, D.B., Bulger, E.M., Knudson, M.M., et al, Death in the operating room: an analysis of a multicenter experience. J Trauma 1994; 37:426–438. 8083904