CHAPTER 80 Hepatitis E
Hepatitis E is a form of acute, icteric, self-limited viral hepatitis caused by the hepatitis E virus (HEV). The disease was first recognized in the 1980s, when sera collected during the first recorded epidemic in Delhi, India, in 1955,1 and during another epidemic in Kashmir, India, in 1978,2 were found to lack serologic markers of hepatitis A and B.3 In retrospect, several epidemics of enterically transmitted hepatitis with epidemiologic features resembling those of hepatitis E outbreaks occurred in Europe in the 18th and 19th centuries. HEV was identified in 1983 by immune electron microscopy4; its genome was cloned in 1990 and fully sequenced shortly thereafter.5,6
VIROLOGY
HEV is a small RNA virus, 32 to 34 nm in diameter, nonenveloped, and icosahedral. HEV RNA is approximately 7.2 kilobases in length, single- and positive-stranded, 5′-capped, and polyadenylated. The crystal structure of HEV-like particles that consist of capsid protein has been found to exhibit the protruding domain that is involved in the binding of HEV to susceptible cells and that contains some neutralization epitopes.7 Other investigators have found that each capsid protein contains 3 linear domains that form distinct structural elements: the continuous capsid (S), 3-fold protrusions (P1), and 2-fold spikes (P2) and have speculated that binding of the susceptible cell receptor to P1 may result in conformational changes that eventually lead to cell membrane penetration and genome release into the infected cell.8
The virus is currently classified in a separate genus named Hepevirus in the family Hepeviridae.9 The HEV genome contains three open reading frames (ORFs) (Fig. 80-1).6 ORF1 encodes nonstructural proteins, ORF2 encodes the viral capsid protein, and ORF3 encodes a protein of unknown function. Details of HEV replication in hepatocytes and its release from infected cells remain unknown; the application of the replicon system to HEV research may generate a better understanding of the intracellular events during virus replication.10 Phylogenetic analysis of HEV isolates shows the existence of four geographically distinct genotypes, termed genotypes 1 to 4 (Table 80-1).11 Genotype 1 includes various isolates from Asia that have a nucleotide sequence homology of 92% to 99% (amino acid sequence homology 95% to 99%) with each other. These isolates have nucleotide homology of 75% (amino acid homology 86%) with genotype 2 isolates, which include a strain from Mexico and some isolates from western Africa. Isolates from the United States, classified as genotype 3, are 92% identical to each other but only 73.5% to 74.5% identical to genotypes 1 and 2.12 Genotype 3 isolates have also been reported from human cases in several European countries (including the United Kingdom, France, the Netherlands, and Spain), Japan, and South America. Genotype 4 consists of isolates from some parts of China, Taiwan, Japan, and Vietnam. All HEV genotypes belong to a single serotype because they share at least one major serologically cross-reactive epitope.
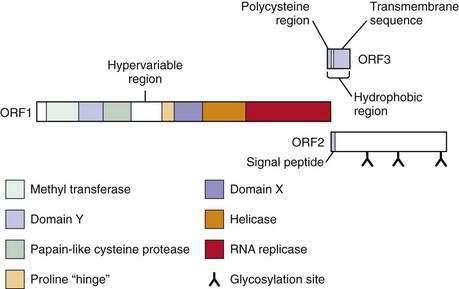
Figure 80-1. The genome of hepatitis E virus. The three open reading frames—ORF1, ORF2, ORF3—are shown.
Table 80-1 Hepatitis E Virus Genotypes and Their Geographic Distribution
| GENOTYPE | HUMAN CASES | ANIMALS |
|---|---|---|
| 1 |
Adapted from Schlauder GG, Frider B, Sookoian S, et al. Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis 2000; 182:294-7.
Swine HEV was identified in pigs in the midwestern United States13; the virus naturally infects pigs and induces transient viremia and antibodies that react with human HEV strains. A comparison of swine HEV with the Burmese and Mexican isolates of human HEV showed approximately 90% to 92% and 79% to 83% identity at the amino acid level in the ORF2 and ORF3 regions, respectively. HEV-like genomic sequences have been isolated from pigs in several parts of the world and occasionally from other animals, including deer and wild boar. In geographical regions where genotype 3 and 4 HEV has been reported among humans, swine isolates of HEV have also belonged to these genotypes. Also, swine HEV strains in Taiwan and Spain have greater genetic similarity to human HEV strains from these regions than to swine and human HEV isolates from other parts of the world, suggesting the possibility of zoonotic transmission. By contrast, swine HEV identified in India is genetically different from the HEV in patients from the same geographic region.14,15 An HEV-like virus has been recovered from chickens and its genome partially sequenced; it appears to be noninfectious to mammals.16
EPIDEMIOLOGY
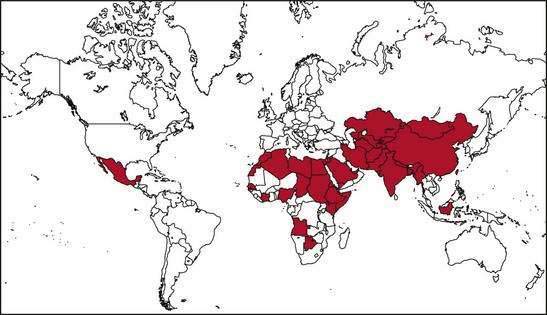
Several epidemics of hepatitis E affecting several hundred to several thousand persons have occurred on the Indian subcontinent and in southeast and central Asia, where this infection is endemic.1,2,17–19 Outbreaks of hepatitis E also have been reported from the northern and western parts of Africa and the Middle East. Two small outbreaks were reported in Mexico in 1986 and 1987 (Fig. 80-2). Overall attack rates range from 1% to 15% and are higher for adults (3% to 30%) than for children (0.2% to 10%). The male-to-female ratio among cases has ranged from 1:1 to 4:1, but the outbreaks have been characterized by particularly high attack and mortality rates among pregnant women. The epidemics vary in nature, ranging from single-peaked, short-lived outbreaks to prolonged, multipeaked epidemics lasting more than a year (Table 80-2). In endemic areas, hepatitis E accounts for up to 50% to 70% of cases of sporadic acute hepatitis; these cases are demographically and clinically similar to those observed during disease outbreaks.
Table 80-2 Epidemiologic Features of Hepatitis E
In nonendemic regions, hepatitis E is related mostly to travel to HEV-endemic regions and accounts for fewer than 1% of cases of acute viral hepatitis. Solitary cases or small series of cases with indigenously-acquired cases of acute hepatitis E have been reported from several countries in Europe, North America, and Australia. A report from the United Kingdom described 40 patients with hepatitis E and no history of travel to an area endemic for this disease. HEV infection was thought to be acquired locally, and the patients were described as a group of “autochthonous” hepatitis E cases.20 Although most patients found to have HEV infection in this series had jaundice, a few had anicteric illness with nonspecific symptoms or asymptomatic aminotransferase elevations. The number of cases peaked in spring and summer, and disease appeared to be more common in residents of coastal and estuarine areas. The disease showed seasonal variations with peaks in spring and summer and no cases occurring during November and December. Case series with similar characteristics have been described from southwest France and the Netherlands.21,22 Table 80-3 highlights differences in the epidemiologic and clinical features of HEV genotypes 1 and 3.
Table 80-3 Comparison of Epidemiologic and Clinical Features Associated with HEV Genotypes 1 and 3
| HEV GENOTYPE 1 | HEV GENOTYPE 3 | |
|---|---|---|
| Epidemiologic patterns | Large epidemics, small outbreaks, and sporadic cases | Only sporadic cases |
| Animal-to-human transmission | Not reported | Demonstrated; a likely mode of transmission |
| Water-borne transmission | Well known to occur; most common route | Unknown |
| Animal reservoir | No | Yes |
| Age group | Young men most commonly affected | Usually elderly |
| Chronic infection | Not known to occur | Reported in transplant recipients receiving immunosuppressive drugs |
| Severity | Variable severity, including fulminant hepatic failure | Severity and poor outcome are related to comorbid conditions |
ROUTES OF TRANSMISSION
HEV infection is transmitted predominantly through the fecal-oral route. Most reported outbreaks have been related to consumption of fecally contaminated drinking water (see Table 80-2). The outbreaks frequently follow heavy rains and floods, but some epidemics occur during the hot summer months, when decreased flow in rivers may increase the risk of water contamination. Person-to-person transmission of hepatitis E seems to be uncommon during both epidemic and sporadic settings,23,24 and secondary attack rates among household contacts are only 0.7% to 2.2%. Therefore, recurrent epidemics in endemic regions are probably related to nearly continuous fecal contamination of water. This conclusion is supported by the demonstration of HEV RNA in waste water, sewage, and drinking water in endemic regions25 and of viable HEV (infectious to primates) in occasional sewage specimens in nonendemic regions.26
Mechanisms postulated to contribute to contamination of water sources by HEV and maintenance of the virus in endemic areas include the occurrence of subclinical HEV infection, animal reservoirs that harbor HEV, and prolonged fecal shedding of the virus by humans. Although data on subclinical HEV infection in humans in endemic regions are scant, viral excretion has been demonstrated in an experimental macaque model of subclinical HEV infection.27 Proven incidents of animal-to-human transmission of HEV have been shown to occur in Japan, where hepatitis E developed after ingestion of uncooked deer meat; the viral genomic sequences obtained from these cases were similar to those retrieved from left-over meat.28 Genomic sequences from human cases of hepatitis E in the United Kingdom have resembled those from swine isolates of HEV, both belonging to genotype 3. HEV genomic sequences were isolated from pig livers sold in grocery stores in Japan and in the United States and were closely related to those from human cases of hepatitis E in those countries.29,30 Zoonotic transmission of HEV infection as a critical factor in HEV transmission, however, is not compatible with the rarity of clinically overt human HEV infection in nonendemic areas despite the high prevalence of antibody to HEV (anti-HEV) among animals (domestic swine, wild rats and mice, cattle, dogs). Moreover, genetic differences between human and animal HEV isolates in India14,15 and failure of epidemic strains of HEV to induce experimental infection in pigs cast doubts on the epidemiologic significance of the zoonotic route of HEV transmission in endemic regions.
Vertical transmission from pregnant mothers to newborn babies is well documented.31 One study showed evidence of transmission of HEV by blood transfusion,32 but further data are needed to determine the frequency of transmission by this mode.
SEROEPIDEMIOLOGY
Prevalence rates of anti-HEV, which is detectable in all geographic areas, are higher among populations in endemic areas (10% to 40%) than among those in nonendemic areas (1% to 5%). Studies from nonendemic areas, however, have reported seroprevalence rates among healthy people of approximately 20% in the United States33 and 16% in the United Kingdom.20 Whether anti-HEV seroreactivity in nonendemic areas reflects subclinical HEV infection, serologic cross-reactivity with other agents, exposure to animal reservoirs of HEV-like viruses, or false-positive serologic results is unclear.
In endemic areas such as India, where antibody to hepatitis A virus is nearly universally detectable by adolescence, anti-HEV is uncommon in children, and the seroprevalence rate increases during young adulthood to reach about 40% in adults.34 These rates of HEV seropositivity appear to be relatively lower than those expected from the frequency of clinical disease and outbreaks. By contrast, in Egypt, anti-HEV is common in children and the seroprevalence rate of anti-HEV reaches 70% or more in young adults,35 although epidemics of disease are not known. These distinctive seroprevalence patterns may be related to infection with an attenuated or animal strain of HEV or differences in environmental conditions.
PATHOGENESIS
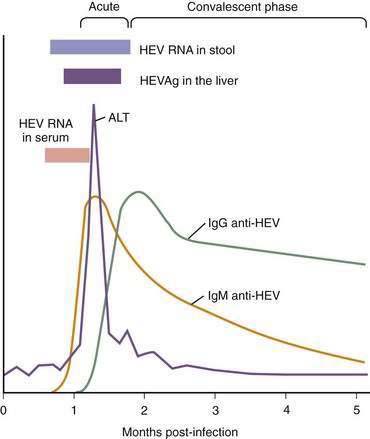
Understanding of virologic, serologic, and pathologic events in the pathogenesis of HEV infection (Fig. 80-3) is based on data from human patients and experimentally infected primates (cynomolgus macaques, rhesus monkeys, and chimpanzees). The virus enters the host primarily through the oral route, although the mechanism(s) by which the virus reaches the liver is unknown. In human volunteers, the incubation period after oral exposure is four to five weeks, and HEV is detected in the stool approximately one week before the onset of illness and for up to two weeks thereafter. In clinical cases, the fecal shedding of the virus is observed until about four weeks after the onset of illness. HEV RNA can be detected in serum for two weeks after the onset of illness in virtually all patients. In experimentally infected primates, HEV RNA has been found in serum, bile, and feces a few days before elevation of the serum alanine aminotransferase (ALT) level. HEV antigen (HEVAg) is expressed in hepatocytes as early as seven days after infection and may be identified in more than 50% of the cells, but the number of HEVAg-positive cells decreases sharply when serum ALT levels increase markedly.36 The onset of ALT elevation in the serum and of histopathologic changes in the liver generally corresponds with the appearance of anti-HEV in serum.
Patients with acute hepatitis E show cellular immune responses to HEV proteins37–39 but the number of cytokine-producing HEV-reactive CD8+ cells is not increased.40 Helper T cell type 1/type 2 (Th1/Th2) cytokine balance is biased toward Th2 in pregnant women,41 in whom hepatitis E is particularly severe. Although the temporal concordance of the HEV-specific immune response with the onset of liver pathology suggests that liver injury is immune mediated, further studies are needed to define the role of the immune response in the induction of liver injury; no evidence for cytopathic properties of HEV itself exists.
Histopathologic features of hepatitis E are similar to those of other forms of acute hepatitis and include ballooned hepatocytes, acidophilic bodies, focal parenchymal necrosis, and inflammatory infiltrates in the lobules and enlarged portal tracts. Nearly one half of patients with hepatitis E have cholestatic hepatitis, characterized by canalicular bile stasis and gland-like transformation of parenchymal cells, with less marked hepatocytic degeneration and necrosis.42 Submassive or massive necrosis and collapse of liver parenchyma are seen in patients with severe liver injury. Chronic HEV viremia with genotype 3 virus has been reported in some renal and liver transplant recipients in Europe, with elevated aminotransferase levels and no evidence of infection with other hepatotropic viruses.43 Viremia and liver enzyme elevation appeared nearly at the same time, and liver biopsies showed evidence of liver injury. A case report has suggested that such infection can lead to chronic hepatitis and cirrhosis.44
CLINICAL FEATURES
The most common recognizable form of HEV infection is acute icteric hepatitis. The clinical manifestations (Table 80-4) are similar to those observed in patients with acute hepatitis A or B.45 The illness usually is insidious in onset. An initial prodromal phase lasts one to four days and is characterized by a varying combination of flu-like symptoms, fever, mild chills, abdominal pain, anorexia, nausea, aversion to smoking, vomiting, clay-colored stools, dark urine, diarrhea, arthralgias, asthenia, and a transient macular skin rash. Most prodromal symptoms tend to diminish with the onset of jaundice (icteric phase), but dark urine, light stool color, and itching persist for a varying amount of time. Physical examination reveals jaundice and a mildly enlarged, soft, and slightly tender liver. Some patients have splenomegaly. Laboratory test abnormalities include bilirubinuria, conjugated hyperbilirubinemia, and marked elevations in serum aminotransferase and gamma glutamyl transpeptidase levels. Elevation of the serum ALT level may precede symptoms, and the magnitude of elevation does not correlate with the severity of liver injury. Mild leukopenia and relative lymphocytosis may occur. As the illness subsides, serum aminotransferase levels return to normal, followed by a decline in the serum bilirubin, which usually returns to normal levels by six weeks. Ultrasonography may demonstrate normal findings or show a mildly enlarged liver, increase in hepatic parenchymal echogenicity, gallbladder wall edema, prominence of portal venules, and a slightly enlarged spleen. The main use of ultrasonography is to exclude biliary obstruction as the cause of jaundice.
Table 80-4 Clinical Features of Hepatitis E
Acute hepatitis E usually is self-limited. A few patients have a prolonged course with marked cholestasis (cholestatic hepatitis), including persistent jaundice lasting 2 to 6 months, prominent itching, and marked elevation of the serum alkaline phosphatase level, ultimately with spontaneous resolution. Reported case-fatality rates have ranged from 0.5% to 4%; however, these rates seem to be overestimated because they are based on data from hospitals. Population surveys during outbreaks have reported lower mortality rates of 0.07% to 0.6%.17
Pregnant women, particularly those in the second or third trimester, are affected more frequently during hepatitis E outbreaks than are others in the population and have a worse outcome, with mortality rates of 5% to 25%. In an epidemic in Kashmir, India, clinical hepatitis E developed in 17.3% of pregnant women, compared with 2.1% of nonpregnant women and 2.8% of men, all ages 15 to 45 years.46 Among the pregnant women, attack rates during the first, second, and third trimesters were 8.8%, 19.4%, and 18.6%, respectively. Fulminant hepatic failure developed in approximately 22% of the affected pregnant women, with an increased frequency of abortions, stillbirths, and neonatal deaths. The pathogenic elements that lead to severe liver damage during pregnancy, especially during the last trimester, remain unknown.
DIAGNOSIS
The diagnosis of human HEV infection is based on detection of either HEV RNA in stool and serum specimens with use of a reverse transcription–polymerase chain reaction assay or demonstration of a virus-specific host immune response (see Fig. 80-3). Tests to detect HEV RNA are available only in research laboratories. Enzyme immunoassays (EIAs) for the detection of immunoglobulin M (IgM) and IgG antibodies to HEV have been developed using recombinant HEV antigens expressed in Escherichia coli or insect cells, synthetic peptides corresponding to immunogenic epitopes of HEV, and protein expressed from a synthetic gene encoding multiple linear antigenic epitopes from the ORF2 and ORF3 regions.47–49 The presence in serum of IgM anti-HEV indicates acute infection, whereas detection of IgG anti-HEV may indicate the convalescent phase or past infection. IgM anti-HEV appears in the early phase of clinical illness, lasts 4 to 5 months, and can be detected in 80% to 100% of cases during outbreaks of acute hepatitis E. IgG anti-HEV appears in serum a few days after IgM anti-HEV, and the titer of IgG anti-HEV increases during the convalescent phase and remains high for at least one to four and one half years; the exact duration of persistence of IgG anti-HEV in serum is not known. Although several commercial kits for the detection of IgM and IgG anti-HEV are available in various countries, none is currently licensed for clinical use in the United States. Use of different target antigens from different HEV strains and of different expression systems to produce recombinant proteins makes comparison of different tests problematic; the sensitivity of various assays has ranged from 17% to 100% when such assays are applied to a panel of coded specimens from nonendemic areas.50
TREATMENT AND PREVENTION
Acute hepatitis E usually is self-limited and requires only supportive care and no specific intervention. Isolation of infected persons is not indicated, because person-to-person transmission is uncommon. Patients with fulminant hepatitis need measures to control cerebral edema and consideration of liver transplantation (see Chapter 93). In pregnant women, no proven benefit to terminating the pregnancy has been documented; postpartum hemorrhage resulting from deranged coagulation requires treatment with fresh-frozen plasma.
Cloning of the HEV genome and subsequent availability of recombinant proteins have led to the development of candidate vaccines. Immunization with recombinant HEV capsid protein in HEV-susceptible primates has shown protection against hepatitis and viremia, although viral excretion is not prevented.51 An experimental HEV DNA vaccine has also been shown to induce serum anti-HEV that is protective against challenge with a heterologous HEV strain in cynomolgus macaques.52
A vaccine using a recombinant truncated form of HEV capsid protein, produced in Spodoptera frugiperda cells infected with a recombinant baculovirus and adsorbed on aluminium hydroxide, has undergone a phase 2, double-blind, randomized, placebo-controlled safety and efficacy trial among human volunteers in Nepal.53 In this trial, nearly 2000 young adults, almost all (>99%) male, were randomly assigned to receive three doses of either the recombinant HEV protein or a matched placebo (at 0, 1, and 6 months). The study subjects underwent active surveillance for clinical acute hepatitis E for a period extending over two years. The vaccine showed 95.5% protective efficacy against hepatitis E disease among those who received three doses and 88.5% efficacy in an intention-to-treat analysis among those who had received only the first dose. A high IgG anti-HEV level was observed in all the vaccinated volunteers at one month after the third vaccine dose, but only 56% of them had this high an antibody titer by the end of the study. More studies are needed to determine the efficacy of the vaccine among pregnant women, the duration of its protective efficacy, its effect on subclinical HEV infection and fecal shedding of the virus, and thus its impact on transmission of the virus. The vaccine may be useful for travelers to HEV-endemic regions and for pregnant women and persons with chronic liver disease who reside in such regions. Finally, cost considerations will determine whether this new and promising vaccine can be used in populations living in developing countries where hepatitis E is endemic and vaccination is likely to be the most beneficial.
Aggarwal R, Krawczynski K. Hepatitis E: An overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9-20. (Ref 17.)
Berke T, Matson DO. Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Arch Virol. 2000;145:1421-36. (Ref 9.)
Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784-90. (Ref 20.)
Fix AD, Abdel-Hamid M, Purcell RH, et al. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519-23. (Ref 35.)
Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-17. (Ref 43.)
Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61-9. (Ref 32.)
Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860-5. (Ref 13.)
Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607-15. (Ref 51.)
Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335-9. (Ref 5.)
Shata MT, Barrett A, Shire NJ, et al. Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. 2007;328:152-61. (Ref 38.)
Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895-903. (Ref 53.)
Srivastava R, Aggarwal R, Jameel S, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56-65. (Ref 40.)
Tsarev SA, Tsareva TS, Emerson SU, et al. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: Identification of HEV infection in primates. J Infect Dis. 1993;168:369-78. (Ref 49.)
Vishwanathan R. Infectious hepatitis in Delhi (1955-56): A critical study: Epidemiology. Indian J Med Res. 1957;45(Suppl 1):1-29. (Ref 1.)
1. Vishwanathan R. Infectious hepatitis in Delhi (1955-56): A critical study: Epidemiology. Indian J Med Res. 1957;45(Suppl 1):1-29.
2. Khuroo MS. Study of an epidemic of non-A, non-B hepatitis: Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-23.
3. Wong DC, Purcell RH, Sreenivasan MA, et al. Epidemic and endemic hepatitis in India: Evidence for a non-A, non-B hepatitis etiology. Lancet. 1980;2:876-9.
4. Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23-31.
5. Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335-9.
6. Tsarev SA, Emerson SU, Reyes GR, et al. Characterization of a prototype strain of hepatitis E virus. Proc Natl Acad Sci U S A. 1992;89:559-63.
7. Yamashita T, Mori Y, Miyazaki N, et al. Biological and immunological characteristics of hepatitis E-like particles based on the crystal structure. Proc Natl Acad Sci U S A. 2009;106:12986-91.
8. Guu TS, Liu Z, Ye Q, et al. Structure of the hepatitis E virus-like particle suggests mechanism for virus assembly and receptor binding. Proc Natl Acad Sci U S A. 2009;106:12992-7.
9. Berke T, Matson DO. Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Arch Virol. 2000;145:1421-36.
10. Graff J, Torian U, Nguyen H, et al. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J Virol. 2006;80:5919-26.
11. Schlauder GG, Frider B, Sookoian S, et al. Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis. 2000;182:294-7.
12. Erker JC, Desai SM, Schlauder GG, et al. A hepatitis E virus variant from the United States: Molecular characterization and transmission in cynomolgus macaques. J Gen Virol. 1999;80:81-90.
13. Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860-5.
14. Arankalle VA, Chobe LP, Joshi MV, et al. Human and swine hepatitis E viruses from Western India belong to different genotypes. J Hepatol. 2002;36:417-25.
15. Shukla P, Chauhan UK, Naik S, et al. Hepatitis E virus infection among animals in northern India: An unlikely source of human disease. J Viral Hepat. 2007;14:310-17.
16. Haqshenas G, Shivaprasad HL, Woolcock PR, et al. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol. 2001;82:2449-62.
17. Aggarwal R, Krawczynski K. Hepatitis E: An overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9-20.
18. Naik SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull WHO. 1992;70:597-604.
19. Zhuang H. Hepatitis E and strategies for its control. In: Wen YM, Xu ZY, Melnick JL, editors. Viral Hepatitis in China: Problems and Control Strategies. Basel: Karger; 1992:126-39.
20. Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784-90.
21. Peron JM, Mansuy JM, Poirson H, et al. Hepatitis E is an autochthonous disease in industrialized countries. Analysis of 23 patients in South-West France over a 13-month period and comparison with hepatitis A. Gastroenterol Clin Biol. 2006;30:757-62.
22. Waar K, Herremans MM, Vennema H, et al. Hepatitis E is a cause of unexplained hepatitis in the Netherlands. J Clin Virol. 2005;33:145-9.
23. Aggarwal R, Naik SR. Hepatitis E: Intrafamilial transmission versus waterborne spread. J Hepatol. 1994;21:718-23.
24. Somani SK, Aggarwal R, Naik SR, et al. A serological study of intrafamilial spread from patients with sporadic hepatitis E virus infection. J Viral Hepat. 2003;10:446-9.
25. Jothikumar N, Aparna K, Kamatchiammal S, et al. Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:2558-62.
26. Pina S, Jofre J, Emerson SU, et al. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol. 1998;64:4485-8.
27. Aggarwal R, Kamili S, Spelbring J, et al. Experimental studies on subclinical hepatitis E virus infection in cynomolgus macaques. J Infect Dis. 2001;184:1380-5.
28. Tei S, Kitajima N, Takahashi K, et al. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371-3.
29. Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351-17.
30. Feagins AR, Opriessnig T, Guenette DK, et al. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88:912-17.
31. Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025-6.
32. Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61-9.
33. Thomas DL, Yarbough PO, Vlahov D, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244-7.
34. Arankalle VA, Tsarev SA, Chadha MS, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447-50.
35. Fix AD, Abdel-Hamid M, Purcell RH, et al. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519-23.
36. Krawczynski K, Bradley DW. Enterically transmitted non-A, non-B hepatitis: Identification of virus-associated antigen in experimentally infected cynomolgus macaques. J Infect Dis. 1989;159:1042-9.
37. Naik S, Aggarwal R, Naik SR, et al. Evidence for activation of cellular immune responses in patients with acute hepatitis E. Indian J Gastroenterol. 2002;21:149-52.
38. Shata MT, Barrett A, Shire NJ, et al. Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. 2007;328:152-61.
39. Aggarwal R, Shukla R, Jameel S, et al. T-cell epitope mapping of ORF2 and ORF3 proteins of human hepatitis E virus. J Viral Hepat. 2007;14:283-92.
40. Srivastava R, Aggarwal R, Jameel S, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56-65.
41. Pal R, Aggarwal R, Naik SR, et al. Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol. 2005;20:1094-101.
42. Gupta DN, Smetana HF. The histopathology of viral hepatitis as seen in the Delhi epidemic (1955-56). Indian J Med Res. 1957;45(Suppl):101-13.
43. Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-17.
44. Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859-60.
45. Vishwanathan R, Sidhu AS. Infectious hepatitis: Clinical findings. Indian J Med Res. 1957;45(Suppl):49-58.
46. Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-5.
47. Dawson GJ, Chau KH, Cabal CM, et al. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J Virol Methods. 1992;38:175-86.
48. Favorov MO, Khudyakov YE, Mast EE, et al. IgM and IgG antibodies to hepatitis E virus (HEV) detected by an enzyme immunoassay based on an HEV-specific artificial recombinant mosaic protein. J Med Virol. 1996;50:50-8.
49. Tsarev SA, Tsareva TS, Emerson SU, et al. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: Identification of HEV infection in primates. J Infect Dis. 1993;168:369-78.
50. Mast EE, Alter MJ, Holland PV, et al. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27:857-61.
51. Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607-15.
52. Kamili S, Spelbring J, Carson D, et al. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258-64.
53. Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895-903.