2
Hepatic, biliary and pancreatic anatomy
The aim of this chapter is to provide the basic anatomical foundation for performing liver, biliary and pancreatic surgery. Surgically unimportant anatomical features are omitted, but anatomical distortions due to pathological processes are included. A key point of hepato-pancreato-biliary (HPB) surgical anatomy is that whilst there is a prevailing pattern of anatomy, i.e. a pattern that is most commonly found, variations from the prevailing pattern termed anomalies are frequent. Every surgical operation in this area should be conducted with this fact in mind.
Liver
Overview of hepatic anatomy and terminology
Modern hepatic anatomy is concerned mainly with internal vascular and biliary structures rather than surface markings. Ramifications of the hepatic artery and bile ducts are regular and virtually identical. The portal vein on the left side of the liver is a vessel with unusual morphology, consequent to its need to perform different functions in the foetus and in the postnatal period. Consequently, the Brisbane 2000 Terminology of Hepatic Anatomy and Resections of the International Hepato-Pancreato-Biliary Association used in this chapter is primarily based on hepatic artery and bile duct ramifications.1
Divisions of the liver based on the hepatic artery
The primary (first-order) division of the proper hepatic artery is into the right and left hepatic arteries (Fig. 2.1). These branches supply arterial inflow to the right and left hemilivers or livers (Fig. 2.2). The plane between the two distinct zones of vascular supply is called a watershed. The border or watershed of the first-order division is called the midplane of the liver. It intersects the gallbladder fossa and the fossa for the inferior vena cava (IVC) (Fig. 2.2). The right liver usually has a larger volume than the left liver (60:40), although this is variable.
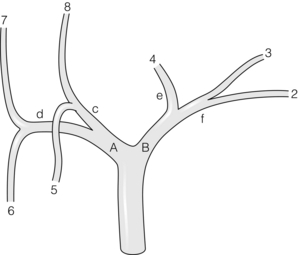
Figure 2.1 Ramification of the hepatic artery in the liver. The prevailing pattern is shown. The first-order division of the proper hepatic artery is into the right (a) and left (b) hepatic arteries, which supply right and left hemilivers (Fig. 2.2), respectively. The second-order division of the hepatic arteries, supplies the four sections (Fig. 2.3). The third-order division, shown in orange, supplies the segments (Fig. 2.4). Since the left medial section and segment 4 are the same, the artery is shown as being both sectional and segmental (red/orange). The caudate lobe is supplied by branches from (a) and (b). Bile duct anatomy and nomenclature is similar to that of the hepatic artery. © Washington University in St Louis.
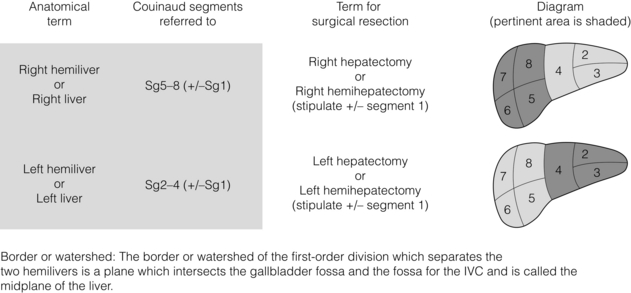
Figure 2.2 Nomenclature for first-order division anatomy (hemilivers or livers) and resections. © Washington University in St Louis.
The second-order divisions (Figs 2.1 and 2.3) of the hepatic artery supply four distinct zones of the liver. Each is referred to as a section. The right liver is divided into two sections, the right anterior section and the right posterior section. These sections are supplied by the right anterior sectional hepatic artery and the right posterior sectional hepatic artery (Fig. 2.1). The plane between these sections is the right intersectional plane. The right intersectional plane does not have any surface markings to indicate its position. The left liver is also divided into two sections, the left medial section and the left lateral section (Fig. 2.3), which are supplied by the left medial sectional hepatic artery and the left lateral sectional hepatic artery (Fig. 2.1). The plane between these sections is referred to as the left intersectional plane. It does have surface markings indicating its position – the umbilical fissure and the line of attachment of the falciform ligament to the anterior surface of the liver.
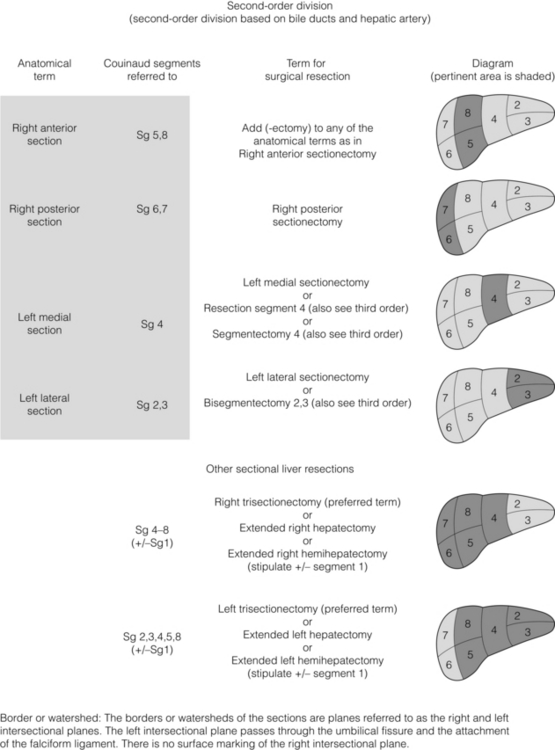
Figure 2.3 Nomenclature for second-order division anatomy (sections) and resections including extended resections. © Washington University in St Louis.
The third-order divisions of the hepatic artery divide the right and left hemilivers into segments (Sg) 2–8 (Figs 2.1 and 2.4). Each of the segments has its own feeding segmental artery. The left lateral section is divided into Sg2 and Sg3. The pattern or ramification of vessels within the left medial section does not permit subdivision of this section into segments, each with its own arterial blood supply. Therefore the left medial section and Sg4 are synonymous. However, Sg4 is arbitrarily divided into superior (4a) and inferior (4b) parts without an exact anatomical plane of separation based on internal ramification of vessels. The right anterior section is divided into two segments, Sg5 and Sg8. The right posterior section is divided into Sg6 and Sg7. The planes between segments are referred to as intersegmental planes. The ramifications of the bile ducts are identical to that described for the arteries, as are the zones of the liver drained by the respective ducts.
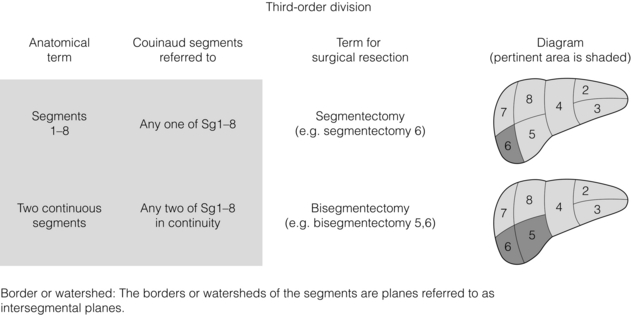
Figure 2.4 Nomenclature for third-order division anatomy (segments) and resections. © Washington University in St Louis.
Segment 1 (caudate lobe) is a distinct portion of the liver, separate from the right and left hemilivers (Fig. 2.5). It is appropriately referred to as a lobe since it is demarcated by visible fissures. It consists of three parts: the bulbous left part (Spiegelian lobe), which grips the left side of the vena cava and is readily visible through the lesser omentum; the paracaval portion, which lies anterior to the vena cava; and the caudate process, on the right. The caudate process merges indistinctly with the right hemiliver. The caudate lobe is situated posterior to the hilum and the portal veins. Lying anterior and superior to the paracaval portion are the hepatic veins, which limit the upper extent of the caudate lobe2,3 (Fig. 2.5). The caudate receives vascular supply from both right and left hepatic arteries (and portal veins). Caudate bile ducts drain into both right and left hepatic ducts.3 The caudate lobe is drained by several short caudate veins that enter the IVC directly from the caudate lobe. Their number and size are variable. Occasionally caudate veins are quite short and wide, and therefore must be isolated and divided cautiously. Commonly, these veins enter the IVC on either side of the midplane of the vessel, an anatomical feature that normally allows the creation of a tunnel behind the liver on the surface of the IVC without encountering the caudate veins. The ‘hanging manoeuvre’ is performed by lifting up on a tape placed through this tunnel (see below).
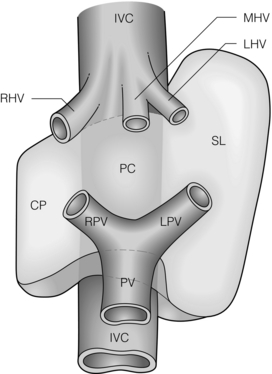
Figure 2.5 Schematic representation of the anatomy of the caudate lobe. The caudate lobe consists of three parts: the caudate process (CP), on the right, the paracaval portion anterior to the vena cava (PC) and the bulbous left part (Spiegelian lobe, SL). IVC, inferior vena cava; PV, portal vein; RHV, MHV, LHV, right hepatic, middle hepatic and left hepatic veins, respectively. © Washington University in St Louis.
Resectional terminology
The terminology of hepatic resections is based upon the terminology of hepatic anatomy. Resection of one side of the liver is called a hepatectomy or hemihepatectomy (Fig. 2.2). Resection of the right side of the liver is a right hepatectomy or hemihepatectomy and resection of the left side of the liver is a left hemihepatectomy or hepatectomy. Resection of a liver section is referred to as a sectionectomy (Fig. 2.3). Resection of the liver to the left side of the umbilical fissure is a left lateral sectionectomy. The other sectionectomies are named accordingly, e.g. right anterior sectionectomy. Resection of the right hemiliver plus Sg4 is referred to as a right trisectionectomy (Fig. 2.3). Similarly, resection of the left hemiliver plus the right anterior section is referred to as a left trisectionectomy.
Resection of one of the numbered segments is referred to as a segmentectomy (Fig. 2.4). Resection of the caudate lobe can be referred to as a caudate lobectomy or resection of Sg1. It is always appropriate to refer to a resection by the numbered segments. For instance, it would be appropriate to call a left lateral sectionectomy a resection of Sg2 and Sg3.
Surgical anatomy for liver resections
Hepatic arteries and liver resections
In the prevailing anatomical pattern, the coeliac artery terminates to divide into splenic and common hepatic arteries. Rarely, the hepatic artery arises directly from the aorta. The common hepatic artery runs for 2–3 cm anteriorly and to the right to ramify into gastroduodenal and proper hepatic arteries. The proper hepatic artery enters the hepatoduodenal ligament and normally runs for 2–3 cm along the left side of the common bile duct and terminates by dividing into the right and left hepatic arteries, the right immediately passing behind the common hepatic duct. The terms “common” and “proper” in respect to hepatic arteries while correct are not intuitive and the arteries are sometimes confused in the literature. The four sectional arteries arise from the right and left arteries 1–2 cm from the liver. While this is the commonest pattern, variations from this pattern are also very common (Fig. 2.6). The surgeon is wise not to make assumptions regarding hepatic arteries based on size or position, but rely instead on complete dissection, trial occlusions and radiological support. When an artery appears unusually large it is especially important to dissect until identification is unquestionable.
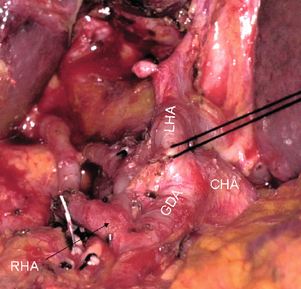
Figure 2.6 A most dangerous arterial anatomy. The right hepatic artery (RHA) arises from the gastroduodenal artery (GDA). There is no proper hepatic artery. The left hepatic artery (LHA) could easily be mistaken for the proper hepatic artery. Ligation of the GDA would lead to arterial devascularisation of the right liver. © Washington University in St Louis.
‘Replaced’ arteries are surgically important anomalies. ‘Replaced’ means that the artery supplying a particular volume of liver is in an unusual location and also that it is the sole supply to that volume of liver. ‘Aberrant’ means the structure is in an unusual location. While the definition of ‘aberrant’ does not state whether the structure provides sole supply, it is usually considered to be synonymous with ‘replaced’ in respect to these arteries. ‘Accessory’ refers to an artery that is additional, i.e. is present in addition to the normal structure and as a result is not the sole supply to a volume. Consequently, ligation of an accessory artery does not result in ischaemia.
In about 25% of patients, part or all of the liver is supplied by a replaced (or aberrant) artery. The replaced right hepatic artery arises from the superior mesenteric artery. It runs from left to right behind the lower end of the common bile duct to emerge and course on its right posterior border. It may supply a segment, section or the entire right hemiliver. Rarely, this artery supplies the entire liver and then it is called a replaced hepatic artery. The replaced left hepatic artery arises from the left gastric artery and courses in the lesser omentum in conjunction with vagal branches to the liver (hepatic nerve). As with the right artery it may supply a segment, section (usually the left lateral section), hemiliver or very rarely the whole liver. Sometimes left hepatic arteries arising from the left gastric artery are actually accessory rather than replaced and exist in conjunction with normally situated left hepatic arteries. Knowledge of these particular arterial variations is of importance not only in hepatobiliary surgery, including transplantation, but also in gastric surgery and pancreatic surgery. Transection of the left gastric artery at its origin during gastrectomy may cause ischaemic necrosis of the left hemiliver if a replaced left artery is present. The same may occur on the right side as a result of injury to a replaced right artery. Also, these vessels need to be preserved and perfused during donor hepatectomy. Sometimes there is no proper hepatic artery because the entire liver is supplied by right or left replaced arteries or both. This anomaly may be suspected when, on opening the peritoneum at the base of the right side of the hepatoduodenal ligament, the portal vein is immediately apparent instead of the hepatic artery.
Replaced arteries may confer an advantage during surgery. For instance, when a replaced left artery supplies the left lateral section it is possible to resect the entire proper hepatic artery when performing a right trisectionectomy for hilar cholangicarcinoma. The replaced right artery is sometimes invaded by pancreatic head tumours and is in danger of injury during pancreato-duodenectomy. This is only a brief description of replaced arteries. There are many variations of replaced arteries, especially on the right, depending on the relationships of the artery to the pancreatic head and neck, the bile duct and the portal vein.4
In performing hepatectomies by the standard technique of isolating individual structures instead of pedicles it is critical to correctly identify the particular artery(ies) supplying the volume of liver to be resected. One important anatomical point is that an artery located to the right side of the bile duct always supplies the right side of the liver, but arteries found on the left side of the bile duct may supply either side of the liver. Therefore, when using the individual vessel ligation method it is important to be aware of the position of the common hepatic duct. A trial occlusion of an artery with an atraumatic clamp should always be performed in order to be sure that there is a good pulse to the side of the liver to be retained.
Bile ducts and liver resections
Prevailing pattern and important variations of bile ducts draining the right hemiliver: Normally only a short portion of the right hepatic duct, about 1 cm, is in an extrahepatic position. The prevailing pattern of bile duct drainage from the right liver is shown in Fig. 2.7a. The segmental ducts from Sg6 and Sg7 (called B6, B7) unite to form the right posterior sectional bile duct and the segmental ducts from Sg5 and Sg8 (B5, B8) unite to form the right anterior sectional bile duct (Fig. 2.7a). The sectional ducts unite to form the right hepatic duct, which unites with the left hepatic duct at the confluence to form the common hepatic duct.
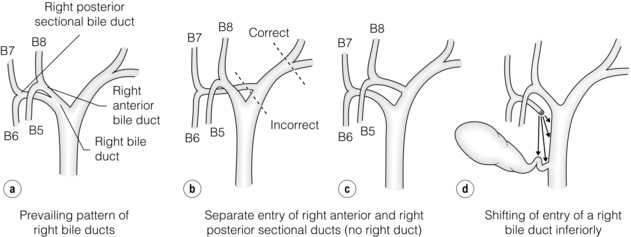
Figure 2.7 Prevailing pattern (a) and important variations (b–d) of bile ducts draining the right hemiliver (see text). © Washington University in St Louis.
There are two important sets of biliary anomalies on the right side of the liver. The first involves insertion of a right sectional duct into the left bile duct. This is a common anomaly. The right posterior sectional duct inserts into the left hepatic duct in 20% of individuals (Fig. 2.7b) and the right anterior bile duct does so in 6% (Fig. 2.7c). In these situations there is no right hepatic duct. A right sectional bile duct inserting into the left hepatic duct is in danger of injury during left hepatectomy if the left duct is divided at its termination. Therefore, when performing left hepatectomy, the left hepatic duct should be divided close to the umbilical fissure to avoid injury to a right sectional duct.
The second important anomaly is insertion of a right bile duct into the biliary tree at a lower level than the prevailing site of confluence. Low union may affect the right hepatic duct, a sectional right duct (usually the anterior one), a segmental duct or a subsegmental duct. A right bile duct unites with the common hepatic duct below the prevailing site of confluence in about 2% of individuals. Sometimes the duct unites with the cystic duct and then with the common hepatic duct. The latter anomaly places the aberrant duct at great risk of injury during laparoscopic cholecystectomy.
Very rarely the right hepatic duct terminates in the gallbladder. This may be congenital or acquired. In the latter case a gallstone has effaced a cystic duct which united with the right hepatic duct, giving the appearance that it joins the gallbladder. An extremely rare anomaly is the absent common hepatic duct. In these cases the right and left hepatic duct enters the gallbladder and the duct emerging from the gallbladder runs downward to join with the duodenum.5 In the presence of these anomalies, which would be extremely difficult to detect, a complete cholecystectomy will result in ductal injury. These ducts should not be confused with ducts of Luschka (see below).
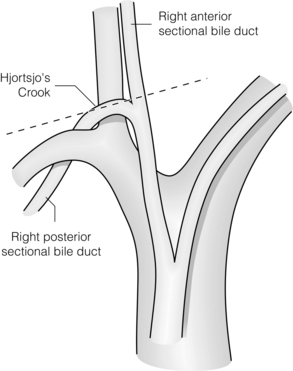
The right posterior sectional duct normally hooks over the origin of the right anterior sectional portal vein (‘Hjortsjo’s crook’),6 where it is in danger of being injured if the right anterior sectional pedicle is clamped too close to its origin (Fig. 2.8).
Prevailing pattern and important variations of bile ducts draining the left hemiliver: The prevailing pattern of bile duct drainage from the left liver is shown in Fig. 2.9a. It is present in only 30% of individuals, i.e. variations (anomalies) are present in the majority of individuals. In the prevailing pattern, the segmental ducts from Sg2 and Sg3 (B2, B3) unite to form the left lateral sectional bile duct. This duct passes behind the umbilical portion of the portal vein and unites with the duct from segment 4 (B4; also called the left medial sectional duct since section and segment are synonymous for this volume of liver). The site of union of these ducts to form the left hepatic duct lies about one-third of the distance between the umbilical fissure and the midplane of the liver. The left hepatic duct continues from this point for 2–3 cm along the base of segment 4 to its confluence with the right hepatic duct. Note that it is in an extrahepatic position and that it has a much longer extrahepatic course than the right bile duct. The extrahepatic position of the left hepatic duct is a key anatomical feature, which makes this section of duct the prime site for high biliary–enteric anastomoses.
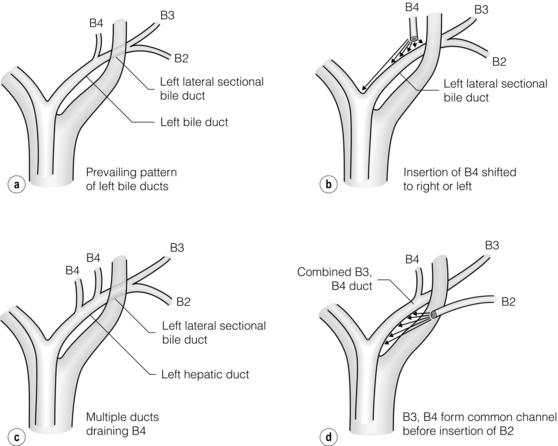
Figure 2.9 Prevailing pattern (a) and important variations (b–d) of bile ducts draining the left hemiliver. © Washington University in St Louis.
The main anomalies of the left ductal system involve variations in site of insertion of B4 (Fig. 2.9b), multiple ducts coming from B4 (Fig. 2.9c) and primary union of B3 and B4 with subsequent union of B2 (Fig. 2.9d). B4 may join the left lateral sectional duct to the left or right of its point of union in the prevailing pattern (Fig. 2.9b); in the former case the insertion of B4 is at the umbilical fissure and in the latter the insertion may occur at any place to the right of the prevailing location up to the point where the left hepatic duct normally unites with the right hepatic duct. In the latter instance, which according to Couinaud is present in 8% of individuals, there is no left hepatic duct. Instead there is a confluence of three ducts, the left lateral sectional duct, B4 and the right hepatic duct to form the common hepatic duct. These variations are important in split liver transplantation and in diagnosis and repair of biliary injuries.
The bile duct to Sg3 has been used to perform biliary bypass and can be isolated by following the superior surface of the ligamentum teres down to isolate the portal pedicle to Sg3. The technique is less commonly used now that internal endoscopic bypass has been developed.
Portal veins and liver resections
On the right side of the liver the portal vein divisions correspond to those of the hepatic artery and bile duct, and they supply the same hepatic volumes. Therefore, there is a right portal vein that supplies the entire right hemiliver (Fig. 2.10). It divides into two sectional and four segmental veins, as do the arteries and bile ducts. On the left side of the liver, however, the left portal vein is quite unusual because of the fact that its structure was adapted to function in utero as a conduit between the umbilical vein and the ductus venosus, whilst postnatally the direction of flow is reversed. The left portal vein consists of a horizontal or transverse portion, which is located under Sg4, and a vertical part or umbilical portion, which is situated in the umbilical fissure (Fig. 2.10). Unlike the right portal vein, neither portion of the left portal vein actually enters the liver, but rather they lie directly on its surface. Often the umbilical portion is hidden by a bridge of tissue passing between left medial and lateral sections. This bridge of liver tissue may be as thick as 2 cm or only be a fibrous band. The junction of the transverse and umbilical portions of the left portal vein is marked by the attachment of a stout cord – the ligamentum venosum. This structure, the remnant of the foetal ductus venosus, runs in the groove between the left lateral section and the caudate lobe and attaches to the left hepatic vein/IVC junction.
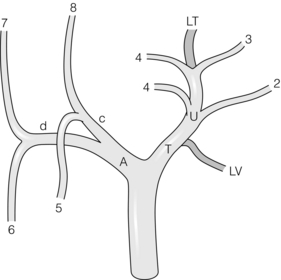
Figure 2.10 Ramification of the portal vein in the liver. The portal vein divides into right (A) and left (T) branches. The branches in the right liver correspond to those of the hepatic artery and bile duct (Fig. 2.1). The branching pattern on the left is unique. The left portal vein has transverse (T) and umbilical portions (U). The transition point between the two parts is marked by the attachment of the ligamentum venosum (LV). All major branches come off the umbilical portion (see text). The vein ends blindly in the ligamentum teres (LT). © Washington University in St Louis.
Ramification of the left portal vein (Figs 2.10 and 2.11): The transverse portion of the left portal vein sends only a few small branches to Sg4. Large branches from the portal vein to the left liver arise exclusively beyond the attachment of the ligamentum venosum, i.e. from the umbilical part of the vein.7 These branches come off both sides of the vein – those arising from the right side pass into Sg4 and those from the left supply Sg2 and Sg3. There is usually only one branch to Sg2 and Sg3, but often there is more than one branch to Sg4. The left portal vein terminates in the ligamentum teres at the free edge of the left liver. Note that the umbilical portion of the portal vein has a unique pattern of ramification. The pattern is similar to an air-conditioning duct that sends branches at right angles from both of its sides to supply rooms (segments), tapering as it does so, finally to end blindly (in the ligamentum teres). Other vascular and biliary structures normally ramify by dividing into two other structures at their termination and not by sending out branches along their length.
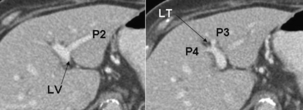
Figure 2.11 Ramification of the left portal vein as seen on CT. Note the branches to segments 2–4 and the ligamentum teres (LT). The arrow points to the ligamentum venosum (LT) and the groove between the left lateral section and the caudate lobe. This is also the site of origin of the ligamentum venosum, where the transverse portion of the left portal vein becomes the umbilical portion of the vein, proving conclusively that the branch to Sg2 is not part of a terminal division of the transverse portion of the vein as might be concluded from case studies (also see Ref. 7). © Washington University in St Louis.
Although the divisions of the portal vein are unusual, for the embryonic reasons described above, it is uncommon to have variations from this pattern. Probably the most common variation is absence of the right portal vein. In these cases the right posterior and right anterior sectional portal veins originate independently from the main portal vein. Under these circumstances the anterior sectional vein is usually quite high in the porta hepatis and may not be obvious. An unsuspecting surgeon may divide the posterior sectional vein thinking that it is the right portal vein and become confused when the anterior sectional vein is subsequently revealed during hepatic transection.
A rare but potentially devastating anomaly is the absent extrahepatic left portal vein (Fig. 2.12). The apparent right vein is really the main portal vein, a structure that enters the liver, gives off branches to the right liver and then loops back within the liver substance to supply the left side. The vein looks like a right vein in terms of position but is larger. Transection results in total portal vein disconnection from the liver. This anomaly should always be searched for on computed tomography (CT) as right hepatectomy is not usually possible when it is present. The presence of the transverse portion of the left vein at the base of Sg4, which then enters the umbilical fissure, precludes the presence of this anomaly.
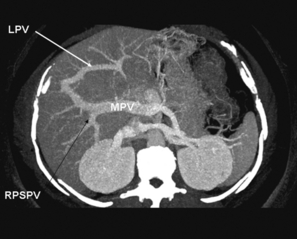
Figure 2.12 Absent extrahepatic left portal vein, a rare and very dangerous anomaly. Three-dimensional reconstruction of CT scan. Note that the main portal vein (MPV) enters the right liver, gives off the right posterior sectional portal vein (RPSPV) and some branches to the right anterior section, then proceeds to the left as an internal left portal vein (LPV). © Washington University in St Louis.
The portal vein branches to Sg4 may be isolated in the umbilical fissure on the right side of the umbilical portion of the left portal vein. The veins here are associated with the bile ducts and the arteries passing to Sg4, i.e. they enter sheaths as they go into the liver substance. Isolation in this location may provide extra margin when resecting a tumour in Sg4 that impinges upon the umbilical fissure. Normally the branches to Sg4 are isolated after dividing the parenchyma of the liver of Sg4 close to the umbilical fissure, an approach that is used to avoid injury to the umbilical portion of the left portal vein. Injury to this vein could, of course, deprive Sg2 and Sg3 of portal vein supply as well as Sg4. For instance, if this occurs when performing a right trisectionectomy, the only portion of the liver to be retained would be devascularised of portal vein flow. However, isolation of these structures within the umbilical fissure does provide an extra margin of clearance on tumours and can be done safely if care is taken to ascertain the position of the portal vein. Likewise, it is possible to isolate the portal vein branches going into Sg2 and Sg3 in the umbilical fissure and to extend a margin when resecting a tumour in the left lateral section. For the same reasons given above, caution must be taken when doing this in order not to injure the umbilical portion of the portal vein. In order to access the portal vein in this location it is usually necessary to divide the bridge of liver tissue, between the left medial and lateral sections. This is done by passing a blunt instrument behind the bridge before dividing it, usually with cautery. Note that arteries and bile ducts passing to the left lateral section are in danger of being injured as one isolates the most posterior–superior portion of the bridge. To facilitate passage of an instrument behind the bridge, the peritoneum at the base of the bridge may be opened in a preliminary step. The instrument being passed behind the bridge should never be forced.
Hepatic veins and liver resection (Fig. 2.13)
There are normally three large hepatic veins. These run in the midplane of the liver (middle hepatic vein), the right intersectional plane (right hepatic vein) and the left intersectional plane (left hepatic vein). The left hepatic vein actually begins in the plane between Sg2 and Sg3 and travels in that plane for most of its length. It becomes quite a large vein even in that location. It leaves the plane between Sg2 and Sg3 and enters the left intersectional plane about 1 cm from where it terminates by uniting with the middle hepatic vein to form a common channel that enters the IVC. It receives the umbilical vein from Sg4 in its short course in the left intersectional plane (Figs 2.13 and 2.14). Note that this is the same plane in which the umbilical portion of the left portal vein lies. It is important not to confuse the ‘umbilical portion of the left portal vein’ with the ‘umbilical vein’. The latter is a tributary of the left hepatic vein that normally drains the most leftward part of Sg4.8,9 The left and middle hepatic veins usually fuse at a distance of about 1–2 cm from the IVC, so that when viewed from within the IVC there are only two hepatic vein openings. Rarely, hepatic veins join the IVC above the diaphragm.
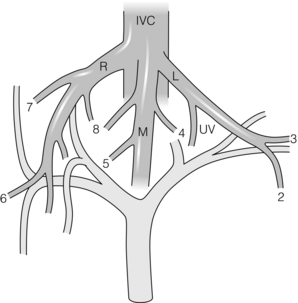
Figure 2.13 Hepatic veins. There are normally three hepatic veins: right (R), middle (M) and left (L). Note the segments drained. UV is the umbilical vein, which normally drains part of Sg4 into the left hepatic vein. The latter is proof that the terminal portion of the left vein lies in the intersectional plane of the left liver. © Washington University in St Louis.
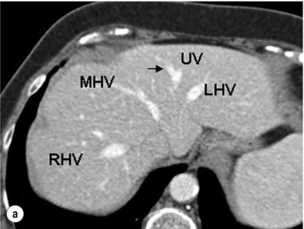
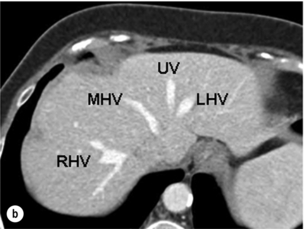
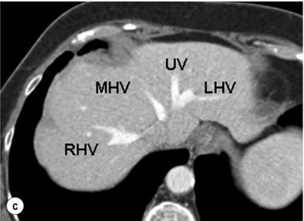
Figure 2.14 Prevailing pattern of the umbilical vein (UV). (a) Umbilical vein forming from tributaries draining both the left lateral section and the left medial section. (b) Umbilical vein coursing in the plane of the umbilical fissure dividing the left lateral and left medial sections. (c) Umbilical vein uniting with the left hepatic vein (LHV), which then runs a short 1-cm course in the plane of the umbilical fissure before joining the IVC. The umbilical vein is normally smaller than in this example. MHV, middle hepatic vein; RHV, right hepatic vein. © Washington University in St Louis.
In about 10% of individuals there is more than one large right hepatic vein. In these people, in addition to the right superior hepatic vein (normally called the right hepatic vein), which enters the IVC just below the level of the diaphragm, there is a right inferior hepatic vein that enters the IVC 5–6 cm below this level. In the presence of this vein, resections of Sg7 and Sg8 may be performed, including resection of the right superior vein, without compromising the venous drainage of Sg5 and Sg6.
The caudate lobe is drained by its own veins – several short veins that enter the IVC directly from the caudate lobe. When performing a classical right hepatectomy, caudate veins are divided in the preliminary stage of the dissection. As dissection moves up the anterior surface of the vena cava to isolate the right hepatic vein, one encounters a bridge of tissue lateral to the IVC referred to as the ‘inferior vena caval ligament’.10 It connects the posterior portion of the right liver to the caudate lobe behind the IVC. This bridge of tissue usually consists of fibrous tissue, but occasionally is a bridge of liver parenchyma. It limits exposure of the right side of the IVC at a point just below the right hepatic vein and must be divided in order to isolate the right vein extrahepatically. This must be done with care as the ligament may contain a large vein and forceful dissection of the ligament may also result in injury to the right lateral side of the IVC. Isolation of the right hepatic vein is also aided by clearing the areolar tissue between the right and middle hepatic veins down to the level of the IVC when exposing these veins from above.
Another approach to right hepatectomy is to leave division of the caudate and right hepatic veins until after the liver is transected. In this case a clamp may be passed up along the anterior surface of the vena from below to emerge between the right and middle hepatic veins. Once an umbilical tape is passed, the liver may be hung to facilitate transection (‘hanging manoeuvre’).11 This is possible since caudate veins usually lie lateral to the midplane of the vena cava, as noted above.
The left and middle veins can also be isolated prior to division of the liver. There are several ways to achieve this anatomically. One method is to divide all the caudate veins as well as the right hepatic vein. This exposes the entire anterior surface of the retrohepatic vena cava and leaves the liver attached to the vena cava only by the middle and left hepatic veins, which are then easily isolated. This is suitable when performing a right hepatectomy or extended right hepatectomy, especially when the caudate lobe is also to be resected. The advantage of having control of these veins during operations on the right liver is that total vascular occlusion is possible without occlusion of the IVC and haemodynamically the effect is not much different from occlusion of the main portal pedicle alone (Pringle manoeuvre).
In performing a left hepatectomy the right hepatic vein is conserved and a different anatomical approach to isolation of the left and middle hepatic veins is required. They may be isolated from the left side by dividing the ligamentum venosum, where it attaches to the left hepatic vein, then dividing the peritoneum at the superior tip of the caudate lobe and gently passing an instrument on the anterior surface of the vena cava to emerge between the middle and right veins and/or between the left and middle veins. Again, care needs to be applied when performing this manoeuvre in order to avoid injury to the structures.
Isolation of the vena cava above and below the hepatic veins is also a technique that should be in the armamentarium of every surgeon performing major hepatic resection. It is not usually necessary when performing standard liver resections but surgeons should be familiar with the anatomical technique of doing so. Isolation of the vena cava superior to the hepatic veins is done by dividing the left triangular ligament and the lesser omentum, being careful to first look for a replaced left hepatic artery. Next the peritoneum on the superior border of the caudate lobe is divided and a finger is passed behind the vena cava to come out just inferior to the crus of the diaphragm. The crus of the diaphragm makes an easily identified column on the right side. This column passes across the right side of the vena cava and dissection of the space inferior to this column and behind the vena cava facilitates passage of the finger from the left side to the right side in the space behind the vena cava. Isolation of the vena cava below the liver is more straightforward but one should be aware of the position of the adrenal vein and in some cases it is necessary to isolate the adrenal vein if bleeding is persisting after occlusion of the vena cava above and below the liver.
Finally, the surgeon should be aware that during transection of the liver large veins will be encountered in certain planes of transection. For instance, in its passage along the midplane the middle hepatic vein usually receives two large tributaries, one from Sg5 inferiorly and the other from Sg8 superiorly (Fig. 2.12). Both are routinely encountered in performing right hepatectomy. The venous drainage of the right side of the liver is highly variable and additional large veins, including one from Sg6, may also enter the middle hepatic vein.
The plate/sheath system of the liver
The system of fibrous plates and sheaths that lies on the ventral surface of the liver and extends into it is of great importance in liver surgery. The plate/sheath system can be understood by first imagining a shirt with the front cut away to leave only the back and the sleeves (Fig. 2.15, inset).12 If the shirt were made of fibrous tissue the back of the shirt would be a plate and the sleeves would be sheaths. The true plate/sheath system is more complex, as there are four plates (hilar, cystic, umbilical and arantian) and several sheaths3 (Fig. 2.15). The hilar plate is the most important plate in liver surgery. It is a mostly flat structure, which lies principally in the coronal plane, posterior to the main bilovascular structures in the porta hepatis. However, its upper border is curved, so that it has the shape of a toboggan when viewed in the sagittal plane. This upper curved edge lies superior to the right and left bile ducts, the most superior structures in the porta hepatis. It is this taut, firm, upper curved edge of the hilar plate that is dissected free from the underside of the liver when ‘lowering the hilar plate’.
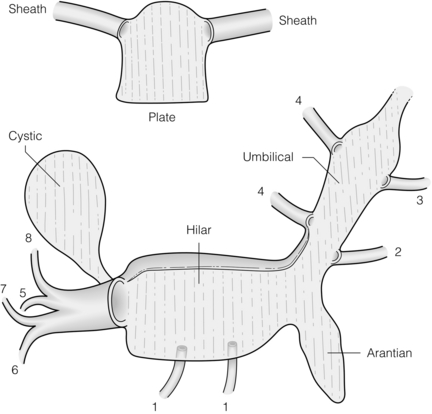
Figure 2.15 Plate/sheath system of the liver with inset showing a schematic of a plate with two sheaths (see text). Reproduced from Strasberg SM, Linehan DC, Hawkins WG. Isolation of right main and right sectional portal pedicles for liver resection without hepatotomy or inflow occlusion. J Am Coll Surg 2008; 206:390–6. With permission of the Journal of the American College of Surgeons.
The sheath of the right portal pedicle extends off the hilar plate like a sleeve into the liver surrounding the portal structures, i.e. portal vein, hepatic artery and bile duct. The combined structure is referred to as a ‘portal pedicle’. As the right portal pedicle enters the liver it divides into a right anterior and right posterior portal pedicle supplying the respective sections, and then segmental pedicles supplying the four segments. On the left side, only the segmental structures are sheathed. There is no sheathed main portal pedicle because the main portal vein, proper hepatic artery and common hepatic duct are not close enough to the liver to be enclosed in a sheath.
The cystic plate is the ovoid fibrous sheet on which the gallbladder lies (Fig. 2.15). When performing a cholecystectomy this plate is normally left behind. In its posterior extent the cystic plate narrows to become a stout cord that attaches to the anterior surface of the sheath of the right portal pedicle. The latter is a point of anatomical importance for the surgeon wishing to expose the anterior surface of the right portal pedicle, since this cord must be divided to do so, as we have described.13 With severe chronic inflammation the cystic plate may become shortened and thickened so that the distance between the top of the cystic plate and the right portal pedicle is likewise much shorter than usual. This places the structures in the right pedicle in danger during cholecystectomy when dissection is performed ‘top down’ as a primary strategy. The other plates are the umbilical and arantian, which underlie the umbilical portion of the left portal vein and the ligamentum venosum, respectively (Fig. 2.15). The other sheaths carry segmental bilovascular pedicles of the left liver and caudate lobe.
In performing a right hepatectomy there are two methods of managing the right-sided portal vessels and bile ducts. The first is to isolate the hepatic artery, portal vein and bile duct individually and either control them or ligate them extrahepatically, and the second is to isolate the entire portal pedicle and staple the pedicle. Isolation of the right portal pedicle can be performed by making hepatotomies above the right portal pedicle in Sg4 and in the gallbladder fossa after removing the gallbladder. A finger is passed through the hepatotomy to isolate the right portal pedicle. This technique usually requires inflow occlusion. It can also be done without inflow occlusion by lowering the hilar plate and coming around the right portal pedicle directly on its surface, as we have described (Fig. 2.16).12 It is advisable to divide caudate veins in the area below the vena caval ligament before performing pedicle isolation, since haemorrhage from these veins can be considerable if they are injured during isolation of the right portal pedicle. The advantage of pedicle isolation over isolation of individual vessels and the bile duct is that sectional resections require isolation of pedicles (Fig. 2.16).12 Furthermore, pedicle isolation is much easier to do laparoscopically than individual structure isolation.
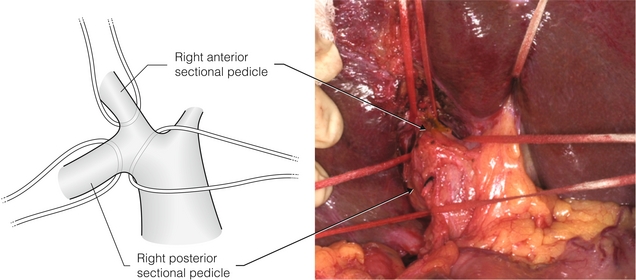
Figure 2.16 Isolation of right portal pedicle and sectional pedicles by technique of dissection on surface of pedicles. No inflow occlusion or separate hepatotomies are used (see Ref. 12). The umbilical tape in the upper right of the photograph is around the bridge of liver tissue over the umbilical fissure. Reproduced from Strasberg SM, Linehan DC, Hawkins WG. Isolation of right main and right sectional portal pedicles for liver resection without hepatotomy or inflow occlusion. J Am Coll Surg 2008; 206:390–6. With permission of the Journal of the American College of Surgeons.
Liver capsule and attachments: The liver is encased in a thin fibrous capsule that covers the entire organ except for a large bare area posteriorly where the organ is in contact with the IVC and with the diaphragm to the right of the IVC. The bare area stretches superiorly to include the termination of the three hepatic veins and ends in a point, which is also where the attachment of the falciform ligament ends. The limit of the bare area, where the peritoneum passes between the body wall and the liver, is called the coronary ligament. It is one of three structures that connect the liver to the abdominal wall ‘dorsally’, the other two being the right and left triangular ligaments. The liver also has another bare area, best thought of as a bare crease, where the hepatoduodenal ligament and the lesser omentum attach on the ‘ventral’ surface. It is through this crease that the portal structures enter the liver at the hilum (hilum = ‘a crease on a seed’). The other ligamentous structures of interest to surgeons are the ligamentum teres, falciform ligament and the ligamentum venosum. The ligamentum teres (teres = ‘round’) is the obliterated left umbilical vein and runs in the free edge of the falciform ligament from the umbilicus to the termination of the umbilical portion of the left portal vein. The falciform (falciform = ‘scythe shaped’) is the filmy fold that runs between the anterior abdominal wall above the umbilicus and attaches to the anterior surface of the liver between the left medial and left lateral sections.
Surface anatomy: Numerous terms for surface anatomy exist. They are of minimal surgical importance. Since the term ‘lobe’ has been used in different ways by various anatomists and surgeons it is best avoided except in reference to the caudate lobe. Fissure and scissure or scissura are similarly confusing terms since they apply only to clefts in casts of the liver. The ligaments of the liver are of surgical importance and are described under capsule and attachments.
Pathological conditions may distort or alter the position of normal hepatic structures. Tumours may push vessels so that they are stretched and curved over the surface of the tumour, narrowing or occluding them by direct pressure. Tumours may partially or completely occlude vessels by mural invasion, by inducing bland thrombi or by entering the lumen and producing tumour thrombi. They may cause bile ducts to dilate to a size many times normal. Atrophy of liver volume will be induced by processes that occlude either the portal vein or bile duct. Since the liver will undergo hyperplasia to maintain a constant volume of liver cells, atrophy of one part of the liver is usually accompanied by growth of another. If the right portal vein is occluded by a tumour, the right liver will atrophy and the left liver will grow. When seen from below, this process will exert a counter-clockwise rotational effect on the porta hepatis, rotating the bile duct posteriorly, the hepatic artery to the right, and the portal vein to the left and anteriorly.
Gallbladder and extrahepatic bile ducts
The gallbladder lies on the cystic plate. The edge of the gallbladder forms one side of the hepatocystic triangle. The other two sides are the right side of the common hepatic duct and the liver. Eponyms covering this anatomy (Calot, Moosman, etc.) are confusing and should be abandoned. The hepatocystic triangle contains the cystic artery and cystic node and a portion of the right hepatic artery, as well as fat and fibrous tissue. Clearance of this triangle along with isolation of the cystic duct and elevation of the base of the gallbladder off the lower portion of the cystic plate gives the ‘critical view of safety’ that we have described for identification of the cystic structures during laparoscopic cholecystectomy.14
A large number of curiosities of the gallbladder, e.g. the phrygian cap, have been described. The following are anomalies of importance to the biliary surgeon.
Agenesis of the gallbladder
Agenesis occurs in about 1 in 8000 patients. It can be difficult to recognise. An ultrasonographer may describe a ‘shrunken’ gallbladder. When agenesis is suspected it may be confirmed by axial imaging. If doubt remains, laparoscopy is definitive.
Double gallbladder
This is also a very rare anomaly but can be the cause of persistent symptoms after resection of one gallbladder. A gallbladder may also be bifid, which usually does not cause symptoms, or have an hourglass constriction, which may cause symptoms due to obstruction of the upper segment.
Cystic duct
This structure is normally 1–2 cm in length and 2–3 mm in diameter. It joins the common hepatic duct at an acute angle to form the common bile duct. The cystic duct normally joins the common hepatic duct approximately 4 cm above the duodenum. However, the cystic duct may enter at any level up to the right hepatic duct and down to the ampulla. The cystic duct may also join the right hepatic duct either when the right duct is in its normal position or in an aberrant location.
There are three patterns of confluence of the cystic duct and common hepatic duct (Fig. 2.17). In the 20% of patients in whom there is a parallel union, the surgeon approaching the common hepatic duct by dissecting the cystic duct is prone to injure the side of the former structure (Fig. 2.17). Also, when making a choledochotomy at this level the incision should be started slightly to the left side of the midplane of the bile duct in order to avoid entering a septum between the two fused cystic/common hepatic ducts. When performing cholecystectomy the cystic duct should be occluded in such a way that there is a visible section of cystic duct below the clip closest to the common bile duct.
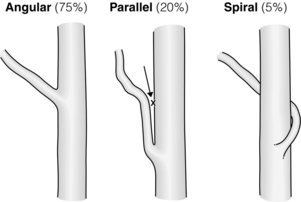
Figure 2.17 The three types of cystic duct/common hepatic duct confluence. The parallel union confluence is shown in the middle. Dissection of this type of cystic duct (arrow) may lead to injury to the side of the common hepatic duct. During laparoscopic cholecystectomy this is often a cautery injury. Adapted from Warrren KW, McDonald WM, Kune GA. Bile duct strictures: New concepts in the management of an old problem. In: Irvine WT (ed.) Modern trends in surgery. London: Butterworth, 1966. With permission from Elsevier.
Although a gallbladder with two cystic ducts has been described, the author has not seen convincing proof that this anomaly actually exists. If it does, it must be an anomaly of extreme rarity. When two ‘cystic ducts’ are identified, it is likelythat the cystic duct is congenitally short or has been effaced by a stone and that the two structures thought to be dual cystic ducts are, in fact, the common bile duct and the common hepatic duct.
Cystic artery
The cystic artery is about 1 mm in diameter and normally arises from the right hepatic artery in the hepatocystic triangle. The cystic artery may arise from a right hepatic artery that runs anterior to the common hepatic duct. The cystic artery may also arise from the right hepatic artery on the left side of the common hepatic duct and run anterior to this duct, while the right hepatic artery runs behind it. Such cystic arteries tend to tether the gallbladder and make dissection of the hepatocystic triangle more difficult. The cystic artery may arise from an aberrant right hepatic artery coming off the superior mesenteric artery (SMA). In this case the cystic artery and not the cystic duct tends to be in the free edge of the fold leading from the hepatoduodenal ligament to the gallbladder. This should be suspected whenever the ‘cystic duct’ looks smaller than the ‘cystic artery’.
Normally the cystic artery runs for 1–2 cm to meet the gallbladder superior to the insertion of the cystic duct. The artery ramifies into an anterior and posterior branch at the point of contact with the gallbladder and these branches continue to divide on their respective surfaces. Sometimes the cystic artery divides into branches before the gallbladder edge is reached. In that case the anterior branch may be mistaken to be the cystic artery proper and the posterior branch will not be discovered until later in the dissection, when it may be divided inadvertently. The artery may ramify into several branches before arriving at the gallbladder, giving the impression that there is no cystic artery. The anterior and posterior branches may arise independently from the right hepatic artery, giving rise to two distinct cystic arteries.
Multiple small cystic veins drain into intrahepatic portal vein branches by passing into the liver around or through the cystic plate. Sometimes there are cystic veins in the hepatocystic triangle that run parallel to the cystic artery to enter the main portal vein.
The cystic plate has been described above. Small bile ducts may penetrate the cystic plate to enter the gallbladder. These ‘ducts of Luschka’ are very small, usually submillimetre accessory ducts. However, when divided during a cholecystectomy postoperative bilomas may occur. Bilomas and haemorrhage may also be caused by penetration of the cystic plate during dissection. In about 10% of patients there is a large peripheral bile duct immediately deep to the plate, disruption of which will cause copious bile drainage. The origin of the middle hepatic vein is also in this location, and if it is injured massive haemorrhage may ensue. There is areolar tissue between the muscularis of the gallbladder and the cystic plate. At the top of the gallbladder the layer is very thin. As one progresses downwards the areolar layer thickens. If dissection from the top of the gallbladder downward is carried out leaving the areolar tissue on the cystic plate one will arrive to the posterior surface of the cystic artery and cystic duct. Conversely, if it is carried out downward on the cystic plate leaving the areolar tissue on the gallbladder one will arrive at the surface of the right portal pedicle. If this is not anticipated, structures in the right portal pedicle may be injured. Therefore, the proper plane of dissection is between the gallbladder and the areolar tissue.
Extrahepatic bile ducts
The common hepatic duct (CHD) is a structure formed by the union of the right and left hepatic ducts. The union normally occurs at the right extremity of the base of Sg4 anterior and superior to the bifurcation of the portal vein. The CHD travels in the right edge of the hepatoduodenal ligament for 2–3 cm, where it joins with the cystic duct to form the common bile duct (CBD). The latter has a supraduodenal course of 3–4 cm and then passes behind the duodenum to run in or occasionally behind the pancreas to enter the second portion of the duodenum. Details of its lower section and relation to the pancreatic duct are described in the final section of this chapter. The external diameter of the common bile duct varies from 5 to 13 mm when distended to physiological pressures. However, the duct diameter at surgery, i.e. in fasting patients with low duct pressures, may be as small as 3 mm. Radiologically, the internal duct diameter is measured on fasting patients. Under these conditions the upper limit is normally about 8 mm. Size should never be used as a sole criterion for identifying a bile duct. Caution is required in situations in which a structure seems larger than expected. Although the cystic duct may be enlarged due to passage of stones, the surgeon should take extra precautions before dividing a ‘cystic duct’ that is greater than 2 mm in diameter because the common bile duct can be 3 mm in diameter and aberrant ducts may be smaller.
Anomalies of extrahepatic bile ducts
As already noted, there are biliary anomalies of the right and left ductal systems that can affect the outcome of hepatic surgery. The same is true for biliary surgery. The most important clinical anomaly is low insertion of right hepatic ducts referred to above. Because of its low location, it may be mistaken to be the cystic duct and be injured. This is even more likely to occur when the cystic duct unites with an aberrant duct as opposed to joining the common hepatic duct. Left hepatic ducts can also join the common hepatic duct at a low level. They are less prone to be injured since the dissection during cholecystectomy is on the right side of the biliary tree.
Extrahepatic arteries
The course of these arteries has been described above. Anomalies of the hepatic artery may be important in gallbladder surgery. Normally the right hepatic artery passes posterior to the bile duct (80%) and gives off the cystic artery in the hepatocystic triangle. However, in 20% of cases the right hepatic artery runs anterior to the bile duct. The right hepatic artery may lie very close to the gallbladder and chronic inflammation can draw the right hepatic artery directly on to the gallbladder, where it lies in an inverse U-loop and is prone to injury. In the ‘classical injury’ in laparoscopic cholecystectomy, in which the common bile duct is mistaken for the cystic duct, an associated right hepatic artery injury is very common.
Blood supply of bile ducts
Many studies, dating back to the 19th century, have examined the blood supply of the extrahepatic bile ducts in cadaveric specimens. A key observation made by Rappaport is that the bile ducts are supplied by the hepatic artery only,15 unlike the liver, which has a dual blood supply from the artery and the portal vein. The arterial blood supply can be thought of as having three anatomical elements. The first consists of afferent vessels from the hepatic artery and its branches (Fig. 2.18a). The second element is longitudinal arteries that run parallel to the long axis of the bile ducts and that receive blood from the afferent vessels (Fig. 2.18b). The third element, an arterial plexus encasing the bile ducts, receives blood from the marginal arteries (Fig. 2.18c). Tiny branches of the plexus pierce the bile duct wall to supply the capillaries of the bile duct.
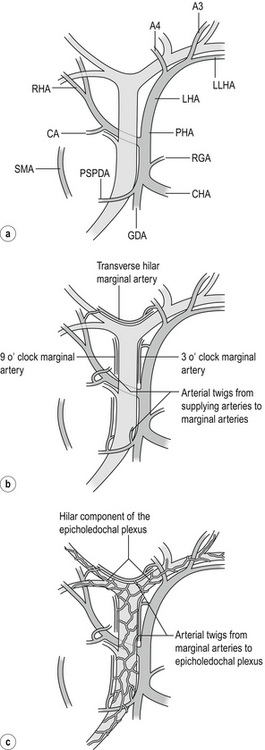
Figure 2.18 (a) The supplying arteries. All arteries shown can all give twigs to the marginal arteries or in some cases directly supply the epicholedochal plexus. A2, A3, A4, arteries to segments 2, 3 and 4; CA, cystic artery; CHA, common hepatic artery; GDA, gastroduodenal artery; LHA, left hepatic artery; LLSA, left lateral sectional artery; PHA, proper hepatic artery; PSPDA, posterior superior pancreaticoduodenal artery, the most important and constant artery; RHA, right hepatic artery. Replaced arteries arising from the superior mesenteric artery may also supply the bile ducts. (b) Marginal arteries. Marginal arteries are disposed at 3 and 9 o’clock (and occasionally at 12 o’clock) on the common bile duct/common hepatic duct. The hilar marginal artery runs across the top of the confluence of the right and left hepatic ducts. (c) Epicholedochal plexus. The epicholedochal plexus is supplied by the marginal arteries. Adapted from Strasberg SM, Helton WS An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB 2011; 13(1):1–14. With permission from John Wiley & Sons.
The afferent vessels are branches of the hepatic arteries and less commonly of the superior mesenteric artery or other upper abdominal arteries. The most constant and important artery supplying the bile duct is the posterior superior pancreatico-duodenal artery, usually the first branch of the gastroduodenal artery. Arterial twigs pass to the duct as the artery winds around the lower end of the duct. These branches supply much of the retroduodenal and intrapancreatic bile duct, but also ascend the bile duct to supply the supraduodenal bile duct. The lowest portion of the duct near the ampulla is also supplied by the anterior superior pancreatic artery from the inferior pancreatico-duodenal artery. Other vessels that commonly send afferents to the supraduodenal duct are the proper hepatic artery, cystic artery and artery to Sg4. Furthermore, body wall collaterals such as phrenic arteries can at times supply the bile ducts (as well as the liver) since bile duct infarction is much more common when there is occlusion of the common hepatic artery after a transplant than it is in an in situ liver. The notion that the extrahepatic bile duct is supplied by arteries that join it only at the bottom and top of its course is incorrect. Supplying arteries from the cystic artery, right and left hepatic arteries and proper hepatic artery may join it in its mid course.
The afferent vessels usually empty into longitudinal or ‘marginal’ arteries that run parallel to the long axis of the bile ducts (also called ‘marginal anastomotic loop’).16 These vessels are disposed at 3 and 9 or, less commonly, at 12 o’clock on the common bile duct/common hepatic duct, or run across the top of the confluence and the right and left bile ducts. This ‘hilar marginal artery’ has been called the ‘caudate arcade’ or ‘communicating arcade’. This artery is of great importance in maintaining blood supply to the liver when one hepatic artery (right or left) is occluded.17
The third element of this system is the ‘epicholedochal plexus’, a fine arterial plexus that lies on and surrounds the entire common bile duct and the left and right bile ducts. The latter is the hilar component of the epicholedochal plexus. The vessels of the plexus tend to run along the long axis of the ducts so that on the common duct many of the vessels are vertical while those around the confluence and the right and left ducts are disposed horizontally. In the portion of the biliary tree that lies adjacent to the hilar plate or which has entered the fibrous sheaths, the epicholedochal plexus lies between the sheath and the wall of the bile duct. Dissection in this plane has the potential to devascularise bile ducts.18
Transection of the bile duct may result in ischaemia of the duct. For instance, if the duct is transected at the level of the duodenum, ischaemia of a portion of the bile duct above this level may occur since blood flow originating from the superior pancreato-duodenal artery and passing up along the marginal artery is cut off. Similarly, in a high transection at the level of the confluence, the lower cut end of the duct may become ischaemic. This problem is thought to be an important contributory cause to the frequent failure of choledocho-choledochotomy as a form of biliary reconstruction. To avoid this problem hepatico-jejunostomy is used and the bile duct is trimmed back to within 1 cm of the confluence.
Pancreas
The pancreas is a retroperitoneal organ lying obliquely across the upper abdomen so that the tail is superior to the head. It is formed by the fusion of ventral and dorsal buds in utero, the ventral pancreas rotating to come behind and fuse with the dorsal pancreas. On average it is 22 cm in length. The head of the pancreas is discoid in shape and terminates inferiorly and medially in the hook-like uncinate process. The neck, body and tail are shaped like a flattened cylinder, sometimes somewhat triangular in cross-section with a flat anterior and pointed posterior surface. These divisions of the organ are somewhat arbitrary; the neck of the pancreas sits anterior to the superior mesenteric and portal veins. Normally the consistency of the gland is soft.
Pancreatic ducts
The prevailing anatomical pattern of the pancreatic duct is the result of union of the ventral duct (Wirsung) with the dorsal duct (Santorini), along with partial regression of the dorsal duct in the head. The ‘genu’ of the duct (genu = knee) is the bend in the duct concave inferiorly where the ventral duct joins the dorsal. In the prevailing pattern both ducts communicate with the duodenum, the dorsal duct entering at the minor papilla about 2 cm above and 5 mm anterior to the major papilla. Other ductal patterns are possible that involve various degrees of dominance or regression of the portions of the ducts in the head of the pancreas. For instance, the ducts may not unite, resulting in separate drainage from the ventral and dorsal pancreas (pancreas divisum), the dorsal duct may lose its connection to the duodenum, or the dorsal duct in the head may lose its connection to the rest of the ductal system and drain only a small section of the head into the duodenum. Alternatively, the ventral duct may regress and the dorsal duct drain more or all of the pancreas through the minor ampulla. The uncinate process is served by its own duct, which joins the main pancreatic duct 1–2 cm from its entry into the duodenum.
The pancreatic duct (and pancreas) are often referred to as proximal (head) and distal (tail). These are confusing terms – as are the terms proximal and distal bile duct. Adding to the confusion is that the ‘distal’ bile duct is near the ampulla, but that site is ‘proximal’ regarding the pancreatic duct. Instead, that part of the bile duct should be referred to as the pancreatic portion or lower bile duct, while that near the confluence should be called the upper extrahepatic or hilar bile duct. In the case of the pancreas, the duct should be referred to as the ‘pancreatic head duct’, ‘pancreatic body duct’, etc.
The ventral duct usually joins the common bile duct to form a common channel several millimetres from the ampulla of Vater, usually within the wall of the duodenum. The bile duct traverses the duodenal wall obliquely and the pancreatic duct at a right angle. Each duct and the common channel have their own sphincters. The sphincters can be palpated from within the duodenum and form part of the raised ‘major papilla’ at whose apex the opening of the common channel can be seen. The common channel may be longer or absent, with both ducts entering the duodenum separately, the pancreatic duct more inferiorly. In performing a sphincteroplasty, it is advisable to open the common opening superiorly (10–12 o’clock position in the mobilised duodenum) to avoid the orifice of the pancreatic duct (4 o’clock). The ampulla is normally at the midpoint of the second part of the duodenum. It is rarely higher but can be as low as the midpoint of the third part of the duodenum. When the dorsal duct has its own communication with the duodenum, it is found at the ‘minor papilla’, about 2 cm proximal and 1 cm anterior to the major papilla.
Blood supply of the pancreas
The arterial supply of the pancreas consists of two vascular systems, one supplying the head and uncinate, and the other the body and tail. The neck is a watershed between these two areas of supply.4 The head and uncinate process are supplied by the pancreatico-duodenal arcade, which consists of two to several loops of vessels that arise from the superior pancreatico-duodenal (branch of gastroduodenal) and inferior pancreatico-duodenal (branch of the SMA). The arcades run on the anterior and posterior surface of the pancreas next to the duodenum, the anterior arcade lying somewhat closer to the duodenum. The second system arises from the splenic artery, which gives rise to three arteries into the dorsal surface of the gland (Fig. 2.19). The dorsal pancreatic artery is the most medial of the three and the most important. It anastomoses with the pancreatico-duodenal arcade in the neck of the pancreas. It is the most aberrant artery in the upper abdomen and may arise from vessels that are routinely occluded during pancreatico-duodenectomy, which may account in part for fistula formation after this procedure.
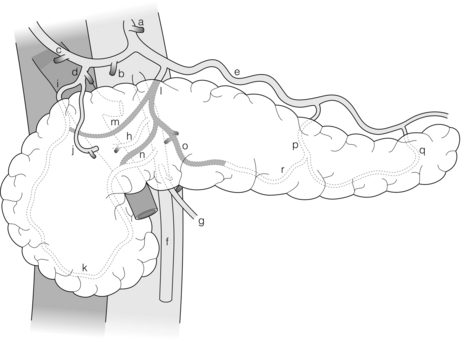
Figure 2.19 Arterial blood supply to the pancreas. The dorsal pancreatic artery is shown shaded. Alternative origins of the artery are shown as black stumps. Key: a, coeliac artery; b, common hepatic artery; c, right hepatic artery; d, gastroduodenal artery; e, splenic artery; f, superior mesenteric artery; g, middle colic artery; h, right hepatic artery (aberrant); i, superior pancreatico-duodenal artery; j, right gastroepiploic artery; k, inferior pancreatico-duodenal artery; l, dorsal pancreatic artery (DPA); m, right anastomotic branch of DPA to superior part of pancreatico-duodenal arcade; o, left anastomotic branch of DPA becomes transverse pancreatic artery; p, pancreatica magna artery; q, caudal pancreatic artery; r, transverse pancreatic artery. © Washington University in St Louis.
Venous drainage generally follows arterial supply. The veins of the body and tail of the pancreas drain into the splenic vein, where it lies partly embedded in the posterior surface of the gland. These veins are short and fragile. The head and uncinate process veins drain into the superior mesenteric vein (SMV) and portal vein on the right lateral side of these structures. Uncinate veins often drain into a large first jejunal tributary vein, which then empties into the SMV. This vein usually passes behind the SMA. A nearly constant posterior superior pancreatico-duodenal vein enters the right lateral side of the portal vein at the level of the duodenum.
Lymphatics of the pancreas
For surgical purposes the lymphatic drainage of the pancreas is best considered in relation to the two main surgical procedures, resection of the pancreatic head and resection on the pancreatic body and tail. The aim of lymphatic resection during these procedures is to resect the primary lymph nodes, i.e. those nodes that receive lymph directly from pancreatic tissue (N1 nodes) as opposed to nodes that receive drainage from N1 nodes (N2 nodes). There is a ring of nodes around the pancreas that drain the adjacent sections of the gland (N1 nodes).19 These in turn drain into nodes along the SMA, coeliac artery and aorta (axial nodes). The axial nodes, which are N2 for most areas of the pancreas, are also N1 for portions of the pancreatic head, body and uncinate process that lie close to the aorta. The lymphatics of the body and tail are shown in Fig. 2.20. The lymphatics of the head and uncinate process drain into a nodal ring for this part of the gland, consisting of lymph nodes in the pancreatico-duodenal groove anteriorly and posteriorly, into subpyloric nodes inferiorly, into nodes adjacent to the common bile duct and hepatic artery superiorly, and into nodes along the SMA medially. These are N1, but as noted above so are some of the axial nodes. The standard node dissection for a Whipple procedure removes all of these nodal groups. This removes the N1 nodes unless there is direct lymphatic drainage to the left side of the SMA or to nodes around the coeliac artery, which does occur in a small proportion of patients.
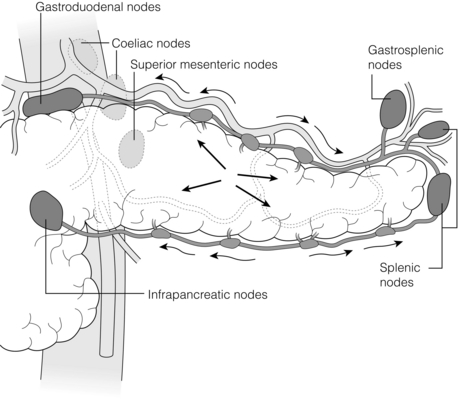
Figure 2.20 Lymphatic drainage of the body and tail of the pancreas. The intraparenchymal lymphatics from the four quadrants empty into lymphatic vessels on the superior and inferior borders of the pancreas (arrows). Small nodes are found along these vessels. The lymph flows to the nodes of the ‘ring’. These are N1 nodes, although some of the lymph may have passed through the smaller nodes as described. The nodes of the ring empty into nodes along the SMA, coeliac artery and aorta. The latter are therefore N2 nodes, but they are also N1 nodes for the more central part of the pancreas. © Washington University in St Louis.
Anatomical relations and ligaments of the pancreas
The pancreas is a deeply seated organ that, unlike the liver and most of the biliary tree, is not obvious when opening the abdomen. The anatomical relations of the pancreas are very important in pancreatic surgery. The structures emphasised in the following section are those that are commonly invaded by tumours.
The pancreas lies in the anterior pararenal space anterior to the anterior renal fascia and behind the peritoneum. Posteriorly, the pancreas is related from right to left to the right kidney and perinephric fat, IVC and right gonadal vein, aorta, left renal vein (slightly inferior), retropancreatic fat, the left adrenal gland and the superior pole of the left kidney. All of the former structures lie in the perirenal space and behind the anterior renal fascia. In the case of oncological resections the plane of dissection should be behind the anterior renal fascia in order to maximise the chance of obtaining negative margins as described for the radical antegrade modular pancreato-splenectomy (RAMPS) procedure.20
The SMV and portal vein are posterior relations of the neck of the pancreas, and splenic vein of the body and tail. The SMA is a posterior relation of the junction of the neck and body of the gland lying posterior and to the right of the SMV. The SMA and SMV are both related to the uncinate process and give branches into (SMA) and receive tributaries from (SMV) the uncinate process. Often the uncinate veins enter a large tributary of the SMV, the first jejunal vein, which also abuts the uncinate process. These short arteries and veins are of importance surgically as they are divided when the head of the pancreas is resected. The coeliac artery rises vertically superior to the SMA close to the superior edge of the pancreas, where it gives off the common hepatic artery and the splenic artery. The former runs anteriorly and to the left in approximation to the superior border of the pancreas. At the point where the artery passes in front of the portal vein, it divides into the gastroduodenal artery, which passes anterior to the neck of the pancreas, sometimes buried within it. It terminates in the right gastroepiploic artery that rises in a fold of tissue toward the pylorus, a fold that also contains the right gastroepiploic vein and subpyloric nodes. The splenic artery snakes along the superior border of the pancreas to leave it 2–3 cm from the termination of the pancreas. The head of the pancreas is wrapped in the first three parts of the duodenum and the tail ends in relation to the splenic hilum. There is variability in the proximity of the tail of the pancreas to the spleen. In some cases the pancreas terminates 2 cm from the splenic substance and in others it abuts it. The anterior surface of the body and tail of the pancreas is covered by peritoneum, which is the posterior wall of the lesser sac, and then by the posterior wall of the stomach anterior to this. The transverse mesocolon is related to the inferior border of the pancreas, and the right and left extremities of the transverse colon are related to the head and tail of the gland. The inferior mesenteric vein is related to the inferior border of the neck of the pancreas and may pass behind it to enter the splenic vein or turn medially to enter the SMV.
The pancreas is normally accessed surgically by entering the lesser sac either by division of the greater omentum below the gastroepiploic arcade or by releasing the greater omentum from its attachment to the transverse colon. When the lesser sac is entered the anterior surface of the neck, body and tail are often visible, but may be obscured by congenital filmy adhesions to the posterior wall of the stomach. To expose the head of the pancreas it is necessary to mobilise the right side of the transverse colon and hepatic flexure inferiorly and to divide the right gastroepiploic vein. The latter crosses the inferior border of the pancreas to join with the middle colic vein to form the gastrocolic trunk, which then enters the SMV. For complete exposure, e.g. for a Frey procedure, the right gastroepiploic artery is also divided and it and the subpyloric nodes are swept upwards off the pancreas. To access the SMV at the inferior border of the pancreas the peritoneum at the inferior border of the neck is divided and the dissection is carried inferiorly and laterally to open a groove between the uncinate process and the mesentery. Division of the right gastroepiploic vein at the inferior border of the pancreas greatly facilitates this manoeuvre. Normally no veins enter the SMV or portal vein from the posterior surface of the neck of the pancreas. Consequently the neck of the pancreas can be separated from the anterior surface of the SMV/portal vein in this avascular plane. The peritoneum at the inferior border of the neck, body and tail of the pancreas is avascular, and there are few vascular connections between the back of the body and tail of the pancreas and retroperitoneal tissues. As a result the pancreas may be readily dissected free from retroperitoneum. The splenic vein is partly embedded in the back of the pancreas from the point that it reaches the gland on the left to about 1 cm from its termination at its confluence with the SMV.
References
1. Terminology Committee of the IHPBA, The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2002;4(2):99. 18332933
2. Healey, J.E.J., Schroy, P.C., Anatomy of the biliary ducts within the human liver; analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. Arch Surg 1953; 66:599–616. 13039731
3. Couinaud, C. Le Foie. Etudes anatomiques et chirugicales. Paris: Masson & Cie; 1957.
4. Michels, N.A. Blood supply and anatomy of the upper abdominal organs. Philadelphia: JB Lippincott; 1955.
5. Moosman, D.A., The surgical significance of six anomalies of the biliary duct system. Surg Obst Gynec 1970; 131:655–660. 4989834
6. Hjortsjo, C.-H., The topography of the intrahepatic duct systems. Acta Anat (Basel) 1951; 11:599–615. 14829155
7. Botero, A.C., Strasberg, S.M., Division of the left hemiliver in man – segments, sectors, or sections. Liver Transpl Surg 1998; 4:226–231. 9563962
8. Masselot, R., Leborgne, J. Anatomical study of hepatic veins. Anat Clin. 1978; 1:109–125.
9. Kawasaki, S., Makuuchi, M., Miyagawa, S., et al, Extended lateral segmentectomy using intraoperative ultrasound to obtain a partial liver graft. Am J Surg 1996; 171:286–288. 8619469
10. Makuuchi, M., Yamamoto, J., Takayama, T., et al, Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology 1991; 38:176–179. 1649789
11. Belghiti, J., Guevara, O.A., Noun, R., et al, Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001; 193:109–111. 11442247
12. Strasberg, S.M., Linehan, D.C., Hawkins, W.G., Isolation of right main and right sectional portal pedicles for liver resection without hepatotomy or inflow occlusion. J Am Coll Surg 2008; 206:390–396. 18222398
13. Strasberg, S.M., Picus, D.D., Drebin, J.A., Results of a new strategy for reconstruction of biliary injuries having an isolated right-sided component. J Gastrointest Surg 2001; 5:266–274. 11360050
14. Strasberg, S.M., Brunt, L.M., Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg 2010; 211:132–138. 20610259
15. Rappaport, A.M., Kawamura, T., Knoblauch, M., et al, Effects of arterial or portal ischemia on survival and metabolism of partially and totally depancreatized dogs. Z Exp Chir 1975; 8:326–342. 1053192
16. Northover, J.M.A., Terblanche, J., A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg 1979; 66:379–384. 466017
17. Gunji, H., Cho, A., Tohma, T., et al, The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg 2006; 192:276–280. 16920417
18. Nery, J.R., Frasson, E., Rilo, H.L., et al, Surgical anatomy and blood supply of the left biliary tree pertaining to partial liver grafts from living donors. Transplant Proc 1990; 22:1492–1496. 2389377
19. O’Morchoe, C.C., Lymphatic system of the pancreas. Microsc Res Tech 1997; 37:456–477. 9220424
20. Mitchem, J.B., Hamilton, N., Gao, F., et al, Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg 2012; 214:46–52. 22192922