Chapter 30 Hemoptysis Caused by Distal Left Main Bronchial Tumor in a Patient with Primary Lung Adenocarcinoma
Case Description
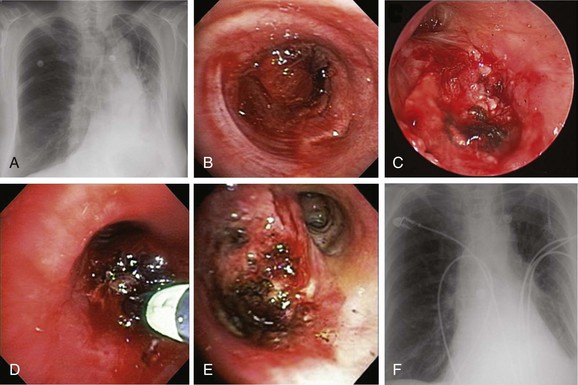
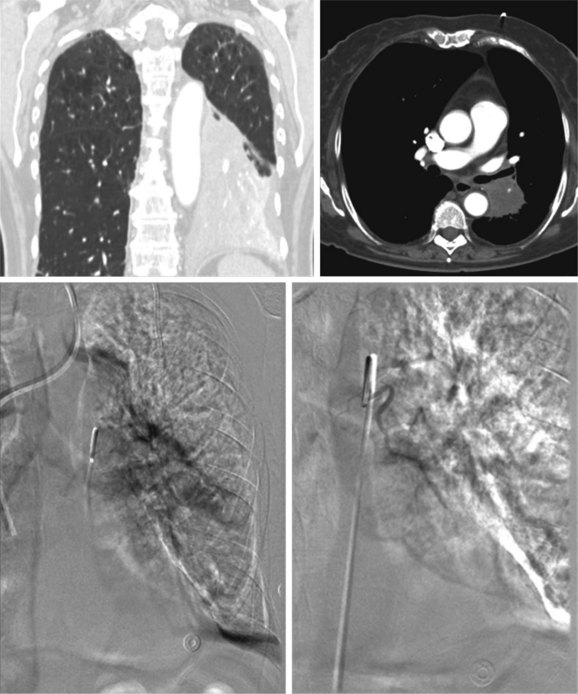
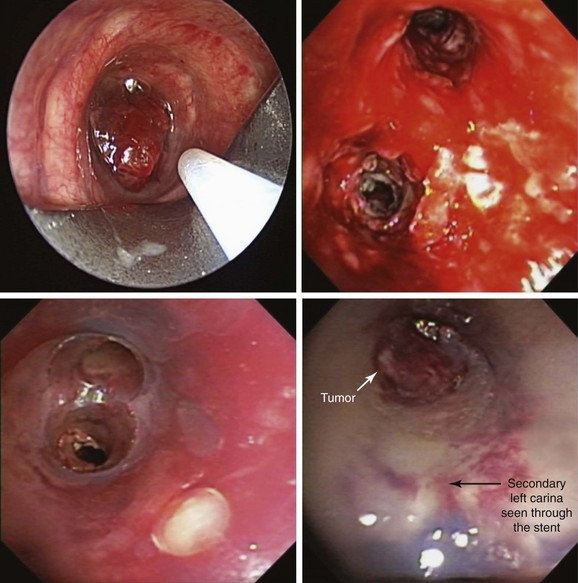
A 62-year-old female who had been diagnosed 6 weeks earlier with stage IV primary adenocarcinoma with rib and brain metastasis came to the emergency department with respiratory distress and hemoptysis. Hemoptysis was described as 4 to 6 tablespoons of bright red blood within 24 hours. She had a history of GERD and COPD requiring 2 L of home oxygen. She had smoked 1 pack per day for 20 years but quit 10 years earlier. At the time of her presentation, she had just completed a cycle of bevacizumab therapy and 10 days of brain radiotherapy. On physical examination, she was awake, alert, and oriented, without neurologic deficits; air entry in the left hemithorax was diminished. Her ECOG (Zubrod score)* before the hemoptysis was 1.1 Her heart rate was 110, blood pressure 160/90, and respiratory rate 28/min. She required 5 L of oxygen by nasal cannula to maintain oxygen saturation of 92%. Chest radiograph showed left-sided tracheal deviation and atelectasis of the left lower lobe and lingula. Aeration in the upper lung field zone was maintained (Figure 30-1, A). The patient consented to bronchoscopy with electrocautery; a large blood clot was noted completely occluding the distal left main bronchus (Figure 30-1, B). After clot removal, patency to the left upper lobe was restored, but active bleeding was noted coming from the tumor that was completely occluding the left lower lobe bronchus (Figure 30-1, C). The tumor was cauterized using an electrocautery probe (Figures 30-1, D and E). During the following hours, this controlled her hemoptysis. Follow-up chest radiograph post procedure shows improved aeration to the left upper lobe and persistent atelectasis in the left lower lobe (Figure 30-1, F).
Discussion Points
1. List two alternative bronchoscopic procedures that can be applied to control this patient’s hemoptysis.
2. List three contraindications to electrocautery performed through the flexible bronchoscope.
3. Describe and justify a diagnostic approach to a patient with lung cancer involving the mediastinum who presents with recurrent hemoptysis after bronchial artery embolization.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient with underlying chronic obstructive pulmonary disease (COPD) and stage IV lung adenocarcinoma presented with respiratory distress likely exacerbated by the left main bronchial obstruction caused by tumor-related bleeding. Several definitions have been put forth for what “major” airway bleeding (hemoptysis) represents, as well as for quantifying the degree and amount of bleeding, but the actual practical and clinical implications of such definitions based on amounts of expectorated blood are unknown. According to one classification schema, bleeding is considered nonmassive for less than 600 mL/24 hr, massive for more than 600 mL/24 hr, exsanguinating for more than 1000 mL/24 hr or for a rate greater than 150 mL/hr, and catastrophic when it causes an immediate threat to life. Based on this system, our patient had a nonmassive hemoptysis (4 to 6 tablespoons is the equivalent of 60 to 90 mL). This is similar to what is considered a significant bronchoscopy-related bleeding in other classification schemas, defined in studies as more than 50 mL.*2 It appears that when it comes to hemoptysis, no uniform cutoff value is agreed upon in the literature to define “massive” (Table 30-1).3–10
Table 30-1 Definitions for “Massive” Hemoptysis Based on the Amount or Rate of Expectorated Blood
| Defining Terms | Amount or Rate of Expectorated blood | Reference |
|---|---|---|
| Massive hemoptysis | ≥200 mL/24 hr | 3 |
| Major hemoptysis | ≥200 mL/24 hr | 4, 5 |
| Severe hemoptysis | ≥150 mL/12 hr | 6 |
| Severe hemoptysis | >400 mL/24 hr | 7 |
| Exsanguinating hemoptysis | ≥1000 mL or ≥150 mL/hr | 8 |
| Life-threatening hemoptysis | ≥600 mL/24 hr | 9 |
| Life-threatening hemoptysis | >200 mL/hr in a patient with normal or nearly normal lung function, or >50 mL/hr in a patient with chronic respiratory failure or >2 episodes of moderate hemoptysis (>30 mL) occurring within 24 hours in spite of administration of intravenous vasopressin |
10 |
Lack of consensus regarding this cutoff volume, the known to be unreliable estimation of expectorated volume (e.g., half a cup, a few teaspoons, two tablespoons), and the indiscriminate use of descriptive criteria independent of common nomenclature, as well as other major determinants of morbidity and mortality such as rate of bleeding, the patient’s ability to maintain patent airways, and the extent and severity of cardiopulmonary comorbidities, have led investigators to propose more clinically relevant definitions for what constitutes a major airway bleed.11 These definitions rely on clinical consequences of hemoptysis such as airway obstruction and hemodynamic instability (Table 30-2).12–15 Based on this classification, our patient had massive hemoptysis, in that she required hospitalization for respiratory distress and hypoxemia. It is estimated that it takes approximately 400 mL of blood in the alveolar space to decrease gas exchange significantly, but less is probably required in someone with previously compromised lung function.16 In fact, the cause of death in patients with hemoptysis is usually asphyxiation rather than exsanguination.17 Regardless of how it is defined, massive hemoptysis assessed subjectively or objectively is a major distressing clinical problem for both the patient and the treating physician. In one survey, 86% of responding chest physicians had treated patients with massive hemoptysis during the previous year, and 28% had seen patients die from hemoptysis.18
Table 30-2 Definitions for “Massive” Hemoptysis Based on Clinical Impact
| Defining Term | Clinical Consequence | Reference |
|---|---|---|
| Massive hemoptysis | Transfusion requirement | 12 |
| Massive hemoptysis | Hospitalization | 13 |
| Massive hemoptysis | Endotracheal intubation | 14 |
| Exsanguinating hemoptysis | Aspiration and airway obstruction | 8 |
| Life-threatening hemoptysis | Hypoxemia (PaO2 <60 mm Hg) | 15 |
PaO2, Partial pressure of oxygen in arterial blood.
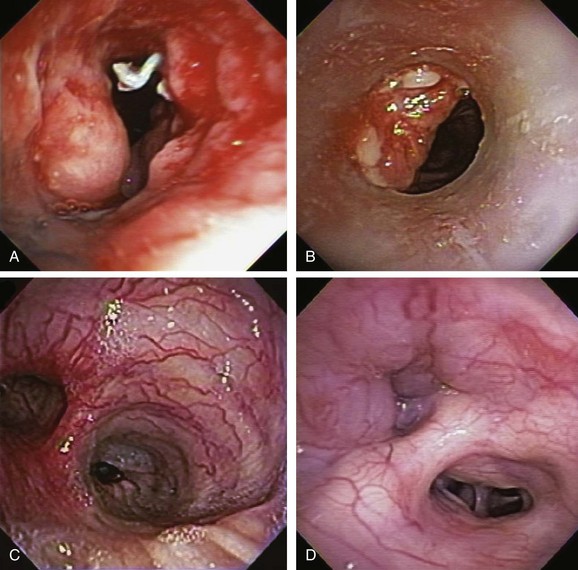
In addition to intrinsic lung disease, increased risks for hemoptysis include a variety of coagulopathic disturbances—congenital, acquired (i.e., uremia), or medication-related (e.g., clopidogrel, bevacizumab). The cause of hemoptysis also tends to be population and region specific. For instance, in Africa and China,* tuberculosis remains a common cause of massive hemoptysis, but this is uncommon in the United States19; in the United States, in fact, the most common causes for hemoptysis are chronic bronchitis, bronchiectasis, and bronchogenic carcinoma, as seen in this patient. These are followed by tuberculosis, fungal infection (e.g., aspergilloma), bacterial pneumonia and abscess, and pulmonary infarction.† The bronchoscopist might encounter hemoptysis caused by granulation tissue overgrowth and erosion of tracheobronchial mucosa from indwelling metal, hybrid, and silicone airway stents (Figure 30-2). Relevant to this patient’s clinical presentation is that hemoptysis occurs in 20% of lung cancer patients at some point during their disease course, with massive episodes developing as the terminal event in 3%.20 Reported mortality rates for massive hemoptysis range from 9% to 38% and depend on rate of bleeding* and cause; the highest mortality rate (38%) was reported in a case series that included a high proportion of patients with advanced carcinoma.†21 In patients with cancer and non–tumor-related hemoptysis (e.g., infection, bronchiectasis), median survival (33 months) was better in comparison with patients who had tumor-related bleeding (2.7 months) following endovascular management.22 These data justify the need to identify the exact cause of hemoptysis in patients in whom cancer has been diagnosed.
Therefore during the initial evaluation of a patient with hemoptysis, a careful bronchoscopic inspection may reveal findings that suggest increased risk for bleeding or findings that could explain the hemoptysis, such as hypervascularization, aberrant vessels, and submucosal arterioles (see Figure 30-2). Pathogenically, the airway inflammation/infiltration associated with carcinoma can cause hypervascularity of bronchial arteries and stimulation of collateral circulation development. Further inflammation or severe coughing causes erosion of these abnormal vascular networks and can result in hemoptysis. Furthermore, in the setting of cancer, angiogenic growth factors may be produced. These promote neovascularization and recruitment of collateral circulation from systemic blood vessels. In this regard, our patient did receive bevacizumab, which was reported to cause hemoptysis (potentially fatal) in 2.3% of patients.*23 Bronchoarterial fistula is of particular concern in patients with tumors invading mediastinal structures. In general, however, a pulmonary arterial origin is responsible for approximately 5% of cases of hemoptysis.24 Large mediastinal vessel rupture secondary to blood vessel wall invasion is very rare but may lead to fatal massive hemoptysis, especially in patients treated with brachytherapy or external beam radiation therapy.25 Multidetector chest computed tomography (MDCT)† with intravenous contrast‡ is very accurate for predicting involvement of nonbronchial systemic arteries in patients with massive bleeding.§ Tumor invasion of the pulmonary artery should not be misread as pulmonary embolism because anticoagulation in this setting could be catastrophic. MDCT is also useful for planning arterial embolization by providing information about bronchial and nonbronchial systemic arteries.24 Although some authors suggest that computed tomography (CT) can replace bronchoscopy as a first-line diagnostic approach in patients with large (<300 mL/24 hr) or massive hemoptysis (>300 mL/24 hr) because of its higher diagnostic yield,7 others find it complementary to bronchoscopy for bleeding site identification.26 MDCT is of considerable diagnostic value for diagnosing underlying disease, and used alone can localize the site of bleeding in 63% to 100% of patients. Combining bronchoscopy and CT increases this yield even further.21 Bronchoscopy is particularly useful in unstable patients with active bleeding who might require endobronchial treatment. Bronchoscopy identifies the site of bleeding 73% to 93% of the time in cases of massive hemoptysis,* but localization rates are significantly lower in cases of mild or moderate hemoptysis.19
Support System
Our patient was divorced; she had two children who were very supportive. They were quite distraught about their mother’s respiratory distress and bleeding and were wondering whether the chemotherapy was helpful for her disease. They had begun inquiries about supportive care measures. They were told that our interpretation of the published evidence was that chemotherapy improves overall survival in patients with advanced non–small cell lung carcinoma (NSCLC) and that those who are fit enough, as their mother had been before this episode (Eastern Cooperative Oncology Group [ECOG] 1), should receive chemotherapy if it is offered. A meta-analysis (2714 patients from 16 randomized controlled studies) of chemotherapy and supportive care versus supportive care alone for NSCLC showed a significant benefit for chemotherapy equivalent to a relative increase in survival of 23%, and an absolute increase in survival of 9% at 12 months. Overall, survival was increased from 20% to 29%, with an absolute increase in median survival of 1.5 months (extending survival from 4.5 months to 6 months).27
Patient Preferences and Expectations
During our conversation, our patient stated that she would undergo any treatments as long as they continued to offer her a good quality of life. She agreed to bronchoscopy with electrocautery to cease the bleeding if possible, but not to intubation and mechanical ventilation, even if respiratory failure was triggered by the bronchoscopy itself. When we asked her why, she said that deep down inside, she believed that intubation was too invasive and was probably “futile.” Sitting at her bedside, surrounded by her family and several physicians-in-training, we asked her what she meant by that. She quoted Star Trek in saying, “resistance is futile,”* and she told us that eventually, death must be accepted. She did not think intubation would prevent her from dying in the very near future, and if anything would probably cause both her and her loved ones to suffer. She insisted that she did not want her daughters to have memories of her on a breathing machine.
According to principles of autonomy and self-determination, patients or their surrogates have the right to refuse procedures regardless of the opinions of their health care providers, so long as they are considered capable of making such decisions, even if the treatment is considered to be life-sustaining. However, the opposite, that is, their right to demand a treatment—especially one that is regarded as “futile” by heath care providers—is much more controversial.28 In this regard, the Texas Advance Directives Act of 1999 provided an extrajudicial conduit for a health care facility to discontinue life-sustaining procedures against patient (or surrogate) wishes.†
Although futility has been described in both qualitative and quantitative terms, today almost all experts would agree that futility cannot be viewed solely as having a single definition but must be considered in context, based on the knowledge, biases, values, and experiences of the individuals involved, and taking into account perceived and accepted goals of care that have been identified for a particular individual. Several succinct definitions have been offered, however, to help clarify the futility issue. One proposed definition for a futile action refers to an action that cannot achieve its goals, no matter how often it is repeated.29 In this sense, however, futility does not refer to something that is impossible to do (as of this writing, systemic chemotherapy is not curative for stage IV lung cancer).
We believe that our patient’s refusal of intubation, regardless of the cause or indication, was likely a reflection of hopelessness and not necessarily a demonstration of her understanding of futility concepts, although her reference to resistance and acceptance can be quite pertinent. Hopelessness is subjective, but futility refers to the objective quality of an action.29 Futility refers to “the expectation of success that is predictably or empirically so unlikely that its exact probability is often incalculable.”29 We respected our patient’s choice, but we took the liberty of explaining to both her and her family that intubation in the setting of hemoptysis causing respiratory distress is not a futile intervention‡ because it could allow us to secure her airway, prevent further damage from ipsilateral or contralateral aspiration, stabilize her respiratory status, and potentially treat her hemoptysis using additional bronchoscopic techniques, or by providing time to perform emergent bronchial artery embolization. We emphasized that these procedures not only would stabilize her condition but could, and in our experience often did, allow extubation and discharge to home.
Procedural Strategies
Indications
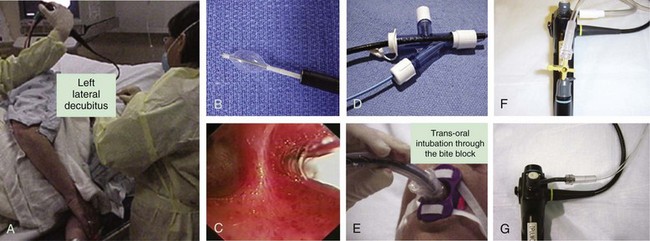
The primary goal of treating an airway bleed is to establish and maintain an open airway to avoid severe hypoxemia and asphyxiation. This is achieved by performing bronchoscopic suction, using large-bore suctioning (e.g., Yankauer suction catheter) of the oral pharynx, and placing the patient in a lateral safety position with the bleeding side down.* This allows face-to-face contact with the patient if the bronchoscopy team is working from the front or side of the patient (Figure 30-3). The lateral position makes it easier for blood and secretions to flow out from the larynx, then into and out of the corner of the mouth. It is easier to suction the mouth and hypopharynx, and the epiglottis does not collapse onto the glottic opening, as it does in the supine position. It also helps avoid excessive collapse of the larynx and prevents upper airway obstruction by the tongue or an edematous upper airway, especially in patients with obstructive sleep apnea (OSA) or in those who have been administered moderate sedation or anxiolytics (these drugs might be beneficial in patients who are fighting for breath, coughing, or becoming increasingly anxious, frightened, and combative because of ongoing hemoptysis).
The second goal of bronchoscopic intervention is to stop the bleeding. This can be attempted by tamponade of the bleeding bronchus using continuous bronchoscopic suction, but in severe, difficult to control cases, unilateral intubation or double-lumen intubation may be necessary.† If intubation is considered, the largest endotracheal tube possible should be inserted. If double-lumen intubation is considered, often times, a flexible bronchoscope with a satisfactorily sized suction channel cannot be inserted, making bronchoscopic aspiration of blood and secretions difficult. Obviously, intubation of the bleeding airway requires practice and additional expertise.
Bleeding can be slowed by using balloons or the rigid bronchoscope (when used) or cotton pledgets for tamponading the airway, or by instilling vasoconstriction agents such as epinephrine (1: 20,000)*30 and cold saline solution.†31 Lavage with normal saline at 4° C in 50 mL aliquots (average volume, 500 mL; range, 300 to 750 mL) stopped the bleeding in 23 patients with massive hemoptysis (>600 mL/24 hr).‡31 Endobronchial instillation of tranexamic acid (500 to 1000 mg) was reported to immediately (within seconds) control airway bleeding (estimated at 600 to 750 mL), which did not respond to initial conservative therapy (cold saline, epinephrine).32 To further enhance clot formation, the lateral decubitus position is probably useful through its gravity effect. Topical hemostatic tamponade using oxidized regenerated cellulose mesh, a sterile kitted fabric, has also been tried successfully in 56 of 57 (98%) patients with “life-threatening” hemoptysis.§15 If a tamponade balloon or a Fogarty catheter (these can be used in segmental airways but usually are too small to occlude a lobar bronchus), a pulmonary artery balloon catheter (these Swan-Ganz catheters also are usually too small), or an endobronchial blocker (see Figure 30-3) is inserted into a bleeding segmental, lobar, or mainstem bronchus, its position should be verified by flexible bronchoscopy and chest radiograph because these items have a tendency to easily migrate out of the target airway. These devices can remain in place for several days if necessary. As has been mentioned, when these devices are considered, the bronchoscopist and assistants should first verify that the balloon diameter will be sufficient to completely occlude the target segmental, lobar, or mainstem bronchial airway, and that the balloon catheter fits through the working channel of the bronchoscope. The Cook (Arndt) bronchial blocker, for instance, does not fit through the working channel of a flexible bronchoscope, but if necessary, it can be placed using a dedicated adapter (see Figure 30-3) by inserting the catheter alongside (![]() Video VI.30.1) or through (
Video VI.30.1) or through (![]() Video VI.30.2) a large endotracheal tube (see videos on ExpertConsult.com). In our experience, after a clot forms, it is wise to not immediately remove it, especially if bleeding has stopped. Inspection bronchoscopy, with or without clot removal, can be performed at a later time.
Video VI.30.2) a large endotracheal tube (see videos on ExpertConsult.com). In our experience, after a clot forms, it is wise to not immediately remove it, especially if bleeding has stopped. Inspection bronchoscopy, with or without clot removal, can be performed at a later time.
The third goal in managing hemoptysis is to prevent and treat respiratory, cardiac, and hemodynamic complications resulting from severe hypoxemia and hypercarbia. During flexible bronchoscopy, overuse of anxiolytics and narcotics should be avoided, if possible, when patients have significant hemoptysis, because these drugs will suppress cough, reduce minute ventilation, and thus worsen gas exchange. In addition, reduced consciousness and cough suppression may contribute to aspiration of upper airway secretions and aspiration pneumonia. Sedation or anxiolysis might warrant intubation even after bleeding is controlled. If intubation is warranted, a large single-lumen endotracheal tube usually can be inserted over the bronchoscope,* preferably through the mouth with the patient wearing a bite block. Haste makes waste, and we have seen on more than one occasion a combative or fearful patient spit out a poorly secured bite block only to bite down on the fragile flexible bronchoscope. Endotracheal tubes are not long enough to extend unilaterally into a mainstem bronchus if placed through the nares. Therefore selective unilateral bronchial intubation is possible only if the oral route is used† (see Figure 30-3). We do not advise selectively intubating the right main bronchus in a case of bleeding originating from the left lung, because this procedure usually occludes the right upper lobe bronchus, allowing ventilation to the right middle lobe and lower lobes only, and potentially further compromising gas exchange. Instead, we suggest that tracheal intubation be performed for left-sided bleeds, followed by insertion of a balloon catheter through or adjacent to the endotracheal tube, with subsequent introduction into the left main bronchus under bronchoscopic visualization.
Expected Results
Most mild to moderate mucosal bleeding can be controlled by using electrocautery via flexible or rigid bronchoscopy when lesions are visible. Several studies have showed an immediate response in controlling hemoptysis in 70% to 100% of patients.34,35 The main disadvantage of using electrocautery for managing hemoptysis is loss of effectiveness due to diffusion of the current across a large surface area and wet tissues. The procedure may be more time-consuming than with laser because it is a contact mode therapy with more superficial tissue coagulation (2 to 3 mm for electrocautery vs. 5 to 10 mm penetration depth for neodymium-doped yttrium aluminum garnet [Nd:YAG] laser). Thus more debridement and probe cleaning are required. With any electrosurgery, there is a need for smoke evacuation. This requires intermittent suctioning, which may decrease tidal volumes and worsen hypoxemia in an already frail patient undergoing flexible bronchoscopy. Airway perforation, endobronchial fires and bronchoscope damage, bronchomalacia and stenosis, and pacemaker and automated implantable cardioverter/defibrillator (AICD) dysfunction are rarely reported complications of electrosurgery.36,37 During the electrocautery application, the bronchoscopist should be aware that cough induced when the probe touches the tumor can promote additional bleeding, and that blood can easily spread throughout the tracheobronchial tree, obscuring the bleeding site and potentially leading to severe hypoxemia and asphyxiation.
Argon plasma coagulation (APC) as a noncontact electrocautery mode could be chosen instead of contact probe electrocautery because it provides easy access to lesions located laterally or around anatomic corners (Figure 30-4).38 In addition, APC allows homogeneous tissue desiccation (see video on ExpertConsult.com) (![]() Video VI.30.3) because it seeks areas with higher water content and less electrical impedance. In a retrospective study, 31 patients with hemoptysis and 25 patients with both airway obstruction and hemoptysis were treated by endobronchial APC therapy. Bleeding was quantified as severe (>200 mL/24 hr) in 6 patients, moderate (50 to 200 mL/24 hr) in 23 patients, and mild (<50 mL/24 hr) but persistent for more than a week in 27 patients. Airway hemorrhage stopped immediately after the procedure in all patients with an endoluminal tumor responsible for the bleeding.38
Video VI.30.3) because it seeks areas with higher water content and less electrical impedance. In a retrospective study, 31 patients with hemoptysis and 25 patients with both airway obstruction and hemoptysis were treated by endobronchial APC therapy. Bleeding was quantified as severe (>200 mL/24 hr) in 6 patients, moderate (50 to 200 mL/24 hr) in 23 patients, and mild (<50 mL/24 hr) but persistent for more than a week in 27 patients. Airway hemorrhage stopped immediately after the procedure in all patients with an endoluminal tumor responsible for the bleeding.38
Team Experience
A bronchoscopy team well experienced in their response to emergency situations should be available. We advise that teams practice procedures using inanimate models or high-fidelity simulation. Communication skills can be practiced, and bronchoscopists and their assistants should be familiar with various instruments and techniques. A hemoptysis protocol can be developed and placed into the policy and procedure manual of an interventional bronchoscopy unit. PowerPoint presentations can be designed for in-services and to ensure that all assistants are familiar with techniques and instruments. Crisis management is best performed when practitioners are familiar with and have practiced management procedures.* Overall, bronchoscopy in a moderately or massively bleeding patient should not be taken lightly. Patients probably should be hospitalized in the intensive care unit (sometimes, a small amount of hemoptysis is a sentinel event, eventually followed by a massive bleed), and the threshold for intubation with a large endotracheal tube should be low if it is believed that the airway needs to be protected.†
Therapeutic Alternatives
• Rigid bronchoscopy: This treatment is indicated for patients with massive hemoptysis resulting in respiratory compromise when the bleeding site is known or is highly suspected to be manageable by bronchoscopic interventions (based on imaging studies or flexible bronchoscopy). For instance, in this patient with known lung cancer and a chest radiograph showing left lower lobe (LLL) atelectasis, LLL obstruction was suspected and rigid bronchoscopy would have been a reasonable treatment alternative. The rigid bronchoscope allows good visualization of the airways, suctioning of blood clots and secretions through its large working channel and large suction catheters, the potential for effective tamponade of accessible bleeding sites using the rigid tube itself or various accessory instruments (see video on ExpertConsult.com) (![]() Video VI.30.4), and isolation of the nonaffected lung by selectively intubating that particular mainstem bronchus. Furthermore, we believe that Nd:YAG laser applications and bronchial blocking techniques using a variety of devices are more readily performed through the wider lumen of a rigid bronchoscope.
Video VI.30.4), and isolation of the nonaffected lung by selectively intubating that particular mainstem bronchus. Furthermore, we believe that Nd:YAG laser applications and bronchial blocking techniques using a variety of devices are more readily performed through the wider lumen of a rigid bronchoscope.
• Laser photocoagulation: Nd:YAG laser coagulation can be an effective treatment option for hemoptysis when the source of bleeding is bronchoscopically visible. It allows photocoagulation of the bleeding abnormal mucosa, causing vasoconstriction of vessels feeding the bleeding site. The 1034 nm wavelength is, however, highly absorbed by dark pigments and therefore is absorbed by blood. This makes laser application ineffective and may cause superficial charring. Charred tissue easily adheres to laser fibers, creating a fire hazard (see video on ExpertConsult.com) (![]() Video VI.30.5). Furthermore, current videotechnology saturates in red, making visibility problematic in the face of a large bleed. When a laser is used, a dry field is necessary. Often, two large suction catheters can be introduced through the rigid tube and placed onto and around the bleeding site. One catheter can be placed directly onto the bleeding vessel itself if visible. As blood rushes into the catheter, laser energy is applied around the bleeding site, blanching tissues and halting the bleed by vasoconstriction. Subsequently, laser energy can be applied directly onto the bleeding site, taking care to avoid overpenetration. Laser also provides an opportunity to vaporize underlying lesions or to assist with debulking after coagulation is optimized. Residual tumor and granulation bleed frequently. Often, the best treatment consists of removal of these tissues, followed by coagulation treatment of underlying mucosa.
Video VI.30.5). Furthermore, current videotechnology saturates in red, making visibility problematic in the face of a large bleed. When a laser is used, a dry field is necessary. Often, two large suction catheters can be introduced through the rigid tube and placed onto and around the bleeding site. One catheter can be placed directly onto the bleeding vessel itself if visible. As blood rushes into the catheter, laser energy is applied around the bleeding site, blanching tissues and halting the bleed by vasoconstriction. Subsequently, laser energy can be applied directly onto the bleeding site, taking care to avoid overpenetration. Laser also provides an opportunity to vaporize underlying lesions or to assist with debulking after coagulation is optimized. Residual tumor and granulation bleed frequently. Often, the best treatment consists of removal of these tissues, followed by coagulation treatment of underlying mucosa.
Hemoptysis is seldom the sole indication for laser treatment because most patients with lung malignancy present with concomitant airway obstruction. Published results of laser photocoagulation for malignancy-induced hemoptysis are variable, but in a larger series of 24 patients, immediate control of hemoptysis was achieved in 67% of cases.39
• Cryotherapy: This technique has proved effective in managing mild hemoptysis in patients with endoluminal tumors and as a temporizing measure in patients with brisk bleeding. Blood clots can also be removed. Cryotherapy can be used to treat tumors because freezing causes vasoconstriction and microthrombi in venules and capillaries, and this results in tumor necrosis,40 but probably has no role in patients with near total obstruction because the effects are delayed.20
• Brachytherapy: Although effective as a palliative treatment for mild, tumor-related hemoptysis in advanced lung cancer, this is not a treatment option for massive bleeding.19
• Airway stent insertion: Reports have described placing covered self-expanding airway stents (i.e., Polyflex, Ultraflex) to control bleeding and allow initiation of palliative radiotherapy.41 We have occasionally used airway stents for tamponade of a bleeding site in patients with massive hemoptysis (especially if stent insertion was additionally indicated to maintain airway patency) (see Figure 30-4).
• Surgical resection: This approach requires clear identification of the bleeding site and a patient able to tolerate thoracotomy,* which was not the case in our patient with stage IV NSCLC and COPD. When performed, surgery has high morbidity and mortality ranging between 20% and 30% but up to 40% in the emergency setting. Because of this, surgical resection for massive hemoptysis has been mostly replaced by less invasive measures such as bronchoscopic treatments and bronchial artery embolization (BAE).† Surgery-related morbidity and mortality during an episode of acute hemorrhage are mostly related to operative bleeding, asphyxia, bronchopleural fistula, and respiratory failure.42 In one survey from the American College of Chest Physicians, most respondents preferred bronchial artery embolization over conservative or surgical management for controlling hemoptysis.43
• Bronchial artery embolization (BAE): Immediate control of hemoptysis with BAE has been reported in 57% to 100% of patients,*19 although bleeding recurrence after BAE has been described in 10% to 29% of patients at 1 month follow-up. The most extensive experience with BAE comes from studies of patients with hemoptysis caused by bronchiectasis, aspergilloma, and pulmonary arteriovenous malformation.19 Hemoptysis related to bronchogenic carcinoma has a higher recurrence rate than that from other causes.44–46 Success rates for malignancy- and aspergillosis-related hemoptysis are lower (58% to 67%) than those for other causes of hemoptysis such as cystic fibrosis (95%). One study of 19 patients with lung cancer and hemoptysis showed that BAE was technically and clinically successful in 100% and 79%, respectively, but had a recurrence rate of 33%.47 Persistent or recurrent hemoptysis following BAE warrants consideration for bleeding of pulmonary artery origin.48 If this is confirmed, PA embolization can be performed. Early recurrence after BAE is usually the result of incomplete embolization; late recurrence probably is caused by recanalization of previously embolized vessels, revascularization, or disease progression.49 Bronchial arteries supply the bronchi and vasa vasorum of the aorta and pulmonary vessels, but also contribute to the esophagus, diaphragmatic and mediastinal visceral pleura, and spinal cord.† Inadvertent occlusion of these branches is responsible for BAE complications. The most common side effects are chest pain and dysphagia, reported in 24% to 91% and 1% to 18%, respectively. Inadvertent embolization of spinal arteries is a rare but significant complication of BAE and may be seen when spinal arteries branch off from the bronchial arteries being embolized.‡19
Cost-Effectiveness
No direct cost-effectiveness analyses have been performed for BAE and bronchoscopy for managing massive hemoptysis. Other published evidence suggests that identification of the bleeding source and isolation of the lobe or lung involved are matters of priority; rigid bronchoscopy is warranted because it is efficient at safeguarding airway patency, ensuring ventilation, and allowing good clearance of blood and debris from the airways.31 A rigid bronchoscopy procedure for airway clearance before surgery may contribute to improving surgical outcome, probably because hemodynamics are stabilized, and airway hygiene is performed because bronchoscopy helps identify the bleeding site before surgery. In another study, a multidisciplinary approach favored BAE as first-line therapy, with surgery undertaken only when BAE failed.* The decision to prefer one specific modality over another is believed to be affected by the underlying cause of the hemoptysis and by the expertise of the thoracic surgery, interventional radiology, and bronchoscopy services at the center providing care.9 In our opinion, care can be individualized, but our protocol pertaining to patients with hemoptysis includes making sure that each of these subspecialty services is informed of the patient’s admission, and that all review patient data and images and participate in the multidisciplinary decision-making process.
Techniques and Results
Anesthesia and Perioperative Care
Before bronchoscopy is performed, cardiac monitoring and supplemental oxygen (the fraction of inspired oxygen [FiO2] will need to be reduced to less than 0.4 during the actual electrocautery application) are necessary. The bronchoscopist should ensure that space in the procedure room is sufficient for staff members to move around should an emergency arise, and that medication and hemodynamic resuscitation equipment are readily available; in particular, airway resuscitation equipment should include a variety of endotracheal tubes, large-bore/Yankauer suction catheters, oral airways, and bite blocks. Because hypoxemia may occur, supplemental oxygen can be provided not only via face mask but also through the bronchoscope. The team should be well versed in the use of all necessary accessories, which ideally should be prepared before the procedure is begun (see Figure 30-3). Performing bronchoscopy in the patient with or at risk for massive hemoptysis provides the perfect case example for a strategy and planning, techniques, and results and response to complications study of team dynamics and procedure-related skills.
Anatomic Dangers and Other Risks
Inserting a flexible bronchoscope into the airway through the transnasal route causes a 17% rise in the functional residual capacity of the lungs, potentially altering gas exchange.50 In a patient with COPD, this may lead to further air trapping and hypoventilation. Therefore, operators should watch for initial signs of hypercarbia. These include agitation, tachycardia, and hypertension, all of which develop before severe acidosis occurs. Once severe respiratory acidosis has been established, the patient’s mental status is altered; bradycardia and hypotension can quickly lead to cardiorespiratory arrest. In our patient, who by the time of the procedure had become extremely fearful and anxious despite the bedside attentions of our nurses, a total of 5 mg of midazolam and 50 mcg of fentanyl was administered intravenously, in addition to 300 mg of 1% topical lidocaine onto the vocal cords and lower airways.
Results and Procedure-Related Complications
With the patient receiving oxygen via face mask, the flexible bronchoscope was advanced through the right nostril. The nasopharynx and the larynx were normal. The vocal cords were normal and symmetric. After local laryngeal anesthesia was obtained, the scope was advanced into the patient’s subglottis, then to the level of the main carina. No tracheal abnormalities were noted, except for bloody secretions, which were easily aspirated. The main carina looked sharp. Two milliliters of lidocaine was instilled into the right and left mainstem bronchi, respectively, followed by inspection of the right bronchial tree. This was performed first to ascertain normal or abnormal airways, before our attention was turned to the left bronchial tree, where we suspected airway obstruction; no mucosal abnormalities were evident on the right, but bloody secretions were present, which were suctioned without difficulty. On the left, hemorrhagic secretions were observed in the mainstem bronchus, and distally, complete obstruction by a large blood clot was noted (see Figure 30-1). Before the clot was removed bronchoscopically, the patient was placed in the left decubitus position. When the patient is turned onto this “safety position” (bleeding side down), the contralateral airway is better protected from spillage of blood into the normal lung. With the use of saline washes, the clot was gently removed. Once this had been done, it was noted that the left upper lobe was completely patent, but the lower lobe was completely closed by infiltrating tumor involving the secondary left carina. Active hemorrhage was evident at the surface of the tumor. Five milliliters of epinephrine (1:20,000) was instilled locally, the FiO2 was reduced to an estimated value of less than 0.4 (3 L via face mask), and electrocautery with a contact probe was initiated at 30 W power using 1 to 3 second bursts (see video on ExpertConsult.com) (![]() Video VI.30.6). After bleeding had been controlled (see Figure 30-1), the airways were re-inspected. No evidence suggested spillage of bloody secretions into the right bronchial tree. The scope was removed and the procedure was terminated. Our patient had no evidence of hemodynamic instability or worsened oxygen desaturation during the procedure. She was transferred to the intensive care unit (ICU) for monitoring.
Video VI.30.6). After bleeding had been controlled (see Figure 30-1), the airways were re-inspected. No evidence suggested spillage of bloody secretions into the right bronchial tree. The scope was removed and the procedure was terminated. Our patient had no evidence of hemodynamic instability or worsened oxygen desaturation during the procedure. She was transferred to the intensive care unit (ICU) for monitoring.
Long-Term Management
Outcome Assessment
The chest radiograph post procedure showed improved aeration in the upper lung zone (see Figure 30-1). We thought that in addition to her airway tumor, the bleeding might have been exacerbated by her bevacizumab treatment. We contacted the patient’s oncologist, who arranged for a follow-up clinic visit to discuss options for further chemotherapy.
Follow-up Tests and Procedures
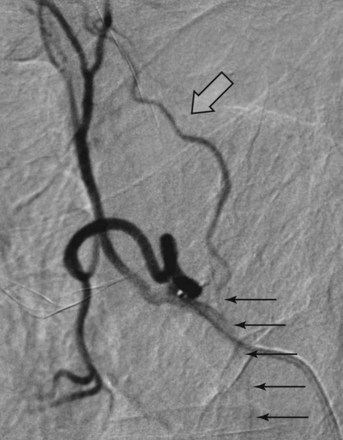
The coagulation effect from electrocautery is superficial (maximum, 2 to 3 mm). We feared that once the charred layer at the surface of the tumor was expectorated, her hemoptysis might recur. We scheduled her for a BAE after consulting with our interventional radiology department. Several hours after her bronchosocopy, she underwent selective left parabronchial artery embolization. A single left bronchial artery was identified, and a selective angiogram revealed abnormal vascularity (Figure 30-5) and a blush in the hilar region of the left lung, corresponding to the hilar mass seen on CT and to the bleeding area seen on bronchoscopy. Selective embolization was performed by slow injection of a mixture of microparticles (embosphere 300 to 500 microns) with water-soluble contrast. Intermittent fluoroscopy was performed during the injection to assess progression of the embolization until satisfactory flow stasis and obliteration of the abnormal vascularity and blush were achieved. Post embolization angiograms showed satisfactory obliteration of the abnormal vascularity (see Figure 30-5). The procedure was terminated, the catheter and the right femoral sheath were removed, and an arteriotomy closure device was placed on the right groin for hemostasis. No immediate complications were reported.
By the next day, the patient’s hemoptysis had completely resolved. Three days post embolization, a flexible bronchoscopy was performed, which revealed hyperemic infiltrated mucosa but no active bleeding. She was discharged home with a plan to undergo follow-up with her pulmonologist and oncologist, and to undergo flexible bronchoscopy on an as needed basis only. Six weeks later, however, she presented again with hemoptysis. She was again transferred to our institution, and we assured her that although immediate control of hemoptysis can be achieved in 73% to 99% of patients treated with BAE, recurrent hemoptysis on long-term follow-up is not uncommon, occurring in 10% to 55.3%. We did not tell her that higher mortality rates have been reported in patients who experienced recurrent bleeding following BAE for massive hemoptysis. In those studies, the following risk factors were identified for recurrence of hemoptysis: (1) residual mild bleeding beyond the first week after BAE; (2) blood transfusion before the procedure; and (3) diagnosis of aspergilloma as the underlying cause.51 Our patient had none of these, but on repeat flexible bronchoscopy, she showed rapid progression of her endobronchial disease with now near complete occlusion of the left main bronchus by tumor and blood clot. Indeed, late recurrence post BAE can result from recanalization of previously embolized vessels, revascularization, or disease progression.49 We proceeded with rigid bronchoscopy, tumor debulking, and Y stent placement at the left secondary carina (Figure 30-6) because we had noted some airway patency to the lower lobe after the distal left main bronchus (LMB) tumor was debulked. During the next 2 months, our patient underwent two additional flexible bronchoscopies to remove tumor overgrowth distal to the limbs of the Y stent (see Figure 30-6), but she had no additional episodes of hemoptysis. We last saw her in our emergency department as she was being admitted for excessive weakness, malnutrition, post obstructive pneumonia, and impending respiratory failure. The patient and her family wanted supportive care and had declined invasive procedures, intubation, noninvasive mechanical ventilation, and ICU admission. She thanked us for having prolonged her life and was admitted to our palliative and end-of-life care service. She passed away quietly several hours later on comfort care measures.
Quality Improvement
We discussed whether a second embolization would have benefited this patient when her hemoptysis recurred. Evidence suggests that BAE can be safely performed in such circumstances.49 However, mucosal bleeding was controlled by electrocautery during repeat flexible bronchoscopy. The cause of her death was progressive cancer-related cachexia and tumor progression with obstruction of the segmental airways distal to the stent; death was not due to hemoptysis. One could propose that the application of delayed effect bronchoscopic interventions such as photodynamic therapy (PDT) or brachytherapy might have maintained distal airway patency. Indeed, PDT was considered during our encounters with the patient, but the need to avoid sun exposure for 6 weeks was against this patient’s goals of care. Brachytherapy was not offered to this patient because of our concerns for causing massive hemoptysis in a territory (left upper lobe [LUL]) at high risk for bronchoarterial fistula formation.25
Discussion Points
1. List two alternative bronchoscopic procedures that can be applied to control this patient’s hemoptysis.
2. List three contraindications to electrocautery performed through the flexible bronchoscope.
3. Describe and justify a diagnostic approach to a patient with lung cancer involving the mediastinum who presents with recurrent hemoptysis after bronchial artery embolization.
Expert Commentary
No algorithm for approaching the patient with hemoptysis has been universally accepted. Early referral for surgery, the historical mainstay of therapy, has fallen out of favor. Published recommendations range from prompt use of bronchoscopy for diagnosis, localization, and pulmonary toilet to no bronchoscopy, with CT recommended as the primary diagnostic modality before BAE. A trend toward multidisciplinary approaches has been noted,9 and most contemporary management strategies incorporate BAE as a strategy for temporizing or definitively treating hemoptysis. New bronchoscopically mediated technologies for achieving hemostasis are promising, but as of this writing, no directly comparative literature exists to guide selection of treatment modality.
Guidelines for performance of bronchial artery embolization are not well defined, and bleeding thresholds vary in the literature. A threshold volume of 300 mL/24 hr is often referenced in the interventional radiology literature. However, some studies have used much lower thresholds, and Mal et al. have used “hemoptysis that poses a threat to life” as the trigger for proceeding to embolization in their practice.10 In general, threshold criteria for performance of interventions should be based on a risk-benefit analysis, whereby a benefit is projected, and the risk of the intervention does not exceed the risk of no intervention.
In a large study of outcomes of hemoptysis, Hirschberg et al. described mortality rates for different volumes of hemoptysis.21 This group defined “trivial” hemoptysis as drops of blood or bloody sputum, moderate as less than 500 mL/24 hr or 1 to 2 cups, and massive as greater than 500 mL/24 hr or more than 2 cups. Most patients with malignancy presented with trivial or moderate hemoptysis. A small number of therapeutic bronchoscopies and surgeries, but no BAE, were performed. In-hospital mortality rates of 2.5% and 6% were reported in the trivial and moderate groups, respectively. A higher total mortality of 21% in the malignancy group was not specifically attributable to bleeding.
Complications from BAE range from femoral artery access complications, as seen with any transarterial procedure, dissection of aorta or bronchial arteries, bronchial infarction, and nontarget embolization. The most feared complication of BAE continues to be inadvertent embolization of the arteries to the spinal cord. The anterior spinal artery may arise from common trunks with the bronchial arteries, or may have well-developed anastomotic connections with the bronchial arteries. Although the risk is considered to be very low (<1% to 2.7%), the complication of paraplegia is devastating and warrants careful consideration of alternatives, as well as thorough discussion with the patient of risks and benefits of the embolization procedure. In all patients, it is imperative to perform careful angiography with close inspection for spinal arteries, especially when interrogating a right intercostobronchial trunk, from which the anterior spinal artery may commonly arise. If any opacification of the spinal artery is observed, many authors recommend against embolization. Super-selective technique using a microcatheter positioned well distal to the origin of the bronchial artery is recommended for improved efficacy and decreased complication rates53 (Figure 30-7). Physicians considering referral of patients for BAE should be made aware of the degree of expertise within their institutions, and of overall complication rates. This information is essential if one is to carefully weigh advantages and disadvantages of the various therapeutic modalities being considered before treating the patient with hemoptysis.
The reported volume of hemoptysis of 4 to 6 tablespoons over a 24 hour period in the patient described in this scenario was below the threshold traditionally considered appropriate for performance of BAE. However, a trend toward more liberal use of BAE has been observed for lower volumes of hemoptysis as a palliative measure, with some evidence of improved survival in patients with malignancy who undergo BAE. In 2000, Witt et al. evaluated a series of 30 patients with malignancy-related pulmonary hemorrhage who underwent BAE and found improved survival of 139 days in this group compared with 62 days in a historical cohort managed conservatively for hemoptysis.54 Such findings encourage use of BAE in this group not only as a palliative measure, but as a therapeutic maneuver that may prolong survival. Little is known about tumor response to embolization beyond cessation of bleeding. However, transarterial embolization has become the standard treatment for hepatic malignancy, and some published research describes the use of pulmonary chemoembolization with evidence of improved control of disease.55
Some authors have described worse prognosis following BAE in patients with malignancy compared with cohorts with other causes of hemoptysis. However, there is a relative paucity of literature specific to the response of tumor-related bleeding to interventional techniques. A large portion of the data on the response of tumor bleeding to embolization is based on subgroup analyses in small retrospective studies. However, results with three series of patients with malignancy undergoing BAE have been published,22,47,54 and early results of embolization are similar to those of historical cohorts of patients with inflammatory and infectious causes of hemoptysis. In the series by Wang et al., cumulative hemoptysis control in patients with tumor-related bleeding was not statistically different from that seen with benign causes of hemoptysis (P = .77).22
1. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655.
2. Mitchell D. Transbronchial lung biopsy with the fiberoptic bronchoscope. Br J Dis Chest. 1981;75:258-262.
3. Knott-Craig CJ, Oostuizen G, Rossouw G, et al. Management and prognosis of massive hemoptysis: recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993;105:394-397.
4. Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987;294:301-309.
5. Brinson GM, Noone PG, Mauro MA, et al. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1951-1958.
6. de Gracia J, de la Rosa D, Catallan E, et al. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir Med. 2003;97:790-795.
7. Khalil A, Soussan M, Mangiapan G, et al. Utility of high-resolution chest CT scan in the emergency management of hemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol. 2007;80:21-25.
8. Garzon AA, Cerruti MM, Golding ME. Exsanguinating hemoptysis. J Thorac Cardiovasc Surg. 1982;84:829-833.
9. Shigemura N, Wan IY, Yu SCH. Multidisciplinary management of life-threatening massive hemoptysis: a 10-year experience. Ann Thorac Surg. 2009;87:849-853.
10. Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115:996-1001.
11. Ibrahim WH. Massive hemoptysis: the definition should be revised. Eur Respir J. 2008;32:1131-1132.
12. Flume PA, Yankaskas JR, Ebeling M, et al. Massive hemoptysis in cystic fibrosis. Chest. 2005;128:729-738.
13. Holsclaw DS, Grand RJ, Shwachman H. Massive hemoptysis in cystic fibrosis. J Pediatr. 1970;76:829-838.
14. Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med. 2003;29:317-320.
15. Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005;127:2113-2118.
16. Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28:1642-1647.
17. Crocco JA, Rooney JJ, Fankushen DS, et al. Massive hemoptysis. Arch Intern Med. 1968;121:495-498.
18. Haponik EF, Fein A, Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000;118:1431-1435.
19. Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration. 2010;80:38-58.
20. Kvale PA, Selecky PA, Prakash UB. American College of Chest Physicians. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl):368S-403S.
21. Hirshberg B, Biran I, Glazer M, et al. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112:440-444.
22. Wang GR, Ensor JE, Gupta S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol. 2009;20:722-729.
23. Cho YJ, Murgu SD, Colt HG. Bronchoscopy for bevacizumab-related hemoptysis. Lung Cancer. 2007;56:465-474.
24. Khalil A, Parrot A, Nedelcu C, et al. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT. Chest. 2008;133:212-219.
25. Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys. 2008;70:701-706.
26. Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol. 2002;179:1217-1224.
27. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 12(5), 2010. CD007309
28. Mayo TWJ. Futility: my life. In: Colt HG, Quadrelli S, Friedman L. The Picture of Health: Medical Ethics and the Movies. New York: Oxford University Press, 2011.
29. Schneiderman LJ, Jecker NS, Jonsen AR. Medical futility: its meaning and ethical implications. Ann Intern Med. 1990;112:949-954.
30. Zavala DC. Pulmonary hemorrhage in fiberoptic transbronchial biopsy. Chest. 1976;70:584-588.
31. Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax. 1980;35:901-904.
32. Wong LT, Lillquist YP, Culham G, et al. Treatment of recurrent hemoptysis in a child with cystic fibrosis by repeated bronchial artery embolizations and long-term tranexamic acid. Pediatr Pulmonol. 1996;22:275-279.
33. Mazkereth R, Paret G, Ezra D, et al. Epinephrine blood concentrations after peripheral bronchial versus endotracheal administration of epinephrine in dogs. Crit Care Med. 1992;20:1582-1587.
34. Homasson JP. Endobronchial electrocautery. Semin Respir Crit Care. 1997;18:535-543.
35. Sutedja G, van Kralingen K, Schramel FM, et al. Fiberoptic bronchoscopic electrosurgery under local anaesthesia for rapid palliation in patients with central airway malignancies: a preliminary report. Thorax. 1994;49:1243-1246.
36. Hooper RG, Jackson FN. Endobronchial electrocautery. Chest. 1988;94:595-598.
37. Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest. 2000;118:516-521.
38. Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781-787.
39. Hetzel MR, Smith SGT. Endoscopic palliation of tracheobronchial malignancies. Thorax. 1991;46:325-333.
40. Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest. 1996;110:718-723.
41. Brandes JC, Schmidt E, Yung R. Occlusive endobronchial stent placement as a novel management approach to massive hemoptysis from lung cancer. J Thorac Oncol. 2008;3:1071-1072.
42. Garzon AA, Cerruti M, Gourin A, et al. Pulmonary resection for massive hemoptysis. Surgery. 1970;67:633-638.
43. Haponik EF, Chin R. Hemoptysis: clinicians’ perspectives. Chest. 1990;97:469-475.
44. Fartoukh M, Khalil A, Louis L, et al. An integrated approach to diagnosis and management of severe haemoptysis in patients admitted to the intensive care unit: a case series from a referral centre. Respir Res. 2007;8:11-20.
45. Swanson KL, Johnson M, Prakash UBS, et al. Bronchial artery embolization, experience with 54 patients. Chest. 2002;121:789-795.
46. Hayakawa K, Tanaka F, Torizuka T. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15:154-159.
47. Park HS, Kim YI, Kim HY, et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol. 2007;30:638-643.
48. Remy J, Lemaitre L, Lafitte JJ, et al. Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. AJR Am J Roentgenol. 1984;143:963-969.
49. Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240-250.
50. Matsushima Y, Jones RL, King EG, et al. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984;86:184-188.
51. Van den Heuvel MM, Els Z, Koegelenberg CF. Risk factors for recurrence of haemoptysis following bronchial artery embolization for life-threatening haemoptysis. Int J Tuberc Lung Dis. 2007;11:909-914.
52. Rabkin JE, Astafjev VI, Gothman LN, et al. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163:361-365.
53. Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8:65-70.
54. Witt CH, Schmidt B, Geisler A, et al. Value of bronchial artery embolisation with platinum coils in tumorous pulmonary bleeding. Eur J Cancer. 2000;36:1949-1954.
55. Vogl TJ, Wetter A, Lindemayr S, et al. Treatment of unresectable lung metastases with transpulmonary chemoembolization: preliminary experience. Radiology. 2005;234:917-922.
* The Eastern Cooperative Oncology Group (ECOG) score, also called the World Health Organization (WHO) or Zubrod score, runs from 0 to 5, with 0 denoting perfect health and 5 death. 0: asymptomatic (fully active; able to carry on all predisease activities without restriction); 1: symptomatic but completely ambulatory (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature); 2: symptomatic, <50% in bed during the day (ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours); 3: symptomatic, >50% in bed, but not bedbound (capable of only limited self-care, confined to bed or chair 50% or more of waking hours); 4: bedbound (completely disabled; cannot carry on any self-care; totally confined to bed or chair); 5: death.
* Using this definition, bronchoscopy-induced hemoptysis is seen in approximately 0.7% of all flexible bronchoscopies, with a higher prevalence of 1.6% to 4.4% for transbronchial lung biopsy. Most agree that routine prebronchoscopy coagulation testing is unjustified, but certain societies such as the British Thoracic Society recommend preoperative verification in patients with known risk factors even if no biopsy is planned.
* Present in 55% of Chinese patients and in 85% of South Africans with massive hemoptysis.
† Less common causes of hemoptysis include mitral stenosis, Goodpasture’s syndrome, endobronchial foreign bodies, broncholithiasis, airway stents, bronchial adenoma, pulmonary arteriovenous (AV) malformations, Behçet’s disease, Wegener’s granulomatosis, drugs (cocaine, anticoagulants, penicillamine), cystic fibrosis, lymphangioleiomyomatosis, laceration of the pulmonary artery by a balloon-tipped catheter, and lung parasites.
* One older study reported a 71% mortality rate in patients who lost more than 600 mL of blood in 4 hours, a mortality rate of 22% in patients losing more than 600 mL within 4 to 16 hours, and of 5% in those with 600 mL of hemoptysis within 16 to 48 hours.17
† Higher mortality has been associated with aspiration of blood in the contralateral lung, massive bleeding requiring single-lung ventilation, and bronchogenic carcinoma.
* It is possible that by antagonizing vascular endothelial growth factor (VEGF), bevacizumab might decrease the renewal capacity of the endothelial cell, which in turn causes endothelial dysfunction in the supporting layers of blood vessels. Consequently, anti-VEGF therapy might present a tendency for bleeding; in lung cancer, this is seen most often with squamous cell carcinoma, for which it is considered contraindicated.
† CT might be superior to chest radiography and comparable with bronchoscopy for detecting the site of bleeding in massive hemoptysis, with correct localization in 70% to 88.5% of cases.19
‡ MDCT may also detect vascular lesions such as aneurysms, arteriovenous malformations, bronchiectases, and lung cavities as alternative explanations.
§ Signs of pulmonary artery bleeding consisted of the following: (1) pulmonary artery (PA) pseudoaneurysm, or (2) aneurysm, or (3) the presence of a pulmonary artery in the inner wall of a cavitary lesion. MDCT findings of associated bronchial arterial hypertrophy predicted the need for simultaneous bronchial artery and PA embolization.
* Although the likelihood of visualizing the site of bleeding was significantly better with early versus delayed fiberoptic bronchoscopy (FOB), the timing of the procedure did not alter therapeutic decisions or clinical outcome in nonmassive hemoptysis.
* Used as the tagline for Star Trek: First Contact (1996), the eighth feature film in the Star Trek science fiction series by Paramount films, and the first to showcase the talents of the actors from Star Trek: The Next Generation television series.
† Texas Advance Directives Act, 1999. Texas Health and Safety Code §166.046. The process is initiated when a physician believes that he is being asked to provide what in the physician’s judgment is futile care.
‡ Schneiderman et al. had defined as futile “in the last 100 cases a medical treatment has been useless.”29
* In the setting of active bleeding, the patient or the table is tilted 45 degrees toward the bleeding side.
† Use of a double-lumen tube permits adequate suctioning of blood, but its placement requires experienced personnel, and tube insertion can be difficult, especially in the actively bleeding patient. It may be wiser to first insert a large endotracheal tube, if necessary, selectively intubating the patient’s good lung while turning the patient onto the lateral decubitus position (bleeding site downward) or using a bronchial blocker.
* Local instillation of topical vasoconstrictive agents in the bleeding airway can be effective in mild to moderate hemoptysis following bronchial brushing and biopsy procedures. This approach is not useful for massive bleeding, however, because the drug is diluted and washed away. Significant cardiovascular effects can occur in the form of acute hypertension and tachyarrhythmias.33 Intravenous vasopressin (0.2 to 0.4 units/min) causes bronchial arterial vasoconstriction, which presents danger if the patient has coronary artery disease and hypertension. In a trial comparing intravenous and endobronchial administration of terlipressin during bronchoscopy-induced bleeding, a similar hemostatic effect was observed for the two routes; however, drug plasma levels were 251-fold higher and diastolic pressure was significantly increased following intravenous administration.
† Saline lavage: Immediate administration of large aliquots of iced saline using a wedged or a partially wedged bronchoscope and continuous or intermittent suction and gravity-dependent clot formation stops bleeding in most cases.
‡ One patient experienced transient sinus bradycardia during the procedure.
§ The oxidized regenerated cellulose mesh was grasped with a biopsy forceps that had already been inserted into a flexible fiberoptic bronchoscope. It was then pulled back into the bronchoscope and was introduced into the bleeding airway, ranging from lobar to subsegmental bronchi.
* A bite block should always be inserted to prevent patients from biting down on the bronchoscope (regardless of level of sedation).
† Nasal intubation will secure the airway by having the tip of the tube in the trachea.
* Many teaching tools, checklists, PowerPoint presentations, and descriptions of techniques and instruments are available on the Bronchoscopy International website at www.Bronchoscopy.org, and in various manuals of the Bronchoscopy Education Project, officially endorsed by numerous national and international bronchology and interventional pulmonology societies, including the American, European, and World Associations for Bronchology and Interventional Pulmonology (AABIP, EABIP, and WABIP) and the South American Association for Respiratory Endoscopy (ASER).
† The debate regarding intubation is ongoing. Some believe that intubation protects the airway; others believe that cough is the better protector, and that the large caliber of a normal trachea is superior to the limited diameter of an endotracheal tube when it comes to clearing blood from the bleeding airway.
* Surgical resection is not an option for patients with poor functional status, moderate to severe lung function impairment, bilateral pulmonary disease, or other comorbidities.
† Surgery also remains the strategy of choice when management is needed for massive hemoptysis caused by diffuse and complex arteriovenous malformations, iatrogenic pulmonary artery rupture, chest trauma, and mycetoma not responding to other therapeutic strategies; when other therapeutic interventions are not readily available; or when all efforts to control bleeding medically (e.g., strict bed rest, no chest percussion or spirometric testing, aggressive cough suppression, bronchoscopic intervention, BAE) are unsuccessful.
* Actual visualization of a bleeding blush during arteriography is rare, but localization is inferential from visualization of the abnormal vascularity of reactive bronchial arterial networks. Vessels greater than 2 mm in diameter are considered abnormal and usually are embolized using Gelfoam, steel coils, polyvinyl alcohol, and isobutyl-2-cyanoacrylate or gelatin cross-linked particles called tris-acryl microspheres.
† Anatomic variations of the numbers and sites of origin of the bronchial arteries have been described in healthy individuals. About 20% of bronchial arteries have an aberrant origin from other systemic nonbronchial arteries. In 5% of patients, a spinal artery originates from a bronchial artery.
‡ The incidence of this complication has significantly decreased since the “super-selective” technique, which enables distal cannulation of the target vessel, beyond the origin of spinal branches, became widely used.
* Old tuberculosis and bronchiectasis were the main underlying diseases in that series, with only 4 of 49 patients given a diagnosis of lung cancer.